Abstract
Background
We aimed to obtain a set of health state utility scores of patients with esophageal cancer (EC) and precancerous lesions in China, and to explore the influencing factors of health‐related quality of life (HRQoL).
Methods
A hospital‐based multicenter cross‐sectional study was conducted. From 2013 to 2014, patients with EC or precancerous lesions were enrolled. HRQoL was assessed using a European quality of life‐5 dimension (EQ‐5D‐3L) instrument. Multivariable linear regression analysis was performed to explore the influencing factors of the EQ‐5D utility scores.
Results
A total of 2090 EC patients and 156 precancer patients were included in the study. The dimension of pain/discomfort had the highest rate of self‐reported problems, 60.5% in EC and 51.3% in precancer patients. The mean visual analog scale (VAS) score for EC and precancer patients were 68.4 ± 0.7 and 64.5 ± 3.1, respectively. The EQ‐5D utility scores for EC and precancer patients were estimated as 0.748 ± 0.009 and 0.852 ± 0.022, and the scores of EC at stage I, stage II, stage III, and stage IV were 0.693 ± 0.031, 0.747 ± 0.014, 0.762 ± 0.015, and 0.750 ± 0.023, respectively. According to the multivariable analyses, the factors of region, occupation, household income in 2012, health care insurance type, pathological type, type of therapy, and time points of the survey were statistically associated with the EQ‐5D utility scores of EC patients.
Conclusions
There were remarkable decrements of utility scores among esophageal cancer patients, compared with precancer patients. The specific utility scores of EC would support further cost‐utility analysis in populations in China.
Keywords: EQ‐5D‐3L, esophageal cancer, health state utility, health‐related quality of life, multivariable linear regression
Introduction
Esophageal cancer (EC) is one of the most prevalent malignant tumors in the upper digestive system. According to GLOBOCAN 2018, it is the seventh most common cancer and the sixth most common cause of death from cancer worldwide, and the estimated numbers of EC new cases and deaths in 2018 were 572 034 and 508 585, respectively.1 In China, EC is the sixth most prevalent cancer type, affecting approximately 17.87 per 100 000 individuals. With a mortality rate of 13.68 per 100 000 people, it has been ranked fourth as a leading cause of cancer death in 2015.2 The prognosis of EC is poor, with a five‐year relative survival of 20.9% in China.3 The postoperative prognosis of patients with early EC is much better, with a five‐year survival rate of ≥90% as reported in the study by Sadiq and Mansour.4 However, most early EC or precancerous lesions which show no typical clinical symptoms cannot be easily detected.5 As the risk of EC development can be reduced after the removal of precancerous lesions and the prognosis improves,6 early detection and treatment is particularly important. Although regular follow‐up has been recommended for most patients with early EC and precancerous lesions, the compliance of patients undergoing long‐term follow‐up visits is relatively poor, which has led to a considerable psychological burden for these patients.5 Furthermore, previous studies have indicated that the incidence rates of EC have decreased in China over the last 20 years, but EC has still been a significant sociopsychological and economic burden for patients.7 It is of great significance to understand EC or precancer patients' health‐related quality of life (HRQoL), which will also benefit healthcare services.
Quality of life (QoL) has been one of the critical indicators in cost‐utility analysis in health economic evaluation.8 QoL reflects one's subjective perceptions, goals, expectations and concerns in relation to his or her living environment,9 meanwhile HRQoL is a subjective assessment of health status.10 It is common to include HRQoL measures in oncology.11 HRQoL has been recognized as an important measure of assessing the outcome of diagnosis and the impact of cancer treatments on patients.12, 13 Health state utility (HSU) is a measure of preference‐based HRQoL often used by health economists, and it is unique because it represents an individual's valuation or preference for being in a particular health state.8 The European quality of life‐5 dimension (EQ‐5D) is an indirect measure of utility for health that generates an index‐based summary score based upon societal preference weights,14 which has been used to assess therapeutic benefits15 and in healthcare surveys across diverse general populations.16, 17 The EQ‐5D‐3L, which is the original version of the EQ‐5D with five dimensions and three levels on each dimension, has been widely applied to measuring general health conditions.18, 19, 20, 21, 22, 23, 24, 25 However, in previous EC studies, due to the absence of an EQ‐5D preference weight set in the Chinese population, Chinese researchers usually tended to choose the English preference weight set to estimate the EQ‐5D utility scores.26, 27 In 2014, Liu et al. successfully developed Chinese utility values for EQ‐5D‐3L health states using the time trade‐off method.28 Therefore, we adopted the Chinese general population‐based algorithm in the current study.
In recent years, HRQoL has become a major measurement of clinical research. Several studies have measured the HRQoL directly in EC patients.29, 30, 31 Liu et al. reported EC significantly impaired Chinese patients' HRQoL in daily life after treatment32; Lin et al. reported that personal characteristics were associated with patients' HRQoL in China.33 More studies from abroad focused on the impact of surgery or other treatments on the HRQoL of EC patients.34, 35, 36 However, as far as we know, published empirical studies on the health condition effects and accurate data on the HRQoL utility values of EC and precancer in China remain scarce. In past decades, early detection and treatment of EC provided the best opportunity for cure.37 To justify the cost of cancer screening techniques or therapeutic methods, policy‐makers need to determine if there is statistical significance of cancer screening methods for cancer survival or the HRQoL of patients.
The objectives of our study were as follows: (i) To obtain a set of health state utility scores of patients with EC and precancerous lesions in China using the EQ‐5D instrument, and (ii) to evaluate the determinants of HRQoL and the relationship between these influencing factors and EQ‐5D utility.
Methods
Study design
The National Cancer Center of China conducted a hospital‐based multicenter cross‐sectional study, namely the Cancer Screening Program in Urban China (CanSPUC), which provides free screening services for Chinese urban residents aged from 40–69 years. The target cancers in this screening program were lung, breast, colorectal, liver, stomach and esophageal cancers. It was a major public health service project supported by the central government of China. This project was initiated in August 2012, and has now covered 29 provinces nationwide. The screening process consisted of three steps: the initial assessment of a high‐risk population, further screening for cancer, and health economic evaluation.
This current EQ‐5D study was one part of the overall project, CanSPUC. We aimed to use the utility instrument EQ‐5D‐3L to systematically evaluate the HRQoL in patients with EC or precancerous lesions in China. From 2013 to 2014, a total of 12 provinces in China were enrolled in the CanSPUC, which were distributed in four geographic regions (East, Central, West, and Northeast).38
Patient selection
We identified cases of esophageal precancerous lesions as low grade intraepithelial lesions (LSIL) and high grade intraepithelial lesions (HSIL); LSIL included mild dysplasia and moderate dysplasia, and HSIL referred to severe dysplasia and carcinoma, in situ.39
In the present study, patients were selected using the method of convenience sampling in each involved medical center. The eligibility criteria for participants were as follows: (i) Patients were 40–69 years of age at the initial diagnosis with EC or precancerous lesions by screening, from September 2013 to December 2014; (ii) residents of the 12 selected provinces; and (iii) able to understand the survey procedure and complete the survey questionnaire. The survey was approved by the Institutional Review Board of the Cancer Hospital of Chinese Academy of Medical Sciences (Approval No. 15‐071/998). Written informed consent was signed before each participant was enrolled into the study.
