Abstract
Background
The aim of this study was to examine the trends in the mortality rate and years of life lost (YLL) rate of lung cancer in Tianjin, China, during the period from 1999 to 2016.
Methods
Lung cancer death data were obtained from Tianjin residents' all‐cause death monitoring system, which covers the whole population of Tianjin. Crude mortality rate, age‐standardized mortality rate, truncated rate (35–64 years), YLL and age‐standardized YLL rate data were calculated and trends examined.
Results
From 1999 to 2016, a total of 93 358 lung cancer deaths were reported in Tianjin, which accounted for 38.0% of all cancer deaths (93 358/245744). The crude mortality rate of lung cancer had increased 58.5% from 1999 (40.15/100000) to 2016 (63.64/100000), average annual percent change (AACP) = 2.9%, P < 0.01. However, the age‐standard YLL rate had decreased to 13.3% in 2016 than in 1999, AACP = –0.8%, P < 0.01, with a stable trend in males (AACP = –0.2%), and noticeable decreasing trend in females (AACP = –1.4%). The lung cancer mortality rate (ASRW) in urban areas was higher than that in rural areas in 1999, with a ratio of 1.99:1. However, it was lower in 2016, with the ratio of 0.98:1. For the truncated rate (35–64 years), it had decreased in urban areas compared with rural areas since the year 2013.
Conclusion
Lung cancer remains the most fatal cancer in Tianjin. However, the age‐standard YLL rate of lung cancer has decreased considerably accompanied by a decline in smoking rate years ago, especially in women and people living in urban areas. Considerable attention is therefore needed in the rural areas where cases of lung cancer are still rapidly increasing.
Keywords: Lung cancer, mortality, risk factors, trend, YLL
Introduction
Worldwide, lung cancer has been the most common cause of cancer deaths over the past few decades.1 According to global cancer statistics in 2018,2 there were 2 096 876 incidence cases of lung cancer, and 1 761 007 deaths worldwide. It is the leading cause of cancer morbidity (11.6%) and mortality (18.4%), respectively. The health care burden and costs attributed to lung cancer is substantial on a global scale.3 The five‐year survival rate (17.8%) is reported to be much lower than that of other leading cancers.4 In 2013, the crude morbidity and mortality of lung cancer in China was 57.7/100000 and 46.9/100000, respectively, accounting for 20.3% of all incidences of cancer and 26.6% of cancer deaths.5
The incidence and mortality rates of lung cancer have been declining in men and rising in women on a global scale in recent decades.6, 7, 8 The reason for this was an uptake and subsequent decline in male smoking prevalence,9 which was followed by a later uptake in smoking by women in many high Human Development Index (HDI) countries. Indoor exposure to fumes from cooking and heating using coal or combustible materials in unventilated stoves has also been reported to increase lung cancer risk in women populations in which there was previously reported to be a low smoking prevalence.10
To better understand the national trends in lung cancer in China, several relevant studies have been conducted, and most of these report an increasing trend.11, 12, 13 However, due to the large differences in living habits and environment across China, the changes in lung cancer mortality rate show different characteristics in different regions and populations. In addition, the years of life lost (YLL) rate is more likely to reflect the death count and the loss of life than the death rate. However, there have been few detailed analyses on YLL rate variation trends of lung cancer. Most previous studies have been partly based on death registries which may have resulted in a loss information compared to a death monitoring system based on the whole population.
Tianjin is the third largest municipal city in China with more than 10 million residents. Its urbanization level, aging population level and air pollution level are all high, and these are all important factors which affect lung cancer. Based on the whole cause of death monitoring system in Tianjin, this paper analyzed the lung cancer mortality rate, YLL level and the change in trends in the last 18 years by birth cohort and region type, in order to provide the basis for formulating relevant future policies.
Methods
Data sources
Lung cancer death data was obtained from Tianjin residents' all‐cause death monitoring system. Medical institutions at all levels in the city are required to report death cases online, and a three‐level quality audit should be carried out by medical institutions, county CDC and municipal CDC.14 This study included lung cancer death data of Tianjin residents from 1999 to 2016, using the disease codes C33–C34 (ICD10). Demographic data were obtained from the Tianjin public security bureau.
