Abstract
Non-steroidal anti-inflammatory drugs (NSAIDs) are the first line pharmacotherapy for patients with axial spondyloarthritis (axSpA). In the recent years, treatment options have expanded with the availability of biologics, including tumor necrosis factor inhibitors (TNFi) and IL-17 inhibitors. However, a treatment strategy that clearly prevents syndesmophyte formation has not been established. Observational studies of patients with ankylosing spondylitis indicated potential disease modifying effects of NSAIDs, but two randomized trials came to different conclusions. More broadly, whether any of the currently available medications for axSpA have an effect on spine radiographic progression, beyond symptom control, remains inconclusive. In this paper, we will review the clinical studies of NSAIDs and biologics on disease modification of axSpA, examine genetic, animal and clinical evidence of NSAID effects on bone formation, and discuss how future studies may investigate the question of disease modification in axSpA.
Introduction
Axial spondyloarthritis (axSpA) is a chronic inflammatory spine condition with a prevalence of 0.9 – 1.4% in the adult population (1). Patients with ankylosing spondylitis (AS), also called radiographic axSpA (r-axSpA), the prototypic form of axSpA, may develop features of new bone formation, such as ankylosis of the sacroiliac joints, syndesmophytes and even fusion of spine (2, 3). Pharmacotherapy for axSpA has significantly broadened beyond non-steroidal anti-inflammatory drugs (NSAIDs) in the past two decades with the availability of biologics, including tumor necrosis factor inhibitors (TNFi) and interleukin-17 (IL-17) inhibitors, and more recently, Janus kinase inhibitors (4).
Despite these new treatments, NSAIDs remain as the first line and cornerstone for management of axSpA, including patients with early disease and patients with well-established AS. In observational cohorts of early axSpA, 73.0 – 92.8% of patients took NSAIDs (5) (6). Similarly, in a prospective cohort of established AS, 70.3% patients were using NSAIDs (7). These frequencies are not surprising given the efficacy of NSAIDs, as shown in many clinical trials. In a recent trial of full dose naproxen in patients with early axSpA with active inflammation of sacroiliac joints, 56.9% patients achieved a moderate response measured by Assessment in Spondyloarthritis International Society 40% response (ASAS40, Table 1 (8)), and 35.3% were in ASAS partial remission (Table 1), after 28 weeks of treatment (9). In an open label study of NSAIDs in patients with axSpA (including both radiographic and nonradiographic axSpA), 35% of participants achieved an ASAS40 response after 4-weeks of NSAID treatment (10). In comparison, in the major phase 3 clinical trials of biologics (TNFi and IL-17 inhibitors) in AS or r-axSpA, 39.4% to 58.1% participants achieved ASAS40 at 12 weeks to 24 weeks (11,12), indicating that the rest of participants, at least 40 – 60%, needed to optimize their NSAIDs use in addition to the study drugs or try a different biologic. Although these results were extracted from different studies, and cannot be compared directly, they support the notion that, for short-term symptom relief, NSAIDs are not only effective as a first line treatment, but are also important as combination therapy with biologics.
Table 1.
Major outcome measures in clinical research of ankylosing spondylitis or radiographic axial spondyloarthritis.
| Measures | Description |
|---|---|
| ASDAS (8) | A composite score, including assessment of total back pain, patient global of disease activity, peripheral pain and swelling, duration of morning stiffness, and C-reactive protein or erythrocyte sedimentation rate. |
| BASDAI (8) | A six-question, self-administered questionnaire, assessing fatigue, spinal pain, peripheral arthritis, enthesitis, intensity and duration of morning stiffness |
| ASAS40 response criteria (8) | On a scale of 10, improvement of >= 40% and >=2 units in at least three of the four domains (patient global, pain, function, inflammation*), and no worsening in any scores. |
| ASAS partial remission (8) | On a scale of 10, the score in each domain (patient global, pain, function and inflammation*) not above 2 units. |
ASDAS: ankylosing spondylitis disease activity score; BASDAI: Bath ankylosing spondylitis disease activity index; ASAS: Assessment of SpondyloArthritis International Society.
average score of severity and duration of morning stiffness in BASDAI.
It is less clear, however, whether NSAIDs slow disease progression in patients with axSpA. In an early retrospective cohort of 40 patients with AS, continuous use of phenylbutazone delayed or arrested radiographic progression, compared to patients who did not use phenylbutazone or used it only intermittently (13). Two randomized trials examined the effects of continuous use versus on-demand use of NSAIDs on disease progression in AS, and came to opposite conclusions (14,15). The uncertainty prompted us to examine the clinical studies of disease modification in AS and the state of knowledge of NSAID effects on bone growth in general, and in axSpA in particular. In this paper, we will review the clinical evidence for NSAID and biologic effects on radiographic progression in patients with AS, examine in vitro and in vivo evidence of NSAID effects on bone formation, and discuss how future studies may evaluate disease modification in axSpA. Biomarkers for radiographic progression in axSpA theoretically may be used as surrogate endpoints for disease modification in clinical studies, however, it is a broad topic in itself, and will not be discussed in this review.
Background
Measurement of disease progression in AS
Syndesmophyte formation and ankylosis of the spine are the key features of AS, and are associated with long-term functional impairment; thus, disease modification to slow or stop syndesmophyte growth is an important goal. The modified Stoke AS Spine Score (mSASSS) has been widely used to describe syndesmophyte growth and radiographic progression. mSASSS is a semi-quantitative scoring system based on features of the anterior vertebral corners on lateral projections of cervical and lumbar spine radiographs. Each of the 24 corners (12 in the cervical spine and 12 in the lumbar spine) is graded as 0 to 3, with 0 being normal, 1 being erosion, sclerosis or squaring, 2 being syndesmophyte, and 3 being bony bridging between adjacent vertebrae, for a total possible score of 0–72 (16). Radiographic progression is usually defined as mSASSS increase of 2 or more units; alternatively, mSASSS absolute change from baseline has been commonly used as a radiographic endpoint (16). When assessed longitudinally, in 2 years, 30 – 40% of patients demonstrate any increase in mSASSS, and about 20% of patients have an increase of mSASSS of 2 or more (16–19). The rate of mSASSS change ranges from about 1.0 unit in two years to 0.98 unit/year (17, 18, 21). The spine has been used as the preferred site to assess radiographic progression, rather than the sacroiliac joints, because it provides a greater range and most patients are eligible to demonstrate change, while changes in joint space or fusion of the SI joints are difficult to detect.
Although the mSASSS has been shown to have face and construct validity, its reliability and sensitivity to change have been challenged. When assessing progression over 2 years, two readers were in agreement in only 54% cases (18). Inter-reader reliability of mSASSS change over 2 years was poor to moderate, ranging from 0.17 to 0.67 (19,20,22,23). Assessing the films in chronological sequence also affects the reading, and results in higher apparent progression (17). In addition, it was estimated that in a 2-year randomized controlled trial, a sample size of 100 in each arm would be needed to detect a difference between arms (24), reflecting the slow progression of the disease and relative insensitivity to change of the method.
Risk factors for disease progression in AS
Using mSASSS as the measure, several risk factors have been associated with disease progression in longitudinal studies, including presence of syndesmophytes at baseline (25–28), elevated inflammatory markers (25,27), smoking (25–27), low bone mineral density (26), and high disease activity measured by the Ankylosing Spondylitis Disease Activity Score (ASDAS, Table 1) (29). In addition, mechanical stress has been associated with worse radiographic outcomes, both in AS cohorts and animal studies (30–32). Certain occupational and physical activities, such as bending, twisting and stretching, as well as exposure to whole body vibration, were associated with worse radiographic outcomes, and physically demanding jobs seem to amplify the radiographic progression (30).
