Age-related macular degeneration (AMD) affects 30 percent of Americans over 80 years of age and is a leading cause of blindness in the elderly.1 While early stages of the disease are characterized by drusen and pigmentary changes, advanced forms of AMD may include retinal pigment epithelium (RPE) and photoreceptor loss in geographic atrophy (GA), or development of choroidal neovascularization (CNV) in exudative AMD. Previous studies on AMD pathogenesis have focused mostly on oxidative stress, complement dysregulation, and lipofuscin or lipid accumulation,2–5 but the advent of optical coherence tomography angiography (OCT-A) has prompted renewed interest in the vascular contribution to this disease.
A hemodynamic model of AMD was first proposed by Friedman, who hypothesized that scleral stiffening results in impaired choroidal blood flow leading to RPE damage and drusen formation.6 Angiographic and histopathologic analyses have also demonstrated decreased choroidal blood flow in eyes with AMD.7–10 More recent studies using OCT-A further supported this hypothesis, demonstrating reduced choriocapillaris density across a spectrum of AMD phenotypes from soft drusen or reticular pseudodrusen11–14 to various forms of CNV.15–17 Choriocapillary loss has also been implicated as a predictor of drusen18 or GA progression.19, 20
While the role of the choroidal vasculature in AMD has been well-established,21, 22 the contribution of retinal vessels to AMD pathogenesis remains unclear. Snyder and colleagues first suggested a possible role of the retinal vasculature in exudative AMD by demonstrating that the presence of a cilioretinal artery may be protective against CNV based on color fundus photographs from the Age-Related Eye Disease Study (AREDS).23 Later OCT-A studies also showed lower retinal vessel density (VD) and larger foveal avascular zones (FAZ) in eyes with non-exudative AMD compared with normal individuals,11, 24, 25 suggesting that a loss in retinal vascularity may be associated with early AMD, although the impact on advanced stages of AMD was unclear.
In this study, we compare retinal VD in non-exudative and exudative AMD eyes using OCT-A to determine if differences in retinal perfusion may be associated with CNV. We hypothesized that eyes with exudative AMD may have reduced retinal capillary perfusion in the central macula. We further evaluate the role of intravitreal anti-angiogenesis treatments and geographic atrophy on the retinal VD in the macular region and speculate on the impact of retinal perfusion on the pathogenesis and management of exudative and non-exudative AMD.
METHODS
Patient Selection
In this retrospective, cross-sectional study, we identified all patients who were seen at the University of California, Davis Eye Center between January 27, 2017 and February 28, 2019 who were diagnosed with AMD (ICD-9-CM code 362.5 or ICD-10-CM code H35.3x) and underwent both spectral-domain OCT and OCT-A imaging. The study was performed in accordance with the Declaration of Helsinki and in compliance with the Health Insurance Portability and Accountability Act. It was approved by the Institutional Review Board at the University of California, Davis. Eyes with any media opacity, retinal vascular conditions, macular disease other than AMD, or prior retinal surgeries were excluded from analysis. Patient demographics and clinical characteristics including age, sex, laterality, best-corrected visual acuity (BCVA, logMAR), refractive error (spherical equivalent), lens status (phakic or pseudophakic), intraocular pressure (IOP, mmHg), type of AMD (exudative or non-exudative) and severity of non-exudative AMD (early, intermediate, or advanced) as diagnosed by the physician, and prior treatments including number and type of previous intravitreal anti-vascular endothelial growth factor (anti-VEGF) treatments, were collected from the visit date when the imaging was performed. Eyes with unclear documentation of total number of prior anti-VEGF injections were excluded from analyses evaluating the impact of anti-VEGF treatments. Additionally, healthy age-matched volunteer subjects without any retinal diseases were recruited as normal controls.
