Abstract
Background
The prognostic value of platelet distribution width (PDW) is different in various malignancies. The purpose of this study was to identify the relationship between preoperative PDW and survival in patients with non‐small cell lung cancer (NSCLC).
Methods
Optimal cutoff values of PDW were confirmed using a receiver operating characteristic (ROC) curve. The Kaplan‐Meier method and log‐rank test were used to estimate disease‐free survival (DFS) and overall survival (OS). Univariate and multivariate Cox regression models were used for prognostic analysis. The likelihood ratio test (LRT) and Akaike information criterion (AIC) were used to compare two models of the TNM staging system and the PDW‐TNM staging system (combination of PDW and the TNM staging system).
Results
Survival analysis indicated that the disease‐free survival (DFS) and overall survival (OS) of patients with PDW > 12.65 were both significantly longer than those of patients with PDW ≤ 12.65. Multivariate analysis showed that PDW was an independent prognostic factor for DFS and OS. After the two prediction models were established, further LRT analysis showed that the PDW‐TNM model had a better ability to assess patient prognosis.
Conclusions
PDW might act as an independent risk factor to predict progression and prognosis. Preoperative PDW combined with the TNM staging system showed a better ability to assess the prognosis of NSCLC patients.
Key points
Our study focused on the prognostic value of preoperative PDW in 750 patients with NSCLC. We also analyzed preoperative PDW in different stages and histological subtypes systematically.
A model built by preoperative PDW combined with the TNM staging system had a better prognostic ability. LRT was used to calculate values of the goodness of fit between the model and the TNM staging system.
Keywords: Non‐small cell lung cancer, platelet distribution width, prognosis
Introduction
Lung cancer is one of the most common causes of cancer‐related death. The five‐year relative survival rate of lung and bronchus cancer is approximately 20.6% in all races (17.2% for males and 24.3% for females), as analyzed by the National Cancer Institute in America from 2009 to 2015.1 In China, lung cancer is the leading cause of cancer death in men and women over 60 years old. It is the most commonly diagnosed cancer in men aged over 75 years and in women aged 60 years or older.2 Although the treatment has greatly improved, the prognosis of lung cancer remains poor due to high rates of recurrence and tumor metastasis. Generally, many factors are used to predict the prognosis of lung cancer patients, including the TNM staging system, serum tumor markers, fibrinogen and so on.3, 4, 5, 6 However, the best risk marker of lung cancer is still not clear. Thus, it is necessary to search for an appropriate and effective method to predict the prognosis of non‐small cell lung cancer (NSCLC) patients.
Extensive scientific research has illustrated that there is a close relationship between blood coagulation and the malignant process of cancer. Francis and Amirkhosravi7 suggested that tumor cells could activate blood coagulation when carrying procoagulants in the circulation. Platelets play an important role in the process of hemostasis and have an impact on cancer occurrence and metastasis. There is evidence which indicates that platelets facilitate tumorigenesis through the association between platelet activation and the development of chronic inflammation.8 Clinical evidence has shown that thrombocytosis is associated with adverse prognosis in various types of cancer, including ovarian cancer, lung cancer, gastric cancer, inflammatory breast cancer and colorectal cancer.9, 10, 11, 12, 13
Platelet distribution width (PDW), an index of platelet size heterogeneity—another platelet index distinguished from mean platelet volume (MPV)—indicates a difference in platelet size.14 As a routine serological indicator, PDW has been considered by many studies as a potential marker to predict prognosis in various types of cancer,15, 16, 17, 18, 19, 20 including NSCLC.20 In early gastric cancer, Cheng et al.15 indicated that low PDW was associated with a poorer DFS. However, Zhang et al.17 and Huang et al.16 reported the opposite results in laryngeal cancer and breast cancer: low PDW was associated with a better OS. Cui et al. revealed that NSCLC patients with elevated PDW had a better prognosis.20
In this study, a large sample of 750 NSCLC patients who underwent complete surgery was analyzed. The purpose of this study was to evaluate the clinical significance of preoperative PDW in patients with NSCLC. Furthermore, we also evaluated the clinical utility of a novel prognostic system based on the combination of PDW and the TNM staging system (PDW‐TNM staging system).
Methods
Patients
A total of 803 NSCLC patients who underwent complete surgical resection at Tianjin Medical University Cancer Institute and Hospital were retrospectively analyzed between January 2008 and December 2014. Among the 803 patients, 53 patients were excluded from this study due to preoperative chemotherapy or radiotherapy (n = 10), previous history of other cancers (n = 13), and insufficient data (n = 27) without routine blood examination. All patients were staged according to the eighth edition of the TNM Classification for Lung Cancer.3 Due to the retrospective nature of the study, the requirement for informed patient consent was waived.
Data collection
The basic information of the patients including age, sex and smoking status were collected before surgery. After surgery, we supplemented other information (resection type, histological subtype, tumor location and pathological TNM classification) related to tumor characteristics. Within two weeks before surgery, venous blood samples were collected in ethylenediaminetetraacetic acid (EDTA)‐containing tubes. Platelet distribution width (PDW) is a measure of the volume of platelets and was measured by an automated hematological analyzer (Sysmex Xs‐800i) in our hospital. Relevant laboratory characteristics were acquired by the medical laboratory in our hospital. The PDW‐TNM staging system was defined as the combination of PDW and the TNM staging system.
