Abstract
Context
Kallmann syndrome (KS) is a rare, genetically heterogeneous Mendelian disorder. Structural defects in KS patients have helped define the genetic architecture of gonadotropin-releasing hormone (GnRH) neuronal development in this condition.
Objective
Examine the functional role a novel structural defect affecting a long noncoding RNA (lncRNA), RMST, found in a KS patient.
Design
Whole genome sequencing, induced pluripotent stem cells and derived neural crest cells (NCC) from the KS patient were contrasted with controls.
Setting
The Harvard Reproductive Sciences Center, Massachusetts General Hospital Center for Genomic Medicine, and Singapore Genome Institute.
Patient
A KS patient with a unique translocation, t(7;12)(q22;q24).
Interventions/Main Outcome Measure/Results
A novel translocation was detected affecting the lncRNA, RMST, on chromosome 12 in the absence of any other KS mutations. Compared with controls, the patient’s induced pluripotent stem cells and NCC provided functional information regarding RMST. Whereas RMST expression increased during NCC differentiation in controls, it was substantially reduced in the KS patient’s NCC coincident with abrogated NCC morphological development and abnormal expression of several “downstream” genes essential for GnRH ontogeny (SOX2, PAX3, CHD7, TUBB3, and MKRN3). Additionally, an intronic single nucleotide polymorphism in RMST was significantly implicated in a genome-wide association study associated with age of menarche.
Conclusions
A novel deletion in RMST implicates the loss of function of a lncRNA as a unique cause of KS and suggests it plays a critical role in the ontogeny of GnRH neurons and puberty.
Keywords: long non-coding RNA, reproduction, human, mutations
The hypothalamic peptide, gonadotropin-releasing hormone (GnRH), is a prime mover of sexual maturation in mammals (1). Human genetics has identified mutations in several genes that cause isolated GnRH deficiency (IGD), a rare Mendelian disorder (2) manifested by abnormal puberty, hypogonadotropism, and infertility (2). Kallmann syndrome (KS) is a phenotypic subset of IGD defined by the association of IGD with anosmia. Discoveries of nearly 20 mutated genes in KS patients have begun to define an emerging genetic architecture governing GnRH neuronal development (3–8).
Following early fate specification as GnRH cells, GnRH precursor cells comigrate with their olfactory companions into the central nervous system, apparently using common guidance mechanism(s) shared with olfactory axons. These still-maturing GnRH neurons permeate the porous cribriform plate guided via as-yet unknown genes on the journey to their final hypothalamic destination from which they oversee the control of the reproductive axis in all mammals (9, 10). During these complex developmental processes, GnRH neurons mature and expand their numbers, eventually giving rise to a population of ~10 000 functioning GnRH neurons in the hypothalamus that ultimately govern human reproduction (3, 10).
Critical biological clues regarding the genetic components that control these basic developmental processes have been provided by the discovery of several distinct structural genomic variations in KS patients. An Xp22.3 contiguous gene syndrome established anosmia 1-ANOS1 (previously known as Kallmann 1-KAL1) as the first KS gene (9, 11–13). Chr8p11 deletions in KS patients with hereditary spherocytosis (14) identified fibroblast growth factor receptor 1-FGFR1 as the first autosomal dominant KS gene (15). Similarly, balanced chromosomal rearrangements on chr10 and chr12 identified WD Repeat Domain 11-WDR11 as a cause of KS (16) and a heterozygous deletion revealed semaphoring 1-SEMA3A as another causal KS gene (17). Studying such informative structural events has been particularly limited by their rarity and the utilization of traditional human genetic methodologies.
We have used next-generation sequencing (NGS) in a KS patient previously reported to harbor a “balanced” t(7;12) chromosomal translocation. NGS can resolve structural defects to the single base-pair level and thus reveal ever subtler defects in unknown disease-causing genes (18–20). Using NGS, we revealed a previously unrecognized breakage on Ch12 that disrupted RMST, a gene not previously linked to IGD. RMST is a long noncoding RNA (lncRNA) that has been previously shown to regulate neurogenesis through its direct binding to the transcription factor Sox2 (21). To assess if the identified defect in RMST in our patient contributes to KS, we performed functional studies using neural crest cells (NCC) generated from healthy control and patient-derived induced pluripotent stem cells (iPSC). These studies demonstrate novel developmental impacts associated with the loss of function of RMST in GnRH neuronal and NCC development.
Materials and Methods
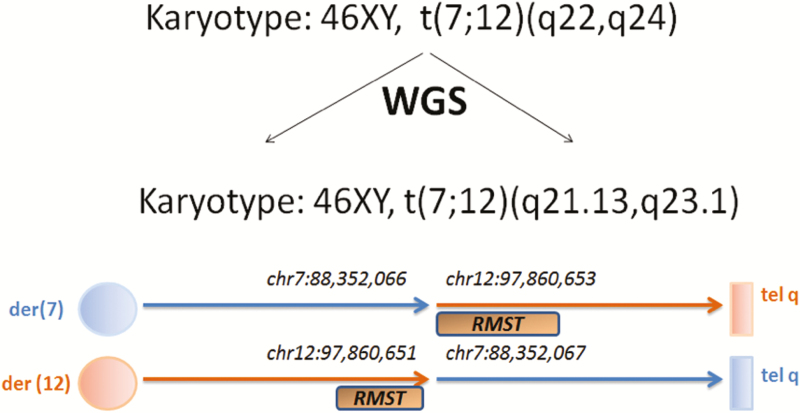
Long insert whole genome sequencing (LiWGS) was performed on the KS patient’s cell lines. DNA samples were obtained from the NIGMS Human Genetic Cell Repository at the Coriell Institute and delineated the breakpoints of a previously identified apparently balanced de novo translocation t(7;12)(q22;q24) (20, 22, 23), as previously described (19, 24–28) (Fig. 1), which were validated by Sanger sequencing.
Figure 1.
Base pair resolution of the “balanced” chromosomal rearrangement demonstrates a breakpoint to be in RMST.
Whole exome sequencing (WES) was performed on the Broad Institute’s Sequencing Platform and rare sequence variants (RSVs) in the 35 known IGD genes were sought (Table S1 (29)).
Targeted sequencing of potential RMST disruption in IGD cohort
The RMST locus on chromosome 12 was captured end to end (introns and exons included) from hg19/b37 genomic coordinates 12:97856799 to 97929544 using the Roche Nimblegen SeqCap Easy Probe kit requiring that >95% of the RMST locus target bases be covered at least 10×.
Copy number variations
A cohort of IGD samples were sequenced for copy number variations (CNVs) using the iPsychCNV pipeline (30) from a cohort of 1386 patients with genotypes from the Illumina PsychChip SNP array (31) and annotated against genecode_v19 (32).