Image Acquisition & Analysis
OCT-A images were acquired using the Zeiss Cirrus HD-OCT 5000 with AngioPlex software (Carl Zeiss Meditec, Dublin, CA, USA; version 11.0), which uses an 840 nm laser with an A-scan rate of 68 kHz. The AngioPlex software uses the OCT-microangiography complex algorithm (OMAG) which identifies changes in both phase and intensity information to quantify motion contrasts. 245 × 245 A-scans images were captured over a 3 × 3 mm area centered on the fovea. OCT-A scans were assessed and manually adjusted to ensure accurate centration over the foveal center, accurate segmentation of the superficial retinal capillary plexus (defined as the layer between the internal limiting membrane to the inner plexiform layer), and accurate delineation of the FAZ. Images with significant segmentation errors, severe atrophy involving the inner retinal layers, or signal strength of 5 or less were excluded from analysis, based on published reliability studies of vessel density measured using the Angioplex OCT-A algorithm and platform.26 Eyes with 6 × 6 mm OCT-A scans were excluded due to reduced accuracy in FAZ measurements.27 Retinal VD was automatically measured from the central 1 mm area (fovea), surrounding 3 mm diameter inner ring (parafovea), and full 3 × 3 mm circular region (full), based on the Early Treatment of Diabetic Retinopathy Study (ETDRS) grid using the AngioPlex review software. The FAZ area, circularity, and perimeter were also measured automatically using the software.
Spectral-domain OCT images captured at the same visit were also analyzed for the presence or absence of GA, defined as complete RPE and outer retinal atrophy (cRORA) as termed by the Classification of Atrophy Meetings (CAM) group,28 in the central 1 mm region (central) or outside the central 1 mm region (non-central). All images were evaluated by two independent graders (SL, ST), with discrepancies in grading resolved by adjudication by a senior image grader (GY).
Statistical Analysis
Differences in demographic, clinical, or ocular characteristics between exudative and non-exudative AMD were assessed by Fisher’s exact tests for categorical variables (sex, laterality, and lens status) and Mann-Whitney U tests for scale variables (age, refractive error, BCVA, and IOP). The association between exudative or non-exudative AMD with foveal, parafoveal, and full VD or FAZ area, perimeter, and circularity were measured using multivariate regression analyses with age and central subfield thickness (CST) as covariates to adjust for age-related and OCT thickness changes in retinal vascularity, and generalized estimating equations to account for more than one eye per subject. Multivariate regression analyses were also used to determine the number of intravitreal injections in exudative AMD, or the association of AMD stage (early, intermediate, or advanced) or presence of central or non-central GA in non-exudative AMD, with retinal vascular measurements. A P-value of < 0.05 was considered statistically significant. Statistical analyses were conducted using SPSS software (IBM Corp., NY, USA; version 22.0).
RESULTS
Demographic & Clinical Characteristics
182 subjects were included in this study. Mean age was 78.8 ± 8.8 years and most subjects (66.5%) were female. Of the 310 eyes that met inclusion criteria for analysis, 168 eyes (54.2%) had non-exudative AMD and 142 eyes (45.8%) had exudative AMD. Patients with eyes with non-exudative AMD were slightly younger than those with exudative AMD (77.6 ± 8.4 vs. 79.7 ± 9.2, P = 0.028), and less likely to be pseudophakic (48.8% vs. 60.6%, P = 0.040) (Table 1). As expected, visual acuity in eyes with non-exudative AMD were superior to those with exudative AMD (logMAR BCVA 0.32 ± 0.32 vs. 0.60 ± 0.48, P < 0.001). Otherwise, there were no significant differences in sex, laterality, refractive error, or IOP between eyes with non-exudative and exudative AMD (Table 1). We also included 33 eyes of 22 age-matched healthy subjects without retinal pathology as normal controls (mean age 69.8 ± 8.7 years and 68% female).
Table.
Demographics & Clinical Characteristics
| Non-exudative AMD | Exudative AMD | P-value | |
|---|---|---|---|
| Eyes, n | 168 | 142 | |
| Age (mean ± SD) | 77.6 ± 8.4 | 79.7 ± 9.2 | 0.028a |
| Sex (female) | 67.3% | 68.3% | 0.903b |
| Laterality (right) | 48.2% | 52.1% | 0.569b |
| BCVA (logMAR) | 0.32 ± 0.32 | 0.60 ± 0.48 | <0.001a |
| Rx (diopters) | +0.05 ± 2.68 | −0.06 ± 1.88 | 0.446a |
| Lens (phakic) | 51.2% | 39.4% | 0.040b |
| IOP (mmHg) | 13.9 ± 3.7 | 13.8 ± 3.7 | 0.607a |
Mann-Whitney U test
Fisher’s exact test
Abbreviations: AMD, age-related macular degeneration; SD, standard deviation; BCVA, best corrected visual acuity; Rx, refractive error; IOP, intraocular pressure.
