Abstract
Myopic children have larger ciliary muscles than non-myopic children, suggesting that the ciliary muscle may have an impact on or be affected by refractive error development. The guinea pig represents an attractive model organism for myopia development research. The purpose of the study was to investigate whether form deprivation-induced myopia in one or more strains of guinea pig causes thickening of the ciliary muscle as seen in human myopia. Thirty-nine guinea pigs were bred from in-house progenitors obtained from Cincinnati Children’s Hospital (Cincinnati) and the United States Army (Strain 13). At 2–4 days of age the right eyes of animals were exposed to form deprivation for 7 days while the fellow eyes served as controls. Refractive error was determined with retinoscopy while vitreous chamber depth (VCD) and axial length (AL) were determined with A-scan ultrasound. Ciliary muscle characteristics (ciliary muscle length, cross-sectional area, volume, cell number, cell size, and smooth muscle actin concentration) were determined histologically with antibody labeling and analyzed according to whether the animal developed axial myopia (anisometropia > −2.00 D with VCD and/or AL differences > 0.1 mm) or was unresponsive. This analysis method yielded four groups with Group 1 having no induced myopia but with axial elongation (n = 11), Group 2 having myopia without vitreous or axial elongation (n = 8), Group 3 having myopia with either vitreous or axial elongation (n = 11), and Group 4 having myopia with both vitreous and axial elongation (n = 8). There were no post-treatment inter-ocular differences between strains or for the overall group of animals for any ciliary muscle variable; however, a higher response group number in multivariate ordinal regression was related to having a treated compared to fellow eye that had a lower smooth muscle actin concentration (p = 0.006), with a shorter ciliary muscle length (p = 0.042), and a less oblate eye shape (p = 0.010). Guinea pig ciliary muscle length and smooth muscle actin concentration were significantly less in the treated eyes of axially myopic animals suggesting that 7 days of form deprivation induced ciliary muscle cellular atrophy or inhibited ciliary muscle growth. Form deprivation myopia in the guinea pig does not result in the increase in ciliary muscle thickness associated with human juvenile and adult myopia.
Keywords: Refractive Error, Myopia, Ciliary Muscle, Histology, Guinea Pigs, Development
1. Introduction
The ciliary muscle’s link to myopia development has long been suspected. Historically, near work (cumulative accommodative effort) was considered a primary risk factor for myopia development (Zylbermann et al., 1993). However, more recently, the relationship between the risk of myopia onset and near work has been questioned (Huang et al., 2015; Ip et al., 2008; Saw et al., 2006; Zadnik et al., 2015). While near work has lost some favor, the accommodative system is still relevant to myopia development because myopic individuals have increased accommodative lags (Gwiazda et al., 2005; Mutti et al., 2006), higher AC/A ratios (Gwiazda et al., 2005; Mutti et al., 2000), and thicker ciliary muscles/bodies (Bailey et al., 2008; Oliveira et al., 2005; Pucker et al., 2013). Subsequently, these associations have led to the hypothesis that ciliary muscle thickening may play a role in producing the relatively less oblate ocular shape found in myopic eyes, specifically that ciliary muscle thickening may cause equatorial eye restriction resulting in axial elongation without proportional equatorial expansion (Atchison et al., 2006; Atchison et al., 2005; Mutti et al., 2007).
A less oblate ocular shape in humans is also associated with a more hyperopic relative peripheral refraction (Atchison et al., 2006; Atchison et al., 2005; Mutti et al., 2007), a feature that is currently believed to be a key regulator of myopia development (Aller and Wildsoet, 2013; Anstice and Phillips, 2011; Liu and Wildsoet, 2012; Sankaridurg et al., 2011; Smith et al., 2009; Walline et al., 2013). Foveal visual cues may be important for regulating refractive error development, but the peripheral retina has been shown to guide eye growth without input from the fovea in animal models (Smith et al., 2009). Smith et al. demonstrated that if one ablates a rhesus monkey’s fovea, peripheral visual stimuli (form deprivation and minus lenses) are still able to induce axial elongation and myopic refractive error at the fovea (Smith et al., 2009; Smith et al., 2007). The success of treating human peripheral hyperopic defocus with orthokeratology and center-distance bifocal contact lenses reinforces the peripheral visual defocus theory (Aller and Wildsoet, 2013; Anstice and Phillips, 2011; Cho et al., 2005; Sankaridurg et al., 2011; Walline et al., 2013; Walline et al., 2009). Yet the myopia community currently lacks a mechanistic understanding of how a less oblate myopic eye shape is created.
The guinea pig is an attractive animal model for investigating this mechanism because: 1) guinea pigs reach adulthood within about 90 days of life, 2) guinea pigs have the ability to accommodate, 3) untreated guinea pig ciliary muscle volume increases 2.5 fold during their first 90 days of life, and 4) guinea pigs have prominent longitudinal ciliary muscle fibers (region of muscle associated with human myopia) (Howlett and McFadden, 2006, 2007, 2009; Ostrin et al., 2014; Pucker et al., 2014; Pucker et al., 2015). However, it is unclear whether induced myopia alters the ciliary muscle growth pattern of guinea pigs, which is a first step toward understanding whether there may be a causal relationship between ciliary thickness and myopia development. Therefore, the purpose of the study was to investigate whether form deprivation-induced myopia in one or more strains of guinea pig causes thickening of the ciliary muscle as seen in human myopia.
