Abstract
Anti‐programmed cell death 1 (PD‐1) and its ligand (PD‐L1) has emerged as a novel immunotherapy for non‐small cell lung cancer (NSCLC). However, the proportion of patients who may benefit from immunotherapy is limited and the factors sensitive or resistant to immunotherapy are not completely clear. Therefore, to identify reliable biomarkers as predictors of clinical response and resistance to anti‐PD‐1/PD‐L1 therapies have become increasingly important. Here, we report a case of a patient with bone metastatic NSCLC, who achieved a pathologic complete response after preoperative pembrolizumab treatment. Postoperative pathological examination found no viable cancer cells in the resected pulmonary nodules and lymph nodes. Several high‐frequency DNA damage response and repair (DDR) gene mutations including two germline mutations were identified in the primary lesion. Moreover, high PD‐L1 expression, Kirsten rat sarcoma viral oncogene homolog (KRAS) combined with tumor protein 53 (TP53) mutations without epidermal growth factor receptor (EGFR)/anaplastic lymphoma kinase (ALK) driver alterations, high infiltration level of CD8‐positive cells and M1 macrophages were observed, which were favorable characteristics for immunotherapy. We explored the possible factors related to an excellent response to immune checkpoint inhibitor in this patient and determined that preoperative use of anti‐PD‐1 therapy might apply to late‐stage lung adenocarcinoma patients with multidimensional advantageous biomarkers for treatment with immune checkpoint inhibitors (ICIs).
Key points
We characterized the genomic features and immune microenvironment signature of a lung adenocarcinoma in a patient with bone metastasis who achieved pathologic complete response after pembrolizumab treatment.
To evaluate multidimensional advantageous biomarkers for immunotherapy.
Keywords: Late‐stage NSCLC, pathologic complete response, preoperative immunotherapy
Introduction
Lung cancer is the most common cancer worldwide, with an estimated 1.8 million new cases and 1.6 million deaths every year.1 The five‐year survival rate is approximately 4%–17% based on different stage and regions.2 The treatment of non‐small cell lung cancer (NSCLC) has changed from traditional cytotoxic therapy to personalized therapy of molecular heterogeneity. It has been shown that epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK) and receptor tyrosine kinase (ROS1) are potential therapeutic targets in more than 69% of patients with advanced NSCLC.3 Further, immune checkpoint inhibitors (ICIs) such as programmed cell death 1 (PD‐1)/ PD ligand 1 (PD‐L1) monoclonal antibodies have dramatically changed the therapeutic landscape in multiple advanced cancers including NSCLC.4, 5, 6, 7, 8, 9 However, the mechanisms of antitumor immunity and sensitivity to immunotherapy are still not fully understood. In addition, the efficacy of ICIs varies greatly among different tumor types and individuals. Therefore, to determine predictive biomarkers for precise cancer immunotherapy is urgently needed. Predictive markers have been identified, and DNA mismatch repair deficiency are the only predictive biomarkers approved by Food and Drug Administration.10 PD‐L1 expression by tumor cells11, 12 and tumor mutation burden (TMB)13 are included in the National Comprehensive Cancer Network (NCCN) guidelines for NSCLC. Furthermore, tumor immunity in the microenvironment (TIME)14 classification and specific gene variations, especially EGFR, Kirsten rat sarcoma viral oncogene homolog (KRAS), tumor protein 53 (TP53) and serine/threonine kinase 11 (STK11) have been confirmed to identify optimal responders to ICIs in lung cancer.15, 16 Meanwhile, with the development of next‐generation sequencing (NGS), analytic tools and other detection methods, multiple indicators should be combined to evaluate the reliable markers of anti‐PD‐1/PD‐L1 inhibitors.
Herein, we describe a patient with metastatic lung adenocarcinoma who achieved a pathologic complete response after pembrolizumab therapy, suggesting that patients with multidimensional advantageous biomarkers for ICIs might be an excellent beneficiary group for immunotherapy.