Heterogeneity in syndesmophyte growth
Increasing evidence suggests that radiographic progression and syndesmophyte growth in AS is a highly heterogeneous process, both temporally and spatially. In a study that evaluated 12-year radiographic progression in patients with AS, new syndesmophytes, detected based on mSASSS change, were observed in about 60% of patients and 40% of time intervals. At the same time, in about 24% of patients and 40% of time intervals, no radiographic progression was observed, indicating high variability in individual patients, and at different time intervals (21). At the syndesmophyte level, within the same intervertebral disk spaces, some syndesmophytes were seen to grow substantially while others did not grow, suggesting that local factors, possibly including mechanical forces and local pro- and anti-proliferation factors, influence syndesmophyte growth (33). This heterogeneity adds further challenges to studying radiographic progression in AS.
Long-term effect of NSAIDs and Biologics on Disease Modification
Observational studies and clinical trials of a disease modification effect of NSAIDs
As there are more than 20 different NSAIDs used in various dosages, an NSAIDs intake index has been developed to quantify the dosage in equivalency and duration of NSAIDs use, with a range of 0 – 100 (34). For example, daily NSAID use equivalent to 150mg diclofenac over the whole study period is scored as 100. Using this index, in the German Spondyloarthritis Inception Cohort (GEPSIC), the odds of mSASSS increase over 2 years was much less (odds ratio = 0.15, 95% confidence interval (CI) 0.02 – 0.96) in patients with high NSAID intake (index >= 50) compared to those with low NSAID intake (index < 50) (total n=88) (35). The results support the hypothesis that NSAIDs have a protective effect on spine radiographic progression in patients with AS. In this study, a similar protective effect was not observed between patients with high versus low AS activity measured by Bath AS Disease Activity Index (BASDAI, Table 1) (35), suggesting that subjective symptoms may not be directly associated with radiographic progression, or that the mechanisms by which NSAIDs may act on progression are other than symptom control.
Two separate randomized trials, one with the COX-2 selective NSAID celecoxib, and the other with the non-selective NSAID diclofenac, examined the efficacy of NSAIDs on radiographic progression (Table 2). In the celecoxib trial, TNFi naïve patients with AS were randomized to continuous use vs. on-demand use of celecoxib 100mg twice daily or higher. After 2 years, patients in the continuous use group had less radiographic progression compared to on-demand group (p=0.002) (14). Post hoc analysis of this trial showed that slowing of progression with continuous treatment was greater in patients with elevated inflammatory markers (erythrocyte sedimentation rate or CRP) (36). The diclofenac trial (Effects of NSAIDs on RAdiographic Damage in Ankylosing Spondylitis (ENRADAS)) used a similar design, in that TNFi naïve patients with AS were randomized to continuous use vs. on-demand use of diclofenac 150mg daily for two years. However, in contrast to the findings in celecoxib study, the continuous group had more progression numerically over two years (p = 0.39). Findings were similar in subgroups of patients with or without syndesmophytes at baseline and in those with or without elevated CRPs (15). The authors stated that despite the fact that “not even a trend for less radiographic progression was seen for the continuous group in our study, it is rather unlikely that inclusion of more patients would have changed the result.”
Table 2.
Comparison of studies on radiographic progression in patients with ankylosing spondylitis.
| Author, year | Study groups | Number of Participants | Study Length | Baseline mSASSS, mean (SD) | Smoking status (%) | Male (%) | HLA-B27 positivity (%) | mSASSS change, mean (SD) |
|---|---|---|---|---|---|---|---|---|
| NSAIDs [Randomized controlled Trials] | ||||||||
| Wanders, 2005 (14) | Continuous celecoxib | 76 | 2 years | 7.9 (14.7) | NR | 66 | 88 | 0.4 (1.7) |
| On-demand celecoxib | 74 | 2 years | 9.3 (15.2) | NR | 70 | 88 | 1.5 (2.5) | |
| Sieper, 2015 (15) | Continuous diclofenac | 62 | 2 years | 10.9 (15.5) | 59* | 71.0 | 88.7 | 1.3 (0.7–1.9) |
| On-demand diclofenac | 60 | 2 years | 16.4 (18.2) | 33* | 66.7 | 91.7 | 0.8 (0.2–1.4) | |
| TNFi [Retrospective analyses of clinical trials/long-term extension of clinical trial data] | ||||||||
| Baraliakos, 2005 (37) | Infliximab | 41 | 2 years | 12.1 | NR | 63 | 90 | 0.4(2.7) |
| GESPIC cohort | 41 | 2 years | 5.9 | NR | 71 | 85 | 0.7(2.8) | |
| van der Heijde, 2008 (19) | Infliximab | 201 | 2 years | 17.7 (17.9) | NR | 78.1 | 86.5 | 0.9 (2.6) |
| OASIS | 192 | 2 years | 15.8 (18.1) | NR | 67.7 | 84.4 | 1.0 (3.2) | |
| van der Heijde, 2008 (38) | Etanercept | 257 | 2 years | 16 (18.3) | NR | 75.5 | 78.2 | 0.91 (2.45) |
| OASIS | 175 | 2 years | 14 (17.6) | NR | 69.1 | 71.1 | 0.95 (3.18) | |
| van der Heijde, 2009 (20) | Adalimumab | 307 | 2 years | 19.8 (19.3) | NR | 76.5 | NR | 0.8 (2.6) |
| OASIS | 169 | 2 years | 15.8 (17.6) | NR | 69.2 | NR | 0.9 (3.3) | |
| Braun, 2014 (39) | Placebo -> Golimumab 50mg | 66 | 208 weeks | 16.1 (18.7) | NR | NR | NR | 2.1 (5.2) |
| Golimumab 50mg | 111 | 208 weeks | 11.7 (16.4) | NR | NR | NR | 1.3 (4.1) | |
| Golimumab 100mg | 112 | 208 weeks | 13.5 (18.9) | NR | NR | NR | 2.0 (5.6) | |
| IL-17A inhibitor [Retrospective analyses of clinical trials/long-term extension of clinical trial data] | ||||||||
| Braun, 2018 (40) | Secukinumab 75mg | 61 | 208 weeks | 10.7 (17.82) | 39.3 | 87.0 | NR | 1.6 (5.67) |
| Secukinumab 75mg -> 150mg | 23 | 208 weeks | 34.8 | 70.5 | NR | 1.8 (4.32) | ||
| Secukinumab 150mg | 71 | 208 weeks | 8.6 (16.23) | 29.6 | 63.4 | NR | 1.2 (3.91) | |
| Braun, 2019 (23) | Secukinumab | 168 | 2 years | NR | 25 | 73.2 | 82.9 | 60.7% * |
| ENRADAS cohort | 69 | 2 years | NR | 44.9 | 66.7 | 88.4 | 52.2% * p = 0.2430 |
|
NSAID: non-steroidal anti-inflammatory drugs; TNFi: tumor necrosis factor inhibitor; IL-17A: interleukin-17A; GESPIC: German Spondyloarthritis Inception Cohort; OASIS: Outcomes in Ankylosing Spondylitis International Study; ENRADAS: Effects of NSAIDs on Radiographic Damage in Ankylosing Spondylitis; SD: standard deviation; NR: not reported.