2. Materials and Methods
2.1. Animals
This study utilized Strain 13 guinea pigs (n = 14; Cavia porcellus) that were obtained from the United States Army Medical Research Institute of Infectious Diseases (Fort Detrick, MD) and a guinea pig strain with unknown origins (Cincinnati strain; n = 25) that is currently being maintained by Cincinnati Children’s Hospital (Cincinnati, OH). Commercially available guinea pigs from Elm Hill Labs (Chelmsford, MA) were excluded because they are known to be resistant to myopia induction (Jiang et al., 2019). The two strains in this study were simultaneously sampled to determine their usefulness in myopia research and to understand if ciliary muscle strain differences exist. All guinea pigs were raised in a 12-hour light/12-hour dark cycle environment. The Ohio State University’s (Protocol Number: 2012A00000012-R1) and the University of Alabama at Birmingham’s (IACUC-20558) Institutional Animal Care and Use Committees authorized this research, and this work was done in accordance with the Association for Research in Vision and Ophthalmology’s Statement for the Use of Animals in Ophthalmic and Vision Research.
All animals were subjected to form deprivation myopia (Howlett and McFadden, 2006). In brief, baseline biometric measurements were collected at two to four days of age (described below), and guinea pigs were hooded at this time to allow them to adapt to the treatment apparatus (Figure 1) (Schaeffel et al., 1988). The hoods were made of durable couch fabric with O-rings attached to the inner side of each eye hole with sewing thread to keep the fabric from rubbing over the animal’s eyes (Stewart J, et al. 67th AALAS National Meeting: Abstract 2544542). An additional O-ring was attached over the right eye with sewing thread to keep the form deprivation diffuser from touching the animal’s eye. Fur around the animal’s eyes was trimmed prior to hood application to also keep the eye safe and from unintended occlusion or abrasion. Form deprivation diffusers were made from roughed human plastic plano spectacle lenses and attached with sewing thread at the superior segment of the lens. Floral wire was used to attach the lower segment of the lens to the hood. Floral wire allows for the lens to be easily detached for eye inspection and cleaning. A draw string was run through the poster segment of the hood, which allowed for easy attachment and removal of the hoods.
Figure 1: Guinea Pig Form Deprivation Mask with Lens Over Right Eye.

The form deprivation diffuser was attached to the hood over the right eye (experimental/treated eye) the following day by taking off the original hood, attaching the diffuser, and reapplying the hood; the left eye (control/fellow eye) was allowed unrestricted vision. The goggles were cleaned daily to maintain a consistent level of exposure to form deprivation. The form deprivation diffusers with their associated hoods were removed after one week of treatment, ocular measurements were repeated, and the eyes were collected for analysis as described below. Animals were grouped according to the increasing severity of their response to form deprivation: Group 1 showed no induced myopia (less than −2.00 D more myopic in the form-deprived eye than the fellow eye); n = 11; Group 2 showed an inter-ocular difference in refractive error that was at least −2.00 D more myopic in the form-deprived eye, but without vitreous or axial elongation of at least 0.1 mm longer than the fellow eye; n = 8; Group 3 were at least −2.00 D more myopic in the form-deprived eye along with either vitreous chamber or axial elongation of at least 0.1 mm; n = 11; and Group 4 were at least −2.00 D more myopic in the form-deprived eye along with both vitreous chamber and axial elongation of at least 0.1 mm; n = 8.
2.2. Biometry & Ocular Shape Measurements
Refractive error, ultrasound, and photographic ocular shape measurements were performed as previously described (Pucker et al., 2014; Pucker et al., 2015). In brief, two trained examiners obtained cycloplegic refractive error measurements (1.0% cyclopentolate) via retinoscopy, and the mean of the two values was reported (Pucker et al., 2014; Pucker et al., 2015). Axial length and vitreous chamber depth measurements were taken with an A-scan ultrasound (10 MHz; Sonomed 5500) after anesthetizing the animals with 1–3% isoflurane and applying one drop of 0.5% proparacaine to each eye (Pucker et al., 2015).
Eyes were then enucleated after the animals were euthanized with carbon dioxide exposure and a secondary pneumothorax. The eyes were stored in phosphate buffered saline until imaging was performed, which happened only a few minutes after enucleation (Pucker et al., 2014). Each eye was photographically analyzed to determine limbal circumference (established preprocessing benchmark for histologically-based ciliary muscle volume measurements used to compensate for histologically-induced changes) and ocular shape (ocular length/equatorial diameter) (Pucker et al., 2014). Refractive error and ultrasound measurements were taken at baseline and at the experimental endpoint while photographic information was only obtained at the experimental endpoint after euthanasia with carbon dioxide.
2.3. Histology
Cell number, cell size, fluorescent labeling intensity (brightness/smooth muscle actin concentration), and histologic dimensions (cross-sectional area, length, volume) were generated using methods similar to those previously described (Pucker et al., 2014; Pucker et al., 2015). In brief, both right and left enucleated eyes were fixed with 4% paraformaldehyde for one hour and transferred to 20% sucrose overnight (Pucker et al., 2014; Pucker et al., 2015). Eyes were divided into nasal and temporal halves, embedded in Optimal Cutting Temperature (OCT) compound, and cut (30 μm thick) into serial sections (Pucker et al., 2014; Pucker et al., 2015). Two slides from the center of each slide deck were labeled with FITC-conjugated, anti-α-smooth muscle actin (SMA) antibody (1:80 dilution; F3777, Sigma, St. Louis, MO) to visualize the concentration of that protein within the smooth muscle fibers, and Draq5 (1:1000 dilution; Thermo Scientific, 62251, Waltham, MA) was used to identify cell nuclei. High resolution 20x magnification images of both labels were then captured with a wide-field epi-fluorescence microscope and a digital camera (Figure 2) (Fischer et al., 2015). All SMA images from a single animal were taken on the same day with the same microscope and camera settings while the Draq5 images were taken in a similar manner, but with exposure settings that allowed for single nucleus resolution (Pucker et al., 2015). Images were merged using Adobe Photoshop™ (San Jose, CA) (Fischer et al., 2015) and coded prior to morphological analysis to prevent investigator bias. Stereo Investigator software (MBF Bioscience, Williston, VT) was used to enumerate cell number and measure ciliary muscle cross-sectional area, mean SMA brightness (measure of smooth muscle actin concentration), and ciliary muscle length via a protocol established by our laboratory (Figure 2) (Pucker et al., 2014; Pucker et al., 2015). Mean cell sizes (cross-sectional area / mean cell number) and ciliary muscle volumes (cross-sectional area * limbal circumference) were derived from the above measurements (Pucker et al., 2014; Pucker et al., 2015).
