Abstract
Background
Immunotherapy plays an important role in advanced non‐small cell lung cancer (NSCLC). However, radiological evaluation is challenging due to the potential inflammatory effects of immunotherapy, which can lead to atypical response patterns. Identifying these atypical responses is critical to making treatment decisions and prognostication.
Methods
We performed a retrospective analysis of consecutive advanced NSCLC patients treated with immunotherapy (alone or in combination). We collected patients' clinical and pathological data, analyzed the proportion of patients who continued immunotherapy beyond progressive disease (PD) per RECIST 1.1, and compared the differences in response patterns between the RECIST 1.1 and iRECIST criteria.
Results
A total of 43 patients treated at the Peking Union Medical College, China from January 2018 to April 2019 were included. Continued immunotherapy beyond PD per RECIST 1.1 was observed in 10 (33.3%, 10/30) patients, of which there were discordant assessments (30%, 3/10) between the RECIST 1.1 and iRECIST, which were evaluated as PD by RECIST 1.1 and immune unconfirmed PD by iRECIST. Among seven patients with immune confirmed PD, one (1/30, 3.3%) had pseudoprogression. Patients who continued immunotherapy beyond PD (n = 10) experienced significantly prolonged overall survival (not reached vs. 8.1 months: hazard ratio, 2.8; 95% confidence interval: 2.7–13.6, P = 0.03) compared with patients who did not continue immunotherapy beyond PD (n = 20).
Conclusions
RECIST 1.1 evaluation underestimated the benefit of immunotherapy. Further research is required to optimize iRECIST and establish some criteria for selecting patients who will benefit from continued immunotherapy beyond PD per RECIST 1.1.
Keywords: Immunotherapy, iRECIST, non‐small cell lung cancer, pseudoprogression, RECIST 1.1
Introduction
Lung cancer is now the leading cause of cancer death worldwide.1 It is often diagnosed at a late stage and has a poor prognosis, and traditional radiotherapy and chemotherapy have limited efficacy. Immune checkpoint inhibitors (ICIs) targeting programmed cell death‐1 (PD‐1) or programmed cell death‐ligand 1 (PD‐L1) play an important role in the treatment of advanced non‐small cell lung cancer (NSCLC) without targeted gene mutations,2, 3 which can significantly prolong the overall survival (OS) of patients with advanced NSCLC compared with traditional chemotherapy.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 At present, nivolumab, pembrolizumab, atezolizumab, and durvalumab have been approved by the United States Food and Drug Administration for the treatment of NSCLC.
The Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 is the conventional radiological response criteria for patients with NSCLC treated with chemotherapy or targeted therapy.17 Immune checkpoint inhibitors stimulate the immune system to attack tumors instead of directly targeting tumor cells, leading to different patterns of response to immunotherapy.18 Due to the potential inflammatory effects of immunotherapy, tumors treated with immunotherapy might develop an atypical response pattern, wherein patients initially meet the criteria for progressive disease as per RECIST 1.1 but later show stable or reduced tumor burden.19, 20, 21, 22 Discontinuation of immunotherapy due to disease progression as per RECIST 1.1 may lead to the underestimation of the efficacy of immunotherapy, leading to the premature stoppage of immunotherapy. Therefore, the RECIST working group set up the immune‐related RECIST (iRECIST) to standardize and validate response criteria in trials evaluating the efficacy of immunotherapeutics.18
The present study aimed to describe the distribution of patients who continued immunotherapy beyond progressive disease (PD) as per RECIST 1.1 in the real world and compare the differences between the RECIST 1.1 and iRECIST criteria assessments.
Patients and methods
Study design
Patients with advanced NSCLC receiving immunotherapy were retrospectively enrolled at the Peking Union Medical College, China from January 2018 to April 2019. Patients without adequate radiological evaluation (without an initial computed tomography [CT] scan after immunotherapy) were excluded. The clinical data for each patient were retrospectively extracted from the CAPTRA‐Lung (NCT03334864) database. The study protocol was approved by the institutional review board of Peking Union Medical College Hospital (approval number: JS‐1410). The study was conducted in accordance with the tenets of the Declaration of Helsinki. All patients provided written informed consent for the collection of their clinical data.