Statistical analysis
The optimal cutoff value of PDW was confirmed on the basis of a receiver operating characteristic (ROC) curve. The areas under the curve (AUCs) of the ROC curves were calculated. The chi‐squared test or Fisher's exact test was used to compare categorical clinical features. Continuous variables are shown as the means ± standard deviations. Differences between continuous variables were analyzed using the Mann‐Whitney U test. Disease‐free survival (DFS) was defined as the time from surgery to the first confirmation of the recurrence of disease or the last follow‐up. Overall survival (OS) was defined as the time from surgery to death or the date the patient was last known to be alive. DFS and OS were compared between groups; they were assessed by Kaplan‐Meier curves, and the log‐rank test was used to calculate the differences between two groups. Relevant clinical variables associated with DFS and OS were examined using univariate and multivariate Cox regression models.
To compare the usefulness of the PDW‐TNM and TNM staging systems in prognostic prediction, comparison of the two ROC curves was performed using the method suggested by DeLong et al.21 In addition, LRT was used to calculate values of the goodness of fit between the different staging systems in predicting DFS and OS.22 In the calculation of LRT, larger χ2 values and smaller AIC values were deemed to illustrate a relatively better fitting model.
Statistical analyses were performed using Statistical Package for the Social Sciences software (SPSS) for Windows version 23.0 (SPSS, Inc., Chicago, IL) and MedCalc (version 15.8; MedCalc Software bvba, Acacialaan, Belgium). In all analyses, P‐values (two‐sided) < 0.05 were considered statistically significant.
Results
Identification of the optimal cutoff value of preoperative PDW
The clinicopathological characteristics of the NSCLC patients are summarized in Table 1. In this study, we used ROC curve analysis to identify the optimal cutoff value of preoperative PDW in predicting the prognosis of NSCLC patients (Fig 1). According to the cutoff value of 12.65, 270 patients (36.0%) had preoperative PDW > 12.65, and 480 patients (64.0%) had preoperative PDW ≤ 12.65. For NSCLC, the AUC of PDW was 0.617, and the 95% confidence interval (CI) was 0.576–0.658 (P < 0.001).
Table 1.
Correlations between the preoperative PDW and clinicopathological variables
| PDW | ||||
|---|---|---|---|---|
| Variables | All | ≤12.65 (n = 480) | >12.65 (n = 270) | P‐value |
| Age | 0.089 | |||
| ≤60 | 369 | 225 (61.0%) | 144 (39.0%) | |
| >60 | 381 | 255 (66.9%) | 126 (33.1%) | |
| Sex | <0.001 | |||
| Female | 275 | 150 (54.5%) | 125 (45.5%) | |
| Male | 475 | 330 (69.5%) | 145 (30.5%) | |
| Smoking status | 0.006 | |||
| Yes | 476 | 322 (67.6%) | 154 (32.4%) | |
| No | 274 | 158 (57.7%) | 116 (42.3%) | |
| Resection type | 0.016 | |||
| Pneumonectomy | 55 | 44 (80%) | 11 (20%) | |
| Lobectomy | 695 | 436 (62.7%) | 259 (37.3%) | |
| Histological subtype | <0.001 | |||
| SqCC | 257 | 185 (72.0%) | 72 (28.0%) | |
| Adenocarcinoma | 401 | 231 (57.6%) | 170 (42.4%) | |
| Others | 92 | 64 (69.6%) | 28 (30.4%) | |
| Tumor location | 0.629 | |||
| Left | 308 | 194 (63.0%) | 114 (37.0%) | |
| Right | 442 | 286 (64.7%) | 156 (35.3%) | |
| T stage | <0.001 | |||
| T1 | 377 | 211 (56.0%) | 166 (44.0%) | |
| T2 | 284 | 199 (70.1%) | 85 (29.9%) | |
| T3 | 65 | 53 (81.5%) | 12 (18.5%) | |
| T4 | 24 | 17 (70.8%) | 7 (29.2%) | |
| N stage | 0.023 | |||
| N0 | 456 | 279 (61.2%) | 177 (38.8%) | |
| N1 | 65 | 38 (58.5%) | 27 (41.5%) | |
| N2 | 229 | 163 (71.2%) | 66 (28.8%) | |
| TNM stage | 0.001 | |||
| Stage I | 352 | 201 | 151 | |
| Stage II | 134 | 90 | 44 | |
| Stage III | 264 | 189 | 75 | |
PDW, platelet distribution width; SqCC, squamous cell carcinoma.
Figure 1.
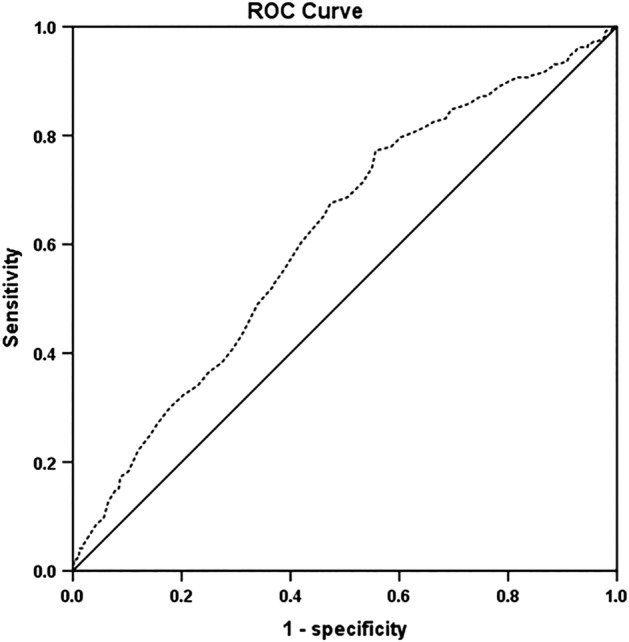
ROC analysis of the preoperative PDW for 750 patients with NSCLC. AUC = 0.617, 95% CI = 0.576–0.658. PDW, platelet distribution width; AUC, area under the curve; CI, confidence interval; NSCLC, non‐small cell lung cancer; ROC, receiver operating characteristic.