Figure 2: Representative Post-Treatment Stained Ciliary Muscle Images (20x objective).
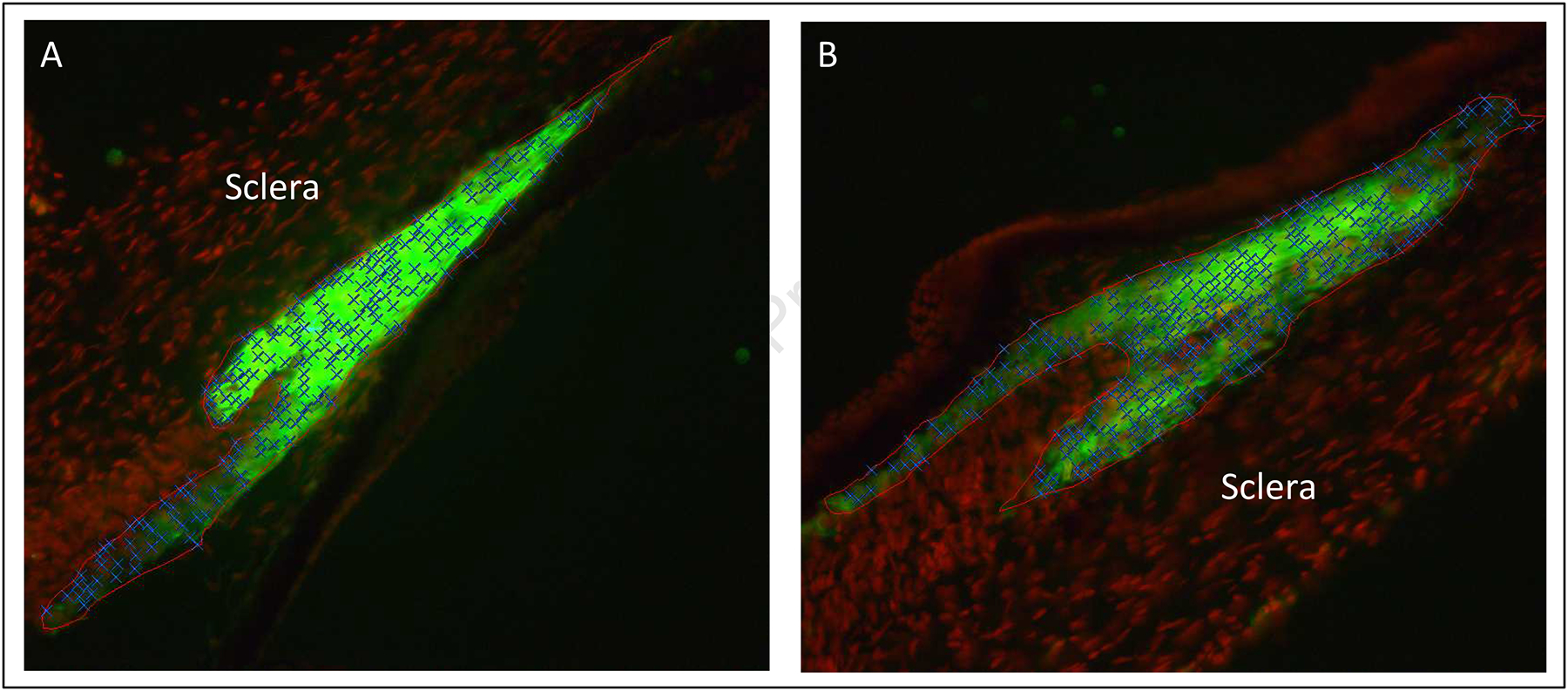
Images are from the treated (A) and fellow (B) eyes of the same animal. The ciliary muscle has been outlined in red, smooth muscle fibers have been stained green (anti-smooth muscle actin conjugated with FITC), and cell nuclei have been stained red (Draq5). Note that ciliary muscle cell nuclei have been labeled with blue Xs.
2.4. Statistical Analysis
Microsoft Excel (Microsoft, Redmond, WA), Stata 15.0 software (StataCorp LP, College Station, TX), and SPSS (v.24; IBM, Armonk, NY) were used to perform all statistical calculations. Descriptive statistics (means and standard deviations) were used to understand general data trends. Unpaired t-tests were used to determine if there were ciliary muscle differences between guinea pig strains. Paired t-tests were used to determine if there were differences between treated and fellow eyes in the sample as a whole and in each of the four response groups. Inter-eye differences in ciliary muscle and ocular variables were analyzed for whether or not they were associated with being in a more severe response group using ordinal regression. Analyzing the four different groups in a single model allowed the investigators to discern if increasing severity of induced myopia and elongation were associated with ciliary muscle or other morphological changes. Morphological values were considered to be significantly different or significant in their association with response group when p < 0.05.
