Abstract
Background Nonunion after open reduction and internal fixation (ORIF) of scaphoid fractures is reported in 5 to 30% of cases; however, predictors of nonunion are not clearly defined.
Objective The purpose of this study is to determine fracture characteristics and surgical factors which may influence progression to nonunion after scaphoid fracture ORIF.
Patients and Methods We performed a retrospective case–control study of scaphoid fractures treated by early ORIF between 2003 and 2017. Inclusion criteria were surgical fixation within 6 months from date of injury and postoperative CT with minimum clinical follow-up of 6 months to evaluate healing. Forty-eight patients were included in this study. Nonunion cases were matched by age, sex, and fracture location to patients who progressed to fracture union in the 1:2 ratio.
Results This series of 48 patients matched 16 nonunion cases with 32 cases that progressed to union. Fracture location was proximal pole in 15% (7/48) and waist in 85% (41/48). Multivariate regression demonstrated that shorter length of time from injury to initial ORIF and smaller percent of proximal fracture fragment volume were significantly associated with scaphoid nonunion after ORIF (63 vs. 27 days and 34 vs. 40%, respectively). Receiver operating curve analysis revealed that fracture volume below 38% and time from injury to surgery greater than 31 days were associated with nonunion.
Conclusion Increased likelihood for nonunion was found when the fracture was treated greater than 31 days from injury and when fracture volume was less than 38% of the entire scaphoid.
Level of Evidence This is a Level III, therapeutic study.
Keywords: postoperative, scaphoid fracture, nonunion, open reduction internal fixation, open reduction and internal fixation
Scaphoid fractures account for 60% of all carpal fractures, 1 and nonunion is a relatively common complication of these injuries, being reported at rates ranging from 5 to 50% for all fracture patterns and treatment modalities. 2 3 Even in the cases of timely surgical treatment, the nonunion rate after surgical fixation has been reported to be between 5 and 30%. 4 5 6 7 Scaphoid nonunion has serious consequences including pain, disability, and carpal collapse and degenerative arthritis in long-term follow-up. 6 Both fracture characteristics and surgical factors have been proposed to contribute to nonunion.
Traditionally, scaphoid fractures have been classified according to anatomic location as proximal pole, waist, and distal pole fractures. Anatomic location of these fractures has been thought to be associated with risk of nonunion and avascular necrosis, as several studies have independently found that location is an important predictor of scaphoid vascularity given the retrograde blood supply to the bone. This is thought to contribute to nonunion being more likely in fractures of the proximal zone. 5 8 9 10 11 In an analysis of a large series of scaphoid nonunions treated operatively, Ramamurthy et al found a 32% nonunion rate in proximal pole fractures versus 23% in waist fractures. 12 Similarly, Lim et al also suggested that fracture fragment size may influence outcomes after scaphoid nonunion surgery. 13 Despite traditional believe that blood supply is linked to risk for nonunion, newer evidence suggests proximal pole vascularity may not play as critical a role in ability to progress to union after nonunion ORIF as has classically been thought. 14
Surgical factors have also been investigated for their role in scaphoid nonunion. Missed diagnosis frequently results in delay in initial treatment and may contribute to increased risk of both nonunion and malunion. 10 12 15 16 17 Additional factors that have been investigated to affect the rate of union following open reduction internal fixation (ORIF) include screw trajectory 18 19 20 21 and implant type. 22 23 24 There are conflicting data regarding optimal screw orientation, with some studies show greater compression with central placement along the longitudinal axis of the scaphoid 25 while others demonstrate the biomechanical advantage of screws placed more perpendicular to the fracture plane. 19 20 26 Still other biomechanical studies suggest screw purchase into the dense 2-mm subchondral shell as a key factor in stability of scaphoid fractures. 21 27 While these studies provide biomechanical data, they are limited in that they do not provide clinical correlates in patient series.
Given the severity of sequela associated with scaphoid nonunion, it is critical to understand the multitude of factors affecting rate of union in the clinical setting. However, the current literature is lacking in data that considers each of these factors in a clinical series. The purpose of this study is to determine the fracture characteristics and surgical factors associated with nonunion following early, primary scaphoid ORIF. We hypothesize that fracture characteristics and surgical factors are associated with a difference in nonunion rate after scaphoid ORIF at a minimum of 1 year following fixation.
Methods
Study Design
This retrospective, case–control study reviewed the imaging database at our institution and identified 2,855 wrist computed tomography (CT) scans with scaphoid reformatting over a period of 14 years (January 2003–March 2017). Inclusion criteria were scaphoid fractures treated with ORIF with screw fixation within 6 months from date of injury, minimum of 6 months of postoperative clinical follow-up, and a postoperative CT to evaluate healing.
