Abstract
Aims
To investigate circulating levels and liver gene expression of 3 hormonal pathways associated with obesity, insulin resistance, and inflammation to identify leads towards potential diagnostic markers and therapeutic targets in patients with nonalcoholic fatty liver disease (NAFLD).
Methods
We compared circulating levels of (1) proglucagon-derived hormones (glucagon-like peptide [GLP]-1, GLP-2, glicentin, oxyntomodulin, glucagon, major proglucagon fragment [MPGF]), (2) follistatins-activins (follistatin-like [FSTL]3, activin B), (3) IGF axis (insulin-like growth factor [IGF]-1, total and intact IGF binding protein [IGFBP]-3 and IGFBP-4, and pregnancy-associated plasma protein [PAPP]-A) in 2 studies: (1) 18 individuals with early stage NAFLD versus 14 controls (study 1; early NAFLD study) and in (2) 31 individuals with biopsy proven NAFLD (15 with simple steatosis [SS] and 16 with nonalcoholic steatohepatitis [NASH]), vs 50 controls (24 lean and 26 obese) (study 2). Liver gene expression was assessed in 22 subjects (12 controls, 5 NASH, 5 NASH-related cirrhosis).
Results
Patients in early stages of NAFLD demonstrate higher fasting MPGF and lower incremental increase of glicentin during oral glucose tolerance test than controls. In more advanced stages, FSTL3 levels are higher in NASH than simple steatosis and, within NAFLD patients, in those with more severe lobular and portal inflammation. The IGF-1/intact IGFBP-3 ratio is lower in patients with liver fibrosis. Genes encoding follistatin, activin A, activin B, and the IGF-1 receptor are higher in NASH.
Conclusion
MPGF and glicentin may be involved in early stages of NAFLD, whereas FSTL3 and IGF-1/intact IGFBP3 in the progression to NASH and liver fibrosis respectively, suggesting potential as diagnostic markers or therapeutic targets.
Keywords: NAFLD, NASH, liver steatosis, biomarkers, GLP-1, follistatin
Nonalcoholic fatty liver disease (NAFLD) affects 25% to 30% of the world population and is characterized by the presence of simple steatosis (SS) that can progress to nonalcoholic steatohepatitis (NASH), liver fibrosis, cirrhosis, and hepatocellular carcinoma (1,2). Currently, there is no approved pharmacologic treatment specifically targeting NAFLD (3). Research efforts are focusing on identifying molecules participating in the pathophysiology of NAFLD, and thus having the potential to serve either as noninvasive diagnostic markers or as therapeutic targets (4,5).
We have investigated 3 hormonal systems that may be implicated in different stages of NAFLD. The first system consists of hormones that derive from the post-translational processing of proglucagon peptide and, specifically, glucagon-like peptide (GLP)-1, GLP-2, oxyntomodulin, and glicentin, secreted primarily after meal intake by the L-cells of the gut, as well as glucagon and major proglucagon fragment (MPGF) secreted by the alpha cells of the pancreas (6). Proglucagon-derived peptides are key regulators of the metabolic response to nutrient intake. In particular, GLP-1 stimulates insulin secretion, reduces appetite, and slows gastric emptying (6,7). Poor postprandial secretion or function of GLP-1 has been associated with obesity and insulin resistance (7) and treatment with GLP-1 receptor agonists (GLP1RAs) has positive effects on liver function and steatosis in patients with NAFLD (8,9). However, it still remains unknown whether patients with NAFLD have a postprandial deficit in GLP-1 or the other proglucagon-derived hormones; thus, these hormones may participate in fundamental pathophysiologic mechanisms of the disease, especially in early stages, and may be targeted therapeutically in the future.
The second hormonal system consists of follistatins and activins (ie, follistatin and follistatin like 3 [FSTL3], activin A and activin B). These hormones, produced by many tissues in health and disease, including gonads, pituitary, immune cells, endothelial cells, skin, and the liver (10,11), are regulated by energy status (eg, energy deficiency due to fasting (12–15) or energy excess in obesity (16–19)) and participate in mechanisms controlling insulin sensitivity and inflammatory procedures (20–23). Thus, activins and follistatins may be relevant for the progression from simple steatosis to NASH. We previously demonstrated that circulating activin A is increased in patients with NASH compared with healthy controls, while follistatin levels were independently associated with NASH, the changes however being related to adiposity (24). No study thus far has investigated whether circulating FSTL3 and activin B levels are different in people with NAFLD, especially in more advanced stages of the disease.
The third hormonal pathway consists of insulin-like growth factor (IGF)-1 and hormones that affect IGF-1 bioavailability. It has been proposed that IGF-1, acting in an autocrine–paracrine manner, improves hepatic inflammation and reduces liver fibrosis by inhibiting the activation of hepatic stellate cells (25). Lower IGF-1 levels were observed in NASH patients and/or those with lobular inflammation and fibrosis than their counterparts with milder or no histologic lesions in the liver (26–28). IGF-binding protein (IGFBP)-3 is the main ligand of IGF-1 in the circulation, rate-limiting IGF-1 binding and activating the IGF-1 receptor. Higher IGFBP-3 levels have been observed in patients with NASH in an untargeted proteomic analysis (29), and a low serum IGF-1 to IGFBP-3 ratio has been associated with hepatic steatosis and high serum alanine transaminase (ALT) in a retrospective study (30). Similarly to IGFBP-3, IGFBP-4 can also bind and alter IGF-1 function. Both IGFBP-3 and IGFBP-4 are susceptible to proteolysis (forming fragments), which increases IGF-1 bioavailability. The cleavage of the IGFBP-4 is performed by the protease pregnancy associated plasma protein (PAPP)-A (31). No studies thus far have investigated whether the circulating total or intact (nonproteolytic, nonfragmented) IGFBPs levels change in NAFLD, thus affecting IGF-1 bioavailability, especially in later stages of NAFLD characterized by the presence of liver fibrosis.
Given the emerging links of the above 3 hormonal systems with NAFLD, the aim of this study, was to evaluate (1) the circulating levels of these hormones in patients with early stages/mild phenotype of NAFLD versus controls (study 1; early NAFLD study), (2) their circulating levels in patients with more advanced stages of the disease (ie, NASH vs NAFLD vs controls) (study 2), and (3) whether circulating and hepatic levels of some of these hormones are associated with the histologic severity of NAFLD.
Patients and Methods
This study includes 3 separate populations: (1) study 1 (early NAFLD study), which includes NAFLD patients (nonhistologically confirmed) and controls and targeted a priori the circulating levels of hormones of the three hormonal systems; (2) study 2, which was not designed for the aim of this study, but for a similar aim (noninvasive markers of NASH and fibrosis (32,33)), thus targeting to evaluate the hormones of the 3 hormonal systems in histologically confirmed NASH patients versus SS patients versus controls; and (3) the biorepository study, which targeted to evaluate the hepatic gene expression of some of the hormones of the three hormonal systems in samples from a biorepository.
