Abstract
This study set out to identify and analyse trends and seasonal variations of monthly global reported cases of the Middle East respiratory syndrome coronavirus (MERS-CoV). It also made a prediction based on the reported and extrapolated into the future by forecasting the trend. Finally, the study assessed contributions of various risk factors in the reported cases. The motivation for this study is that MERS-CoV remains among the list of blueprint priority and potential pandemic diseases globally. Yet, there is a paucity of empirical literature examining trends and seasonality as the available evidence is generally descriptive and anecdotal. The study is a time series analysis using monthly global reported cases of MERS-CoV by the World Health Organisation between January 2015 and January 2018. We decomposed the series into seasonal, irregular and trend components and identified patterns, smoothened series, generated predictions and employed forecasting techniques based on linear regression. We assessed contributions of various risk factors in MERS-CoV cases over time. Successive months of the MERS-CoV cases suggest a significant decreasing trend (P = 0.026 for monthly series and P = 0.047 for Quarterly series). The MERS-CoV cases are forecast to wane by end 2018. Seasonality component of the cases oscillated below or above the baseline (the centred moving average), but no association with the series over time was noted. The results revealed contributions of risk factors such as camel contact, male, old age and being from Saudi Arabia and Middle East regions to the overall reported cases of MERS-CoV. The trend component and several risk factors for global MERS-CoV cases, including camel contact, male, age and geography/region significantly affected the series. Our statistical models appear to suggest significant predictive capacity and the findings may well inform healthcare practitioners and policymakers about the underlying dynamics that produced the globally reported MERS-CoV cases.
Key words: Forecasting, MERS-COV cases, prediction, risk factors, seasonality, trend
Introduction
This study set out to identify trends and seasonal variations; made a prediction based on the globally reported cases of the Middle East respiratory syndrome coronavirus (MERS-CoV), extrapolated into the future by forecasting the trend and assessed contributions of various risk factors for the MERS-CoV cases. Specifically, we consider the questions: (1) what is the underlying growth or trend of the globally reported MERS-CoV cases? (2) Are there seasonal variations present in globally reported MERS-CoV cases over time and how do they affect the series? (3) What are the contributions of various risk factors for MERS-CoV cases?
The motivation for this study is that to date, the World Health Organisation (WHO) places the MERS-CoV among the list of blueprint priority diseases. Although a survey of the literature shows a rapid increase in research activities related to MERS-CoV [1], yet there is still a general paucity of empirical literature examining trends and seasonality. To the best of our knowledge, there is a single study, which anecdotally examined seasonality and time series patterns of MERS-CoV to date [2]. Given MERS-CoV remains a potential pandemic disease globally, it is important to understand the dynamics of the underlying growth or trend of the globally reported cases.
The motivation for this study also comes from the aetiology of MERS-CoV, especially its causes, spread, the complexity of its diagnosis and mortality. MERS-CoV is a virus that causes severe viral pneumonia in humans, known to have a high mortality rate [3–7] and has clinical symptoms similar to severe acute respiratory syndrome coronavirus [8, 9]. It was first reported in Saudi Arabia [10] and after that, the virus exhibited outbreaks in several regions of the world, particularly Saudi Arabia and the Republic of Korea [10, 11]. Additionally, the complexity of MERS-CoV and its diagnosis of infection have been acknowledged in the literature [12, 13]. According to WHO, 2160 laboratory-confirmed cases of MERS-CoV were reported at the end of January 2018, including 773 associated deaths (case–fatality rate: 35.8%) that were reported globally [14]. The majority of these cases were reported in Saudi Arabia.
Methods
This is a time series analysis using publicly reported MERS-CoV monthly global cases. The WHO receives confirmed MERS-CoV cases from countries across the world. To date, new cases continue to be identified and reported to the WHO, specifically from the Middle East region. These data are available at http://www.who.int/csr/don/archive/disease/coronavirus_infections/en/. The latest report included one case from Malaysia on 8 January 2018. We used time series of MERS-CoV cases reported between January 2015 and January 2018, where WHO began using standard case report. A research assistant retrieved data from WHO webpage and reviewed for quality by the second study author. The data retrieved include patient and clinical data such as age, gender, healthcare worker, comorbidity, the source of infection and geographical regions. The main outcome was the number of cases reported on a monthly basis from January 2015 to January 2018.
