Abstract
PURPOSE
Head and neck squamous cell carcinoma (HNSCC) incidence is high in South America, where recent data on survival are sparse. We investigated the main predictors of HNSCC survival in Brazil, Argentina, Uruguay, and Colombia.
METHODS
Sociodemographic and lifestyle information was obtained from standardized interviews, and clinicopathologic data were extracted from medical records and pathologic reports. The Kaplan-Meier method and Cox regression were used for statistical analyses.
RESULTS
Of 1,463 patients, 378 had a larynx cancer (LC), 78 hypopharynx cancer (HC), 599 oral cavity cancer (OC), and 408 oropharynx cancer (OPC). Most patients (55.5%) were diagnosed with stage IV disease, ranging from 47.6% for LC to 70.8% for OPC. Three-year survival rates were 56.0% for LC, 54.7% for OC, 48.0% for OPC, and 37.8% for HC. In multivariable models, patients with stage IV disease had approximately 7.6 (LC/HC), 11.7 (OC), and 3.5 (OPC) times higher mortality than patients with stage I disease. Current and former drinkers with LC or HC had approximately 2 times higher mortality than never-drinkers. In addition, older age at diagnosis was independently associated with worse survival for all sites. In a subset analysis of 198 patients with OPC with available human papillomavirus (HPV) type 16 data, those with HPV-unrelated OPC had a significantly worse 3-year survival compared with those with HPV-related OPC (44.6% v 75.6%, respectively), corresponding to a 3.4 times higher mortality.
CONCLUSION
Late stage at diagnosis was the strongest predictor of lower HNSCC survival. Early cancer detection and reduction of harmful alcohol use are fundamental to decrease the high burden of HNSCC in South America.
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) incidence is high in South America, with higher rates in Argentina, Brazil, French Guyana, and Uruguay.1 The main risk factors for HNSCC continue to be smoking and alcohol consumption, 2 potentially modifiable lifestyle factors. However, high-risk human papillomavirus (HPV) infection has become an important risk factor for a subset of HNSCC, mostly in the oropharynx.2 In the United States, where aggressive tobacco control programs have resulted in reduced tobacco use, a marked decline in the incidence rates of larynx cancer (LC), hypopharynx cancer (HC), and oral cavity cancer (OC) has been observed over the past 3 decades. In the same period, the incidence of HPV-related oropharynx cancer (OPC) has increased substantially.3 In South America, the prevalence of HPV-related OPC seems to be much lower than that in the United States (60%) or Europe (31%). The reported prevalence of HPV-related OPC has been reported to be as low as 4% in Sao Paulo, Brazil.4
Despite progress in diagnostic procedures and therapeutic management, survival after HNSCC diagnosis remains relatively low in Europe5,6 and North America,7 whereas recent survival data are scarce in South America. In this study, we aimed to examine the survival of patients with HNSCC in several South American institutions and investigate the influence of sociodemographic, lifestyle, and clinical factors on survival.
CONTEXT
Key Objective
What are the main predictors of survival in patients with head neck squamous cell carcinoma (HNSCC) from South America?
Knowledge Generated
Three-year survival for HNSCC is low in South America, especially for patients with oropharynx and hypopharynx cancer. Late stage at diagnosis is a strong independent predictor of worse survival for all HNSCC sites. Former and current drinkers diagnosed with laryngeal or hypopharyngeal cancers had over two-fold increased hazard of death compared with never drinkers.
Relevance
Early cancer detection and reduction of harmful alcohol use need to be prioritized to reduce the high burden of HNSCC in South America.
PATIENTS AND METHODS
Patients
We used data prospectively collected from the InterCHANGE study, a multicenter case-control study that was initiated to investigate the risk factors for HNSCC onset and outcomes. Patients were followed up to investigate the main predictors of survival after HNSCC. The International Agency for Research on Cancer (IARC; Lyon, France) was responsible for the overall coordination of the study, which included 8 institutions from the following 4 countries: Argentina, Brazil, Colombia, and Uruguay. Data from Argentina were mostly from the Angel Roffo Institute of Oncology (Buenos Aires; public health system), with a few patients from the Italiano Hospital (Buenos Aires; private). Data from Brazil are from the following 4 centers: National Cancer Institute (INCA) in Rio de Janeiro (public), Araujo Jorge Hospital in Goiania (private), A.C. Camargo Cancer Center in Sao Paulo (private), and Santa Rita de Cassia Hospital in Vitoria (private). Uruguay was represented by the Dr Manuel Quintela Hospital in Montevideo (public), whereas data from Colombia were obtained from the Santa Fé de Bogotá University Hospital (Bogotá; private).
Inclusion criteria included age ≥ 18 years and a diagnosis of a primary HNSCC from 2011 to 2017. HNSCC was classified according to the International Classification of Diseases for Oncology, third edition,8 and included LC (C32.0-C32.9), HC (C12.9 and 13C.0-13C.9), OC (C00.3-C00.9, C02.0-C02.3, C03.0-C03.9, C04.0-C04.9, C05.0, and C06.0-C06.9), and OPC (C01.0-C01.9, C02.4, C05.1-C05.2, C09.0-C09.9, and C10.0-10.9). All tumors were confirmed by histology.
