Abstract
Background:
Morning stiffness is a hallmark symptom of rheumatoid arthritis (RA), but its etiology is poorly understood. We sought to determine whether any histologic features of synovium associate with this symptom.
Methods:
Patient reports of morning stiffness duration, stiffness severity and disease activity scores (DAS28) were collected from 176 patients with RA undergoing arthroplasty. Histopathology of synovium was scored for 10 features: synovial lining hyperplasia, lymphocytes, plasma cells, Russell bodies, binucleate plasma cells, fibrin, synovial giant cells detritus, neutrophils and mucin. Fibrinolysis of clots seeded with various cell types was measured in turbidimetric lysis assays.
Results:
Stiffness severity and morning stiffness duration were both significantly associated with DAS28 (p=0.0001 and p=0.001 respectively). None of the synovial features examined were associated with patient-reported stiffness severity. The presence of neutrophils and fibrin in RA synovial tissue were significantly (P<0.0001) associated with patient report of greater than one hour of morning stiffness, such that 73% of patients with both synovial fibrin and neutrophils report more than one hour of morning stiffness. Further, neutrophils and fibrin deposits colocalized along the synovial lining. In in vitro analyses, fibrin clots seeded with necrotic neutrophils were more resistant to fibrinolysis than those seeded with living neutrophils or no cells (p=0.008). DNase1 treatment of necrotic neutrophils abrogated the delay in fibrinolysis.
Conclusion:
In RA, prolonged morning stiffness may be related to impaired fibrinolysis of neutrophil enmeshed fibrin deposits along the synovial membrane. Our findings also suggest that morning stiffness severity and duration may reflect distinct pathophysiological phenomena.
Introduction
Rheumatoid arthritis (RA) is characterized by symptoms of joint swelling, pain and stiffness in the morning[1], which improves as the day progresses. Morning stiffness interferes with activities of daily living such as bathing and dressing, making it difficult for RA patients to get to work on time, and is therefore the most commonly cited reason for early retirement [2, 3]. Morning stiffness lasting more than one hour was one of the 1987 American College of Rheumatology classification criteria for RA[4]. This symptom was removed from the classification criteria update in 2010[1], in part due to reports noting the lack of specificity for RA, as well as conflicting reports of its association with disease activity [5–7] [8]. Though morning stiffness is no longer included in the classification criteria, the symptom is important to patients [9] and is still routinely used by clinical rheumatologists to distinguish inflammatory arthritis from degenerative arthritis. If prolonged morning stiffness is a characteristic of inflammatory arthritis, synovial inflammation might be increased in patients who report morning stiffness. However, histologic descriptions of synovial pathology from RA patients with and without morning stiffness are lacking. We hypothesized that inflammatory features of RA synovium might associate with morning stiffness, and reasoned that a more granular understanding of which synovial features correlate with this symptom might provide insights into the mechanism underlying this problem.
We recently demonstrated that 10 synovial features, identified by histologic analysis of hematoxylin and eosin (H&E) stained synovial tissue sections, can be used to predict inflammatory gene expression subtypes[10]. These include scores of cellular and other histologic features including synovial lining hyperplasia, lymphocytes, plasma cells, Russell bodies, binucleate plasma cells, synovial giant cells, detritus, mucin, fibrin and neutrophils. Here, we report our analysis of histology features and patient reports of stiffness in a cohort of 176 RA patients undergoing arthroplasty. We discovered that synovial fibrin and neutrophils correlated with duration of morning stiffness. We also found that neutrophils could be detected within fibrin clots along the lining of the synovial membrane and embedded in synovial fluid rice bodies. Based on this observation, we performed in vitro coagulation and lysis assays and found that clots containing necrotic neutrophils were resistant to plasmin mediated fibrinolysis and this effect could be abrogated by treatment with DNase1. Taken together our results demonstrate that neutrophil laden fibrin deposits are present in RA synovium and are associated with prolonged morning stiffness in patients with RA.