Generation of pluripotent stem cells from proband/controls and differentiation of KS and wild-type iPSCs into NCCs
To test RMST expression in neural crest cells, the previously published stepwise differentiation of human pluripotent stem cells to multipotent NCCs was used (33). The resulting iPSCs were then treated for 11 days with the Wnt agonist (CHIR99021) under dual-SMAD inhibition for the first 4 days (34). Efficiency of NCC induction was monitored based on SOX10 immunostaining, which marks early multipotent NC stem cells.
RMST expression in iPSCs and NCCs
Expression of RMST and all genes presented in Figs 2, 3A–B was quantified via quantitative reverse transcription-polymerase chain reaction primers previously described (21).
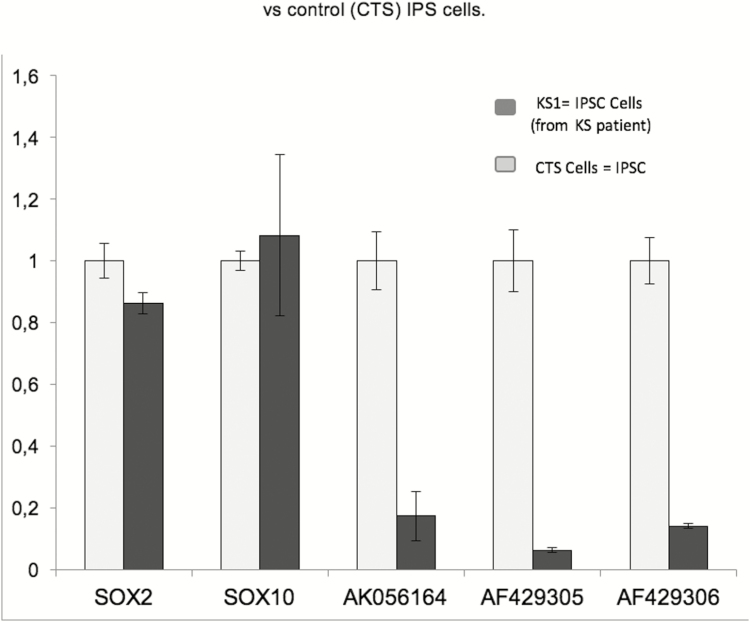
Figure 2.
RMST levels in both the KS patient’s IPSC (KS1) and healthy control’s iPSCs; the patient’s iPSCs expressed only 6% to 18% of the total RMST transcripts (AK056164, AF429305, and AF429306) that were expressed by healthy iPSCs but with no significant differences in SOX2 and SOX10 expression in both cell lines.
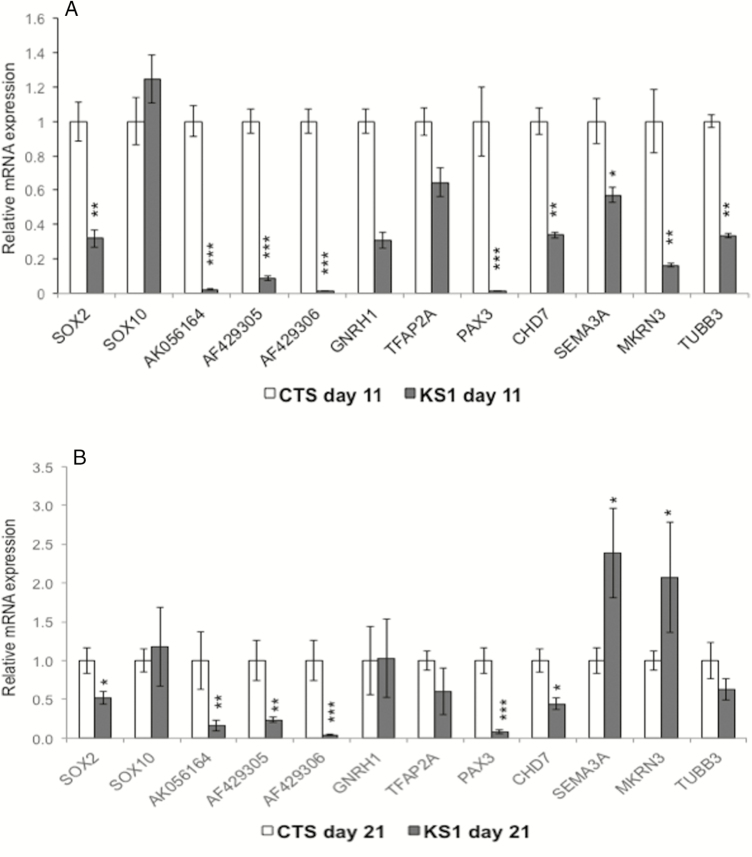
Figure 3.
(A, B) Expression of SOX2, SOX10, RMST transcripts (AK056164, AF429305, and AF429306), PAX3, GNRH1, TFAP2A, CHD7, SEMA3A, MKRN3, and TUBB3 in patient’s neural crest cells compared with controls after induction of NC differentiation for days 11 and 21. Abbreviations: CTS, control cells; KS1, patient cells.
SOX10 RNA-immunoprecipitation (day 21 NCC lysates)
RNA immunoprecipitation (RIP) was performed as described (21) on day 11 with NCCs harvested in RIP buffer. Cells lysates were precleared with protein G magnetic beads before incubating with specific antibodies against SOX10 or an immunoglobulin G control. For each assay, 5 mg of antibodies was used, incubated with precleared lysate at room temperature for 4 hours, and incubated with protein G magnetic beads for 2 hours. The resulting bound proteins were washed three times in RIP buffer and eluted in Trizol reagent for RNA extraction.
Chromatin immunoprecipitation sequencing analysis
Chromatin immunoprecipitation sequencing (ChIP-seq) analysis was performed to identify SOX2 binding sites in human neural stem cell lines as previously described (21). In brief, redundant reads that could result from the overamplification of ChIP DNA were removed and peak enrichment then calculated relative to the genome background. A threshold of P = 10–5 was used to call significant peaks. An “input” sample was also included to eliminate nonrandom enrichment (35). To filter for high-confidence ChIP-seq peaks, the difference between the SOX2 libraries and input mapped reads were plotted vs. random SOX2 peaks. Peaks with a greater peak height in comparison to random peaks were then selected. For motif discovery, the HOMER 4.1 algorithm (36) was applied to each set of ChIP-seq libraries for de novo binding motif discovery and known motif analysis with default parameters. All significant peaks were used for motif analysis in both sets of SOX2 ChIP-seq experiments. The HOMER 4.1 software annotated the peaks using the hg18 genome assembly RefSeq genes transcription start site (defined as from -1 kb to +100 bp), transcription termination site (by default defined from -100 bp to +1 kb), exons, 50 UTR, 30 UTR, introns, and intergenic regions.
Statistical analysis
All assays were performed in three independent experiments (biological replicates). Student t test was performed to assess statistical significance. Throughout the manuscript, * denotes P < 0.05, ** denotes P < 0.01, and *** denotes P < 0.001. To test for excess of rare variants, IGD cases were compared with control cohorts; we used the gnomAD database (37) using Fisher’s exact test. P < 0.05 was considered significant.