Statistical analysis
Statistical analysis was performed to calculate the crude mortality rate, age‐standardized rate adjusted to the world population (ASRW), truncated rate (35–64 years), years of life lost (YLL) and age‐standardized YLL rate using Segi's world standard population age composition (ASRW of YLL) of lung cancer in Tianjin. We employed joinpoint regression analysis to examine the trend of mortality and YLL rate, and to calculate the average annual percentage change (AAPC) using joinpoint statistical software version 4.3. The AAPC was calculated as a geometrically weighted average of the various annual percent changes (APCs) from the joinpoint regression analysis, with weights being equivalent to the length of each segment during the specified time interval.15 The statistical significance of AAPC was ascertained comparing its magnitude with zero, and all insignificant AAPCs were regarded as having “stable trends”.
| (1) |
The truncated rate (35–64) was the age‐standard rate of people aged between 35 to 64 years old by Segi's World standard population age composition, as there was usually a high mortality rate in this age group.
| (2) |
Where:
N(s,a,t) was the number of deaths due to lung cancer for the given age (a) and sex (s) in year (t).
L(s,a) was a standard loss function specifying years of life lost for a death at age (a) for sex (s).
We chose the standard loss function specifying years of life lost from WHO Standard Life Table for Years of Life Lost16
(3) YLL rate = YLL/ number of population; age‐standard YLL rate (ASRW Of YLL) was the YLL rate modified by Segi's World standard population age composition. (3)
Results
General lung cancer mortality rate
From 1999 to 2016, a total of 93 358 lung cancer deaths were reported in Tianjin, which accounted for 38.0% of all cancer deaths (93 358/245744), with a total of 54 800 men and 38 558 women. The crude mortality rate of lung cancer was 40.15/100000 to 63.64/100000 from 1999 to 2016, which had increased 58.5%, AACP = 2.9%, P < 0.01. However, there was a stable trend of the ASRW, AACP = –0.1%, P > 0.05. For the age‐standard YLL rate (ASRW of YLL), there was a decreasing trend, AACP = –0.8%, P < 0.01, which had decreased 13.3% in 2016 from the rate reported in 1999 (Table 1).
Table 1.
Age‐standardized rate adjusted to the world population (ASRW), Years of life lost (YLL) and YLL ASRW of lung cancer in Tianjin, China from 1999 to 2016
| Years | Crude mortality rate (1/105) | ASRW (1/105) | Truncated rate (35–64 years) (1/105) | YLL (person years) | YLL ASRW (person years/1000) |
|---|---|---|---|---|---|
| 1999 | 40.15 | 36.63 | 38.23 | 94 857 | 9.27 |
| 2000 | 44.02 | 38.95 | 39.48 | 102 845 | 9.77 |
| 2001 | 44.95 | 38.60 | 38.30 | 104 475 | 9.65 |
| 2002 | 41.75 | 34.60 | 33.34 | 96 494 | 8.57 |
| 2003 | 47.02 | 37.53 | 34.02 | 107 367 | 9.21 |
| 2004 | 47.17 | 36.57 | 34.42 | 109 109 | 9.04 |
| 2005 | 46.19 | 34.98 | 33.18 | 106 667 | 8.63 |
| 2006 | 50.06 | 36.44 | 32.22 | 114 188 | 8.84 |
| 2007 | 52.76 | 37.15 | 32.04 | 119 444 | 8.90 |
| 2008 | 55.63 | 37.87 | 32.45 | 126 314 | 9.06 |
| 2009 | 59.48 | 39.18 | 33.81 | 135 718 | 9.38 |
| 2010 | 59.75 | 38.35 | 31.65 | 134 433 | 9.00 |
| 2011 | 57.37 | 35.96 | 30.24 | 130 572 | 8.49 |
| 2012 | 59.73 | 36.68 | 29.41 | 134 206 | 8.54 |
| 2013 | 62.92 | 38.11 | 29.71 | 142 820 | 8.74 |
| 2014 | 65.43 | 38.31 | 29.81 | 149 372 | 8.78 |
| 2015 | 65.14 | 36.58 | 30.20 | 149 979 | 8.44 |
| 2016 | 63.64 | 34.69 | 29.03 | 147 818 | 8.04 |
| AAPC (%) | 2.90 | −0.10 | −1.60 | ‐ | −0.70 |
| P | <0.01 | 0.80 | <0.01 | ‐ | <0.01 |
Temporal trends of lung cancer mortality and YLL rate by gender
Lung cancer mortality rate (ASRW) in men was higher than in women, with a ratio of 1.62:1 men to women in 2016.