Case report
Our study was approved by the General Hospital of Southern Theater Command of PLA. In October 2018, a 68‐year‐old Chinese man presented to the clinic who had developed a productive cough and hemoptysis over a week's duration. He had extremely severe mixed ventilatory dysfunction and a 20‐year history of psoriasis. Positron emission tomography‐computed tomography (PET‐CT) scan showed a 1.8 x 1.5 x 1.8 cm shadow in the upper lobe of the right lung together with an abnormal hypermetabolic lesion in the fifth lumbar vertebra (Fig 1a). Based on pathological examination of the puncture biopsy (Fig 1b), the patient was diagnosed with stage IV (T1bN0M1b), poorly differentiated lung adenocarcinoma accompanied by unilateral bone metastasis, without lymph node or bronchial stump involvement. EGFR, ALK and ROS1 genomic aberrations were not identified by NGS test in the primary lesion, while the PD‐L1 expression level assessed by immunohistochemical (IHC) staining with SP142 was up to 70% (Fig 1c). Given this, treatment was commenced with pembrolizumab (200 mg/day) for two cycles in November 2018. A partial response (PR) was evaluated using PET‐CT scan according to RECIST 1.1 criteria, which showed a reduction in the size of the tumor (1.4 x 1.0 x 1.5 cm) in his right upper lung lobe with a sharp decrease in standard uptake value max (SUVmax) from 13.0 to 2.3 and also in bone metastasis from 13.8 to 1.8 (Fig 1a). Following this, he underwent thoracoscopic wedge resection of the right upper lung nodule and mediastinal lymph node dissection in December 2018. Surprisingly, no viable cancer cells were observed in the resected pulmonary and lymph nodes under postoperative pathological examination (Fig 1b), indicating that he had a pathologic complete response after immunotherapy. Subsequently, the patient received radiotherapy for bone metastasis in January 2019 with pembrolizumab as an adjuvant therapy from 24 January 2019. After seven days, immunotherapy and radiotherapy were discontinued due to tachypnea, loss of consciousness and grade 3 elevated transaminase. The immune‐related adverse events (irAEs) were under control through symptomatic interventions. The patient was still alive without recurrence at recent follow‐up in October 2019. The entire course of his clinical treatment is illustrated in Fig 1d.
Figure 1.
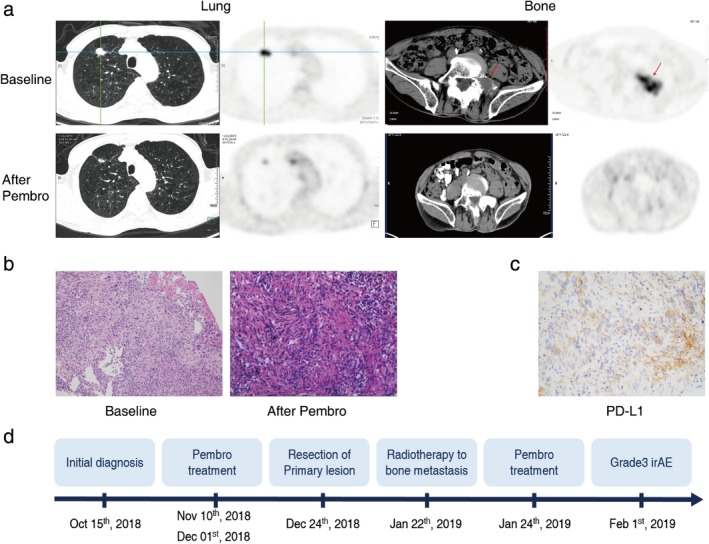
Clinical outcome of the patient with metastatic lung adenocarcinoma who achieved a pathologic complete response to preoperative immunotherapy. (a) Following treatment with pembrolizumab for two cycles, a partial response (PR) was seen on positron emission tomography‐computed tomography (PET‐CT) scan with a reduction in the size of the tumor in his right upper lung lobe and also in bone metastasis. (b) Pathological results of the resected pulmonary specimens after treatment with pembrolizumab. Magnification, ×200. (c) PD‐L1 assay of the primary lesion on needle biopsy by immunohistochemical (IHC) staining with SP142. Magnification, ×200. (d) Illustrating the timeline of the treatment course of the patient. Pembro, pembrolizumab.
This patient's primary tumor specimens of needle biopsy were further analyzed through another NGS test targeting 543 cancer‐associated genes (all exons or hotspots) at a CAP‐certified laboratory (GeneCast Biotechnology Co., Beijing). The genetic mutations of certain genes in cancer‐related pathways are summarized in Table 1. Three pathogenic or likely pathogenic mutations including KRAS p.G12C (35.17%), TP53 p.R267P (23.12%) and BRAF p.G596D (9.47%) were detected. Notably, there were multiple high‐frequency alterations in DDR genes such as RAD51C, BRCA2, RAD50 and FANCA. Among them, RAD51C p.G112R (19.48%) is considered as a pathogenic mutation in public database (COSMIC), while little is known about the other mutations. In addition, no copy number variations (CNV) were present in our results and his TMB value was 11.44/Mb. TIME signature of this patient's primary lesion was examined by multiplex immunohistochemistry (mIHC). Consistent with previous PD‐L1 assay results, PD‐L1 expression in the tumor and stroma regions had increased to to 41.78% and 57.18%, respectively (Fig 2a,b). Importantly, CD8+ tumor infiltrating lymphocytes (TILs) were rich in the tumor (14.83%) and stroma (28.68%) regions (Fig 2a,b). In addition, the infiltration of CD68‐positive cells (macrophages) and CD68+CD163− cells (M1 macrophages) in the stroma region were 9.49% and 8.93%, respectively (Fig 2b,c). Moreover, CD68+PD‐L1+ cells accounted for 77.6% of CD68+ cells in the stroma region and 71.1% in the tumor region (Fig 2b).