Retinal Vessel Density in Age-Related Macular Degeneration
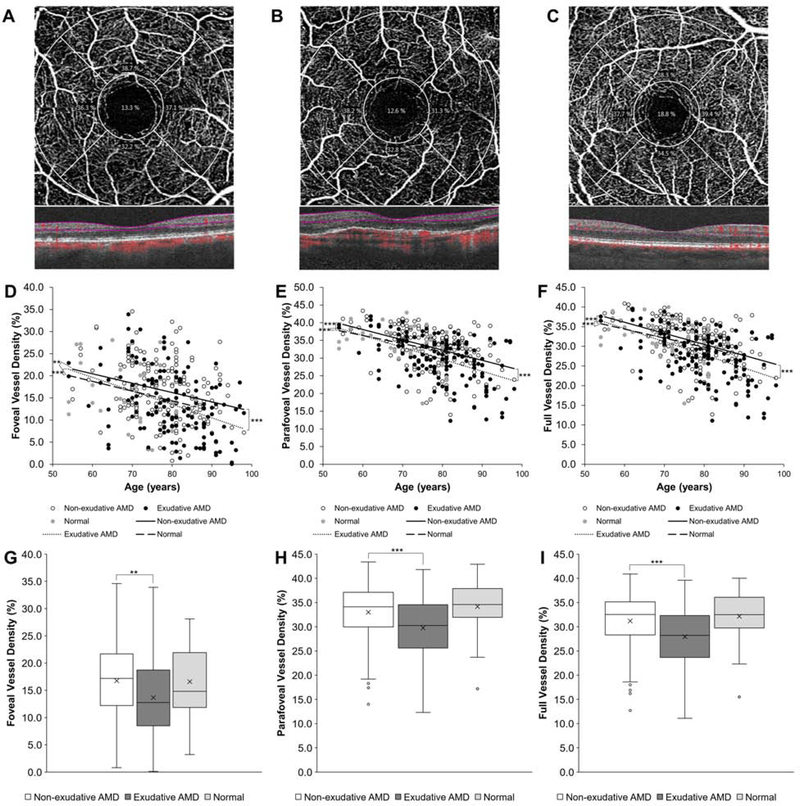
Across all eyes with AMD, the superficial retinal VD decreased with age, including the foveal (β = −0.211, P < 0.001), parafoveal (β = −0.305, P < 0.001), and full 3 mm macular regions (β = −0.295, P < 0.001) (Figures 1A–1F), consistent with prior studies.29–32 Retinal VD also increased with CST for the foveal (β = 0.047, P < 0.001) and full (β = 0.013, P = 0.020) regions but did not differ for the parafoveal region (β = 0.009, P = 0.125). Using multivariate linear regression analyses that adjusted for age and CST, we found that eyes with exudative AMD demonstrated a lower VD in the foveal (13.6 ± 7.2% vs. 16.7 ± 6.7%, P = 0.002), parafoveal (29.8 ± 6.3% vs. 33.0 ± 5.7%, P < 0.001), and full macular regions (27.9 ± 6.2% vs. 31.2 ± 5.5%, P < 0.001) as compared to eyes with non-exudative AMD (Figures 1G–1I). Due to the variability in FAZ size between individuals, VD measurements in the foveal 1 mm region were predictably more variable than the parafoveal ring or full region, as shown on scatterplot analyses (Figures 1D–1F). Normal eyes showed similar mean retinal VD as non-exudative AMD and published studies,29–32 with no statistical difference with either type of AMD in the foveal (16.6 ± 6.6%, P = 0.406 – 0.664), parafoveal (34.2 ± 5.4%, P = 0.248 – 0.400), and full macular regions (32.1 ± 5.4%, P = 0.277 – 0.365) after adjusting for age and CST (Figures 1G–1I), likely due to the smaller number of healthy eyes for comparison.
Figure 1. Retinal Vessel Density in Non-exudative & Exudative Age-Related Macular Degeneration.