The ASRW of lung cancer mortality in men had a stable trend from 1999 to 2016, which was 43.63/100000 and 42.88/100000 in 1999 and 2016, respectively, AACP = 0.2%, P > 0.05. The ASRW of YLL also showed a stable trend, AACP = −0.2%, P > 0.05. There were decreasing trends for ASRW, and ASRW of YLL of women, the AACP was −0.4, −1.4, respectively. The ASRW of YLL had decreased 25.2% in 2016 compared to 1999 (Table 2).
Table 2.
Age‐standardized rate adjusted to the world population (ASRW), truncation rate and age‐standardized years of life lost (YLL) rate of lung cancer by gender in Tianjin, China, from 1999 to 2016
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Years | ASRW (1/105) | Truncated rate (35–64 years) (1/105) | YLL ASR (person years/1000) | ASRW (1/105) | Truncated rate (35–64 years) (1/105) | YLL ASRW (person years/1000) |
| 1999 | 43.63 | 43.44 | 10.86 | 29.63 | 33.06 | 7.67 |
| 2000 | 45.50 | 46.05 | 11.41 | 32.40 | 32.98 | 8.13 |
| 2001 | 45.60 | 45.60 | 11.43 | 31.60 | 31.12 | 7.88 |
| 2002 | 41.18 | 40.17 | 10.15 | 28.02 | 26.58 | 6.99 |
| 2003 | 46.06 | 43.42 | 11.40 | 29.00 | 24.77 | 7.02 |
| 2004 | 43.47 | 42.35 | 10.78 | 29.67 | 26.57 | 7.30 |
| 2005 | 41.61 | 40.09 | 10.25 | 28.35 | 26.33 | 7.01 |
| 2006 | 43.23 | 40.68 | 10.55 | 29.65 | 23.84 | 7.13 |
| 2007 | 45.05 | 41.77 | 10.84 | 29.26 | 22.41 | 6.96 |
| 2008 | 45.72 | 43.44 | 11.10 | 30.02 | 21.57 | 7.03 |
| 2009 | 47.01 | 44.49 | 11.46 | 31.35 | 23.24 | 7.31 |
| 2010 | 46.11 | 42.54 | 11.03 | 30.59 | 20.90 | 6.97 |
| 2011 | 43.04 | 40.26 | 10.44 | 28.88 | 20.31 | 6.54 |
| 2012 | 45.25 | 40.65 | 10.84 | 28.10 | 18.31 | 6.24 |
| 2013 | 46.81 | 40.88 | 11.05 | 29.42 | 18.73 | 6.43 |
| 2014 | 46.80 | 39.83 | 10.98 | 29.82 | 20.00 | 6.59 |
| 2015 | 44.90 | 41.18 | 10.66 | 28.26 | 19.44 | 6.23 |
| 2016 | 42.88 | 41.24 | 10.34 | 26.51 | 17.04 | 5.73 |
| AAPC (%) | 0.20 | −0.50 | −0.20 | −0.40 | −3.60 | −1.40 |
| P‐value | 0.30 | 0.02 | 0.40 | 0.05 | <0.01 | <0.01 |
Temporal trends of lung cancer mortality and YLL rate by region
From 1999 to 2016, the ASRW of lung cancer mortality in urban areas had decreased by 26.6% (from 46.61/100000 to 34.19/100000), AACP = –1.4%, P < 0.01. The ASRW of YLL had decreased 34.0% in 2016 compared to 1999. In rural areas, the ASRW of lung cancer mortality had increased from 23.46/100000 to 35.01/100000, AACP = 2.46%, P < 0.01. The ASRW of YLL had increased 37.6% in 2016 compared to 1999, AACP = 1.74%, P < 0.01.