Data collection
A total of 2090 patients with EC and 156 patients with precancerous lesions were included in the present study. Each patient was interviewed with a structured questionnaire for sociodemographic, clinical, and HRQoL information. The HRQoL was assessed using the EQ‐5D‐3L questionnaire. In the EQ‐5D questionnaire, respondents were at particular levels in particular dimensions, and the three levels of each dimension were categorized into “no problems” (level 1) and “any problem” (levels 2 and 3).40 Utility values were defined on a scale from 0–1, with 0 representing death and 1 representing perfect health. We applied a preference weight set for the Chinese population to estimate the mean EQ‐5D utility score.28 In addition, further patient information including sociodemographics and clinical characteristics, such as age, sex, region of residence, education, occupation, marital status, income, health care insurance type, age at diagnosis, pathological type, type of therapy, time points of the survey, and clinical stage for EC according to the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer staging system,41 were also collected.
Quality control
First, study protocol and data collection training were provided by the National Cancer Center of China for all the principle staff from the abovementioned 12 sites. Second, all the participating physicians in local centers/hospitals were trained for in‐person interviewing using a structured questionnaire with an EQ‐5D instrument (Chinese version). A face‐to‐face interview was either administered by a trained interviewer, or alternatively, self‐administered by capable patients in the presence of the project staff member who could answer any doubts the patients had about the interview. Furthermore, data input and basic data checks were also performed in the 15 collaborative sites. The National Cancer Center of China conducted multiple rounds of data logistical checks by interacting with local staff, and was responsible for the database building, data cleaning, and data analyses.
Statistical analysis
Frequencies and percentages were used for categorical variables, and the mean ± standard deviation (SD) was used for continuous variables. Means of EQ VAS scores and EQ‐5D utility scores were analyzed (overall, by sex and by age), using the Kruskal‐Wallis test, which is appropriate for non‐normal data. The Kruskal‐Wallis test was also used to compare the means of EQ‐5D utility scores across different categories of sociodemographics (region, education, occupation, marital status, household income, health care insurance type, and age at diagnosis) and clinical characteristics (pathological type, type of therapy, and time points of the survey). Finally, we conducted a multivariate linear ordinary least square (OLS) regression analysis to explore the impact of important variables, by inputting variables with statistical significance confirmed in the univariate analysis, and using a stepwise approach. OLS regression analysis is known to be the most commonly used and optimal method for HSU multivariate analyses so far,42, 43, 44, 45 and it is sensitive to non‐normal data.46 P‐values <0.05 were considered statistically significant. All hypothetical tests were two‐sided.
Data were entered into the EpiData software (version 3.1, EpiData Association, Odense, Denmark), using a double entry method. Logical check and statistical analyses were performed using SAS statistical software for Windows, version 9.3 (SAS Institute, Cary, NC, USA).
Results
Demographics of the participants
The sociodemographic and clinical characteristics of the patients are summarized in Table 1. The participant rate of EC patients was 87.1% and that of precancer was 90.2%.
Table 1.
Sociodemographic and clinical characteristics of patients
| Variables | Patients with esophageal precancerous lesions (N = 156) | Patients with esophageal cancer (N = 2090) |
|---|---|---|
| Age, mean ± SD | 57.29 ± 12.86 | 62.64 ± 9.06 |
| Age, years, n (%) | ||
| 40–44 | 26 (16.67) | 43 (2.06) |
| 45–49 | 8 (5.13) | 118 (5.65) |
| 50–54 | 23 (14.74) | 227 (10.86) |
| 55–59 | 28 (17.95) | 355 (16.99) |
| 60–64 | 29 (18.59) | 490 (23.44) |
| 65–69 | 42 (26.92) | 857 (41.00) |
| Sex, n (%) | ||
| Male | 105 (67.31) | 1675 (80.14) |
| Female | 51 (32.69) | 415 (19.86) |
| Region, n (%) | ||
| East | 79 (50.64) | 823 (39.38) |
| Central | 2 (1.28) | 465 (22.25) |
| West | 38 (24.36) | 559 (26.75) |
| Northeast | 37 (23.72) | 243 (11.63) |
| Education, n (%) | ||
| Primary school or below | 49 (31.41) | 1004 (48.04) |
| Junior high school | 58 (37.18) | 631 (30.19) |
| Senior high school | 30 (19.23) | 333 (15.93) |
| Undergraduate or over | 19 (12.18) | 120 (5.74) |
| Occupation, n (%)a | ||
| Farmer | 56 (35.90) | 1103 (52.78) |
| Enterprise or company employee/worker | 33 (21.15) | 290 (13.88) |
| Self‐employed or unemployed | 18 (11.54) | 224 (10.72) |
| Retiree | 14 (8.97) | 127 (6.08) |
| Public sector employee | 33 (21.15) | 341 (16.32) |
| Other | 2 (1.28) | 5 (0.24) |
| Marital status, n (%) | ||
| Married | 147 (94.23) | 1979 (94.69) |
| Other | 9 (5.77) | 111 (5.31) |
| Household income in 2012, CNY, n (%)a | ||
| <20 000 | 16 (10.26) | 469 (22.44) |
| 20 000– | 23 (14.74) | 563 (26.94) |
| 40 000– | 31 (19.87) | 513 (24.55) |
| 60 000– | 33 (21.15) | 271 (12.97) |
| ≥80 000 | 53 (33.97) | 271 (12.97) |
| Health care insurance type, n (%) | ||
| Urban employee basic medical insurance | 61 (39.10) | 543 (25.98) |
| Urban residents basic medical insurance | 24 (15.38) | 261 (12.49) |
| New rural cooperative medical scheme | 66 (42.31) | 1201 (57.46) |
| Self‐pay | 2 (1.28) | 19 (0.91) |
| Other | 3 (1.92) | 66 (3.16) |
| Age at diagnosis, yearsa | ||
| <45 | 26 (16.67) | 54 (2.58) |
| 45–54 | 35 (22.44) | 351 (16.79) |
| 55–64 | 49 (31.41) | 794 (37.99) |
| ≥65 | 38 (24.36) | 831 (39.76) |
| Pathological typea | ||
| Low grade squamous intraepithelial tumors | 34 (21.79) | — |
| High grade squamous intraepithelial neoplasms | 36 (23.08) | — |
| Adenoma | — | 142 (6.79) |
| Squamous cell carcinoma | — | 1417 (67.80) |
| Other types of cancer | — | 361 (17.27) |
| Stagea | ||
| Stage I | — | 194 (9.28) |
| Stage II | — | 761 (36.41) |
| Stage III | — | 679 (32.49) |
| Stage IV | — | 302 (14.45) |
| Type of therapya | ||
| Surgery | 63 (40.38) | 611 (29.23) |
| Symptomatic treatment | 62 (39.74) | 264 (12.63) |
| Radiotherapy | — | 303 (14.50) |
| Chemotherapy | — | 385 (18.42) |
| Surgery & postoperative chemotherapy | — | 201 (9.62) |
| Neoadjuvant chemotherapy & surgery | — | 42 (2.01) |
| Concurrent chemoradiotherapy | — | 205 (9.81) |
| Other | — | 19 (0.91) |
| Time points of the surveya | ||
| Pretreatment | 38 (24.36) | 263 (12.58) |
| In treatment | 31 (19.87) | 1284 (61.44) |
| Post‐treatment | 51 (32.69) | 365 (17.46) |
| Follow‐up | 23 (14.74) | 114 (5.45) |
aThe sum of the numbers for some characteristic variables is less than the total due to missing values.