3. Results
3.1. Biometric Measurements
All guinea pigs were able to successfully wear the form-deprivation hoods. The lenses stayed on all hoods, though four of guinea pigs had their hood fall off between one and two times. Hood removal did not result in a discernible link to being responsive to the treatment. There was no substantial variation in the percentages of animals in each response group between the two strains. The Cincinnati guinea pigs were between 50% and 73% of the animals in each of the four response groups, similar to the 63% (24/38) of that strain in the study (Table 1; p = 0.79, chi-square df = 3). Histological data from one unresponsive Cincinnati guinea pig was excluded due to a tissue processing malfunction. There were no post-treatment inter-ocular differences between strains for any ocular biometric variable (Table 2, p-values between 0.25 and 0.99), so data from the two strains were analyzed together from this point forward.
Table 1:
Included Guinea Pigs by Strain and Group
| Group | Strain 13 n (%) | Cincinnati n (%) | Total |
|---|---|---|---|
| 1) Non-myopic | 4 (36) | 7 (64) | 11 |
| 2) Myopic but no elongation | 4 (50) | 4 (50) | 8 |
| 3) Myopic with elongation in VCD or AL | 3 (27) | 8 (73) | 11 |
| 4) Myopic with elongation in both VCD and AL | 3 (37.5) | 5 (62.5) | 8 |
| Total | 14 (37) | 24 (63) | 38 |
Chi-square df = 3; p-value = 0.79; VCD = Vitreous Chamber Depth; AL = Axial Length
Table 2:
Post-Treatment Guinea Pig Ciliary Muscle Characteristics by Strain
| Strain 13 Inter-ocular difference (mean ± SD) | Cincinnati Inter-ocular difference (mean ± SD) | Strain difference (mean ± SEM) | p-Value | |
|---|---|---|---|---|
| Ciliary Muscle Length (μm) | −3.54 ± 106.0 | 12.8 ± 110.5 | −16.35 ± 36.62 | 0.66 |
| Ciliary Muscle Cross-Sectional Area (mm2) | 0.0011 ±.010 | 0.0012 ± 0.014 | −0.000060 ± 0.0043 | 0.99 |
| Ciliary Muscle Volume (mm3) | 0.034 ± 0.22 | −0.016 ± 0.27 | 0.050 ± 0.087 | 0.57 |
| Ciliary Muscle Cell Number (cells) | −2.16 ± 36.21 | 8.41 ± 55.31 | −10.57 ± 16.57 | 0.53 |
| Ciliary Muscle Cell Cross-Sectional Area (μm2/cell) | 8.35 ± 44.8 | −13.8 ± 64.3 | 22.18 ± 19.51 | 0.26 |
| SMA Concentration (Brightness) | 3.74 ± 19.75 | −2.27 ± 25.8 | 6.01 ± 8.00 | 0.46 |
| Refractive Error (D) | −2.31 ± 1.49 | −2.38 ± 1.70 | 0.068 ± 0.55 | 0.90 |
| Vitreous Chamber Depth (mm) | 0.057 ± 0.14 | 0.093 ± 0.12 | −0.036 ± 0.044 | 0.42 |
| Axial Length (mm) | 0.17 ± 0.31 | 0.047 ± 0.31 | 0.12 ± 0.10 | 0.25 |
| Limbal Circumference (mm) | 0.15 ± 0.55 | −0.083 ± 0.86 | 0.24 ± 0.26 | 0.37 |
| Ocular Shape (Ratio) | 0.0090 ± 0.047 | 0.0093 ± 0.028 | −0.00025 ± 0.013 | 0.98 |
At baseline, there were no statistically significant differences in refractive errors (p = 0.93), crystalline lens thickness (p = 0.98), axial lengths (p = 0.083), or vitreous chamber depths (p = 0.46) between treated and fellow eyes for the sample as a whole, and there were not any significant differences within any of the four response groups (p-values between 0.26 and 0.48). Treated eyes were more myopic than fellow eyes by −2.36 ± 1.61 D (p<0.0001) for the sample as a whole, and had longer vitreous chamber depths by 0.080 ± 0.13 mm (p = 0.001) at the completion of the study (Table 3). No other biometric inter-ocular difference was significantly different after treatment (Table 3; p-values between 0.077 and 0.91). By definition, biometric variables differed by response group. Group 1 animals showed axial elongation by 0.27 ± 0.23 mm (Table 4; p<0.01) but no induced myopia. Group 2 animals were more myopic in treated compared to fellow eyes by −3.41 ± 1.70 D (p<0.01) with paradoxically shorter axial lengths by −0.14 ± 0.17 mm (p<0.05). Group 3 animals were more myopic in treated compared to fellow eyes by −3.02 ± 0.97 D (p<0.001) with longer vitreous chamber depths of 0.15 ± 0.10 mm (p<0.001) but with no significant axial elongation. Group 4 animals were more myopic in treated compared to fellow eyes by −3.03 ± 0.70 D (p<0.001), had longer vitreous chamber depths by 0.16 ± 0.056 mm (p<0.001), and longer axial lengths by 0.36 ± 0.24 mm (p<0.01). Group 4 animals also had a less oblate shape ratio by 0.033 ± 0.037 (p<0.05).