The ASRW of lung cancer mortality in urban areas was higher than that in rural areas in 1999, with a ratio of 1.99:1. However, it decreased in 2016, with a ratio of 0.98:1. For the truncated rate (35–64 years), it had decreased in urban areas compared with rural areas since 2013 (Table 3).
Table 3.
Age‐standardized rate adjusted to the world population (ASRW), truncation rate and age‐standardized years of life lost (YLL) rate of lung cancer by region in Tianjin, China, from 1999 to 2016
| Urban area | Rural area | |||||
|---|---|---|---|---|---|---|
| Years | ASRW (1/105) |
Truncated rate (35–64 years) (1/105) |
YLL ASRW (person years/1000) | ASRW(1/105) | Truncated rate (35–64 years) (1/105) | YLL ASRW (person years/1000) |
| 1999 | 46.61 | 48.45 | 11.77 | 23.46 | 25.71 | 6.01 |
| 2000 | 47.72 | 45.24 | 11.70 | 27.26 | 32.53 | 7.21 |
| 2001 | 47.36 | 44.78 | 11.68 | 26.69 | 26.17 | 6.52 |
| 2002 | 40.10 | 34.35 | 9.60 | 27.05 | 31.95 | 7.11 |
| 2003 | 48.67 | 41.97 | 11.70 | 22.94 | 24.45 | 6.00 |
| 2004 | 44.02 | 38.43 | 10.60 | 26.81 | 29.67 | 7.01 |
| 2005 | 42.18 | 36.09 | 10.09 | 25.53 | 29.76 | 6.73 |
| 2006 | 43.93 | 36.19 | 10.30 | 26.64 | 27.53 | 6.92 |
| 2007 | 45.57 | 35.38 | 10.60 | 26.30 | 28.20 | 6.77 |
| 2008 | 44.10 | 34.86 | 10.21 | 29.90 | 29.80 | 7.61 |
| 2009 | 44.87 | 35.63 | 10.34 | 32.05 | 31.85 | 8.21 |
| 2010 | 41.81 | 33.16 | 9.68 | 33.09 | 29.90 | 8.01 |
| 2011 | 39.60 | 31.70 | 9.20 | 30.39 | 28.52 | 7.45 |
| 2012 | 39.74 | 31.11 | 9.15 | 31.88 | 27.22 | 7.63 |
| 2013 | 39.41 | 28.80 | 8.85 | 35.66 | 30.80 | 8.46 |
| 2014 | 39.46 | 28.79 | 8.84 | 36.02 | 30.50 | 8.52 |
| 2015 | 37.19 | 30.05 | 8.54 | 35.61 | 30.41 | 8.28 |
| 2016 | 34.19 | 27.88 | 7.77 | 35.01 | 30.22 | 8.27 |
| AAPC (%) | −1.40 | −2.90 | −2.00 | 2.46 | 0.40 | 1.74 |
| P‑value | <0.01 | <0.01 | <0.01 | <0.01 | 0.28 | <0.01 |
Temporal trends of lung cancer mortality by age groups
In the 0–44 age group, the lung cancer mortality rate in urban men (AACP = –7.4%, P < 0.01) and women (AACP = –3.4%, P < 0.01) all had decreasing trends and became lower than for rural men and women. For rural men and women, there were stable trends, and the AACP was −1.4 and −1.0, respectively, P > 0.05 (Fig 1).
Figure 1.
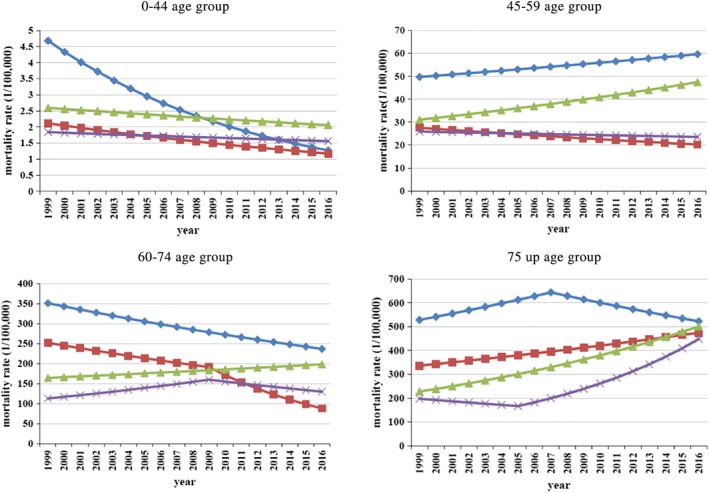
Trends of lung cancer mortality by age groups in urban and rural areas from 1999 to 2016 in Tianjin, China. ( ) urban male, (
) urban male, ( ) urban female, (
) urban female, ( ) rural male, (
) rural male, ( ) rural female.
