SUMMARY
We conducted prospective, community-wide surveillance for acute respiratory illnesses (ARIs) in Rochester, NY and Marshfield, WI during a 3-month period in winter 2011. We estimated the incidence of ARIs in each community, tested for viruses, and determined the proportion of ARIs associated with healthcare visits. We used a rolling cross-sectional design to sample participants, conducted telephone interviews to assess ARI symptoms (defined as a current illness with feverishness or cough within the past 7 days), collected nasal/throat swabs to identify viruses, and extracted healthcare utilization from outpatient/inpatient records. Of 6492 individuals, 321 reported an ARI within 7 days (4·9% total, 5·7% in Rochester, 4·4% in Marshfield); swabs were collected from 208 subjects. The cumulative ARI incidence for the entire 3-month period was 52% in Rochester [95% confidence interval (CI) 42–63] and 35% in Marshfield (95% CI 28–42). A specific virus was identified in 39% of specimens: human coronavirus (13% of samples), rhinovirus (12%), RSV (7%), influenza virus (4%), human metapneumovirus (4%), and adenovirus (1%). Only 39/200 (20%) had a healthcare visit (2/9 individuals with influenza). ARI incidence was ~5% per week during winter.
Key words: Acute respiratory illnesses (ARIs), influenza, respiratory syncytial virus (RSV)
INTRODUCTION
Seasonal influenza disease causes substantial morbidity in the United States – 200 000 annual hospitalizations, thousands of annual deaths [1], and many emergency department (ED) and outpatient visits [2–4]. Most recent studies of the burden of influenza disease have focused on healthcare visits; few have examined the community-wide burden of influenza in the general population [5, 6]. Because many individuals with influenza disease do not seek medical care but are nevertheless ill enough to miss work or school [7–12], the high morbidity documented in studies of influenza-related illness may underestimate the burden of influenza. With vaccination coverage hovering at only 50% (43% in the 2010–2011 season, http://www.cdc.gov/flu/fluvaxview/coverage_1011estimates.htm) [13] despite universal vaccination recommendations [14], it is important to understand the full impact of this virus at the population level.
Other viruses also cause acute respiratory illnesses (ARIs) and it is important to document their population-wide impact. A Healthy People 2020 goal is to prevent disease, disability, and death from infectious disease. Population-level surveillance is an integral step in assessing morbidity from ARIs. Also vaccines are in development for several viral pathogens including respiratory syncytial virus (RSV) [15], parainfluenza viruses (PIVs) [16], and human metapneumovirus (hMPV, first identified in the early 2000s) which are significant causes of hospitalizations, ED visits and outpatient visits [2, 17–19]. Longitudinal community-based surveillance studies conducted in the 1960s–1980s found that RSV and PIVs accounted for many ARIs [6, 20–24] yet few recent studies have assessed their population-wide burden and little is known about the incidence of hMPV in the general population. Finally, while rhinoviruses (RVs) [25], coronaviruses (CoVs) [26], and other viruses [2] are known to cause medically attended ARI-related visits, studies on their population-wide burden are lacking. Such knowledge could guide future vaccine development.
We conducted a prospective, population-wide surveillance study in two distinct geographical areas to assess the incidence of ARIs during the winter months. Our objectives were to (1) estimate, at the population level, the incidence of ARIs across the age spectrum; (2) identify the common viruses currently causing ARIs throughout the community and their relative frequency; (3) compare the types of viruses causing ARIs in child vs. adult populations; and (4) estimate the proportion of individuals in the community with ARIs who make a healthcare visit.
MATERIAL AND METHODS
The study was approved by institutional review boards at the University of Rochester and Marshfield Clinic Research Foundation.
Study design
Our prospective, population-wide surveillance study used a previously described rolling cross-sectional (RCS) study design [27] in two communities (Rochester, NY and Marshfield, WI) in the 2011 winter season to identify individuals with ARIs (defined as a current illness with feverishness or cough within the past 7 days) and document viruses associated with these ARIs. The RCS design was first described and used by political scientists in the early 1980s for studies of voter preferences and election results; it consists of a series of cross-sectional samples in which each sample is representative of the source population [28]. A random sample of subjects was selected each week and then interviewed by telephone to identify those with ARIs and to obtain demographic and disease-specific information. Consenting individuals provided nasal and throat swabs which were tested in a research laboratory by real-time polymerase chain reaction (rPCR) for a wide spectrum of viruses.
Study setting and sampling frame
We performed the study in two communities labelled ‘Rochester’ and ‘Marshfield’: (a) Monroe County, NY surrounding the city of Rochester (population ~744 000); and (b) the Marshfield, Wisconsin area (population ~49 000). The Rochester sampling frame comprised 12 primary-care practices (six internal medicine, four paediatric, one family medicine, and one medicine-paediatrics practice) serving 90 245 patients whose age, race/ethnicity, and health insurance mirrored the demographics of Monroe County. These practices were from the Greater Rochester Practice-based Research Network [29]. The Marshfield sampling frame consisted of 49 000 residents of the Marshfield Epidemiologic Study Area (MESA), a population-based cohort of residents living in 14 zip codes surrounding Marshfield; >90% of MESA resident receive their healthcare at Marshfield Clinic [30].