Explanatory Variables
All patients participated in a standardized interview conducted within 6 months of diagnosis, during which information on sociodemographic, lifestyle, and clinical factors was collected. Sociodemographic variables included residence at time of recruitment, sex (male or female), age at diagnosis (≤ 50, 51-60, 61-70, or ≥ 71 years), education level (categorized as illiterate, fundamental incomplete, fundamental complete, medium complete, or superior), and self-identified race or ethnicity (white, black, mulatto, or other/unknown). Mulattos are considered to be people of mixed African and European descent. As a result of small numbers, Asians, indigenous, and mestizo (mixed indigenous and European descent people from Colombia) patients were grouped in the race or ethnicity category of other.
Lifestyle variables included comprehensive tobacco and alcohol histories. Tobacco use included cigarettes, cigars, and pipes, and the following 3 aspects were investigated: overall smoking history (never-smoker, former smoker, or current smoker), (2) smoking duration (categorized as never, 1-39, or ≥ 40 years), and (3) smoking intensity (categorized as never, < 30, or ≥ 30 pack-years). Alcohol intensity was examined as overall alcohol history (never-drinker, former drinker, or current drinker) and alcohol intensity (categorized as ≤ 2 or > 2 drinks per day). Alcohol use included all types of alcohol, such as cachaça, vodka, whisky, tequila, rum, gin, beer, and wine.
Clinicopathologic data were obtained from medical records and pathologic reports. Stage at diagnosis was based on the seventh edition of the TNM classification of the American Joint Committee on Cancer (AJCC)9 and classified into four categories (stage I-IV). Institutions from Buenos Aires, Goiania, and Vitoria provided additional information on HPV-16 E6 serology (a highly specific biomarker of HPV-16 infection) for patients with OPC, which corresponded to nearly 50% of patients with OPC. Patients with high seroreactivity (mean fluorescent intensity, ≥ 1,000) were considered to have HPV-related OPC.10
Follow-Up
The primary outcome was overall survival, which was measured from the date of HNSCC diagnosis until death from any cause, the end of the study (June 31, 2018), or loss to follow-up, whichever occurred first. Patients contribute person-years of follow-up from their date of diagnosis to the date of death, loss to follow-up, or end of study. Therefore, there was a maximum of 7.5 years of follow-up (January 1, 2011, to June 31, 2018). Information on vital status was obtained via routine follow-up visits and/or by linking the patient’s data with vital statistics, cancer registry files, and medical records, depending on the available sources at each center. IARC provided an online database to enter follow-up information. For the main analysis, we assumed that a patient was lost to follow-up when he or she could not be tracked by any source of information in the last 24 months of the study completion.
Statistical Analyses
Analyses were performed separately by anatomic cancer site. Because of the relatively small sample size, patients with HC were pooled with patients with LC for multivariable regression analysis. We estimated the probability of 3-year survival using the Kaplan-Meier method and compared differences in survival curves across strata for each variable using the log-rank test.
To evaluate the association between survival and the explanatory variables, we used hazard ratios (HRs) and 95% CIs estimated by means of Cox proportional hazards models. Variables included in the models were those that had an a priori hypothesized or previously observed association with HNSCC survival based on a review of the literature. These include age and stage at diagnosis, sex, education, race or ethnicity, and smoking and alcohol histories. Univariable and multivariable models (including all variables) were fitted. All multivariable models were adjusted for calendar year of diagnosis (categorized as 2011-2013 and 2014-2017) and center to account for possible changes in therapeutic management over time and different treatment approaches, respectively. The model with combined LC and HC was additionally adjusted for tumor site as a result of the potential differences in prognosis between the 2 types of cancer. The model for OPC also included HPV status because differences in prognosis between HPV-related and HPV-unrelated OPC have been reported.
The proportional hazards assumption, assessed by examining log-log survival plots and Schoenfeld residuals, was met for all variables in the multivariable models. Likelihood ratio tests were used to obtain overall P values for each variable in the multivariable models, considering comparison with the model excluding the variable in question. A small amount of data was missing for some explanatory variables, and all analyses were restricted to patients with complete data on all variables used in the multivariable models, thus assuming that the data were missing completely at random. All statistical analyses were performed using Stata 14.2 statistical software (StataCorp, College Station, TX). A 2-sided P < .05 was considered statistically significant.
Ethics Approval
The InterCHANGE study was approved by the ethical review boards of the IARC and of each participating center. All patients provided written informed consent for their participation in this study.
RESULTS
From 2011 through 2017, a total of 1,829 patients newly diagnosed with HNSCC were enrolled in the InterCHANGE study. Patients with missing (n = 18), overlapping (n = 101), or “other” (n = 65) topography codes and those with missing (n = 100) or inconsistent date of known last vital status (at or before date of diagnosis, n = 7) were excluded from the study. Less than 5% of patients (n = 75) had missing data for ≥ 1 of the explanatory variables and were excluded. The proportions of individuals with missing data were 3.1% for stage, 1.5% for education, 0.4% for smoking history, and 0.5% for alcohol history. The proportion of patients lost to follow-up was 17.4%.
