Abstract
Objective:
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a common and heterogeneous inflammatory condition, for which the drivers of the underlying inflammation are not yet fully understood. The use of biologic therapies to target specifically relevant effector cells or cytokines in CRSwNP is a growing field of interest. The objectives of this review are to provide an update on the existing studies of biologics in CRSwNP and to identify potential future areas for further research.
Data Sources:
An initial literature review of biologic therapies in CRS was performed through publications gathered from a PubMed search for title/abstract containing “biologic” and “chronic rhinosinusitis”. Further manuscripts describing scientific premise for each biologic were then reviewed.
Study Selections:
A detailed review of all studies describing biologic therapies targeting inflammation in CRSwNP was performed.
Results:
Biologic therapies targeting IL-4Ra, IL-5, IL-5Ra, IL-33, IgE, and TSLP have all been developed and have been investigated for treatment in CRSwNP or current research suggests that they may have utility in this area. Thus far only dupilumab, which inhibits IL-4Ra, has gained FDA approval for the treatment of adults with inadequately controlled CRSwNP.
Conclusion:
Recent advances in our understanding of the fundamental drivers of the chronic respiratory inflammation in CRSwNP has led to the identification of several potential therapeutic targets for this disease. Future clinical success will rely on the availability of biomarker-based endotyping and responder analyses so that clinicians can precisely match each patient to the appropriate biologic, thereby optimizing the proper treatment strategy.
Trial Registration: not applicable
Introduction
Chronic rhinosinusitis (CRS) describes a heterogeneous spectrum of persistent inflammatory diseases affecting the paranasal sinuses, consisting of ill-defined subtypes with variable responses to existing therapies. The underlying pathophysiology of CRS remains elusive, but recent advances in our understanding of the distinct inflammatory mechanisms involved have led to encouraging progress in the development of targeted biologic pharmacotherapies. The use of immunomodulatory therapies (‘biologics’) for the treatment of CRS has not yet become clinical standard-of-care, due to the lack of clear evidence to guide treatment selection and to the need for additional research to further elucidate their therapeutic benefit. Several biologics approved for the management of severe asthma have demonstrated therapeutic potential for the treatment of CRS, with particular focus on patients with CRS and nasal polyposis (CRSwNP).
CRS is defined both by symptoms of mucopurulent drainage, nasal obstruction, facial pain, and/or decreased sense of smell lasting more than 12 weeks, with corresponding radiologic or endoscopic visualization of inflammation of the sinuses.1 It is one of the most common chronic diseases, with a prevalence of 1–16% in the US, and is more prevalent in patients with comorbidities including asthma and environmental allergies.2 The primary aim of our treatments is to achieve a state of clinical control, which was defined by the most recent European Guidelines on Sinusitis and Nasal Polyposis in 2012 as a clinical state in which patients no longer have bothersome symptoms combined with a healthy or almost healthy mucosa upon examination, and the need for local medication only.3 The burden of CRS is high, and patient-reported outcome surveys have shown that CRS negatively affects many aspects of quality of life and has a more detrimental effect on social functioning than back pain, chronic heart failure, or chronic obstructive pulmonary disease.4 Unfortunately, many patients are unable to achieve sufficient control with the standard medical and surgical therapies and there is a need for treatment modalities that extend beyond the use of revision sinus surgery and increasing doses of corticosteroids to curb the inflammation for patients with recalcitrant CRS.
Advances in Clinical and Biomarker-based Endotypes
Clinical Classification: CRSwNP vs CRSsNP
A primary subclassification of CRS is the division of patients into CRSsNP vs CRSwNP depending on whether or not the patients have nasal polyps (NP). This is a simple clinical classification, though is likely insufficient to fully explain the underlying pathophysiologic differences between the phenotypes. Proper diagnosis regarding the presence or absence of NP generally requires examination with rhinoscopy or a sinus CT scan. In general, patients with CRSwNP are considered to have a clinically more severe disease than those without NP, though there is a lot of overlap in the therapeutic options offered to both groups.
Clinical Classification: AERD
CRSwNP can also be further subclassified, and one relevant clinical syndrome within this category is aspirin-exacerbated respiratory disease (AERD), which is characterized by chronic rhinosinusitis with recurrent eosinophilic NP, asthma, and respiratory reactions induced by aspirin and any NSAIDs that inhibit the cyclooxygenase-1 enzyme. The prevalence of AERD is about 30% of patients with asthma and CRSwNP,7 and for patients who present with adult-onset asthma, recurrent NP, and a reliable history of two or more NSAID-induced reactions that caused respiratory symptoms, the diagnosis can be made clinically and based on history alone. The sinus disease in AERD tends to develop with severe and quickly growing nasal polyposis, and most patients present with anosmia. It is important to diagnose AERD in a timely manner in order to provide accurate prognostic information, and appropriate education and treatment. For example, recurrence of NP after sinus surgery and requirement for repeat surgery is more commonly reported in patients with AERD than in aspirin-tolerant patients.8–10 Furthermore, patients with AERD report that despite the comorbid presence of often severe asthma, their loss of sense of smell and chronic nasal symptoms are the two aspects that most severely affect their quality of life, suggesting that targeting the upper respiratory inflammation and symptomatology in these patients is particularly important.11
Patient education and safety is also key. A study by our group showed that even after being diagnosed with AERD, nearly 25% patients had accidentally ingested an NSAID and developed a reaction, indicating the need for improved education about NSAID avoidance.12 Furthermore, aspirin desensitization and initiation of high-dose daily aspirin treatment is a disease-specific therapy for AERD that can help tremendously to slow polyp regrowth and delay sinus disease recurrence following surgery.13
Several ongoing studies of biologics for the treatment of CRSwNP include patients with AERD, and plan to additionally analyze the data separately for the subset of patients with AERD.