Methods
Patient Cohort
176 patients diagnosed with RA undergoing arthroplasty, as previously described[11], who had responded to questions about stiffness were included in this analysis. Disease activity was measured at the presurgical screening visit, typically 2 weeks prior to arthroplasty, or on the day of surgery, using the DAS28-ESR. DAS28 scores were categorized as low (<3.2), moderate (<5.1) or high (≥5.1). The rheumatoid arthritis disease activity index (RADAI) [12], which includes the questions: “Were your joints stiff when you woke up today? If yes, how long did the stiffness last?” and the answers options including “no stiffness, <30 minutes, 30–60 minutes, 1–2 hours, 2–3 hours, 3–4 hours, >4 hours, all day” were used to measure stiffness duration and the Outcomes Measures in Rheumatology (OMERACT) RA Flare Questionnaire[13], which includes the question, “How severe was your stiffness over the past week” with a 10-point numerical rating scale to respond between no stiffness and severe stiffness, was used to mesure stiffness severity.
Histology Scoring
Synovial samples were preferentially taken from grossly inflamed (dull and opaque) synovium. If no inflammation was apparent, samples were taken from standardized locations: the femoral aspects of the medial and lateral gutters, and the central supratrochlear region in the suprapatellar pouch. Tissue samples were stained with Harris modified hematoxylin solution (Sigma-Aldrich) and eosin Y (Sigma-Aldrich). Ten features (synovial lining hyperplasia, lymphocytes, plasma cells, Russell bodies, binucleate plasma cells, fibrin, synovial giant cells detritus, neutrophils and mucin) were scored as previously described[10], www.hss.edu/pathology-synovitis.asp.
Clotting Assay
Normal donor plasma was collected using ethylenediaminetetraacetic acid (EDTA) tubes (Becton Dickinson), which anticoagulates blood by chelating calcium. Plasma was filtered using a 0.45μm syringe filter (Whatman). 90μl plasma was plated in 4–10 technical replicates with either no cells, live neutrophils (PMN) or necrotic PMN (necrosis was induced by five successive cycles of freezing and thawing of PMN pellets) at 1.5 million cells per milliliter, in 96 well plates. For experiments using DNase1 pretreatment, necrotic PMN were incubated with 5U DNase1 (Promega) for 30 minutes at 37°C in HBSS with 6mM CaCl2. Clotting was induced with addition of 6mM calcium chloride (Sigma-Aldrich) diluted in phosphate buffered saline (PBS) (EMD Millipore). Plates were incubated either rotating at 300 revolutions per minute or without movement. Clotting was confirmed by comparing optical density prior to addition of calcium.
Fibrinolysis Assay
Clots were incubated at 65°C for 30 minutes to denature enzyme activity and then 570nM human plasmin (Haematologic Technologies) was added to the clots (1:20) and plates were incubated on a rotator at 37°C and optical density was measured serially over time up to 270 minutes.
Statistical Methods
Shapiro-Wilk normality test was used to assess the normality of distribution of clinical features. Difference in the presence of stiffness across the DAS28 scores ranges of low, moderate or high was tested using a chi-squared test. Kruskal-Wallis tests were used to test for differences in morning stiffness duration and stiffness severity across DAS28 groups. Morning stiffness duration was modeled as a binary outcome using logistic regression classified as either < 1 hour vs. ≥ 1 hour and severity was modeled as a continuous outcome using linear regression. Simple regression models were performed first to examine the association between DAS28-ESR and morning stiffness duration as well as stiffness severity. Multivariable regression models were performed with the main variable of interest (DAS28-ESR) and the following clinical variables: age, gender, BMI, duration since RA diagnosis, anti-CCP, and RF. Mann-Whitney or Kruskal-Wallis tests were used to compare stiffness severity according to 10 histology feature scores. Chi-squared or Fisher’s exact test was used to test the association between the 10 histology features and the binary classification of greater or less than 1 hour of morning stiffness duration. Bonferroni-adjusted p-values are reported to correct for multiple testing of the histology features. The Cochrane-Armitage Trend test was used to test the significance of neither, fibrin only, neutrophils only or both fibrin and neutrophils and morning stiffness duration. Unpaired t-test was used to test significance of EDTA plasma before and after addition of calcium. Mixed-effects model for repeated measures with clot condition, time, and clot condition-by-time interaction was used to measure the effect of various cells on fibrinolysis. ANOVA with Dunnett’s correction for multiple comparisons was used to test effect of clot condition at the final time point. SAS Studio Version 3.7 was used for the statistical analysis.
Results
Clinical characteristics
Patient characteristics are presented in Supplemental Table 1. The majority of patients were female, and 41% and 70% were seropositive for RF and CCP, respectively. Median disease duration was 11 years, the average DAS28 was 3.7 (moderate) and 52% of patients were treated with a biologic agent (until one dose interval prior to surgery).