Proportion of patients with no radiographic progression (least squares mean change of mSASSS <= 0)
The results from both studies might suggest that only COX-2 selective NSAIDs have a disease modification effect, but several caveats should be considered. First, absence of a dose effect makes the trial results difficult to interpret. When using mSASSS change as the study outcome, at least two years are proposed to detect a significant change with a sample size of 100 patients in each treatment arm. Because it would be unethical to conduct placebo-controlled trials lasting 2 years, both NSAIDs trials compared continuous use versus on-demand use, to approximate the ideal placebo-controlled study, with the intention to see whether the difference in NSAID intake between the two groups was correlated with the difference in mSASSS increase. The result from the celecoxib trial did show a lower rate of mSASSS increase with continuous use, however, a dose effect of NSAIDs was not demonstrated. The average dose in the continuous group (243mg) was only modestly higher than the on-demand group (201mg), despite the significant difference in the outcome (mean mSASSS change +0.4 in the continuous group vs. +1.5 in the on-demand group). The diclofenac trial groups had a difference in NSAID dose, with NSAIDs indices of 77 in the continuous group vs. 44 in the on-demand group, but did not show a corresponding decrease in radiographic progression (mean mSASSS change +1.3 in the continuous group vs. +0.8 in the on-demand group).
Further, in the event of imbalance in randomization, risk factors that are associated with spine radiographic progression could lead to bias when assessing treatment effects. For example, in the diclofenac study, the continuous use group had a significantly higher proportion of current smokers at baseline, compared to the on-demand group. Whether the difference in smoking between the groups was enough to overwhelm a potential inhibitory effect of continuous diclofenac use is unclear (15).
A third clinical trial (CONSUL trial, NCT02758782), which evaluates the effect of celecoxib with golimumab compared to golimumab alone on radiographic progression in patients with AS, is ongoing.
Disease modification effects of biologics
With regard to biologics, two strategies have been taken to retrospectively analyze radiographic data from long-term extensions of clinical trials. The first strategy was to compare the radiographic progression among participants in biologic trials to that of biologic-naïve, historical cohorts (Table 2) (19,20,23,37,38). Most of these studies used mSASSS change over 2 years as the primary radiographic endpoint, and did not find any significant difference in radiographic progression between groups. One of the studies, secukinumab vs. ENRADAS cohort used the proportion of patients with no radiographic progression (defined as least square mean change of mSASSS <= 0) as the endpoint, and reported a suggestion toward more non-progressors in the secukimunab group (60.7% vs 52.2%, p = 0.2430). Notably, the inter-reader agreement for mSASSS change of this study was poor (k = 0.17), and ENRADAS cohort had a much higher percentage of smokers (23).
The second strategy, similar to the NSAIDs trials, was to compare different dosing regimens with the question of whether there was a dose effect (Table 2) (39,40). However, this approach has not shown associations between the dose of biologics and progression. In the 4-year secukinumab study (Braun 2018), although the 150mg group had marginally less radiographic progression than the 75mg groups by mSASSS, the 75mg groups had higher mSASSS at baseline, which is a risk factor for radiographic progression (40). In addition, similar proportions of patients in each dosing group (78.9% vs. 78.6%) had no radiographic progression (mSASSS change <= 2)., in the open label extension of Certolizumab study, 80.6% patients had no radiographic progression at 4 years, although there was no comparison group (41).
Two observational studies examined the effect of TNFi on spine radiographic progression, with somewhat conflicting results. In a prospective cohort of 334 patients with AS in North America, after adjustment for baseline mSASSS and propensity to receive TNFi, patients who were on a TNFi had a 50% lower odds of progression compared to those who never received TNFi. Also, patients who took TNFi for a larger proportion of their disease course had less mSASSS progression (42). In contrast, in a recent observational study of 432 patients with AS from the Swiss Clinical Quality Management Cohort, no contemporaneous association between TNFi use and radiographic progression (43). Instead, treatment with TNFi prior to the radiographic interval was protective, as was longer duration of prior use of TNFi. The data did not detect an association with TNFi during the radiographic interval, perhaps indicating that prolonged treatment is needed to see an effect (43).
In summary, current evidence for a disease-modifying effect of biologics, including TNFi and IL-17 inhibitors, is lacking; and the effect of the different classes of biologics on radiographic progression has not been compared directly. A direct comparison of secukinumab to an adalimumab biosimilar on radiographic progression is on-going ( NCT03259074).
NSAIDs and Bone Formation
The inconclusive results from AS clinical studies of the effect of NSAIDs on radiographic disease progression prompts a review of pre-clinical, biological evidence that would support an inhibitory effect of NSAIDs on syndesmophyte formation in AS.
At the genetic level, in an experiment-wide genetic association study that examined genes related to radiographic severity in AS, a single nucleotide polymorphism (SNP) rs1236913 was found to have a protective association with the degree of radiographic damage (44). This SNP lies in the PTGS-1 gene, encoding Prostaglandin-Endoperoxide Synthase 1, also known as COX-1. Although extensive functional studies are not available, the association at least suggests that COX-1 might be involved in radiographic progression in AS.
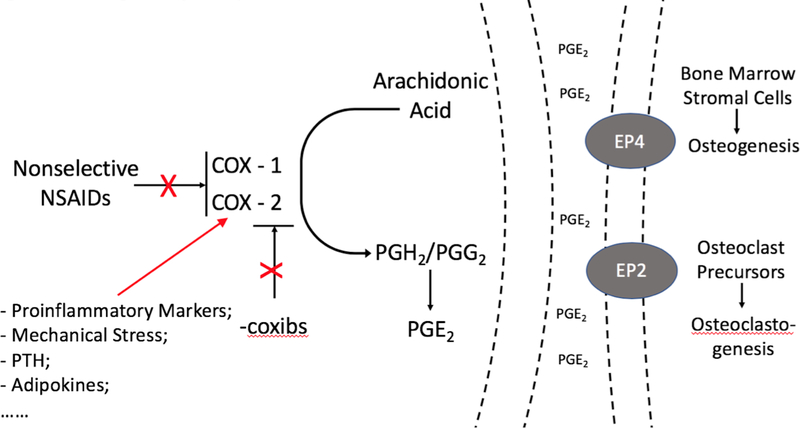
NSAIDs are COX inhibitors, blocking the synthesis of prostaglandin (PG) G2/H2 from arachidonic acid, the main precursor of prostanoids. Arachidonic acid is first hydrolyzed by secretory or cytoplasmic phospholipase A2, then oxygenated to PGG2/H2 by COX, which is then further converted to PGD2, PGE2, PGF2, PGI2 or thromboxane A2 by different synthases (45). Among them, PGE2 is the most studied prostanoid involved in inflammation and bone formation. Local administration of PGE2 into long bones in rats stimulates bone formation by increasing osteoblast number and activity, and systemic administration of PGE2 has been shown to increase the osteogenic capacity of bone marrow in ex vivo culture systems (46, 47). However, in some experimental systems, PGE2 is also a potent stimulator of bone resorption, by inducing receptor activator of nuclear factor kappa B ligand (RANKL) expression in primary osteoblastic cell cultures via the EP2/EP4 receptor (48,49). Figure 1 illustrates the prostaglandin pathway and effects on bone metabolism.
Figure 1. Prostaglandin pathway and bone metabolism.