Subject identification
The study focused on individuals (not households). Participants were identified at random from the two source populations. In Rochester, we created a denominator of all eligible individuals by merging the practice-level patient databases across 12 practices. We used random digit-dialling to call the primary contact telephone number (mobile or land-line) and called 490–825 people/week (based on previous power calculations), enrolling 118–247 subjects/week. In Marshfield, we randomly called 500–800 individuals/week and enrolled 250–400 subjects/week. In both communities, a person was eligible if he/she was aged ⩾6 months as of 1 January, had at least one healthcare encounter in the previous 24 months, and was a resident of Monroe County (Rochester) or a member of MESA (Marshfield) for ⩾12 continuous months prior to 1 January or since birth for those aged <1 year. Rochester subjects received a letter from their primary-care physician explaining the study and that an interviewer would telephone them for consent to participate.
Surveillance timelines
Using protocols developed by the CDC-funded New Vaccine Surveillance Network [2] and Influenza Vaccine Effectiveness (VE) Network [31], the two sites initiated telephone calls when the University laboratories (Rochester) or the Marshfield Laboratories and Wisconsin State Laboratory of Hygiene identified ⩾2 positive influenza specimens within 1 week or one positive specimen/week for 2 consecutive weeks, and stopped calls when laboratories failed to meet this threshold. The surveillance periods were 11 January 2011 to 1 April 2011 (Rochester) and 17 January 2011 to 8 April 2011 (Marshfield), identical to surveillance in a parallel study of influenza vaccine effectiveness [31].
Telephone interview process
We used trained telephone interviewers to perform structured interviews. From the randomly selected list of individuals to call each week, interviewers called 6 days/week (Monday–Saturday, Rochester) or 7 days/week (Marshfield) during daytime and evening hours. On Monday–Friday in both communities, study enrollers were given a list of new patients to call and attempted to contact individuals on their assigned day for verbal consent and an interview. Up to three calls (morning, afternoon, early evening) were made per day to telephone numbers listed on practice patient lists. If contact was unsuccessful on the assigned day, interviewers continued calls for 2 days, up to three calls per day, with times staggered. Individuals from the source population could be eligible more than once in subsequent weeks (96% of those who agreed to participate were enrolled once).
Content of telephone interviews
Telephone interviewers screened subjects for ARI symptoms in the identified person (subject or child) over the past 7 days, described the study, and obtained verbal consent for a survey that included a symptom assessment. Subjects with ARI symptoms in the past 7 days were defined as ARI positive; the remainder were defined as ARI negative.
In Rochester, if the sampled person was a child aged ⩽17 years, the interviewer spoke with a parent or guardian. The interviewer conducted a brief computer-assisted interview to determine if the subject had new onset of feverishness or cough within 7 days.
Telephone procedures in Marshfield were the same except: parents were interviewed if subjects were aged ⩽12 years; subjects aged 13–18 years were interviewed but with parental consent.
If a potential subject with an ARI was identified, the telephone interviewer obtained the date of symptom onset and specific symptoms experienced (using a checklist of symptoms), and asked for verbal consent for an in-person visit to conduct an interview and collect nasal/throat specimens for viral testing; they were compensated $20 for a home visit or $30 for a clinic visit (or community site). Subjects with no ARI symptoms were compensated with a $5 gift card.
In-person interviews and specimen collection
In-person visits were conducted within 7 days of the onset of illness symptoms to (1) obtain written consent for specimens and access to subjects’ medical records for ARI-related information, (2) complete a brief interview to gather additional health-related information (ARI symptoms ⩽7 days, and in Rochester – sites for medical care for the ARI), and (3) obtain nasal/throat swabs for testing for viruses. Research staff met subjects at their home or a convenient location in Rochester or at clinics in Marshfield. Enrollers collected one nasal and one throat swab for testing.
Medical chart (electronic medical record) reviews
Trained abstractors reviewed medical records of ARI-positive cases for: (1) ARI-related healthcare visits (primary care, speciailty, ED, urgent care, or hospitalization) occurring within 7 days before or after the interview; (2) procedures obtained during visits (chest radiograph, bloodwork, nasopharyngeal or respiratory cultures, rapid antigen testing); and (3) primary diagnosis for outpatient and ED visits and all diagnoses for hospitalizations.