Association of severity and duration of morning stiffness with disease activity
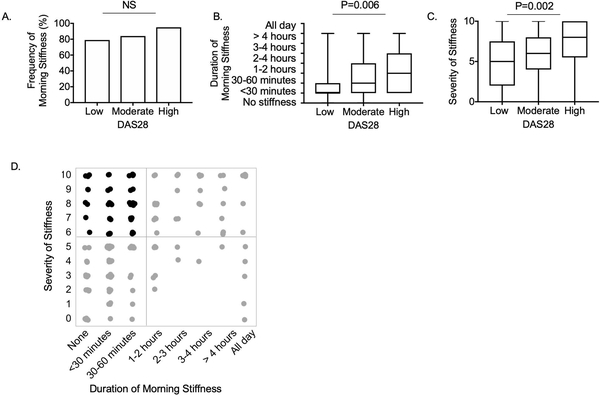
Given inconsistencies in previous reports evaluating the association of morning stiffness with RA related disease activity, we first evaluated whether the severity or duration of morning stiffness was associated with disease activity in our cohort. The vast majority of patients (83%) responded that they were stiff that morning, and there was no significant difference in frequency of any morning stiffness between individuals having low, moderate or high DAS28 scores (Figure 1A). However, the duration of morning stiffness was significantly longer in those with high DAS28 (Figure 1B). We also evaluated whether morning stiffness duration was associated with disease activity using logistic regression and found morning stiffness duration of greater than one hour was significantly associated with DAS28 (P =0.001), and this association remained significant after incorporating covariates including age, gender, body mass index (BMI), duration of diagnosis, anti-CCP, RF and smoking status (Table 1). None of these additional covariates were significantly associated with morning stiffness duration.
Figure 1:
Association of morning stiffness severity and duration with disease activity
A. Frequency of patients reporting any morning stiffness stratified by DAS28 score severity. B. Duration of morning stiffness stratified by DAS28 score severity. C. Severity of stiffness stratified by DAS28 score severity. D. Distribution of responses to questions regarding duration of morning stiffness and severity of stiffness. Each dot represent one patient response. The black dots represent the 43% of patients with discordant responses (less than one hour of morning stiffness but stiffness severity ≥6/10). P values represent results of chi-squared (A) and Kruskal-Wallis (B and C) tests.
Table 1.
Logistic regression model of morning stiffness duration (1 hour cutoff) and DAS28-ESR
| Initial Model | Final Model | |||
|---|---|---|---|---|
| Variables | Odds ratio (95% CI) | P-value | Odds ratio (95% CI) | P-value |
| DAS28-ESR | 1.63 (1.22, 2.19) | 0.001 | 1.85 (1.29, 2.65) | 0.001 |
| Age (years) | 1.0 (0.96, 1.04) | 0.91 | ||
| BMI | 0.98 (0.92, 1.03) | 0.40 | ||
| Duration of diagnosis (years) | 1.03 (1.0, 1.07) | 0.09 | ||
| Gender | ||||
| M (reference) | ||||
| F | 0.86 (0.27, 2.71) | 0.80 | ||
| CCP | ||||
| 0 (reference) | ||||
| 1 | 0.36 (0.12, 1.13) | 0.08 | ||
| 2 | 1.71 (0.57, 5.15) | 0.34 | ||
| RF | ||||
| 0 (reference) | ||||
| 1 | 0.76 (0.29, 1.98) | 0.58 | ||
| Current smoker | ||||
| No (reference) | ||||
| Yes | 0.38 (0.05, 2.93) | 0.36 | ||
Stiffness severity also varied significantly across DAS28 groups (Figure 1C). We further evaluated the relationship of stiffness severity with DAS28 using linear regression and found stiffness severity was significantly associated with DAS28 (Table 2) (P =0.0001), and this association remained significant after incorporating the additional clinical features into the model. Duration of disease was also significantly associated with stiffness severity (P=0.003). BMI, gender, CCP or RF were not significantly associated with stiffness severity or duration.
Table 2.