Table 3:
Summary of Post-Treatment Biometric Measurements for all Animals.
| Treated (Right Eye) (Mean ± SD) | Fellow (Left Eye) (Mean ± SD) | Mean Difference (± SD) | P-Value | |
|---|---|---|---|---|
| Refractive Error (D) | −2.23 ± 3.33 | 0.13 ± 3.26 | −2.36 ± 1.61 | <0.0001 |
| Vitreous Chamber Depth (mm) | 2.87 ± 0.14 | 2.79 ± 0.13 | 0.080 ± 0.13 | 0.001 |
| Axial Length (mm) | 7.08 ± 0.37 | 6.99 ± 0.27 | 0.092 ± 0.31 | 0.077 |
| Limbal Circumference (mm) | 18.9 ± 1.9 | 18.9 ± 1.9 | 0.014 ± 0.75 | 0.91 |
| Ocular Shape (Ratio) | 0.92 ± 0.044 | 0.91 ± 0.052 | 0.0092 ± 0.036 | 0.15 |
Ocular shape ratios less than 1.00 indicate an oblate ocular shape.
Table 4:
Summary of Post-Treatment Biometric Measurements by Animal Group.
| Group 1 Inter-ocular difference (mean ± SD) |
Group 2 Inter-ocular difference (mean ± SD) |
Group 3 Inter-ocular difference (mean ± SD) |
Group 4 Inter-ocular difference (mean ± SD) |
|
|---|---|---|---|---|
| Refractive Error (D) | −0.43 ± 0.66 | −3.41 ± 1.70** | −3.02 ± 0.97*** | −3.03 ± 0.70*** |
| Vitreous Chamber Depth (mm) | 0.032 ± 0.13 | −0.031 ± 0.12 | 0.15 ± 0.10*** | 0.16 ± 0.056*** |
| Axial Length (mm) | 0.27 ± 0.23** | −0.14 ± 0.17* | −0.11 ± 0.25 | 0.36 ± 0.24** |
| Limbal Circumference (mm) | 0.022 ± 0.89 | −0.17 ± 0.55 | −0.046 ± 0.83 | 0.26 ± 0.73 |
| Ocular Shape (Ratio) | −0.0072 ± 0.032 | 0.0053 ± 0.037 | 0.0066 ± 0.035 | 0.033 ± 0.037* |
p<0.05;
p<0.01;
p<0.001;
Ocular shape ratios less than 1.00 indicate an oblate ocular shape.
3.2. Between Group Morphological Ciliary Muscle Differences
After combining the two strains, there were no inter-ocular difference between treated and fellow eyes for any ciliary muscle variable for the overall group or by animal group (Tables 5 and 6; p-values between 0.55 and 0.99). Ciliary muscle inter-ocular differences were related to response group (Table 7). A higher response group number in multivariate ordinal regression was related to having a treated compared to fellow eye that was less bright (lower smooth muscle actin concentration; p = 0.006), with a shorter ciliary muscle length (p = 0.042) and a less oblate ocular shape ratio (p = 0.010). The coefficients from the multivariate ordinal regression are shown in Table 7 (Nagelkerke R2 = 0.39). Regression analysis subsequently found that a shorter ciliary muscle length was associated with a lower number of ciliary muscle cells (r = 0.38, p = 0.02) and a smaller ciliary muscle cross-sectional area (r = 0.45. p = 0.004).
Table 5:
Comparison of Treated (Right Eye) and Untreated (Left Eye) Ciliary Muscle Differences for All Animals.
| Treated (Right Eye) (Mean ± SD) | Fellow (Left Eye) (Mean ± SD) | Mean Difference (± SD) | P-Value | |
|---|---|---|---|---|
| Ciliary Muscle Length (μm) | 718.4 ± 157.9 | 711.7 ± 147.0 | 6.8 ± 107.7 | 0.70 |
| Ciliary Muscle Cross-Sectional Area (mm2) | 0.049 ± 0.022 | 0.047 ± 0.019 | 0.0011 ± 0.012 | 0.58 |
| Ciliary Muscle Volume (mm3) | 1.00 ± 0.27 | 0.99 ± 0.30 | 0.0048 ± 0.25 | 0.91 |
| Ciliary Muscle Cell Number (cells) | 180.9 ± 44.5 | 176.4 ± 58.9 | 4.5 ± 48.9 | 0.57 |
| Ciliary Muscle Cell Cross-Sectional Area (μm2/cell) | 271.2 ± 74.5 | 276.9 ± 64.0 | −5.7 ± 58.2 | 0.55 |
| SMA Concentration (Brightness) | 51.9 ± 32.1 | 52.0 ± 32.7 | −0.057 ± 23.7 | 0.99 |
SMA = Smooth muscle actin
Table 6:
Comparison of Treated (Right Eye) and Untreated (Left Eye) Ciliary Muscle Differences by Group.
| Group 1 Inter-ocular difference (mean ± SD) | Group 2 Inter-ocular difference (mean ± SD) | Group 3 Inter-ocular difference (mean ± SD) | Group 4 Inter-ocular difference (mean ± SD) | |
|---|---|---|---|---|
| Ciliary Muscle Length (μm) | 37.5 ± 97.7 | 62.6 ± 100.5 | −45.0 ± 88.8 | −20.1 ± 127.4 |
| Ciliary Muscle Cross-Sectional Area (mm2) | 0.0050 ± 0.014 | 0.0018 ± 0.011 | −0.0041 ± 0.013 | 0.0023 ± 0.0097 |
| Ciliary Muscle Volume (mm3) | 0.045 ± 0.24 | 0.040 ± 0.25 | −0.097 ± 0.29 | 0.056 ± 0.21 |
| Ciliary Muscle Cell Number (cells) | 16.1 ± 49.5 | −4.3 ± 42.2 | −13.8 ± 57.7 | 22.7 ± 37.4 |
| Ciliary Muscle Cell Cross-Sectional Area (μm2/cell) | 0.33 ± 90.0 | 16.15 ± 29.28 | −14.38 ± 41.86 | −23.70 ± 43.63 |
| SMA Concentration (Brightness) | 8.17 ± 19.2 | 0.32 ± 23.8 | 2.33 ± 22.2 | −15.03 ± 28.0 |
p-values range from 0.12 to 0.99;
SMA = Smooth muscle actin
Table 7:
Factors Significantly Associated with Induced-Myopia
| Inter-ocular variable | Coefficient (standard error) | p-value |
|---|---|---|
| SMA Concentration | −0.047 (0.017) | 0.006 |
| Ciliary Muscle Length | −0.007 (0.004) | 0.042 |
| Ocular Shape | 27.8 (10.8) | 0.010 |
Nagelkerke R2 = 0.39;
SMA = Smooth muscle actin
4. Discussion
An association between thicker human ciliary bodies/muscles and myopic refractive errors in children and adults is well established in the literature, yet the mechanism leading to a thicker ciliary body/muscle is currently unknown, motivating the need to study ciliary muscle development in animal models (Bailey et al., 2008; Oliveira et al., 2005; Pucker et al., 2013). The purpose of the study was to investigate whether form deprivation-induced myopia in one or more strains of guinea pig causes thickening of the ciliary muscle as seen in human myopia.