Laboratory procedures
Nasal/throat specimens were combined in transport media, processed and stored at −80 °C. Laboratory testing was performed at the University of Rochester. Total nucleic acid was extracted according to the manufacturer's protocol using the QIAamp Viral RNA Mini kit on a QIAcube robotic instrument (Qiagen, USA). Specimens were tested by TaqMan Array Card (TAC) methodology (Life Technologies, USA), which allows simultaneous, singleplex, rPCR to be performed in a 384-well microfluidic card format [32]. We tested specimens for influenza virues A and B, RSV, PIV1–4, RV, adenovirus, hMVP, and CoVs 229E, NL63, OC43 and HKU1 by TAC assay. All primer and probe oligonucleotide sequences used for PCR assays were prepared and optimized by CDC. TAC assays demonstrated high sensitivity (75–95%) for influenza, RSV, PIV2–4, RV, and hMPV, and moderate sensitivity (54–56%) for PIV1 and adenovirus compared with individual-virus PCRs [33]. The four CoV rPCR assays were highly sensitive and specific for the detection of CoV 229E, NL63, OC43 and HKU1, with positive predictive values in the TAC assay of 94%, 97%, 96% and 88%, respectively [34].
Statistical analyses
We assessed the frequency of each virus and also multiple viruses and tabulated results for all subjects, by age [child (6 months–18·9 years) vs. adult], setting (Rochester vs. Marshfield), and whether subjects had sought medical care for ARIs. We used tabular analyses to summarize diagnoses/procedures for subjects with ARI-related medical visits. We used Pearson's χ2 tests to compare individual viruses between adult and child ARI positive subjects. In instances where expected values were 1–5 we corrected the χ2 test statistic with the N – 1 correction, and used Fisher's exact test when expected cell sizes were <1 [35]. We estimated population-wide ARI and individual virus cumulative incidence during the 3-month study period by summing the percent of the population that had a new ARI (symptoms for ⩽7 days) each week (i.e. weekly incidence). For instance, if we had run a 3-week RCS, and weekly incidences were 2%, 3% and 4%, we would calculate the cumulative incidence as 9%.
We used a stratified biased corrected and accelerated bootstrap procedure to calculate 95% confidence intervals (CIs) for population-wide proportions, with 1000 replications [36].
RESULTS
Incidence of ARIs during the influenza season (Fig. 1)
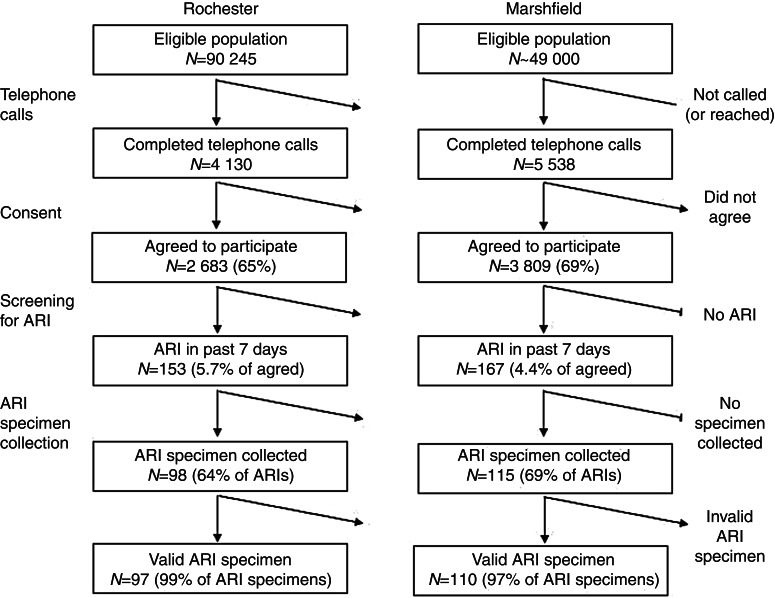
Fig. 1.
Flow chart for numbers of individuals contacted, with acute respiratory illnesses (ARIs), and number of ARI specimens.