Study 1 (early NAFLD study)
Thirty-two individuals (16 males and 16 females) were recruited from the Second Propaedeutic Department of Internal Medicine, Ippokration Hospital, Aristotle University of Thessaloniki, Greece, from January till May 2017 and divided into 2 groups: (a) control group (n = 14) and (b) NAFLD group (n = 18). The control group consisted of individuals with a fatty liver index (FLI) <30 or FLI < 60 and normal liver ultrasound imaging (34). The NAFLD group consisted of individuals with a FLI ≥ 30 and ultrasound imaging indicating fatty liver. No subject demonstrated an FLI ≥ 60 and normal ultrasound imaging and no subject had a fibrosis-4 score (FIB-4) >3.25 (35). Exclusion criteria were the presence of any secondary cause of fatty liver, such as alcoholic or drug-induced hepatitis (according to medical history), and viral and autoimmune hepatitis (according to blood examination), both in the control and the NAFLD groups. All the participants underwent a 75-g oral glucose tolerance test (OGTT) after an overnight fasting and blood was collected every 30 minutes for up to 120 minutes.
Study 2
Eighty-three individuals were recruited from the Second Medical Clinic of Aristotle University of Thessaloniki and the Department of Endocrinology of 424 General Military Hospital, Thessaloniki, Greece. In this analysis, 81 of them (19 males and 62 females) were included (2 were excluded due to limited amount of serum) and were divided into 4 groups: (1) SS group (n = 15) and (2) NASH group (n = 16), classified according to the criteria of NASH Clinical Research Network (36), (3) lean controls (n = 24), and (4) obese controls (n = 26). Inclusion and exclusion criteria are described in supplementary material (37). The obese control group was selected to have similar body mass index (BMI) and waist circumference with SS and NASH patients. The lean control group consisted of individuals with lower BMI and waist circumference than the SS and NASH patients. Liver biopsy was performed under computed tomography guidance by 1 experienced radiologist and histology was interpreted by 2 independent pathologists according to the criteria by Brunt et al. (38). Morning blood (8–9 am) was collected after an overnight fasting and 1 to 2 hours prior to liver biopsy.
ClinicalTrials.gov Identifier: Study 1 has the NCT03986684 in ClinicalTrials.gov. Study 2 (observational case–control study) was performed in an earlier timepoint when registration was not obligatory. Both studies were in accordance with the declaration of Helsinki and were approved from the ethics committee of Aristotle University of Thessaloniki, Greece (IRB identifiers 2113/23-3-2016 and A13750/31-8-2010, respectively). Written informed consent was obtained from all the participants.
Biochemical and clinical measurements
GLP-1, GLP-2, glucagon, MPGF, oxyntomodulin, FSTL3, activin B, total IGF-1, total and intact IGFBP-3, total and intact IGFBP-4, and picoPAPP-A were measured with enzyme-linked immunosorbent assay (ELISA) obtained from Ansh Laboratories (Webster, TX, USA). The serum samples of the Study 1 and 2 were frozen for approximately 1.5 and 7 years, respectively, before being thawed for the measurements used in this study. Biochemical parameters were measured with automated analyzers. Details about the assays and the clinical measurements are provided in detail in supplementary material (37).
qPCR
De-identified coded “normal” (n = 12) and “NASH” (n = 5 with NASH and n = 5 with NASH-related cirrhosis) human liver specimens were obtained through the Liver Tissue Cell Distribution System (Minneapolis, Minnesota), which was funded by NIH Contract # HSN276201200017C (subjects’ information) (37). According to the Institutional Review Board of Beth Israel Deaconess Medical Center (BIDMC), this study is not considered human research (exempted). qPCR has been performed as previously presented (39) and is described in detail in supplementary material (37).
Statistical analysis
Study 1 had 86% power to detect a 20% difference between 2 groups with SD 20% in MPGF (2-tailed), at α = 0.05. Statistical analysis was performed with SPSS v25.0 (IBM Corp., Armonk, NY, USA) for Windows and with Graphpad prism 7 (GraphPad Software Inc., La Jolla, CA, USA) and is presented in detail in supplementary material (37).
Results
Study 1
Comparative data between groups and correlations of proglucagon-derived hormones with parameters of liver function and adiposity.
Comparative data between groups of study 1 are presented in supplementary material (37). Individuals in the NAFLD group had higher BMI, waist and hip circumferences, as well as glucose, insulin, HOMA-IR, and HbA1c compared with controls. They also had higher aspartate AST, ALT, GGT, and CK-18 fragment, albeit within normal range, indicating a mild NAFLD phenotype. Additionally, only 4 patients had ultrasonographic signs of severe steatosis and none of the subjects had a high (>3.25) FIB-4 index, which would have been suggestive of advanced liver fibrosis (35).
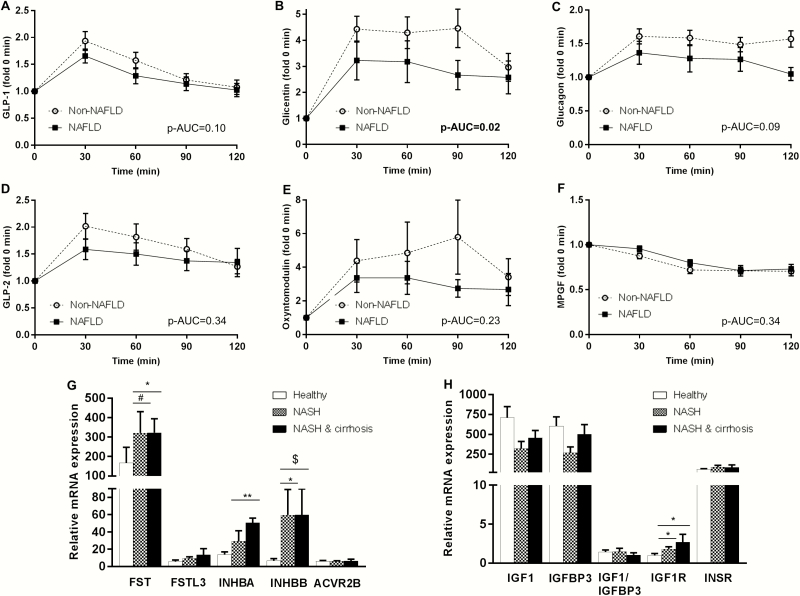
Regarding the proglucagon-derived hormones, fasting MPGF was higher in NAFLD group (Table 1). During OGTT, NAFLD patients demonstrated a lower increase in circulating glicentin than controls, indicating a worst postprandial response (Figure 1). There were no differences between groups in the other proglucagon-derived hormones, including GLP-1 or glucagon. The significance for MPGF and for the AUC of glicentin was lost after adjusting for insulin or HOMA-IR (37). Finally, we did not observe any significant differences in the circulating levels of the other 2 hormonal systems between our groups.
