Abstract
Background
Tumor recurrence or residual tumor after targeted therapy is common in patients with advanced non‐small cell lung cancer (NSCLC). There is a lack of high‐level evidence on which type of treatment should be employed for these patients and the role of salvage surgery has not been well reported in the literature.
Methods
A retrospective analysis of patients who underwent salvage surgery in our center between January 2016 and June 2019 for advanced NSCLC after targeted therapy was performed.
Results
A total number of nine patients were identified, including five males and four females, with a median age of 56 years (range, 40–65 years), all diagnosed with lung adenocarcinoma stage IIIa–IVb. All patients had received targeted therapy according to individual positive mutation of driver gene(s). Salvage surgery was performed for tumor recurrence or residual tumor after a duration of 2–46 months of targeted therapy. A negative surgical margin was achieved in all cases. Postoperative complication rate was 11.1% (1/9). All patients were alive at the time of this analysis and two patients had disease progression. After a median follow‐up of 17 months (range: 5–44 months), the median event‐free survival and postoperative survival was 14 months (range: 2–44 months) and 17 months (range: 5–44 months) respectively.
Conclusions
Salvage surgery may be a feasible and promising therapeutic option for tumor recurrence or residual tumor in advanced NSCLC in selective patients after targeted therapy.
Key points
Salvage surgery is feasible in selected patients with advanced NSCLC and provides promising survival outcomes after targeted therapy failure.
Salvage surgery provides precise molecular and pathological information which is most important for subsequent therapy.
Keywords: Non‐small cell lung cancer, salvage surgery, survival, targeted therapy
Introduction
Lung cancer is the most common malignancy and is the first killer of cancer‐related death worldwide.1 Nearly 85% of lung cancers are non‐small cell lung cancer (NSCLC). To date, surgery is still the cornerstone of NSCLC treatment strategy since it provides the opportunity of cure. Unfortunately, 70%–85% of NSCLC patients are at an advanced stage at the time of diagnosis and thus lose the opportunity of radical surgery.2 For patients with advanced NSCLC, platinum‐based chemotherapy, with or without local radiotherapy, is usually administered as first‐line treatment, although effectiveness is far from satisfactory. Five‐year survival rates of patients with stage III or IV diseases treated by chemotherapy and radiotherapy are only 30% and 5%, respectively.3
In the previous two decades, targeted therapy which employs a new class of drugs that specifically target certain molecular pathways has developed remarkably and dramatically changed the treatment strategy of lung cancer.4 Many patients with advanced lung cancer have benefited from targeted therapy with longer survival, fewer adverse effects and a better quality of life, compared with conventional chemotherapy and radiotherapy.5, 6 With the developments in molecular biology, targeted drugs are being constantly improved and updated to provide better therapeutic effect for lung cancer.7 However, tumor recurrence or residual tumor during targeted therapy has been commonly reported6, 8 and there is a lack of high‐level evidence on which type of treatment should be employed for these patients.9, 10 Conventionally, alternative medical treatment or radiotherapy may be administrated as second‐ or third‐line therapy, although the effect is usually unpredictable and poor.11, 12, 13 On the other hand, there are some patients having had their NSCLC converted from an advanced to resectable stage as a result of targeted therapy, that salvage surgery may be another alternative option for tumor recurrence or residual tumor after targeted therapy.14
Salvage surgery already plays a role in the treatment of esophageal cancer, colorectal cancer and other tumors.15, 16, 17 In recent years, salvage surgery has also been introduced into the treatment strategy of advanced lung cancer after chemotherapy/radiotherapy failure.18, 19, 20, 21, 22 In a study conducted by Hidetaka et al. a complete pathological response was found in nearly half the cases who received salvage surgery after chemotherapy/radiotherapy.23 However, the role of salvage surgery in patients with advanced lung cancer after targeted therapy has still not been well reported in the literature.24, 25
Here, we present our single‐center study and analyze the feasibility and efficiency of salvage surgery for advanced NSCLC after targeted therapy.