Clinical Classification: Infectious CRS
Within patients with CRSsNP, further clinical subclassification based on infectious history may be helpful. There is a spectrum of inflammation in patients with CRSsNP, with some patients experiencing acute exacerbations with viral or bacterial infections, but with infection playing a less clear role in the chronic respiratory inflammation. Recurrent bacterial infections do occur in subsets of patients with CRSsNP,14 though there is data to suggest that the chronic inflammation in the absence of acute infection may be due to the ongoing presence of bacterial biofilm.15–24 For example, the presence of bacterial biofilm found on surgically removed sinus tissue is associated with persistent inflammation after surgery,21 and the presence of biofilm containing Staphylococcus aureus specifically is associated with more severe disease and a poorer postsurgical course.25
Clinical Classification: Allergic fungal sinusitis
The role of fungus in CRS may be important as well. Allergic fungal rhinosinusitis (AFS), often related to Aspergillus, is not a true fungal infection but is due to an inflammatory Type 2 reaction to a fungal antigen.5 There are clear geographic variations in the frequency of AFS, with very low incidence of disease in the northern United States, and the highest incidence found in southern states where nearly one-quarter of all endoscopic sinus procedures were performed for treatment of AFS.6 There are currently no known clinical trials for the use of biologics to treat AFS, and furthermore, most of the recent Phase 2 and Phase 3 trials of biologics in CRS explicitly excluded patents with AFS. As AFS presents as a unique clinical disorder that likely has distinct causative immunologic triggers, future studies will need to be designed expressly to investigate treatment options for these patients.
Biomarker Classification: Cells, cytokines, and antibodies
CRSwNP is usually a Th2-dominant inflammatory process, characterized by extensive tissue eosinophilia. This is generally accompanied by an increase in tissue mast cells, ILC2 cells, local IgE, and Th2 cytokines.26–30 The tissue immunopathology of CRSwNP and current therapeutic targets under investigation are shown graphically in Figure 1. Blood and tissue eosinophilia have often been used as diagnostic biomarkers to classify CRSwNP patients as “Type 2 high”, which is a convenient and likely useful distinction. However, we may need to resist the impulse to then assume that eosinophils themselves should be directly targeted with therapeutics, as anti-eosinophil trials have not shown great success for CRSwNP.31,32 Interestingly, immunoglobulin levels are also globally increased within nasal polyp tissue, including IgE and autoantibodies of all isotypes.33,30,34,35 Furthermore, although plasma IgE levels are also increased in patients with CRSwNP, there is no association of these IgE levels with the presence of atopy or environmental allergies.36 It is not yet clear what clinical benefit may be seen if CRSwNP patients are treated with an anti-IgE agent.
Figure 1. Immunopathology of CRSwNP and current therapeutic targets under investigation.
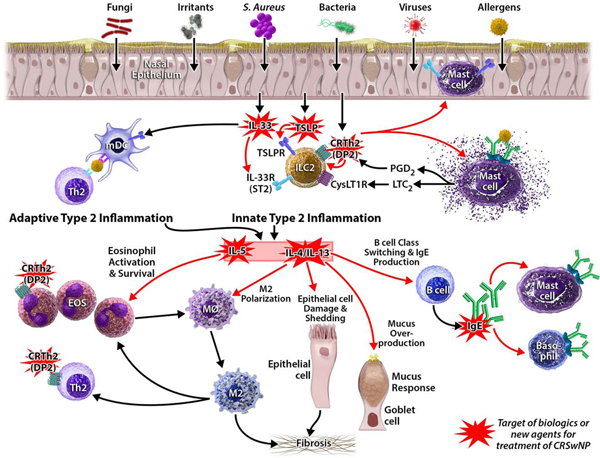
Red starbursts highlight the targets of biologics or new agents for CRSwNP.
Three type 2 cytokines, interleukin (IL)-4, IL-5, and IL-13 have been studied extensively regarding their role in controlling the eosinophilic respiratory inflammation in CRSwNP.8,37 IL-5 is a key survival and activation factor for eosinophils. IL-4 and IL-13 contribute to fibrosis and remodeling, goblet cell hyperplasia and mucous production, and class switching for IgE production.38–40 These type 2 cytokines can be produced by Th2 cells, mast cells, and ILC2s, and their induction can be triggered by both adaptive and innate signaling events. Specifically, IL-33 and thymic stromal lymphopoietin (TSLP) are two innate cytokines that can drive Th2 cytokine production and likely play a role in the development and/or maintenance of the type 2 inflammation in CRSwNP. Levels of these cytokines are elevated in CRSwNP, and perhaps more so in patients with the AERD endotype, though the mechanisms driving the local antibody production and their clinical consequences are still not understood.41–44
In contrast to the largely eosinophilic and Type 2 inflammation-dominant CRSwNP seen in the US, Europe, and Japan, there are mixed inflammatory patterns found in CRSwNP in other parts of Asia, including non-eosinophilic neutrophil-dominant polyps.45,46 There are currently no known clinical trials for the use of biologics to specifically treat neutrophilic CRSwNP. However, many ongoing trials have not specified that the NP must be eosinophilic for inclusion, so there may be data forthcoming from existing trials that can help further our understanding of the differences in pathologic mechanisms between eosinophilic vs neutrophilic NP (Table 1).