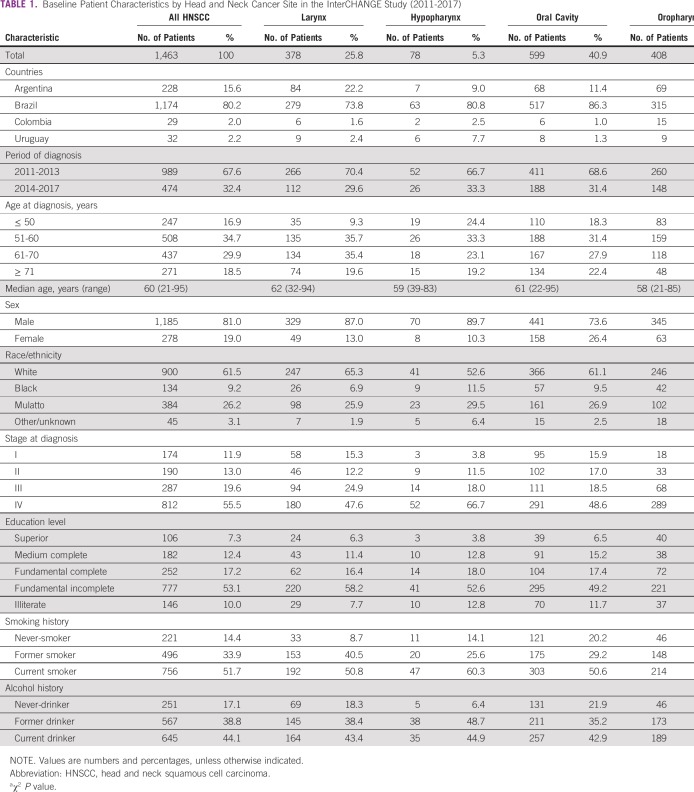
Analyses were based on 1,463 patients with a complete set of information. Of these patients, 378 (25.8%) had LC, 78 (5.3%) had HC, 599 (40.9%) had OC, and 408 (27.9%) had OPC. Most patients were from Brazil (n = 1,174, 80.3%), followed by Argentina (n = 228, 15.6%), Uruguay (n = 32, 2.2%), and Colombia (n = 29, 2.0%). Approximately 55.5% of patients were diagnosed with stage IV tumors, varying from 47.6% for LC to 70.8% for OPC. The baseline characteristics of the participants are listed in Table 1.
TABLE 1.
Baseline Patient Characteristics by Head and Neck Cancer Site in the InterCHANGE Study (2011-2017)
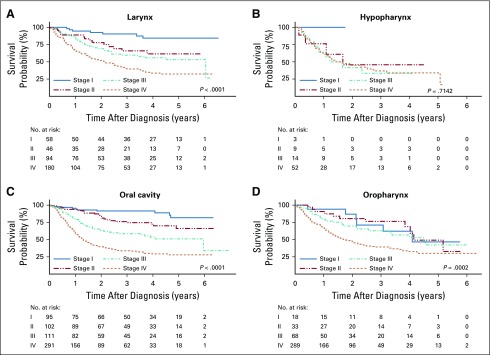
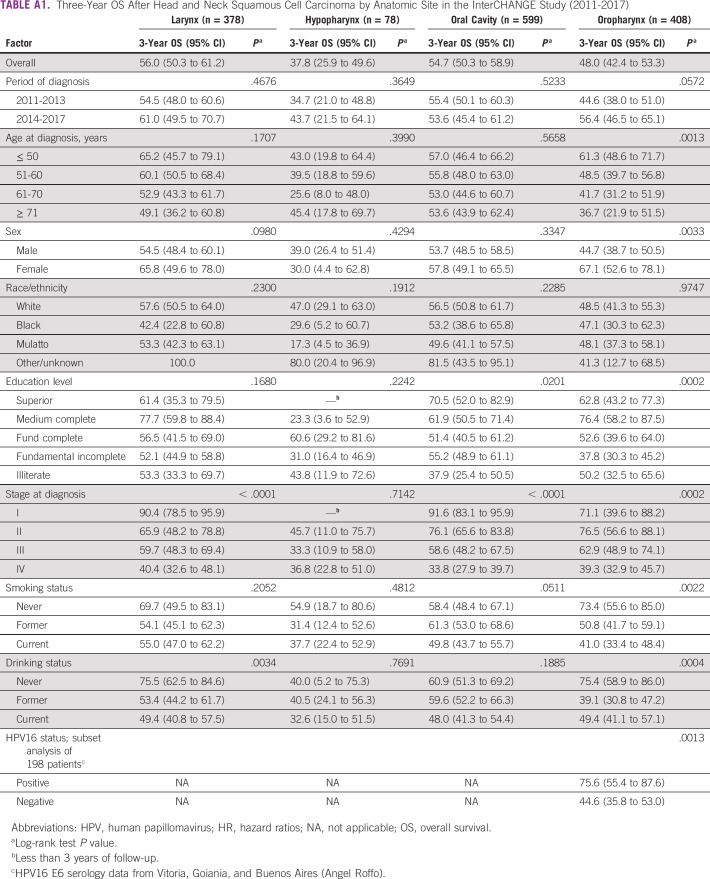
By the end of the follow-up period, 672 patients (45.9%) had died, and the median follow-up time was 1.7 year (range, 0.1-6.9 years). On the basis of Kaplan-Meier analyses, 3-year survival was estimated to be highest for LC (56.0%; 95% CI, 50.3% to 61.2%) and OC (54.7%; 95% CI, 50.3% to 58.9%), intermediate for OPC (48.0%; 95% CI, 42.4% to 53.3%), and lowest for HC (37.8%; 95% CI, 25.9% to 49.6%). On the basis of the log-rank test, worse survival was associated with advanced stage at diagnosis (Fig 1), lower education (OC and OPC), smoking (OC and OPC), alcohol consumption (LC and OPC), male sex (OPC), and older age at diagnosis (OPC; Appendix Table A1).