The main findings from this study suggest that axial elongation from form deprivation-induced myopia results in inhibition of ciliary muscle growth and not the increase in thickness or volume seen in human myopia. The length of the ciliary muscle was significantly shorter and the brightness of the cells was significantly less (indicates lower concentration of ciliary muscle fibers) in the treated eyes of axially myopic animals. This ciliary muscle length difference is likely the result of their being fewer ciliary muscle cells and the muscle having a smaller cross-sectional area. This result is consistent with work from Browrey et al. who found that defocus-induced myopia in the guinea pig appears to accelerate peri-papillary elongation with little change in the periphery (Bowrey et al., 2017). Perhaps form deprivation has a similar effect of sparing the ocular periphery from changes that might have accelerated ocular elongation and myopic refractive error through restriction of equatorial growth. The unexpected inhibition of ciliary muscle growth or atrophy of the ciliary muscle from form deprivation myopia argues against its use as a model of the ciliary muscle in human myopia. Potential ciliary muscle differences between humans and guinea pigs that may have produced these contrary results could be that humans (well-developed circular, radial, and longitudinal ciliary muscle fibers) and guinea pigs (primarily longitudinal ciliary muscle fibers) have differing ciliary muscle morphologies and that humans and guinea pigs have different eye locations within the skull (anterior vs lateral, respectively). Another possible reason for the difference between guinea pig and human may be that form-deprivation myopia does not mimic the process of human myopia development that involves the ciliary muscle; this difference suggests the need for a future study involving lens-induced myopia. Nevertheless, the effects on eye length and refractive error of visual or other manipulations that do produce ciliary muscle hypertrophy in the guinea pig would still be of interest. While the primary findings of this study were in the opposite direction than the association noted in humans, the results of this study are still important because they used an established myopia animal model (guinea pig) to provide evidence that ciliary muscle morphology can be influenced by a change in the visual environment that affects refractive error and eye length (Howlett and McFadden, 2006, 2007; Pucker et al., 2014; Pucker et al., 2015).
The findings from this study suggest that seven days’ of form deprivation inhibited ciliary muscle growth or induced ciliary muscle atrophy (smaller cells with less smooth muscle actin) in eyes that developed axial myopia. This study also found multiple non-significant trends that perhaps may have become significant if the treatment were applied for a longer duration, if a greater amount of myopia was induced, or if the sample size were increased. Past work has demonstrated in humans that there are no significant between-eye ciliary muscle differences with low amounts of anisometropia (> 1.00 D), while there are significant between-eye differences with high amounts of anisometropia (> 5.00 D) (Kuchem et al., 2013; Muftuoglu et al., 2009). Furthermore, this study should be repeated with defocus-induced myopia to determine if the two myopia induction procedures produce similar or discordant results (Howlett and McFadden, 2009). This step may prove to be important in understanding the relationship between myopia and the ciliary muscle because the two myopia induction techniques have previously produced discordant results (e.g., a monkey’s ocular growth response to high light conditions) (Smith et al., 2013; Smith et al., 2012).
The current study also attempted to understand if two different United States-based guinea pig strains were susceptible to form deprivation myopia. This approach was necessary because the only commercially available (Elm Hill Laboratory) pigmented guinea pigs in the United States are known to be resistant to myopia induction. Jiang et al. 2019 specifically found that unresponsive Elm Hill guinea pigs had a thicker, multilayered choroid while responsive New Zealand guinea had thinner, unilayered choroids (structural differences that may play a role in induced-myopia susceptibility) (Jiang et al., 2019). The current study found that only about half of the guinea pigs (both strains) used in this study were susceptible to form deprivation-induced myopia, that the Cincinnati and Strain 13 guinea pig strains had a similar susceptibility to form deprivation-induced myopia, and overall, both guinea pig strains had similar histological ciliary muscle characteristics. While not specifically tested in this experiment, structural choroidal variations may be responsible for the variability seen related to the amount of induced myopia (Jiang et al., 2019). The choroid or potentially the cornea or crystalline lens may also be responsible for the non-significantly longer axial lengths seen in the myopic animals with the inconsistent vitreous or axial elongation response (Groups 2 and 3). The ciliary muscle was the primary emphasis of the study and lack of complete biometry was a study limitation. Nevertheless, a future study will be needed to fully evaluate the underlying ocular characteristics resulting in the induced-myopia variability seen in this study. It is likewise currently unknown if a similar susceptibility to myopia or if a similar ciliary muscle response would be seen in both strains if defocus induced myopia was employed. Again, it might be worthwhile exploring defocus lens induced myopia because it has the potential to result in a more dependable myopia animal model. When employing these experiments, it might be best to select a single guinea pig strain since they had a similar susceptibility to induced myopia, especially if studying genetics because other strain differences may exist.