) rural female.
In the 45–59 age group, there were increasing trends in urban men (AACP = 1.1%, P < 0.01) and rural men (AACP = 2.5%, P < 0.01). There was a decreasing trend in urban women (AACP = –1.8%, P < 0.01), but a stable trend in rural women (AACP = –0.6%, P > 0.05) (Fig 1).
In the 60–74 age group, the lung cancer mortality rate in both men and women in urban areas decreased rapidly, and the AACP was −2.3 and −6.0, respectively, P < 0.01, but there was an increased trend for men in rural areas (AACP = 1.1%, P < 0.01) and stable trend for women in rural areas (AACP = 0.8%, P > 0.05). (Fig 1).
In the 75 and upwards age group, there was a great increase in lung cancer mortality rates in rural areas in both men (AACP = 4.7%, P < 0.01) and women (AACP = 4.9%, P < 0.01) which became almost the same as that in urban areas (Fig 1).
Discussion
The results of this study show that there was a really high disease burden of lung cancer in Tianjin, which accounted for 38.0% of all cancer deaths, in comparison with 18.4% worldwide, and 26.6% in China [5,17] In 2013, the world age standardized rates for men and women in Tianjin were 46.81/100000 and 29.42/100000, respectively, which was higher than the national average (40.3/100000 for men, 17.21/100000 for women).17 Compared with other countries and regions of the world in 2012,7 the mortality rate of lung cancer in women in Tianjin was almost the same as Denmark (28.40/100000) which was ranked as second in the world. For men, it was similar to Greece (45.4/100000), which was ranked as twenty‐third in the world.
The prevalence of lung cancer in different regions is closely related to smoking rate, passive smoking and indoor and outdoor air pollution. Studies have established that smokers have a greatly increased risk of lung cancer 18, 19 and that lung cancer incidence and mortality increase in a dose‐dependent manner with smoking.20, 21 The proportion of lung cancer deaths attributable to smoking vary across populations, ranging from >80% in the United States22 and France23 to 61% in a pooled analysis of 21 Asian cohorts24 and 40% in sub‐Saharan Africa.25 Previous studies havereported that the smoking‐attributed fraction for lung cancer was 50.2% in men and 32.7% in women in Tianjin.26
In several studies, the high mortality rate in China, despite a relatively lower prevalence of female smoking compared to more developed countries, was probably due to higher exposure to secondhand smoke and indoor air pollution from unventilated combustion of coal used for heating and cooking purposes.10, 27 A meta‐study showed that indoor air pollution from cooking was also a high risk to lung cancer for non‐smoking Chinese women, with ORs (95% CI) as follows: kitchen smog while cooking 2.21 (1.27, 2.96), frequency of deep frying food per week 2.24 (1.61, 3.12), compared to passive smoking (at workplace in adult period 1.47 [1.28, 1.69]).28 In addition, outdoor air pollution has also been reported to play an important role in lung cancer.29, 30 The First National Smoking Prevalence Survey in 1984 showed that the smoking prevalence was 7.04% in women in China, while the prevalence of 27.4% in Tianjin women was the highest.31 The air PM2.5 concentrations in Tianjin ranked in the top 10 among 70 monitored cities. The reason why Tianjin women had a high mortality rate because of lung cancer was mainly the result of the interaction of three factors; smoking, passive smoking and indoor and outdoor air pollution. Neighboring Hebei province, which has the same air pollution exposure and women who smoke as Tianjin, also has a higher death rate of lung cancer among women (24.64/100000).32
Globally, the ASRW of lung cancer mortality of men in most developed countries has decreased from the 1970s to the 1990s, but in the meantime has increased for women.7, 33 In the United States, lung cancer incidence has decreased since 1990 (ACP = –0.9 [95% CI, −1.0%, −0.8%]), and escalated from 2007 to 2015 (ACP = –2.6 [−2.9%, −2.2%]).34 In 36 selected countries of different socioeconomic status over the world, there was one country with increasing trends, 30 countries with decreasing trends, and five countries with stable trends in men. There were 16 countries with increasing mortality trends, six countries with decreasing trends and 14 countries with stable trends in women.8