Table 1.
Genetic mutations of cancer‐related pathways in the patient's primary lesion
| Pathway | Gene | Mutations | Germline | Frequency (%) |
|---|---|---|---|---|
| DDR pathway | RAD51C | p.G112R | N | 19.48 |
| BRCA2 | p.E2364V | N | 22.01 | |
| RAD50 | p.A171S | Y | 41.82 | |
| FANCA | p.S1088F | Y | 55.41 | |
| RTK/RAS pathway | RET | p.Q681E | Y | 39.7 |
| KRAS | p.G12C | N | 35.17 | |
| FLT3 | p.D358V | Y | 37 | |
| ROS1 | p.D1776H | Y | 38.11 | |
| BRAF | p.G596D | N | 9.47 | |
| Notch pathway | KDM5A | p.A633T | Y | 69.43 |
| NOTCH3 | p.P2126L | Y | 36.82 | |
| Hippo pathway | NF2 | p.E204Kfs*5 | N | 25 |
| FAT1 | p.R26Q | Y | 40.2 | |
| PI3K pathway | INPP4B | p.L471S | N | 24.99 |
| p53 pathway | TP53 | p.R267P | N | 23.12 |
Y, yes; N, no.
Figure 2.
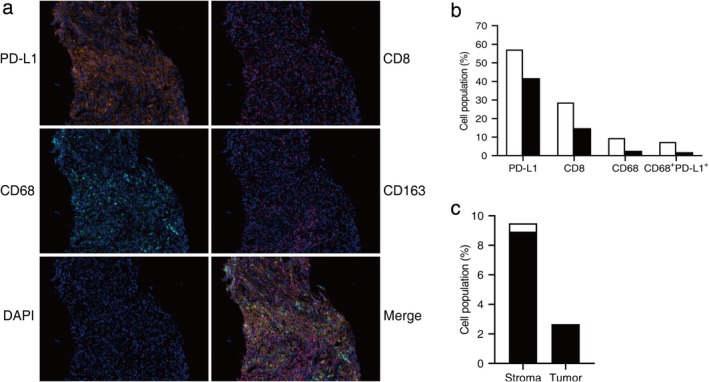
Tumor immune microenvironment signature of the patient's pulmonary lesion on needle biopsy. (a) Images show five‐color mIHC (PD‐L1, CD8, CD68, CD163, DAPI) in the primary lesion. Nuclei were counterstained with DAPI. Magnification, ×200. (b) Quantitative mIHC results of PD‐L1, CD8, CD68, ( ) Stroma region, (
) Stroma region, ( ) Tumor region. (c) The proportion of CD68+CD163− cells (
) Tumor region. (c) The proportion of CD68+CD163− cells ( ) and CD68+CD163+ cells (
) and CD68+CD163+ cells ( ) in CD68+ cells from six random vision fields.
) in CD68+ cells from six random vision fields.
Discussion
The efficacy and feasibility of neoadjuvant immunotherapy has previously been demonstrated in patients with early‐stage resectable NSCLC.17 Here, we describe a case of pathologic complete response in a stage IV lung adenocarcinoma patient with bone metastasis after preoperative treatment with pembrolizumab. The results showed that this individual had multiple favorable characteristics for immunotherapy such as high expression level of PD‐L1 (70%), concomitant KRAS together with TP53 mutations without EGFR/ALK driver alterations, and high level of CD8+ TILs. However, whether it is sufficient to account for the unexpected and significant benefits from ICI treatment remains to be explored, especially in an advanced NSCLC patient with distant metastasis. Previous studies revealed that compared with other combinations of KRAS, TP53, EGFR and STK11 alterations, infiltration of CD8 positive T cells was more significant in those lung adenocarcinoma patients harboring KRAS and/or TP53 mutations without EGFR or STK11 mutations.16 In addition, some high‐frequency DDR genetic mutations including two germline mutations were identified in this patient, which were classified as pathogenic mutation or variants of uncertain significance (VUS). Importantly, the alterations of DDR genes exhibited potential value of predicting response to ICIs for NSCLC patients.18 Moreover, the known or likely deleterious DDR alterations were demonstrated to be associated with clinical benefit from treatment with ICIs in patients with advanced urothelial cancer.19 Thus, we hypothesized that DDR mutations detected above in this case may also contribute to his outstanding response to pembrolizumab, which requires further study. Additionally, co‐expression of CD68 and PD‐L1 was observed in most CD68 positive cells in this individual, which was in line with the finding that PD‐L1 expression in macrophages was associated with good prognosis in NSCLC patients receiving ICIs.20
In summary, we characterized the genomic features and immune microenvironment signature of a metastatic lung adenocarcinoma patient who achieved a pathologic complete response after pembrolizumab treatment. Our observations revealed that preoperative immunotherapy with ICIs might be applicable to late‐stage NSCLC patients with multidimensional advantageous biomarkers for ICIs.