Statistical analysis
The analysis was performed using STATA 12 (STATA Corp., Texas, USA) and Microsoft Excel 10. Using the classical multiplicative time series model, we decomposed the original series into seasonal, irregular and trend components and examined their effects. Additionally, we identified patterns, smoothened series, generated predictions and employed forecasting techniques based on linear regression. Finally, we assessed contributions of various risk factors in MERS-CoV cases over time. P-values <0.05 were considered statistically significant.
Results
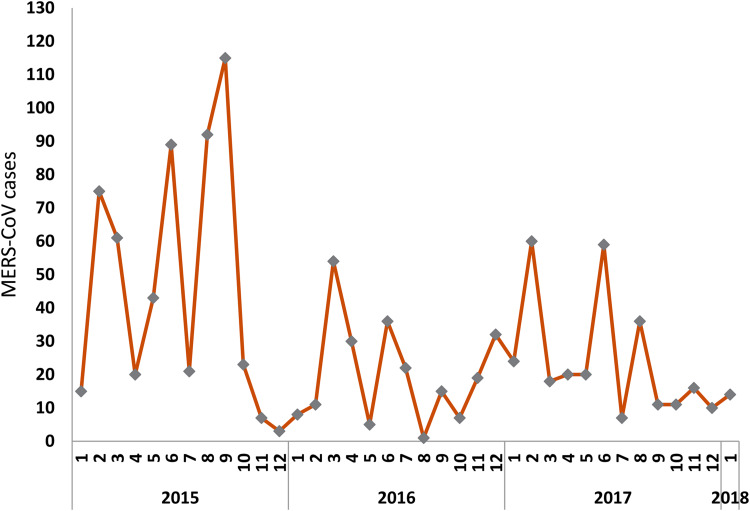
Figure 1 shows that although cases of MERS-CoV are decreasing in the range period selected, the series exhibits peaks and spikes.
Fig. 1.
Time series of globally reported MERs-COV cases (January 2015 to January 2018).
Figure 1 also reveals a negative trend of MERS-CoV series from January 2015 to January 2018.
We investigated direction and significance of this trend, as well as stationarity of the series. While a unit root test for non-stationarity confirmed the MERS-CoV cases had a negative and statistically significant trend, the series was found to be stationary. The negative and statistically significant trend was also confirmed by subsequent regressions.
We collapsed the monthly series into quarters and then smoothened out the series using a centred moving average in order to understand underlying growth component. We assumed the classical multiplicative time series model by decomposing original series into seasonal, irregular, and trend components (Table 1)
Table 1.
Decomposing reported MERS-CoV cases
| Time | year | Quarter | MERS-CoV Cases | Moving average (4) | Centred moving average (4) | Seasonality with irregularity(St, It) | Seasonality, (St) | Deseasonalised | Trend | Predicted/Forecast |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2015 | Q1 | 151 | 1.05 | 144 | 164.74 | 173 | |||
| 2 | Q2 | 152 | 1.11 | 137 | 153.91 | 170 | ||||
| 3 | Q3 | 228 | 0.69 | 330 | 143.07 | 99 | ||||
| 4 | Q4 | 33 | 141 | 131 | 0.25 | 0.59 | 56 | 132.23 | 78 | |
| 5 | 2016 | Q1 | 73 | 122 | 111 | 0.66 | 1.05 | 70 | 121.39 | 127 |
| 6 | Q2 | 71 | 101 | 78 | 0.92 | 1.11 | 64 | 110.55 | 122 | |
| 7 | Q3 | 38 | 54 | 57 | 0.67 | 0.69 | 55 | 99.72 | 69 | |
| 8 | Q4 | 58 | 60 | 64 | 0.91 | 0.59 | 99 | 88.88 | 52 | |
| 9 | 2017 | Q1 | 102 | 67 | 71 | 1.44 | 1.05 | 97 | 78.04 | 82 |
| 10 | Q2 | 99 | 74 | 76 | 1.30 | 1.11 | 89 | 67.20 | 74 | |
| 11 | Q3 | 54 | 78 | 76 | 0.71 | 0.69 | 78 | 56.36 | 39 | |
| 12 | Q4 | 37 | 73 | 62 | 0.60 | 0.59 | 63 | 45.53 | 27 | |
| 13 | 2018 | Q1 | 14 | 51 | 1.05 | 13 | 34.69 | 36 | ||
| 14 | Q2* | 1.11 | 23.85 | 26.41 | ||||||
| 15 | Q3* | 0.69 | 13.01 | 8.99 | ||||||
| 16 | Q4* | 0.59 | 2.17 | 1.28 |
Q1, Q2, Q3, Q4 are the Quarters of the year and * = implies forecast.