FIG 1.
Overall survival by stage at diagnosis of head and neck squamous cell carcinoma for each anatomic site in the InterCHANGE study (2011-2017).
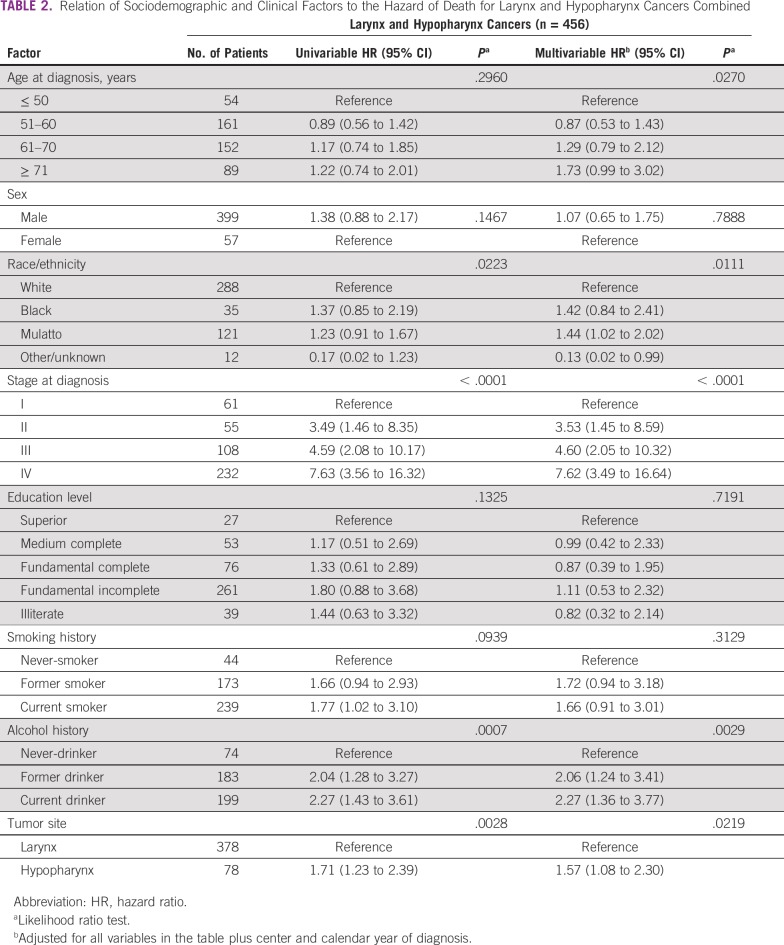
For LC and HC combined, univariable analyses showed that advanced stage at diagnosis and alcohol consumption were associated with higher mortality rates. The mortality hazard in patients with HC was 1.7 times that in patients with LC (Table 2). In multivariable-adjusted models, the hazard for mortality was higher among older patients (≥ 71 v ≤ 50 years: HR, 1.73; 95% CI, 0.99 to 3.02), mulattos (v whites: HR, 1.44; 95% CI, 1.02 to 2.02), those with advanced stage (IV v I: HR, 7.62; 95% CI, 3.49 to 16.64), and ever-drinkers (current v never: HR, 2.27; 95% CI, 1.36 to 3.77; and former v never: HR, 2.06; 95% CI, 1.24 to 3.41). After multivariable adjustment, the mortality hazard in patients with HC was approximately 1.6 times that in patients with LC (HR, 1.57; 95% CI, 1.08 to 2.30; Table 2).
TABLE 2.
Relation of Sociodemographic and Clinical Factors to the Hazard of Death for Larynx and Hypopharynx Cancers Combined
For patients with OC, univariable analyses revealed that advanced stage at diagnosis, lower education level, and smoking history were associated with higher mortality rates (Table 3). In multivariable-adjusted models, mortality hazards were significantly higher among patients age ≥ 61 years (61-70 v ≤ 50 years: HR, 1.46; 95% CI, 0.99 to 2.14; and ≥ 71 v ≤ 50 years: HR, 1.82; 95% CI, 1.18 to 2.78) and those with advanced-stage disease (IV v I: HR, 11.7; 95% CI, 6.09 to 22.43; Table 3).
TABLE 3.