Among 199 patients who met these criteria, fractures were then classified according to progression to union. Nonunion was defined as either a fracture without signs of healing on CT or radiograph at 6 months after ORIF or a fracture that required a revision ORIF after initial fixation. Union was determined if two criteria were met: (1) evidence of healing without mention of progression toward nonunion on the postoperative CT as determined by the report of a musculoskeletal radiologist, and (2) no secondary surgery for scaphoid nonunion at minimum 1 year following ORIF. Conversely, cases with CT reported of evidence of nonunion and those that underwent revision scaphoid surgery were classified as nonunion. Sixteen patients were identified in the nonunion group, while fractures in the remaining 183 patients united. The sixteen nonunion patients (case group) were matched by age (within a 10-year range), sex, and fracture location in the 1:2 fashion to patients with united fractures, by random number assignment. Fracture location was defined in a three-zone fashion as proximal, waist, or distal based on the description given by a musculoskeletal fellowship trained radiologist in the CT report. Our control group consisted of 32 matched union patients (control group; Fig. 1 ). Chart review was performed to gather data on patient demographics, comorbidities, mechanism of injury, surgical details, and postoperative follow-up. Qualitative displacement of the fracture based on preoperative imaging was available for 38 of the 48 patients. Follow-up phone calls were made to all patients in the control group to ensure no further surgery was performed with a change in provider.
Fig. 1.
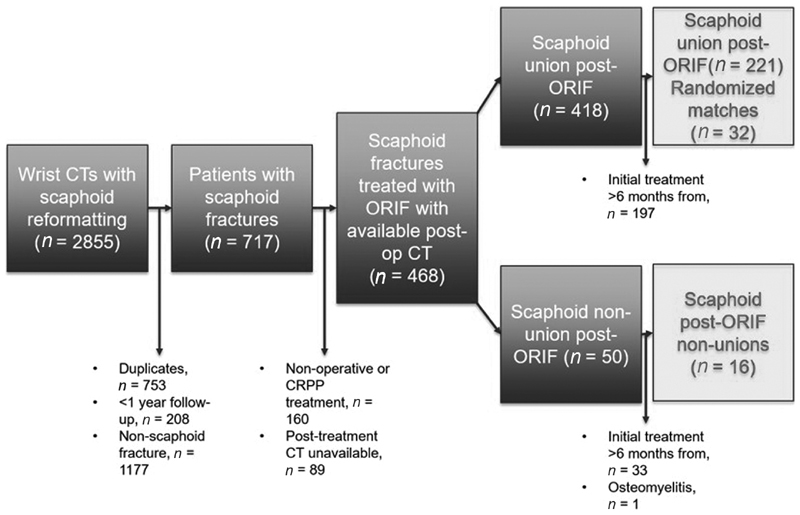
Study recruitment shown in exclusion flowchart.
Description of Three-Dimensional Computer Analysis
Three-dimensional models were created using postoperative CT scans. Mimics software (Materialise, Leuven, Belgium) was used to segment the scaphoid, fracture fragments, and implants ( Fig. 2 ) into surface meshes. Volumes of the scaphoid and its fragments were calculated from volume-filled masks of the surface meshes. The masks were created by calculating three-dimensional objects, calculating polylines, and cavity-filling the meshes. The masks were imported as stereolithography files into Geomagic Design X (3D Systems, Rock Hill, SC) for further measurements. Virtual reduction was manually performed by transforming the distal fragment toward the proximal fragment. 28 29
Fig. 2.
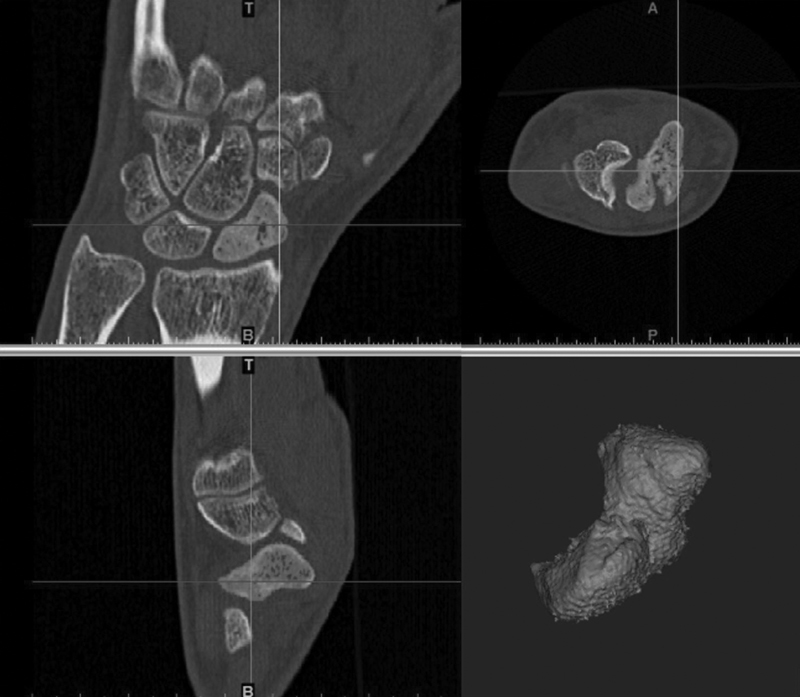
Segmentation of scaphoid was done from CT scans. CT, computed tomography.