Relationship between preoperative PDW and clinicopathological features
Table 1 indicates the correlations between preoperative PDW and clinicopathological variables. Sex (P < 0.001), smoking status (P = 0.006), resection type (P = 0.016), histological subtype (P < 0.001), T stage (P < 0.001), N stage (P = 0.023) and TNM stage (P = 0.001) were found to have a significant correlation with PDW.
Table 2 indicates the differences in clinical laboratory variables grouped by PDW in 750 NSCLC patients. PDW was correlated with maximum tumor diameter (P < 0.001), WBC count (P < 0.001), platelet count (P < 0.001), hemoglobin (P = 0.041), albumin (P < 0.001), globulin (P = 0.002), and fibrinogen (P < 0.001).
Table 2.
Correlations between the preoperative PDW and clinicolaboratory variables
| Variables | PDW ≤ 12.65 (n = 480) | PDW > 12.65 (n = 270) | P‐value |
|---|---|---|---|
| Age (year) | 60.6 ± 8.9 | 60.2 ± 9.5 | 0.622 |
| Maximum tumor diameter (cm) | 4.0 ± 2.1 | 3.3 ± 1.9 | <0.001 |
| WBC count (×103 mm−3) | 6.9 ± 1.8 | 6.3 ± 1.6 | <0.001 |
| Platelet count (×104 mm−3) | 26.4 ± 7.4 | 20.8 ± 5.2 | <0.001 |
| hemoglobin (g/L) | 138.7 ± 15.5 | 141.0 ± 13.7 | 0.041 |
| LDH (U/L) | 181.7 ± 49.0 | 184.6 ± 50.0 | 0.431 |
| Albumin (g/dL) | 4.4 ± 0.4 | 4.5 ± 0.4 | <0.001 |
| Globulin (g/dL) | 3.1 ± 0.5 | 3.0 ± 0.5 | 0.002 |
| Fibrinogen (g/L) | 3.5 ± 1.0 | 3.2 ± 0.8 | <0.001 |
LDH, lactate dehydrogenase; PDW, platelet distribution width; WBC, white blood cell.
Prognostic value of preoperative PDW
The results of the univariate analysis are shown in Table 3. DFS and OS were found to be significantly correlated with PDW (P < 0.001 and P < 0.001, respectively), resection type (P < 0.001 and P < 0.001, respectively), TNM stage (P < 0.001 and P < 0.001, respectively), tumor size (P < 0.001 and P < 0.001, respectively), adjuvant chemotherapy (P < 0.001 and P < 0.001, respectively), WBC count (P = 0.003 and P = 0.003, respectively), platelet count (P < 0.001 and P < 0.001, respectively), hemoglobin (P = 0.043 and P = 0.036, respectively), LDH (P < 0.001 and P < 0.001, respectively), fibrinogen (P < 0.001 and P < 0.001, respectively), albumin (P = 0.044 and P = 0.022, respectively), and globulin (P < 0.001 and P < 0.001, respectively).
Table 3.
Univariate analysis of DFS and OS for 750 NSCLC patients
| DFS | OS | |||||
|---|---|---|---|---|---|---|
| P‐value | HR | 95% CI | P‐value | HR | 95% CI | |
| Age (≤61, >61) | 0.692 | 1.048 | 0.832 to 1.319 | 0.646 | 1.056 | 0.838 to 1.329 |
| Sex (female, male) | 0.763 | 1.037 | 0.818 to 1.315 | 0.931 | 1.011 | 0.797 to 1.281 |
| Smoking status (yes, no) | 0.451 | 0.912 | 0.717 to 1.160 | 0.365 | 0.895 | 0.703 to 1.138 |
| Tumor location (left, right) | 0.702 | 0.955 | 0.756 to 1.207 | 0.878 | 0.982 | 0.777 to 1.240 |
| Resection type (pneumonectomy, lobectomy) | <0.001 | 2.330 | 1.628 to 3.334 | <0.001 | 2.347 | 1.640 to 3.359 |
| Histological subtype (squamous, adenocarcinoma, others) | 0.820 | 1.022 | 0.850 to 1.228 | 0.926 | 1.009 | 0.839 to 1.213 |
| TNM stage (I, II, III) | <0.001 | 1.889 | 1.653 to 2.158 | <0.001 | 1.882 | 1.648 to 2.150 |
| Stage I versus stage II | <0.001 | 1.916 | 1.352 to 2.715 | <0.001 | 1.929 | 1.361 to 2.735 |
| Stage II versus stage III | <0.001 | 1.862 | 1.360 to 2.548 | <0.001 | 1.862 | 1.361 to 2.549 |
| Stage I versus stage III | <0.001 | 1.891 | 1.653 to 2.164 | <0.001 | 1.879 | 1.643 to 2.150 |
| Tumor size | <0.001 | 1.148 | 1.099 to 1.199 | <0.001 | 1.151 | 1.102 to 1.202 |
| Adjuvant chemotherapy (yes, no) | <0.001 | 1.858 | 1.453 to 2.377 | <0.001 | 1.808 | 1.414 to 2.312 |
| WBC count (×103 mm−3) | 0.003 | 1.438 | 1.133 to 1.824 | 0.003 | 1.436 | 1.132 to 1.822 |
| Platelet count (×104 mm−3) | <0.001 | 1.736 | 1.332 to 2.264 | <0.001 | 1.735 | 1.331 to 2.262 |
| hemoglobin (g/L) | 0.043 | 0.788 | 0.625 to 0.992 | 0.036 | 0.781 | 0.620 to 0.984 |
| LDH (U/L) | <0.001 | 1.776 | 1.410 to 2.238 | <0.001 | 1.800 | 1.429 to 2.267 |
| Fibrinogen (g/L) | <0.001 | 1.984 | 1.569 to 2.509 | <0.001 | 2.009 | 1.588 to 2.540 |
| Albumin (g/dL) | 0.044 | 0.786 | 0.621 to 0.994 | 0.022 | 0.759 | 0.600 to 0.960 |
| Globulin (g/dL) | <0.001 | 1.970 | 1.563 to 2.483 | <0.001 | 2.007 | 1.592 to 2.530 |
| PDW (>12.65, ≤12.65) | <0.001 | 0.428 | 0.325 to 0.563 | <0.001 | 0.429 | 0.326 to 0.564 |
CI, confidence interval; DFS, disease‐free survival; HR, hazard ratio; LDH, lactate dehydrogenase; OS, overall survival; PDW, platelet distribution width; WBC, white blood cell.