Table 1.
Hormonal parameters at baseline (fasting state) of study 1.
| Hormone | Non-NAFLD (n = 14) | NAFLD (n = 18) | P-value* |
|---|---|---|---|
| GLP-1 (pg/mL) | 86.8 (80.2, 142.2) | 133.2 (88.3. 175.9) | .13 |
| GLP-2 (ng/mL) | 0.93 (0.68, 0.93) | 1.14 (0.82, 1.89) | .12 |
| Glicentin (pmol/L) | 8.4 (5.8, 15.3) | 14.3 (7.8, 17.2) | .12 |
| Oxyntomodulin (pg/mL) | 270.5 ± 187.9 | 246.9 ± 179.0 | .73 |
| Glucagon (pg/mL) | 15.0 (10.8, 20.1) | 21.3 (12.1, 24.9) | .22 |
| MPGF (ng/mL) | 0.65 (0.54, 0.85) | 1.11 (0.76, 1.41) | .006 |
| FSTL3 (ng/mL) | 10.6 ± 1.7 | 12.0 ± 2.3 | .07 |
| Activin B (pg/mL) | 93.4 ± 27.4 | 111.8 ± 29.5 | .09 |
| Total IGF-1 (ng/mL) | 184.3 ± 78.9 | 172.6 ± 54.6 | .62 |
| Total IGFBP-3 (ng/mL) | 5467 ± 2260 | 4735 ± 1676 | .30 |
| Intact IGFBP-3 (ng/mL) | 1401 ± 307 | 1455 ± 234 | .58 |
| Total IGFBP-4 (ng/mL) | 165.4 ± 31.3 | 174.0 ± 29.6 | .44 |
| Intact IGFBP-4 (ng/mL) | 53.7 ± 35.6 | 44.9 ± 24.3 | .41 |
| Total IGF-1/Total IGFBP-3 | 0.037 ± 0.017 | 0.041 ± 0.016 | .54 |
| Total IGF-1/Intact IGFBP-3 | 0.139 ± 0.041 | 0.120 ± 0.037 | .18 |
| CK-18 fragment (U/L) | 84.9 ± 49.9 | 133.6 ± 69.4 | .04 |
Data are presented as mean ± SD if normally distributed and as median with (first and third quartile) if not normally distributed.
*Independent sample t-test or Mann–Whitney test between healthy versus NAFLD.
The parameters with statistically significant difference are highlighted in bold. Baseline characteristics of study 1 (ie, age, height, weight, BMI, waist circumference, hip circumference, transaminases, lipids, HOMA-IR, ultrasound result and FIB-4) are presented in supplementary material (37).
Figure 1.
Circulating concentrations of proglucagon-derived hormones after oral glucose tolerance tests (OGTT) in groups with (n = 18) and without NAFLD (n = 14); each time point shows the change in the specific parameter in fold compared with baseline (A–F). Mann–Whitney test was performed between the AUCs of NAFLD vs non-NAFLD for GLP-1, GLP-2, glicentin, oxyntomodulin (non-normally distributed), whereas independent t-test was performed for glucagon and MPGF (normally distributed). mRNA expression of genes related to the follistatins/activins and the IGF-hormonal pathway in 12 subjects with normal liver in histology (healthy), 5 subjects with NASH and 5 with NASH-related cirrhosis (G,H). p-AUC: p value for the comparisons of AUCs between NAFLD and the controls (Mann–Whitney test). *P < .05; **P < .001; #P = .060; $P = .086. ANOVA or Kruskal–Wallis test was used (depending on distribution of data) to assess gene expression, and post hoc Dunnett’s or Dunn’s test, respectively, was performed to compare the controls with NASH or with NASH-related cirrhosis (see ref. (37) for absolute values and variability of the baseline.
As shown in supplementary material (37), fasting glicentin and GLP-2 levels were positively associated with ALT and GGT, respectively. Similarly, MPGF was positively associated with AST as well as with BMI and waist circumference. In contrast, postprandial glicentin (AUC) was negatively associated with ALT, AST and BMI.
Study 2
Comparative data between groups, correlations and adjustment for confounders.
Anthropometric and biochemical data between groups of study 2 are shown in supplementary material (37) and have been previously published (40).
FSTL3 levels differ between groups and are higher in patients with NASH compared to lean or obese controls and SS patients. Activin B levels are also higher in NASH patients than in controls (lean and obese), but not compared with SS patients. Regarding the IGF-hormonal system, there were no differences between groups for total IGFBP-3, total IGFBP-4, and PAPP-A. Higher levels of IGF-1 and intact IGFBP-4 were observed in obese than in lean controls. Additionally, higher levels of intact IGFBP-3 and intact IGFBP-4 were observed in patients with NASH than in lean controls. The ratio of total IGF-1 to total IGFBP-3 was higher in obese than in lean controls. The total IGF-1 to intact IGFBP-3 ratio was significantly lower in NASH patients than in both lean and obese controls.
Hormones of study 2 having provided significant trend between groups (Table 2) were sequentially adjusted for the most important confounders (Table 2). The analysis demonstrated that only FSTL3 and total IGF-1/intact IGFBP-3 ratio remained significantly different between groups after adjustment in all models (predefined P-value corrected for multiple comparisons <.007) (Table 2).
Table 2.
Comparative hormonal data between the four groups of study 2 and P-values (ANCOVA) after comparison of the circulating levels of different hormones between the four groups of study 2 sequentially adjusted for potential confounders.