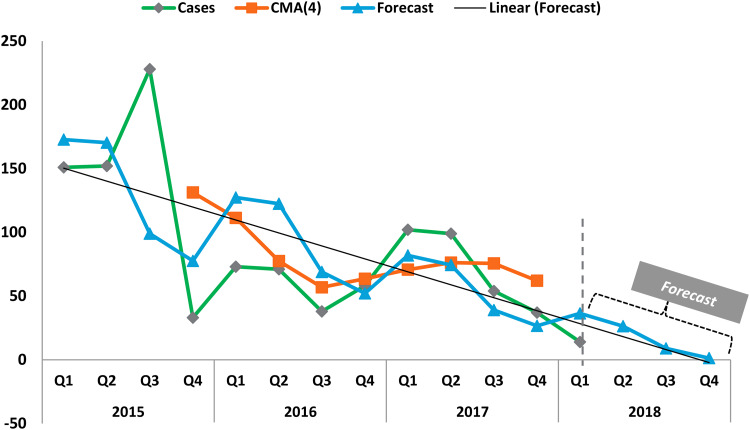
Our decomposition of the MERS-CoV cases shows that in 2016Q2, the seasonality and irregularity components of the series were 8% below the baseline (the centred moving average). Decomposing further, seasonality component was 11% above the baseline in 2016Q2, while it was 41% below the baseline in 2016Q4. Our analysis also shows the de-seasonalised series of the original MERS-CoV by removing seasonality and irregularity components. Using a linear regression, we then estimated the effect of time on the deseasonalised series to capture the underlying growth or trend component using a linear regression in order to make predictions. Since the last available data was in January 2018, we also made a forecast of three more quarters (2018Q2, Q3, Q4), an additional 9 months into the future. The forecast of the series revealed that MERS-CoV cases would approach zero by end of 2018 or beginning of 2019, making further extrapolation into the horizon infeasible.
Figure 2 shows decomposition of MERS-CoV series of the original series, centred moving average (smoothed series), forecast and linear forecast. Together, after accounting for irregular and trend components of the series, seasonality was found to range between 41% below the baseline i.e. the centred moving average (of order 4) in some Quarters and 11% above the baseline in other Quarters. The average seasonality component was found to be 14% below the baseline. However, regression estimation revealed that, unlike the trend component, seasonality was not statistically significant, a fact also backed by our statistical test, which showed the monthly global MERS-CoV cases series were stationary.
Fig. 2.
Decomposition of MERS-CoV series, showing original series, centred moving average (CMA), linear trend and forecast. The forecast indicates the waning of MERS-CoV cases by end of 2018.
Regressions
Table 2 shows results of the effect of trend and seasonal on MERS-CoV cases. We compared the seasonal dummy variables, interpreted by comparing them with Quarter 4 (Q4) (base season) while holding time constant. Time, in this analysis, was represented by successive months and was interpreted as the effect of the linear trend on MERS-CoV cases over time, holding the effect of the seasons constant.
Table 2.
Effect of trend and seasonality (n = 37)
| Dependent variable = MERS-CoV cases | Coefficient | Robust s.e. | t value | P-value | (95% Conf. Interval) | |
|---|---|---|---|---|---|---|
| Intercept | 34.158 | 11.025 | 3.100 | 0.004 | 11.701 | 56.615 |
| Time | −0.867 | 0.420 | −2.070 | 0.047a | −1.722 | −0.012 |
| Quarter_1 | 13.970 | 8.998 | 1.550 | 0.130 | −4.358 | 32.299 |
| Quarter_2 | 16.355 | 9.647 | 1.700 | 0.100 | −3.295 | 36.005 |
| Quarter_3 | 18.733 | 12.321 | 1.520 | 0.138 | −6.363 | 43.829 |
Significant at <5% level.
The regression results revealed that after accounting for the trend, MERS-CoV cases Quarter 1 (Q1) each year averaged about 14 cases more than Q4 cases, although the effect was not found to be statistically significant. Similarly, after adjusting for the trend, cases in Quarter 2 (Q2) averaged around 16 cases more than Q4 cases. Quarter 3 (Q3) cases averaged 19 cases more than Q4 cases after accounting for the trend component time. It is important to note that the effects of seasonality were not statistically significant. However, what was revealed statistically significant is the negative trend. Consistent with the negative trend shown in Figure 1, the regression results revealed that each additional quarter registered approximately an average decrease of one case, after adjusting for the season. In other words, the MERS-CoV cases decreased, on average, by four (4 × −0.8667627) per quarter year to year. The model as a whole appears to suggest statistically significant predictive capacity.