We then included all the variables that were significant in the univariate analysis in the multivariate analysis (Table 4). In the multivariate analyses, we found that PDW was an independent prognostic factor for DFS (HR = 0.536, 95% CI: 0.403–0.711, P < 0.001) and OS (HR = 0.532, 95% CI: 0.401–0.707, P < 0.001) in patients with NSCLC. Patients with PDW > 12.65 had a significantly better five‐year DFS rate than patients with PDW ≤ 12.65 (74.9% vs. 52.6%, P < 0.001, Fig 2a). The five‐year OS rate had the same trend: 77.0% for the PDW > 12.65 group and 55.6% for the PDW ≤ 12.65 group (P < 0.001, Fig 2b).
Table 4.
Multivariate analysis of DFS and OS for 750 NSCLC patients
| DFS | OS | |||||
|---|---|---|---|---|---|---|
| P‐value | HR | 95% CI | P‐value | HR | 95% CI | |
| Resection type (pneumonectomy, lobectomy) | 0.084 | 1.403 | 0.955 to 2.062 | 0.089 | 1.394 | 0.950 to 2.046 |
| TNM stage (I, II, III) | <0.001 | 1.576 | 1.361 to 1.826 | <0.001 | 1.581 | 1.366 to 1.831 |
| WBC count (×103 mm−3) | 0.518 | 1.092 | 0.837 to 1.425 | 0.427 | 1.114 | 0.853 to 1.454 |
| Platelet count (×104 mm−3) | 0.541 | 1.098 | 0.814 to 1.481 | 0.541 | 1.099 | 0.812 to 1.488 |
| hemoglobin (g/L) | 0.297 | 0.872 | 0.674 to 1.128 | 0.236 | 0.856 | 0.662 to 1.107 |
| LDH (U/L) | <0.001 | 1.557 | 1.231 to 1.971 | <0.001 | 1.578 | 1.248 to 1.995 |
| Fibrinogen (g/L) | 0.413 | 1.129 | 0.845 to 1.508 | 0.414 | 1.129 | 0.844 to 1.509 |
| Albumin (g/dL) | 0.856 | 0.976 | 0.755 to 1.262 | 0.688 | 0.949 | 0.735 to 1.225 |
| Globulin (g/dL) | 0.001 | 1.536 | 1.188 to 1.985 | 0.001 | 1.554 | 1.202 to 2.008 |
| Tumor size | 0.909 | 1.014 | 0.796 to 1.293 | 0.849 | 1.024 | 0.804 to 1.303 |
| Adjuvant chemotherapy (yes, no) | 0.045 | 1.303 | 1.006 to 1.688 | 0.050 | 1.294 | 1.000 to 1.674 |
| PDW (>12.65, ≤12.65) | <0.001 | 0.536 | 0.403 to 0.711 | <0.001 | 0.532 | 0.401 to 0.707 |
CI, confidence interval; DFS, disease‐free survival; HR, hazard ratio; LDH, lactate dehydrogenase; OS, overall survival; PDW, platelet distribution width; WBC, white blood cell.
Figure 2.
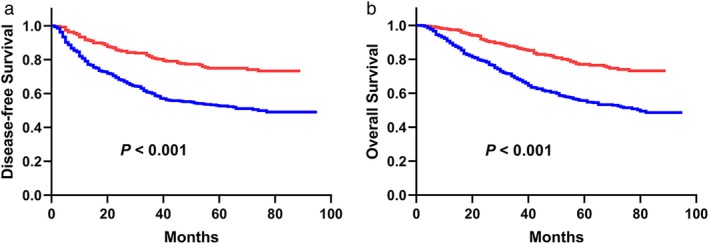
(a) The DFS analysis for 750 patients with NSCLC (P < 0.001). (b) The OS analysis for 750 patients with NSCLC (P < 0.001).  ≤12.65,
≤12.65,  >12.65. PDW, platelet distribution width; DFS, disease‐free survival; OS, overall survival; NSCLC, non‐small cell lung cancer.
>12.65. PDW, platelet distribution width; DFS, disease‐free survival; OS, overall survival; NSCLC, non‐small cell lung cancer.
Relationships between preoperative PDW and the TNM staging system as well as histological subtype
In terms of the TNM stage, 352 patients with NSCLC were categorized as stage I, 134 as stage II, and 264 as stage III. In stage I, there were 151 (42.9%) patients with PDW > 12.65 and 201 (57.1%) patients with PDW ≤ 12.65. In stage II, there were 44 (32.8%) patients with PDW > 12.65 and 90 (67.2%) patients with PDW ≤ 12.65. In stage III, there were 75 (28.4%) patients with PDW > 12.65 and 189 (71.6%) patients with PDW ≤ 12.65. Survival analyses are shown in Fig 3 according to TNM stage. The DFS and OS of the PDW > 12.65 group were better than those of the PDW ≤ 12.65 group in stage I (P < 0.001 for DFS, P < 0.001 for OS, Fig 3a,b), stage II (P = 0.034 for DFS, P = 0.024 for OS, Fig 3c,d), and stage III (P = 0.006 for DFS, P = 0.006 for OS, Fig 3e,f) patients.