Relation of Sociodemographic and Clinical Factors to the Hazard of Death for Oral Cavity Cancer
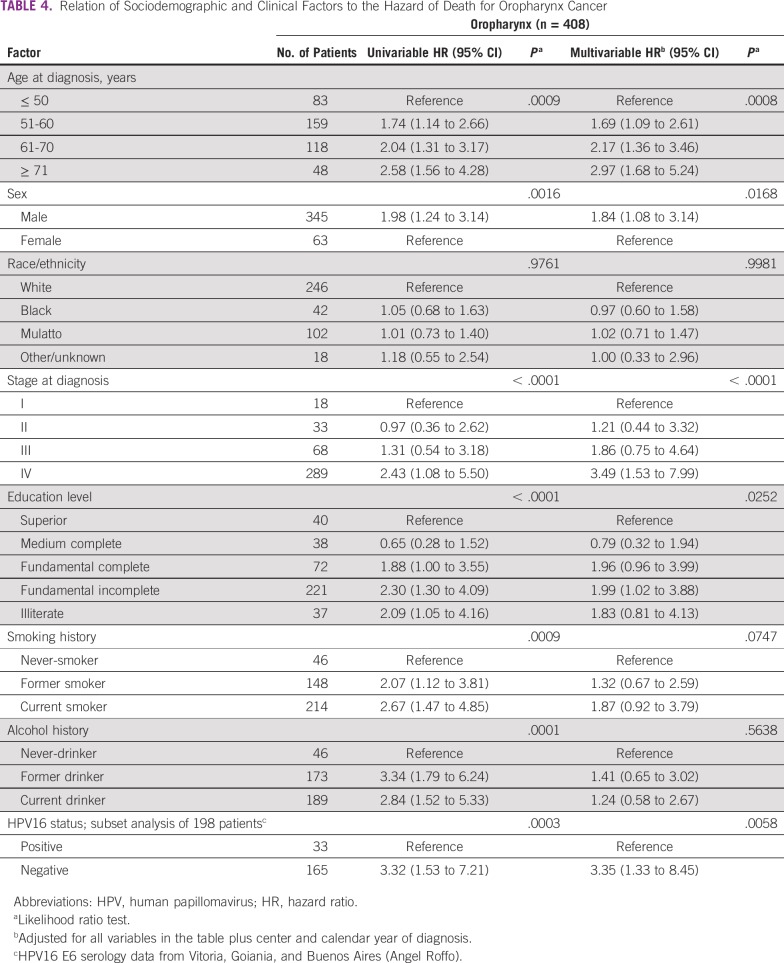
Among patients with OPC, univariable analyses showed that older age, male sex, lower education, advanced stage at diagnosis, ever-smoker status, and ever-drinker status were associated with higher mortality rates (Table 4). After adjustment, mortality rates remained higher among men (v women: HR, 1.84; 95% CI, 1.08 to 3.14), patients age ≥ 61 years (61-70 v ≤ 50 years: HR, 2.17; 95% CI, 1.36 to 3.46; and ≥ 71 v ≤ 50 years: HR, 2.97; 95% CI, 1.68 to 5.24), and patients with advanced-stage disease (IV v I: HR, 3.49; 95% CI, 1.53 to 7.99; Table 4). In a subset analysis of 198 patients with OPC with available HPV-16 status (48.5% of all patients with OPC), 3-year survival was lower among patients with HPV-unrelated versus HPV-related OPC (44.6% v 75.6%, respectively; Fig 2). Patients with HPV-unrelated OPC had a mortality rate 3.4 times that of patients with HPV-related OPC after multivariable adjustment (HR, 3.35; 95% CI, 1.33 to 8.45; Table 4).
TABLE 4.
Relation of Sociodemographic and Clinical Factors to the Hazard of Death for Oropharynx Cancer
FIG 2.
Three-year overall survival (and 95% CI) from oropharynx cancer by human papillomavirus (HPV) type 16 status in the InterCHANGE study (2011-2017).
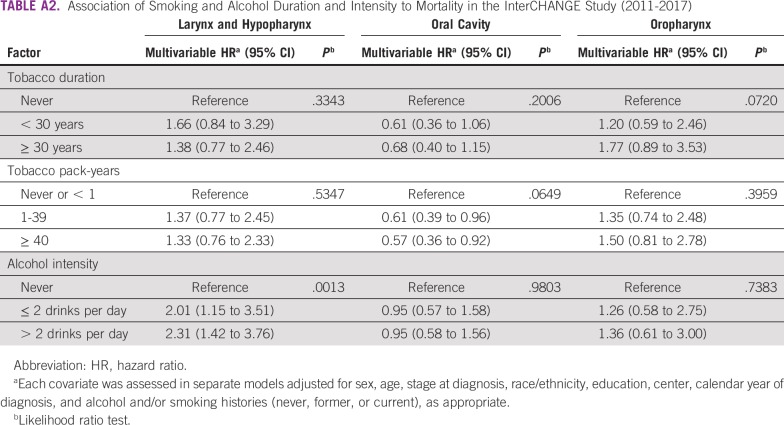
Similar associations between mortality and smoking duration and intensity and alcohol intensity were observed when compared with overall histories for all cancer sites (Appendix Table A2). When we performed an analysis restricted to centers with > 100 patients (INCA in Rio de Janeiro, A.C. Camargo Cancer Center in São Paulo, Santa Rita de Cassia in Vitória, Araújo Jorge in Goiania, and Angel Roffo in Buenos Aires; total of 1,400 patients), 3-year survival did not differ substantially from results across centers.
DISCUSSION
The InterCHANGE study provided relevant information on the predictors of survival after larynx, hypopharynx, oral cavity, and oropharynx squamous cell carcinoma using up-to-date information (2011-2017) from 8 centers in 4 countries in South America. Three-year overall survival was < 50% for HC and OPC and slightly > 50% for LC and OC. Late stage at diagnosis was a strong independent predictor of worse survival for all HNSCC sites. Other predictors of lower survival were older age at diagnosis for all anatomic sites, alcohol consumption (former or current drinkers) for LC/HC, male sex and low education level for OPC, and mulatto race/ethnicity for LC/HC. In addition, in a subset analysis, HPV-unrelated OPC was associated with significantly inferior survival compared with HPV-related OPC.