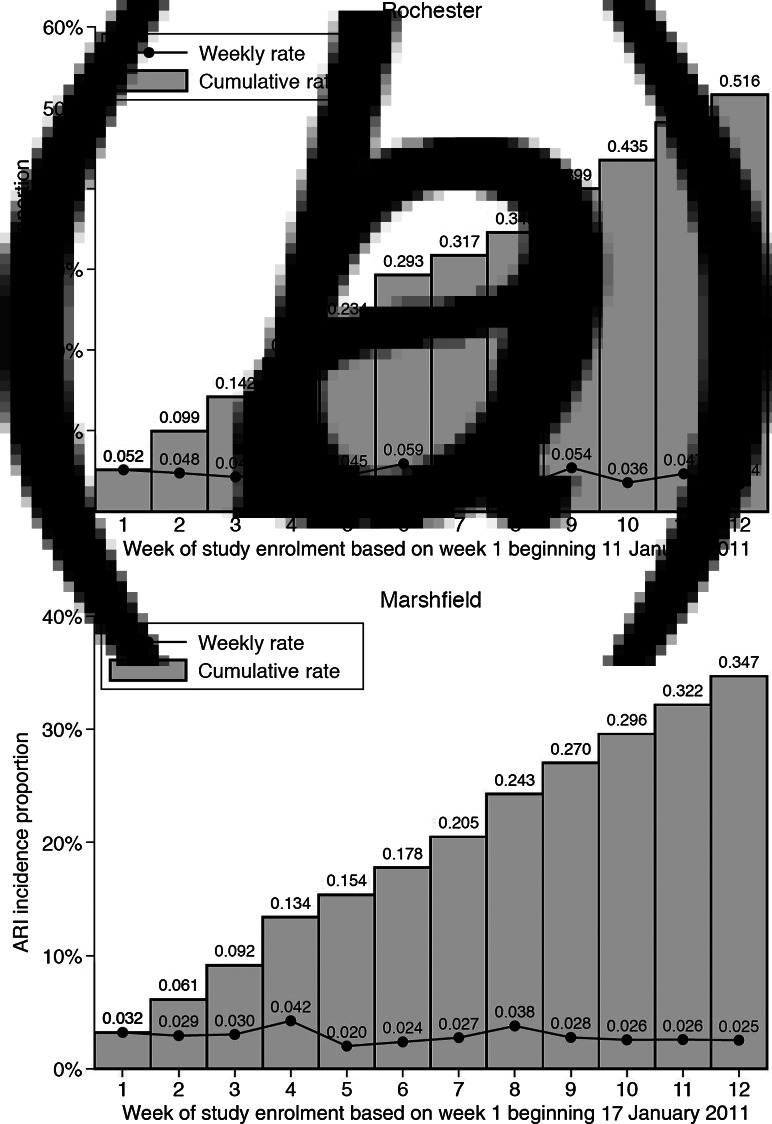
In Rochester, 17 485 telephone calls were made, 4130 calls were completed (i.e. direct contact with a household member), 2683 (65%) agreed to participate in symptom assessment and were enrolled, of whom 2263 (84%) consented for medical chart review for influenza vaccination dates. Altogether, 98 nasal/throat specimens were collected (64% of ARI subjects); one specimen was invalid with no ribonucleoprotein (RNP) human cellular control detected. Of individuals contacted by telephone, 153 had an ARI with feverishness or cough for a weekly (i.e. ARI within the previous 6 days) mean ARI incidence of 5·7%. The cumulative incidence for ARI during the 3-month study period was 52% (95% CI 42–63) as shown in Figure 2. It was 21% when restricted to subjects for whom a viral respiratory pathogen was detected (95% CI 15–28).
Fig. 2.
Number of acute respiratory illness (ARI) cases by week of surveillance, by geographical site. (a) Rochester, (b) Marshfield.
In Marshfield, 20 530 telephone calls were made, 5538 calls were completed, 3809 (69%) subjects agreed to participate, 3261 (86%) consented for a medical chart review. Altogether 115 nasal/throat specimens were collected (69% of ARI subjects); five specimens were invalid with no RNP human cellular control detected. Of individuals contacted by telephone, 167 had an ARI for a weekly mean ARI incidence of 4·4%. The cumulative ARI incidence during the 3-month study period was 35% (95% CI 28–42) as shown in Figure 2; it was 14% when restricted to virus-positive ARIs (95% CI 10–19).
Across both sites and all ages, 43·1% of individuals had an ARI during the 3-month study period (78·5% of children, 33·7% of adults). In Rochester, none of the subjects qualified on the basis of fever alone. In Marshfield, five of the 46 children aged <19 years and one of the 69 adults qualified on the basis of fever alone.
The gender distribution of ARI cases was slightly different than the source population (e.g. in Rochester 42% of ARI cases were female, vs. 44% from the source; in Marshfield 61% were female vs. 51%). ARI cases were slightly older (e.g. in Rochester 75% of ARI cases were adults aged >19 years, vs. 70% from the source population; in Marshfield 60% of ARI cases were adults vs. 77% from the source).
Viruses associated with ARIs during the influenza season (Table 1)
Table 1.
Viruses associated with acute respiratory illnesses
| Rochester (n = 97)* | Marshfield (n = 110)* | Total (N = 207)* | |
|---|---|---|---|
| Influenza | 5 (5%) | 4 (4%) | 9 (4%) |
| Influenza A | 4 | 4 | 8 |
| Influenza B | 1 | 0 | 1 |
| Respiratory syncytial virus | 7 (7%) | 7 (6%) | 14 (7%) |
| Parainfluenza (PIV) | 1 (1%) | 3 (3%) | 4 (2%) |
| PIV-1 | 0 | 0 | 0 |
| PIV-2 | 0 | 0 | 0 |
| PIV-3 | 1 | 3 | 4 |
| PIV-4 | 0 | 0 | 0 |
| Rhinovirus | 17 (18%) | 8 (7%) | 25 (12%) |
| Human coronavirus (HCV) | 8 (8%) | 20 (18%) | 28 (14%) |
| HCV-1 (229E) | 0 | 0 | 0 |
| HCV-2 (NL63) | 2 | 9 | 11 |
| HCV-3 (OC43) | 5 | 6 | 11 |
| HCV-4 (HKU1) | 1 | 5 | 6 |
| Adenovirus | 1 (1%) | 1 (1%) | 2 (1%) |
| Human metapneumovirus | 5 (5%) | 4 (4%) | 9 (4%) |
| At least 1 virus identified | 38 (39%) | 43 (39%) | 81 (39%) |
| 1 virus identified | 33 (34%) | 39 (35%) | 72 (35%) |
| ⩾2 viruses identified | 5 (5%) | 4 (4%) | 9 (4%) |
| No viruses identified | 59 (61%) | 67 (61%) | 126 (61%) |
The sum of the virus-specific percentages and the percentages of ‘no virus identified’ range between 104% and 106% because more than one virus present in some subjects.