A total of 2090 patients with EC were included in the analysis (1675 males and 415 females), with a mean age of 62.64 ± 9.06 years. Most of the patients were 65–69 years of age and of all the cancer patients in the analysis, 39.38% were from the eastern region. A majority of the EC patients were married (94.69%). Among 1920 patients with available pathological information, 1417 patients were diagnosed with squamous cell carcinoma and 142 patients were diagnosed with adenoma. Among 1936 patients with available stage information, 761 patients were diagnosed with stage II cancer, whereas only 194 had stage I cancer. Of all the EC patients, 611 (29.23%) patients underwent surgery, 385 (18.42%) patients received chemotherapy and 303 (14.50%) patients received radiotherapy.
There was a total of 156 patients with esophageal precancerous lesions (105 males and 51 females), with a mean age of 57.29 ± 12.86 years. The sociodemographic characteristics were similar to those of the EC patients. Among 70 patients with available pathological information, 36 patients were diagnosed with high grade squamous intraepithelial neoplasms and 34 patients with low grade squamous intraepithelial tumors. A total of 63 patients underwent surgery and 62 patients received symptomatic treatment.
Health status of the participants
Figure 1 shows the results of the five dimensions. Compared to the patients with precancerous lesions, cancer patients tended to report more problems in all dimensions, with all P‐values <0.05 (Kruskal‐Wallis test results). Figures 2 and 3 present further detailed mean VAS and EQ‐5D utility scores for different subgroups. The overall VAS means among patients with EC and precancerous lesions were 68.4 ± 0.7 and 64.5 ± 3.1 (Fig 2a1), respectively, but there was no significant difference between the two groups. For cancer patients, significant differences of VAS were detected among subgroups by age (Fig 2a3) and by cancer stages (Fig 2b1) (both P‐values <0.05). The mean EQ‐VAS scores were 69.5 ± 2.2 at stage I, 69.8 ± 2.1 at stage II, 70.5 ± 2.1 at stage III, and 64.8 ± 1.8 at stage IV. After applying the preference weights of the Chinese population, the overall EQ‐5D utility scores of cancer patients and patients with precancerous lesions were 0.748 ± 0.009 and 0.852 ± 0.022, respectively. Compared with EC patients, patients with precancerous lesions were statistically associated with higher EQ‐5D utility scores (Fig 3a1) (P < 0.0001). A significant difference of EQ‐5D utility scores was detected among four cancer stages (Fig 3b1) (P = 0.0055). The corresponding mean EQ‐5D utility scores were 0.693 ± 0.031 at stage I, 0.747 ± 0.014 at stage II, 0.762 ± 0.015 at stage III, and 0.750 ± 0.023 at stage IV.
Figure 1.

Distribution of self‐reported EQ‐5D problems. ( ) Patients with esophageal precancerous lesions and (
) Patients with esophageal precancerous lesions and ( ) patients with esophageal cancer.
) patients with esophageal cancer.
Figure 2.
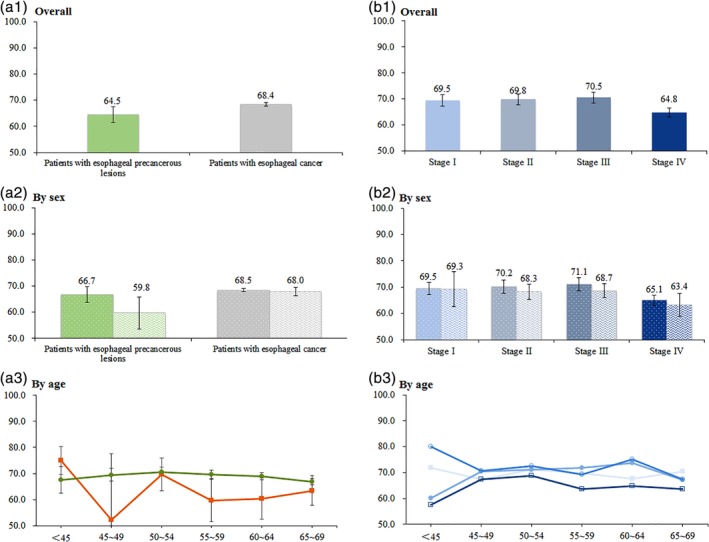
Mean of VAS scores, overall and by sex and age. (a2) ( ) Male and (
) Male and ( ) female. (b2) (
) female. (b2) ( ) Male and (
) Male and ( ) female. (a3) (
) female. (a3) ( ) Patients with esophageal precancerous lesions and (
) Patients with esophageal precancerous lesions and ( ) patients with esophageal cancer. (b3) (
) patients with esophageal cancer. (b3) ( ) Stage I, (
) Stage I, ( ) Stage II, (
) Stage II, ( ) Stage III and (
) Stage III and ( ) Stage IV.
) Stage IV.
Figure 3.
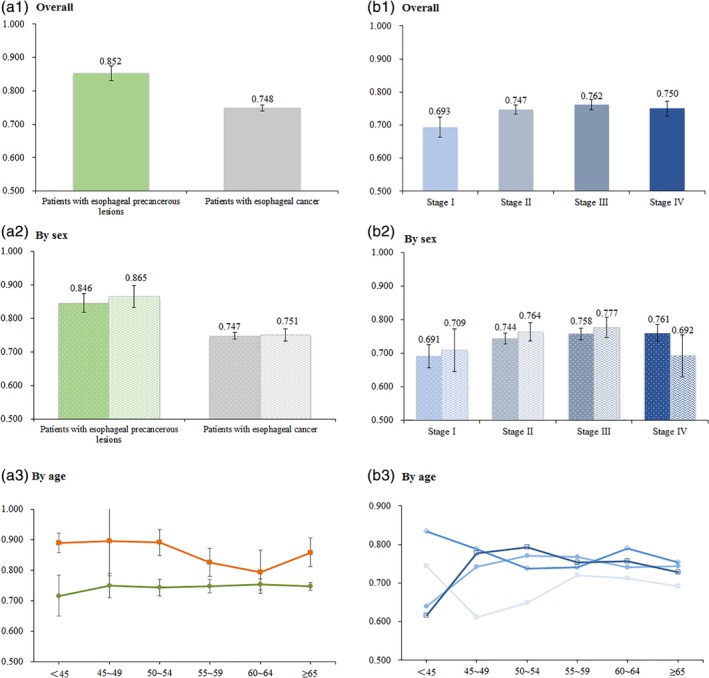
Mean of EQ‐5D index scores, overall and by sex and age. (a2) ( ) Male and (
) Male and ( ) female. (b2) (
) female. (b2) ( ) Male and (
) Male and ( ) female. (a3) (
) female. (a3) ( ) Patients with esophageal precancerous lesions and (
) Patients with esophageal precancerous lesions and ( ) patients with esophageal cancer. (b3) (
) patients with esophageal cancer. (b3) ( ) Stage I, (
) Stage I, ( ) Stage II, (
) Stage II, ( ) Stage III and (
) Stage III and ( ) Stage IV.
) Stage IV.