Figure 3.
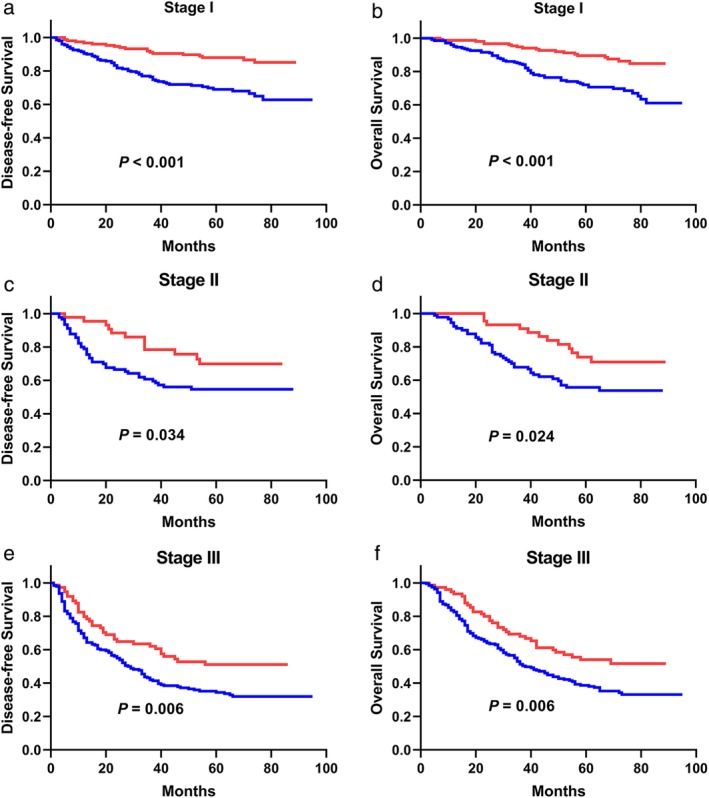
(a) The DFS analysis in patients with stage I NSCLC (P < 0.001). (b) The OS analysis in patients with stage I NSCLC (P < 0.001). (c) The DFS analysis in patients with stage II NSCLC (P = 0.034). (d) The OS analysis in patients with stage II NSCLC (P = 0.024). (e) The DFS analysis in patients with stage III NSCLC (P = 0.006). (f) The OS analysis in patients with stage III NSCLC (P = 0.006).  ≤12.65,
≤12.65,  >12.65. PDW, platelet distribution width; DFS, disease‐free survival; OS, overall survival; NSCLC, non‐small cell lung cancer.
>12.65. PDW, platelet distribution width; DFS, disease‐free survival; OS, overall survival; NSCLC, non‐small cell lung cancer.
Next, we performed statistical analyses for squamous cell carcinoma and adenocarcinoma, which account for the two main pathological types of NSCLC, in 750 patients (Fig 4). The DFS and OS of the PDW > 12.65 group were better than those of the PDW ≤ 12.65 group in squamous cell carcinoma (P < 0.001 for DFS, P < 0.001 for OS, Fig 4a,b) and adenocarcinoma (P = 0.001 for DFS, P = 0.001 for OS, Fig 4c,d) patients.
Figure 4.
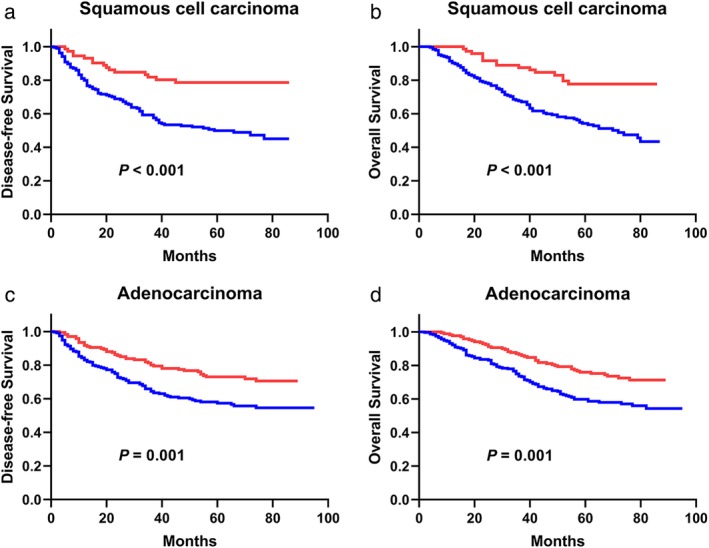
(a) The DFS analysis in patients with squamous cell carcinoma (P < 0.001). (b) The OS analysis in patients with squamous cell carcinoma (P < 0.001). (c) The DFS analysis in patients with adenocarcinoma (P = 0.001). (d) The OS analysis in patients with adenocarcinoma (P = 0.001).  ≤12.65,
≤12.65,  >12.65. PDW, platelet distribution width; DFS, disease‐free survival; OS, overall survival.
>12.65. PDW, platelet distribution width; DFS, disease‐free survival; OS, overall survival.