Two doctors, specialized in immunotherapy evaluation (one senior, one junior), centrally reviewed all consecutive CT scans to reach a consensus. At the baseline, the sums of the longest diameters of target lesions (maximum five measurable target lesions >10 mm, maximum two per organ) and nontarget lesions were determined, following the RECIST 1.1 guidelines.17 In this study, we did not require confirmatory CT scans to assess for progressive disease because RECIST version 1.1 does not require the confirmation of progressive disease. Follow‐up scans were performed periodically according to study protocols or clinical routine. The cutoff date for data collection was 9 September 2019.
Patterns of response
The distribution of stable disease (SD), partial response (PR), and complete response (CR) were identical for both guidelines. For RECIST 1.1, PD was defined as at least a 20% increase from nadir in the sum of the longest diameter (SLD) of the target lesions and/or the appearance of new lesions. For the iRECIST guideline, new measurable lesions were evaluated separately; suspicion of progression was recorded as immune unconfirmed progressive disease (iUPD). Immune confirmed PD (iCPD) was defined as an additional increase in the size of target lesions; additional qualitative worsening of nontarget lesions; an increase in the sum of new measurable target lesions >5 mm; qualitative worsening of nonmeasurable new lesions; or the appearance of new lesions. If progression was not confirmed, the response status was evaluated compared with the baseline or nadir as iCR, iPR, iSD, or iUPD.18 Death or discontinuation of immunotherapy due to clinical progression was also considered as confirmation of progression. We defined progression‐free survival 1 (PFS1) as the time from initial immunotherapy to RECIST 1.1‐defined first progressive disease or death, progression‐free survival 2 (PFS2) as the time from RECIST 1.1‐defined first progressive disease to iRECIST‐defined first progressive disease or death, and immune‐related progression‐free survival (iPFS) as the first date at which progression criteria were met (i.e., the date of iUPD) provided that iCPD was confirmed at the next assessment. For patients with tumor progression evaluated per the RECIST 1.1, pseudoprogression (PsPD) was defined among patients who met the conventional response criteria for progressive disease but later showed reduced tumor burden.21, 23
Statistical analysis
Continuous variables are expressed as means ± standard deviations. Categorical variables were analyzed using Fisher's exact test. OS curves were drawn using the Kaplan‐Meier method. Univariate analysis of OS was performed using the Kaplan‐Meier method and differences in survival compared using the log‐rank test. All statistical analyses were performed using SPSS version 21.0 (IBM Corp., Armonk, NY, USA). All tests were two‐sided, and P ≤ 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 11 of 54 patients with advanced lung cancer treated with immunotherapy were excluded due to the absence of evaluation CT scans after immunotherapy. Ultimately, a total of 43 patients were eligible for inclusion in this study (Fig 1). Among these patients, the most common pathological type was adenocarcinoma (22 cases), followed by squamous cell carcinoma (17 cases), adenosquamous carcinoma (three cases), and large cell carcinoma (one case). Anti‐PD1 was the most common treatment (34 cases), followed by immunotherapy combined with chemotherapy (four cases), double‐immunotherapy (four cases), and anti‐PD‐L1 (one case). A summary of the characteristics of patients at the baseline is provided in Table 1.
Figure 1.
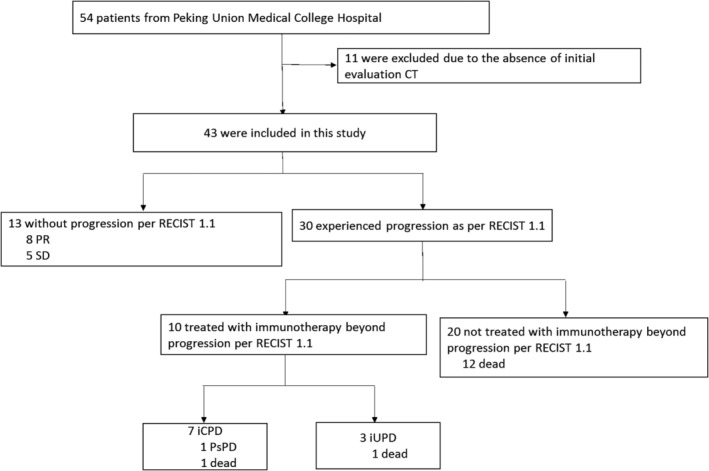
Study profile of the pooled population. CT, computed tomography; iCPD, immune‐related confirmed progressive disease; iUPD, immune‐related unconfirmed progressive disease; PR, partial response; PsPD, pseudoprogression; SD, stable disease.