Valid acute respiratory illness specimens.
In 61% of ARI cases, no viruses were identified, in 35% a single virus was identified, and in 4% of cases 2–3 viruses were identified. The most common viruses were CoVs (14% of all ARI cases), followed by RVs (12%), RSV (7%), influenza viruses (4%), hMPV (4%), and adenovirus (1%). The distribution of viruses varied – subjects from Marshfield experienced more ARIs from CoVs and those from Rochester had more from RVs.
Types of viruses associated with ARIs in children vs. adults (Table 2)
Table 2.
Distribution of viruses for children versus adults
| Children (n = 84) | Adults (n = 123) | P value | |
|---|---|---|---|
| Influenza | 1 (1) | 8 (7) | 0·06* |
| Respiratory syncytial virus | 9 (11) | 5 (4) | 0·06 |
| Parainfluenza | 3 (4) | 1 (1) | 0·16* |
| Rhinovirus | 14 (17) | 11 (9) | 0·09 |
| Human coronavirus | 11 (13) | 17 (14) | 0·88 |
| Adenovirus | 2 (2) | 0 (0) | 0·16† |
| Human metapneumovirus | 5 (6) | 4 (3) | 0·35* |
| At least 1 virus identified | 38 (45) | 43 (36) | 0·14 |
| No viruses identified | 46 (55) | 80 (64) | 0·14 |
| ⩾2 viruses identified | 7 (8) | 2 (2) | 0·02* |
P values from the Pearson χ2 test.
Uses the N – 1 χ2 test.
Uses Fisher's exact test.
Influenza viruses were identified in 1% of ARIs in children and 7% in adults. RSV was identified in a greater proportion of ARIs in children, while CoVs and RVs were identified in a high proportion of ARIs in both children and adults. At least one virus was identified in 45% of ARIs in children and 37% in adults; two viruses were identified in 8% of children with ARIs and 2% of adults.
Healthcare utilization in individuals with ARIs (Table 3)
Table 3.
Healthcare utilization in subjects with ARIs
| Rochester (n = 90) | Marshfield (n = 110) | Total (N = 200) | |
|---|---|---|---|
| Did not have any healthcare visits | 71 (79%) | 90 (82%) | 161 (81%) |
| Had a healthcare visit for the ARI* | 19 (21%) | 20 (18%) | 39 (20%) |
| Primary care office | 14 (78%) | 16 (80%) | 30 (79%) |
| ED or urgent care | 4 (22%) | 1 (5%) | 5 (13%) |
| Hospitalization | 0 | 3 (15%) | 3 (8%) |
ARI, Acute respiratory illness; ED, emergency department.
Based on medical chart reviews, ARI-related healthcare visit within 7 days prior to the telephone interview and 7 days post-interview.
Out of the 200 subjects with ARIs and who also agreed to a medical record review (seven from Rochester did not agree), 39 (19%) had a healthcare visit, five (2·5%) had an ED visit, and three (1·5%) had a hospitalization, all within 7 days of illness onset. Of the 39 with a healthcare visit, 30 (79%) were to primary care.
Of 84 participating children with ARIs, healthcare utilization data were available from medical charts for 79 children, and 20 (25%) had a healthcare visit. Of 123 adults with ARIs, healthcare utilization was available for 118 adults; 19 (16%) had a healthcare visit.
Given the small number of specific viral infections detected, it was not possible to compare healthcare utilization patterns by virus. The single child with an influenza infection did have a healthcare visit; 1/8 adults with influenza had a healthcare visit.
DISCUSSION
This RCS study is one of the few recent epidemiological studies to assess the incidence and causes of ARIs in the general population. We performed the study during the respiratory season (January to early April), when influenza detections were occurring weekly. Our study has several important findings. First, the overall incidence of ARI with feverishness or cough per week was 4·9% (ranging from 4·4% in Marshfield to 5·7% in Rochester), for a cumulative incidence during a 3-month respiratory season of 52% in Rochester and 35% in Marshfield. Second, despite use of advanced molecular techniques, we were able to identify viruses from only 39% of individuals with ARI symptoms. Third, influenza viruses accounted for only 11% of virus-positive cases, or 4% of all ARI cases. Fourth, the distribution of viruses was similar for children vs. adults. Finally, only one-fifth of ARI cases (and 2/9 influenza cases) had a healthcare visit for the ARI.