Combination of preoperative PDW with the TNM staging system to assess prognosis
Patients in the PDW > 12.65 group scored zero, and those in the PDW ≤ 12.65 group scored one. In addition, we scored NSCLC patients based on the TNM staging system. Patients in stage I scored one, stage II scored two and stage III scored three. The PDW and TNM scores for each patient were then summed. All 750 patients with NSCLC were divided into four groups, with scores from 1 to 4. Then, survival curves were reanalyzed and calculated on the basis of the PDW‐TNM staging system (Fig 5).
Figure 5.
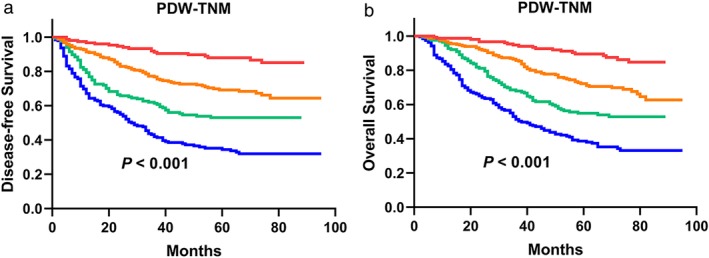
According to the novel model, there were 151 patients who scored 1, 245 patients who scored 2, 165 patients who scored 3 and 189 patients who scored 4. (a ‐ b) The DFS analysis and the OS analysis based on the PDW‐TNM staging system in patients with NSCLC (P < 0.001, respectively).  1,
1,  2,
2,  3,
3,  4. PDW, platelet distribution width; DFS, disease‐free survival; OS, overall survival; NSCLC, non‐small cell lung cancer.
4. PDW, platelet distribution width; DFS, disease‐free survival; OS, overall survival; NSCLC, non‐small cell lung cancer.
Compared with the TNM staging system, the Kaplan‐Meier curves of the PDW‐TNM staging system showed an obvious difference among patients in different groups (P < 0.001 for DFS, P < 0.001 for OS, Fig 5a,b). In the PDW‐TNM staging system, the five‐year DFS rate was 88.0% for the scored 1 group, 69.1% for the scored 2 group, 53.1% for the scored 3 group and 34.4% for the scored 4 group. The five‐year OS rate was 89.6% for the scored 1 group, 72.2% for the scored 2 group, 54.9% for the scored 3 group and 38.0% for the scored 4 group.
Comparison of the TNM and PDW‐TNM staging systems in the value of prognosis prediction
In the present study, the values of the TNM and PDW‐TNM staging systems were compared by analyzing their ROC curves (Fig 6). The PDW‐TNM staging system ROC curve was significantly different from the TNM staging system ROC curve (P < 0.001). The AUC of the TNM staging system was 0.682 (95% CI = 0.643–0.722, P < 0.001), and the AUC of the PDW‐TNM staging system was 0.716 (95% CI = 0.679–0.753, P < 0.001). In terms of the results of the ROC curves, the AUC of the PDW‐TNM staging system was larger than the AUCs of PDW and TNM (P < 0.001).
Figure 6.
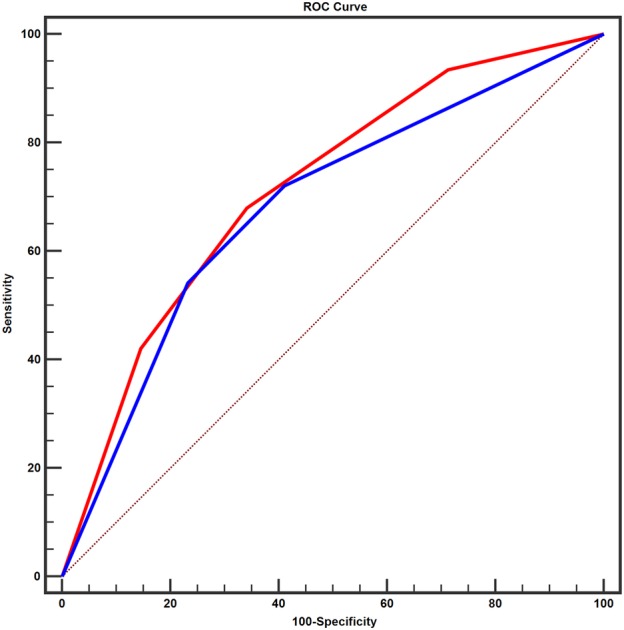
ROC analysis was used in the TNM staging system (AUC = 0.682, 95%CI = 0.643–0.722, P < 0.001) and in the PDW‐TNM staging system (AUC = 0.716, 95%CI = 0.679–0.753, P < 0.001) for all patients. The AUC of PDW‐TNM was larger than the AUC of TNM. (z = 3.941, P = 0.001).  TNM,
TNM,  PDW‐TNM. AUC, area under the curve; NSCLC, non‐small cell lung cancer; ROC, receiver operating characteristic; CI, confidence interval.
PDW‐TNM. AUC, area under the curve; NSCLC, non‐small cell lung cancer; ROC, receiver operating characteristic; CI, confidence interval.
To further measure the goodness of fit of the PDW‐TNM staging system model that we established, the LRT was used. In Table 5, the LRT showed that the χ2 value of the PDW‐TNM staging system was larger than that of the TNM staging system, and the opposite trend was observed in the AIC value (χ2 = 423.991 and 275.034; AIC = 5052.476 and 5080.982 for the PDW‐TNM and TNM staging systems, respectively). The P‐values between these models were all significantly different (P < 0.001).
Table 5.
Comparisons of TNM staging system and PDW‐TNM system in the values of prognosis prediction
| Staging system | Likelihood ratio test χ2 | AIC | P‐value |
|---|---|---|---|
| TNM | 275.034 | 5080.982 | <0.001 |
| PDW‐TNM | 423.991 | 5052.476 | <0.001 |
AIC, Akaike information criterion; PDW, platelet distribution width.