PG: prostaglandin; COX: cyclooxygenase; NSAIDs: non-steroidal anti-inflammatory drugs; EP2: prostaglandin E receptor 2; EP4: prostaglandin E receptor 4; PTH: parathyroid hormone.
Prostaglandin pathway and bone metabolism: In vitro evidence
Two isoenzymes, COX-1 and COX-2, are traditionally considered the main rate-limiting enzymes in the generation of PGE2. COX-1 is constitutively expressed, while COX-2 expression is induced under certain conditions. Proinflammatory cytokines, including interleukin-1 (IL-1), TNF, and IL-17, have been shown to induce COX-2 expression and PGE2 production in bone marrow cultures and osteoclast precursors, osteoblastic cells, and synoviocytes (50–52). In addition, mechanical loading of human osteoblastic cell line and primary bone cell cultures derived from the iliac crest triggered expression of COX-2 and prostaglandin synthesis, and induced bone nodule formation (53, 54). In human periodontal ligament cells, cyclic tension force increased PGE2 expression as well as RANKL mRNA expression, but not osteoprotegerin expression, in a COX-2 dependent manner, suggesting a potential for increased osteoclastogenesis (55, 56). However, as the skeletal system constantly undergoes remodeling, and COX-2, triggered by proinflammatory cytokines and mechanical force, regulates PGE2 expression in osteoblasts as well as osteoclasts, these in vitro experiments did not address the net effect of these factors on osteogenesis and osteoclastogenesis.
Cyclooxygenases inhibition and bone formation: In vivo evidence
COX-1−/− and COX-2−/− mice are useful tools to study the net effect of inhibition of COX on bone formation. Using fracture healing models in these mice, COX-2 was shown to be critical for fracture healing, but COX-1 was not (57, 58). Consistent with these findings, both non-selective COX inhibitors, e.g. diclofenac, indomethacin, ketorolac, and selective COX-2 inhibitors, e.g. celecoxib, rofecoxib, valdecoxib, exerted a delayed or inhibitory effect on fracture healing in rat or rabbit models when given over 4–10 weeks (58 – 63). Interestingly, when treated with one to two weeks of COX-selective NSAIDs (rofecoxib, valdecoxib), or one week of diclofenac, the inhibitory effect of NSAIDs on bone growth was either reversible or less profound (64–66), suggesting a temporal effect of NSAIDs on bone formation.
The effect of NSAIDs on fracture healing in humans has been assessed in randomized controlled trials, case control studies and cohort studies, with somewhat different conclusions (table 3). Dodwell et al reported an increased risk of nonunion among NSAID- treated patients (odds ratio 3.0, 95% CI 1.6 – 5.6) in the pooled effect of all of the 11 studies, but no effect was observed when only including seven high-quality studies (67). Another meta-analysis by Wheatley et al also showed that NSAID use was associated with an increased risk of nonunion or delayed union (odds ratio 2.07, 95% CI 1.19 – 3.61), but similar to the temporal effect observed in the animal studies, no association was found in studies with a short duration of NSAID treatment (68). In addition, no association was observed in studies with low dose NSAIDs or in the pediatric groups (68). Neither of these two meta-analyses examined the difference in effects of COX-2 selective versus non-selective NSAIDs.
Table 3.
Meta-analyses of NSAIDs effect on fracture healing and heterotopic ossification.
| Study | Condition | Study type | Length of follow up | Type of NSAIDs | number of studies and participants | Result |
|---|---|---|---|---|---|---|
| Dodwellet al, 2010 [67] | Fracture Healing | Mostly retrospective cohort; one prospective cohort study | 5 month to 3.8 years | Diclofenac, indomethacin, ibuprofen, ketorolac, and not defined. | 11 studies/2067 patients exposed to NSAIDs | Increased risk for non-union: OR = 3.0 (1.6 – 5.6); in 7 high-quality studies, no statistical significant risk, OR = 2.2 (0.8 – 6.3). |
| Wheatly, 2018 [68] | Fracture Healing | RCTs, cohort studies, and case-control studies | > 6 months | All type of NSAIDs | 16 studies/3283 exposed bones to NSAIDs | Increased risk for delayed union or nonunion: OR = 2.07 (1.19 to 3.61). No risk for low dose (< 125mg/d diclofenac, 150mg/d of indomethacin or 120mg/d ketorolac) or shorter duration (< 1 week): OR = 1.68 (0.63 to 4.46) |
| Ma, 2018 [69] | Heterotopic Ossification | RCTs | 1.5 to 12 months | Naproxen vs. Placebo | 4 RCTs/total of 269 patients | Decreased risk for HO: RR = 0.21 (0.12 to 0.35) at 12 months. |
| Joice, 2018 [70] | Heterotopic Ossification | RCTs | 3 – 24 months | Non-selective NSAIDs vs. Placebo | 17 RCTs/ total of 4979 patients | Decreased risk for HO: Log OR = −1.35 (−1.83 to −0.86) |
| Selective NSAIDs vs. Placebo | 5 RCTs/ total of 628 patients | Decreased risk for HO: Log OR = −1.58 (−2.41 to −0.75) | ||||
| Non-selective vs. selective NSAIDs | 7 RCTs/ total of 1096 patients | No difference in risk for HO: Log OR = 0.22 (−0.36 to 0.79) |
NSAIDs: non-steroidal anti-inflammatory drugs; OR: odds ratio; RCT: randomized controlled trial; RR: relative risk.
NSAID effects on bone formation have also been examined in the prevention of heterotopic ossification (HO), an abnormal localized growth of bone in muscles and tendons. Systematic reviews and meta-analysis of RCTs (table 3) have shown that post-operative use of NSAIDs, including indomethacin, naproxen, as well as COX-2 selective NSAIDs, was effective in preventing severe HO after total hip arthroplasty (69,70). In the most recent meta-analysis of NSAID effects on HO prevention, the effect of non-selective and COX-2 selective NSAIDs were directly compared, based on 7 RCTs and 1096 participants, and no difference was found between these classes in preventing post-operative HO (70).
The animal studies and clinical evidence from studies of fracture healing and HO formation support the notion that NSAIDs have an inhibitory effect on new bone formation, with no difference between selective and non-selective NSAIDs, at least as clinically evident in humans. A temporal effect of NSAIDs on bone formation has been suggested in animal and human fracture healing studies, i.e. that short duration of NSAID use had less profound or reversible effect on bone growth, but definitive proof is needed.
Future studies on disease modification in patients with axSpA
Whether NSAIDs or biologics have a disease modification effect, or more fundamentally, whether suppressing inflammation is sufficient to prevent bone formation in AS, remains unclear. Further studies on disease modification are needed to identify treatment strategies that effectively prevent radiographic progression with the least side effects. How can we improve future studies?