The associations between EQ‐5D utility scores and population characteristics are shown in Table 2. Region (P = 0.005), level of education (P = 0.031), and age at diagnosis (P = 0.041) were significantly associated with EQ‐5D utility scores among patients with esophageal precancerous lesions in univariate analyses. However, the region (P < 0.0001), occupation (P = 0.021), health care insurance type (P = 0.034), pathological type (P = 0.0011), type of therapy (P < 0.0001), and time points of the survey (P < 0.0001) were statistically associated with the EQ‐5D utility scores among cancer patients.
Table 2.
Mean of EQ‐5D index scores by sociodemographic and clinical characteristics
| Patients with esophageal precancerous lesions | Patients with esophageal cancer | |||
|---|---|---|---|---|
| Variable | Mean (95% CI) | P‐value | Mean (95% CI) | P‐value |
| Region | ||||
| East | 0.885 (0.85–0.92) | 0.005* | 0.741 (0.73–0.76) | <0.0001* |
| Central | 0.399 (−3.22–4.02) | 0.771 (0.75–0.79) | ||
| West | 0.813 (0.76–0.86) | 0.776 (0.76–0.79) | ||
| Northeast | 0.849 (0.79–0.91) | 0.662 (0.62–0.70) | ||
| Education | ||||
| Primary school or below | 0.870 (0.81–0.93) | 0.031* | 0.756 (0.74–0.77) | 0.138 |
| Junior high school | 0.818 (0.78–0.86) | 0.729 (0.71–0.75) | ||
| Senior high school | 0.889 (0.84–0.94) | 0.764 (0.74–0.79) | ||
| Undergraduate or over | 0.856 (0.77–0.94) | 0.738 (0.69–0.79) | ||
| Occupation | ||||
| Farmer | 0.837 (0.79–0.88) | 0.729 | 0.752 (0.74–0.77) | 0.021* |
| Enterprise or company employee/worker | 0.863 (0.82–0.91) | 0.779 (0.76–0.80) | ||
| Self‐employed or unemployed | 0.851 (0.78–0.93) | 0.758 (0.73–0.79) | ||
| Retiree | 0.831 (0.72–0.94) | 0.758 (0.71–0.81) | ||
| Public sector employee | 0.873 (0.81–0.94) | 0.697 (0.67–0.73) | ||
| Marital status | ||||
| Married | 0.853 (0.83–0.88) | 0.875 | 0.751 (0.74–0.76) | 0.068 |
| Other | 0.850 (0.74–0.96) | 0.700 (0.65–0.75) | ||
| Household income in 2012, CNY | ||||
| <20 000 | 0.845 (0.79–0.90) | 0.733 | 0.731 (0.71–0.75) | 0.144 |
| 20 000–39 999 | 0.854 (0.76–0.95) | 0.751 (0.73–0.77) | ||
| 40 000–69 999 | 0.847 (0.79–0.91) | 0.762 (0.74–0.78) | ||
| 60 000–79 999 | 0.848 (0.79–0.90) | 0.741 (0.71–0.77) | ||
| ≥80 000 | 0.860 (0.82–0.91) | 0.752 (0.72–0.78) | ||
| Health care insurance type | ||||
| New rural cooperative medical scheme | 0.832 (0.79–0.87) | 0.051 | 0.755 (0.74–0.77) | 0.034* |
| Urban employee basic medical insurance | 0.891 (0.85–0.93) | 0.746 (0.73–0.77) | ||
| Urban residents basic medical insurance | 0.800 (0.72–0.88) | 0.711 (0.68–0.74) | ||
| Self‐pay | 0.957 (0.41–1.50) | 0.831 (0.72–0.94) | ||
| Age at diagnosis, years | ||||
| <45 | 0.890 (0.85–0.93) | 0.041* | 0.738 (0.67–0.81) | 0.909 |
| 45–54 | 0.895 (0.85–0.94) | 0.744 (0.72–0.77) | ||
| 55–64 | 0.813 (0.77–0.86) | 0.752 (0.74–0.77) | ||
| ≥65 | 0.891 (0.85–0.94) | 0.743 (0.73–0.76) | ||
| Pathological type | ||||
| Low grade squamous intraepithelial tumors | 0.854 (0.81–0.90) | 0.7977 | — | |
| High grade squamous intraepithelial neoplasms | 0.850 (0.79–0.91) | — | ||
| Adenoma | — | 0.723 (0.69–0.76) | 0.0011* | |
| Squamous cell carcinoma | — | 0.737 (0.72–0.75) | ||
| Other types of cancer | — | 0.782 (0.76–0.81) | ||
| Type of therapy | ||||
| Surgery | 0.885 (0.85–0.92) | 0.052 | 0.661 (0.64–0.68) | <0.0001* |
| Symptomatic treatment | 0.865 (0.83–0.90) | 0.734 (0.70–0.77) | ||
| Radiotherapy | — | 0.791 (0.77–0.81) | ||
| Chemotherapy | — | 0.821 (0.80–0.84) | ||
| Surgery and postoperative chemotherapy | — | 0.746 (0.71–0.78) | ||
| Neoadjuvant chemotherapy and surgery | — | 0.792 (0.74–0.84) | ||
| Concurrent chemoradiotherapy | — | 0.798 (0.77–0.83) | ||
| Other | — | 0.804 (0.73–0.88) | ||
| Time points of the survey | ||||
| Pretreatment | 0.833 (0.78–0.89) | 0.217 | 0.813 (0.79–0.84) | <0.0001* |
| In treatment | 0.838 (0.79–0.89) | 0.738 (0.73–0.75) | ||
| Post‐treatment | 0.889 (0.85–0.93) | 0.736 (0.71–0.76) | ||
| Follow‐up | 0.894 (0.84–0.95) | 0.720 (0.67–0.77) | ||
Significant difference P < 0.05.
Multiple linear regression analyses for EQ‐5D utility scores
Table 3 illustrates the results of multivariable linear regression analyses for EQ‐5D utility scores. Compared with the western region, people from the east or the northeast had higher EQ‐5D utility scores for patients with precancerous lesions, but lower scores for cancer patients. Compared with the new rural cooperative medical scheme, patients who had urban employee basic medical insurance obtained higher EQ‐5D utility scores for patients with precancerous lesions. However, patients who had urban residents' basic medical insurance had lower scores for cancer patients. For patients with esophageal precancerous lesions, the variables of education and age at diagnosis were also retained in the final model (F = 3.988, P < 0.001, R 2 = 0.279). Additionally, for cancer patients, the factors of occupation, household income in 2012, pathological type, type of therapy, and time points of the survey were statistically associated with EQ‐5D utility scores in the multivariate analysis (F = 11.194, P < 0.001, R 2 = 0.141).
Table 3.