Table 1.
All studies listed/ongoing in Clinicaltrials.gov for biologics or other novel mechanisms in chronic rhinosinusitis (CRS) with or without nasal polyps (CRSwNP, CRSsNP)
| Mechanism | Drug | # of CRS patients in trial | Indication | Primary endpoints | Clinicaltrials.gov # | Phase |
|---|---|---|---|---|---|---|
| Anti-IL-5 | mepolizumab | 413 | CRSwNP | NPS*, VAS** | NCT03085797 | 3 |
| Anti-IL-5Rα | benralizumab | 409 | CRSwNP | NPS, NB*** | NCT03401229 | 3 |
| Anti-IgE | omalizumab | 127 and 138 | CRSwNP | NPS, NCS**** | NCT03280537 NCT03280550 | 3 |
| Anti-TSLP | tezepelumab / AMG 157 | No CRS trials yet | Asthma | AERR^ | NCT03927157 | 3 |
| Anti-IL-33 | AMG 282 | 41 | CRSwNP | Safety and tolerability, and immunogenicity | NCT02170337 | 1 |
| Anti-IL-33 | PF-06817024 | CRSwNP | AEs# | NCT02743871 | 1 | |
| Anti-IL-33 | Etokimab/ANB020 | 100 | CRSwNP | NPS, SNOT-22@ | NCT03614923 | 2 |
| CRTH2 antagonist | GB001 | 100 | CRSwNP & CRSsNP | SNOT-22 | NCT03956862 | 2 |
| CRTH2 antagonist | ACT-774312 | 24 | CRSwNP | NPS | NCT03688555 | 2 |
| CRTH2 antagonist | Fevipiprant | 93 | CRSwNP & asthma | NPS | NCT03681093 | 3 |
| TP antagonist | Ifetroban | 76 | AERD | SNOT-22 | NCT03028350 | 2 |
NPS: Total endoscopic nasal polyp score
VAS: Change from baseline in mean nasal obstruction visual analogue scale
NB: Patient reported nasal blockage
NCS: Change From Baseline in Average Daily Nasal Congestion Score
AERR: Annualized asthma exacerbation rate
AEs: Number of Participants With Treatment Emergent Treatment-Related Adverse Events
SNOT-22: Change From Baseline in Sino-Nasal Outcome Test −22 Score
Data for use of available biologics in CRS
Anti-IL-4Rα
Dupilumab, a fully human monoclonal antibody targeting IL-4α, a shared receptor subunit between IL-4 and IL-13, is now FDA approved for treatment of inadequately controlled nasal polyposis in the United States. A phase 2 study of 60 subjects with CRSwNP refractory to intranasal corticosteroids demonstrated reduced endoscopic polyp burden in the patients treated with dupilumab compared to placebo. Thirty-five of the 60 subjects in this study had comorbid asthma. Subjects treated with dupilumab also had improvement in sinonasal imaging scores, sinonasal symptom scores, and sense of smell.47 In two international phase 3 follow-up studies including 724 patients with CRSwNP treated with dupilumab vs. placebo, patients that received dupilumab had improved nasal polyp size (LS mean change from baseline in bilateral nasal polyp score), reduced need for systemic corticosteroids and sinus surgery, improved sinonasal symptoms/nasal congestion, and improved sense of smell compared to placebo.48 Although most patients in the CRSwNP trials of dupilumab showed improvement, in a sub-study of patients with AERD, who had greater sinus opacification, worse sense of smell, and poorer lung function at baseline than did the aspirin-tolerant patients with CRSwNP, dupilumab specifically showed dramatic improvement in both upper and lower airway outcomes in the AERD patients, suggesting particular efficacy in this difficult-to-treat subgroup of patients.49 Sinus CT images from a patient with AERD prior to initiating dupilumab (Figure 2, left) and after four months of dupilumab treatment (Figure 2, right) show an example of the response we have seen in many patients with AERD.
Figure 2. Response to dupilumab in a patient with AERD.
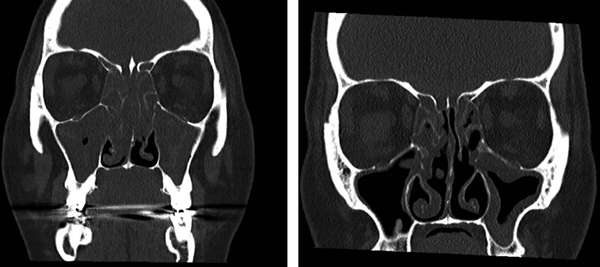
Representative sinus CT scan images are shown for a patient with AERD prior to starting treatment with dupilumab (left) and again after four months of every-other-week dupilumab treatment (right).