After modeling the fracture fragments, relevant indices were modeled including longitudinal axis of the scaphoid, fracture plane and area, and screw trajectory and screw distance from subchondral bone. To approximate the longitudinal axis of the scaphoid, a cylinder best fit vector was created for the scaphoid mask 30 ( Fig. 3 ). The fracture plane was modeled using a best fit plane created on the proximal fragment. The angle between the fracture plane and longitudinal axis was measured, depicting the smallest angle between the plane and axis. 28
Fig. 3.
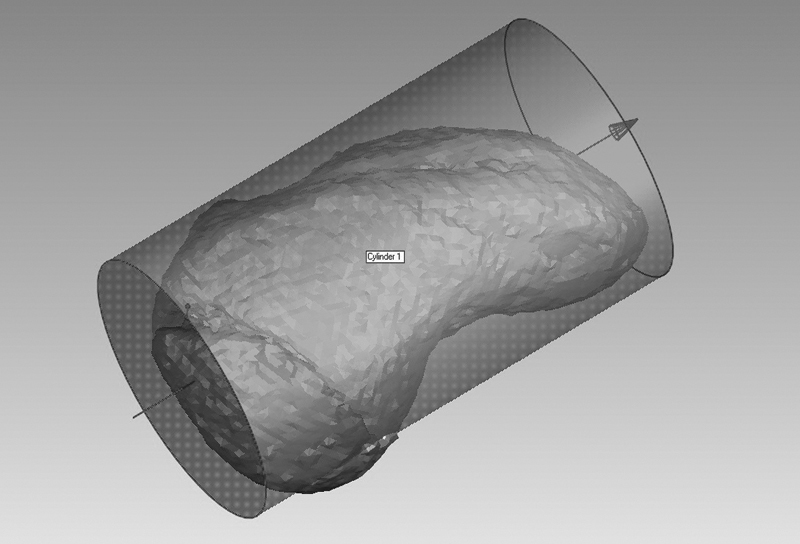
A cylinder best fit algorithm approximated the scaphoid longitudinal axis.
After delineation of fracture characteristics, fixation constructs were evaluated. The angle between the screw axis and the scaphoid longitudinal axis was measured. Additionally, the angle between the screw axis and the fracture plane was measured ( Fig. 4 ). This value subtracted from 90 degrees is the angle between the screw and perpendicular axis to the fracture. 20 Finally, to determine screw distance from subchondral bone, a plane was created at the distal end of the screw perpendicular to the screw longitudinal axis. The distance between this plane and the end of the distal scaphoid fragment was measured digitally.
Fig. 4.
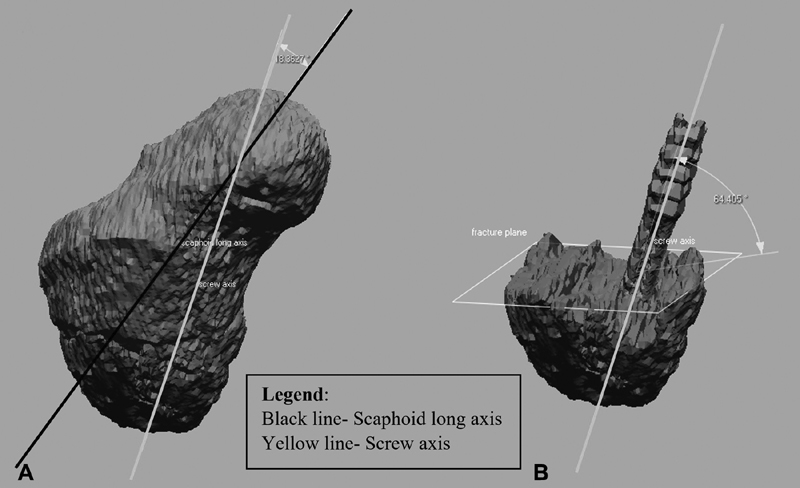
The angle of screw axis to scaphoid longitudinal axis ( A ) and angle of screw axis to fracture plane ( B ) were measured.
Statistical Analysis
Descriptive statistics were reported as means and standard deviations for continuous variables, while discrete variables were reported as frequencies and percentages. Assumption of normality of continuous variables was found to be violated using Shapiro–Wilk's tests. Differences in continuous variables between patients with union versus nonunion were evaluated using Mann–Whitney's U tests. Fisher's exact tests were used to compare differences in discrete variables between study groups.
Because of the limited sample size available, variables in the univariate analysis that achieved a p value of 0.20 or less were considered as candidate variables eligible for evaluation in a conditional logistic regression model. Conditional regression modeling was used to account for the matched design of the nonunion and union patients in the study to identify potential risk factors associated with nonunion. To prevent overfitting of variables in the model, stepwise iterations were performed until a final model converged. Variables that achieved a p value of 0.20 or less were retained in the final model, while those that achieved a p value of 0.05 or less were called statistically significant. Results from the regression analysis are reported as odds ratio (OR) and 95% confidence intervals (CI). Receiver operating characteristic (ROC) curve was generated to determine if there was any potential threshold in fracture volume or time from injury to treatment that would best predict fracture nonunion. All analyses were performed using SPSS version 23.0 (IBM Corp., Armonk, NY).