Radiographic outcome measures: new imaging modalities
In recent years, three-dimensional imaging modalities, including full-dose computed tomography (CT) and low dose CT (ldCT) of spine, have been evaluated to improve the measurement of radiographic progression of AS (Table 4) (71,72). The thoracic spine has been omitted from radiographic studies because of the difficulty in seeing vertebral changes on conventional radiographs. In contrast, CT scan methods provide a 360-degree evaluation of the entire vertebral body, and a better visualization of thoracic spine. Using CT scanning, it has been shown that syndesmophytes develop more commonly at thoracolumbar junction and thoracic spine, rather than lumbar spine, offering the potential to detect more patients with abnormalities (73,74). In the full dose CT scan method, syndesmophyte volume was directly quantified and compared over time using computer algorithm (75). In the ldCT method, a CT Syndesmophyte Score (CTSS) has been developed to measure the radiographic damage in the entire spine by human readers, with moderate inter-reader ICC for change score (74). Both CT methods have shown to be more sensitive to change than the mSASSS (74,75), which makes it possible to detect a treatment effect in a clinical trial with fewer participants and/or shorter study length. Notedly, the full dose CT scan method has a radiation exposure of 8 millisievert per scan, which is comparable to 3 years of natural background radiation, and about one third of a PET/CT scan, while the ldCT has a radiation exposure of 4 millisievert per scan. To date, no RCTs data have been reported using these new measurements for disease modification effects.
Table 4.
Comparison of radiographic measures for new bone formation in ankylosing spondylitis, or radiographic axial spondyloarthritis.**
| mSASSS | CTSS | Quantitative Syndesmophyte height volume | |
|---|---|---|---|
| Imaging modality | spine radiograph | low dose CT | full dose CT |
| Spine segments | cervical and lumbar spine | cervical, thoracic and lumbar spine | thoracic and lumbar spine |
| Number of scored intervertebral disk spaces (IDS) | 12 (C2/C3 to C7/T1, T12/L1 to L5/S1) |
23 (C2/C3 to L5/S1) |
13 (T3/T4 to L3/L4) |
| Number of scoring sites per IDS | 2 | 8 | circumferential |
| Total score | 0 – 72 | 0 – 552 | not applicable |
| Training human readers | needed | needed | not needed |
| Sensitivity to change (in 2 years) | mSASSS increase in 30 – 40% patients | any net change in 61 –76% patients; the SDC in 37 – 43% patients | volume increase in more than 70% patients |
| Inter-reader ICC of change scores | 0.17 – 0.67 | whole spine: 0.77 spine segments: 0.32–0.75 |
Not applicable: measured by computer algorithm, no human readers involved. |
| Radiation | 1.5mSV* | 4 mSV | 8 mSV |
mSASSS: modified Stoke AS Spine Score; CTSS: computed tomography syndesmophyte score; CT: computed tomography; SDC: smallest detectable change; ICC: Intraclass correlation coefficient; mSV: millisievert.
2 views.
MRI scoring method is not included here because it does not measure new bone formation.
Magnetic Resonance Imaging (MRI) is a useful imaging tool to detect bone marrow edema in the spine and pelvis. However, it is less sensitive to signals from calcification, so is not as useful to measure new bone formation. A sequential process in which bone marrow edema proceeds the development of fat metaplasia and new bone formation has been proposed, but with mixed evidence (76,77). It is unclear whether presence of bone marrow edema or its resolution closely correlates with future development of syndesmophytes.
Participants: identifying subsets of disease
Studies have consistently shown that 30–40% of patients have an increase of mSASSS in a 2-year study. A handful of risk factors for radiographic progression have been identified from previous longitudinal studies, including male sex, HLA-B27 positivity, smoking status, elevated CRP, and presence of syndesmophytes at baseline. These risk factors can be used to identify the subset of potential candidates who are more likely to have disease progression, and hence, a higher chance of detecting a difference in radiographic progression in a given time frame. The trial design most likely to detect an NSAID effect would be a study of patients with high risk for progression treated with either minimal doses or full-dose NSAIDs and assessed with spinal CT.
Identifying novel risk factors and potential interactions
Another methodological aspect is to identify potential risk factors and their interactions, and include them as covariates in future observational studies. Recent cross-sectional studies have shed light on several new possible risk factors for disease progression, such as crystals, PTH and Vitamin D, and adipokines, but these have not been examined in longitudinal studies. Monosodium urate (MSU) microcrystals have been shown to induce COX-2 expression in human monocytes and osteoblast-like cells, and have a synergistic effect with IL-1 on osteoblasts to overexpress COX-2 (78,79). In patients with AS but not a clinical diagnosis of gout, urate crystal deposition at the sacroiliac joint was associated with progression of sacroiliac joint fusion (80). Both PTH and Vitamin D have direct and indirect effects on cyclooxygenase expression, and hence PGE2 level. A systematic review of cross-sectional studies showed that patients with AS commonly have low vitamin D levels (81). In a cross-sectional study, serum PTH levels were found to be significantly higher in patients with AS than in healthy controls (82). Consistent with this finding, PTH was reported to modulate the response to mechanical stress in osteoblast-like cells (76). Syndesmophyte formation and its association with serum adipokine levels have been investigated in several studies, but the results were inconsistent (84, 85).
Conclusion
Despite new therapies that are effective in relieving symptoms in patients with axSpA, treatments to prevent radiographic progression remains elusive. Observational studies have suggested that NSAIDs might slow syndesmophyte formation in patients with AS, however, two clinical trials had inconsistent results. Genetic and animal studies suggested potential effects on NSAIDs on bone formation, and clinical studies have indicated that NSAIDs may potentially modify disease progression, particularly in patients with higher risk of syndesmophyte growth. Better quantification of syndesmophyte growth, disease subsets with higher risk for radiographic progression, and potential risk factors and their interactions should be considered when designing future studies on disease progression in patients with axSpA.
Acknowledgments
The authors have no financial or commercial conflicts of interest related to this work. Dr. Runsheng Wang is supported by Rheumatology Research Foundation Scientist Development Award. Dr. Ward is supported by the Intramural Research Program, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health.
Contributor Information
Runsheng Wang, Columbia University College of Physicians and Surgeons..
Joan M. Bathon, Columbia University College of Physicians and Surgeons.
Michael M. Ward, Intramural Research Program, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health..