In our study, there was a stable trend for men and significant decreasing trend for women, especially for women under 75 years old. It is quite different to other places in China which had stable trends of both gender of the whole country during 2000 to 2011,35 increasing trends of Shandong province in both gender from 1970 to 2013,13 increasing trends of Hebei province from 1973–1975 to 2010–2011,32 decreasing trends from 2005 to 2014 in men (APC = −2.68%, P = 0.009) but not in women (APC = −0.91%, P = 0.305) of Xuhui district of Shanghai.36 The changing trends of lung cancer of Tianjin were closely related to the change of smoking rates. Men born before 1980 in Tianjin all had high smoking rates (more than 58%), but the smoking rates of women born after 1950 in Tianjin dramatically declined (Fig 2). This was another case proving that lung cancer mortality rate is deeply affected by the smoking rate.
Figure 2.
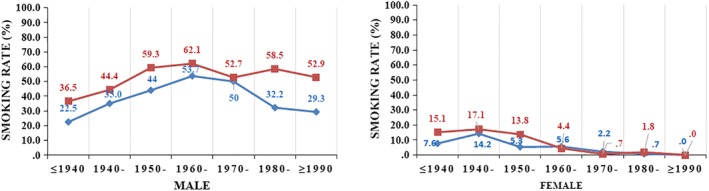
Current smoking rate by birth cohort, region and gender of 2015 in Tianjin, China. ( ) Urban, (
) Urban, ( ) Rural.
) Rural.
Another finding in our study was that the lung cancer mortality rate (ASRW) in urban areas exceeded that of urban areas in 2016. Also, the truncated rate (35–64 years) was lower in urban areas than in rural areas since 2013. More attention is needed from key decision makers in rural areas where people have a lower health knowledge and poor medical resources compared to the urban areas.37 The same trend had been found in the capital city Beijing nearby Tianjin, where the incidence rate of lung cancer was reported to have increased slightly in urban areas but increased significantly in rural areas during 2000–2012.38 Changes in smoking rates between urban and rural populations can partly explain these changes. Among young men in Tianjin born after 1970, smoking rates declined significantly in urban areas but remained high in rural areas. Accordingly, the mortality of male lung cancer in the corresponding urban areas showed a downward trend, while the mortality of males lung cancer in rural areas showed an upward trend in all age groups above 45 years old. It is essential that tobacco control, health education and necessary screening of key population in rural areas is carried out. In 2012, Tianjin implemented smoke free regulations, and the second‐hand smoke exposure rate of residents decreased significantly. However, its effect on lung cancer mortality is not as yet evident. Tougher enforcement is needed, especially in the rural areas.
Compared to the mortality rate, the age standard YLL rate can reflect both the death count and the loss of life, which could better indicate the disease burden. In our study, the age standard YLL rate had generally decreased considerably from 1999 to 2016, while the ASRW was stable, which indicates that the problem of lung cancer in Tianjin is in a better situation.
In conclusion, the mortality rate of lung cancer in Tianjin remains very high, and taking into account the aging population statistics, the crude mortality rate of lung cancer and YLL are still rising rapidly. However, the age standard YLL rate has seen a decreasing trend, especially in women and people in urban areas which means the age standard lung cancer disease burden decreases without taking into account the aging factor. Much attention is therefore needed since the ASRW and age standard YLL rate in rural areas both have increasing trends, and have exceeded that of urban areas. The trends of lung cancer mortality by gender, region and age groups are closely related to the trends of smoking rates many decades previously.
Disclosure
The authors declare that they have no competing interests.