Methods
Study design and patients
All consecutive NSCLC patients receiving surgery after targeted therapy in the sixth medical center of the Chinese PLA General Hospital between January 2016 and June 2019 were collected. Patients were eligible for inclusion if they met the following criteria: (i) Pathologically identified NSCLC in clinical TNM stage of IIIa–IVb at the time of diagnosis, according to the eighth edition of the TNM staging system26; (ii) targeted therapy had been administered according to the positive mutation of corresponding driver gene(s), and (iii) comprehensive preoperative reassessment had been performed. Patients undergoing surgical biopsy for diagnostic purposes were excluded.
Demographic and clinical data including gender, age, clinical stage, histology, driver‐gene status, targeted therapy agents, duration of targeted therapy before surgery, surgical procedure, operation time, intraoperative blood loss, postoperative complications, length of hospital stay after surgery, pathological diagnosis, postoperative therapy, and survival data were reviewed from the electronic medical records library.
The study was approved by the Ethics Committee of the sixth medical center of the Chinese PLA General Hospital (No. 2019112507).
Follow‐up
Follow‐up was performed every three months for each patient, and included physical status, and contrast computed tomography (CT) of the chest and upper abdomen. Contrast magnetic resonance imaging (MRI) of the brain and nuclear bone scan were performed every six months. Positron emission tomography (PET) scan was utilized if required.
Event‐free survival (EFS) was calculated from surgery to recurrence or progression, postoperative survival (POS) was calculated from surgery to death for any reason, and overall survival (OS) was calculated from first diagnosis to death for any reason.
Results
Patient characteristics
Between January 2016 and June 2019, 962 patients had undergone surgery for lung cancer. Among these, 13 patients had received preoperative targeted therapy. Four patients were ruled out of the analysis: three patients who had lost the opportunity of surgical treatment due to multiple pulmonary metastasis underwent wedge resection to secure intact histological and genomic information to enable guidance on further treatment; one patient had stage II disease at the time of first diagnosis. A total of nine patients were eventually enrolled into our study.
The baseline demographic and clinical characteristics of eligible patients are shown in Table 1. There were five males and four females with a median age of 56 years (range, 40–65 years). All patients were diagnosed with lung adenocarcinoma, staged from IIIa–IVb at the time of first diagnosis. Metastasis was found in the mediastinal lymph nodes, pleural membrane, lung and brain. Epidermal growth factor receptor (EGFR) mutation was discovered in eight patients and anaplastic lymphoma kinase (ALK) translocation was discovered in one patient. Corresponding targeted therapy agents included EGFR‐targeted agents (Erlotinib, Icotinib or Gefitinib) and ALK‐targeted agent (Crizotinib). The duration of targeted therapy before surgery ranged from two to 46 months.
Table 1.
Characteristics of patients undergoing salvage surgery after targeted therapy
| Case | Gender | Age | Histology | Clinical stage | Metastasis | Driver gene | Targeted therapy agent | Duration of targeted therapy | Other treatment | Therapeutic effect |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 63 | Ad | cT2N0M1a, IVa | Pleura effusion | EGFR, exon 19 deletion | Icotinib | 46 months | None | PR |
| 2 | Male | 51 | Ad | cT2N3M0, IIIb | LNs | EGFR, exon 21 L858R | Gefitinib | 12 months | six cycles of chemotherapy | PR |
| 3 | Male | 45 | Ad | cT2N0M1a, IVa | Pleura effusion | EGFR, exon 19 deletion | Gefitinib | six months | None | PR |
| 4 | Female | 65 | Ad | cT2N0M1b, IVb | lung | EGFR, exon 21 L858R mutation | Icotinib | six months | None | PR |
| 5 | Male | 40 | Ad |
cT2bN3M1, IVb |
LNs, lung | EGFR, exon 19 deletion | Icotinib | 14 months | None | PR |
| 6 | Male | 42 | Ad | cT2N2M0, IIIa | LNs | EGFR, exon 21 L858R mutation | Tarceva | two months | None | SD |
| 7 | Male | 57 | Ad | cT2N2M0, IIIa | LNs | EGFR, exon 21 L861 mutation | Icotinib | three months | None | SD |
| 8 | Female | 56 | Ad | cT2N2M1a, IVa | LNs, pleura effusion | ALK translocation | Crizotinib | eight months | None | PR |
| 9 | Female | 59 | Ad | cT2N2M1b, IVb | LNs, brain | EGFR, exon 19 deletion | Icotinib | two months | Gamma knife for brain metastasis | SD |
Ad, adenocarcinoma; ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; LNs, lymph nodes; PR, partial response; SD, stable disease.