Multiple linear regression analysis for EQ‐5D index scores
| Patients with esophageal precancerous lesions | Patients with esophageal cancer | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Coefficient (95% CI) | S.E. | t | P‐value | Coefficient (95% CI) | S.E. | t | P‐value |
| Intercept | 0.854 (0.77–0.94) | 0.044 | 19.41 | <0.001* | 0.741 (0.70–0.78) | 0.018 | 39.76 | <0.001* |
| Region (Ref = West) | ||||||||
| East | 0.073 (0.02–0.12) | 0.026 | 2.82 | 0.006* | −0.059 (−0.08–0.03) | 0.013 | −4.31 | <0.001* |
| Central | 0.040 (−0.22–0.30) | 0.132 | 0.30 | 0.764 | −0.005 (−0.03–0.02) | 0.015 | 0.02 | 0.982 |
| Northeast | 0.090 (0.03–0.15) | 0.031 | 2.90 | 0.004* | −0.126 (−0.16–0.09) | 0.018 | −6.74 | <0.001* |
| Education (Ref = Primary school or below) | ||||||||
| Junior high school | −0.088 (−0.14− −0.03) | 0.028 | −3.11 | 0.002* | — | — | — | — |
| Senior high school | −0.052 (−0.12– 0.01) | 0.034 | −1.55 | 0.124 | — | — | — | — |
| Undergraduate or over | −0.087 (−0.17 − −0.01) | 0.040 | −2.19 | 0.030* | — | — | — | — |
| Occupation (Ref = Farmer) | ||||||||
| Enterprise or company employee/worker | — | — | — | — | 0.018 (−0.02–0.06) | 0.019 | 0.93 | 0.351 |
| Self‐employed or unemployed | — | — | — | — | 0.021 (−0.01–0.06) | 0.018 | 1.10 | 0.269 |
| Public sector employee | — | — | — | — | −0.065 (−0.10– –0.03) | 0.019 | −3.32 | 0.001* |
| Retiree | — | — | — | — | 0.013 (−0.04–0.06) | 0.025 | 0.44 | 0.660 |
| Other | — | — | — | — | 0.090 (−0.10–0.28) | 0.096 | 0.95 | 0.341 |
| Household income in 2012, CNY (Ref = <20 000) | ||||||||
| 20 000~ | — | — | — | — | 0.041 (0.01–0.07) | 0.014 | 3.02 | 0.003* |
| 40 000~ | — | — | — | — | 0.049 (0.02–0.08) | 0.015 | 3.32 | 0.001* |
| 60 000~ | — | — | — | — | 0.016 (−0.02–0.05) | 0.018 | 1.13 | 0.259 |
| ≥80 000 | — | — | — | — | 0.033 (0.00–0.07) | 0.018 | 1.96 | 0.050 |
| Health care insurance type (Ref = New rural cooperative medical scheme) | ||||||||
| Urban employee basic medical insurance | 0.077 (0.03–0.13) | 0.024 | 3.16 | 0.002* | 0.008 (−0.03–0.04) | 0.018 | 0.36 | 0.719 |
| Urban residents basic medical insurance | −0.054 (−0.12–0.01) | 0.034 | −1.61 | 0.109 | −0.050 (−0.08– ‐0.01) | 0.018 | −2.60 | 0.009* |
| Other insurance | 0.117 (−0.07–0.30) | 0.093 | 1.25 | 0.213 | 0.005 (−0.05–0.06) | 0.030 | 0.40 | 0.688 |
| Self‐pay | 0.140 (−0.06–0.34) | 0.099 | 1.42 | 0.159 | 0.059 (−0.05–0.17) | 0.056 | 1.06 | 0.288 |
| Age at diagnosis, years (ref = <45) | ||||||||
| 45–49 | 0.021 (−0.05 to 0.09) | 0.035 | 0.58 | 0.560 | — | — | — | — |
| 50–54 | −0.066 (−0.13 to 0.00) | 0.033 | −1.98 | 0.050 | — | — | — | — |
| 55–59 | −0.005 (−0.08 to 0.07) | 0.037 | −0.14 | 0.887 | — | — | — | — |
| Pathological type (Ref = squamous cell carcinoma) | ||||||||
| Adenoma | — | — | — | — | −0.047 (−0.08– −0.01) | 0.019 | −2.47 | 0.014* |
| Other types of cancer | — | — | — | — | 0.040 (0.02–0.06) | 0.012 | 3.41 | 0.001* |
| Type of therapy (Ref = symptomatic treatment) | ||||||||
| Surgery | — | — | — | — | −0.065 (−0.10– ‐0.03) | 0.017 | −3.89 | <0.001* |
| Radiotherapy | — | — | — | — | 0.051 (0.01–0.09) | 0.019 | 2.73 | 0.006* |
| Chemotherapy | — | — | — | — | 0.077 (0.04–0.11) | 0.017 | 4.28 | 0.000* |
| Surgery & postoperative chemotherapy | — | — | — | — | 0.037 (0.00–0.08) | 0.021 | 1.75 | 0.080 |
| Neoadjuvant chemotherapy & surgery | — | — | — | — | 0.030 (−0.04–0.10) | 0.036 | 0.77 | 0.442 |
| Concurrent chemoradiotherapy | — | — | — | — | 0.066 (0.03–0.11) | 0.021 | 3.23 | <0.001* |
| Other | — | — | — | — | 0.039 (−0.06–0.14) | 0.051 | 0.70 | 0.485 |
| Time points of the survey (Ref = in treatment) | ||||||||
| Pretreatment | — | — | — | — | 0.064 (0.04–0.09) | 0.015 | 4.30 | <0.001* |
| Post‐treatment | — | — | — | — | 0.027 (0.00–0.05) | 0.013 | 1.84 | 0.066 |
| Follow‐up | — | — | — | — | 0.014 (−0.03–0.06) | 0.022 | 0.56 | 0.574 |
Note. Model of patients with esophageal precancerous lesions: F = 3.988 P < 0.001 R2 = 0.279.
Model of patients with esophageal cancer: F = 11.194 P < 0.001 R2 = 0.141.
Estimate of partial regression coefficient.
Significant difference P < 0.05.
Discussion
This current work is a unique large‐scale multicenter study focusing on the HRQoL of patients with EC or precancerous lesions assessed by an internationally comparable and utility instrument, the EQ‐5D questionnaire. Our results provided reliable utility estimates for HRQoL in patients with EC or precancerous lesions using a well‐validated method, which will be informative for future detailed cost‐effectiveness evaluations.