Table 1.
Characteristics of 43 patients at the baseline
| Variables | No. (%) |
|---|---|
| Age | |
| Median (range) | 62 (37–77) |
| Gender | |
| Male | 31 (72.1%) |
| Female | 12 (27.9%) |
| Smoking history | |
| Never | 15 (34.9%) |
| Ever | 28 (65.1%) |
| Pathology | |
| Nonsquamous | 26 (60.5%) |
| Squamous | 17 (39.5%) |
| Stage | |
| III | 2 (4.6%) |
| IV | 41 (95.4%) |
| EGFR status | |
| Wild‐type | 22 (51.2%) |
| Mutant type | 5 (11.6%) |
| Unknown | 16 (37.2%) |
| ALK status | |
| Wild‐type | 35 (81.4%) |
| Rearrangement type | 0 (0.0%) |
| Unknown | 8 (18.6%) |
| Treatment line | |
| First‐line | 7 (16.3%) |
| Second‐line | 31 (72.1%) |
| Third‐line | 3 (7.0%) |
| Fourth‐line | 2 (4.6%) |
| ECOG PS | |
| 0–1 | 40 (93.0%) |
| >1 | 3 (7.0%) |
| Treatment | |
| Anti‐PD1 | 34 (79.1%) |
| Anti‐PDL1 | 1 (2.3%) |
| Anti‐PD1 + Anti‐PDL1 | 4 (9.3%) |
| Anti‐PD1 + Chemo | 4 (9.3%) |
ALK, anaplastic lymphoma kinase; Chemo, chemotherapy; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; IO, immunotherapy; NSCLC, non‐small cell lung cancer; PD, progression disease; PD1, programmed cell death‐1; PD‐L1, programmed cell death‐ligand 1; PS, performance status.
Response patterns evaluated as per RECIST 1.1
At the time of the analysis, the results of best response were as follows: 15 patients achieved PR, nine had SD, and 19 had PD. The total objective response rate (ORR; defined as the proportion of patients with CR and PR) was 34.9%. A total of 30 PFS events (69.8%) and 14 OS events (32.6%) had occurred (Fig 2). The median PFS duration was 5.4 months. The median OS was not reached. Among 30 patients with tumor progression, the most common pattern of progression was target lesion progression (15 cases), followed by nontarget lesion progression (13 cases) and new lesions (nine cases). Four patients had both nontarget lesion progression and new lesions. Two patients had target lesion progression, nontarget lesion progression, and new lesions simultaneously. One patient had both target lesion progression and nontarget lesion progression.
Figure 2.