The proportion of stage IV HNSCCs was strikingly high (56%) in our cohort. If we combine stages III and IV, approximately 75% of patients were diagnosed at later stages. HNSCC diagnosis includes clinical and pathologic information.11 Although immunohistochemical HPV testing has prognostic significance, this test is not currently mandatory to make therapeutic decisions for patients with OPC not included in deintensification trials.10 Reasons for late HNSCC diagnosis in South America are often multifactorial and may include lack of awareness of cancer signs and symptoms (in both patients and health care providers), lack of access to appropriate health care, and shortage of medical resources as a result of fragmented health systems.12-14 Nearly half of the participants in our study (n = 677, 46.3%) were treated at public hospitals, where patients frequently face a significant delay in cancer diagnosis.15 Whenever a delay in diagnosis occurs, the potential curability of HNSCC decreases considerably.12,16 Importantly, we found that stage at diagnosis was strongly associated with worse survival for all HNSCC sites, regardless of the center of treatment.
Although not directly comparable, results from the United States and Europe also show a high proportion of advanced-stage disease (regional or distant) at diagnosis. For instance, data from the American Cancer Society showed that, during 2007-2013, 66% of patients with OC and OPC were diagnosed with advanced disease.17 In Europe, a study that investigated survival after HNSCC from 86 cancer registries during 1999-2007 revealed that 54% of patients were diagnosed at an advanced stage (regional or metastatic).6 These results highlight the unmet need of early HNSCC diagnosis, not only in South America but also in several high-income countries.
We observed that older age at diagnosis was independently associated with lower survival for all cancer sites, consistent with previous studies in Europe5 and North America.18 Relevant factors that influence HNSCC prognosis among elderly patients are the presence and severity of comorbidities and therapeutic approaches. Relevant comorbidities include cardiovascular and respiratory diseases, which are strongly associated with smoking and alcohol consumption.19 These comorbidities likely contribute to treatment decisions.20,21 Although older patients with HNSCC may have limited capacity to endure multimodality treatment (surgery, chemotherapy, and/or radiotherapy), a US study showed that elderly patients with advanced disease who received multimodality treatment had better survival than older patients who received single-modality therapy.18 A careful assessment of risk and potential benefit of different treatment approaches is crucial to decrease HNSCC mortality and morbidity among elderly patients.
We observed higher mortality rates among ever-smokers with OPC in the univariable analysis, but this association was attenuated after adjustment for covariates. For patients with LC or HC, alcohol consumption was significantly associated with lower survival. Current and former drinkers had approximately 2.3 and 2.1 times higher mortality rates, respectively, compared with never-drinkers. The small difference between former and current drinkers may be partially related to cumulative years of drinking. Mortality rates were also higher among drinkers with OPC, although the association was attenuated after adjustment for confounders. Our findings are similar to those from a recent study in Uruguay,22 which found alcohol use to be an independent predictor of worse survival for HNSCC. Alcohol consumption has also been associated with lower survival in patients with HNSCC in a number of studies in North America,23 Asia,24 and Europe.25 In Latin America, harmful alcohol consumption is a major health problem and one of the leading risk factors for cancer, indicating a need for stronger policies to decrease alcohol misuse.26
Our study supports the role of HPV as a prognostic factor for OPC, with patients with HPV-related OPC experiencing better survival than patients with HPV-unrelated cancer, consistent with preceding studies in Europe and North and South America.27,28 Given the significant epidemiology changes in OPC observed over the past few decades, studies looking at the potential benefit of primary prevention (HPV vaccination) and secondary prevention (screening for oral HPV infection) are warrented.29
This study was limited because we lacked data on patient comorbidities, as well as detailed information on treatment approaches by stage at diagnosis. Because data were collected on patients diagnosed before 2018, we did not use the AJCC 8th edition staging system, which was fully implemented in January 2018. Consequently, we could not evaluate changes in staging classification, particularly for OPC. In addition, at the time of this study, tissue biomarkers for OPC (such as p16 expression) were not available, and this will be the subject of a future research project. However, several studies have demonstrated that HPV-16 E6 antibody is a highly specific biomarker for HPV positivity with false-positive rates < 1%.10,30-32 Data were missing for some explanatory variables. A complete-records analysis was used, which assumes that data are missing completely at random. Deviations from this assumption could result in bias in the estimated associations. However, < 5% of individuals were excluded as a result of missing covariate data; therefore, it is unlikely that this had a significant impact on the results. The extent to which our cohort is representative of patients with HNSCC in South America is unclear, and additional studies including more South American centers are warranted to confirm our findings.
The InterCHANGE project has major strengths. This study represents a multicollaborative research effort to provide actual information on a disease with great mortality and morbidity. We obtained recent (2011-2017), good-quality, prospective data from several hospitals specializing in cancer treatment in South America, providing relevant findings to the literature, where data are scarce. Our study followed a standardized protocol, with all cancers confirmed by histology. We had nearly complete information on stage at diagnosis, which is a strong predictor of HNSCC outcome and is often missing in epidemiologic studies. We collected detailed information on tobacco and alcohol use, which are modifiable risk factors that not only cause HNSCC but also have been associated with cancer prognosis. In addition, we provided information on patients’ race or ethnicity and education level, allowing us to account for social determinants of health, elements that may greatly influence early diagnosis and cancer survival.33
In summary, 3-year survival after HNSCC diagnosis was low in the South American centers we studied, especially for OPC and HC. Elderly patients had higher mortality rates, which highlights the need of careful assessment of risk and potential benefit of therapeutic management. Our findings of worse survival among former and current drinkers diagnosed with LC or HC emphasize the need for health policies aimed at decreasing alcohol consumption in the population. This is especially relevant in light of a recent a systematic analysis of 195 countries that showed that the total attributable burden of alcohol use was greater than demonstrated in previous studies. Moreover, cancer, including HNSCC, accounted for a substantial proportion of total alcohol-attributable deaths.34 Finally, late stage at diagnosis was the strongest predictor of HNSCC survival. Efforts to detect cancer at an earlier stage are fundamental to improve survival after HNSCC in South America.