Several groundbreaking longitudinal cohort studies of respiratory illnesses were conducted from 1948 to 1976: the Cleveland Family Study, the Houston Family Study, the New York Virus Watch, the Tecumseh Study, and the Seattle Virus Watch [5, 10, 22–24, 37]. In these early studies, overall annual ARI incidence rates per person-year ranged from 4·5 to 8·0 for children aged <5 years of age and from 1·3 to 6·2 for individuals aged ⩾5 years. Because of differences in ARI symptom criteria, our focus on wintertime incidence only, and differences in study designs, findings from our study cannot be directly compared with many of these classic studies. However, we estimated that one-third of adults and nearly four-fifths of children in our two communities developed ⩾1 ARI during the 3-month respiratory period. A recent household cohort study from Michigan [38] found that the mean number of ARIs per individual over a 6-month period that coincided with our 3-month period was 1·1 for children aged <5 years and 0·6 for adults aged 18–49 years – these results are similar to ours, despite the differences in study design. Further studies over multiple years would be helpful to better understand the current patterns of ARI incidence in the community setting.
The weekly (or by extrapolation, 3-month) incidence of ARIs reported by our subjects in two US communities was in the range of the incidence of ARIs reported in a telephone survey of residents of Australia during the 2008–2009 season, reported in this journal [39]. In that telephone-based survey, 20% of subjects self-reported ARI symptoms (based on very similar definitions to ours) within the past 4 weeks. Our study extends findings from the Australian study by identifying viruses associated with ARIs and healthcare utilization patterns of individuals with ARIs.
We found that during the ‘flu season’ of 2010–2011, influenza caused only 1% of ARIs in children and 7% in adults. These findings contrast with early longitudinal studies [5, 10, 22–24, 37] and the recent Michigan cohort study [38] in which influenza accounted for a higher proportion of ARIs. Data from previously published New Vaccine Surveillance Network studies (which included the Rochester site) found that during peak influenza season, influenza-related ARIs accounted for up to one-quarter of ED visits in children aged <5 years [3].
The low rates of influenza detection in subjects with ARI in our community-based study were likely influenced by several factors. Across the United States, the 2010–2011 influenza season was less severe than the 2009–2010 pandemic year or the 2007–2008 seasonal influenza season, but more severe than the 2008–2009 influenza season, with overall hospitalization rates around 19–20/100 000 individuals of all ages [40]. Further, despite suboptimal influenza vaccination rates, influenza vaccination may have lowered the relative disease burden from this virus, although the tremendous variability in the timing, intensity, and duration of influenza circulation from year-to-year makes such assessments difficult. Vaccination rates were 59% and 41% in the Rochester and Marshfield study populations, respectively, and it was estimated that vaccine effectiveness in preventing medically attended influenza visits in a four-community study that included both Rochester and Marshfield was 60% during the same influenza season as assessed in our study [31]. Moreover, while our criteria for definition of ARIs comprised a list of symptoms used by other studies of medically attended ARI-related visits, these criteria may have been too broad and some patients labelled with ARI in this study may not have had a respiratory virus. For example, we included feverishness as one ARI criteria.
Our data highlight several additional aspects of the current epidemiology of ARI. While we confirm the high prevalence of RV, RSV, and PIV as aetiological agents of ARI, we also documented high rates of ARIs associated with CoVs and hMPV, a finding also highlighted by the recent Michigan cohort study [38]. Our previous studies of medically attended ARI visits have noted that these two viruses are associated with ARI-related medical visits [19, 26]. Together, these findings highlight the potential value of future vaccines against these two viruses.
We noted that the pattern of viruses was generally similar for children and adults, although there was a trend toward a greater proportion of ARIs being attributable to RSV in children than in adults. These mirror findings from cohort studies [5, 10, 22–24, 37, 38].
We found that only one-fifth of ARI cases had a healthcare visit, and that four-fifths of these medical visits were to a primary-care physician. A similar pattern was noted in both children and adults, and in both communities. Our study strongly suggests that the burden of ARIs is vastly greater than the burden demonstrated by studies limited to ARI-associated healthcare visits. Thus, efforts to develop vaccines to reduce ARIs have the potential for substantial benefit in reducing the disease burden from community-based ARIs as well as reducing medically attended healthcare visits. Future studies should consider collecting additional data on the morbidity of ARIs that do not result in visits to healthcare providers.