We analysed fluctuations of reported MERS-CoV cases from period to another by graphing the residuals (generated from the regression of trend and seasonality on MERS-CoV cases) against time, as is the convention with time series analysis. The results indicated no clear patterns, suggesting that correlated errors are not a problem with this model. Other diagnostic tests also revealed neither violation of the classical linear assumptions nor correlation between the reported MERS-CoV cases in each month with cases reported in earlier months.
We further examined the effects of various risk factors of MERS-CoV cases such as camel contact, healthcare worker contact, exposure, gender and region. Table 3 shows regression that adjusts for these factors. The results reveal camel contact, Saudi Arabia and the Middle East regions, as well as being male significantly contribute to the global reported MERS-CoV cases (Table 3). The model in this estimation also appears to suggest statistically significant predictive capacity.
Table 3.
Effects of risk factors of MERS-CoV (n = 36)
| MERS-CoV Cases | Coefficient | Robust s.e. | t value | P > t | (95% Conf. Interval) | |
|---|---|---|---|---|---|---|
| Intercept | 0.400 | 0.434 | 0.920 | 0.365 | −0.049 | 1.292 |
| Camel exposure | 0.166 | 0.065 | 2.580 | 0.016a | 0.034 | 0.299 |
| Comorbidities | −0.114 | 0.099 | −1.140 | 0.264 | −0.318 | 0.091 |
| Healthcare worker | 0.004 | 0.070 | 0.060 | 0.951 | −0.140 | 0.148 |
| Saudi Arabia | 0.149 | 0.034 | 4.430 | <0.001a | 0.080 | 0.217 |
| Middle East | 0.511 | 0.134 | 3.810 | 0.001a | 0.235 | 0.788 |
| Male | 0.372 | 0.093 | 4.020 | <0.001a | 0.182 | 0.563 |
| Age 30–59 years | 0.596 | 0.094 | 6.350 | <0.001a | 0.403 | 0.789 |
| Age ⩾ 60 years | 0.493 | 0.164 | 3.010 | 0.006a | 0.156 | 0.831 |
| Exposure to MERS-CoV cases | 0.239 | 0.055 | 4.360 | <0.001a | 0.126 | 0.352 |
Significant at <5% level.
Discussion
Using linear time series models and their application to the modelling and prediction of the globally reported MERS-CoV data, the present study identified trends, analysed seasonality, predicted and forecast evolution of MERS-CoV cases and assessed the contribution of various risk factors. The decomposition of the time series of MERS-CoV cases into trend and seasonality components and making predictions have not hitherto been studied in the context of MERS-CoV pandemic. In this study, we set out to understand the dynamics of its growth over time.
The results of our time series analysis of globally reported MERS-CoV cases suggest a significant negative trend that is forecast to be eradicated in the near future unless something unexpected happens.
Our study showed that although seasonality oscillated below or above the baseline i.e. the centred moving average (of order 4) over time, the average seasonality component was found to be 14% below the baseline. Even then, our analysis showed that, unlike the trend component, seasonality did not affect the series over time. Many risk factors are associated with MERS-CoV cases, mortalities, or complications. Our results indicate those aged under 30 years (reference category) are less likely to be a MERS-CoV case than those aged over 30, consistent with several studies that associated MERS-COV with elderly patients [15, 16]. Surprisingly, comorbidity did not show a statistically significant contribution to MERS-CoV cases. However, there are studies that showed MERS-CoV cases were associated with patients with comorbidities [17–19]. A recent systematic study, for example, suggests the prevalence of comorbidities of MERS-CoV cases, diabetes, hypertension and cardiac diseases [20]. While our analysis suggests males contribute to the global reported MERS-CoV cases, gender was reported to have a mixed effect on MERS-CoV mortalities in the literature. Some studies showed men as high risk [7, 18] and MERS-CoV infects more males than females [5, 21–23]. Other literature indicated that the frequency of deaths was less in men [24].
The literature showed that MERS-CoV can be spread via human–human [25–29], or healthcare facilities [23, 30–32]. Other studies revealed animal to human [33, 34] as the primary culprit of MERS-CoV virus transmission. Specifically, the literature showed camels act as a direct source of human MERS-CoV infection [31, 35], while healthcare workers were reported to be at higher risk [7, 16, 19, 24]. The results of our study in this regard were mixed. While our study indicated that the effect of camel cases on overall MERS-CoV reported cases are positive and significant, the contribution of healthcare workers was not. Our analysis also showed evidence of geographical contributions to MERS-CoV cases such as Saudi Arabia and greater Middle East compared with South Korea. This can be seen as somewhat consistent with earlier studies that demonstrated a link between mortality associated with MERS-CoV and geography [15]. This finding is also intuitive in that Saudi Arabia and Middle East, in general, remain the global epicentre of MERS-CoV, hence the name.