| Hormone | Lean controls | Obese controls | SS | NASH | P-value for trend* | ||||
|---|---|---|---|---|---|---|---|---|---|
| FSTL3 (ng/mL) | 10.2 ± 1.9 | 11.0 ± 2.5 | 12.8 ± 1.4a | 15.3 ± 2.7a,b,c | <.001 | ||||
| Activin B (pg/mL) | 77.2 ± 21.7 | 80.6 ± 21.3 | 87.8 ± 27.0 | 115.6 ± 42.0a,b | .002 | ||||
| Total IGF-1 (ng/mL) | 181.8 ± 47.9 | 260.0 ± 80.8a | 208.9 ± 63.7 | 206.6 ± 106.4 | .01 | ||||
| Total IGFBP-3 (ng/mL) | 6298 (4961, 8351) | 5671 (4597, 6935) | 5733 (4571, 7259) | 6066 (3926, 7666) | .56 | ||||
| Intact IGFBP-3 (ng/mL) | 849.2 ± 325.8 | 1160.0 ± 334.6 | 1099.0 ± 274.5 | 1326.7 ± 687.9a | .01 | ||||
| Total IGFBP-4 (ng/mL)# | 161.7 (144.4, 200.7) | 164.6 (141.6, 193.2) | 144.7 (132.0, 180.9) | 182.4 (144.4, 193.4) | .41 | ||||
| Intact IGFBP-4 (ng/mL)# | 12.4 (9.1, 22.8) | 22.8 (14.5, 32.5)a | 15.5 (12.6, 29.6) | 24.8 (18.2, 49.5)a | .002 | ||||
| PAPP-A (ng/mL)# | 1.00 (0.94, 1.14) | 0.89 (0.74, 0.97) | 0.85 (0.78, 1.20) | 0.92 (0.74, 1.05) | .14 | ||||
| Total IGF-1/Total IGFBP-3# | 0.026 (0.020, 0.035) | 0.048 (0.034, 0.064)a | 0.034 (0.024, 0.040) | 0.029 (0.025, 0.050) | .004 | ||||
| Total IGF-1/Intact IGFBP-3# | 0.224 (0.199, 0.272) | 0.223 (0.165, 0.312) | 0.197 (0.146, 0.230) | 0.115 (0.110, 0.154)a,b | <.001 | ||||
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | Model 8 | Model 9 | |
| Adjusting factors | none (unadjusted) | BMI | BMI, age | BMI, age, gender | WC, age, gender | Glucose, age, gender | Insulin, age, gender | HOMA-IR, age, gender | HOMA-IR, age, gender, BMI |
| Hormones | |||||||||
| FSTL3 (ng/mL) | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <0.001 | 0.001 |
| Activin B (pg/mL) | .002 | .02 | .02 | .02 | .04 | .002 | .05 | 0.04 | 0.05 |
| Total IGF-1 (ng/mL) | .01 | .02 | .05 | .05 | .06 | .04 | .04 | 0.05 | 0.06 |
| Intact IGFBP-3 (ng/mL) | .01 | .39 | .18 | .12 | .20 | .004 | .05 | 0.09 | 0.36 |
| Intact IGFBP-4 (ng/mL) | .001 | .01 | .01 | .01 | .03 | .001 | <.001 | <0.001 | 0.009 |
| Total IGF-1/Total IGFBP-3 | .006 | .004 | .006 | .01 | .02 | .02 | .008 | 0.009 | 0.02 |
| Total IGF-1/Intact IGFBP-3 | <.001 | .003 | .003 | .003 | .004 | <.001 | .002 | 0.002 | 0.007 |
Data are presented as mean ± SD if normally distributed, and as median with (first and third quartile) if not normally distributed.
*One-way ANOVA or Kruskal-Wallis test, followed by multiple pairwise comparisons with Tukey’s or Dunn’s correction, respectively, if needed; superscript letters indicate statistical significance in the adjusted pairwise comparisons.
a P < 0.05 compared to lean control group.
b P < 0.05 compared to obese control group.
c P < 0.05 compared to SS group.
All parameters, except for FSTL3, activin B, total IGF-1, intact IGFBP-3 and gender, were not normally distributed and were therefore logarithmically transformed.
#Parameters that are not normally distributed.
The parameters provided statistically significant difference were highlighted in bold.
Similarly, more robust correlations with parameters of adiposity (BMI, waist circumference) or liver function tests (AST, ALT, or GGT) were observed for FSTL3 and for the IGF-1/intact IGFBP-3 ratio (for the whole population and separately for controls and NAFLD (37)).
Comparative data of histologic lesions within patients with NAFLD and predictive value of FSTL3 and the ratio of IGF-1 to intact IGFBP3 levels in NAFLD.
Since FSTL3 and total IGF-1/intact IGFBP-3 ratio were the 2 parameters that differed between groups independently of potential confounders, we further investigated whether they are associated with histologic lesions in patients with NAFLD. Circulating FSTL3 levels were higher in patients with higher steatosis grade, and lobular or portal inflammation (Table 3). Total IGF-1/intact IGFBP-3 ratio was lower in patients with fibrosis (Table 3).
Table 3.
Circulating levels of FSTL3 and of total IGF-1/intact IGFBP-3 ratio in histologic lesions within NAFLD subjects (n = 31).
| Histologic lesion | N | FSTL3 (ng/ml) | Total IGF-1 to Intact IGFBP-3 ratio | |
|---|---|---|---|---|
| Steatosis grade | ≤ 33% | 20 | 13.3 ± 1.8 | 0.160 (0.139, 0.229) |
| >33% | 11 | 15.6 ± 2.8 | 0.136 (0.110, 0.277) | |
| P-value* | .02 | .22# | ||
| Lobular inflammation | Absent | 19 | 13.1 ± 1.4 | 0.177 ± 0.068 |
| Present | 12 | 15.9 ± 2.9 | 0.150 ± 0.064 | |
| P-value* | .003 | .34 | ||
| Portal inflammation | None to minimal | 18 | 13.0 ± 1.6 | 0.183 ± 0.068 |
| Greater than minimal | 13 | 15.7 ± 2.7 | 0.143 ± 0.060 | |
| P-value* | .005 | .15 | ||
| Ballooning | Absent | 6 | 13.0 ± 1.4 | 0.219 ± 0.075 |
| Present | 25 | 14.5 ± 2.6 | 0.156 ± 0.061 | |
| P-value* | .23 | .08 | ||
| Fibrosis | Absent | 10 | 13.4 ± 1.9 | 0.205 ± 0.074 |
| Present | 21 | 14.6 ± 2.7 | 0.147 ± 0.055 | |
| P-value* | .25 | .04 |
Data are presented as mean ± SD if normally distributed, and as median with (first and third quartile) if not normally distributed.
#P-value referring to the comparison of parameters that are not normally distributed.
*Independent sample t-test or Mann–Whitney test.
Subsequently, a binary logistic regression for the presence of SS or NASH, as well as for the histologic lesions was performed. FSTL3 was significantly associated with the presence of NASH, lobular and portal inflammation, and higher steatosis grade. These associations were maintained after sequentially entering BMI, age, and gender into the model (Table 4). The ratio of IGF-1 to intact IGFBP3 did not remain robustly associated with NASH or liver fibrosis (P = .06 unadjusted and .08 after adjusting for BMI and age). In an exploratory analysis, FSTL3 showed the highest sensitivity and specificity at differentiating NAFLD from healthy status and secondarily NAFL from NASH (see (37)).
Table 4.