Disclosure
The authors report no conflict of interest.
Acknowledgments
The authors wish to gratefully acknowledge the patient and his family for allowing us to publish his clinical case.This study was granted by the National Key Sci‐Tech Special Project of China (No. 2018ZX10302207) and Guangdong science and technology infrastructure construction projects of China (No.2018A030321017).
References
- 1. Ferlay J, Soerjomataram I, Dikshit R et al Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–86. [DOI] [PubMed] [Google Scholar]
- 2. American Cancer Society . Cancer Facts & Figures 2015. American Cancer Society, Atlanta, GA: 2015. [Cited 5 Dec 2019.] http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. [Google Scholar]
- 3. Tsao AS, Scagliotti GV, Bunn PA Jr et al Scientific advances in lung cancer 2015. J Thorac Oncol 2016; 11: 613–38. [DOI] [PubMed] [Google Scholar]
- 4. Garon EB, Rizvi NA, Hui R et al Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 2015; 372: 2018–28. [DOI] [PubMed] [Google Scholar]
- 5. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus Docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brahmer J, Reckamp KL, Baas P et al Nivolumab versus Docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015; 373: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fehrenbacher L, Spira A, Ballinger M et al Atezolizumab versus docetaxel for patients with previously treated non‐small‐cell lung cancer (POPLAR): A multicentre, open‐label, phase 2 randomised controlled trial. Lancet 2016; 387: 1837–46. [DOI] [PubMed] [Google Scholar]
- 8. Herbst RS, Baas P, Kim DW et al Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016; 387: 1540–50. [DOI] [PubMed] [Google Scholar]
- 9. Reck M, Rodriguez‐Abreu D, Robinson AG et al Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016; 375: 1823–33. [DOI] [PubMed] [Google Scholar]
- 10. Le DT, Durham JN, Smith KN et al Mismatch repair deficiency predicts response of solid tumors to PD‐1 blockade. Science 2017; 357: 409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herbst RS, Soria JC, Kowanetz M et al Predictive correlates of response to the anti‐PD‐L1 antibody MPDL3280A in cancer patients. Nature 2014; 515: 563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shukuya T, Carbone DP. Predictive markers for the efficacy of anti‐PD‐1/PD‐L1 antibodies in lung cancer. J Thorac Oncol 2016; 11: 976–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rizvi NA, Hellmann MD, Snyder A et al Cancer immunology. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science 2015; 348: 124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim TK, Herbst RS, Chen L. Defining and understanding adaptive resistance in cancer immunotherapy. Trends Immunol 2018; 39: 624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dong ZY, Zhong WZ, Zhang XC et al Potential predictive value of TP53 and KRAS mutation status for response to PD‐1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res 2017; 23: 3012–24. [DOI] [PubMed] [Google Scholar]
- 16. Biton J, Mansuet‐Lupo A, Pecuchet N et al TP53, STK11, and EGFR mutations predict tumor immune profile and the response to anti‐PD‐1 in lung adenocarcinoma. Clin Cancer Res 2018; 24: 5710–23. [DOI] [PubMed] [Google Scholar]
- 17. Forde PM, Chaft JE, Smith KN et al Neoadjuvant PD‐1 blockade in Resectable lung cancer. N Engl J Med 2018; 378: 1976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. 2019 ASCO Abstract 9077 . [Cited 5 Dec 2019.] Available from URL: https://meetinglibrary.asco.org/record/174379/abstract.
- 19. Teo MY, Seier K, Ostrovnaya I et al Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD‐1/PD‐L1 blockade in advanced Urothelial cancers. J Clin Oncol 2018; 36: 1685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. 2019 AACR Abstract 2674 . [Cited 5 Dec 2019.] Available from URL: https://cancerres.aacrjournals.org/content/79/13_Supplement/2674.