Discussion
In this study, we investigated the prognostic value of PDW in 750 patients with NSCLC. To the best of our knowledge, some researchers have analyzed the prognostic value of preoperative PDW in some solid tumors.15, 16, 17, 18, 19, 20 However, the clinical significance of preoperative PDW in NSCLC patients is not yet clear. Thus, we reviewed the clinical information of 750 patients with NSCLC in our institute. In the entire retrospective cohort study, we systematically analyzed the relationship between preoperative PDW and the prognosis of NSCLC after surgery. Our study found that PDW might be a potential prognostic marker in NSCLC patients.
PDW as a predictive factor has been investigated in many cancers,15, 16, 17, 18, 19, 20 including NSCLC.20 To the best of our knowledge, only one study has reported the prognostic value of PDW in NSCLC. Cui et al.20 investigated 270 clinical samples and revealed that patients with decreased PDW were associated with poor OS. The study of Cheng et al.15 in early gastric cancer also indicated that decreased PDW was positively correlated with poorer DFS. However, the prognostic value of PDW in various malignancies was different. In laryngeal cancer and breast cancer, Zhang et al.17 and Huang et al.16 revealed that the OS of patients with increased PDW was markedly poorer than that of patients with decreased PDW. It seems that the role of PDW may be tissue dependent and related to cancer‐associated inflammation. Therefore, further studies are needed to clarify the role of PDW in cancer.
We included a larger sample size to investigate the clinical significance of preoperative PDW in NSCLC patients who underwent surgery. In the multivariate analyses, we illustrated that PDW was an independent prognostic factor. The results of the survival analysis indicated that elevated PDW was associated with better DFS and OS in patients with NSCLC, which was consistent with our initial results.20 Next, we performed subgroup analyses according to the different TNM stages and different histological subtypes. Subgroup analyses also showed similar results in that patients with high PDW had better DFS and OS. To further assess the value of PDW, the PDW‐TNM staging system model was constructed in this study and had an AUC greater than the AUC of either TNM or PDW alone. Furthermore, survival analysis of the PDW‐TNM staging system showed an obvious discrimination among the four regrouped kinds of patients. The results calculated by the LRT indicated that the PDW‐TNM staging system was much better at predicting the prognosis of patients with NSCLC than PDW or the TNM staging system alone.
Extensive studies have reported the role of platelets in the progression of cancer. According to a recent review by Gay et al. numerous studies showed that coagulation and hemostasis were the basic biological functions of platelets.23 In the process of cohesion, coagulation and immune evasion, activated platelets, together with leukocytes, fibrinogen and a variety of cytokines, interact with tumor cells to promote invasion and metastasis while preventing death.23 Moreover, growing tumors can also increase the production and activation of platelets by producing interleukin (IL)‐6, forming a positive feedback loop to enhance tumor growth.24 Platelets have the ability to interact with coagulation factors to protect tumor cells within the bloodstream. In addition, selectins on platelets can enhance metastasis through tumor cell binding to platelets.23 Soluble P‐selectin, CD40 ligand and β‐TG levels were significantly higher in patients with cancer, indicating the occurrence of sustained platelet activation in these patients compared with the group of healthy people.25 A study revealed that the TGFβ signaling pathway derived from platelets could enhance the metastasis of cancer, while the decreased expression of TGFβ1 in platelets inhibited tumor metastasis in vivo.26 Numerous experimental studies of drugs that inhibit some components during platelet activation, such as inhibitors of adenosine diphosphate (ADP), have been confirmed to reduce tumor metastasis.27, 28 Platelet‐derived microvesicles were also indicated to promote cell proliferation by mitosis stimulation in lung cancer cell lines.29 Overall, platelets play a crucial role in promoting tumor cell metastasis.
Several limitations of the study are noteworthy. First, this was a single‐center retrospective study, and the data source may reflect selection bias. Further multicenter prospective studies are warranted to verify our results. Moreover, only Chinese patients were included in the study sample. The conclusions need to be further confirmed in people of different races.
To summarize, PDW is easily available with a hematological test. Preoperative PDW might be a potential marker to predict the prognosis of NSCLC patients. Compared with the TNM staging system, the PDW‐TNM staging system showed a better correlation with the outcome of NSCLC patients who underwent surgery. Further studies are warranted to clarify the mechanism of PDW involvement in NSCLC progression.
Disclosure
The authors declare no conflict of interest, including specific financial interests and relationships and affiliations relevant to the subject of their manuscript.
Acknowledgments
This work was supported by National Key Research and Development Program of China (2016YFC0905501, 2016YFC0905500); and National Natural Science Foundation of China (81772484); and Tianjin Cancer Hospital Clinical Trial Project (C1705); and Doctoral Start‐up Fund of Tianjin Medical University Cancer Institute and Hospital (B1709 to H Zhang).