No other anticancer therapy was administered to these patients, with the exception of case 2, who had received six cycles of chemotherapy, and case 9 who had received gamma knife therapy for brain metastasis. After targeted therapy, partial response (PR) was reached in six patients and stable disease (SD) was achieved in three patients, according to the preoperative assessment.
Perioperative factors
As shown in Table 2, seven patients underwent salvage surgery because of residual tumor and two patients because of slight disease relapse.
Table 2.
Perioperative factors of patients undergoing salvage surgery after targeted therapy
| Case | Reason for salvage surgery | y‐stage | Interval (days) | Surgical procedure | Surgical accident | Operation time (minutes) | Intraoperative Blood loss (mL) |
|---|---|---|---|---|---|---|---|
| 1 | Relapse | T2bN0M0, IIa | 9 | (open) RUL + LND | Bleeding | 130 | 840 |
| 2 | Residual tumor | T2aN0M0, Ib | 7 | (VATS) LUL + LND | None | 110 | 100 |
| 3 | Relapse | T2aN0M0, Ib | 14 | (open) LUL + LND | Extensive pleural adhesion | 170 | 250 |
| 4 | Residual tumor | T2aN0M0, Ib | 5 | (VATS) RLL + LND | None | 90 | 120 |
| 5 | Residual tumor | T1bN0M0, Ib | 7 | (VATS) LUL + LND | None | 110 | 100 |
| 6 | Residual tumor | T2aN2M0, IIIa | 7 | (open) RUL + LND | Extensive pleural adhesion | 150 | 220 |
| 7 | Residual tumor | T2aN2M0, IIIa | 7 | (VATS) LUL + LND | None | 90 | 100 |
| 8 | Residual tumor | T1aN0M0, Ia | 6 | (VATS) RLL + LND | None | 80 | 140 |
| 9 | Residual tumor | T2aN2M0, IIIa | 5 | (VATS) RLL + LND | None | 90 | 100 |
LND, lymph node dissection; LUL, left upper lobectomy; open, open surgery; RLL, right lower lobectomy; RUL, right upper lobectomy; VATS, video‐assisted thoracoscopic surgery; y‐stage, the clinical stage after targeted therapy.
The median duration of targeted therapy before surgery was six months (range, 2–46 months). Of note, there were three cases who received targeted therapy before salvage surgery for a relatively short duration. Case 6, a 42‐year‐old male patient, was assessed with unresectable lung adenocarcinoma in a county‐level hospital and received Tarceva therapy there because of EGFR mutation. After two months of observation, there was no obvious tumor shrinkage and the patient was transferred to our center. Reassessment found that the tumor was actually resectable even at the time of first diagnosis, and therefore the patient received salvage surgery in our center. Cases 7 and 9 were two patients with resectable tumor at the time of first diagnosis who all refused surgical treatment, only receiving targeted therapy at the start of treatment. After three and two months, respectively, they changed their minds because there had been no obvious response to targeted therapy and they subsequently received salvage surgery in our center.
All patients were reassessed for resectability. The interval between targeted therapy and surgery ranged from 5–14 days. Lobectomy procedures plus systematic lymph node dissection were performed on all nine patients, including six who underwent video‐assisted thoracoscopic surgeries (VATS). In three cases, operations had to be transferred from VATS to open surgery due to accidental bleeding (case 1) or extensive pleural adhesion (cases 3 and 6). The median operation time and intraoperative blood loss were 110 minutes (range, 80–170 minutes) and 120 mL (range, 100–840 mL), respectively.