REFERENCE
- 1.Reveille JD, Witter JP, Weisman MH. Prevalence of Axial Spondylarthritis in the United States: Estimates From a Cross-Sectional Survey. Arthritis Care Res (Hoboken) 2012;64:905–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taurog JD, Chhabra A, Colbert RA. Ankylosing Spondylitis and Axial Spondyloarthritis. Longo DL, ed. New England Journal of Medicine 2016;374:2563–2574. [DOI] [PubMed] [Google Scholar]
- 3.Boel A, Molto A, van der Heijde D, Ciurea A, Dougados M, Gensler LS, et al. Do patients with axial spondyloarthritis with radiographic sacroiliitis fulfil both the modified New York criteria and the ASAS axial spondyloarthritis criteria? Results from eight cohorts. Annals of the Rheumatic Diseases 2019:annrheumdis-2019–215707. Available at: https://ard.bmj.com/content/early/2019/07/29/annrheumdis-2019-215707. [DOI] [PubMed] [Google Scholar]
- 4.Ward MM, Deodhar A, Gensler LS, Dubreuil M, Yu D, Khan MA, et al. 2019. Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis & Rheumatology 0 Available at: https://onlinelibrary.wiley.com/doi/abs/10.1002/art.41042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molto A, Paternotte S, van der Heijde D, Claudepierre P, Rudwaleit M, Dougados M. Evaluation of the validity of the different arms of the ASAS set of criteria for axial spondyloarthritis and description of the different imaging abnormalities suggestive of spondyloarthritis: data from the DESIR cohort. Annals of the rheumatic diseases 2015;74:746–51. [DOI] [PubMed] [Google Scholar]
- 6.Fongen C, Dagfinrud H, Berg IJ, Ramiro S, van Gaalen F, Landewé R, et al. Frequency of Impaired Spinal Mobility in Patients with Chronic Back Pain Compared to Patients with Early Axial Spondyloarthritis. J Rheumatol 2018;45:1643–1650. [DOI] [PubMed] [Google Scholar]
- 7.Dau JD, Lee M, Ward MM, Gensler LS, Brown MA, Learch TJ, et al. Opioid Analgesic Use in Patients with Ankylosing Spondylitis: An Analysis of the Prospective Study of Outcomes in an Ankylosing Spondylitis Cohort. J Rheumatol 2018;45:188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sieper J, Rudwaleit M, Baraliakos X, Brandt J, Braun J, Burgos-Vargas R, et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Annals of the rheumatic diseases 2009;68 Suppl 2:ii1–44. [DOI] [PubMed] [Google Scholar]
- 9.Sieper J, Lenaerts J, Wollenhaupt J, Rudwaleit M, Mazurov VI, Myasoutova L, et al. Efficacy and safety of infliximab plus naproxen versus naproxen alone in patients with early, active axial spondyloarthritis: results from the double-blind, placebo-controlled INFAST study, Part 1. Ann Rheum Dis 2014;73:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baraliakos X, Kiltz U, Peters S, Appel H, Dybowski F, Igelmann M, et al. Efficiency of treatment with non-steroidal anti-inflammatory drugs according to current recommendations in patients with radiographic and non-radiographic axial spondyloarthritis. Rheumatology (Oxford) 2017;56:95–102. [DOI] [PubMed] [Google Scholar]
- 11.van der Heijde D, Kivitz A, Schiff MH, Sieper J, Dijkmans BAC, Braun J, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: Results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis & Rheumatism 2006;54:2136–2146. [DOI] [PubMed] [Google Scholar]
- 12.van der Heijde D, Da Silva JC, Dougados M, Geher P, van der Horst-Bruinsma I, Juanola X, et al. Etanercept 50 mg once weekly is as effective as 25 mg twice weekly in patients with ankylosing spondylitis. Annals of the Rheumatic Diseases 2006;65:1572–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boersma J. Retardation of ossification of the lumbar vertebral column in ankylosing spondylitis by means of phenylbutazone. Scand J Rheumatol 1975;5:60–64. [PubMed] [Google Scholar]
- 14.Wanders A, van der Heijde D, Landewé R, Béhier J-M, Calin A, Olivieri I, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: A randomized clinical trial. Arthritis & Rheumatism 2005;52:1756–1765. [DOI] [PubMed] [Google Scholar]
- 15.Sieper J, Listing J, Poddubnyy D, Song I-H, Hermann K-G, Callhoff J, et al. Effect of continuous versus on-demand treatment of ankylosing spondylitis with diclofenac over 2 years on radiographic progression of the spine: results from a randomised multicentre trial (ENRADAS). Ann Rheum Dis 2015:annrheumdis-2015–207897. [DOI] [PubMed] [Google Scholar]
- 16.Creemers MCW. Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Annals of the Rheumatic Diseases 2005;64:127–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wanders A, Landewé R, Spoorenberg A, de Vlam K, Mielants H, Dougados M, et al. Scoring of radiographic progression in randomised clinical trials in ankylosing spondylitis: a preference for paired reading order. Ann Rheum Dis 2004;63:1601–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baraliakos X, Listing J, Rudwaleit M, Haibel H, Brandt J, Sieper J, et al. Progression of radiographic damage in patients with ankylosing spondylitis: defining the central role of syndesmophytes. Ann Rheum Dis 2007;66:910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Heijde D, Landewé R, Baraliakos X, Houben H, van Tubergen A, Williamson P, et al. Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum 2008;58:3063–3070. [DOI] [PubMed] [Google Scholar]
- 20.van der Heijde D, Salonen D, Weissman BN, Landewé R, Maksymowych WP, Kupper H, et al. Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis Res Ther 2009;11:R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramiro S, Stolwijk C, van Tubergen A, van der Heijde D, Dougados M, van den Bosch F, et al. Evolution of radiographic damage in ankylosing spondylitis: a 12 year prospective follow-up of the OASIS study. Annals of the Rheumatic Diseases 2015;74:52–59. [DOI] [PubMed] [Google Scholar]
- 22.Braun J, Baraliakos X, Deodhar A, Baeten D, Sieper J, Emery P, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis 2017;76:1070–1077. [DOI] [PubMed] [Google Scholar]
- 23.Braun J, Haibel H, de Hooge M, Landewé R, Rudwaleit M, Fox T, et al. Spinal radiographic progression over 2 years in ankylosing spondylitis patients treated with secukinumab: a historical cohort comparison. Arthritis Res Ther 2019;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wanders AJB, Landewé RBM, Spoorenberg A, Dougados M, van der Linden S, Mielants H, et al. What is the most appropriate radiologic scoring method for ankylosing spondylitis? A comparison of the available methods based on the Outcome Measures in Rheumatology Clinical Trials filter. Arthritis Rheum 2004;50:2622–2632. [DOI] [PubMed] [Google Scholar]
- 25.Poddubnyy D, Haibel H, Listing J, Märker‐Hermann E, Zeidler H, Braun J, et al. Baseline radiographic damage, elevated acute-phase reactant levels, and cigarette smoking status predict spinal radiographic progression in early axial spondylarthritis. Arthritis & Rheumatism 2012;64:1388–1398. [DOI] [PubMed] [Google Scholar]
- 26.Kim HR, Hong YS, Park S-H, Ju JH, Kang KY. Low bone mineral density predicts the formation of new syndesmophytes in patients with axial spondyloarthritis. Arthritis Res Ther 2018;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deminger A, Klingberg E, Geijer M, Göthlin J, Hedberg M, Rehnberg E, et al. A five-year prospective study of spinal radiographic progression and its predictors in men and women with ankylosing spondylitis. Arthritis Res Ther 2018;20:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Tubergen A, Ramiro S, van der Heijde D, Dougados M, Mielants H, Landewé R. Development of new syndesmophytes and bridges in ankylosing spondylitis and their predictors: a longitudinal study. Annals of the Rheumatic Diseases 2012;71:518–523. [DOI] [PubMed] [Google Scholar]