Study approval
Approval from the tribal and Indian Health Service institutional review board and the Massachusetts General Hospital institutional review boards were obtained.
Results
A KS patient with a de novo chromosomal translocation
A Native American (Chippewa/French) man diagnosed with KS presented at age 22 with a prepubertal status, hypogonadotropism and anosmia, unfused epiphyses, shortened metacarpals with clubbed distal ends, a sharply outlined occipital region, and delayed mental development confirmed by formal testing (20, 22, 23). He never underwent treatment for his hypogonadism. By age 44, he had developed hypertension and type 2 diabetes. He was 175 cm tall, weighed 89.5 kg, had a span of 156 cm (difficult with prior orthopedic surgeries implying skeletal dysplasia), and an upper/lower segment ratio of 0.68. He remained undervirilized with no axillary and sparse pubic hair. His penis was underdeveloped and testes were prepubertal at <4 mL. He had no neurologic deficits. He was the oldest of 6 full siblings (5 female and 1 male) and 3 half-siblings (2 male and 1 female), all without evidence of KS or other reproductive or skeletal disorders. Laboratory confirmed hypogonadotropic hypogonadism and a pyelogram showed 2 normal kidneys. His initial karyotype revealed a reciprocal translocation (7;12)(q22;q24) that was absent in both parents and an additional, paternally inherited pericentric polymorphic inversion (9)(p12q13), which is a common variation (38–42).
Precise mapping of chromosomal breakpoints
No genes previously associated with KS mapped to the breakpoints on Ch7 and Ch12 that were observed in this patient. Therefore, we used NGS to more accurately determine the genetic variations of this patient. Applying LiWGS (Fig. 1), we precisely determined the genomic breakpoints at t(7;12)(q21.13,q23.1). This sequencing revealed a 2-bp deletion on Chr12 that disrupted intron 2 of the longest transcript of a previously identified lncRNA (AK056164 [2.5 kb]; chr12:97 860 651-97 860 653) of the rhabdomyosarcoma 2 associated transcript (OMIM#607045)], RMST. The disruption led the gene to span into 2 different chromosomes, implying the complete disruption of this lncRNA and/or separation of the second piece of gene from its regulatory region. The other breakpoint on Ch7 did not disrupt any annotated gene (43). Neither breakpoint spans known DNase I hypersensitivity sites nor disrupts any open reading frames of RMST. The Database of Genomic Variants revealed few duplications spanning this region and extremely rare microdeletions were reported by the 1000 Genome Project (44). Additional searches of ~13 991 controls (19) confirmed the absence of any CNVs spanning this region.
We next performed WES of the proband to identify any coding variants. WES revealed a rare missense variant in the gene of leptin receptor, LEPR: p.V658I predicted to be benign by Polyphen (45) and SiFT (46) with a minor allele frequency (MAF) (37) of 0.0001733 in ExAC and a MAF of 0.001732 in ExAC’s African subpopulation. No loss-of-function (LoF) RSVs in any of the 35 known IGD genes existed (Table S1 (29)). Thus, the unique de novo disruption in RMST was the only genetic abnormality evident by LiWGS and WES in the genome of this KS patient.
Sequencing the RMST locus in additional IGD patients
Given the finding of a novel RMST breakage in the proband, we searched for RMST genetic variations in a cohort of 622 IGD individuals (292 KS and 330 normosmic idiopathic hypogonadotropic hypogonadism [nIHH]). For each patient DNA sample, the RMST locus was captured with primers and sequenced with at least 10× coverage. A total of 17 RSVs in RMST were detected (13 KS and 18 nIHH), all of which were unique or had an MAF frequency <1% (37) (Table 1). One KS patient carried 3 RSVs in RMST. No rare noncoding exonic homozygous RSVs in RMST exist in GnomAD. Ethnicity-based burden testing was performed to test for statistical excess of RSVs in IGD probands vs. ethnically matched controls (Table 2). An excess of such RSVs was seen in Caucasian/non-Finish Europeans and African American control cohorts, whereas Asian IGD patients showed a nonsignificant excess compared with Asian controls (47). No other structural variants (CNVs) at the RMST site of any others were found in 1386 IGD probands.
Table 1.
Rare sequencing variants (RSVs) in RMST
| Chr | bp | Ref | Alt | Annotation | Location | Dx | Gender | Other genes | Zygosity | gnomAD |
|---|---|---|---|---|---|---|---|---|---|---|
| 12 | 97 858 842 | A | G | Exon 1 | KS | Male | KLB c.820A > G p.I274V het; FGFR1 c.1097C > T p.P366L het | Het | 3.23E-05 | |
| 12 | 97 858 843 | T | C | Exon 1 | nIHH | Male | Het | Not seen | ||
| 12 | 97 885 730 | G | A | Exon 1 of MIR1251 | KS | Male | homozygous | Not seen | ||
| 12 | 97 887 656 | T | A | Exon 5 | KS | Male | KAL1 c.1759G > T p.V587L hem | Het | 3.23E-05 | |
| 12 | 97 887 663 | G | A | n.256-6G > A | Exon 5 | nIHH | Male | Het | 0.000226 | |
| 12 | 97 887 706 | T | A | Exon 5 | nIHH | Male | Het | Not seen | ||
| 12 | 97 887 763 | G | A | Exon 5 | KS | Male | Het | 3.23E-05 | ||
| 12 | 97 888 490 | C | T | Exon 6 | KS | Male | Het | 0.00097 | ||
| 12 | 97 888 657 | A | G | n.1521A > G | Exon 6 | Het | 0.000807 | |||
| 12 | 97 927 340 | T | C | Exon 9 | Het | 0.000486 | ||||
| 12 | 97 889 772 | C | A | Exon 7 | KS | Male | Het | 0.000129 | ||
| 12 | 97 926 848 | C | T | Exon 9 | KS | Male | PROKR2 c.254G > A p.R85H het; | Het | 0.000388 | |
| 12 | 97 926 848 | C | G | Exon 9 | KS | Male | Het | Not seen | ||
| 12 | 97 962 885 | G | A | Exon 9 | nIHH | Female | IL17RD c.1697C > T p.P566L het; | Het | Not seen | |
| nIHH | Male | Het | ||||||||
| KS | Male | OTUD4 c.755T > C p.V252A het; | Het | |||||||
| nIHH | Male | CHD7 c.2831G > A p.R944H het; PROKR2 c.991G > A p.V331M het; FGFR1 c.289G > A p.G97S het | Het | |||||||
| nIHH | Male | Het | ||||||||
| KS | Male | KLB c.1825A > G p.T609A het | Het | |||||||
| nIHH | Female | NR0B1 c.376G > A p.V126M het; PROK2 c.218G > A p.R73H het | Het | |||||||
| nIHH | Male | CHD7 c.120A > C p.Q40H het; CHD7 c.4565A > T p.D1522V het; PROKR2 c.949G > C p.V317L het | Het | |||||||
| 12 | 97 927 223 | C | T | Exon 9 | KS | Male | KAL1 c.1056_1060delGGATG p.T352TfsX2 hem; CHD7 c.8416C > G p.L2806V het; | Het | 0.000259 | |
| nIHH | Male | Het |
All the rare sequencing variants (RSVs) (defined as variants with allele frequenting <0.1% the gnomAD database) including their chromosomal position, nucleic acid change, annotations (only the splice region changes have been annotated), exonal position, diagnosis of the affected proband, and RSVs in other IGD genes. Note that all RSVs were found to be in heterozygous state.