Anti-IgE
Monoclonal antibodies targeting IgE are of interest in the treatment of CRSwNP given elevations in tissue IgE in nasal polyposis as described above. An early observational study of 19 subjects with CRSwNP and comorbid asthma showed improvement in nasal polyp size and reduction in nasal topical steroid use following treatment with omalizumab.50 Following this, in a phase 2 placebo-controlled study of 24 adult subjects with CRSwNP and co-morbid asthma, subjects receiving omalizumab had improvement in endoscopic sinus scores compared to those receiving placebo. There were also significant improvements in upper and lower airway symptoms including nasal congestion, rhinorrhea, sense of smell, cough, dyspnea and wheezing. Interestingly, non-atopic subjects with CRSwNP had greater improvement in Lund-Mackay CT score and the AQLQ compared to atopic subjects with CRSwNP, suggesting that although the inhibition of IgE was of value, the presence of allergic disease was not a driver of disease.51 Two phase 3 studies of omalizumab for CRSwNP were recently completed (NCT03280537, NCT03280550) and will provide further information as to the efficacy of omalizumab for treatment of nasal polyposis.
As previously discussed, subjects with AERD have greater tissue IgE levels compared to aspirin-tolerant patients with CRSwNP30 and targeting IgE with omalizumab may therefore be of particular interest in this severe subset of CRSwNP patients. In a study of 21 adult patients with AERD and allergic sensitization to at least one perennial aeroallergen, Hayashi et al. showed that treatment with omalizumab for 12 months induced significant reductions in urinary levels of leukotriene E4 (LTE4) and the prostaglandin D2 metabolite 9a,11b- prostaglandin F2 (PGD2M), both markers of mast cell activation. In addition, the subjects also had reductions in exacerbations, hospitalizations, systemic corticosteroids, and nasal and asthma symptom scores.52 A recent small, placebo-controlled study of 11 subjects with AERD looked at the effect of omalizumab on aspirin-induced respiratory reactions in subjects with AERD. Of the seven subjects treated with omalizumab, only two of seven (29%) had respiratory symptoms upon ingestion of aspirin compared to all four (100%) subjects on placebo. The subjects on omalizumab who did not have a reaction to aspirin had significantly lower levels of urinary LTE4 at the time of the reaction compared to those taking placebo.53 Together these studies suggest a role for omalizumab for treatment of asthma and nasal polyposis in AERD, as well as a potential use for omalizumab prior to aspirin desensitization.
Anti-IL5/Rα
Given the profound tissue eosinophilia and elevated IL-5 levels in many patients with CRSwNP, targeting IL-5 and the relevant receptor, IL-5Rα, makes sense as possible treatments of CRSwNP. Mepolizumab, a humanized monoclonal antibody targeting IL-5, has shown promise in in CRSwNP. A double-blind, placebo-controlled study of subjects with corticosteroid refractory nasal polyposis, showed statistically significant reductions in endoscopic total nasal polyp scores in 12 of 20 patients receiving mepolizumab 750 mg intravenous (IV) for 8 weeks. Only one of 10 subjects in the placebo group had reduction in nasal polyp size. In addition to reduction in nasal polyp size, the mepolizumab group showed a trend toward improvement in sense of smell, postnasal drip and congestion.32 A larger follow-up double-blind, placebo-controlled study of 105 subjects with CRSwNP receiving mepolizumab 750 mg IV every 4 weeks or placebo, on a background of intranasal glucocorticoids, showed reduced need for surgery in the mepolizumab group after 25 weeks of treatment. The primary composite endpoint, the number of patients no longer requiring surgery, was based on the endoscopic nasal polyp scores and patient-reported symptom severity scoring. In addition to improvement in nasal polyp size and reduction in number of patients needing sinus surgery, subjects treated with mepolizumab also had reduction in the individual symptom scores including rhinorrhea, mucus in throat, nasal blockage, loss of smell, as well as total SNOT-22 scores.54 There was no sub-analysis for response to mepolizumab in either study for the subset of subjects with AERD. However, a small retrospective study of subjects with AERD receiving mepolizumab 100 mg subcutaneously for treatment of asthma compared sinonasal symptom scores pre- and post-treatment with mepolizumab. Following treatment with mepolizumab, there were significant reductions in total SNOT-22 score, and the individual scores for sense of smell and nasal congestion were also reduced.55
Another humanized monoclonal antibody targeting IL-5, reslizumab, has also been studied for treatment of CRSwNP. In a randomized, double-blind, placebo-controlled study of 24 subjects, a single dose of IV reslizumab at 1 mg/kg or 3 mg/kg reduced the total nasal polyp score in half of the subjects receiving reslizumab. A responder analysis showed increased nasal secretion IL-5 levels in responders versus non-responders. Twelve weeks after withdrawal of reslizumab, there was a deterioration in nasal polyp score in responders.56
Benralizumab, a drug targeting the specific receptor subunit for IL-5, IL-5Ra, is now available for treatment of severe eosinophilic asthma and are under investigation for treatment of CRSwNP (NCT03450083 and NCT03401229). A post-hoc pooled analysis of two Phase 3 studies of benralizumab for treatment of severe asthma57,58 showed that benralizumab had increased clinical efficacy for treatment of asthma in subjects with both asthma and co-morbid nasal polyposis compared to asthmatics without nasal polyposis. Compared to placebo, subjects receiving benralizumab had a 42% reduction in asthma exacerbation rates, while subjects with asthma and nasal polyps had a 54% reduction in asthma exacerbation rates.59 These results suggest that IL-5 is an especially pertinent pathway for patients who present with eosinophilic asthma and comorbid CRSwNP.