ACKNOWLEDGMENT
We thank Hélène Renard and Priscilia Chopard for support in recruitment logistics within InterCHANGE.
Appendix
TABLE A1.
Three-Year OS After Head and Neck Squamous Cell Carcinoma by Anatomic Site in the InterCHANGE Study (2011-2017)
TABLE A2.
Association of Smoking and Alcohol Duration and Intensity to Mortality in the InterCHANGE Study (2011-2017)
Where authors are identified as personnel of the International Agency for Research on Cancer or WHO, the authors alone are responsible for the views expressed in this article, and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer or WHO.
PRIOR PRESENTATION
Presented at the 6th Annual Symposium on Global Cancer Research, New York, NY, March 15, 2018.
SUPPORT
Supported by the International Agency for Research on Cancer and the European Union’s Horizon 2020 research and innovation program HEADSpAcE under Grant Agreement No. 825771; and R.A. was supported in part by the National Research Service Award (NRSA) for Primary Medical Care (Award No. T32HP300370401).
AUTHOR CONTRIBUTIONS
Conception and design: Sandra Perdomo, Fernando Luis Dias, Luiz P. Kowalski, Mauricio Cuello, José Antonio Hakim, Paul Brennan, Maria Paula Curado
Provision of study materials or patients: Luis Felipe Ribeiro Pinto, José Roberto V. de Podestá, Marta Vilensky, Raul Eduardo Giglio, Luiz P. Kowalski, Mauro K. Ikeda, Andres Munyo, Paula A. Rodríguez-Urrego, David Alfonso Suarez-Zamora, Federico Cayol, Javier Oliver, Paul Brennan
Collection and assembly of data: Sandra Perdomo, Luis Felipe Ribeiro Pinto, Flávia Nascimento de Carvalho, José Roberto V. de Podestá, Sandra Ventorin von Zeidler, Priscila Marinho de Abreu, Marta Vilensky, Raul Eduardo Giglio, José Carlos Oliveira, Matinair Siqueira Mineiro, Luiz P. Kowalski, Mauro K. Ikeda, Mauricio Cuello, Andres Munyo, Paula A. Rodríguez-Urrego, David Alfonso Suarez-Zamora, Federico Cayol, Marcelo Fernando Figari, Javier Oliver, Valerie Gaborieau, Paul Brennan, Maria Paula Curado
Data analysis and interpretation: Renata Abrahão, Sandra Perdomo, Mauricio Cuello, Ruth H. Keogh, Paul Brennan, Maria Paula Curado
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Sandra Perdomo
Consulting or Advisory Role: Bristol-Myers Squibb
Raul Eduardo Giglio
Consulting or Advisory Role: MSD
Speakers' Bureau: MSD, Biotoscana, Merck KGaA, Bristol-Myers Squibb
Travel, Accommodations, Expenses: MSD, Bristol-Myers Squibb
José Antonio Hakim
Honoraria: Johnson & Johnson
Federico Cayol
Research Funding: Merck KGaA
Travel, Accommodations, Expenses: Roche, MSD Oncology, Merck KGaA
No other potential conflicts of interest were reported.
REFERENCES
- 1.Perdomo S, Martin Roa G, Brennan P, et al. Head and neck cancer burden and preventive measures in Central and South America. Cancer Epidemiol. 2016;44(suppl 1):S43–S52. doi: 10.1016/j.canep.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Lewis A, Kang R, Levine A, et al. The new face of head and neck cancer: The HPV epidemic. Oncology (Williston Park) 2015;29:616–626. [PubMed] [Google Scholar]
- 3.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anantharaman D, Abedi-Ardekani B, Beachler DC, et al. Geographic heterogeneity in the prevalence of human papillomavirus in head and neck cancer. Int J Cancer. 2017;140:1968–1975. doi: 10.1002/ijc.30608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abrahão R, Anantharaman D, Gaborieau V, et al. The influence of smoking, age and stage at diagnosis on the survival after larynx, hypopharynx and oral cavity cancers in Europe: The ARCAGE study. Int J Cancer. 2018;143:32–44. doi: 10.1002/ijc.31294. [DOI] [PubMed] [Google Scholar]
- 6.Gatta G, Botta L, Sánchez MJ, et al. Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: The EUROCARE-5 population-based study. Eur J Cancer. 2015;51:2130–2143. doi: 10.1016/j.ejca.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 7.Pulte D, Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: A period analysis. Oncologist. 2010;15:994–1001. doi: 10.1634/theoncologist.2009-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fritz A, Percy C, Jack A, et al: International Classification of Diseases for Oncology (ed 3). Geneva, Switzerland, World Health Organization, 2013. [Google Scholar]
- 9. doi: 10.1245/s10434-010-0985-4. Edge SB, Compton CC: The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17:1471-1474, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Lang Kuhs KA, Anantharaman D, Waterboer T, et al. Human papillomavirus 16 E6 antibodies in individuals without diagnosed cancer: A pooled analysis. Cancer Epidemiol Biomarkers Prev. 2015;24:683–689. doi: 10.1158/1055-9965.EPI-14-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amin M, Edge S, Greene F, et al: AJCC Cancer Staging Manual (ed 8). New York, NY, Springer, 2017. [Google Scholar]
- 12.Strasser-Weippl K, Chavarri-Guerra Y, Villarreal-Garza C, et al. Progress and remaining challenges for cancer control in Latin America and the Caribbean. Lancet Oncol. 2015;16:1405–1438. doi: 10.1016/S1470-2045(15)00218-1. [DOI] [PubMed] [Google Scholar]