Postoperative factors
As shown in Table 3, there was one patient with a minor postoperative complication of atrial fibrillation and the postoperative complication rate was 11.1% (1/9). There was no in‐hospital death. The median time of hospital stay after surgery was five days (range, 4–8 days). Pathological examination on surgical specimens identified three patients whose pathological results were different from their preoperative results: one patient with mixed histological types (case 2) and two patients with gene mutation of T790M (case 3 and 7). A negative surgical margin was achieved in all cases. The postoperative pathological stages of four patients (cases 2, 3, 6 and 8) were confirmed to be different from their preoperative y‐stages, due to unexpected lymph node metastasis.
Table 3.
Postoperative factors of patients undergoing salvage surgery after targeted therapy
| Case | Postoperative complication | Hospital stay (days) | Histology | Pathological stage | Driver gene | Postoperative treatment | POS (months) | OS (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | None | 7 | AD | T2bN0M0, IIa | EGFR, exon 19 deletion | Icotinib | 44 | 89 |
| 2 | None | 5 | SCC + AD+SC | T2aN2M0, IIIa | EGFR, exon 21 L858R mutation | Gefitinib, radiotherapy | 30 | 42 |
| 3 | None | 8 | AD | T2aN2M0, IIIa | EGFR exon 20, T790M mutation | Osimertinib | 27 | 33 |
| 4 | None | 4 | AD | T2aN0M0, Ib | EGFR, exon 21 L858R mutation | Icotinib, chemotherapy | 19 | 25 |
| 5 | None | 5 | AD | T1cN0M0, Ia | EGFR, exon 19 deletion | Icotinib | 17 | 31 |
| 6 | Atrial fibrillation | 8 | AD | T1cN1M0, IIa | EGFR, exon 21 L858R mutation | Icotinib | 14 | 16 |
| 7 | None | 4 | AD | T2aN2M0, IIIa | EGFR, exon 20, T790M mutation | Osimertinib | 7 | 10 |
| 8 | None | 5 | AD | T1aN2M0, IIIa | ALK translocation | Crizotinib | 6 | 14 |
| 9 | None | 5 | AD | T1aN2M0, IIIa | EGFR, exon 19 deletion | Icotinib | 5 | 7 |
AD, adenocarcinoma; ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; OS, overall survival calculated from the first diagnosis; POS, post‐operative survival; p‐stage, pathological stage; SC, sarcomatoid carcinoma; SCC, squamous cell carcinoma.
Survival
All patients were alive at the time of the analysis. The median follow‐up time of patients in this study was 17 months (range, 5–44 months). There were two patients with disease progression and the median EFS was 14 months (range, 2–44 months) as shown in Figure 1. The median postoperative survival (POS) was 17 months (range, 5–44 months) and median overall survival (OS) was 25 months (range, 7–89 months), as shown in Table 3.
Figure 1.
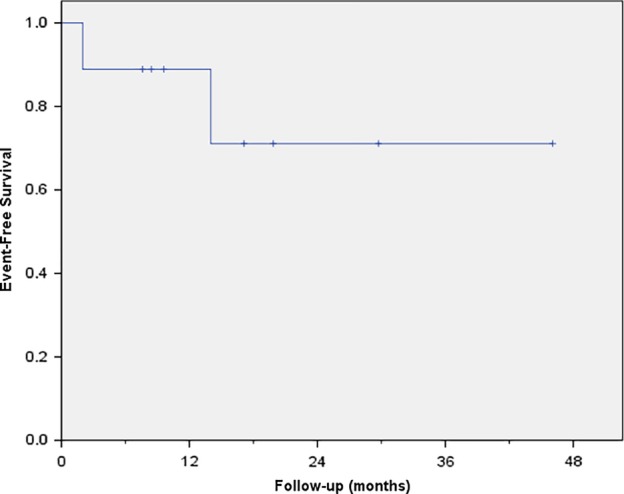
The event‐free survival curve.
Discussion
In this case series, we retrospectively analyzed the feasibility and efficiency of salvage surgery in patients with advanced lung cancer after targeted therapy. To the best of our knowledge, this is the first case series report on this subject.