While guinea pig strains with variable susceptibility to myopia induction may seem like a limitation, variable susceptibility may actually prove to be a useful quality because these guinea pigs could potentially be used to determine what genetic or histological features make some of these animals susceptible to myopia and others not susceptible to myopia. Chen et al. used a similar approach in chicks to demonstrate that genetics play an integral part in induced-myopia susceptibility (Chen et al., 2011). Repeating such an experiment in a mammal could prove to be medically important because it could lead to a new myopia treatment or prevention strategy that is more easily translated to humans than one developed in chicks. Additional research with guinea pigs will determine if such a strategy is fruitful.
In conclusion, this study found that seven days’ worth of form deprivation resulted in shorter ciliary muscle length and decreased SMA concentration in guinea pigs that became axially myopic. This ciliary muscle morphological change most likely resulted from ciliary muscle atrophy or inhibited ciliary muscle growth, an induced change that provides some evidence that a visual manipulation that affects ocular growth and refractive error can also affect ciliary muscle characteristics. The information collected in this experiment can also be used to help guide the selection of animal models for studying myopia, and may help provide a better understanding of how the anterior eye responds to ocular elongation. Since responsiveness to induced myopia and induced ciliary muscle characteristics between the two guinea pigs strains were equivocal, the authors simply suggest that future experiments use one of the two strains to decrease potential variability. Overall, gaining a better understanding of the relationship between the ciliary muscle and myopia, as well as other key areas of myopia research, such as time outdoors, are vital to fully understanding the mechanism(s) underlying myopia development and developing novel therapeutic intervention strategies (Bailey et al., 2008; Cho et al., 2005; Ip et al., 2008; Jones et al., 2007; Oliveira et al., 2005; Pucker et al., 2013; Walline et al., 2013; Walline et al., 2009).
Highlights:
Induced axial myopia results in concomitant ciliary muscle morphological changes, but ones that differ from the thickening seen in human myopia
Ciliary muscle changes suggest atrophy or inhibited muscle growth (shorter muscle length and lower smooth muscle actin concentration)
New guinea pig strains were described for myopia development research
5. ACKNOWLEDGEMENTS
The authors thank the National Eye Institute (K08EY023264; P30EY003039) and the University of Alabama at Birmingham (Investment Pool for Action Funds) for financial and equipment support during this experiment. The authors also thank the United States Army Medical Research Institute of Infectious Diseases and Cincinnati Children’s Hospital for providing the guinea pigs strains used in this study. This work was completed in partial fulfillment of ADP’s PhD dissertation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. REFERENCES
- Aller T, Wildsoet C, 2013. Optical control of myopia has come of age: or has it? Optom Vis Sci 90, e135–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstice NS, Phillips JR, 2011. Effect of dual-focus soft contact lens wear on axial myopia progression in children. Ophthalmology 118, 1152–1161. [DOI] [PubMed] [Google Scholar]
- Atchison DA, Pritchard N, Schmid KL, 2006. Peripheral refraction along the horizontal and vertical visual fields in myopia. Vision Res 46, 1450–1458. [DOI] [PubMed] [Google Scholar]
- Atchison DA, Pritchard N, Schmid KL, Scott DH, Jones CE, Pope JM, 2005. Shape of the retinal surface in emmetropia and myopia. Invest Ophthalmol Vis Sci 46, 2698–2707. [DOI] [PubMed] [Google Scholar]
- Bailey MD, Sinnott LT, Mutti DO, 2008. Ciliary body thickness and refractive error in children. Invest Ophthalmol Vis Sci 49, 4353–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowrey HE, Zeng G, D YT, A JL, Wu Y, To CH, C FW, McFadden SA, 2017. The Effect of Spectacle Lenses Containing Peripheral Defocus on Refractive Error and Horizontal Eye Shape in the Guinea Pig. Invest Ophthalmol Vis Sci 58, 2705–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YP, Hocking PM, Wang L, Povazay B, Prashar A, To CH, Erichsen JT, Feldkaemper M, Hofer B, Drexler W, Schaeffel F, Guggenheim JA, 2011. Selective breeding for susceptibility to myopia reveals a gene-environment interaction. Invest Ophthalmol Vis Sci 52, 4003–4011. [DOI] [PubMed] [Google Scholar]
- Cho P, Cheung SW, Edwards M, 2005. The longitudinal orthokeratology research in children (LORIC) in Hong Kong: a pilot study on refractive changes and myopic control. Curr Eye Res 30, 71–80. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Zelinka C, Milani-Nejad N, 2015. Reactive retinal microglia, neuronal survival, and the formation of retinal folds and detachments. Glia 63, 313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwiazda J, Thorn F, Held R, 2005. Accommodation, accommodative convergence, and response AC/A ratios before and at the onset of myopia in children. Optom Vis Sci 82, 273–278. [DOI] [PubMed] [Google Scholar]
- Howlett MH, McFadden SA, 2006. Form-deprivation myopia in the guinea pig (Cavia porcellus). Vision Res 46, 267–283. [DOI] [PubMed] [Google Scholar]
- Howlett MH, McFadden SA, 2007. Emmetropization and schematic eye models in developing pigmented guinea pigs. Vision Res 47, 1178–1190. [DOI] [PubMed] [Google Scholar]