Anti-IL-33
IL-33 has emerged as a mediator that drives the pathogenesis of allergic disease, with data to suggest that it can exacerbate eosinophil-mediated airway inflammation, induce mucous production and goblet cell hyperplasia, and predispose to viral-induced asthma exacerbations.60–63 In addition to its likely role in allergic asthma, increased expression of IL-33 mRNA and protein has been found in the sinus tissue of patients with CRSwNP compared to controls42 and elevated expression of ST2, the ligand-binding chain of the IL-33 receptor, was observed in sinus mucosa from CRSwNP compared with CRSsNP and healthy control subjects.64 This alarmin may be of particular importance in AERD, as there is markedly increased epithelial expression of IL-33 in the nasal polyps of patients with AERD than in those from aspirin-tolerant CRSwNP patients, and in a mouse model of AERD, aspirin-induced reactions induce mast cell activation that is IL-33 dependent.41 Several anti-IL-33 biologics have been developed and initially studied for their efficacy in asthma (NCT03469934, NCT03112577), with promising early results65,66 and consideration for the application of this class of agents for the treatment of CRSwNP is underway.
Anti-TSLP
TSLP is another innate cytokine that has been linked to the pathogenesis of a variety of allergic diseases and is known to trigger type 2 inflammatory responses. Earlier work in asthma showed that TSLP expression is increased in asthmatic airways and correlates with both asthma disease severity and with the expression of Th2 chemokines.67 Following this, a clinical study of allergen-induced asthmatic responses showed that treatment with an anti-TSLP antibody lowered sputum eosinophil counts and FeNO levels and reduced allergen-induced bronchoconstriction.68 In a larger Phase 2 study in asthma, the anti-TSLP antibody tezepelumab was shown to reduce asthma exacerbations and it provided some increase in lung function. Interestingly, these effects were seen even in patients who did not have elevated blood eosinophil levels, suggesting that this treatment modality could be efficacious even in the non-eosinophilic subgroup of patients with uncontrolled asthma.69 More recent work in CRSwNP has shown increased expression and/or activity of TSLP in nasal polyp tissue compared to healthy sinus tissue or that from patients with CRSsNP.44,70 As with IL-33, TSLP may be particularly important to the pathogenesis of AERD, as the nasal polyp tissue from patients with AERD has even higher levels of TSLP protein than does that from patients with aspirin-tolerant CRSwNP, and it may drive PGD2 production from mast cells in these patients.43
Conclusion
CRSwNP is a complex and heterogeneous inflammatory condition for which there is substantial unmet medical need. Both our grasp of the mechanisms that underlie the pathogenesis of the disease, and our understanding of the differing clinical endotypes have improved over the last decade, largely due to extensive translational research efforts in this field. The use of monoclonal antibodies to specifically suppress relevant inflammatory pathways has emerged as a viable means to provide biomarker-driven therapies for these patients. With one biologic agent, dupilumab, already FDA-approved for the treatment of nasal polyposis, and several other biologics currently in clinical trials, there are likely to be promising new therapeutic options for patients with recalcitrant CRSwNP (Table 1). However, these trials will need to be designed from the outset to include careful investigation of surrogate biomarkers of response in order that clinicians can more precisely determine the patient criteria that will best guide future treatment practices.
Key Messages:
Chronic rhinosinusitis represents a heterogenous spectrum of inflammatory diseases of the paranasal sinuses, with many patients failing current standard-of-care treatment.
Type 2 cytokines including interleukin (IL)-4, 5 and 13 have a role in eosinophilic respiratory inflammation in chronic rhinosinusitis with nasal polyps.
Several cytokines, cytokine receptors, and other immunologic effector pathways are targets for currently available treatments and treatments under investigation for nasal polyposis, including IL-4Rα, IL-5, IL-5Rα, IL-33, IgE, and TSLP.
Future studies focused on biomarker-based endotyping and responder analyses will allow for optimization of personalized treatment for chronic rhinosinusitis with nasal polyp patients.
Acknowledgments
Funding: This work was supported by the National Institutes of Health (NIH grant nos R01HL128241 and K23AI139352 and by generous contributions from the Vinik and Kaye Families.