Linear regression model of morning stiffness severity and DAS28-ESR
| Initial Model | Final Model | |||
|---|---|---|---|---|
| Variables | Coefficient estimate |
P-value | Coefficient estimate | P-value |
| Intercept | 3.52 | <0.0001 | 2.50 | 0.20 |
| DAS28-ESR | 0.66 | 0.0001 | 0.48 | 0.008 |
| Age (years) | −0.01 | 0.62 | ||
| BMI | 0.03 | 0.32 | ||
| Duration of diagnosis (years) | 0.06 | 0.003 | ||
| Gender | ||||
| M (reference) | ||||
| F | 0.91 | 0.15 | ||
| CCP | ||||
| 0 (reference) | 0.87 | |||
| 1 | −0.17 | |||
| 2 | −0.33 | |||
| RF | ||||
| 0 (reference) | ||||
| 1 | −0.29 | 0.57 | ||
| Current smoker | ||||
| No (reference) | ||||
| Yes | 1.70 | 0.10 | ||
| R2 | 0.09 | 0.18 | ||
Though reports of stiffness severity tend to increase with duration of morning stiffness, there was notable discordance between reports of severity and duration of stiffness (Figure 1D). 43% of patients with less than one hour of morning stiffness considered their stiffness severe (severity greater than or equal to six out of ten). The finding that stiffness severity, but not morning stiffness duration, was associated with disease duration along with the notable discordance in patient reports of severity and duration suggest that the two questions may capture distinct manifestations of RA.
Neutrophils and fibrin are associated with duration of morning stiffness
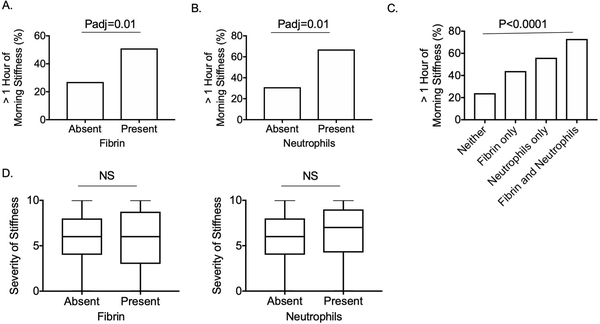
We next tested whether there was an association of any of the 10 histology features, or a histology summary score, and reports of morning stiffness. Histologic detection of fibrin and neutrophils were significantly associated with more than one hour of morning stiffness (Figures 2A and 2B). Fibrin deposition was identified in 41% of cases, while neutrophils were only identified in 15% of cases. We found a significant trend of increasing likelihood for prolonged morning stiffness comparing synovial samples with neither, fibrin only, or neutrophils only, to samples with both fibrin and neutrophils (Figure 2C). None of the 10 synovial histology features assessed, including neutrophils and fibrin, which were associated with stiffness duration, were associated with stiffness severity (Figure 2D).
Figure 2:
Fibrin and neutrophils associate with duration of morning stiffness
Percent of patients with more than one hour of morning stiffness according to presence of synovial fibrin (A) and neutrophils (B). P values represent results of Fisher’s exact tests, using the Bonferroni correction to account for multiple comparisons. C. Percentages of patients with more than one hour of morning stiffness according to having synovium with neither fibrin nor neutrophils, fibrin only, neutrophils only, or both fibrin and neutrophils. P values represent results of Cochran-Armitage trend test. D. Severity of stiffness according to presence of neutrophils and fibrin. P values represent results of Mann-Whitney tests.
Neutrophils colocalize with fibrin deposition along the RA synovial lining
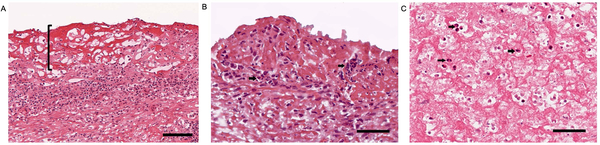
A shared feature of synovial fibrin deposits and neutrophils is that they are both typically found in RA synovial fluid. Fibrin clots can be found in synovial fluid as “rice bodies”, which appear macroscopically as grains of rice floating in synovial fluid[14] and neutrophils are the most abundant white blood cell type in RA synovial fluid[15] and are comparatively sparse in synovial tissue. Both fibrin (Figure 3A) and neutrophils (Figure 3B) can be found along the synovial lining, at the interface with synovial fluid. Further, we histologically observed neutrophils intermixed with synovial lining fibrin deposits (Figure 3B). We also identified neutrophils enmeshed in fibrinous “rice bodies” (Figure 3C).