Post hoc power analysis demonstrated group sample sizes of 16 and 32 achieve 74% power to detect a difference in length of time to initial treatment and a 30% power to detect a difference in average proximal fragment percent of total scaphoid volume.
Results
The mean period of follow-up to determine if additional surgery was performed was 3.2 years (range = 1–10.4). Of the 48 total patients, there were six female and 42 male patients with no difference between union and nonunion patients ( p > 0.99). The mean age was 30.8 (range = 17–57) years and mean BMI was 25.1 kg/m 2 (range = 19.4–39.6) with no statistical difference between study groups. A total of 23% patients were nonwhite. Low energy trauma accounted for 73% of all fractures, with 66% in the union group and 88% in the nonunion group. There were seven proximal pole and 41 waist fractures, as one fracture was reclassified from its initial radiology read based on the consensus of all authors ( Table 1 ). Among the 38 fractures that had preoperative imaging available, 95% were read as nondisplaced and 5% were minimally displaced. Of the two fractures that were minimally displaced, one went on to union and one went on to nonunion. Qualitative displacement was excluded from statistical analysis due to incomplete data and small sample size.
Table 1. Demographics by union versus nonunion.
| Variable | Total | Union | Nonunion | p -Value | |||
|---|---|---|---|---|---|---|---|
| Mean or n | SD or % | Mean or n | SD or % | Mean or n | SD or % | ||
| Age (at time of surgery) | 30.8 | 11.7 | 30.5 | 11.9 | 31.5 | 11.6 | 0.66 |
| BMI | 25.1 | 3.8 | 24.3 | 2.8 | 26.6 | 5.1 | 0.17 a |
| Sex | |||||||
| Female | 6 | 13% | 4 | 13% | 2 | 13% | 1.00 |
| Male | 42 | 88% | 28 | 88% | 14 | 88% | |
| Smoking status | |||||||
| Former/nonsmoker | 47 | 98% | 32 | 100% | 15 | 94% | 0.33 |
| Current smoker | 1 | 2% | 0 | 0% | 1 | 6% | |
| Race | |||||||
| Caucasian | 37 | 77% | 25 | 78% | 12 | 75% | 1.00 |
| Non-Caucasian | 11 | 23% | 7 | 22% | 4 | 25% | |
| Mechanism of trauma | |||||||
| Low energy | 35 | 73% | 21 | 66% | 14 | 88% | 0.17 a |
| High energy | 13 | 27% | 11 | 34% | 2 | 13% | |
Abbreviations: BMI, body mass index; SD, standard deviation.
Variables that achieved a p value of 0.20 or less in the univariate analysis which were included in the multivariate regression.
Length of time from injury to initial ORIF was significantly higher in the nonunion group (63.3 vs. 27 days, p = 0.02). The mean proximal fragment volume percent of total scaphoid volume was 37.9% for all fractures, and 40% for the union group and 33.8% for the nonunion group ( p = 0.12). The screw obliquity to the longitudinal axis of the scaphoid was greater for the nonunion group than the union group, but this was not statistically significant (19.6 vs. 14.4 degrees, p = 0.18; Table 2 ).
Table 2. Fracture characteristics by union versus nonunion.
| Variable | Total | Union | Nonunion | p -Value | |||
|---|---|---|---|---|---|---|---|
| Mean or n | SD | Mean or n | SD | Mean or n | SD | ||
| Fracture location | |||||||
| Proximal | 7 | 15% | 4 | 13% | 3 | 19% | 0.67 |
| Waist | 41 | 85% | 28 | 88% | 13 | 81% | |
| Avg. % of fragment volume (proximal) | 37.9 | 15.0 | 40.0 | 14.3 | 33.8 | 16.0 | 0.12 a |
| Long axis-fracture plane angle (obliquity) | 47.0 | 16.6 | 49.1 | 14.7 | 43.2 | 19.6 | 0.36 |
Abbreviation; SD, standard deviation.
Variables that achieved a p value of 0.20 or less in the univariate analysis that were included in the multivariate regression.
The majority (94%) of patients in the cohort was treated by dorsal approach and 48% were treated with bone graft. The length of implant in the union group was significantly longer than the length in the nonunion group (21.8 vs. 19.8 mm, p = 0.02). The caliber of implant, surgical approach and use of bone graft were similar between the groups. Neither the angle of the screw to the fracture plane, the distance of the screw to subchondral bone, nor the caliber of the implant were significantly different between the groups ( Table 3 ).