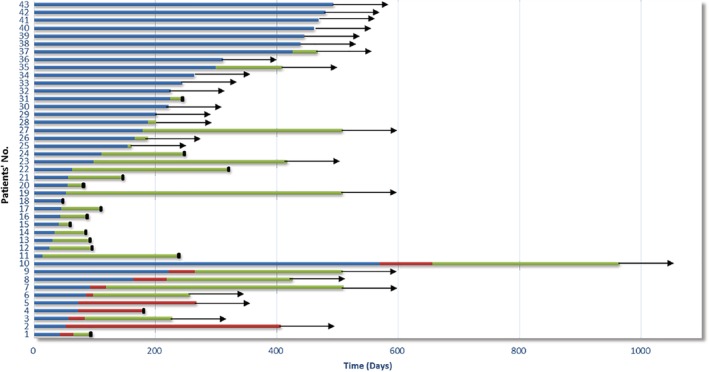
Progression‐free survival (PFS) and survival follow‐up in patients with non‐small cell lung cancer (NSCLC) treated with immunotherapy. PFS1, time from initial immunotherapy to RECIST 1.1‐defined first progressive disease or death; PFS2, time from RECIST 1.1‐defined first progressive disease to iRECIST‐defined first progressive disease or death. ( ), PFS1, (
), PFS1, ( ) PFS2, (
) PFS2, ( ) follow‐up time after immunotherapy discontinued, (
) follow‐up time after immunotherapy discontinued, ( ) dead, (
) dead, ( ) survival
) survival
Response patterns evaluated as per iRECIST 1.1
At the time of the analysis, the results of best response were as follows: 15 patients achieved iPR, nine had iSD, and 19 had iUPD. The total ORR (defined as the proportion of patients with iCR and iPR) was 34.9%, the median iPFS was 6.2 months, and the median OS was not reached. Finally, among 30 patients with iUPD, 10 (33.3%) patients continued immunotherapy beyond PD as per RECIST 1.1, of which seven (70%, 7/10) had iCPD and three (30%, 3/10) had continued iUPD without a decrease in tumor burden. Interestingly, among seven patients with iCPD, one (1/30, 3.3%) patient presented with PsPD later. A total of 27 iCPD events (62.8%) and 14 OS events (32.6%) had occurred (Fig 2).
Table 2 summarizes the clinicopathological features of 10 patients who continued to receive immunotherapy beyond PD as per RECIST 1.1. There were 7 patients confirmed to have disease progression at the first assessment after iUPD. The most common pattern of first progression was target lesion progression (six patients), followed by new lesions (four patients), and nontarget lesion progression (three patients). One patient had target lesion progression, nontarget lesion progression, and new lesion simultaneously; one patient had both nontarget lesion progression and new lesions. Among seven patients with iCPD, the most common pattern of second progression was target lesion progression (five cases), followed by nontarget lesion progression (two cases), new lesions (two cases), and new nontarget lesion progression (one case). One patient had both nontarget lesion progression and new lesions, and one patient had target lesion progression, nontarget lesion progression, and new lesions.
Table 2.
Treatment details and outcomes among 10 patients treated with immunotherapy beyond progression
| No. | Age/sex | Smoking history | H | EGFR | ALK | ROS1 | ECOG PS | Treatment | Line | Best response | PFS mo | Reason for first PD | BR after first PD | Reason for confirmed PD | iPFS mo | OS outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 55/M | Ever | A | Wild | Wild | Fusion | 0 | Anti‐PD1 | 2 | PR | 5.4 | T, NT, NEW | iCPD | T, NT, NEW | 5.4 | Alive |
| 2† | 69/M | Ever | S | Wild | Wild | NA | 0 | Anti‐PD1 | 4 | PD | 2.4 | T | iUPD | — | 8.6 | Death |
| 3 | 68/M | Ever | A | L858R | Wild | NA | 0 | Anti‐PD1 | 3 | PD | 3.1 | NEW | iCPD | T | 3.1 | Alive |
| 4 | 62/M | Ever | A | Wild | Wild | NA | 0 | Anti‐PD1 | 2 | SD | 1.7 | NT | iUPD | — | 13.5 | Alive |
| 5 | 54/F | Never | A | 19del | Wild | Wild | 0 | Anti‐PD1 | 3 | PD | 2.8 | NT, NEW | iCPD | NT, NEW | 2.8 | Alive |
| 6 | 73/M | Ever | S | NA | Wild | NA | 0 | Anti‐PD1 | 2 | PD | 1.9 | T | iCPD | T | 1.9 | Alive |
| 7 | 72/M | Ever | A | Wild | Wild | NA | 2 | Anti‐PD1 | 2 | PD | 2.4 | T | iUPD | — | 8.9 | Alive |
| 8 | 68/M | Ever | S | NA | Wild | NA | 1 | Anti‐PD1 | 2 | PD | 1.4 | NEW | iCPD | NEW‐NT | 1.4 | Death |
| 9 | 65/M | Ever | S | NA | NA | NA | 0 | Anti‐PD1 | 2 | PR | 7.4 | T | iCPD | T | 7.4 | Alive |
| 10‡ | 64/M | Ever | S | NA | Wild | NA | 1 | Anti‐PDL1 | 1 | PR | 18.7 | T | iCPD | T | 18.7 | Alive |
—, Did not progress.