Figure 3:
Neutrophils colocalize with fibrin deposits along the synovial lining
A. Representative image of H&E stained RA synovial tissue, 20x magnification. Bracket highlights eosinophilic synovial fibrin deposition along the synovial membrane. The scale bar indicates 100μm. B. Representative image of H&E stained RA synovial tissue, 40x magnification. The arrows highlight neutrophils intermixed within fibrin, which appear as pink fibrillary material along the synovial lining. The scale bar indicates 50μm. C. Image of H&E stained rice body, 40x magnification. The arrows highlight neutrophils intermixed within fibrin. The scale bar indicates 50μm.
Neutrophil DNA impedes fibrinolysis
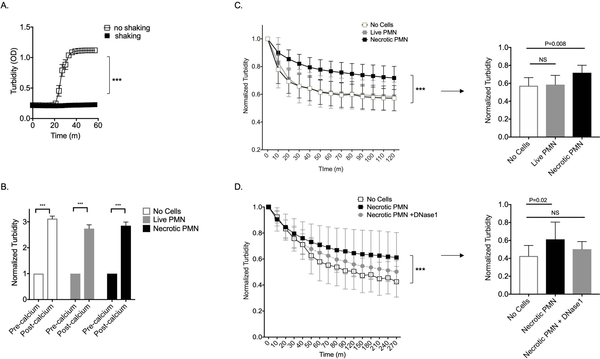
We considered the possibility that fibrin could accumulate along the synovial membrane while patients are relatively sedentary overnight and that this may improve as the day progresses as fibrin clots are lysed by plasmin. Recalcifying EDTA anticoagulated plasma in vitro induced clotting in samples that were incubated without shaking but not in those that were shaken (Figure 4A), which is consistent with extensive clinical data noting immobilization is a risk for thrombosis. We further hypothesized that neutrophils might enhance the stability of fibrin deposits, thereby contributing to the sensation of prolonged morning stiffness. To test whether neutrophils impede plasmin mediated fibrinolysis, we generated fibrin clots in vitro by recalcifying EDTA anticoagulated plasma seeded with either no cells, live PMN, or necrotic PMN. After one hour, all samples were clotted (Figure 4B); however, the peak optical density was variable across groups. We next added plasmin to the various clots and measured fibrinolysis over two hours. We normalized to the peak optical density for any given well, to control for the variability of peak optical density seen in Figure 4B. We observed a significant difference in the various clot conditions over time (condition*time p<0.0001). Clots seeded with live PMN were lysed with kinetics that were similar to clots with no cells, while clots seeded with necrotic PMN were resistant to fibrinolysis (Figure 4C), and this effect could be abrogated by pretreatment of the necrotic PMN lysate with DNase1 (Figure 4D), demonstrating that DNA derived from necrotic PMN can render fibrin resistant to fibrinolysis.
Figure 4:
Necrotic neutrophils impede fibrinolysis
A. Clotting as measured by turbidity (OD=optical density) of EDTA plasma incubated with calcium while shaking or not shaking over time. Data presented are mean and standard deviation of the optical density (absorbance at 405nm) and represent one of two experiments. (m=minutes) B. Clotting as measured by turbidity of EDTA plasma alone or seeded with either live PMN, or necrotic PMN, before and after incubation with calcium for one hour. Data presented are mean and standard deviation of the optical density (absorbance at 405nm), normalized to the value obtained just prior to addition of calcium. For A and B, *** indicates P<0.0001 in unpaired t-tests. C. Left panel: Fibrinolysis as measured by normalized turbidity of clots alone or seeded with either live PMN or necrotic PMN and treated with plasmin. Right panel: Normalized turbidity of fibrin clots from left panel at 120 minutes. Data represent one of six independent experiments. D. Left panel: Fibrinolysis as measured by normalized turbidity of clots alone or seeded with either necrotic PMN or necrotic PMN pretreated with DNase1 and then lysed with plasmin. Right panel: Normalized turbidity of fibrin clots from left panel at 270 minutes. Data represent one of three independent experiments. For C and D, data presented are mean and standard deviation of optical density (absorbance at 405nm), normalized to the value obtained at time 0, just after addition of plasmin. P values in left panels represent results of mixed-effects model for clot conditions over time (*** indicates condition*time P<0.0001) and right panel represents ANOVA with Dunnett’s multiple comparisons test, using the no cells group as the reference. NS= not significant.