Binary logistic regression analysis investigating FSTL3 and IGF-1 to intact IGFBP3 as independent associates of NASH and histologic lesions within NAFLD patients (n = 31).
| Logistic regression Model variables | Model 1 (unadjusted) | Model 2 (BMI) | Model 3 (BMI, age) | Model 4 (BMI, age, gender) | Model 5 (HOMA-IR) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hormones | FSTL3 | IGF-1 to iIGFBP3 | FSTL3 | IGF-1 to iIGFBP3 | FSTL3 | IGF-1 to iIGFBP3 | FSTL3 | IGF-1 to iIGFBP3 | FSTL3 | IGF-1 to iIGFBP3 | |
| SS vs NASH | Exp(B) | 4.89 | 0.36 | 4.97 | 0.39 | 7.78 | 0.40 | 9.00 | 0.42 | 5.82 | 0.44 |
| 95% CI | 1.2–20.1 | 0.1–1.0 | 1.2–21.1 | 0.1–1.1 | 1.3–44.7 | 0.1–1.1 | 1.3–61.3 | 0.2–1.2 | 0.9–35.9 | 0.2–1.2 | |
| P-value* | .03 | .06 | .03 | .08 | .02 | .08 | .03 | 0.10 | 0.06 | 0.12 | |
| Steatosis | Exp(B) | 3.09 | 0.66 | 3.07 | 0.52 | 3.26 | 0.51 | 3.36 | 0.62 | 2.42 | 0.90 |
| (≤33% or >33%) | 95% CI | 1.1–8.8 | 0.3–1.7 | 1.1–8.9 | 0.2–1.4 | 1.0–10.7 | 0.2–1.4 | 1.0–11.6 | 0.2–1.8 | 0.8–7.6 | 0.4–2.3 |
| P-value* | .04 | .38 | .04 | .21 | .05 | .20 | .06 | 0.39 | 0.13 | 0.82 | |
| Lobular inflammation | Exp(B) | 4.58 | 0.64 | 4.52 | 0.74 | 11.4 | 0.75 | 12.1 | 0.88 | 4.11 | 0.82 |
| (absent vs present) | 95% CI | 1.3–16.1 | 0.3–1.6 | 1.3–16.1 | 0.3–1.9 | 1.7–77.3 | 0.3–1.9 | 1.6–91.0 | 0.3–2.4 | 0.9–18.7 | 0.3–2.1 |
| P-value* | .02 | .33 | .02 | .53 | .01 | .53 | .02 | 0.88 | 0.07 | 0.67 | |
| Portal inflammation | Exp(B) | 4.30 | 0.48 | 4.72 | 0.55 | 8.77 | 0.53 | 9.31 | 0.54 | 3.90 | 0.63 |
| (none to minimal vs | 95% CI | 1.2–14.9 | 0.2–1.3 | 1.3–17.5 | 0.2–1.6 | 1.5–51.1 | 0.2–1.6 | 1.5–59.5 | 0.2–1.6 | 1.1–14.1 | 0.2–1.7 |
| greater than minimal) | P-value* | .02 | .15 | .02 | .26 | .02 | .26 | .02 | 0.28 | 0.04 | 0.35 |
| Ballooning | Exp(B) | 2.36 | 0.38 | 2.34 | 0.40 | 2.65 | 0.39 | 2.64 | 0.35 | 2.36 | 0.39 |
| (absent vs present) | 95% CI | 0.6–9.9 | 0.1–1.2 | 0.6–9.7 | 0.1–1.3 | 0.6–11.9 | 0.1–1.3 | 0.6–11.9 | 0.1–1.3 | 0.4–12.9 | 0.1–1.3 |
| P-value* | .24 | .11 | .24 | .13 | .21 | .13 | .21 | 0.12 | 0.32 | 0.14 | |
| Fibrosis | Exp(B) | 1.78 | 0.37 | 1.78 | 0.39 | 1.93 | 0.39 | 2.00 | 0.42 | 1.44 | 0.42 |
| (absent vs present) | 95% CI | 0.7–4.8 | 0.1–1.0 | 0.7–4.8 | 0.1–1.1 | 0.7–5.6 | 0.1–1.1 | 0.6–6.3 | 0.1–1.3 | 0.4–4.9 | 0.1–1.3 |
| P-value* | .25 | .06 | .25 | .08 | .23 | .08 | .23 | 0.12 | 0.56 | 0.12 |
Age and HOMA IR values were not normally distributed and were therefore logarithmically transformed before entering into the analysis.
*Exp(B): corresponds to one SD increase. P-values refer to FSTL3 or IGF-1 to iIGFBP3 (intact IGFBP3).
Gene expression profile in NASH with or without cirrhosis
Subjects with NASH without cirrhosis demonstrated higher INHBB gene (encoding the INHβB protein, which is dimerized to activin B), IGF-1R (encoding the IGF-1 receptor) and a trend to higher FST expression than controls (Fig. 1G and 1H). Despite a trend towards higher INHBA in NASH patients without cirrhosis versus controls, this difference was not statistically significant in adjusted pairwise comparisons. Subjects with NASH with cirrhosis had higher FST, INHBA (encoding the INHβA protein, which is dimerized to activin A), IGF1R and a trend to higher INHBB expression. FSTL3 and ACVR2B (encoding the ACVR2B protein, which is the main receptor of activins) expression was generally low in the liver, whereas INSR expression was high but no differences were observed between the three groups in all three genes.
Discussion
Our study demonstrates higher fasting MPGF levels and lower glicentin levels during OGTT in early stages of NAFLD, which probably reflects the deterioration of insulin sensitivity observed in these patients. Second, in patients with more advanced stages of the disease, FSTL3 levels are higher than controls independently of adiposity or insulin resistance. Most importantly FSTL3 levels are higher in NASH than in SS, as well as in higher steatosis grade, and lobular and portal inflammation, thus FSTL3 may possibly serve as a surrogate marker in the noninvasive diagnosis of NASH and hepatic inflammation. Third, the ratio of total IGF-1 to intact IGFBP3 is lower in NASH patients with fibrosis, indicating possible decrease of IGF-1 bioavailability when the disease advances to NASH and liver fibrosis. Altogether, molecules of these 3 hormonal systems may be implicated in different stages of the disease, warranting their evaluation as diagnostic markers or therapeutic targets in larger cohorts and in future mechanistic studies.
Proglucagon-derived hormonal pathway
The first study consisted of subjects with early stages of NAFLD, indicated by the normal liver enzymes, the intermediate to moderate ultrasound findings and the lack of patients with advanced fibrosis according to FIB-4 score. We observed changes in proglucagon-derived hormones, but not in the other 2 hormonal systems. A 10% lower AUC for GLP-1 has been previously reported in individuals with NAFLD than in controls after OGTT (41), which was also observed in our study without reaching statistical significance though. In contrast, we observed a 30% lower AUC for glicentin in NAFLD patients compared to controls after OGTT and approximately 70% higher fasting MPGF levels. Interestingly, both the AUC of glicentin during OGTT and the fasting MPGF levels were associated (the AUC negatively and the MPGF positively) with parameters of liver function and adiposity.