Table 2.
Burden testing in IGD vs. gnomAD cohort
| Chr | bp | Ref | Alt | Annotation | Location | Daignosis | Gender | Additional features | Other genes | Ethnicity | GnomAD MAF based in subpopulation ethnicity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 | 97 858 842 | A | G | Exon 1 | KS | Male | KLB c.820A > G p.I274V het; FGFR1 c.1097C > T p.P366L het | Asian | None | ||
| 12 | 97 858 843 | T | C | Exon 1 | nIHH | Male | Caucasian | None | |||
| 12 | 97 886 308 | G | C | Exon 3 | KS | Male | External ear defect | Not assessed | 0.003094 (East Asian) | ||
| KS | Female | Eye defects, speech impairement & cerebellar ataxia | Asian | None | |||||||
| 12 | 97 887 656 | T | A | Exon 5 | KS | Male | Synkinesia | KAL1 c.1759G > T p.V587L hem | Caucasian | None | |
| 12 | 97 887 663 | G | A | n.256-6G > A | Exon 5 | nIHH | Male | No | Caucasian | 0.0002665 (non-Finish Europeans); 0.0002862 (Finish Europeans) | |
| 12 | 97 887 706 | T | A | Exon 5 | niHH | Male | No | Caucasian | None | ||
| 12 | 97 887 763 | G | A | Exon 5 | KS | Male | No | Not assessed | 0.00006666 (non-Finish European) | ||
| 12 | 97 888 490 | C | T | Exon 6 | KS | Male | No | African american | 0.003212 | ||
| 12 | 97 888 657 | A | G | n.1521A > G | Exon 6 | 0.002749 | |||||
| 12 | 97 927 340 | T | C | Exon 9 | 0.001375 | ||||||
| 12 | 97 889 772 | C | A | Exon 7 | KS | Male | Flat feet, kyphosis, and hypermobility | Caucasian | 0.0002667 | ||
| 12 | 97 926 773 | G | T | Exon 9 | nIHH | Male | No | Caucasian | None | ||
| nIHH | Male | Flat feet and eye defects | GLCE c.427G > A p.V143M het; POLR3B c.1-1C > T het; CHD7 c.7315G > A p.E2439K het; PNPLA6 c.1484C > T p.P495L het | Caucasian | |||||||
| 12 | 97 926 848 | C | T | Exon 9 | KS | Male | Deviated septum and high arched palate | PROKR2 c.254G > A p.R85H het | Caucasian | 0.0006676 (non-Finish European); 0.0002862 (Finish) | |
| 12 | 97 926 848 | C | G | Exon 9 | KS | Male | Clinodactyly and neurologic defects | Asian | None | ||
| 12 | 97 962 885 | G | A | Exon 9 | nIHH | Female | CL/CP | IL17RD c.1697C > T p.P566L het | African American | None | |
| nIHH | Male | No | Not assessed | ||||||||
| KS | Male | Flat feet and pectus excavatum | OTUD4 c.755T > C p.V252A het | Asian | |||||||
| nIHH | Male | No | CHD7 c.2831G > A p.R944H het; PROKR2 c.991G > A p.V331M het; FGFR1 c.289G > A p.G97S het | Asian | |||||||
| nIHH | Male | Ataxia | Not assessed | ||||||||
| KS | Male | Synkinesia | KLB c.1825A > G p.T609A het | Asian | |||||||
| nIHH | Female | No | PROK2 c.218G > A p.R73H het | Asian | |||||||
| nIHH | Male | Crowded teeth and protruding ears | CHD7 c.120A > C p.Q40H het; CHD7 c.4565A > T p.D1522V het; PROKR2 c.949G > C p.V317L het | Caucasian | |||||||
| 12 | 97 927 223 | C | T | Exon 9 | KS | Male | Excessive joint mobility, high arched palate, synkinesia | KAL1 c.1056_1060delGGATG p.T352TfsX2 hem; CHD7 c.8416C > G p.L2806V het | Not assessed | 0.0003339 (non-Finish European); 0.0002862 (Finish Europeans); 0.001196 (Latino); 0.001018 (other) | |
| nIHH | Male | No | Caucasian | ||||||||
| 12 | 97 927 489–97 927 490 | GC | G | Exon 9 | nIHH | Female | No | TACR3 c.623G > A p.W208X het | Caucasian | 0.001671 (non-Finish Europeans); 0.0002864 (Finish Europeans) | |
| KS | Male | Curved spine, foreshortened arm/leg, and bone deformities | CHD7 c.5051-4C > T het; LEPR c.2246T > C p.L749S het; FGFR1 c.745 + 7G > A het; FGFR1 c.1809C > A p.C603X het | Caucasian | |||||||
| nIHH | Male | No | GNRHR c.892_893insA p.N298KfsX22 hom; KISS1R c.998C > T p.A333V het | Caucasian | |||||||
| nIHH | Male | No | IL17RD c.2012C > T p.S671L het; POLR3B c.543A > C p.Q181H het; | Caucasian | |||||||
| nIHH | Male | No | Caucasian | ||||||||
| nIHH | Male | No | Caucasian | ||||||||
| nIHH | Male | No | CHD7 c.2819C > T p.P940L het; also WES data not back | Caucasian |
Table shows prevalence of rare variation (MAF < 1%) and very rare variation (MAF < 0.1%) in the IGD cohort and the reference-control database of the gnomAD. The statistical differences were calculated between cohort of similar genetic background. Abbreviations: MAF: minor allele frequency; N/A: not available.
RMST expression in cell-based models of NCC development
RMST is highly expressed in the murine hypothalamus, at lower levels in whole brain, and is undetectable in mature (GT1-7) and immature (GN11) murine isogenic GnRH-producing cell lines (data not shown). RMST expression has previously been demonstrated to be critical for neuronal development (21). Hence, its role in the development of specific subsets of neurons derived from neural crest cells (ie, those presumably contributing to GnRH neurogenesis) was examined. To address this issue, an in vitro model of NCC development was developed to begin to define RMST’s role in neurogenesis in the patient vs control (N = 6) cell lines.