Table 3. Surgical factors by union versus nonunion.
| Variable | Total | Union | Nonunion | p -Value | |||
|---|---|---|---|---|---|---|---|
| Mean or n | SD or % | Mean or n | SD or % | Mean or n | SD or % | ||
| Angle of screw to long axis (degrees) | 16.2 | 9.1 | 14.4 | 7.2 | 19.6 | 11.7 | 0.18 a |
| Angle of screw to fracture plane (degrees) | 54.1 | 17.4 | 53.0 | 19.2 | 56.3 | 13.1 | 0.62 |
| Distance from screw to distal cortex (mm) | 1.4 | 1.6 | 1.3 | 1.4 | 1.8 | 1.9 | 0.65 |
| Length of implants (mm) | 21.1 | 2.6 | 21.8 | 2.4 | 19.8 | 2.5 | 0.02 a |
| Caliber of implants (mm) | 2.2 | 0.6 | 2.2 | 0.6 | 2.4 | 0.6 | 0.38 |
| Length of time to initial treatment (d) | 39.1 | 41.0 | 27.0 | 31.5 | 63.3 | 47.9 | 0.02 a |
| Initial treatment type | |||||||
| Operative - ORIF Dorsal Approach | 45 | 94% | 31 | 97% | 14 | 88% | 0.25 |
| Operative - ORIF Volar Approach | 3 | 6% | 1 | 3% | 2 | 13% | |
| Bone graft during initial surgery | |||||||
| No | 25 | 52% | 18 | 56% | 7 | 44% | 0.41 |
| Yes | 23 | 48% | 14 | 44% | 9 | 56% | |
Abbreviation: ORIF, open reduction and internal fixation.
Variables that achieved a p value of 0.20 or less in the univariate analysis that were included in the multivariate regression.
The variables that achieved a p value of 0.20 or less in the univariate analysis were considered as candidate variables for analysis in the conditional logistic regression model. These variables were BMI, mechanism of trauma, average percent of fragment volume, angle of screw to long axis, length of time to initial surgery, and length of implants. After stepwise iterations to achieve a final model, the only variables that were retained in the final model and identified as potential risk factors with fracture nonunion were lower average percent of fragment volume and increased length of time from injury to surgery ( Table 4 ). For every 1% increase in fracture volume, there was a 10% decrease in risk of nonunion (OR: 0.90, 95% CI: 0.81–1.01). Conversely, for every day delayed in initial treatment, the risk of nonunion increases by 4% (OR: 1.04, 95% CI: 1.01–1.07). Receiver operating curve analysis revealed that fracture volume below 38% and time from injury to surgery greater than 31 days were associated with nonunion (78% specificity and 44% sensitivity; and 63% sensitivity, and 28% false positive rate, respectively; Fig. 5 ).
Table 4. Multivariate regression.
| Model | Variable | Odds ratio | 95% CI | p -Value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Full | BMI | 0.50 | 0.00 | 180.39 | 0.817 |
| Mechanism of trauma | 0.00 | 0.00 | 7.62E + 21 | 0.607 | |
| Average % of fragment volume (proximal) | 1.31 | 0.45 | 3.87 | 0.621 | |
| Angle of screw to long axis | 0.99 | 0.12 | 8.36 | 0.994 | |
| Length of implants | 8.34 | 0.00 | 2.35E + 04 | 0.601 | |
| Length of time to initial treatment (d) | 1.21 | 0.70 | 2.11 | 0.493 | |
| Final | Average % of fragment volume (proximal) | 0.90 | 0.81 | 1.01 | 0.05 |
| Length of time to initial treatment (d) | 1.04 | 1.01 | 1.07 | 0.02 | |
Abbreviations: BMI, body mass index; CI, confidence interval.
Note: Dependent variable: nonunion.
Fig. 5.
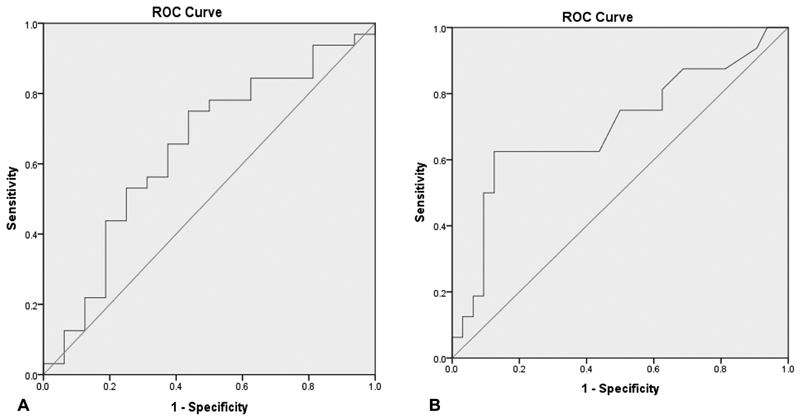
Receiver operating characteristic curve analysis was completed to find the ( A ) average percent of fragment volume (proximal) on union and ( B ) length of time to initial treatment (days) on nonunion.