Discussion
Morning stiffness is a hallmark clinical symptom experienced by RA patients. Here we report that in a cohort of 176 patients with RA undergoing arthroplasty, 83% experienced morning stiffness, and both morning stiffness duration and stiffness severity were significantly associated with disease activity.
We found that synovial neutrophils and fibrin were significantly associated with patient reports of greater than one hour of morning stiffness. Fibrin, the final product of the clotting cascade, is a gel-like substance frequently observed along the surface of inflamed synovium in RA[10]. Neutrophils are short-lived cells programmed to die within hours after infiltrating tissue. Therefore, the association of synovial neutrophils with prolonged morning stiffness suggests acute synovial inflammation or ongoing neutrophil recruitment may play a role in mediating this symptom. Such a possibility is consistent with our finding that patient reports of stiffness were significantly associated with disease activity. Indeed, one of the principle mechanisms of corticosteroids is to inhibit access of neutrophils to sites of inflammation [16], and a clinical trial of delayed release prednisone found this treatment produced a significant reduction in duration of morning stiffness [17]. We further demonstrate that neutrophils can be found enmeshed in fibrin deposits along the synovial membrane. It is not unexpected that neutrophils can be found in this location as it is well established from extensive clinical experience that inflamed synovial fluid is laden with neutrophils, and since neutrophils reach the synovial fluid by extravasating through blood vessels in synovium, they must cross the synovial lining to reach the synovial fluid space. We report here that necrotic neutrophil DNA can render fibrin deposits resistant to fibrinolysis. This result is consistent with prior reports demonstrating that clots containing histones and DNA have thicker fibers, increased clot stability and greater rigidity [18, 19]. It is plausible that when neutrophils infiltrate the joint, their DNA confers increased stability and rigidity to fibrin deposits along the synovial surface, contributing to the sensation of stiffness by altering the mechanics of articular movement. Interestingly, no plasma cell features or RA associated autoantibodies were significantly associated with duration of morning stiffness. The lack of association of markers typical of an adaptive immune response (plasma cells and autoantibodies), along with the fact that fibrin deposition is a finding common to a spectrum of inflammatory states, raises the possibility that neutrophil enmeshed fibrin could represent a generic cause of stiffness that may also mediate morning stiffness in other rheumatologic diseases, such as ankylosing spondylitis or psoriatic arthritis.
Another interesting finding of our study was that RA patients who rated their stiffness severity as high did not necessarily experience prolonged duration of morning stiffness. It is possible that the discrepancy between these two reported symptoms is that the stiffness severity question was worded to capture stiffness over the past week rather than that particular day, while the stiffness severity question was worded to capture symptoms that day. Alternatively, severity and duration of morning stiffness may reflect distinct pathophysiological mechanisms of inflammation and damage, and thereby representing different clinical symptom constructs. This hypothesis is supported by our finding that stiffness severity associates with disease duration but not with any of the synovial histology features assessed in this study.
An important limitation of this work is that we only assessed a small section of a relatively large synovial membrane of each patient. There could be variability within any given synovium that was not assessed in this study. Further, there are other important structures in joints that might affect the sensation of morning stiffness such as the joint capsule, tendons, ligaments, vasculature, cartilage and bone and none of these were included in this analysis. Finally, it is important to note that the joints studied in this analysis were limited to large joints (hips and knees) from patients with relatively long disease duration (median 11 years). It is not known whether neutrophils and fibrin are also associated with morning stiffness duration in small joints such as hands and feet, or in patients with early arthritis.
In summary, we investigated the association of morning stiffness with clinical and synovial histologic features of RA in a cohort of patients undergoing arthroplasty. We found that both synovial tissue neutrophils and fibrin are associated with duration of morning stiffness and that neutrophil derived DNA renders fibrin resistant to fibrinolysis.
Our observations identify histologic findings that are significantly associated with patient report of morning stiffness, and thereby provide insights into potential mechanisms mediating a vexing clinical symptom that is frequently experienced by patients with RA and other types of inflammatory arthritis.