Footnotes
Conflict of Interest: T Laidlaw has served on scientific advisory boards for GlaxoSmithKline, Sanofi-Genzyme, and OptiNose. K Buchheit has served on scientific advisory boards for Genentech, Regeneron, and AstraZeneca.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2015;152(2 Suppl):S1–S39. [DOI] [PubMed] [Google Scholar]
- 2.Halawi AM, Smith SS, Chandra RK. Chronic rhinosinusitis: epidemiology and cost. Allergy and asthma proceedings : the official journal of regional and state allergy societies. 2013;34(4):328–334. [DOI] [PubMed] [Google Scholar]
- 3.Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50(1):1–12. [DOI] [PubMed] [Google Scholar]
- 4.Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1995;113(1):104–109. [DOI] [PubMed] [Google Scholar]
- 5.Steinke JW, Borish L. Chronic rhinosinusitis phenotypes. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2016;117(3):234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferguson BJ, Barnes L, Bernstein JM, et al. Geographic variation in allergic fungal rhinosinusitis. Otolaryngol Clin North Am. 2000;33(2):441–449. [DOI] [PubMed] [Google Scholar]
- 7.Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: A meta-analysis of the literature. The Journal of allergy and clinical immunology. 2015;135(3):676–681 e671. [DOI] [PubMed] [Google Scholar]
- 8.Morse JC, Shilts MH, Ely KA, et al. Patterns of olfactory dysfunction in chronic rhinosinusitis identified by hierarchical cluster analysis and machine learning algorithms. International forum of allergy & rhinology. 2019;9(3):255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith KA, Orlandi RR, Oakley G, Meeks H, Curtin K, Alt JA. Long-term revision rates for endoscopic sinus surgery. International forum of allergy & rhinology. 2019;9(4):402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendelsohn D, Jeremic G, Wright ED, Rotenberg BW. Revision rates after endoscopic sinus surgery: a recurrence analysis. Ann Otol Rhinol Laryngol. 2011;120(3):162–166. [DOI] [PubMed] [Google Scholar]
- 11.Ta V, White AA. Survey-Defined Patient Experiences With Aspirin-Exacerbated Respiratory Disease. J Allergy Clin Immunol Pract. 2015;3(5):711–718. [DOI] [PubMed] [Google Scholar]
- 12.Kiladejo A, Palumbo M, Laidlaw TM. Accidental ingestion of aspirin and nonsteroidal anti-inflammatory drugs is common in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White AA, Stevenson DD. Aspirin-Exacerbated Respiratory Disease. The New England journal of medicine. 2018;379(11):1060–1070. [DOI] [PubMed] [Google Scholar]
- 14.Hamilos DL. Chronic sinusitis. The Journal of allergy and clinical immunology. 2000;106(2):213–227. [DOI] [PubMed] [Google Scholar]
- 15.Hamilos DL. Drivers of chronic rhinosinusitis: Inflammation versus infection. The Journal of allergy and clinical immunology. 2015;136(6):1454–1459. [DOI] [PubMed] [Google Scholar]
- 16.Hamilos DL. Biofilm Formations in Pediatric Respiratory Tract Infection : Part 1: Biofilm Structure, Role of Innate Immunity in Protection Against and Response to Biofilm, Methods of Biofilm Detection, Pediatric Respiratory Tract Diseases Associated with Mucosal Biofilm Formation. Current infectious disease reports. 2019;21(2):6. [DOI] [PubMed] [Google Scholar]
- 17.Hamilos DL. Biofilm Formations in Pediatric Respiratory Tract Infection Part 2: Mucosal Biofilm Formation by Respiratory Pathogens and Current and Future Therapeutic Strategies to Inhibit Biofilm Formation or Eradicate Established Biofilm. Current infectious disease reports. 2019;21(2):8. [DOI] [PubMed] [Google Scholar]
- 18.Cryer J, Schipor I, Perloff JR, Palmer JN. Evidence of bacterial biofilms in human chronic sinusitis. ORL; journal for oto-rhino-laryngology and its related specialties. 2004;66(3):155–158. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson BJ, Stolz DB. Demonstration of biofilm in human bacterial chronic rhinosinusitis. American journal of rhinology. 2005;19(5):452–457. [PubMed] [Google Scholar]
- 20.Harvey RJ, Lund VJ. Biofilms and chronic rhinosinusitis: systematic review of evidence, current concepts and directions for research. Rhinology. 2007;45(1):3–13. [PubMed] [Google Scholar]
- 21.Hochstim CJ, Masood R, Rice DH. Biofilm and persistent inflammation in endoscopic sinus surgery. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2010;143(5):697–698. [DOI] [PubMed] [Google Scholar]
- 22.Perloff JR, Palmer JN. Evidence of bacterial biofilms on frontal recess stents in patients with chronic rhinosinusitis. American journal of rhinology. 2004;18(6):377–380. [PubMed] [Google Scholar]
- 23.Sanclement JA, Webster P, Thomas J, Ramadan HH. Bacterial biofilms in surgical specimens of patients with chronic rhinosinusitis. The Laryngoscope. 2005;115(4):578–582. [DOI] [PubMed] [Google Scholar]
- 24.Singhal D, Psaltis AJ, Foreman A, Wormald PJ. The impact of biofilms on outcomes after endoscopic sinus surgery. American journal of rhinology & allergy. 2010;24(3):169–174. [DOI] [PubMed] [Google Scholar]