Discussion
This study was performed to assess both fracture-specific and surgical factors that affect progression to nonunion after scaphoid ORIF. We found that timeliness of surgery following injury and fracture volume are critical factors associated with progression to nonunion. Specifically, patients undergoing fixation greater than 1 month from injury have greater risk for nonunion after ORIF. Additionally, our results suggest that when the fracture volume is less than 38% of the entire scaphoid, there is an increased risk for nonunion after surgery. We did not find volar versus dorsal surgical approach, use of bone graft, fracture obliquity, nor screw angle relationship to the fracture plane or scaphoid longitudinal axis to be significantly different between the union and nonunion cohorts.
Previous investigations have cited time to treatment as a critical factor in union after scaphoid fracture. Ramamurthy et al evaluated 126 scaphoid nonunions presenting after initial nonoperative management with an average time to operative treatment of 42 months (3 months–16 years). The study also demonstrated that time between injury and surgery to be significant ( p = 0.02) in a stepwise multivariate logistic regression. 12 Nakamura et al found that functional outcome, including motion, wrist pain, and strength was worse when surgery was performed more than 5 years from injury regardless of bony union. 15 The current study also confirms the impact of time to treatment on scaphoid union but is unique in its identification of the importance of time to surgery even when scaphoid fracture is treated within 6 months of injury. This novel finding, emphasizing the importance of early ORIF, may suggest the advantage of earlier identification and treatment of scaphoid fractures in the acute setting instead of a trial of nonoperative management.
Several studies have suggested that a larger proximal pole fracture fragments are associated with better outcomes. 9 10 In a study of 222 patients presenting with acute scaphoid fractures managed by cast immobilization, Leslie and Dickson demonstrated that fracture location had the greatest influence on union rates with proximal pole fractures demonstrating the greatest delay to radiological and clinical union as well as the greatest rate of nonunion. 31 Similarly, Herbert found that proximal pole fractures have the greatest rate of nonunion and avascular necrosis and suggested that all proximal pole fractures should be managed operatively in the acute setting. 32 Our study further demonstrates that even in the setting of acute operative management, fractures with smaller proximal pole fragments, have increased rates of nonunion. While in the current study, we controlled for qualitative fracture location (proximal, waist, or distal), we nonetheless found quantitatively that when the fracture fragment was less than 38% of the total volume of the scaphoid, risk for nonunion was greatest.
Several limitations are inherent in our study. Because scaphoid nonunion is a relatively rare event following early management by ORIF, our cohort of nonunion cases is small. We intentionally limited the series to cases treated within 6 months from time of injury to specifically investigate factors impacting nonunion in fractures that were recognized early, thus excluding fractures that had may have progressed to nonunion prior to initiation of treatment. Furthermore, we only included cases if postoperative CT imaging was available to accurately characterize fracture segments, volumes, and angles and to provide consistent verification of fracture union postoperatively. This resulted in exclusion of patients if they were lost to follow up or were not imaged with CT postoperatively. While there is a large difference in proportion of nonunion to union in the initial population and the study population, this case–control study carefully matched patients to precisely define these exposures and outcomes. Second, as our primary aim was to report on the radiographic outcome of union after ORIF, we do not report any patient outcome data. As a retrospective review, our ability to evaluate history, presenting symptoms and signs as well as postoperative outcomes was limited to the medical record. Given heterogeneity in data recording, functional metrics such as pain and range of motion were not reported in our manuscript. Additionally, we are unable to analyze the effect of displacement at the time of injury on nonunion risk due to incomplete preoperative imaging records. In the 38 of 48 patients with preoperative imaging, there was a similar distribution of initial fracture displacement, which did not lead to difference in rate of nonunion. However, as we were underpowered for this analysis, we did not report fracture displacement at the time of injury as a factor in our models. This factor may represent an important consideration for future study. These inherent limitations to our retrospective review are considered versus the value of the highly quantitative and objective data provided by three-dimensional (3D) CT analysis of several fracture and surgical parameters. Finally, with regard to 3D analysis, postoperative CT scans were used to calculate all computer-based metrics, which may have led to variation and underreporting of fragment volume given bone loss. In spite of these limitations, computer modeling from CT DICOM files more accurately reflects fracture plane in relationship to the long axis of the scaphoid and to hardware when compared with conventional radiographs. 28
Using 3D CT imaging and modeling of fracture pattern and screw fixation, our study investigated both fracture and surgical characteristics for their impact on scaphoid union after early treatment by ORIF. In contradistinction to prior biomechanical studies, in this clinical series of fractures treated within 6 months of injury, we were not able to demonstrate with statistical significance a difference between the union and nonunion groups with respect to screw obliquity versus the fracture plane or longitudinal axis of the scaphoid. These biomechanical relationships may be clinically relevant at extremes and more data are required to study specific predictors of nonunion among scaphoid waist fractures.
We found that fractures treated with surgery greater than 1 month after injury were associated with nonunion, a novel finding, as the study includes only fractures treated within 6 months of injury. Even when matching for fracture location by traditional three-zone classification, smaller fragment volume was also found to be significantly associated with scaphoid nonunion. Specifically, quantitative analysis showed that fracture fragment volume less than 38% of the entire scaphoid carried increased likelihood for nonunion following early treatment by ORIF. Further studies with larger cohorts and prospectively collected data would be beneficial in informing optimal management of these fractures to decrease risk for nonunion as a complication following early scaphoid ORIF.