Died due to hemoptysis without iCPD; OS, overall survival.
Immunotherapy beyond iCPD, and the target lesion continued to regress.
A, adenocarcinoma; ALK, anaplastic lymphoma kinase; BR, best response; ECOG PS, performance status according to Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; iCPD, confirmed progression disease according to iRECIST; iUPD, unconfirmed progression disease according to iRECIST; H, histologic type; NA, not available; NEW, new lesion; NT, nontarget lesion; PD, progressive disease; S, squamous carcinoma; T, target lesion.
Comparison of the RECIST 1.1 and iRECIST criteria
Patients who continued immunotherapy beyond PD (n = 10) had significantly prolonged OS (not reached vs. 8.1 months: hazard ratio = 2.8, 95% confidence interval: 2.7–13.6, P = 0.03) compared with patients who did not continue immunotherapy beyond PD (n = 20) (Fig 3). Among the 10 confirmatory CT scans, there were three discordant assessments (30%) between the RECIST and iRECIST, which were confirmed as PD using RECIST 1.1, but not by with iRECIST (which identified them as iUPD, allowing treatment continuation).
Figure 3.
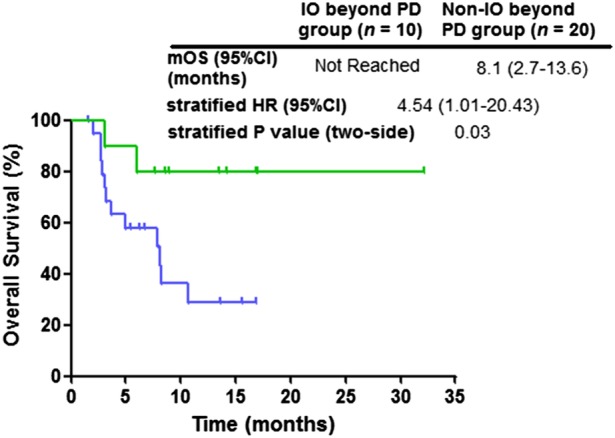
Kaplan‐Meier curves of overall survival (OS) stratified by continued immunotherapy beyond progress disease (PD) per RECIST 1.1. CI, confidence interval; HR, hazard ratio; IO, immunotherapy; mOS, median overall survival; PD, progression disease. ( ) Immunotherapy beyond PD, (
) Immunotherapy beyond PD, ( ) non‐immunotherapy beyond PD
) non‐immunotherapy beyond PD
Patients who continued immunotherapy beyond PD per RECIST 1.1 were all previously treated. For patients who had progressed per RECIST 1.1, only three patients received first‐line immunotherapy. It is immature to analyze the OS curve. However, the total ORR of patients who received first‐line immunotherapy was 100% (3/3), the total ORR of patients who did not receive first‐line immunotherapy was only 14.8% (4/27).
Discussion
In this retrospective analysis, we found that in the real world, 10 patients (33.3%, 10/30) continued to receive immunotherapy beyond progression. Three patients (30%, 3/10) showed continued response to immunotherapy, of which two patients benefited from subsequent immunotherapy and one patient died because of massive hemoptysis. However, no patient experienced decreased tumor burden in our study. Interestingly, among seven patients with iCPD, one later presented with PsPD. Patients who continued immunotherapy beyond PD experienced significantly prolonged OS compared with patients who did not continue immunotherapy beyond PD. These results suggested that the RECIST 1.1 evaluation underestimated the efficacy of immunotherapy. In the era of immunotherapy, iRECIST may be better used to evaluate the efficacy.