- 25.Foreman A, Wormald PJ. Different biofilms, different disease? A clinical outcomes study. The Laryngoscope. 2010;120(8):1701–1706. [DOI] [PubMed] [Google Scholar]
- 26.Van Zele T, Claeys S, Gevaert P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61(11):1280–1289. [DOI] [PubMed] [Google Scholar]
- 27.Baba S, Kondo K, Suzukawa M, Ohta K, Yamasoba T. Distribution, subtype population, and IgE positivity of mast cells in chronic rhinosinusitis with nasal polyps. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2017;119(2):120–128. [DOI] [PubMed] [Google Scholar]
- 28.Stevens WW, Ocampo CJ, Berdnikovs S, et al. Cytokines in Chronic Rhinosinusitis. Role in Eosinophilia and Aspirin-exacerbated Respiratory Disease. American journal of respiratory and critical care medicine. 2015;192(6):682–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poposki JA, Klingler AI, Tan BK, et al. Group 2 innate lymphoid cells are elevated and activated in chronic rhinosinusitis with nasal polyps. Immun Inflamm Dis. 2017;5(3):233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buchheit KM, Dwyer DF, Ordovas-Montanes J, et al. IL-5Rα marks nasal polyp IgG4 and IgE-secreting cells in aspirin-exacerbated respiratory disease. BioRxiv Preprint. 2019:527762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laidlaw TM, Prussin C, Panettieri RA, et al. Dexpramipexole depletes blood and tissue eosinophils in nasal polyps with no change in polyp size. Laryngoscope. 2018. [DOI] [PubMed] [Google Scholar]
- 32.Gevaert P, Van Bruaene N, Cattaert T, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. The Journal of allergy and clinical immunology. 2011;128(5):989–995 e981–988. [DOI] [PubMed] [Google Scholar]
- 33.Tan BK, Li QZ, Suh L, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. The Journal of allergy and clinical immunology. 2011;128(6):1198–1206 e1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hulse KE, Norton JE, Suh L, et al. Chronic rhinosinusitis with nasal polyps is characterized by B-cell inflammation and EBV-induced protein 2 expression. The Journal of allergy and clinical immunology. 2013;131(4):1075–1083, 1083 e1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Schryver E, Devuyst L, Derycke L, et al. Local immunoglobulin e in the nasal mucosa: clinical implications. Allergy Asthma Immunol Res. 2015;7(4):321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vizuete J, Picado C, Plaza V, Rodrigo G, Juliá B, Mullol J. IgE plasma level in asthmatics with nasal polyps is independent of atopy. European Respiratory Journal. 2016;48. [Google Scholar]
- 37.Ordovas-Montanes J, Dwyer DF, Nyquist SK, et al. Allergic inflammatory memory in human respiratory epithelial progenitor cells. Nature. 2018;560(7720):649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Li YY, Andiappan AK, et al. Role of IL-13Ralpha2 in modulating IL-13-induced MUC5AC and ciliary changes in healthy and CRSwNP mucosa. Allergy. 2018;73(8):1673–1685. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Derycke L, Holtappels G, et al. Th2 cytokines orchestrate the secretion of MUC5AC and MUC5B in IL-5-positive chronic rhinosinusitis with nasal polyps. Allergy. 2019;74(1):131–140. [DOI] [PubMed] [Google Scholar]
- 40.Wise SK, Laury AM, Katz EH, Den Beste KA, Parkos CA, Nusrat A. Interleukin-4 and interleukin-13 compromise the sinonasal epithelial barrier and perturb intercellular junction protein expression. International forum of allergy & rhinology. 2014;4(5):361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu T, Kanaoka Y, Barrett NA, et al. Aspirin-Exacerbated Respiratory Disease Involves a Cysteinyl Leukotriene-Driven IL-33-Mediated Mast Cell Activation Pathway. Journal of immunology. 2015;195(8):3537–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim DK, Jin HR, Eun KM, et al. The role of interleukin-33 in chronic rhinosinusitis. Thorax. 2017;72(7):635–645. [DOI] [PubMed] [Google Scholar]
- 43.Buchheit KM, Cahill KN, Katz HR, et al. Thymic stromal lymphopoietin controls prostaglandin D2 generation in patients with aspirin-exacerbated respiratory disease. The Journal of allergy and clinical immunology. 2016;137(5):1566–1576 e1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagarkar DR, Poposki JA, Tan BK, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. The Journal of allergy and clinical immunology. 2013;132(3):593–600 e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kato A Immunopathology of chronic rhinosinusitis. Allergology international : official journal of the Japanese Society of Allergology. 2015;64(2):121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wen W, Liu W, Zhang L, et al. Increased neutrophilia in nasal polyps reduces the response to oral corticosteroid therapy. The Journal of allergy and clinical immunology. 2012;129(6):1522–1528 e1525. [DOI] [PubMed] [Google Scholar]