We found that pain/discomfort posed a major problem, followed by anxiety/depression, problem of usual activity, problem of mobility, and problem of self‐care in sequential order. This finding is consistent with the results of another HRQoL analysis of EC using the EQ‐5D instrument in Anhui Province, China.26 Chen et al. reported the proportion of five dimensions in 209 EC patients in Anhui, where the percentages of respondents reporting problems of pain/discomfort, anxiety/depression, usual activity, mobility, and self‐care were 38.3%, 25.4%, 22.0%, 18.2% and 12.0%.26 As expected, there were more cancer patients reporting problems than patients with precancerous lesions. A large‐scale survey, which included the EQ‐5D instrument, was previously conducted based on a national representative sample in 2013 (N = 188 720), that was the Chinese National Health Services Survey (NHSS), where the percentages of respondents reporting problems of pain/discomfort, anxiety/depression, usual activity, mobility, and self‐care in the population aged 45–64 years were found to be approximately 14.0%, 5.8%, 3.6%, 4.8%, and 2.3%, respectively.47 The current analysis found that 60.5% of cancer patients and 51.3% of patients with precancerous lesions in this survey were suffering pain and discomfort. The proportion was dramatically high when compared with the value of 14.0% of the sampled general population across mainland China.47 We also found that a higher proportion of study participants (46.8% of cancer patients and 29.5% of the patients with precancerous lesions) were suffering from anxiety and depression than those in the Chinese general population.47 In contrast to the two dimensions mentioned above, the dimensions of self‐care and usual activity in our study population were even more affected by the disease according to comparisons with the results of the Chinese general population.47 This situation was due partly to the psychological changes resulting from the function of social role and loss of self‐care ability. During the screening procedure, subjects may experience side effects such as doubt and distress, which will affect their health status. Once the subjects are detected as having cancer, they will be labeled as cancer patients and treated earlier than without screening. Wang et al. reported that the worst status of quality of life in cancer patients occurred when patients were receiving treatment.48 Therefore, the psychological intervention, including social support and psychological support, is needed to help patients overcome their mental problems.27, 49
In this study, we used the VAS method to derive values for the health state. In the mentioned NHSS study, the average VAS in the Chinese general population aged 45–64 years was found to be 79.3.47 In our study, the measurement of VAS scores suggested that the HRQoL of patients was substantially lowered. As we expected, the VAS score in cancer stage IV was the lowest, which is consistent with the results of the HRQoL analysis in Anhui Province mentioned previously.26 Therefore, early diagnosis and treatment of EC is crucial to improving a cancer patient's quality of life. Although the results demonstrated that significant differences of VAS were detected among these age groups of cancer patients, our age curve of VAS scores in patients with EC showed a flat “inverted U" pattern, while the VAS scores of the <45‐year‐old group and ≥ 65‐year‐old group were the lowest. A potential reason for this curve was that young patients with EC are liable to loss of appetite,50 whereas young individuals require more energy for fast metabolism, so they rated their health states as low scores. However, with increasing age, the physiological functions51 and cognitive functions52 of the elderly declined at different levels.
As expected, the cancer patients (0.748) suffered a larger decrease in quality of life than patients with precancerous lesions (0.852). However, there were no significant differences of both EQ‐5D utility scores and VAS scores between males and females, which suggested that sex was not an influencing factor of HRQoL. The scores differed notably among cancer stages. There is some debate about the association between EQ‐5D utility score and the stage of EC. It was surprising to see that the utility score for cancer stage I was the lowest, and it was inconsistent with the results of previous studies.26, 27, 53 For example, Wildi et al reported that the utility scores were negatively associated with the severity of EC.54 However, as for other types of cancer, Wong et al. reported worse HRQoL in patients with earlier stage of colorectal cancer compared with those at stage III and IV.55 Thus, further studies are needed to explore the relationship between utility score and cancer stage.
For patients with EC, compared with the western region, people from the east or the northeast had lower EQ‐5D utility scores, inconsistent with the results mentioned above.47 In China, the socioeconomic status of eastern areas is better than western areas. Previous studies have suggested that respondents with lower socioeconomic status might have lower expectations of health, and under the same health conditions, they might assess their own health status as higher than respondents in a higher socioeconomic status.56 It is not surprising that patients with higher household incomes tended to have higher scores. Patients who owned urban resident basic medical insurance had lower scores in comparison with the patients who owned new rural cooperative medical insurance. Such findings were consistent with the results of the previous HRQoL studies in China.26, 57 The New Rural Cooperative Medical Scheme is a main medical security form of Chinese rural citizens, which has made remarkable achievements since 2003 when it was initiated, and many insurers have received benefits from it.58 Zhou et al. have also reported that having new rural cooperative medical insurance significantly improved the HRQoL of residents.59 We concluded that patients with adenoma received lower scores, compared with squamous cell carcinoma. Studies have indicated that the prognosis of adenoma is poorer than that of squamous cell carcinoma.60 Our findings also suggested the type of therapy might impact the HRQoL of patients. Patients who underwent surgery received the lowest EQ‐5D utility scores, while patients who received chemotherapy, radiotherapy, and concurrent chemoradiotherapy received higher scores than those who received symptomatic treatment. It was identical to the results of the HRQoL analysis in Anhui Province mentioned previously.26 Lagergren et al. reported that esophagectomy for cancer had a temporary negative impact on most aspects of self‐reported HRQoL, which typically recovered within the first postoperative year.61 We conducted this cross‐sectional survey just one or two years after cancer screening, so surgery had a negative influence on the HRQoL. The time points of this survey were also an important influencing factor. The participants were interviewed at one of the following four time points: pretreatment (before the commencement of the treatment process); in‐treatment (under the treatment process); post‐treatment (participants who had completed the main treatment process and were soon leaving hospital); follow‐up (more than one month after the end of treatment). It is understandable that patients who completed the survey before treatment had higher scores than those in treatment. In a previous study, persistent deterioration in physical function and increased breathlessness, diarrhea, and reflux were observed during treatment and within 6–12 months after surgery.61
For patients with esophageal precancerous lesions, our finding that the western patients suffered lower EQ‐5D utility scores than the eastern and northeastern patients could be explained by the unequal economic development between China's eastern and western areas. The economic conditions and healthcare services in the western areas lag behind the eastern areas because of the limitations of geographical location. Our results suggested that patients with diplomas of junior high school or undergraduate or above had lower EQ‐5D utility scores that patients with diplomas of primary school or below, which may be because the highly‐educated patients suffered higher stress from jobs and from living a faster‐paced life.
The ability to place a value on the physical and emotional experiences of patients undergoing screening is important in the quality‐adjustment of any survival advantage that might be afforded by cancer screening.62 Our findings determined the underlying relevant factors that impacted the HRQoL or utility values of patients, which will be informative for future EC prevention and control efforts.
One potential limitation is that this study was a cross‐sectional survey rather than a longitudinal study. It did not provide a comprehensive description of the temporal trend of HRQoL. However, as a key part of the CanSPUC, we will continue with the long‐term follow‐up survey in subsequent years. In addition, EQ‐5D‐5L, a new version of the EQ‐5D questionnaire has been developed with a higher sensitivity,63 and the Chinese value set of EQ‐5D‐5L was published in 2017.64 The comparison between the three‐level and five‐level instruments is worth noting.65
In conclusion, the section of our study based on the EQ‐5D instrument substantially provided the detailed HRQoL utility values of EC patients across mainland China, which will be informative for future cost‐utility analyses. We also provided information about the variables affecting the HRQoL of EC patients. Psychological intervention, social support, early diagnosis, and early treatment, and critical illness insurance are needed to help to improve the quality of life in patients. The findings of influencing factors are beneficial for future EC prevention and control efforts.
Disclosure
The authors confirm that there is no conflict of interest.
Acknowledgments
This study was supported by the National Key Research and Development Program of China (No.: 2016YFC0901405), Ministry of Science and Technology of the P. R. China; the National Natural Science Foundation of China (No.: 81773521); the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (No.: 2018251644), and the National Key Research and Development Program of China (No.: 2018YFC1313100). We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
[Correction added on 13 March 2020, after first online publication: sequence of the last three authors has been reordered.]
Contributor Information
Jufang Shi, Email: shijf@cicams.ac.cn.