Using episomal reprogramming vectors, patient-specific iPSC were generated from lymphoblastoid cells derived from the proband. These patient-derived iPSC (KS1-iPSC) were confirmed to be pluripotent by expression of Oct4, Nanog, Sox2, and Tra1-81 (pluripotency markers) and their ability to generate all 3 germ layers by in vitro differentiation (Fig. S1A & B (29)). The presence of t(7;12) chromosomal translocation was confirmed in KS1-iPSC by polymerase chain reaction amplification (Fig. S1C (29)) and DNA sequencing (data not shown); the RMST breakpoints were identical to those of the patient’s DNA. NCC were subsequently derived from the KS1-iPSC and healthy control iPSC (CTS-iPSC) by directed differentiation using established protocols involving the inhibition of bone morphogenetic protein, transforming growth factor-β, and WNT signaling pathways. Differentiation was assessed by expression of Sox10, a marker of early, multipotent NC stem cells.
Although RMST expression was detected in both the KS1-iPSC and healthy CTS- iPSCs, the KS1-iPSCs had significantly decreased expression of all RMST transcripts (~6% to 18% vs. healthy iPSCs; P < 0.0018) (Fig. 2). During NCC development, RMST transcripts increased for control iPSCs as differentiation progressed coincident with the appearance of known neural crest markers, p75NGFR and AP2a (Fig. S2 (29)). In contrast, RMST transcripts in KS1-iPSC during this same period following NCC differentiation (day 11) showed significant reductions in all isoforms of RMST (AK056164, AF429305, and AF429306) that were <10% of healthy NCC (P < 0.0001) (Fig. 3A), confirming reduced RMST expression in the patient’s iPSC and NCC.
RMST binds to SOX10 in iPSC-derived NCC
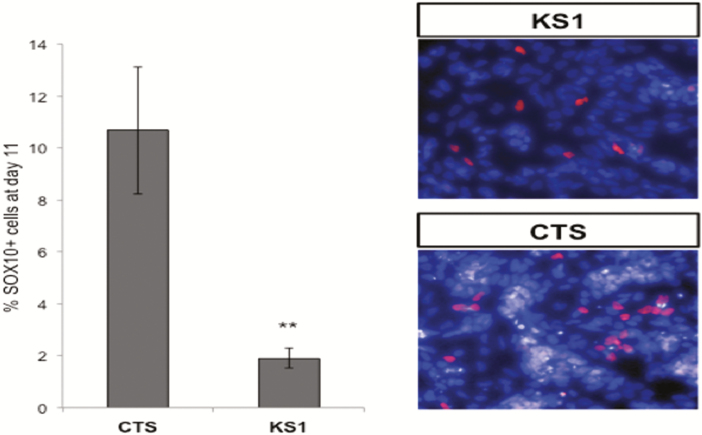
RMST has been shown to interact directly with SOX2 during neurogenesis. Therefore, we explored whether RMST interacts directly with SOX10. Sox10-specific antibodies were used in RIP experiments in NCC derived in vitro from iPSC. Robust enrichment of RMST was observed in SOX10 RIP (Fig. 4). Because both RMST and SOX2 are essential for neuronal development, it is plausible that RMST-SOX10 interactions are similarly critical for NCC differentiation. To confirm that RMST interactions with SOX10 and SOX2 are similar, both KS and healthy iPSCs were exposed to NCC differentiation conditions and the SOX10 expression in NCC cells quantified. The efficiency of NCC differentiation in healthy iPSCs was 10% vs only 2% of the KS1 iPSCs that became SOX10+ NCC cells (Fig. 5).
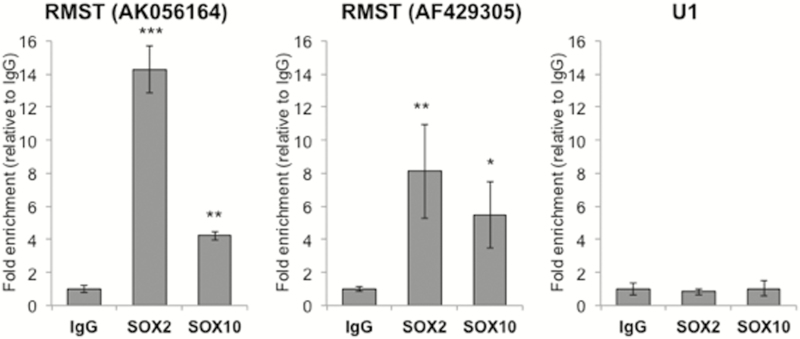
Figure 4.
Interaction of RMST transcripts AK056164 and AF429305 with SOX2 and SOX10: RNA-immunoprecipitation was performed with isotype IgG as negative control and SOX2 as positive control. Significant enrichment of RMST transcripts AK056164 and AF429305 in SOX10 pulldown samples indicated interaction between SOX10 and RMST.
Figure 5.
RMST transcript quantification was repeated again on day 11 of differentiation, when the iPSCs have differentiated into neural crest cells (NCS). Patient NCCs expressed <20% of the RMST transcripts present in healthy NC.
Evidence of direct RMST-SOX2 interactions
: Using Chip-Seq analysis (21) and small interfering RNA depletion of RMST in neuronal stem cells, SOX2 binding sites were assessed at 35 known IGD genes. Of the 15 IGD genes containing a SOX2 binding site (Table 3), RMST deletion induced loss of occupancies in 12/15, several of which are known major and/or candidate IGD genes affecting sexual maturation including: FGFR1, TAC3, NROB1, LEPR, SOX10, RNF216, OTUD4, FEZF1, MKRN3, IL17RD) and DUSP6. Intriguingly, SOX2 binding sites on TACR3, TUBB3, and FLRT3 were not altered upon deletion of RMST and, in PROKR2, a novel SOX2 binding site was acquired upon RMST depletion.
Table 3.
SOX2 binding sites in known IGD genes and changes after depletion of RMST
| SOX2 binding site | SOX2 binding site (absence of RMST) | |
|---|---|---|
| KAL1 | No | No |
| NROB1(DAX1) | chrX:30237372:30237587 | No |
| GNRHR | No | No |
| PCSK1 | No | No |
| LEP | No | No |
| FGFR1 | chr8:38444431:38445018 | No |
| KISS1R | No | No |
| NSFM(NELF) | No | No |
| PROKR2 | No | chr20:5243291:5244788 |
| PROK2 | No | No |
| LEPR | chr1:65658276:65658800 | No |
| FGF8 | No | No |
| CHD7 | No | No |
| GNRH1 | No | No |
| TAC3 | chr12:55696425:55696910 | No |
| TACR3 | chr4:104859805:104860418 | chr4:104859537:104861056 |
| WDR11 | No | No |
| HS6ST1 | No | No |
| POLR3B | No | No |
| KISS1 | No | No |
| SEMA3A | No | No |
| FGF17 | No | No |
| IL17RD | chr3:57173766:57174183 | No |
| SPRY4 | No | No |
| DUSP6 | chr12:88270267:88270887 | No |
| FLRT3 | chr20:14266025:14266376 | chr20:14265925:14266584 |
| SOX10 | chr22:36709222:36709997 | No |
| OTUD4 | chr4:146320233:146320452 | No |
| RNF216 | chr7:5787630:5788029 | No |
| TUBB3 | chr16:88516780:88517669 | chr16:88517627:88518915 |
| FEZF1 | chr7:121731148:121731552 | No |
| PNPLA6 | No | No |
| STUB1 | No | No |
| AXL | No | No |
| MKRN3 | chr15:21361791:21362052 | No |
From the 35 known IGD genes, 15 contain a SOX2 binding site. Upon deletion of RMST, the SOX2 binding sites disappear in 12 of 15 genes including FGFR1, TAC3, NROB1, LEPR, SOX10, RNF216, OTUD4, FEZF1, MKRN3, MCM4, IL17RD, and DUSP6. On the other hand, SOX2 binding sites of TACR3, TUBB3, and FLRT3 were not altered upon deletion of RMST and 1, PROKR2, gained 1 SOX2 binding site.