Supplementary Material
Acknowledgments
This study was supported by Rockefeller University grant # UL1 TR001866 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program, grant #UL1-TR000457–06, grant # 1UH2AR067691 from the National Institutes of Health (NIH) Accelerating Medicine Partnership program, the Weill Cornell Clinical Translational Science Center (CTSC) (UL1-TR000457–06), and The Block Family Foundation. SLM is affiliated to the Leeds Biomedical Research Centre, part of the UK National Institute for Health Research (NIHR). The views expressed are those of the authors and not necessarily those of the NIHR or the UK Department of Health and Social Care.
References
- 1.Aletaha D, et al. , 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum, 2010. 62(9): p. 2569–81. [DOI] [PubMed] [Google Scholar]
- 2.Mattila K, Buttgereit F, and Tuominen R, Impact of morning stiffness on working behaviour and performance in people with rheumatoid arthritis. Rheumatol Int, 2014. 34(12): p. 1751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mattila K, Buttgereit F, and Tuominen R, Influence of rheumatoid arthritis-related morning stiffness on productivity at work: results from a survey in 11 European countries. Rheumatol Int, 2015. 35(11): p. 1791–7. [DOI] [PubMed] [Google Scholar]
- 4.Arnett FC, et al. , The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum, 1988. 31(3): p. 315–24. [DOI] [PubMed] [Google Scholar]
- 5.Hazes JM, Hayton R, and Silman AJ, A reevaluation of the symptom of morning stiffness. J Rheumatol, 1993. 20(7): p. 1138–42. [PubMed] [Google Scholar]
- 6.Boers M, et al. , What Is the Relationship Between Morning Symptoms and Measures of Disease Activity in Patients With Rheumatoid Arthritis? Arthritis Care Res (Hoboken), 2015. 67(9): p. 1202–9. [DOI] [PubMed] [Google Scholar]
- 7.Khan NA, et al. , Reevaluation of the role of duration of morning stiffness in the assessment of rheumatoid arthritis activity. J Rheumatol, 2009. 36(11): p. 2435–42. [DOI] [PubMed] [Google Scholar]
- 8.Yazici Y, et al. , Morning stiffness: how common is it and does it correlate with physician and patient global assessment of disease activity? J Rheumatol, 2001. 28(6): p. 1468–9. [PubMed] [Google Scholar]
- 9.Orbai AM, et al. , “Stiffness has different meanings, I think, to everyone”: examining stiffness from the perspective of people living with rheumatoid arthritis. Arthritis Care Res (Hoboken), 2014. 66(11): p. 1662–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orange DE, et al. , Identification of Three Rheumatoid Arthritis Disease Subtypes by Machine Learning Integration of Synovial Histologic Features and RNA Sequencing Data. Arthritis Rheumatol, 2018. 70(5): p. 690–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodman SM, et al. , Flares in Patients with Rheumatoid Arthritis after Total Hip and Total Knee Arthroplasty: Rates, Characteristics, and Risk Factors. J Rheumatol, 2018. 45(5): p. 604–611. [DOI] [PubMed] [Google Scholar]
- 12.Stucki G, et al. , A self-administered rheumatoid arthritis disease activity index (RADAI) for epidemiologic research. Psychometric properties and correlation with parameters of disease activity. Arthritis Rheum, 1995. 38(6): p. 795–8. [DOI] [PubMed] [Google Scholar]
- 13.Bykerk VP, et al. , Identifying flares in rheumatoid arthritis: reliability and construct validation of the OMERACT RA Flare Core Domain Set. RMD Open, 2016. 2(1): p. e000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popert AJ, et al. , Frequency of occurrence, mode of development, and significance or rice bodies in rheumatoid joints. Ann Rheum Dis, 1982. 41(2): p. 109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dougados M, Synovial fluid cell analysis. Baillieres Clin Rheumatol, 1996. 10(3): p. 519–34. [DOI] [PubMed] [Google Scholar]
- 16.Fauci AS, Dale DC, and Balow JE, Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med, 1976. 84(3): p. 304–15. [DOI] [PubMed] [Google Scholar]
- 17.Buttgereit F, et al. , Targeting pathophysiological rhythms: prednisone chronotherapy shows sustained efficacy in rheumatoid arthritis. Ann Rheum Dis, 2010. 69(7): p. 1275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longstaff C, et al. , Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J Biol Chem, 2013. 288(10): p. 6946–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varju I, et al. , DNA, histones and neutrophil extracellular traps exert anti-fibrinolytic effects in a plasma environment. Thromb Haemost, 2015. 113(6): p. 1289–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.