Glicentin consists of the first 69 amino acids of the proglucagon peptide (6) and its effects in metabolism have not been adequately investigated to date. It has been supported that glicentin stimulates insulin secretion, inhibits gastric acid secretion, and affects gut mobility and growth (42). A specific receptor for glicentin has not been identified, thus it is unclear whether it acts through the GLP-1 receptor or other receptors. MPGF consists of the 72 to 158 amino acids of the proglucagon peptide (ie, the whole sequence of GLP-1 and GLP-2) and is secreted primarily from the pancreas (6). To date, MPGF has been considered a byproduct of the proglucagon peptide with unknown functional relevance or an intermediate step for the final synthesis of GLP-1 and GLP-2.
Given the high interest for developing combination treatments with gut and pancreatic hormones against metabolic diseases, including diabetes and NAFLD, our results emphasize the need for the investigation of the effects of other hormones beyond GLP-1 and glucagon, such as glicentin and MPGF, especially in early stages of NAFLD during which excess fat deposition and progressive reduction of insulin sensitivity still play the dominant role.
FSTL3/Activin B hormonal pathway
In the second study, subjects with more advanced NAFLD were studied. In these subjects, we focused on follistatins–activins and IGFs, since both hormonal systems seem to be involved in inflammatory processes. FSTL3 demonstrates significant structural and functional homology with follistatin and antagonizes the actions of activin A and activin B. Rodent knockout of FSTL3 have profound liver steatosis, despite the higher whole body and hepatic insulin sensitivity, the improved glucose profile and the normal serum triglyceride levels and body weight (18,43,44). The liver steatosis was attributed to changes promoting lipogenesis and gluconeogenesis and were partially resulting from increased activin bioavailability (18,43,44). In contrast, overexpression of FSTL3 in skeletal muscle reduced liver fat content in diet-induced obese mice (16).
Our study is the first to show that FSTL3 is increased in humans with NAFLD and its levels are higher with higher severity of the disease (from controls to SS and to NASH). FSTL3 gene expression was low in the liver of both healthy and NASH patients; thus the higher circulating FSTL3 levels in NAFLD may probably not derive from increased FSTL3 secretion by the liver, but possibly from other organs, in which FSTL3 is commonly expressed, such as the adipose tissue, muscle, and gonads (45). Alternatively, the higher circulating levels of FSTL3 may result from reduced clearance of the hormone. Taking into consideration findings from preclinical studies, the higher FSTL3 in humans with NAFLD may also serve as a compensatory mechanism aiming to prevent disease progression; however, this speculation needs validation from further mechanistic studies. Interestingly, in our study, circulating FSTL3 levels were also independently associated with hepatic inflammation, both lobular and portal, but not ballooning. The role of FSTL3 in inflammation has only partially been investigated so far. A previous study has shown that plasma FSTL3 correlates positively with plasma tumor necrosis factor (TNF)-α and interleukin (IL)-6 levels, whereas infusion of lipopolysaccharide and TNF-α stimulated circulating FSTL3 (17). Additionally, FSTL3 concentrations are elevated in serum and synovial fluid of patients with osteoarthritis and are correlated with the severity of the disease (46). Given also the emerging role of the, highly homologous to FSTL3, FSTL1 in inflammatory conditions, future research should probably focus on more precise investigation of the role of FSTL3 in inflammation, both hepatic and systematic. Finally, the differences we observe in circulating FSTL3 in the different stages of NAFLD remain robust after adjusting for potential confounding factors. This supports the notion that FSTL3 could serve as a surrogate marker in the diagnosis of advanced stages of NAFLD, namely NASH and hepatic inflammation; these findings need validation from independent longitudinal cohort studies. In contrast to FSTL3, the changes we observed in activin B were primarily related to adiposity, similarly to our previous findings for activin A, thus only marginally not being able to differentiate SS from NASH; interestingly though, the genes encoding activins (INHBA, INHBB) as well as follistatin (FST) are not only highly expressed in the liver, but they are further upregulated in patients with NASH. Thus, future studies with larger sample size (ie, higher power) are required to clarify whether, in addition to FSTL3, activin A, activin B, and follistatin may have a diagnostic or even therapeutic potential for NAFLD, as well as to further elucidate their role in the pathogenesis and progression of the disease.
IGF hormonal pathway
Previous studies have reported a reduction of IGF-1 in NAFLD and the different stages of it, ranging from 8% to 22% (26–28). We did not observe significant differences, except for the ratio of total IGF-1/intact IGFBP-3, which was reduced in patients with NASH compared with lean or obese controls. IGFBP-3 is the main binding protein of IGF-1. The IGF-1 to IGFBP-3 ratio has been previously suggested as a marker of IGF-1 bioavailability (47). Direct measurements of free IGF-1 need be considered given that IGF-1 binds to other IGFBPs, as well as that IGFBP-3 also binds IGF-2 (48). Here, we were able to quantify both total (intact and fragmented) as well as intact IGFBP-3 levels, but only the total IGF-1/intact IGFBP-3 was reduced in patients with NASH compared with healthy individuals, indicating reduced IGF-1 bioavailability. The differences between groups were maintained after adjusting for potential confounders (Table 3). Interestingly, we also observe an upregulation of IGF-1 receptor expression in the liver, which may be a compensatory mechanism aiming to maintain the beneficial anti-inflammatory and anti-fibrotic effects of IGF-1 in the liver despite its reduced bioavailability. In line with the above findings, the total IGF-1/intact IGFBP-3 was lower in patients with fibrosis, indicating that this hormonal system should be investigated in more advanced stages of the disease, NASH with advanced fibrosis, and possibly NASH-related cirrhosis.
Strengths and limitations
The strength of this study is its originality, since most of the measured parameters had not been previously investigated in NAFLD. Both study 1 and 2 are limited by their sample size, which, albeit relatively small, was sufficient to show statistically significant differences in the hormones of interest. Additionally, an exploratory analysis for the evaluation of sensitivity and specificity of FSTL3 and total IGF1/intact IGFBP3 at differentiating between healthy versus NAFLD, NAFL versus NASH, and the presence of liver fibrosis or not was performed. Although some of these findings (especially for FSTL3) are promising, they should be further confirmed in focused studies of diagnostic accuracy with larger sample sizes and compared with established composite biomarkers. Furthermore, the observational nature of the findings does not allow conclusions regarding causality, including whether the observed hormone differences are primarily related to alterations in the secretion sites of the hormones or their clearance. Another limitation was the lack of performance of liver biopsies in study 1 and in the controls of study 2, the latter due to obvious ethical considerations. Measurements were performed by personnel blinded to the study hypotheses and laboratory errors, if any, would have been random, which may lead to suppression of effect estimates, but could never account for falsely significant results.