Acknowledgments
We thank all the staff who were involved in the Tianjin CDRS, local CDCs, hospitals, and community health service centers. We have not used copyrighted material and copyrighted surveys, instruments, or tools in our study. This study was implemented as a register‐based study based on anonymous data at TJCDC and was approved by the TJCDC Ethics Committee. Register‐based studies on anonymous data do not require written consent in Tianjin.Founding: Smoke‐free environment regulatory performance, enforcement effectiveness and health effects evaluation research project.
References
- 1. Ferlay J, Soerjomataram I, Ervik M et al GLOBOCAN 2012v1.0, Cancer Incidence and Mortality Worldwide. IARC Cancer Base No. 11. International Agency for Research on Cancer, Lyon: 2013. [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68 (6): 394–424. 10.3322/caac.21492 Epub 2018 Sep 12. [DOI] [PubMed] [Google Scholar]
- 3. Vos T, Allen C, Arora M et al Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the global burden of disease study 2015. Lancet 2016; 388 (10053): 1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. National Institutes of Health. National Cancer Institute. SEER Cancer Statistics Review; 1975–2011. [Cited 2 Dec 2016.] Available from URL: http://www.seer.cancer.gov/.
- 5. He J, Chen WQ. Chinese Cancer Registry Annual Report 2016. Tsinghua University Press, Beijing: 2017; 139. [Google Scholar]
- 6. Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010; 19: 1893–907. [DOI] [PubMed] [Google Scholar]
- 7. Islami F, Torre LA, Jemal A. Global trends of lung cancer mortality and smoking prevalence. Transl Lung Cancer Res 2015; 4 (4): 327–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. MCS W, Lao XQ, Ho KF, Goggins WB, Tse SLA. Incidence and mortality of lung cancer: Global trends and association with socioeconomic status. Sci Rep 2017; 7: 14300; Published online 2017 Oct 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Torre LA, Siegel RL, Ward E, Jemal A. Global cancer incidence and mortality rates and trends – An update. Cancer Epidemiol Biomarkers Prev 2016; 25: 16–27. [DOI] [PubMed] [Google Scholar]
- 10. Sisti J, Boffetta P. What proportion of lung cancer in never‐smokers can be attributed to known risk factors? Int J Cancer 2012; 131: 265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen W, Zhang S, Zou X. Estimation and projection of lung cancer incidence and mortality in China. Chin J Lung Cancer 2010; 13: 488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fang J, Dong H, Wu K, Du P, Xu Z, Lin K. Characteristics and prediction of lung cancer mortality in China from 1991 to 2013. Asian Pac J Cancer Prev 2015; 16: 5829–34. [DOI] [PubMed] [Google Scholar]
- 13. Fu Z, Li Y, Lu Z et al Lung cancer mortality clusters in Shandong Province, China: How do they change over 40 years? Oncotarget 2017; (51); 8: 88770–88 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang GH, Zhang H, Li W et al Study on smoking‐attributed mortality by using all causes of death surveillance system in Tianjin. Chin J Epidemiol 2016; 37 (3): 381–3. [DOI] [PubMed] [Google Scholar]
- 15. Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual percent change in trend analysis. Stat Med 2009; 28: 3670–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. WHO . WHO methods and data sources for global burden of disease estimates 2000‐2015 In: Global Health Estimates Technical Paper WHO/HIS/IER/GHE/2017, Vol. 1 WHO, Geneva: 2017. [Google Scholar]
- 17. Chen WQ, Zuo TT, Zheng RS et al Lung cancer incidence and mortality in China in 2013. Zhonghua Zhong Liu Za Zhi 2017; 39 (10): 795–800. [DOI] [PubMed] [Google Scholar]
- 18. Agudo A, Ahrens W, Benhamou E et al Lung cancer and cigarette smoking in women: A multicenter case‐control study in Europe. Int J Cancer 2000; 88: 820–7. [DOI] [PubMed] [Google Scholar]
- 19. Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ 2004; 328: 1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peto R, Darby S, Deo H et al Smoking, smoking cessation, and lung cancer in the UKsince 1950: Combination of national statistics with two case‐control studies. BMJ 2000; 321: 323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Svensson C, Pershagen G, Klominek J. Smoking and passive smoking in relation to lung cancer in women. Acta Oncol 1989; 28: 623–9. [DOI] [PubMed] [Google Scholar]
- 22. National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health . The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Centers for Disease Control and Prevention (US), Atlanta, GA: 2014. [PubMed] [Google Scholar]
- 23. Ribassin‐Majed L, Hill C. Trends in tobacco‐attributable mortality in France. Eur J Public Health 2015; 5(5): 824–828. [DOI] [PubMed] [Google Scholar]
- 24. Zheng W, McLerran DF, Rolland BA et al Burden of total and cause‐specific mortality related to tobacco smoking among adults aged ≥45 years in Asia: A pooled analysis of 21 cohorts. PLOS Med 2014; 11: e1001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Institute for Health Metrics and Evaluation (IHME) . GBD Cause Patterns [Cited 28 Jul 2015.] Available from URL: http://vizhub.healthdata.org/gbd-cause-patterns/.