References
- 1. Howlader N, Noone AM, Krapcho M e a, eds. SEER Cancer Statistics Review, 1975–2016. National Cancer Institute, Bethesda, MD, Available from URL: https://seer.cancer.gov/csr/1975_2016/, based on November 2018 SEER data submission, posted to the SEER web site, April 2019. [Google Scholar]
- 2. Chen W, Zheng R, Baade PD e a. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66 (2): 115–32. [DOI] [PubMed] [Google Scholar]
- 3. Detterbeck FC, Chansky K, Groome P e a. The IASLC lung cancer staging project: Methodology and validation used in the development of proposals for revision of the stage classification of NSCLC in the forthcoming (eighth) edition of the TNM classification of lung cancer. J Thorac Oncol 2016; 11 (9): 1433–46. [DOI] [PubMed] [Google Scholar]
- 4. Diez M, Torres A, Maestro ML e a. Prediction of survival and recurrence by serum and cytosolic levels of CEA, CA125 and SCC antigens in resectable non‐small‐cell lung cancer. Br J Cancer 1996; 73 (10): 1248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cedres S, Nunez I, Longo M e a. Serum tumor markers CEA, CYFRA21‐1, and CA‐125 are associated with worse prognosis in advanced non‐small‐cell lung cancer (NSCLC). Clin Lung Cancer 2011; 12 (3): 172–9. [DOI] [PubMed] [Google Scholar]
- 6. Zhong H, Qian Y, Fang S, Wang Y, Tang Y, Gu W. Prognostic value of plasma fibrinogen in lung cancer patients: A meta‐analysis. J Cancer 2018; 9 (21): 3904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Francis JL, Amirkhosravi A. Effect of antihemostatic agents on experimental tumor dissemination. Semin Thromb Hemost 2002; 28 (1): 29–38. [DOI] [PubMed] [Google Scholar]
- 8. Contursi A, Sacco A, Grande R, Dovizio M, Patrignani P. Platelets as crucial partners for tumor metastasis: From mechanistic aspects to pharmacological targeting. Cell Mol Life Sci 2017; 74 (19): 3491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stone RL, Nick AM, McNeish IA e a. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med 2012; 366 (7): 610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maraz A, Furak J, Varga Z, Kahan Z, Tiszlavicz L, Hideghety K. Thrombocytosis has a negative prognostic value in lung cancer. Anticancer Res 2013; 33 (4): 1725–9. [PubMed] [Google Scholar]
- 11. Wang YH, Kang JK, Zhi YF e a. The pretreatment thrombocytosis as one of prognostic factors for gastric cancer: A systematic review and meta‐analysis. Int J Surg (London, England) 2018; 53: 304–11. [DOI] [PubMed] [Google Scholar]
- 12. Harano K, Kogawa T, Wu J e a. Thrombocytosis as a prognostic factor in inflammatory breast cancer. Breast Cancer Res Treat 2017; 166 (3): 819–32. [DOI] [PubMed] [Google Scholar]
- 13. Gu D, Szallasi A. Thrombocytosis portends adverse prognosis in colorectal cancer: A meta‐analysis of 5,619 patients in 16 individual studies. Anticancer Res 2017; 37 (9): 4717–26. [DOI] [PubMed] [Google Scholar]
- 14. Kaito K, Otsubo H, Usui N e a. Platelet size deviation width, platelet large cell ratio, and mean platelet volume have sufficient sensitivity and specificity in the diagnosis of immune thrombocytopenia. Br J Haematol 2005; 128 (5): 698–702. [DOI] [PubMed] [Google Scholar]
- 15. Cheng S, Han F, Wang Y e a. The red distribution width and the platelet distribution width as prognostic predictors in gastric cancer. BMC Gastroenterol 2017; 17 (1): 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang Y, Cui MM, Huang YX e a. Preoperative platelet distribution width predicts breast cancer survival. Cancer Biomark 2018; 23 (2): 205–11. [DOI] [PubMed] [Google Scholar]
- 17. Zhang H, Liu L, Fu S e a. Higher platelet distribution width predicts poor prognosis in laryngeal cancer. Oncotarget 2017; 8 (29): 48138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Artunc Ulkumen B, Pala HG, Calik E, Oruc Koltan S. Platelet distribution width (PDW): A putative marker for threatened preterm labour. Pak J Med Sci 2014; 30 (4): 745–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li N, Diao Z, Huang X e a. Increased platelet distribution width predicts poor prognosis in melanoma patients. Sci Rep 2017; 7 (1): 2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cui MM, Li N, Liu X e a. Platelet distribution width correlates with prognosis of non‐small cell lung cancer. Sci Rep 2017; 7 (1): 3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988; 44 (3): 837–45. [PubMed] [Google Scholar]
- 22. Cai XR, Chen ZH, Liu MM e a. Modified CLIP score with the albumin‐bilirubin grade retains prognostic value in HBV‐related hepatocellular carcinoma patients treated with trans‐catheter arterial chemoembolization therapy. J Cancer 2018; 9 (13): 2380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gay LJ, Felding‐Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer 2011; 11 (2): 123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin RJ, Afshar‐Kharghan V, Schafer AI. Paraneoplastic thrombocytosis: The secrets of tumor self‐promotion. Blood 2014; 124 (2): 184–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mantur M, Kemona H, Kozlowski R, Kemona‐Chetnik I. Effect of tumor stage and nephrectomy on CD62P expression and sP‐selectin concentration in renal cancer. Neoplasma 2003; 50 (4): 262–5. [PubMed] [Google Scholar]
- 26. Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial‐mesenchymal‐like transition and promotes metastasis. Cancer Cell 2011; 20 (5): 576–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gebremeskel S, LeVatte T, Liwski RS, Johnston B, Bezuhly M. The reversible P2Y12 inhibitor ticagrelor inhibits metastasis and improves survival in mouse models of cancer. Int J Cancer 2015; 136 (1): 234–40. [DOI] [PubMed] [Google Scholar]
- 28. Mezouar S, Darbousset R, Dignat‐George F, Panicot‐Dubois L, Dubois C. Inhibition of platelet activation prevents the P‐selectin and integrin‐dependent accumulation of cancer cell microparticles and reduces tumor growth and metastasis in vivo. Int J Cancer 2015; 136 (2): 462–75. [DOI] [PubMed] [Google Scholar]
- 29. Janowska‐Wieczorek A, Wysoczynski M, Kijowski J e a. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer 2005; 113 (5): 752–60. [DOI] [PubMed] [Google Scholar]