- 29.Ramiro S, van der Heijde D, van Tubergen A, Stolwijk C, Dougados M, van den Bosch F, et al. Higher disease activity leads to more structural damage in the spine in ankylosing spondylitis: 12-year longitudinal data from the OASIS cohort. Annals of the rheumatic diseases 2014;73:1455–61. [DOI] [PubMed] [Google Scholar]
- 30.Ward MM, Reveille JD, Learch TJ, Davis JC, Weisman MH. Occupational physical activities and long-term functional and radiographic outcomes in patients with ankylosing spondylitis. Arthritis & Rheumatism 2008;59:822–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramiro S, Landewé R, van Tubergen A, Boonen A, Stolwijk C, Dougados M, et al. Lifestyle factors may modify the effect of disease activity on radiographic progression in patients with ankylosing spondylitis: a longitudinal analysis. RMD Open 2015;1:e000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacques P, Lambrecht S, Verheugen E, Pauwels E, Kollias G, Armaka M, et al. Proof of concept: enthesitis and new bone formation in spondyloarthritis are driven by mechanical strain and stromal cells. Annals of the Rheumatic Diseases 2014;73:437–445. [DOI] [PubMed] [Google Scholar]
- 33.Tan S, Yao J, Flynn JA, Yao L, Ward MM. Dynamics of syndesmophyte growth in AS as measured by quantitative CT: heterogeneity within and among vertebral disc spaces. Rheumatology (Oxford) 2015;54:972–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dougados M, Simon P, Braun J, Burgos-Vargas R, Maksymowych WP, Sieper J, et al. ASAS recommendations for collecting, analysing and reporting NSAID intake in clinical trials/epidemiological studies in axial spondyloarthritis. Annals of the rheumatic diseases 2011;70:249–51. [DOI] [PubMed] [Google Scholar]
- 35.Poddubnyy D, Rudwaleit M, Haibel H, Listing J, Märker-Hermann E, Zeidler H, et al. Effect of non-steroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Annals of the Rheumatic Diseases 2012;71:1616–1622. [DOI] [PubMed] [Google Scholar]
- 36.Kroon F, Landewé R, Dougados M, van der Heijde D. Continuous NSAID use reverts the effects of inflammation on radiographic progression in patients with ankylosing spondylitis. Annals of the Rheumatic Diseases 2012;71:1623–1629. [DOI] [PubMed] [Google Scholar]
- 37.Baraliakos X, Listing J, Rudwaleit M, Brandt J, Sieper J, Braun J. Radiographic progression in patients with ankylosing spondylitis after 2 years of treatment with the tumour necrosis factor alpha antibody infliximab. Ann Rheum Dis 2005;64:1462–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Heijde D, Landewé R, Einstein S, Ory P, Vosse D, Ni L, et al. Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum 2008;58:1324–1331. [DOI] [PubMed] [Google Scholar]
- 39.Braun J, Baraliakos X, Hermann K-GA, Deodhar A, van der Heijde D, Inman R, et al. The effect of two golimumab doses on radiographic progression in ankylosing spondylitis: results through 4 years of the GO-RAISE trial. Ann Rheum Dis 2014;73:1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braun J, Baraliakos X, Deodhar A, Poddubnyy D, Emery P, Delicha EM, et al. Secukinumab shows sustained efficacy and low structural progression in ankylosing spondylitis: 4-year results from the MEASURE 1 study. Rheumatology (Oxford) 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Heijde D, Baraliakos X, Hermann K-GA, Landewé RBM, Machado PM, Maksymowych WP, et al. Limited radiographic progression and sustained reductions in MRI inflammation in patients with axial spondyloarthritis: 4-year imaging outcomes from the RAPID-axSpA phase III randomised trial. Ann Rheum Dis 2018;77:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haroon N, Inman RD, Learch TJ, Weisman MH, Lee M, Rahbar MH, et al. The impact of tumor necrosis factor alpha inhibitors on radiographic progression in ankylosing spondylitis. Arthritis and rheumatism 2013;65:2645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molnar C, Scherer A, Baraliakos X, de Hooge M, Micheroli R, Exer P, et al. TNF blockers inhibit spinal radiographic progression in ankylosing spondylitis by reducing disease activity: results from the Swiss Clinical Quality Management cohort. Ann Rheum Dis 2018;77:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cortes A, Maksymowych WP, Wordsworth BP, Inman RD, Danoy P, Rahman P, et al. Association study of genes related to bone formation and resorption and the extent of radiographic change in ankylosing spondylitis. Annals of the Rheumatic Diseases 2015;74:1387–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: Structural, Cellular, and Molecular Biology. Annual Review of Biochemistry 2000;69:145–182. [DOI] [PubMed] [Google Scholar]
- 46.Weinreb M, Suponitzky I, Keila S. Systemic administration of an anabolic dose of PGE2 in young rats increases the osteogenic capacity of bone marrow. Bone 1997;20:521–526. [DOI] [PubMed] [Google Scholar]
- 47.Suponitzky I, Weinreb M. Differential effects of systemic prostaglandin E2 on bone mass in rat long bones and calvariae. J Endocrinol 1998;156:51–57. [DOI] [PubMed] [Google Scholar]
- 48.Li X, Pilbeam CC, Pan L, Breyer RM, Raisz LG. Effects of prostaglandin E2 on gene expression in primary osteoblastic cells from prostaglandin receptor knockout mice. Bone 2002;30:567–573. [DOI] [PubMed] [Google Scholar]
- 49.Miyaura C, Inada M, Suzawa T, Sugimoto Y, Ushikubi F, Ichikawa A, et al. Impaired Bone Resorption to Prostaglandin E2 in Prostaglandin E Receptor EP4-knockout Mice. J Biol Chem 2000;275:19819–19823. [DOI] [PubMed] [Google Scholar]
- 50.Min YK, Rao Y, Okada Y, Raisz LG, Pilbeam CC. Regulation of prostaglandin G/H synthase-2 expression by interleukin-1 in human osteoblast-like cells. J Bone Miner Res 1998;13:1066–1075. [DOI] [PubMed] [Google Scholar]
- 51.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest 1999;103:1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.LeGrand A, Fermor B, Fink C, Pisetsky DS, Weinberg JB, Vail TP, et al. Interleukin-1, tumor necrosis factor alpha, and interleukin-17 synergistically up-regulate nitric oxide and prostaglandin E2 production in explants of human osteoarthritic knee menisci. Arthritis Rheum 2001;44:2078–2083. [DOI] [PubMed] [Google Scholar]
- 53.Joldersma M, Burger EH, Semeins CM, Klein-Nulend J. Mechanical stress induces COX-2 mRNA expression in bone cells from elderly women. J Biomech 2000;33:53–61. [DOI] [PubMed] [Google Scholar]
- 54.Siddhivarn C, Banes A, Champagne C, Riché EL, Weerapradist W, Offenbacher S. Prostaglandin D2 pathway and peroxisome proliferator-activated receptor gamma-1 expression are induced by mechanical loading in an osteoblastic cell line. J Periodont Res 2006;41:92–100. [DOI] [PubMed] [Google Scholar]
- 55.Shimizu N, Ozawa Y, Yamaguchi M, Goseki T, Ohzeki K, Abiko Y. Induction of COX-2 expression by mechanical tension force in human periodontal ligament cells. J Periodontol 1998;69:670–677. [DOI] [PubMed] [Google Scholar]