- 26. Li W, Jiang G, Wang D et al Smoking and mortality in Tianjin, China: A death registry based case‐control study of 180 000 adult deaths from 2010 to 2014. Prev Chronic Dis 2018; 15: 170577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Secretan B, Straif K, Baan R et al A review of human carcinogens‐part E: Tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol 2009; 10: 1033–4. [DOI] [PubMed] [Google Scholar]
- 28. Yu YW, Wang CP, Han YF et al Meta‐analysis on related risk factors regarding lung cancer in non‐smoking Chinese women. Zhonghua Liu Xing Bing Xue Za Zhi 2016; 37 (2): 268–72. 10.3760/cma.j.issn.0254-6450 [Article in Chinese]. [DOI] [PubMed] [Google Scholar]
- 29. Hamra GB, Guha N, Cohen A et al Outdoor particulate matter exposure and lung cancer: A systematic review and meta‐analysis. Environ Health Perspect 2014; 122: 906–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Loomis D, Grosse Y, Lauby‐Secretan B et al The carcinogenicity of outdoor air pollution[J]. Lancet Oncol 2013; 14: 1262–3. [DOI] [PubMed] [Google Scholar]
- 31. Weng XZ, Hong ZG, Chen DY. Smoking prevalence in Chinese aged 15 and above. Chin Med J 1987; 100 (11): 886–92. [PubMed] [Google Scholar]
- 32. He Y, Li D, Song G et al Lung cancer burden has increased during the last 40 years in Hebei Province, China. Thorac Cancer 2016; 7 (3): 323–32. Published online 2016 Jan 26. 10.1111/1759-7714.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Torre LA, Siegel RL, Ward EM, Jemal A. International variation in lung cancer mortality rates and trends among women. Cancer Epidemiol Biomarkers Prev 2014; 23 (6): 1025–36. [DOI] [PubMed] [Google Scholar]
- 34. Zhong YJ, Wen YF, Wong HM, Yin G, Lin R, Yang SY. Trends and patterns of disparities in burden of lung cancer in the United States, 1974–2015. Front Oncol 2019; 9 (5): 1–11. 10.3389/fonc.2019.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen W, Zheng R, Baade PD et al Cancer statistics in China. CA Cancer J Clin 2016; 2015; 66 (2): 115–32. [DOI] [PubMed] [Google Scholar]
- 36. Liang F, Wu C, Gu H et al Lung cancer incidence in female rises significantly in urban sprawl of Shanghai after introduction of LDCT screening. Lung Cancer 2019; 132: 114–8. [DOI] [PubMed] [Google Scholar]
- 37. Yang J, Zhu J, Zhang YH et al Lung cancer in a rural area of China: Rapid rise in incidence and poor improvement in survival. Asian Pac J Cancer Prev 2015; 16 (16): 7295–302. [DOI] [PubMed] [Google Scholar]
- 38. Yuan YN, Yang L, Liu S, Li HC, Wang N. Analyses on the difference and trend of lung cancer incidence in Beijing, 2000–2012. Zhonghua Yu Fang Yi Xue Za Zhi 2018; 52 (7): 691–6. [DOI] [PubMed] [Google Scholar]