Effect of RMST LoF on downstream target genes
: Previous depletion of RMST by small interfering RNA in neural stem cell lines decreased the levels of the known IGD- associated genes CHD7, TUBB3, and SEMA3A and increased MKRN3 (21). To determine these effects of our patient’s RMST depletion on these same IGD genes, their levels were measured in the KS1 and control NCC on days 11 and 21 of differentiation (Fig. 3A). At day 11, KS1 cells expressed reduced levels of SOX2, TUBB3, and CHD7 but no change in SOX10 and SEMA3A. However, by day 21, a 2.4-fold increase in SEMA3A and 2-fold increase in MKRN3 expression levels had become evident (Fig. 3B).
Discussion
LiWGS in a KS patient with a known de novo chromosomal rearrangement identified a novel disruption in the lncRNA, RMST. RMST’s association with neuronal development is based on its strong, early neuronal localization and expression in the Wnt and transforming growth factor-β/bone morphogenetic protein-rich domains of the embryonic dorsal forebrain (48). In addition, RMST expression increases during critical stages of neuronal differentiation (49, 50) when it binds directly to SOX2 (23,61), a critical transcriptional regulator of GnRH ontogeny. RMST knockdowns in neuronal stem cells also reduce expression of CHD7, SEMA3, and TUBB3, all of which are validated IGD-causing genes (51–53). However, the biology of lncRNAs in general and RMST specifically remains largely unclear in part from the lack of any functional assay to assess their loss of function. Thus, to address the potential role of RMST depletion in our KS patient, we generated iPSC and NCC from this unique KS patient with a translocation-disrupting deletion in RMST.
RMST expression increases during normal neuronal differentiation and binds directly to and potentially amplifies SOX2 and likely SOX10’s effects on early GnRH neuronal development. In contrast, in the presence of this translocation, RMST expression was significantly lower compared with healthy iPSC cells and the NCC development was severely disrupted. A large number of IGD-causing genes, particularly those already identified to be SOX2-dependent and hence playing a critical role in NCC development (eg, CHD7, TUBB3), failed to develop in the RMST-deficient iPSC and NCC cells.
We hypothesize that RMST plays an essential role as a cofactor for SOX2 (and presumably SOX10) in GnRH neuronal differentiation by binding to the promoter regions of several important neurogenic genes. These include many known IGD-causing genes (FGFR1, TAC3, NROB1, LEPR, SOX10, RNF216, OTUD4, FEZF1, MKRN3, MCM4, IL17RD, and DUSP6). RMST also shares binding with SOX2 to the promoters of the same neurogenic transcription factor genes such as Neurogenin 2, underscoring its partnership with SOX2.
SOX2 has a known role in the early development of the hypothalamic-pituitary-gonadal axis and differentiation of GnRH neurons in addition among other neurogenic progenitors during development of olfactory and vomeronasal receptors (54). Patients with heterozygous mutations in SOX2 exhibit IGD with or without ocular defects (55). Thus, the binding partners, RMST and SOX2, acting in concert oversee a common pathway of downstream SOX2–dependent (and now “RMST-dependent”) genes critical for GnRH neuronal differentiation as validated by the fact that most of these genes also cause KS when mutated (17, 21, 52, 53, 56).
RMST depletion led to initial decreases on day 11 followed by further increases on day 21 relative to controls in MKRN3, a paternally imprinted gene in the Prader-Willi/Angelman critical region whose LoF mutations are associated with precocious puberty (57). Mkrn3 is highly expressed in the arcuate nucleus of the hypothalamus during the infantile and early juvenile periods with subsequent reductions at postnatal days 12 through 15 (ie, just before GnRH-induced sexual maturation in mice) (57, 58). Collectively, these observations suggest that MKRN3 plays a “braking” function in the prepubertal inhibition of GnRH secretion and that any sustained increase in its expression could lead to a potentially severe delay of pubertal development such as occurs in KS and IGD.
These associative data demonstrate for the first time that RMST is an lncRNA implicated in GnRH deficiency and has a key role as a cogovernor with SOX2 in regulating downstream KS genes, making it the potential cause of the KS phenotypic expression in our subject. It remains to be seen if other structural or genetic abnormalities in RMST and/or other noncoding genes in this domain (or others) can also cause KS. Future mammalian modeling (eg, RMST knock-outs) will hopefully provide more detailed mechanistic and developmental insights into the mechanisms by which RMST governs GnRH neurogenesis. The previous inherent difficulties of examining functional consequence of alterations in noncoding regions has been a formidable generic limitation of studying mutations in this new class of disease-causing genes. However, using IPSC and NCC cells begins to address these biological limitations in a convincing way, at least as RMST relates to neurogenesis and the ontogeny of GnRH neurons.
Even though rare RMST variants were discovered in both KS and nIHH patients, rare variants were also found in excess in control population. Given the noncoding nature of the gene, the functional effect of these point variants can be difficult to examine. The rarity of KS (1:30 000 in men; 1:125 000 in women) (59) challenges efforts to seek replication of these findings in other humans. Importantly, no CNVs were detected in IGD cohort of 1386 probands and no CNVs were detected in a large cohort of controls either (N = 13 991), highlighting the rarity of occurrence of structural events in this genomic region. Similarly, structural defects causing human disease are rare but quite critical in revealing novel biological roles in such areas as autism. Thus, given the large body of coherent biologic evidence from these current studies, we believe these observations from this single KS patient lacking any other cause of his disease by WGS are coherent and call attention to a novel role of a LncRNA, RMST, as a genomic region worthy of further study. Future accurate WGS will continue to resolve ever smaller potential defects in other coding and noncoding genomic regions in important human disease models like IGD with relevance to developmental biology and pathophysiology.
The complexity of analyzing the genetic changes in noncoding areas led to identification of additional studies that implicated RMST to pubertal regulation. An association between the SNP rs76369685 located in the second intron of RMST and the age of menarche in the UK Biobank was detected (60). The same SNP was also associated with gestational and type 1 diabetes in databases of 102 000 and 146 000 individuals, respectively (data not shown). Those associations highlight the importance of the intronic regions of this lncRNA and can explain the lack of enrichment of exonic RMST genetic changes in the IGD cohort compared with controls.