- 56.Kanzaki H, Chiba M, Shimizu Y, Mitani H. Periodontal ligament cells under mechanical stress induce osteoclastogenesis by receptor activator of nuclear factor kappaB ligand up-regulation via prostaglandin E2 synthesis. J Bone Miner Res 2002;17:210–220. [DOI] [PubMed] [Google Scholar]
- 57.Zhang X, Schwarz EM, Young DA, Puzas JE, Rosier RN, O’Keefe RJ. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest 2002;109:1405–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simon AM, Manigrasso MB, O’Connor JP. Cyclo-Oxygenase 2 Function Is Essential for Bone Fracture Healing. J Bone Miner Res 2002;17:963–976. [DOI] [PubMed] [Google Scholar]
- 59.Goodman S, Ma T, Trindade M, Ikenoue T, Matsuura I, Wong N, et al. COX-2 selective NSAID decreases bone ingrowth in vivo. Journal of Orthopaedic Research 2002;20:1164–1169. [DOI] [PubMed] [Google Scholar]
- 60.Altman RD, Latta LL, Keer R, Renfree K, Hornicek FJ, Banovac K. Effect of nonsteroidal antiinflammatory drugs on fracture healing: a laboratory study in rats. J Orthop Trauma 1995;9:392–400. [DOI] [PubMed] [Google Scholar]
- 61.Gerstenfeld LC, Thiede M, Seibert K, Mielke C, Phippard D, Svagr B, et al. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. Journal of Orthopaedic Research 2003;21:670–675. [DOI] [PubMed] [Google Scholar]
- 62.Beck A, Krischak G, Sorg T, Augat P, Farker K, Merkel U, et al. Influence of diclofenac (group of nonsteroidal anti-inflammatory drugs) on fracture healing. Arch Orthop Trauma Surg 2003;123:327–332. [DOI] [PubMed] [Google Scholar]
- 63.Bergenstock M, Min W, Simon AM, Sabatino C, O’Connor JP. A comparison between the effects of acetaminophen and celecoxib on bone fracture healing in rats. J Orthop Trauma 2005;19:717–723. [DOI] [PubMed] [Google Scholar]
- 64.Gerstenfeld LC, Al-Ghawas M, Alkhiary YM, Cullinane DM, Krall EA, Fitch JL, et al. Selective and nonselective cyclooxygenase-2 inhibitors and experimental fracture-healing. Reversibility of effects after short-term treatment. J Bone Joint Surg Am 2007;89:114–125. [DOI] [PubMed] [Google Scholar]
- 65.Goodman SB, Ma T, Mitsunaga L, Miyanishi K, Genovese MC, Smith RL. Temporal effects of a COX-2-selective NSAID on bone ingrowth. Journal of Biomedical Materials Research Part A 2005;72A:279–287. [DOI] [PubMed] [Google Scholar]
- 66.Krischak GD, Augat P, Sorg T, Blakytny R, Kinzl L, Claes L, et al. Effects of diclofenac on periosteal callus maturation in osteotomy healing in an animal model. Arch Orthop Trauma Surg 2007;127:3–9. [DOI] [PubMed] [Google Scholar]
- 67.Dodwell ER, Latorre JG, Parisini E, Zwettler E, Chandra D, Mulpuri K, et al. NSAID Exposure and Risk of Nonunion: A Meta-Analysis of Case–Control and Cohort Studies. Calcified Tissue International 2010;87:193–202. [DOI] [PubMed] [Google Scholar]
- 68.Wheatley BM, Nappo KE, Christensen DL, Holman AM, Brooks DI, Potter BK. Effect of NSAIDs on Bone Healing Rates: A Meta-analysis. J Am Acad Orthop Surg 2018. [DOI] [PubMed] [Google Scholar]
- 69.Ma R, Chen G-H, Zhao L-J, Zhai X-C. Efficacy of naproxen prophylaxis for the prevention of heterotopic ossification after hip surgery: a meta-analysis. J Orthop Surg Res 2018;13:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Joice M, Vasileiadis GI, Amanatullah DF. Non-steroidal anti-inflammatory drugs for heterotopic ossification prophylaxis after total hip arthroplasty: a systematic review and meta-analysis. The Bone & Joint Journal 2018;100-B:915–922. [DOI] [PubMed] [Google Scholar]
- 71.Tan S, Yao J, Flynn JA, Yao L, Ward MM. Quantitative measurement of syndesmophyte volume and height in ankylosing spondylitis using CT. Ann Rheum Dis 2014;73:544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Bruin F, de Koning A, van den Berg R, Baraliakos X, Braun J, Ramiro S, et al. Development of the CT Syndesmophyte Score (CTSS) in patients with ankylosing spondylitis: data from the SIAS cohort. Ann Rheum Dis 2018;77:371–377. [DOI] [PubMed] [Google Scholar]
- 73.Tan S, Yao J, Flynn JA, Yao L, Ward MM. Zygapophyseal Joint Fusion in Ankylosing Spondylitis Assessed by Computed Tomography: Associations with Syndesmophytes and Spinal Motion. The Journal of Rheumatology 2017;44:1004–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Koning A, de Bruin F, van den Berg R, Ramiro S, Baraliakos X, Braun J, et al. Low-dose CT detects more progression of bone formation in comparison to conventional radiography in patients with ankylosing spondylitis: results from the SIAS cohort. Ann Rheum Dis 2018;77:293–299. [DOI] [PubMed] [Google Scholar]
- 75.Tan S, Yao J, Flynn JA, Yao L, Ward MM. Quantitative syndesmophyte measurement in ankylosing spondylitis using CT: longitudinal validity and sensitivity to change over 2 years. Ann Rheum Dis 2015;74:437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baraliakos X, Heldmann F, Callhoff J, Listing J, Appelboom T, Brandt J, et al. Which spinal lesions are associated with new bone formation in patients with ankylosing spondylitis treated with anti-TNF agents? A long-term observational study using MRI and conventional radiography. Ann Rheum Dis 2014;73:1819–1825. [DOI] [PubMed] [Google Scholar]
- 77.Maksymowych WP, Chiowchanwisawakit P, Clare T, Pedersen SJ, Østergaard M, Lambert RGW. Inflammatory lesions of the spine on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis: Evidence of a relationship between inflammation and new bone formation. Arthritis & Rheumatism 2009;60:93–102. [DOI] [PubMed] [Google Scholar]
- 78.Bouchard L, de Médicis R, Lussier A, Naccache PH, Poubelle PE. Inflammatory microcrystals alter the functional phenotype of human osteoblast-like cells in vitro: synergism with IL-1 to overexpress cyclooxygenase-2. J Immunol 2002;168:5310–5317. [DOI] [PubMed] [Google Scholar]
- 79.Pouliot M, James MJ, McColl SR, Naccache PH, Cleland LG. Monosodium urate microcrystals induce cyclooxygenase-2 in human monocytes. Blood 1998;91:1769–1776. [PubMed] [Google Scholar]
- 80.Zhu J, Li A, Jia E, Zhou Y, Xu J, Chen S, et al. Monosodium urate crystal deposition associated with the progress of radiographic grade at the sacroiliac joint in axial SpA: a dual-energy CT study. Arthritis Research & Therapy 2017;19:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao S, Duffield SJ, Moots RJ, Goodson NJ. Systematic review of association between vitamin D levels and susceptibility and disease activity of ankylosing spondylitis. Rheumatology (Oxford) 2014;53:1595–1603. [DOI] [PubMed] [Google Scholar]
- 82.Orsolini G, Adami G, Rossini M, Ghellere F, Caimmi C, Fassio A, et al. Parathyroid hormone is a determinant of serum Dickkopf-1 levels in ankylosing spondylitis. Clin Rheumatol 2018. [DOI] [PubMed] [Google Scholar]
- 83.Ryder KD, Duncan RL. Parathyroid hormone modulates the response of osteoblast-like cells to mechanical stimulation. Calcif Tissue Int 2000;67:241–246. [DOI] [PubMed] [Google Scholar]
- 84.Hartl A, Sieper J, Syrbe U, Listing J, Hermann K-G, Rudwaleit M, et al. Serum levels of leptin and high molecular weight adiponectin are inversely associated with radiographic spinal progression in patients with ankylosing spondylitis: results from the ENRADAS trial. Arthritis Res Ther 2017;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park J-H, Lee S-G, Jeon Y-K, Park E-K, Suh Y-S, Kim H-O. Relationship between serum adipokine levels and radiographic progression in patients with ankylosing spondylitis. Medicine (Baltimore) 2017;96. [DOI] [PMC free article] [PubMed] [Google Scholar]