Our study has several strengths, including rigorously defined protocols across both communities, large numbers of individuals contacted by random sampling, trained staff to obtain nasal/throat specimens and conduct chart reviews, case definitions that mirrored the definitions used in studies of medically attended ARIs, and detection of viral infections by advanced molecular techniques. However, we caution against over-interpretation of our findings due to several important limitations. First, we studied only two communities, during a single respiratory season over a 3-month period which missed the peak season for PIV and RV. Geographical and seasonal variability in the viral aetiologies of ARI are substantial [2, 7, 10], for example, it is difficult to draw inferences about ARI incidence or virus burden from a single season of surveillance. Also, the relative contribution of some viruses might be different had we included a longer study period. Second, while our strict definition of ARIs (current illness with feverishness or cough) likely excluded other causes of stuffiness such as allergies, we may have missed some ARIs. Third, we interviewed only two-thirds of persons contacted, and obtained nasal/throat specimens for only two-thirds of ARI cases. We are aware of some selection bias in subjects agreeing to be interviewed. For example, Rochester subjects who agreed were more likely than the original sampling frame to be male and adult. While others have noted lower ARI reports in men [41], our ARI findings do not vary by gender; also we stratify findings by age group. It is possible that subjects from whom specimens were collected differed by viral aetiology or other characteristics. Fourth, despite making >38 000 telephone calls, we were able to collect only 208 nasal/throat swabs from subject with ARIs. Our sample sizes for individual viruses are small. Fifth, we may have missed some subjects who would have tested positive for a viral respiratory pathogen because we performed nasal swabs and used TAC detection methods rather than nasopharyngeal swabs and more sensitive conventional PCR assays. Sixth, we selected subjects with symptoms for ⩽7 days, but it is possible that some were no longer shedding virus, especially if they underestimated their days of symptoms prior to being contacted. Finally, we simply summed estimated weekly ARI incidence to derive a cumulative incidence, using a RCS design. This method has not yet been validated against more standard methods to calculate cumulative incidence in traditional cohort studies.
CONCLUSION
We conclude that in the 2011 winter respiratory season, about one in 20 individuals within two communities had an ARI during any single week, and one-third to one-half had an ARI during the winter respiratory season. Of those with ARIs, influenza infection accounted for only 4% of cases, while RSV, PIV, RV, CoV, and hMPV accounted for the bulk of ARIs. In individuals with ARIs, four-fifths did not make a visit to a healthcare provider because of their symptoms. ARIs due to both influenza and other viruses cause substantial morbidity undetected by the healthcare delivery system. In an era when actively following large cohorts of consenting subjects for disease surveillance purposes is expensive and resource intensive, the use of RCS designs similar to ours may offer a reasonable alternative strategy for conducting surveillance for more common illnesses. New vaccines for several common respiratory viruses may hold promise for further reducing the population-wide disease burden from ARIs.
ACKNOWLEDGEMENTS
This study was funded by the Centers for Disease Control and Prevention through cooperative agreements with the University of Rochester (U01 IP000172) and Marshfield Clinic Research Foundation (U01 IP000183). The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
We thank Dean Erdman, MD (CDC), for his assistance and invaluable advice on laboratory assays and Edward Belongia, MD, for his overall guidance on the study methods.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.CDC. Estimates of deaths associated with seasonal influenza,United States, 1976–2007. Morbidity and Mortality Weekly Report 2010; 59: 1057–1062. [PubMed] [Google Scholar]
- 2.Iwane MK, et al. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics 2004; 113: 1758–1764. [DOI] [PubMed] [Google Scholar]
- 3.Poehling KA, et al. The underrecognized burden of influenza in young children. New England Journal of Medicine 2006; 355: 31–40. [DOI] [PubMed] [Google Scholar]
- 4.Thompson WW, et al. Influenza-associated hospitalizations in the United States. Journal of American Medical Association 2004; 292: 1333–1340. [DOI] [PubMed] [Google Scholar]
- 5.Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiologic Reviews 1994; 16: 351–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monto AS. Epidemiology of viral respiratory infections. American Journal of Medicine 2002; 112: 4S–12S. [DOI] [PubMed] [Google Scholar]
- 7.Monto AS, Sullivan KM. Acute respiratory illness in the community. Frequency of illness and the agents involved. Epidemiology and Infection 1993; 110: 145–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molinari NA, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25: 5086–5096. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan KM, Monto AS, Longini IM. Jr. Estimates of the US health impact of influenza. American Journal of Public Health 1993; 83: 1712–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall CE, Cooney MK, Fox JP. The Seattle virus watch. IV. Comparative epidemiologic observations of infections with influenza A and B viruses, 1965–1969, in families with young children. American Journal of Epidemiology 1973; 98: 365–380. [DOI] [PubMed] [Google Scholar]
- 11.Gaglia MA Jr., et al. Patient knowledge and attitudes about antiviral medication and vaccination for influenza in an internal medicine clinic. Clinical Infectious Diseases 2007; 45: 1182–1188. [DOI] [PubMed] [Google Scholar]