In our analysis, we focused on patients with advanced (stage III or IV) NSCLC. It is our understanding that the treatment strategy for patients with early‐stage (stage I or II) NSCLC is relatively consistent (surgery is generally considered as the first choice) and the prognosis of patients is relatively satisfactory. By contrast, the prognosis of patients with relatively advanced NSCLC is far from satisfactory due to the complicated nature of the disease at this stage and unclarified treatment strategy. Therefore, we only included those patients with stage III or IV NSCLC and excluded those with early‐stage NSCLC.
Salvage surgery after effective targeted therapy gives full access to the advantages of surgery and targeted therapies. This is an idea of multidisciplinary therapy and individualized therapy.26 Similar to the role of surgery in neoadjuvant targeted therapy for lung cancer, the purpose of salvage surgery is to remove the residual or recurrent tumors when effective targeted therapy has transferred unresectable disease to resectable disease in some patients.27, 28 Moreover, an intact tumor specimen after salvage surgery can provide accurate and individual molecular pathological information in every patient. In our case 2, a mixed histological type of lung cancer was diagnosed, while in cases 3 and 7, T790M mutation was identified which is related to drug‐resistance of first‐generation agents targeting EGFR mutation.29 In concordance with the findings of Yoshida et al. these postoperative findings were key information which led to consequential treatment.13
Given the fact that the effect of targeted therapy is not static in one patient but will enter the plateau stage sooner or later,4, 6 we tended to carry out salvage surgery earlier (eg cases 2, 4, 5 and 8 in our series), in case the opportunity of surgical intervention was lost due to possible disease progression when drug resistance occurred over time. For patients who started with no apparent response to targeted therapy for more than two months, we held a similar view and suggested that they received salvage surgery if fit (eg cases 6, 7 and 9 in our series). There were also some patients receiving salvage surgery only when drug resistance of targeted therapy was proven by disease recurrence because they were afraid of surgical trauma and the inherent risks (eg cases 1 and 3 in our series). Since the number of cases reports in the literature is so small, whether salvage surgery should be carried out and how long is sufficient to judge the effect of targeted therapy is still inconclusive.
In terms of survival, the effect of salvage surgery is promising. All nine patients in our group were alive at the time of this analysis. After a mean follow‐up duration of 18.9 months, their median EFS reached 14 months, median POS 17 months, and OS reached 25 months. By contrast in the literature, the median progression‐free survival (PFS) of patients treated by targeted therapy with first generation drugs was only about 10.0 months and the PFS of patients who failed primary chemotherapy was only 3–6 months.30, 31, 32
Surgical trauma and risks are usually the major concerns of salvage surgery. The possible existence of lymph node metastasis, tumor invasion, pleural adhesion or destruction of hilar structures may make surgery more difficult. The physical status of patients may also deteriorate due to the side effects of preoperative treatment. However, according to our results, the process of salvage surgery is not so difficult or risky as imagined. Although pleural adhesion and fibrosis had developed in some patients in our group, radical surgery was achieved in all of them. There were six patients out of nine who received salvage surgery via minimally invasive thoracoscopic approaches. The intraoperative blood loss, operation time, postoperative hospital stay and postoperative complication rate in our group were similar to those for regular surgery. This was consistent with other reports on salvage surgery for lung cancer.28 Therefore, in our experience, it is feasible to perform surgery after targeted therapy. Undoubtedly, serious preoperative assessment on tumor resectability and patient fitness is essential to ensure feasibility and safety.21
Notably in our series there was a deviation between the preoperative clinical stage (y‐stage) and the postoperative pathological stage (p‐stage) in some patients, mainly due to the difference in lymph node status. The reason for this might be that preoperative targeted therapy had decreased the size and proportion of positive lymph nodes and thus the preoperative findings of PET‐CT or CT scan were not always consistent with the real status of the lymph nodes.33, 34 However, preoperative CT or PET‐CT is useful to assess the tumor resectability after targeted therapy.
The limitations of this study included the small sample size, its retrospective nature, the heterogeneity of baseline characteristics of enrolled patients, and the relatively short median follow‐up time. Given this, the results of our study should be interpreted with caution and it is hard to draw any definitive conclusion from our findings. However, this study highlighted salvage surgery as a feasible and promising therapeutic option for advanced NSCLC after targeted therapy in some selective fit patients. We look forward to future prospective studies to provide high‐level clinical evidence on this subject.