Conclusions
We identify glicentin and MPGF as the 2 molecules most closely associated with early stages of NAFLD among the proglucagon-derived hormones. We identify FSTL3 as a potential biomarker for diagnosing NAFLD and most importantly for differentiating between SS and NASH as well as for identifying NAFLD patients with more severe steatosis, and portal and lobular inflammation. Finally, we identify the ratio of total IGF-1 to intact IGFBP3 as potential marker of liver fibrosis in patients with NAFLD. Future larger, ideally prospective cohort studies or clinical trials are needed to confirm the role of glicentin, MPGF, FSTL3, follistatin, activin A, activin B, IGF-1, intact IGFBP3, in the pathophysiology of NAFLD, their diagnostic utility including their ability to improve the sensitivity and specificity of already existing diagnostic scores as well as their therapeutic potential in NAFLD patients.
Acknowledgments
Financial Support: CSM was funded by National Institutes of Health K24DK081913. NP was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) –389891681 (PE 2431/2-1).
Clinical Trials Information: Study 1 has the NCT03986684 in ClinicalTrials.gov. Study 2 (observational case-control study) was performed in an earlier time-point when registration was not obligatory. Both studies were in accordance with the declaration of Helsinki and were approved from the ethics committee of Aristotle University of Thessaloniki, Greece (IRB identifiers 2113/23-3-2016 and A13750/31-8-2010, respectively). Written informed consent was obtained from all the participants.
Glossary
Abbreviations
- ALT
alanine transaminase
- FIB-4
fibrosis-4 score
- FLI
fatty liver index
- FSTL3
follistatin like 3
- GLP
glucagon-like peptide
- GLP1RA
GLP-1 receptor agonists
- MPGF
major proglucagon fragment
- IGF
insulin-like growth factor
- IGFBP
IGF-binding protein
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- OGTT
oral glucose tolerance test
- PAPP
pregnancy associated plasma protein
- SS
simple steatosis
Additional Information
Disclosure Summary: C.S.M. is advisor to Ansh Labs LLC, consultant to Intarcia, grant recipient through BIDMC, and consultant to Novo Nordisk. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Polyzos SA, Mantzoros CS. Nonalcoholic fatty future disease. Metabolism. 2016;65(8):1007–1016. [DOI] [PubMed] [Google Scholar]
- 2. Younossi Z, Tacke F, Arrese M, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69(6):2672–2682. [DOI] [PubMed] [Google Scholar]
- 3. Polyzos SA, Mantzoros CS. Adiponectin as a target for the treatment of nonalcoholic steatohepatitis with thiazolidinediones: a systematic review. Metabolism. 2016;65(9):1297–1306. [DOI] [PubMed] [Google Scholar]
- 4. Stefan N, Häring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019;7(4):313–324. [DOI] [PubMed] [Google Scholar]
- 5. Boutari C, Perakakis N, Mantzoros CS. Association of adipokines with development and progression of nonalcoholic fatty liver disease. Endocrinol Metab (Seoul). 2018;33(1):33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drucker DJ, Habener JF, Holst JJ. Discovery, characterization, and clinical development of the glucagon-like peptides. J Clin Invest. 2017;127(12):4217–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holst JJ, Madsbad S. Mechanisms of surgical control of type 2 diabetes: GLP-1 is key factor. Surg Obes Relat Dis. 2016;12(6):1236–1242. [DOI] [PubMed] [Google Scholar]
- 8. Feng W, Gao C, Bi Y, et al. Randomized trial comparing the effects of gliclazide, liraglutide, and metformin on diabetes with non-alcoholic fatty liver disease. J Diabetes. 2017;9(8):800–809. [DOI] [PubMed] [Google Scholar]
- 9. Petit JM, Cercueil JP, Loffroy R, et al. Effect of liraglutide therapy on liver fat content in patients with inadequately controlled type 2 diabetes: the Lira-NAFLD Study. J Clin Endocrinol Metab. 2017;102(2):407–415. [DOI] [PubMed] [Google Scholar]
- 10. Hansen JS, Rutti S, Arous C, et al. Circulating follistatin is liver-derived and regulated by the glucagon-to-insulin ratio. J Clin Endocrinol Metab. 2016;101(2):550–560. [DOI] [PubMed] [Google Scholar]
- 11. Hedger MP, de Kretser DM. The activins and their binding protein, follistatin—diagnostic and therapeutic targets in inflammatory disease and fibrosis. Cytokine Growth Factor Rev. 2013;24(3):285–295. [DOI] [PubMed] [Google Scholar]
- 12. Anastasilakis AD, Polyzos SA, Skouvaklidou EC, et al. Circulating follistatin displays a day-night rhythm and is associated with muscle mass and circulating leptin levels in healthy, young humans. Metabolism. 2016;65(10):1459–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moragianni VA, Aronis KN, Chamberland JP, Mantzoros CS. Short-term energy deprivation alters activin a and follistatin but not inhibin B levels of lean healthy women in a leptin-independent manner. J Clin Endocrinol Metab. 2011;96(12): 3750–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perakakis N, Upadhyay J, Ghaly W, et al. Regulation of the activins-follistatins-inhibins axis by energy status: Impact on reproductive function. Metabolism: clinical and experimental 2018;85:240–249. [DOI] [PMC free article] [PubMed]
- 15. Vamvini MT, Aronis KN, Chamberland JP, Mantzoros CS. Energy deprivation alters in a leptin- and cortisol-independent manner circulating levels of activin A and follistatin but not myostatin in healthy males. J Clin Endocrinol Metab. 2011;96(11):3416–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brandt C, Hansen RH, Hansen JB, et al. Over-expression of Follistatin-like 3 attenuates fat accumulation and improves insulin sensitivity in mice. Metabolism. 2015;64(2):283–295. [DOI] [PubMed] [Google Scholar]
- 17. Brandt C, Pedersen M, Rinnov A, et al. Obesity and low-grade inflammation increase plasma follistatin-like 3 in humans. Mediators Inflamm. 2014;2014:364209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown ML, Bonomi L, Ungerleider N, et al. Follistatin and follistatin like-3 differentially regulate adiposity and glucose homeostasis. Obesity (Silver Spring). 2011;19(10):1940–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perakakis N, Mougios V, Fatouros I, et al. Physiology of activins/follistatins: associations with metabolic and anthropometric variables and response to exercise. J Clin Endocrinol Metab. 2018;103(10):3890–3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hansen J, Rinnov A, Krogh-Madsen R, et al. Plasma follistatin is elevated in patients with type 2 diabetes: relationship to hyperglycemia, hyperinsulinemia, and systemic low-grade inflammation. Diabetes Metab Res Rev. 2013;29(6):463–472. [DOI] [PubMed] [Google Scholar]