- 12.CDC. Self-Reported influenza-like illness during the 2009 H1N1 influenza pandemic – United States, September 2009–March 2010. Morbidity and Mortality Weekly Report 2011; 60: 37–41. [PubMed] [Google Scholar]
- 13.CDC. Flu vaccination coverage: United States, 2012–13 influenza season (http://www.cdc.gov/flu/fluvaxview/coverage-1213estimates.htm). Morbidity and Mortality Weekly Report. [PMC free article] [PubMed]
- 14.Fiore AE, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. Morbidity and Mortality Weekly Reports. Recommendations and Reports 2010; 59: 1–62. [PubMed] [Google Scholar]
- 15.Castilow EM, Olson MR, Varga SM. Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunologic Research 2007; 39: 225–239. [DOI] [PubMed] [Google Scholar]
- 16.Sato M, Wright PF. Current status of vaccines for parainfluenza virus infections. Pediatric Infectious Disease Journal 2008; 27: S123–125. [DOI] [PubMed] [Google Scholar]
- 17.Hall CB, et al. The burden of respiratory syncytial virus infection in young children. New England Journal of Medicine 2009; 360: 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinberg GA, et al. Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. Journal of Pediatrics 2009; 154: 694–699. [DOI] [PubMed] [Google Scholar]
- 19.Edwards KE, et al. The burden of human metapneumovirus infection in young children. New England Journal of Medicine 2013; 368: 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandt CD, et al. Epidemiology of respiratory syncytial virus infection in Washington, D.C. 3. Composite analysis of eleven consecutive yearly epidemics. American Journal of Epidemiology 1973; 98: 355–364. [DOI] [PubMed] [Google Scholar]
- 21.Kim HW, et al. Influenza A and B virus infection in infants and young children during the years 1957–1976. American Journal of Epidemiology 1979; 109: 464–479. [DOI] [PubMed] [Google Scholar]
- 22.Cooney MK, Fox JP, Hall CE. The Seattle Virus Watch. VI. Observations of infections with and illness due to parainfluenza, mumps and respiratory syncytial viruses and Mycoplasma pneumoniae. American Journal of Epidemiology 1975; 101: 532–551. [DOI] [PubMed] [Google Scholar]
- 23.Glezen WP, et al. Risk of primary infection and reinfection with respiratory syncytial virus. American Journal of Diseases of Children 1986; 140: 543–546. [DOI] [PubMed] [Google Scholar]
- 24.Hall CE, et al. The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. IX. A comparison of infections with several respiratory pathogens in New York and New Orleans families. American Journal of Epidemiology 1971; 94: 367–385. [DOI] [PubMed] [Google Scholar]
- 25.Miller EK, et al. Rhinovirus-associated hospitalizations in young children. Journal of Infectious Diseases 2007; 195: 773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talbot HK, et al. Coronavirus infection and hospitalizations for acute respiratory illness in young children. Journal of Medical Virology 2009; 81: 853–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donahue JG, et al. Can the rolling cross-sectional survey design be used to estimate the effectiveness of influenza vaccines? Vaccine 2014; 32: 6440–6444. [DOI] [PubMed] [Google Scholar]
- 28.Brady HE, Johnston R. Capturing Campaign Effects. Ann Arbor: University of Michigan Press, 2006, pp. 395. [Google Scholar]
- 29.Gibson K, et al. Physician perspectives on incentives to participate in practice-based research: a greater rochester practice-based research network (GR-PBRN) study. Journal of the American Board of Family Medicine 2010; 23: 452–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenlee RT. Measuring disease frequency in the Marshfield Epidemiologic Study Area (MESA). Clinical Medicine and Research 2003; 1: 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Treanor JJ, et al. Effectiveness of seasonal influenza vaccines in the United States during a season with circulation of all three vaccine strains. Clinical Infectious Diseases 2012; 55: 951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kodani M, et al. Application of TaqMan low-density arrays for simultaneous detection of multiple respiratory pathogens. Journal of Clinical Microbiology 2011; 49: 2175–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinberg GA, et al. Field evaluation of TaqMan Array Card (TAC) for the simultaneous detection of multiple respiratory viruses in children with acute respiratory infection. Journal of Clinical Virology 2013; 57: 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dare RK, et al. Human coronavirus infections in rural Thailand: a comprehensive study using real-time reverse-transcription polymerase chain reaction assays. Journal of Infectious Diseases 2007; 196: 1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell I. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Statistics in Medicine 2007; 26: 3661–3675. [DOI] [PubMed] [Google Scholar]
- 36.Efron B, Tibshirani R. An Introduction to the Bootstrap. New York: Chapman & Hall, 1993: pp. xvi, 436 p. [Google Scholar]
- 37.Dingle JH, et al. A study of illness in a group of Cleveland families. I. Plan of study and certain general observations. American Journal of Tropical Medicine and Hygiene 1953; 58: 16–30. [DOI] [PubMed] [Google Scholar]
- 38.Monto AS, et al. Frequency of acute respiratory illnesses and circulation of respiratory viruses in households with children over 3 surveillance seasons. Journal of Infectious Diseases 2014; 210: 1792–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Kirk MD. Incidence of acute respiratory infections in Australia. Epidemiology and Infection 2014; 142: 1355–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.CDC. Update: influenza activity – United States, 2010–11 season, and composition of the 2011–12 influenza vaccine. Morbidity and Mortality Weekly Report 2011; 60: 705–712. [PubMed] [Google Scholar]
- 41.Thompson MG, et al. Subjective social status predicts wintertime febrile acute respiratory illness among women healthcare personnel. Health Psychology 2014; 33: 282–291. [DOI] [PubMed] [Google Scholar]