Disclosure
All authors declare no conflict of interest.
Acknowledgments
This study was supported by the Novel Technique Program Foundation of the Sixth Medical Center of Chinese PLA General Hospital (NTPF 20160926).
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69: 7–34. [DOI] [PubMed] [Google Scholar]
- 2. Ramnath N, Dilling TJ, Harris LJ et al Treatment of stage III non‐small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest 2013; 143: e314S–e40S. [DOI] [PubMed] [Google Scholar]
- 3. Goldstraw P, Chansky K, Crowley J et al The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016; 11: 39–51. [DOI] [PubMed] [Google Scholar]
- 4. Shi Q, Guan M, Wang Y et al Survival analysis of patients with advanced non‐small cell lung cancer receiving tyrosine kinase inhibitor (TKI) treatment: A multi‐center retrospective study. Thorac Cancer 2018; 9: 278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cafarotti S, Lococo F, Froesh P, Zappa F, Andrè D. Target therapy in lung cancer. Adv Exp Med Biol 2016; 893: 127–36. [DOI] [PubMed] [Google Scholar]
- 6. Sculier JP, Berghmans T, Meert AP. Advances in target therapy in lung cancer. Eur Respir Rev 2015; 24: 23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tan AC, Teh YL, Lai GGY, Tan DSW. Third generation EGFR TKI landscape for metastatic EGFR mutant non‐small cell lung cancer (NSCLC). Expert Rev Anticancer Ther 2019; 19: 431–5. [DOI] [PubMed] [Google Scholar]
- 8. Nan X, Xie C, Yu X, Liu J. EGFR TKI as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer. Oncotarget 2017; 8: 75712–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chong CR, Janne PA. The quest to overcome resistance to EGFR‐targeted therapies in cancer. Nat Med 2013; 19: 1389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu SG, Shih JY. Management of acquired resistance to EGFR TKI‐targeted therapy in advanced non‐small cell lung cancer. Mol Cancer 2018; 17: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu B, Gu X, Zhang Q. Cost‐effectiveness of Osimertinib for EGFR mutation‐positive non‐small cell lung cancer after progression following first‐line EGFR TKI therapy. J Thorac Oncol 2018; 13: 184–93. [DOI] [PubMed] [Google Scholar]
- 12. Dudek AZ. Management of resistance to EGFR TKI‐targeted therapy of lung cancer: Lessons in monitoring cancer evolution. Oncology (Williston Park) 2016; 30: 616–8. [PubMed] [Google Scholar]
- 13. Yoshida T, Kuroda H, Oya Y et al Clinical outcomes of platinum‐based chemotherapy according to T790M mutation status in EGFR‐positive non‐small cell lung cancer patients after initial EGFR‐TKI failure. Lung Cancer 2017; 109: 89–91. [DOI] [PubMed] [Google Scholar]
- 14. Martin LW, Mehran RJ. Perspectives on the effect of nodal downstaging and its implication of the role of surgery in stage IIIA (N2) non‐small cell lung cancer. J Thorac Dis 2017; 9: E646–E52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marin C, Robles R, López Conesa A, Torres J, Flores DP, Parrilla P. Outcome of strict patient selection for surgical treatment of hepatic and pulmonary metastases from colorectal cancer. Dis Colon Rectum 2013; 56: 43–50. [DOI] [PubMed] [Google Scholar]
- 16. Yoo C, Park JH, Yoon DH et al Salvage esophagectomy for locoregional failure after chemoradiotherapy in patients with advanced esophageal cancer. Ann Thorac Surg 2012; 94: 1862–8. [DOI] [PubMed] [Google Scholar]
- 17. Petrella F, Leo F, Veronesi G et al "Salvage" surgery for primary mediastinal malignancies: Is it worthwhile? J Thorac Oncol 2008; 3: 53–8. [DOI] [PubMed] [Google Scholar]