The contribution of our study is that it adduces empirical evidence by making inferences and predictions based on the globally reported cases of MERS-CoV and extrapolated into the future by forecasting the trend. Unlike previous studies that descriptively analysed seasonality patterns of MERS-CoV and influenza in the Middle East [2], our study presents statistically significant results of trends of global MERS-CoV cases, consistent with regularities underlying the empirical dynamics and classical time series analysis. However, there are limitations of this study. First, the data used for this study comprised 37 months (January 2015 to January 2018). While this was just enough for several years’ worth of monthly observations to appropriately model seasonality, time series analysis can be sensitive to the number of observations. Hence, sufficiently large number of observations might have provided a better fit and results. Additionally, the analysis utilised WHO open source globally reported data, which may lack harmonisation from the various country sources.
Conclusion
This study contributes to the time series analysis of MERS-CoV literature. In particular, our analysis of trends and seasonality components the series, the prediction based on the globally reported cases of MERS-CoV and extrapolation into the future by forecasting the trend is envisaged to help in understanding the dynamics of the reported cases over time. The study findings suggest a significant negative trend of the monthly and quarterly data from 2015 to 2018. However, a further extrapolation into the future reveals that the MERS-CoV cases are forecast to be zero by end 2018 or beginning of 2019 unless something unexpected happens. Seasonality component of the series oscillated below or above the baseline, i.e. the centred moving average but did not affect the series over time. The results demonstrated that camel contact, exposure, gender, age and geography/region significantly contributed to the overall global reported MERS-CoV cases. The findings may well inform healthcare practitioners and policymakers about the underlying dynamics that produced the globally reported MERS-CoV cases.
Acknowledgements
The authors are grateful for the collegiality and research support at the College of Public Health and Health Informatics, King Saud bin Abdulaziz University for Health Sciences. The information and opinions contained in this work do not necessarily reflect the views or policy of these institutions. This research is supported by King Abdullah International Medical Research Centre (KAIMRC), King Saud bin Abdulaziz University for Health Sciences, National Guard Health Affairs, Riyadh, Saudi Arabia.
Data
These dataset used and/or analysed are available at http://www.who.int/csr/don/archive/disease/coronavirus_infections/en/.
Author contributions
OBD analysed the data and wrote manuscript. AEA retrieved the data from the WHO /registry website. AEA reviewed analysis and manuscript. All authors approved final manuscript for submission.
Ethical standards
Not applicable.
Conflict of interest
None declared.
References
- 1.Zyoud SH (2016) Global research trends of Middle East respiratory syndrome coronavirus: a bibliometric analysis. BMC Infectious Diseases 16, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He D et al. (2015) Differences in the seasonality of MERS-CoV and influenza in the Middle East. International Journal of Infectious Diseases 40, 15–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed AE (2017) The predictors of 3-and 30-day mortality in 660 MERS-CoV patients. BMC Infectious Diseases 17, 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed AE et al. (2018) Development of a risk-prediction model for Middle East respiratory syndrome coronavirus infection in dialysis patients. Hemodialysis International. 2018 Apr 14. doi: 10.1111/hdi.12661. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assiri AJ et al. (2013) Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infectious Diseases 13, 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahallawi WH et al. (2018) MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine 104, 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherbini NA et al. (2017) Middle East respiratory syndrome coronavirus in Al-Madinah City, Saudi Arabia: demographic, clinical and survival data. Journal of Epidemiology and Glob Health 7, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hui DS, Memish ZA and Zumla A (2014) Severe acute respiratory syndrome vs. the Middle East respiratory syndrome. Current Opinion in Pulmonary Medicine 20, 233–241. [DOI] [PubMed] [Google Scholar]
- 9.Corman VM et al. (2016) Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clinical Infectious Diseases 62, 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization (2018) Middle East respiratory syndrome coronavirus (MERS-CoV). Fact sheet. Retrieved April 8, 2018.