Lingbin Du, Email: dulb@zjcc.org.cn.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2. Sun K, Zheng R, Zhang S et al Report of cancer incidence and mortality in different areas of China, 2015. China Cancer 2019; 28: 1–11. [Google Scholar]
- 3. Zeng H, Zheng R, Guo Y et al Cancer survival in China, 2003‐2005: A population‐based study. Int J Cancer 2014; 136 (8): 1921–30. [DOI] [PubMed] [Google Scholar]
- 4. Sadiq A, Mansour KA. Esophageal cancer: Recent advances. Thorac Cancer 2011; 2: 75–83. [DOI] [PubMed] [Google Scholar]
- 5. Wu Y, Zhang H, Zhou B, Han S, Zhang Y. Clinical efficacy of endoscopic submucosal dissection in the treatment of early esophageal cancer and precancerous lesions. J Cancer Res Ther 2018; 14(1):52–56 [DOI] [PubMed] [Google Scholar]
- 6. Ning B, Abdelfatah MM, Othman MO. Endoscopic submucosal dissection and endoscopic mucosal resection for early stage esophageal cancer. Ann Cardiothorac Surg 2017; 6: 88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zeng HM, Zheng RS, Zhang SW et al Analysis and prediction of esophageal cancer incidence trend in China. Chin J Prev Med 2012; 46 (7): 593–7. [PubMed] [Google Scholar]
- 8. Torrance GW. Measurement of health state utilities for economic appraisal. J Health Econ 1986; 5 (1): 1–30. [DOI] [PubMed] [Google Scholar]
- 9. Canavarro MC, Serra AV, Simoes MR et al Development and psychometric properties of the World Health Organization quality of life assessment instrument (WHOQOL‐100) in Portugal. Int J Behav Med 2009; 16 (2): 116–24. [DOI] [PubMed] [Google Scholar]
- 10. Guyatt GH, Feeny DH, Patrick DL. Measuring health‐related quality of life. Ann Intern Med 1993; 118 (8): 622–9. [DOI] [PubMed] [Google Scholar]
- 11. Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ‐5D utility and VAS scores in cancer. Health Qual Life Outcomes 2007; 5: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nayfield SG, Ganz PA, Moinpour CM, Cella DF, Hailey BJ. Report from a National Cancer Institute (USA) workshop on quality of life assessment in cancer clinical trials. Qual Life Res 1992; 1 (3): 203–10. [DOI] [PubMed] [Google Scholar]
- 13. Osoba D. The quality of life committee of the clinical trials group of the National Cancer Institute of Canada: Organization and functions. Qual Life Res 1992; 1 (3): 211–8. [DOI] [PubMed] [Google Scholar]
- 14. Pickard AS, Wilke CT, Lin HW, Lloyd A. Health utilities using the EQ‐5D in studies of cancer. Pharmacoeconomics 2007; 25 (5): 365–84. [DOI] [PubMed] [Google Scholar]
- 15. Rabin R, de Charro F. EQ‐5D: A measure of health status from the EuroQol Group. Ann Med 2001; 33 (5): 337–43. [DOI] [PubMed] [Google Scholar]
- 16. Badia X, Schiaffino A, Alonso J, Herdman M. Using the EuroQoI 5‐D in the Catalan general population: Feasibility and construct validity. Qual Life Res 1998; 7 (4): 311–22. [DOI] [PubMed] [Google Scholar]
- 17. Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: Results from a United Kingdom national questionnaire survey. BMJ 1998; 316 (7133): 736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park SS, Yoon YS, Oh SW. Health‐related quality of life in metabolic syndrome: The Korea National Health and Nutrition Examination Survey 2005. Diabetes Res Clin Pract 2011; 91 (3): 381–8. [DOI] [PubMed] [Google Scholar]
- 19. Lang HC, Chuang L, Shun SC, Hsieh CL, Lan CF. Validation of EQ‐5D in patients with cervical cancer in Taiwan. Support Care Cancer 2010; 18 (10): 1279–86. [DOI] [PubMed] [Google Scholar]
- 20. Hoi le V, Chuc NT, Lindholm L. Health‐related quality of life, and its determinants, among older people in rural Vietnam. BMC Public Health 2010; 10: 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kang EJ, Ko SK. A catalogue of EQ‐5D utility weights for chronic diseases among noninstitutionalized community residents in Korea. Value Health 2009; 12 (Suppl. 3): S114–7. [DOI] [PubMed] [Google Scholar]
- 22. Trippoli S, Vaiani M, Lucioni C, Messori A. Quality of life and utility in patients with non‐small cell lung cancer. Pharmacoeconomics 2001; 19 (8): 855–63. [DOI] [PubMed] [Google Scholar]
- 23. Polsky D, Keating NL, Weeks JC, Schulman KA. Patient choice of breast cancer treatment: Impact on health state preferences. Med Care 2002; 40 (11): 1068–79. [DOI] [PubMed] [Google Scholar]
- 24. Essink‐Bot ML, de Koning HJ, Nijs HG, Kirkels WJ, van der Maas PJ, Schröder FH. Short‐term effects of population‐based screening for prostate cancer on health‐related quality of life. J Natl Cancer Inst 1998; 90 (12): 925–31. [DOI] [PubMed] [Google Scholar]
- 25. Anderson H, Palmer MK. Measuring quality of life: Impact of chemotherapy for advanced colorectal cancer. Experience from two recent large phase III trials. Br J Cancer 1998; 77 (Suppl. 2): 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen S, Chen J, Wang L, Jiang Q, Tang Z. The study of health related quality of life of patients with esophagus cancer. J Nanjing Med Univ 2016; 16 (1): 24–8. [Google Scholar]
- 27. Zhao ZM, Pan XF, Huang WZ et al Quality of life among patients with esophageal/gastric cardia precancerous lesion or cancer: A one‐year follow‐up study. Mod Prev Med 2015; 42: 4417–20. [Google Scholar]
- 28. Liu GG, Wu H, Li M, Gao C, Luo N. Chinese time trade‐off values for EQ‐5D health states. Value Health 2014; 17 (5): 597–604. [DOI] [PubMed] [Google Scholar]
- 29. Blazeby JM, Alderson D, Winstone K et al Development of an EORTC questionnaire module to be used in quality of life assessment for patients with oesophageal cancer. Eur J Cancer 1996; 32A (11): 1912–7. [DOI] [PubMed] [Google Scholar]
- 30. Blazeby JM, Williams MH, Brookes ST, Alderson D, Farndon JR. Quality of life measurement in patients with oesophageal cancer. Gut 1995; 37 (4): 505–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dearden G, Laurillard D. A prospective comparison of laser therapy and intubation in endoscopic palliation for malignant dysphagia. Gastroenterology 1991; 100 (1): 1303–10. [PubMed] [Google Scholar]
- 32. Liu Q, Zeng H, Xia R et al Health‐related quality of life of esophageal cancer patients in daily life after treatment: A multicenter cross‐sectional study in China. Cancer Med 2018; 7 (11): 5803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin JY, Wang MS, Dong LP et al Influence of personal character on quality of life of patients with esophageal cancer in North Henan province and influencing factors. Asian Pac J Cancer Prev 2012; 13: 5415–20. [DOI] [PubMed] [Google Scholar]
- 34. Cavallin F, Pinto E, Saadeh LM et al Health related quality of life after oesophagectomy: Elderly patients refer similar eating and swallowing difficulties than younger patients. BMC Cancer 2015; 15: 640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Safieddine N, Xu W, Quadri SM et al Health‐related quality of life in esophageal cancer: Effect of neoadjuvant chemoradiotherapy followed by surgical intervention. J Thorac Cardiovasc Surg 2009; 137: 36–42. [DOI] [PubMed] [Google Scholar]