- 47.Bachert C, Mannent L, Naclerio RM, et al. Effect of Subcutaneous Dupilumab on Nasal Polyp Burden in Patients With Chronic Sinusitis and Nasal Polyposis: A Randomized Clinical Trial. JAMA. 2016;315(5):469–479. [DOI] [PubMed] [Google Scholar]
- 48.Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019. [DOI] [PubMed] [Google Scholar]
- 49.Laidlaw TM, Mullol J, Fan C, et al. Dupilumab improves nasal polyp burden and asthma control in patients with CRSwNP and AERD. J Allergy Clin Immunol Pract. 2019;7(7):2462–2465 e2461. [DOI] [PubMed] [Google Scholar]
- 50.Vennera Mdel C, Picado C, Mullol J, Alobid I, Bernal-Sprekelsen M. Efficacy of omalizumab in the treatment of nasal polyps. Thorax. 2011;66(9):824–825. [DOI] [PubMed] [Google Scholar]
- 51.Gevaert P, Calus L, Van Zele T, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. The Journal of allergy and clinical immunology. 2013;131(1):110–116 e111. [DOI] [PubMed] [Google Scholar]
- 52.Hayashi H, Mitsui C, Nakatani E, et al. Omalizumab reduces cysteinyl leukotriene and 9alpha,11beta-prostaglandin F2 overproduction in aspirin-exacerbated respiratory disease. The Journal of allergy and clinical immunology. 2016;137(5):1585–1587 e1584. [DOI] [PubMed] [Google Scholar]
- 53.Lang DM, Aronica MA, Maierson ES, Wang XF, Vasas DC, Hazen SL. Omalizumab can inhibit respiratory reaction during aspirin desensitization. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2018;121(1):98–104. [DOI] [PubMed] [Google Scholar]
- 54.Bachert C, Sousa AR, Lund VJ, et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: Randomized trial. The Journal of allergy and clinical immunology. 2017;140(4):1024–1031 e1014. [DOI] [PubMed] [Google Scholar]
- 55.Tuttle KL, Buchheit KM, Laidlaw TM, Cahill KN. A retrospective analysis of mepolizumab in subjects with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract. 2018;6(3):1045–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gevaert P, Lang-Loidolt D, Lackner A, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. The Journal of allergy and clinical immunology. 2006;118(5):1133–1141. [DOI] [PubMed] [Google Scholar]
- 57.Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–2127. [DOI] [PubMed] [Google Scholar]
- 58.FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141. [DOI] [PubMed] [Google Scholar]
- 59.Harrison T, Werkstrom V, Wu Y, Gopalan G, Zangrilli J. Clinical Efficacy of Benralizumab in Patients With Severe, Uncontrolled Eosinophilic Asthma and Nasal Polyposis: Pooled Analysis of the SIROCCO and CALIMA Trials. Journal of Allergy and Clinical Immunology. 2018;141(2):AB12. [Google Scholar]
- 60.Stolarski B, Kurowska-Stolarska M, Kewin P, Xu D, Liew FY. IL-33 exacerbates eosinophil-mediated airway inflammation. Journal of immunology. 2010;185(6):3472–3480. [DOI] [PubMed] [Google Scholar]
- 61.Ishinaga H, Kitano M, Toda M, et al. Interleukin-33 induces mucin gene expression and goblet cell hyperplasia in human nasal epithelial cells. Cytokine. 2017;90:60–65. [DOI] [PubMed] [Google Scholar]
- 62.Werder RB, Zhang V, Lynch JP, et al. Chronic IL-33 expression predisposes to virus-induced asthma exacerbations by increasing type 2 inflammation and dampening antiviral immunity. The Journal of allergy and clinical immunology. 2018;141(5):1607–1619 e1609. [DOI] [PubMed] [Google Scholar]
- 63.Ravanetti L, Dijkhuis A, Dekker T, et al. IL-33 drives influenza-induced asthma exacerbations by halting innate and adaptive antiviral immunity. The Journal of allergy and clinical immunology. 2019;143(4):1355–1370 e1316. [DOI] [PubMed] [Google Scholar]
- 64.Shaw JL, Fakhri S, Citardi MJ, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. American journal of respiratory and critical care medicine. 2013;188(4):432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.PRNewswire. Regeneron and Sanofi Announce Positive Topline Phase 2 Results for IL-33 Antibody in Asthma. https://www.prnewswire.com/news-releases/regeneron-and-sanofi-announce-positive-topline-phase-2-results-for-il-33-antibody-in-asthma-300872459.html. Published 2019. Accessed.
- 66.AnaptysBio. AnaptysBio presents updated data from etokimab Phase 2a proof-of-concept clinical trial in severe eosinophilic asthma https://ir.anaptysbio.com/news-releases/news-release-details/anaptysbio-presents-updated-data-etokimab-phase-2a-proof-concept. Published 2019. Accessed.
- 67.Ying S, O’Connor B, Ratoff J, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. Journal of immunology. 2005;174(12):8183–8190. [DOI] [PubMed] [Google Scholar]
- 68.Gauvreau GM, O’Byrne PM, Boulet LP, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. The New England journal of medicine. 2014;370(22):2102–2110. [DOI] [PubMed] [Google Scholar]
- 69.Corren J, Parnes JR, Wang L, et al. Tezepelumab in Adults with Uncontrolled Asthma. The New England journal of medicine. 2017;377(10):936–946. [DOI] [PubMed] [Google Scholar]
- 70.Kimura S, Pawankar R, Mori S, et al. Increased expression and role of thymic stromal lymphopoietin in nasal polyposis. Allergy Asthma Immunol Res. 2011;3(3):186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]