Knowledge of these associations may aid in counseling and decision-making with regard to management of patients with these fractures in the acute setting. Finally, these data may also support the earlier use of advanced imaging to look for signs of nonunion if a trial of conservative management has been initiated.
Acknowledgments
The authors thank the Scaphoid Nonunion Consortium, Krystle Hearns, Ryan Breighner, Kate Meyers, and the HSS Department of Biomechanics.
Scaphoid Nonunion Consortium: Edward Athanasian, MD, Aaron Daluiski, MD, Robert Hotchkiss, MD, Lana Kang, MD, Steve Lee, MD, Daniel Osei, MD, Andrew Weiland, MD, Scott Wolfe, MD—Division of Hand and Upper Extremity Surgery, Hospital for Special Surgery, Department of Orthopedic Surgery; Manjula Bansal, MD—Department of Pathology and Laboratory Medicine, Hospital for Special Surgery; Keith Crivello, MD, Mercer-Bucks Orthopaedics, P.C.; Edward McCarthy MD—Department of Pathology, The Johns Hopkins Hospital; and Hollis G. Potter MD—Department of Radiology and Imaging Hospital for Special Surgery.
Conflict of Interest None declared.
Ethical Approval
This study was approved by the Institutional Review Board at the Hospital for Special Surgery. Work was performed at the Hospital for Special Surgery, New York City. There were no outside sources of funding.
References
- 1.Hove L M. Epidemiology of scaphoid fractures in Bergen, Norway. Scand J Plast Reconstr Surg Hand Surg. 1999;33(04):423–426. doi: 10.1080/02844319950159145. [DOI] [PubMed] [Google Scholar]
- 2.Freedman D M, Botte M J, Gelberman R H. Vascularity of the carpus. Clin Orthop Relat Res. 2001;(383):47–59. doi: 10.1097/00003086-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Mack G R, Bosse M J, Gelberman R H, Yu E. The natural history of scaphoid non-union. J Bone Joint Surg Am. 1984;66(04):504–509. [PubMed] [Google Scholar]
- 4.Daly K, Gill P, Magnussen P A, Simonis R B. Established nonunion of the scaphoid treated by volar wedge grafting and Herbert screw fixation. J Bone Joint Surg Br. 1996;78(04):530–534. [PubMed] [Google Scholar]
- 5.Inoue G, Shionoya K, Kuwahata Y. Herbert screw fixation for scaphoid nonunions. An analysis of factors influencing outcome. Clin Orthop Relat Res. 1997;(343):99–106. [PubMed] [Google Scholar]
- 6.Kawamura K, Chung K C. Treatment of scaphoid fractures and nonunions. J Hand Surg Am. 2008;33(06):988–997. doi: 10.1016/j.jhsa.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warren-Smith C D, Barton N J. Non-union of the scaphoid: Russe graft vs Herbert screw. J Hand Surg [Br] 1988;13(01):83–86. doi: 10.1016/0266-7681_88_90060-5. [DOI] [PubMed] [Google Scholar]
- 8.Gelberman R H, Menon J. The vascularity of the scaphoid bone. J Hand Surg Am. 1980;5(05):508–513. doi: 10.1016/s0363-5023(80)80087-6. [DOI] [PubMed] [Google Scholar]
- 9.Shah J, Jones W A. Factors affecting the outcome in 50 cases of scaphoid nonunion treated with Herbert screw fixation. J Hand Surg [Br] 1998;23(05):680–685. doi: 10.1016/s0266-7681(98)80028-4. [DOI] [PubMed] [Google Scholar]
- 10.Steinmann S P, Adams J E. Scaphoid fractures and nonunions: diagnosis and treatment. J Orthop Sci. 2006;11(04):424–431. doi: 10.1007/s00776-006-1025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taleisnik J, Kelly P J. The extraosseous and intraosseous blood supply of the scaphoid bone. J Bone Joint Surg Am. 1966;48(06):1125–1137. [PubMed] [Google Scholar]
- 12.Ramamurthy C, Cutler L, Nuttall D, Simison A JM, Trail I A, Stanley J K. The factors affecting outcome after non-vascular bone grafting and internal fixation for nonunion of the scaphoid. J Bone Joint Surg Br. 2007;89(05):627–632. doi: 10.1302/0301-620X.89B5.18183. [DOI] [PubMed] [Google Scholar]
- 13.Lim T K, Kim H K, Koh K H, Lee H I, Woo S J, Park M J. Treatment of avascular proximal pole scaphoid nonunions with vascularized distal radius bone grafting. J Hand Surg Am. 2013;38(10):1906–120. doi: 10.1016/j.jhsa.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Rancy S K, Swanstrom M M, DiCarlo E F, Sneag D B, Lee S K, Wolfe S W; Scaphoid Nonunion Consortium.Success of scaphoid nonunion surgery is independent of proximal pole vascularity J Hand Surg Eur Vol 2018430132–40. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura R, Horii E, Watanabe K, Tsunoda K, Miura T. Scaphoid non-union: factors affecting the functional outcome of open reduction and wedge grafting with Herbert screw fixation. J Hand Surg [Br] 1993;18(02):219–224. doi: 10.1016/0266-7681(93)90114-u. [DOI] [PubMed] [Google Scholar]