- 18. Dickhoff C, Dahele M, Paul MA et al Salvage surgery for locoregional recurrence or persistent tumor after high dose chemoradiotherapy for locally advanced non‐small cell lung cancer. Lung Cancer 2016; 94: 108–13. [DOI] [PubMed] [Google Scholar]
- 19. Casiraghi M, Maisonneuve P, Piperno G et al Salvage surgery after definitive Chemoradiotherapy for non‐small cell lung cancer. Semin Thorac Cardiovasc Surg 2017; 29: 233–41. [DOI] [PubMed] [Google Scholar]
- 20. Schreiner W, Dudek W, Lettmaier S, Fietkau R, Sirbu H. Long‐term survival after salvage surgery for local failure after definitive Chemoradiation therapy for locally advanced non‐small cell lung cancer. Thorac Cardiovasc Surg 2018; 66: 135–41. [DOI] [PubMed] [Google Scholar]
- 21. Hamaji M, Chen F, Matsuo Y, Ueki N, Hiraoka M, Date H. Treatment and prognosis of isolated local relapse after stereotactic body radiotherapy for clinical stage I non‐small‐cell lung cancer: Importance of salvage surgery. J Thorac Oncol 2015; 10: 1616–24. [DOI] [PubMed] [Google Scholar]
- 22. Hino H, Nishimura T, Usuki C et al Salvage surgery for primary lung cancer after chemotherapy in octogenarians. Thorac Cancer 2017; 8: 271–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Uramoto H, Akiyama H, Nakajima Y et al The long‐term outcomes of induction chemoradiotherapy followed by surgery for locally advanced non‐small cell lung cancer. Case Rep Oncol 2014; 7: 700–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hishida T, Nagai K, Mitsudomi T et al Salvage surgery for advanced non‐small cell lung cancer after response to gefitinib. J Thorac Cardiovasc Surg 2010; 140: e69–71. [DOI] [PubMed] [Google Scholar]
- 25. Hashimoto K, Horinouchi H, Ohtsuka T et al Salvage surgery for a super‐responder by gefitinib therapy for advanced lung cancer. Gen Thorac Cardiovasc Surg 2012; 60: 851–4. [DOI] [PubMed] [Google Scholar]
- 26. Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest 2017; 151: 193–203. [DOI] [PubMed] [Google Scholar]
- 27. Situ D, Du Y, Li Y, Chen J, Yang H. Neoadjuvant target therapy followed by video‐assisted thoracoscopic surgery lobectomy plus lymph node clearance for locally advanced lung cancer. J Thorac Dis 2019; 11: 246–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhong WZ, Chen KN, Chen C et al Erlotinib versus gemcitabine plus Cisplatin as Neoadjuvant treatment of stage IIIA‐N2 EGFR‐mutant non‐small‐cell lung cancer (EMERGING‐CTONG 1103): A randomized phase II study. J Clin Oncol 2019; 37:2235–45. [DOI] [PubMed] [Google Scholar]
- 29. Bell DW, Gore I, Okimoto RA et al Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nat Genet 2005; 37: 1315–6. [DOI] [PubMed] [Google Scholar]
- 30. Maruyama R, Nishiwaki Y, Tamura T et al Phase III study, V‐15‐32, of gefitinib versus docetaxel in previously treated Japanese patients with non‐small‐cell lung cancer. J Clin Oncol 2008; 26: 4244–52. [DOI] [PubMed] [Google Scholar]
- 31. Mok TS, Wu YL, Thongprasert S et al Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–57. [DOI] [PubMed] [Google Scholar]
- 32. Morita S, Okamoto I, Kobayashi K et al Combined survival analysis of prospective clinical trials of gefitinib for non‐small cell lung cancer with EGFR mutations. Clin Cancer Res 2009; 15: 4493–8. [DOI] [PubMed] [Google Scholar]
- 33. Gupta NC, Graeber GM, Rogers JS, Bishop HA. Comparative efficacy of positron emission tomography with FDG and computed tomographic scanning in preoperative staging of non‐small cell lung cancer. Ann Surg 1999; 229: 286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nambu A, Kato S, Motosugi U et al Thin‐section CT of the mediastinum in preoperative N‐staging of non‐small cell lung cancer: Comparison with FDG PET. Eur J Radiol 2010; 73: 510–7. [DOI] [PubMed] [Google Scholar]