In conclusion, RMST’s high expression during normal neural crest cell differentiation; its direct association with key neural transcription factors, SOX2 and SOX10; its ability to regulate downstream genes that control the reproductive axis such as CHD7, TUBB3 and MKRN3; and its association with the age of menarche all support a previously unappreciated role for RMST as a regulator of neural crest development potentially affecting GnRH precursors.
Acknowledgments
The authors thank the surviving patient family members who generously provided permissions to perform these studies.
Financial Support: Funded by National Institute of Health grants P50HD028138, R01HD081256, GM061354, and K99DE026824 and Singapore’s Agency for Science, Technology and Research. Dr. Talkowski was supported as the Desmond and Ann Heathwood MGH Research Scholar. Dr. Stamou was funded by the Daland Fellowship of the American Philosophical Society. The DGAP project was supported by GM061354.
Author Contributions:
WFC, LWS, and MIS conceived and designed the study, participated in coordination, molecular genetics, data analyses, and drafting the manuscript; SYN generated the NCC lines, performed the functional studies on them including ChIP-seq, biochemistry, RIP, and participated in the manuscript drafting; HB performed molecular studies including liWGS and CNV analyses and participated in the manuscript drafting; HZW analyzed CNV data; LP performed/oversaw Sanger confirmations and molecular studies, WES and WGS analyses, and drafting the manuscript; LGB phenotyped and cared for the patient, oversaw all consenting of pedigree members, and participated in drafting the manuscript; SH generated the patient-specific iPSC line; MLH and KCC participated in design, execution, and data analysis of targeted RMST sequencing in IGD cohort; RB and MET participated in the experimental design, coordination of the molecular genetics, and drafting of manuscript; and JG participated in analysis of the genetic translocation as part of the DGAP project. All authors read and approved the final manuscript.
Additional Information
Current Affiliation: Current address for Lawrence W. Stanton is Qatar Biomedical Research Institute, Doha, Qatar.
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;202(4368):631–633. [DOI] [PubMed] [Google Scholar]
- 2. Stamou MI, Cox KH, Crowley WF Jr. Discovering genes essential to the hypothalamic regulation of human reproduction using a human disease model: adjusting to life in the “-Omics” era. Endocr Rev. 2015;2016(1):4–22. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3. Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;338(6211):161–164. [DOI] [PubMed] [Google Scholar]
- 4. Wray S, Grant P, Gainer H. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci U S A. 1989;86(20):8132–8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Whitlock KE, Illing N, Brideau NJ, Smith KM, Twomey S. Development of GnRH cells: setting the stage for puberty. Mol Cell Endocrinol. 2006;254–255:39–50. [DOI] [PubMed] [Google Scholar]
- 6. Forni PE, Taylor-Burds C, Melvin VS, Williams T, Wray S. Neural crest and ectodermal cells intermix in the nasal placode to give rise to GnRH-1 neurons, sensory neurons, and olfactory ensheathing cells. J Neurosci. 2011;31(18):6915–6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schlosser G. Making senses development of vertebrate cranial placodes. Int Rev Cell Mol Biol. 2010;283:129–234. [DOI] [PubMed] [Google Scholar]
- 8. Pingault V, Bodereau V, Baral V, et al.. Loss-of-function mutations in SOX10 cause Kallmann syndrome with deafness. Am J Hum Genet. 2013;92(5):707–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bick D, Curry CJ, McGill JR, Schorderet DF, Bux RC, Moore CM. Male infant with ichthyosis, Kallmann syndrome, chondrodysplasia punctata, and an Xp chromosome deletion. Am J Med Genet. 1989;33(1):100–107. [DOI] [PubMed] [Google Scholar]
- 10. Schwanzel-Fukuda M, Crossin KL, Pfaff DW, Bouloux PM, Hardelin JP, Petit C. Migration of luteinizing hormone-releasing hormone (LHRH) neurons in early human embryos. J Comp Neurol. 1996;366(3):547–557. [DOI] [PubMed] [Google Scholar]
- 11. Ballabio A, Parenti G, Tippett P, et al. X-linked ichthyosis, due to steroid sulphatase deficiency, associated with Kallmann syndrome (hypogonadotropic hypogonadism and anosmia): linkage relationships with Xg and cloned DNA sequences from the distal short arm of the X chromosome. Hum Genet. 1986;72(3):237–240. [DOI] [PubMed] [Google Scholar]
- 12. Petit C, Levilliers J, Weissenbach J. Long-range restriction map of the terminal part of the short arm of the human X chromosome. Proc Natl Acad Sci U S A. 1990;87(10):3680–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Franco B, Guioli S, Pragliola A, et al. A gene deleted in Kallmann’s syndrome shares homology with neural cell adhesion and axonal path-finding molecules. Nature. 1991;353(6344):529–536. [DOI] [PubMed] [Google Scholar]
- 14. Vermeulen S, Messiaen L, Scheir P, De Bie S, Speleman F, De Paepe A. Kallmann syndrome in a patient with congenital spherocytosis and an interstitial 8p11.2 deletion. Am J Med Genet. 2002;108(4):315–318. [DOI] [PubMed] [Google Scholar]
- 15. Dodé C, Fouveaut C, Mortier G, et al. Novel FGFR1 sequence variants in Kallmann syndrome, and genetic evidence that the FGFR1c isoform is required in olfactory bulb and palate morphogenesis. Hum Mutat. 2007;28(1):97–98. [DOI] [PubMed] [Google Scholar]
- 16. Kim HG, Ahn JW, Kurth I, et al. WDR11, a WD protein that interacts with transcription factor EMX1, is mutated in idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. Am J Hum Genet. 2010;87(4):465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Young J, Metay C, Bouligand J, et al. SEMA3A deletion in a family with Kallmann syndrome validates the role of semaphorin 3A in human puberty and olfactory system development. Hum Reprod. 2012;27(5):1460–1465. [DOI] [PubMed] [Google Scholar]
- 18. Talkowski ME, Ordulu Z, Pillalamarri V, et al. Clinical diagnosis by whole-genome sequencing of a prenatal sample. N Engl J Med. 2012;367(23):2226–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Talkowski ME, Rosenfeld JA, Blumenthal I, et al. Sequencing chromosomal abnormalities reveals neurodevelopmental loci that confer risk across diagnostic boundaries. Cell. 2012;149(3):525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Redin C, Brand H, Collins RL, et al. The genomic landscape of balanced cytogenetic abnormalities associated with human congenital anomalies. Nat Genet. 2017;49(1):36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ng SY, Bogu GK, Soh BS, Stanton LW. The long noncoding RNA RMST interacts with SOX2 to regulate neurogenesis. Mol Cell. 2013;51(3):349–359. [DOI] [PubMed] [Google Scholar]
- 22. Best LG, Wasdahl WA, Larson LM, Sturlaugson J. Chromosome abnormality in Kallmann syndrome. Am J Med Genet. 1990;35(3):306–309. [DOI] [PubMed] [Google Scholar]
- 23. Higgins AW, Alkuraya FS, Bosco AF, et al. Characterization of apparently balanced chromosomal rearrangements from the developmental genome anatomy project. Am J Hum Genet. 2008;82(3):712–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Talkowski ME, Ernst C, Heilbut A, et al. Next-generation sequencing strategies enable routine detection of balanced chromosome rearrangements for clinical diagnostics and genetic research. Am J Hum Genet. 2011;88(4):469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brand H, et al. Cryptic and complex chromosomal aberrations in early-onset neuropsychiatric disorders. Am J Hum Genet. 2014;95(4):454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hanscom C, Talkowski M. Design of large-insert jumping libraries for structural variant detection using Illumina sequencing. Curr Protoc Hum Genet. 2014;80:7221–7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brand H, Pillalamarri V, Collins RL, et al. Paired-duplication signatures mark cryptic inversions and other complex structural variation. Am J Hum Genet. 2015;97(1):170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Collins RL, Brand H, Redin CE, et al. Defining the diverse spectrum of inversions, complex structural variation, and chromothripsis in the morbid human genome. Genome Biol. 2017;18(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stamou M, Ng S-Y, Brand H, et al. Supplementary appendix. Dataset posted on 16.05.2019, 22:06 by, 2019.