- 13.Carvalho AL, Pintos J, Schlecht NF, et al. Predictive factors for diagnosis of advanced-stage squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2002;128:313–318. doi: 10.1001/archotol.128.3.313. [DOI] [PubMed] [Google Scholar]
- 14.Frenk J, Gómez-Dantés O. Health systems in Latin America: The search for universal health coverage. Arch Med Res. 2018;49:79–83. doi: 10.1016/j.arcmed.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Felippu AW, Freire EC, Silva Rde A, et al. Impact of delay in the diagnosis and treatment of head and neck cancer. Braz J Otorhinolaryngol. 2016;82:140–143. doi: 10.1016/j.bjorl.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kowalski LP, Carvalho AL. Influence of time delay and clinical upstaging in the prognosis of head and neck cancer. Oral Oncol. 2001;37:94–98. doi: 10.1016/s1368-8375(00)00066-x. [DOI] [PubMed] [Google Scholar]
- 17.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 18.Moye VA, Chandramouleeswaran S, Zhao N, et al. Elderly patients with squamous cell carcinoma of the head and neck and the benefit of multimodality therapy. Oncologist. 2015;20:159–165. doi: 10.1634/theoncologist.2013-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paleri V, Wight RG, Silver CE, et al. Comorbidity in head and neck cancer: A critical appraisal and recommendations for practice. Oral Oncol. 2010;46:712–719. doi: 10.1016/j.oraloncology.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Sanabria A, Carvalho AL, Vartanian JG, et al. Comorbidity is a prognostic factor in elderly patients with head and neck cancer. Ann Surg Oncol. 2007;14:1449–1457. doi: 10.1245/s10434-006-9296-1. [DOI] [PubMed] [Google Scholar]
- 21.Sanabria A, Carvalho AL, Vartanian JG, et al. Factors that influence treatment decision in older patients with resectable head and neck cancer. Laryngoscope. 2007;117:835–840. doi: 10.1097/MLG.0b013e3180337827. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez AD, Munyo-Estefan A, Borche G, et al. Cáncer de cabeza y cuello en Uruguay: Análisis de sobrevida en dos centros de referencia. Rev Med Urug (Montev) 2018;34:21–28. [Google Scholar]
- 23.Mayne ST, Cartmel B, Kirsh V, et al. Alcohol and tobacco use prediagnosis and postdiagnosis, and survival in a cohort of patients with early stage cancers of the oral cavity, pharynx, and larynx. Cancer Epidemiol Biomarkers Prev. 2009;18:3368–3374. doi: 10.1158/1055-9965.EPI-09-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawakita D, Oze I, Hosono S, et al. Prognostic value of drinking status and aldehyde dehydrogenase 2 polymorphism in patients with head and neck squamous cell carcinoma. J Epidemiol. 2016;26:292–299. doi: 10.2188/jea.JE20140240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leoncini E, Vukovic V, Cadoni G, et al. Clinical features and prognostic factors in patients with head and neck cancer: Results from a multicentric study. Cancer Epidemiol. 2015;39:367–374. doi: 10.1016/j.canep.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 26. Pan American Health Organization, WHO: Health in Americas, 2017 Edition: Regional Look and Country Profiles. Washington, DC, Pan American Health Organization, 2017. [Google Scholar]
- 27.D’Souza G, Anantharaman D, Gheit T, et al. Effect of HPV on head and neck cancer patient survival, by region and tumor site: A comparison of 1362 cases across three continents. Oral Oncol. 2016;62:20–27. doi: 10.1016/j.oraloncology.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillison ML, Chaturvedi AK, Anderson WF, et al. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33:3235–3242. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anantharaman D, Gheit T, Waterboer T, et al. Human papillomavirus infections and upper aero-digestive tract cancers: The ARCAGE study. J Natl Cancer Inst. 2013;105:536–545. doi: 10.1093/jnci/djt053. [DOI] [PubMed] [Google Scholar]
- 31.Kreimer AR, Johansson M, Waterboer T, et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol. 2013;31:2708–2715. doi: 10.1200/JCO.2012.47.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ribeiro KB, Levi JE, Pawlita M, et al. Low human papillomavirus prevalence in head and neck cancer: Results from two large case-control studies in high-incidence regions. Int J Epidemiol. 2011;40:489–502. doi: 10.1093/ije/dyq249. [DOI] [PubMed] [Google Scholar]
- 33. doi: 10.1177/1757975916686913. McDaniel JT, Nuhu K, Ruiz J, et al: Social determinants of cancer incidence and mortality around the world: An ecological study. Glob Health Promot 26:41-49, 2019. [DOI] [PubMed] [Google Scholar]
- 34. doi: 10.1016/S0140-6736(18)31310-2. GBD 2016 Alcohol Collaborators: Alcohol use and burden for 195 countries and territories, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 392:1015-1035, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]