In previous reports, the proportion of patients with NSCLC who received continued immunotherapy beyond PD assessed using RECIST 1.1 ranged from 30%–90%,21, 24, 25 similar to our results. In clinical trials, the incidence of PsPD in NSCLC was 0.6–5.8%.7, 26, 27, 28, 29, 30, 31 Consistent with previous reports, one case (3.3%, 1/30) with PsPD was found in this study. In addition, we found that prolonged disease stabilization occurred in 30% (3/10) of the patients after PD as per RECIST 1.1, and continuation of immunotherapy beyond PD significantly prolonged patients' OS. This result suggested that although the current reported incidence of PsPD has never exceeded 10%, for some patients, especially without symptoms, continued immunotherapy beyond PD may still have significant survival benefits, even if there is no reduction in tumor burden. Due to patients who continued immunotherapy beyond PD all being previously treated, whether this result was affected by the treatment line needs to be confirmed by larger sample studies.
In addition, in our study, one patient still experienced a decrease in tumor burden after continuing immunotherapy beyond iCPD as per iRECIST. Similar to this case, Nishino et al.32 reported that a patient who received anti‐PD1 had achieved a reduction in tumor burden five months after iRECIST confirmed disease progression. Therefore, it is necessary to consider whether the currently recommended diagnosis of confirmed PD for at least four weeks is sufficient to detect all patients with delayed tumor shrinkage. Therefore, in our opinion, if patients have good ECOG performance status and have not experienced serious toxicity, immunotherapy should be continued even beyond progression, which can avoid premature discontinuation, while avoiding unnecessary prolonged treatment and missing other alternative therapies.
Obviously, current radiological evaluation is not yet able to distinguish between atypical responses and genuine disease progression, and further research is needed to describe the clinical, pathological, and molecular characteristics of patients undergoing atypical responses. Based on previous reports, clinical symptom assessment,33 positron emission tomography/CT,34 serum interleukin‐8 levels,35, 36, 37, 38 circulating tumor DNA,39, 40, 41 tumor mutation burden evaluated by next generation sequence,42 and histopathological biopsy28, 43 may be helpful in the screening of patients suitable for continued immunotherapy beyond PD per RECIST 1.1. Further research is needed to investigate these biomarkers.
The major limitations of our study were its retrospective nature and small sample size. The findings of this research need to be validated via more large‐scale prospective studies.
In conclusion, RECIST 1.1 based on radiological assessment underestimates the effectiveness of immunotherapy, while iRECIST still needs to be optimized by choosing the appropriate time to confirm PD, for instance. The future direction of research is to build a comprehensive evaluation system to screen suitable patients who would benefit from continuing immunotherapy after PD, including changes in tumor size, clinical system assessment, positron emission tomography/CT evaluation, tumor mutation burden evaluation, and histopathological biopsy.
Disclosure
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by the CAMS Innovation Fund for Medical Sciences (grant number: 2018‐12M‐1‐003), the “13th Five‐Year” National Science and Technology Major Project for New Drugs (grant number: 2019ZX09734001‐002), and the National Natural Science Foundation of China (grant number: 81702292).
Contributor Information
Hongge Liang, Email: honggeliang2017@163.com.
Mengzhao Wang, Email: mengzhaowang@sina.com.