- 16.Schuind F, Haentjens P, Van Innis F, Vander Maren C, Garcia-Elias M, Sennwald G. Prognostic factors in the treatment of carpal scaphoid nonunions. J Hand Surg Am. 1999;24(04):761–776. doi: 10.1053/jhsu.1999.0761. [DOI] [PubMed] [Google Scholar]
- 17.Trezies A JH, Davis T RC, Barton N J. Factors influencing the outcome of bone grafting surgery for scaphoid fracture non-union. Injury. 2000;31(08):605–607. doi: 10.1016/s0020-1383(00)00059-0. [DOI] [PubMed] [Google Scholar]
- 18.Luchetti T J, Hedroug Y, Fernandez J J, Cohen M S, Wysocki R W. The morphology of proximal pole scaphoid fractures: implications for optimal screw placement. J Hand Surg Eur Vol. 2018;43(01):73–79. doi: 10.1177/1753193417739546. [DOI] [PubMed] [Google Scholar]
- 19.Luria S, Hoch S, Liebergall M, Mosheiff R, Peleg E. Optimal fixation of acute scaphoid fractures: finite element analysis. J Hand Surg Am. 2010;35(08):1246–1250. doi: 10.1016/j.jhsa.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Swanstrom M M, Morse K W, Lipman J D, Hearns K A, Carlson M G. Effect of screw perpendicularity on compression in scaphoid waist fractures. J Wrist Surg. 2017;6(03):178–182. doi: 10.1055/s-0036-1596059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swanstrom M M, Morse K W, Lipman J D, Hearns K A, Carlson M G. Variable bone density of scaphoid: importance of subchondral screw placement. J Wrist Surg. 2018;7(01):66–70. doi: 10.1055/s-0037-1605381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grewal R, Assini J, Sauder D, Ferreira L, Johnson J, Faber K. A comparison of two headless compression screws for operative treatment of scaphoid fractures. J Orthop Surg Res. 2011;6(01):27. doi: 10.1186/1749-799X-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hart A, Harvey E J, Rabiei R, Barthelat F, Martineau P A. Fixation strength of four headless compression screws. Med Eng Phys. 2016;38(10):1037–1043. doi: 10.1016/j.medengphy.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 24.Mandaleson A, Tham S K, Lewis C, Ackland D C, Ek E T. Scaphoid fracture fixation in a nonunion model: a biomechanical study comparing 3 types of fixation. J Hand Surg Am. 2018;43(03):221–228. doi: 10.1016/j.jhsa.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 25.McCallister W V, Knight J, Kaliappan R, Trumble T E. Central placement of the screw in simulated fractures of the scaphoid waist: a biomechanical study. J Bone Joint Surg Am. 2003;85(01):72–77. doi: 10.2106/00004623-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Hart A, Harvey E J, Lefebvre L P, Barthelat F, Rabiei R, Martineau P A. Insertion profiles of 4 headless compression screws. J Hand Surg Am. 2013;38(09):1728–1734. doi: 10.1016/j.jhsa.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dodds S D, Panjabi M M, Slade J F., III Screw fixation of scaphoid fractures: a biomechanical assessment of screw length and screw augmentation. J Hand Surg Am. 2006;31(03):405–413. doi: 10.1016/j.jhsa.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 28.Luria S, Schwarcz Y, Wollstein R, Emelife P, Zinger G, Peleg E. 3-dimensional analysis of scaphoid fracture angle morphology. J Hand Surg Am. 2015;40(03):508–514. doi: 10.1016/j.jhsa.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Schwarcz Y, Schwarcz Y, Peleg E, Joskowicz L, Wollstein R, Luria S. Three-dimensional analysis of acute scaphoid fracture displacement: proximal extension deformity of the scaphoid. J Bone Joint Surg Am. 2017;99(02):141–149. doi: 10.2106/JBJS.16.00021. [DOI] [PubMed] [Google Scholar]
- 30.Leventhal E L, Wolfe S W, Walsh E F, Crisco J J. A computational approach to the “optimal” screw axis location and orientation in the scaphoid bone. J Hand Surg Am. 2009;34(04):677–684. doi: 10.1016/j.jhsa.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Leslie I J, Dickson R A. The fractured carpal scaphoid. Natural history and factors influencing outcome. J Bone Joint Surg Br. 1981;63-B(02):225–230. doi: 10.1302/0301-620X.63B2.7217146. [DOI] [PubMed] [Google Scholar]
- 32.Herbert T J. The fractured scaphoid. St. Louis quality. Medical Publishing. 1990:57. [Google Scholar]