- 21. de Kretser DM, O’Hehir RE, Hardy CL, Hedger MP. The roles of activin A and its binding protein, follistatin, in inflammation and tissue repair. Mol Cell Endocrinol. 2012;359(1-2):101–106. [DOI] [PubMed] [Google Scholar]
- 22. Perakakis N, Kokkinos A, Peradze N, et al. Follistatins in glucose regulation in healthy and obese individuals. Diabetes Obes Metab. 2019;21(3):683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tao R, Wang C, Stöhr O, et al. Inactivating hepatic follistatin alleviates hyperglycemia. Nat Med. 2018;24(7):1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Polyzos SA, Kountouras J, Anastasilakis AD, Triantafyllou GΑ, Mantzoros CS. Activin A and follistatin in patients with nonalcoholic fatty liver disease. Metabolism. 2016;65(10):1550–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takahashi Y. The role of growth hormone and insulin-like growth factor-I in the liver. Int J Mol Sci 2017;18:E1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dichtel LE, Corey KE, Misdraji J, et al. The association between IGF-1 levels and the histologic severity of nonalcoholic fatty liver disease. Clin Transl Gastroenterol. 2017;8(1):e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. García-Galiano D, Sánchez-Garrido MA, Espejo I, et al. IL-6 and IGF-1 are independent prognostic factors of liver steatosis and non-alcoholic steatohepatitis in morbidly obese patients. Obes Surg. 2007;17(4):493–503. [DOI] [PubMed] [Google Scholar]
- 28. Sumida Y, Yonei Y, Tanaka S, et al. Lower levels of insulin-like growth factor-1 standard deviation score are associated with histological severity of non-alcoholic fatty liver disease. Hepatol Res. 2015;45(7):771–781. [DOI] [PubMed] [Google Scholar]
- 29. Miller MH, Walsh SV, Atrih A, Huang JT, Ferguson MA, Dillon JF. Serum proteome of nonalcoholic fatty liver disease: a multimodal approach to discovery of biomarkers of nonalcoholic steatohepatitis. J Gastroenterol Hepatol. 2014;29(10):1839–1847. [DOI] [PubMed] [Google Scholar]
- 30. Völzke H, Nauck M, Rettig R, et al. Association between hepatic steatosis and serum IGF1 and IGFBP-3 levels in a population-based sample. Eur J Endocrinol. 2009;161(5):705–713. [DOI] [PubMed] [Google Scholar]
- 31. Hjortebjerg R. IGFBP-4 and PAPP-A in normal physiology and disease. Growth Horm IGF Res. 2018;41:7–22. [DOI] [PubMed] [Google Scholar]
- 32. Polyzos SA, Kountouras J, Papatheodorou A, et al. Adipocytokines and cytokeratin-18 in patients with nonalcoholic fatty liver disease: Introduction of CHA index. Ann Hepatol. 2013;12(5):749–757. [PubMed] [Google Scholar]
- 33. Polyzos SA, Kountouras J, Slavakis A, et al. A novel noninvasive index for nonalcoholic steatohepatitis: a pilot study. Biomarkers: biochemical indicators of exposure, response, and susceptibility to chemicals 2013; 18:607–613. [DOI] [PubMed] [Google Scholar]
- 34. Bedogni G, Bellentani S, Miglioli L, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sterling RK, Lissen E, Clumeck N, et al. ; APRICOT Clinical Investigators Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–1325. [DOI] [PubMed] [Google Scholar]
- 36. Kleiner DE, Brunt EM, Van Natta M, et al. ; Nonalcoholic Steatohepatitis Clinical Research Network Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. [DOI] [PubMed] [Google Scholar]
- 37. Perakakis N. Supplemental material related to the article entitled “Targeted analysis of three hormonal systems identifies molecules associated with the presence and severity of NAFLD.” figshare. Journal contribution. 2019. https://doi.org/10.6084/m9.figshare.9120437 [DOI] [PMC free article] [PubMed]
- 38. Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94(9):2467–2474. [DOI] [PubMed] [Google Scholar]
- 39. Tuccinardi D, Farr OM, Upadhyay J, et al. Lorcaserin treatment decreases body weight and reduces cardiometabolic risk factors in obese adults: a six-month, randomized, placebo-controlled, double-blind clinical trial. Diabetes Obes Metab. 2019;21(6):1487–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Polyzos SA, Kountouras J, Anastasilakis AD, Geladari EV, Mantzoros CS. Irisin in patients with nonalcoholic fatty liver disease. Metabolism. 2014;63(2):207–217. [DOI] [PubMed] [Google Scholar]
- 41. Bernsmeier C, Meyer-Gerspach AC, Blaser LS, et al. Glucose-induced glucagon-like Peptide 1 secretion is deficient in patients with non-alcoholic fatty liver disease. Plos One. 2014;9(1):e87488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Raffort J, Lareyre F, Massalou D, Fénichel P, Panaïa-Ferrari P, Chinetti G. Insights on glicentin, a promising peptide of the proglucagon family. Biochem Med (Zagreb). 2017;27(2):308–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mukherjee A, Sidis Y, Mahan A, et al. FSTL3 deletion reveals roles for TGF-beta family ligands in glucose and fat homeostasis in adults. Proc Natl Acad Sci U S A. 2007;104(4):1348–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ungerleider NA, Bonomi LM, Brown ML, Schneyer AL. Increased activin bioavailability enhances hepatic insulin sensitivity while inducing hepatic steatosis in male mice. Endocrinology. 2013;154(6):2025–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.The Human Protein Atlas. https://www.proteinatlas.org/ENSG00000070404-FSTL3/tissue. Accessed 21 September 2019.
- 46. Liu G, Chen S, Deng S, Ma X, Hao Y, Bu G. Association of follistatin-like 3 concentrations in serum and synovial fluid with the radiographic severity of knee osteoarthritis. Int J Clin Exp Med. 2015;8(10):18884–18888. [PMC free article] [PubMed] [Google Scholar]
- 47. Kucera R, Topolcan O, Pecen L, et al. Reference values of IGF1, IGFBP3 and IGF1/IGFBP3 ratio in adult population in the Czech Republic. Clin Chim Acta. 2015;444:271–277. [DOI] [PubMed] [Google Scholar]
- 48. Bach LA, Headey SJ, Norton RS. IGF-binding proteins–the pieces are falling into place. Trends Endocrinol Metab. 2005;16(5):228–234. [DOI] [PubMed] [Google Scholar]
- 49. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]