- Howlett MH, McFadden SA, 2009. Spectacle lens compensation in the pigmented guinea pig. Vision Res 49, 219–227. [DOI] [PubMed] [Google Scholar]
- Huang HM, Chang DS, Wu PC, 2015. The Association between Near Work Activities and Myopia in Children-A Systematic Review and Meta-Analysis. PLoS One 10, e0140419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip JM, Rose KA, Morgan IG, Burlutsky G, Mitchell P, 2008. Myopia and the urban environment: findings in a sample of 12-year-old Australian school children. Invest Ophthalmol Vis Sci 49, 3858–3863. [DOI] [PubMed] [Google Scholar]
- Jiang L, Garcia MB, Hammond D, Dahanayake D, Wildsoet CF, 2019. Strain-Dependent Differences in Sensitivity to Myopia-Inducing Stimuli in Guinea Pigs and Role of Choroid. Invest Ophthalmol Vis Sci 60, 1226–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LA, Sinnott LT, Mutti DO, Mitchell GL, Moeschberger ML, Zadnik K, 2007. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci 48, 3524–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchem MK, Sinnott LT, Kao CY, Bailey MD, 2013. Ciliary muscle thickness in anisometropia. Optom Vis Sci 90, 1312–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wildsoet C, 2012. The effective add inherent in 2-zone negative lenses inhibits eye growth in myopic young chicks. Invest Ophthalmol Vis Sci 53, 5085–5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muftuoglu O, Hosal BM, Zilelioglu G, 2009. Ciliary body thickness in unilateral high axial myopia. Eye 23, 1176–1181. [DOI] [PubMed] [Google Scholar]
- Mutti DO, Hayes JR, Mitchell GL, Jones LA, Moeschberger ML, Cotter SA, Kleinstein RN, Manny RE, Twelker JD, Zadnik K, 2007. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci 48, 2510–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutti DO, Jones LA, Moeschberger ML, Zadnik K, 2000. AC/A ratio, age, and refractive error in children. Invest Ophthalmol Vis Sci 41, 2469–2478. [PubMed] [Google Scholar]
- Mutti DO, Mitchell GL, Hayes JR, Jones LA, Moeschberger ML, Cotter SA, Kleinstein RN, Manny RE, Twelker JD, Zadnik K, 2006. Accommodative lag before and after the onset of myopia. Invest Ophthalmol Vis Sci 47, 837–846. [DOI] [PubMed] [Google Scholar]
- Oliveira C, Tello C, Liebmann JM, Ritch R, 2005. Ciliary body thickness increases with increasing axial myopia. Am J Ophthalmol 140, 324–325. [DOI] [PubMed] [Google Scholar]
- Ostrin LA, Garcia MB, Choh V, Wildsoet CF, 2014. Pharmacologically stimulated pupil and accommodative changes in Guinea pigs. Invest Ophthalmol Vis Sci 55, 5456–5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucker AD, Carpenter AR, McHugh KM, Mutti DO, 2014. Guinea pig ciliary muscle development. Optom Vis Sci 91, 730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucker AD, Jackson AR, Morris HJ, Fischer AJ, McHugh KM, Mutti DO, 2015. Ciliary Muscle Cell Changes During Guinea Pig Development. Invest Ophthalmol Vis Sci 56, 7691–7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucker AD, Sinnott LT, Kao CY, Bailey MD, 2013. Region-specific relationships between refractive error and ciliary muscle thickness in children. Invest Ophthalmol Vis Sci 54, 4710–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaridurg P, Holden B, Smith E 3rd, Naduvilath T, Chen X, de la Jara PL, Martinez A, Kwan J, Ho A, Frick K, Ge J, 2011. Decrease in rate of myopia progression with a contact lens designed to reduce relative peripheral hyperopia: one-year results. Invest Ophthalmol Vis Sci 52, 9362–9367. [DOI] [PubMed] [Google Scholar]
- Saw SM, Shankar A, Tan SB, Taylor H, Tan DT, Stone RA, Wong TY, 2006. A cohort study of incident myopia in Singaporean children. Invest Ophthalmol Vis Sci 47, 1839–1844. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Glasser A, Howland HC, 1988. Accommodation, refractive error and eye growth in chickens. Vision Res 28, 639–657. [DOI] [PubMed] [Google Scholar]
- Smith EL 3rd, Hung LF, Arumugam B, Huang J, 2013. Negative lens-induced myopia in infant monkeys: effects of high ambient lighting. Invest Ophthalmol Vis Sci 54, 2959–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL 3rd, Hung LF, Huang J, 2009. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vision Res 49, 2386–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL 3rd, Hung LF, Huang J, 2012. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci 53, 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL 3rd, Ramamirtham R, Qiao-Grider Y, Hung LF, Huang J, Kee CS, Coats D, Paysse E, 2007. Effects of foveal ablation on emmetropization and form-deprivation myopia. Invest Ophthalmol Vis Sci 48, 3914–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walline JJ, Greiner KL, McVey ME, Jones-Jordan LA, 2013. Multifocal contact lens myopia control. Optom Vis Sci 90, 1207–1214. [DOI] [PubMed] [Google Scholar]
- Walline JJ, Jones LA, Sinnott LT, 2009. Corneal reshaping and myopia progression. Br J Ophthalmol 93, 1181–1185. [DOI] [PubMed] [Google Scholar]
- Zadnik K, Sinnott LT, Cotter SA, Jones-Jordan LA, Kleinstein RN, Manny RE, Twelker JD, Mutti DO, Collaborative Longitudinal Evaluation of, E., Refractive Error Study, G., 2015. Prediction of Juvenile-Onset Myopia. JAMA Ophthalmol 133, 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylbermann R, Landau D, Berson D, 1993. The influence of study habits on myopia in Jewish teenagers. J Pediatr Ophthalmol Strabismus 30, 319–322. [DOI] [PubMed] [Google Scholar]