- 11.Khan MA et al. (2017) Middle East respiratory syndrome corona virus alert verification in Mirpur, Azad Kashmir. Journal Ayub Medical College Abbottabad 29, 173–175. [PubMed] [Google Scholar]
- 12.Al Johani S and Hajeer AH (2016) MERS-CoV diagnosis: an update. Journal of Infection and Public Health 9, 216–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sung H et al. (2016) Comparative evaluation of three homogenization methods for isolating Middle East respiratory syndrome coronavirus nucleic acids from sputum samples for real-time reverse transcription PCR. Annals Laboratory Medicine 36, 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization, Regional Office for the Eastern Mediterranean (2018) Epidemic and pandemic-prone diseases. MERS situation update, January 2018. Available at http://www.emro.who.int/pandemic-epidemic-diseases/mers-cov/mers-situation-update-january-2018.html.
- 15.Ahmed AE (2018) Estimating survival rates in MERS-CoV patients 14 and 45 days after experiencing symptoms and determining the differences in survival rates by demographic data, disease characteristics and regions: a worldwide study. Epidemiology and Infection 146, 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Turaiki I, Alshahrani M and Almutairi T (2016) Building predictive models for MERS-CoV infections using data mining techniques. Journal of Infection and Public Health 9, 744–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi WS et al. (2016) Clinical presentation and outcomes of Middle East respiratory syndrome in the Republic of Korea. Infection & Chemotherapy 48, 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banik GR et al. (2016) Risk factors for severity and mortality in patients with MERS-CoV: analysis of publicly available data from Saudi Arabia. Virologica Sinica 31, 81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivers CM, Majumder MS, Lofgren ET (2016) Risks of death and severe disease in patients with Middle East respiratory syndrome coronavirus, 2012–2015. American Journal of Epidemiology 184, 460–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badawi A and Ryoo SG (2016) Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. International Journal of Infectious Diseases 49, 129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Who Mers-Cov Research Group (2013) State of knowledge and data gaps of Middle East respiratory syndrome coronavirus (MERS-CoV) in humans. PLoS Currents 5, 1–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arabi YM et al. (2014) Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Annals of Internal Medicine 160, 389–397. [DOI] [PubMed] [Google Scholar]
- 23.Assiri A et al. (2013) Hospital outbreak of Middle East respiratory syndrome coronavirus. New England Journal of Medicine 369, 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al Ghamdi M et al. (2016) Treatment outcomes for patients with Middle Eastern respiratory syndrome coronavirus (MERS CoV) infection at a coronavirus referral center in the Kingdom of Saudi Arabia. BMC Infectious Diseases 16, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Health Protection Agency (HPA) UK Novel Coronavirus Investigation team (2013) Evidence of person-to-person transmission within a family cluster of novel coronavirus infections, United Kingdom, February 2013. Euro Surveillance 18, 20427. [DOI] [PubMed] [Google Scholar]
- 26.Alsahafi AJ and Cheng CA (2016) The epidemiology of Middle East respiratory syndrome coronavirus in the Kingdom of Saudi Arabia, 2012–2015. International Journal of Infectious Diseases 45, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drosten C et al. (2013) Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet Infectious Diseases 13, 745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drosten C et al. (2015) An observational, laboratory-based study of outbreaks of Middle East respiratory syndrome coronavirus in Jeddah and Riyadh, Kingdom of Saudi Arabia, 2014. Clinical Infectious Diseases 60, 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou J et al. (2015) Middle East respiratory syndrome coronavirus infection: virus-host cell interactions and implications on pathogenesis. Virology Journal 12, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harriman K, Brosseau L and Trivedi K (2013) Hospital-associated Middle East respiratory syndrome coronavirus infections. New England Journal of Medicine 369, 1761. [DOI] [PubMed] [Google Scholar]
- 31.Memish ZA, Al-Tawfiq JA and Assiri A (2013) Hospital-associated Middle East respiratory syndrome coronavirus infections. New England Journal of Medicine 369, 1761–1762. [DOI] [PubMed] [Google Scholar]
- 32.Oboho IK et al. (2015) 2014 MERS-CoV outbreak in Jeddah–a link to health care facilities. New England Journal of Medicine 372, 846–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gossner C et al. (2016) Human-Dromedary camel interactions and the risk of acquiring zoonotic Middle East respiratory syndrome coronavirus infection. Zoonoses and Public Health 63, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed AE et al. (2018) Early identification of pneumonia patients at increased risk of Middle East respiratory syndrome coronavirus infection in Saudi Arabia. International Journal of Infectious Diseases 70, 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Memish ZA et al. (2014) Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerging Infectious Diseases 20, 1012–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]