Jing Zhao, Email: pumchzj@sina.com.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holt GE, Podack ER, Raez LE. Immunotherapy as a strategy for the treatment of non‐small‐cell lung cancer. Therapy 2011; 8: 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rizvi NA, Mazieres J, Planchard D et al Activity and safety of nivolumab, an anti‐PD‐1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non‐small‐cell lung cancer (CheckMate 063): A phase 2, single‐arm trial. Lancet Oncol 2015; 16: 257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gettinger SN, Horn L, Gandhi L. Overall survival and long‐term safety of nivolumab (Anti‐programmed death 1 antibody, BMS‐936558, ONO‐4538) in patients with previously treated advanced non‐small‐cell lung cancer. J Clin Oncol 2015; 33: 2004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spigel DR, Reckamp KL, Rizvi NA et al A phase III study (CheckMate 017) of nivolumab (NIVO; anti‐programmed death‐1 [PD‐1]) vs docetaxel (DOC) in previously treated advanced or metastatic squamous (SQ) cell non‐small cell lung cancer (NSCLC). J Clin Oncol 2015; 33: 8009. [Google Scholar]
- 7. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Horn L, Spigel DR, Vokes EE et al Nivolumab versus docetaxel in previously treated patients with advanced non‐small‐cell lung cancer: Two‐year outcomes from two randomized, open‐label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol 2017; 35: 3924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu YL, Lu S, Cheng Y et al Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J Thorac Oncol 2019; 14 (5): 867–75. [DOI] [PubMed] [Google Scholar]
- 10. Herbst RS, Baas P, Kim DW et al Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016; 387: 1540–50. [DOI] [PubMed] [Google Scholar]
- 11. Reck M, Rodriguez‐Abreu D, Robinson AG et al Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016; 375: 1823–33. [DOI] [PubMed] [Google Scholar]
- 12. Gandhi L, Rodriguez‐Abreu D, Gadgeel S et al Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med 2018; 378: 2078–92. [DOI] [PubMed] [Google Scholar]
- 13. Paz‐Ares L, Luft A, Vicente D et al Pembrolizumab plus chemotherapy for squamous non‐small‐cell lung cancer. N Engl J Med 2018; 379: 2040–51. [DOI] [PubMed] [Google Scholar]
- 14. Rittmeyer A, Barlesi F, Waterkamp D et al Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): A phase 3, open‐label, multicentre randomised controlled trial. Lancet 2017; 389: 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Socinski MA, Jotte RM, Cappuzzo F et al Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018; 378: 2288–301. [DOI] [PubMed] [Google Scholar]
- 16. Antonia SJ, Villegas A, Daniel D et al Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018; 379: 2342–50. [DOI] [PubMed] [Google Scholar]
- 17. Eisenhauer EA, Therasse P, Bogaerts J et al New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 18. Seymour L, Bogaerts J, Perrone A et al iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017; 18: e143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hodi FS, Hwu WJ, Kefford R et al Evaluation of immune‐related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol 2016; 34: 1510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nishino M, Giobbie‐Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS. Developing a common language for tumor response to immunotherapy: Immune‐related response criteria using unidimensional measurements. Clin Cancer Res 2013; 19: 3936–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tazdait M, Mezquita L, Lahmar J et al Patterns of responses in metastatic NSCLC during PD‐1 or PDL‐1 inhibitor therapy: Comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer 2018; 88: 38–47. [DOI] [PubMed] [Google Scholar]
- 22. Beaver JA, Hazarika M, Mulkey F et al Patients with melanoma treated with an anti‐PD‐1 antibody beyond RECIST progression: A US Food and Drug Administration pooled analysis. Lancet Oncol 2018; 19: 229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fujimoto D, Yoshioka H, Kataoka Y et al Pseudoprogression in previously treated patients with non‐small cell lung cancer who received nivolumab monotherapy. J Thorac Oncol 2019; 14: 468–74. [DOI] [PubMed] [Google Scholar]