- 36. Homs MY, Essink Bot ML, Borsboom GJ et al Quality of life after palliative treatment for oesophageal carcinoma‐a prospective comparison between stent placement and single dose brachytherapy. Eur J Cancer 2004; 40: 1862–71. [DOI] [PubMed] [Google Scholar]
- 37. O'Rourke I, Tait N, Bull C, Gebski V, Holland M, Johnson DC. Oesophageal cancer: Outcome of modern surgical management. Aust N Z J Surg 1995; 65 (1): 11–6. [DOI] [PubMed] [Google Scholar]
- 38. Shi JF, Huang HY, Guo LW et al Quality‐of‐life and health utility scores for common cancers in China: A multicentre cross‐sectional survey. Lancet 2016; 388 (Suppl. 1): S29. [Google Scholar]
- 39. Wang LD, Zhou Q, Hong JY, Qiu SL, Yang CS. p53 protein accumulation and gene mutations in multifocal esophageal precancerous lesions from symptom free subjects in a high incidence area for esophageal carcinoma in Henan, China. Cancer 1996; 77 (7): 1244–9. [PubMed] [Google Scholar]
- 40. Matalqah LM, Radaideh KM, Yusoff ZM, Awaisu A. Health‐related quality of life using EQ‐5D among breast cancer survivors in comparison with age‐matched peers from the general population in the state of Penang, Malaysia. J Public Health 2011; 19 (5): 475–80. [Google Scholar]
- 41. Rice TW, Blackstone EH, Rusch VW. 7th Edition of the AJCC cancer staging manual: Esophagus and esophagogastric junction. Ann Surg Oncol 2010; 17 (7): 1721–4. [DOI] [PubMed] [Google Scholar]
- 42. Ruiz MA, Gutiérrez LL, Monroy M, Rejas J. Mapping of the OAB‐SF questionnaire onto EQ‐5D in Spanish patients with overactive bladder. Clin Drug Investig 2016; 36 (4): 267–79. [DOI] [PubMed] [Google Scholar]
- 43. Menezes RM, Andrade MV, Noronha KV, Kind P. EQ‐5D‐3L as a health measure of Brazilian adult population. Qual Life Res 2015; 24 (11): 2761–76. [DOI] [PubMed] [Google Scholar]
- 44. Diels J, Hamberg P, Ford D, Price PW, Spencer M, Dass RN. Mapping FACT‐P to EQ‐5D in a large cross‐sectional study of metastatic castration‐resistant prostate cancer patients. Qual Life Res 2015; 24 (3): 591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kontodimopoulos N, Pappa E, Chadjiapostolou Z, Arvanitaki E, Papadopoulos AA, Niakas D. Comparing the sensitivity of EQ‐5D, SF‐6D and 15D utilities. Eur J Health Econ 2010; 13 (1): 111–20. [DOI] [PubMed] [Google Scholar]
- 46. Walker E. Applied regression analysis and other multivariable methods. Dent Tech 1989; 74 (1): 117–8. [Google Scholar]
- 47. Yao Q, Liu C, Zhang Y et al Changes in health‐related quality of life of Chinese populations measured by the EQ‐5D‐3 L: A comparison of the 2008 and 2013 National Health Services Surveys. Health Qual Life Outcomes 2019; 17 (1): 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang JP, Cui JN, Chen ZG et al Quality of life and factors that influence it among cancer patients in China. Chin J Clin Psych 2000; 8 (1): 23–5. [Google Scholar]
- 49. Junxiu L, Zhenyan L. Study on the emotion regulation manner and related factors in patients with esophageal cancer. Hebei Med 2016; 22 (6): 906–9. [Google Scholar]
- 50. Xiao HE, Zhimin Z, Jie XU et al Study on the quality of life among esophageal cancer patients of different ages at diagnosis in North Henan. Mod Prev Med 2013; 40 (20): 3771–74. [Google Scholar]
- 51. Cai MJ, Shu‐Ling WU, Rui‐Yi LI, et al Investigation on health state and health behavior of aged people in community. Chin J Gen Pract 2010; 8 (8): 88–90. [Google Scholar]
- 52. Yao S, Zeng H, Sun S. Investigation on status and influential factors of cognitive function of the community‐dwelling elderly in Changsha City. Arch Gerontol Geriatr 2009; 49 (3): 329–34. [DOI] [PubMed] [Google Scholar]
- 53. Yamashita H, Omori M, Okuma K, Kobayashi R, Igaki H, Nakagawa K. Longitudinal assessments of quality of life and late toxicities before and after definitive chemoradiation for esophageal cancer. Jpn J Clin Oncol 2014; 44 (1): 78–84. [DOI] [PubMed] [Google Scholar]
- 54. Wildi SM, Cox MH, Clark LL et al Assessment of health state utilities and quality of life in patients with malignant esophageal dysphagia. Am J Gastroenterol 2004; 99: 1044–9. [DOI] [PubMed] [Google Scholar]
- 55. Wong CK, Lam CL, Poon JT et al Clinical correlates of health preference and generic health‐related quality of life in patients with colorectal neoplasms. PLOS One 2013; 8: e58341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Symon Z, Daignault S, Symon R, Dunn RL, Sanda MG, Sandler HM. Measuring patients' expectations regarding health‐related quality‐of‐life outcomes associated with prostate cancer surgery or radiotherapy. Urology 2006; 68 (6): 1224–9. [DOI] [PubMed] [Google Scholar]
- 57. Levenstein S, Li Z, Almer S et al Cross‐cultural variation in disease‐related concerns among patients with inflammatory bowel disease. Am J Gastroenterol 2001; 96 (6): 1822–30. [DOI] [PubMed] [Google Scholar]
- 58. Babiarz KS, Miller G, Yi H, Zhang L, Rozelle S. New evidence on the impact of China's new rural cooperative medical scheme and its implications for rural primary healthcare: Multivariate difference‐in‐difference analysis. BMJ 2010; 341: c5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhou ZL, Zhou ZY, Dan LI. Analyzing the health‐related quality of life of urban and rural residents in Shaanxi: Estimation based on the EQ‐5D value sets. Chin Health Econ 2015; 34: 13. [Google Scholar]
- 60. Sun S, Chen J, Johannesson M et al Population health status in China: EQ‐5D results, by age, sex and socio‐economic status, from the National Health Services Survey 2008. Qual Life Res 2011; 20 (3): 309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lagergren P, Avery KNL, Hughes R et al Health‐related quality of life among patients cured by surgery for esophageal cancer. Cancer 2007; 110 (3): 686–93. [DOI] [PubMed] [Google Scholar]
- 62. Havrilesky LJ, Broadwater G, Davis DM et al Determination of quality of life‐related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol 2009; 113 (2): 216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Oppe M, Devlin NJ, van Hout B et al A program of methodological research to arrive at the new international EQ‐5D‐5L valuation protocol. Value Health 2014; 17 (4): 445–53. [DOI] [PubMed] [Google Scholar]
- 64. Luo N, Liu G, Li M, Guan H, Jin X, Rand‐Hendriksen K. Estimating an EQ‐5D‐5L value set for China. Value Health 2017; 20 (4): 662–9. [DOI] [PubMed] [Google Scholar]
- 65. Wang L, Shi JF, Zhu J, et al Health‐related quality of life and utility scores of patients with breast neoplasms in China: A multicenter cross‐sectional survey. The Breast 2018; 53–62. [DOI] [PubMed] [Google Scholar]