- 30. Stamouli S, Anderlid BM, Willfors C, et al. Copy number variation analysis of 100 twin pairs enriched for neurodevelopmental disorders. Twin Res Hum Genet, 2018;21(1):1–11. [DOI] [PubMed] [Google Scholar]
- 31. Logue MW, Amstadter AB, Baker DG, et al. The psychiatric genomics consortium posttraumatic stress disorder workgroup: posttraumatic stress disorder enters the age of large-scale genomic collaboration. Neuropsychopharmacology. 2015;40(10):2287–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harrow J, Frankish A, Gonzalez JM, et al. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res. 2012;22(9):1760–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mica Y, Lee G, Chambers SM, Tomishima MJ, Studer L. Modeling neural crest induction, melanocyte specification, and disease-related pigmentation defects in hESCs and patient-specific iPSCs. Cell Rep. 2013;3(4):1140–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chambers SM, Qi Y, Mica Y, et al. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat Biotechnol. 2012;30(7):715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Feng J, Liu T, Qin B, Zhang Y, Liu XS. Identifying ChIP-seq enrichment using MACS. Nat Protoc. 2012;7(9):1728–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heinz S, Benner C, Spann N, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38(4):576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kaiser P, Pericentric inversions. Problems and significance for clinical genetics. Hum Genet. 1984;68(1):1–47. [DOI] [PubMed] [Google Scholar]
- 39. Hsu LY, Benn PA, Tannenbaum HL, Perlis TE, Carlson AD. Chromosomal polymorphisms of 1, 9, 16, and Y in 4 major ethnic groups: a large prenatal study. Am J Med Genet. 1987;26(1):95–101. [DOI] [PubMed] [Google Scholar]
- 40. Teo SH, Tan M, Knight L, Yeo SH, Ng I. Pericentric inversion 9--incidence and clinical significance. Ann Acad Med Singapore. 1995;24(2):302–304. [PubMed] [Google Scholar]
- 41. Yamada K, Population studies of INV(9) chromosomes in 4,300 Japanese: incidence, sex difference and clinical significance. Jpn J Hum Genet. 1992;37(4):293–301. [DOI] [PubMed] [Google Scholar]
- 42. Kim JJ, Rhee HS, Chung YT, Park SY, Choi SK. Prenatal detection of de novo inversion of chromosome 9 with duplicated heterochromatic region and postnatal follow-up. Exp Mol Med. 1999;31(3):134–136. [DOI] [PubMed] [Google Scholar]
- 43. Chan AS, Thorner PS, Squire JA, Zielenska M. Identification of a novel gene NCRMS on chromosome 12q21 with differential expression between rhabdomyosarcoma subtypes. Oncogene. 2002;21(19):3029–3037. [DOI] [PubMed] [Google Scholar]
- 44. MacDonald JR, Ziman R, Yuen RK, Feuk L, Scherer SW. The Database of Genomic Variants: a curated collection of structural variation in the human genome. Nucleic Acids Res. 2014;42(Database issue):D986–D992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4(7):1073–1081. [DOI] [PubMed] [Google Scholar]
- 47. Jiang Y, Epstein MP, Conneely KN. Assessing the impact of population stratification on association studies of rare variation. Hum Hered. 2013;76(1):28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Caronia-Brown G, Anderegg A, Awatramani R. Expression and functional analysis of the Wnt/beta-catenin induced mir-135a-2 locus in embryonic forebrain development. Neural Dev. 2016;11:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Uhde CW, Vives J, Jaeger I, Li M. Rmst is a novel marker for the mouse ventral mesencephalic floor plate and the anterior dorsal midline cells. PLoS One. 2010;5(1):e8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hart RP, Goff LA. Long noncoding RNAs: central to nervous system development. Int J Dev Neurosci. 2016;55:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chew S, Balasubramanian R, Chan W-M, et al. A novel syndrome caused by the E410K amino acid substitution in the neuronal beta-tubulin isotype 3. Brain. 2013;136(Pt 2):522–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hanchate NK, Giacobini P, Lhuillier P, et al. SEMA3A, a gene involved in axonal pathfinding, is mutated in patients with Kallmann syndrome. PLoS Genet. 2012;8(8):e1002896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim HG, Kurth I, Lan F, et al. Mutations in CHD7, encoding a chromatin-remodeling protein, cause idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. Am J Hum Genet. 2008;83(4):511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tucker ES, Lehtinen MK, Maynard T, et al. Proliferative and transcriptional identity of distinct classes of neural precursors in the mammalian olfactory epithelium. Development. 2010;137(15):2471–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kelberman D, Rizzoti K, Avilion A, et al. Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. J Clin Invest. 2006;116(9):2442–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Abreu AP, Macedo DB, Brito VN, Kaiser UB, Latronico AC. A new pathway in the control of the initiation of puberty: the MKRN3 gene. J Mol Endocrinol. 2015;54(3):R131–R139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Abreu AP, Trarbach EB, de Castro M, et al. Loss-of-function mutations in the genes encoding prokineticin-2 or prokineticin receptor-2 cause autosomal recessive Kallmann syndrome. J Clin Endocrinol Metab. 2008;93(10):4113–4118. [DOI] [PubMed] [Google Scholar]
- 58. Laitinen EM, Vaaralahti K, Tommiska J, et al. Incidence, phenotypic features and molecular genetics of Kallmann syndrome in Finland. Orphanet J Rare Dis. 2011;6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kichaev G, Bhatia G, Loh PR, et al. Leveraging polygenic functional enrichment to improve GWAS power. Am J Hum Genet. 104(1):65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Koch CA, Vedanarayanan V. Micropenis SOX. Poster Board Number: SAT-461. 97th Endocrine Society meeting. March 7, 2015, San Diego.