- 24. Nishino M, Ramaiya NH, Chambers ES et al Immune‐related response assessment during PD‐1 inhibitor therapy in advanced non‐small‐cell lung cancer patients. J Immunother Cancer 2016; 4: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kazandjian D, Keegan P, Suzman DL, Pazdur R, Blumenthal GM. Characterization of outcomes in patients with metastatic non‐small cell lung cancer treated with programmed cell death protein 1 inhibitors past RECIST version 1.1‐defined disease progression in clinical trials. Semin Oncol 2017; 44: 3–7. [DOI] [PubMed] [Google Scholar]
- 26. Gettinger S, Rizvi NA, Chow LQ et al Nivolumab monotherapy for first‐line treatment of advanced non‐small‐cell lung cancer. J Clin Oncol 2016; 34: 2980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim HK, Heo MH, Lee HS et al Comparison of RECIST to immune‐related response criteria in patients with non‐small cell lung cancer treated with immune‐checkpoint inhibitors. Cancer Chemother Pharmacol 2017; 80: 591–8. [DOI] [PubMed] [Google Scholar]
- 28. Tanizaki J, Hayashi H, Kimura M et al Report of two cases of pseudoprogression in patients with non‐small cell lung cancer treated with nivolumab‐including histological analysis of one case after tumor regression. Lung Cancer 2016; 102: 44–8. [DOI] [PubMed] [Google Scholar]
- 29. Kumagai T, Kimura M, Inoue T, Tamiya M, Nishino K, Imamura F. Delayed pseudoprogression of lung adenocarcinoma accompanied with interstitial lung disease during chemotherapy after nivolumab treatment. Thorac Cancer 2017; 8: 275–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kolla BC, Patel MR. Recurrent pleural effusions and cardiac tamponade as possible manifestations of pseudoprogression associated with nivolumab therapy‐ a report of two cases. J Immunother Cancer 2016; 4: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Izumida T, Kawagishi Y, Tsuji H. Pseudoprogression in lung adenocarcinoma during treatment with nivolumab. BMJ Case Rep 2017; 2017 10.1136/bcr-2017-219919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nishino M, Dahlberg SE, Cardarella S et al Volumetric tumor growth in advanced non‐small cell lung cancer patients with EGFR mutations during EGFR‐tyrosine kinase inhibitor therapy: Developing criteria to continue therapy beyond RECIST progression. Cancer 2013; 119: 3761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baxi SS, Dunn LA, Burtness BA. Amidst the excitement: A cautionary tale of immunotherapy, pseudoprogression and head and neck squamous cell carcinoma. Oral Oncol 2016; 62: 147–8. [DOI] [PubMed] [Google Scholar]
- 34. Tan AC, Emmett L, Lo S et al FDG‐PET response and outcome from anti‐PD‐1 therapy in metastatic melanoma. Ann Oncol 2018; 29: 2115–20. [DOI] [PubMed] [Google Scholar]
- 35. Sanmamed MF, Carranza‐Rua O, Alfaro C et al Serum interleukin‐8 reflects tumor burden and treatment response across malignancies of multiple tissue origins. Clin Cancer Res 2014; 20: 5697–707. [DOI] [PubMed] [Google Scholar]
- 36. Waugh DJ, Wilson C. The interleukin‐8 pathway in cancer. Clin Cancer Res 2008; 14: 6735–41. [DOI] [PubMed] [Google Scholar]
- 37. Zarogoulidis P, Katsikogianni F, Tsiouda T, Sakkas A, Katsikogiannis N, Zarogoulidis K. Interleukin‐8 and interleukin‐17 for cancer. Cancer Invest 2014; 32: 197–205. [DOI] [PubMed] [Google Scholar]
- 38. Sanmamed MF, Perez‐Gracia JL, Schalper KA et al Changes in serum interleukin‐8 (IL‐8) levels reflect and predict response to anti‐PD‐1 treatment in melanoma and non‐small‐cell lung cancer patients. Ann Oncol 2017; 28: 1988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee JH, Long GV, Menzies AM et al Association between circulating tumor DNA and pseudoprogression in patients with metastatic melanoma treated with anti‐programmed cell death 1 antibodies. JAMA Oncol 2018; 4: 717–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee JH, Long GV, Boyd S et al Circulating tumour DNA predicts response to anti‐PD1 antibodies in metastatic melanoma. Ann Oncol 2017; 28: 1130–6. [DOI] [PubMed] [Google Scholar]
- 41. Cabel L, Riva F, Servois V et al Circulating tumor DNA changes for early monitoring of anti‐PD1 immunotherapy: A proof‐of‐concept study. Ann Oncol 2017; 28: 1996–2001. [DOI] [PubMed] [Google Scholar]
- 42. Rizvi H, Sanchez‐Vega F, La K et al Molecular determinants of response to anti‐programmed cell death (PD)‐1 and anti‐programmed death‐ligand 1 (PD‐L1) blockade in patients with non‐small‐cell lung cancer profiled with targeted next‐generation sequencing. J Clin Oncol 2018; 36: 633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chiou VL, Burotto M. Pseudoprogression and immune‐related response in solid tumors. J Clin Oncol 2015; 33: 3541–3. [DOI] [PMC free article] [PubMed] [Google Scholar]