Representative optical coherence tomography-angiography (OCT-A) and spectral-domain OCT scans of eyes with non-exudative (A) and exudative (B) age-related macular degeneration (AMD) and a normal control (C) with grid overlay demonstrating the central fovea, parafoveal inner ring, and full 3 mm macular region for vessel density (VD) measurements. Scatterplots with linear regression trendlines showing foveal (D), parafoveal (E), and full (F) VD in eyes with non-exudative and exudative AMD and normal eyes across different patient ages. Box-and- whisker plots comparing foveal (G), parafoveal (H), and full (I) VD in eyes with non-exudative and exudative AMD and normal eyes. **, P<0.01; ***, P<0.001
FAZ Measurements in Age-Related Macular Degeneration
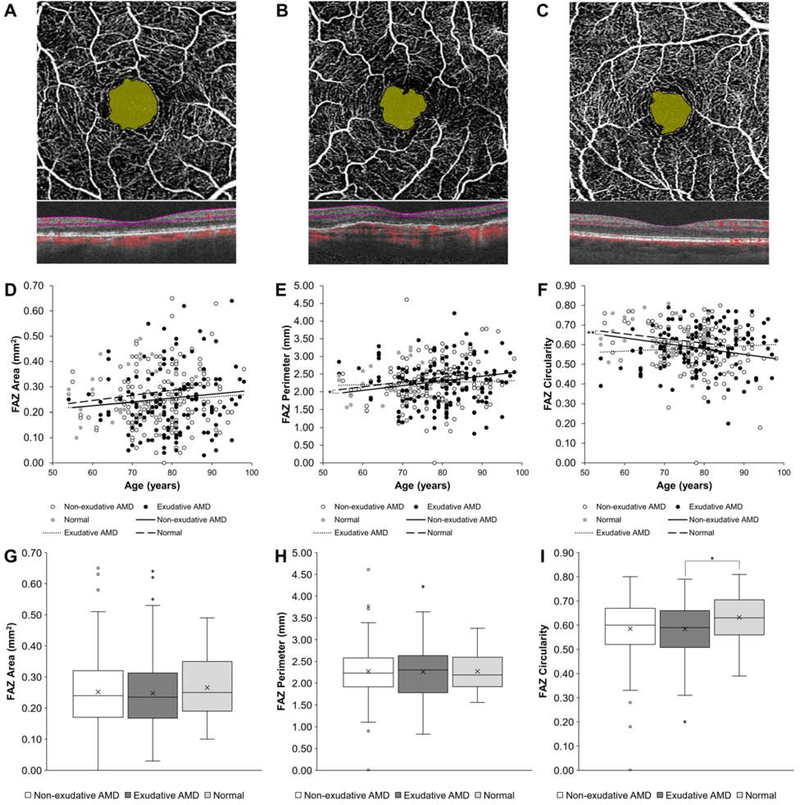
In our cohort of eyes with AMD, FAZ area (β = 0.001, P = 0.411), perimeter (β = 0.004, P = 0.287), and circularity (β = −0.001, P = 0.363) did not show any relationship with age (Figures 2A–2F). Even after adjusting for age and CST, we found no significant difference in FAZ area (β = 0.009, P = 0.503), perimeter (β = 0.042, P = 0.523), or circularity (β = −0.002, P = 0.907) between eyes with non-exudative and exudative AMD (Figures 2G–2I). We also found no significant difference between normal eyes and eyes with either type of AMD in FAZ area (P = 0.261 – 0.528) and FAZ perimeter (P = 0.757 – 0.782) after age and CST adjustments. FAZ circularity was slightly greater in normal eyes compared with eyes with exudative AMD (β = 0.052, P = 0.035), but not significantly different from non-exudative AMD (P = 0.257).
Figure 2. FAZ Measurements in Non-exudative and Exudative Age-Related Macular Degeneration.
Representative OCT-A and spectral-domain OCT scans of eyes with non-exudative (A) and exudative (B) AMD and a normal control (C) with foveal avascular zone (FAZ) area highlighted. Scatterplots with linear regression trendlines showing FAZ area (D), perimeter (E), and circularity (F) in eyes with non-exudative and exudative AMD and normal eyes across different patient ages. Box-and-whisker plots comparing FAZ area (G), perimeter (H), and circularity (I) in eyes with non-exudative and exudative AMD and normal eyes. *, P<0.05; **, P<0.01
Retinal Vascular Measurements after Anti-Vascular Endothelial Growth Factor Treatments
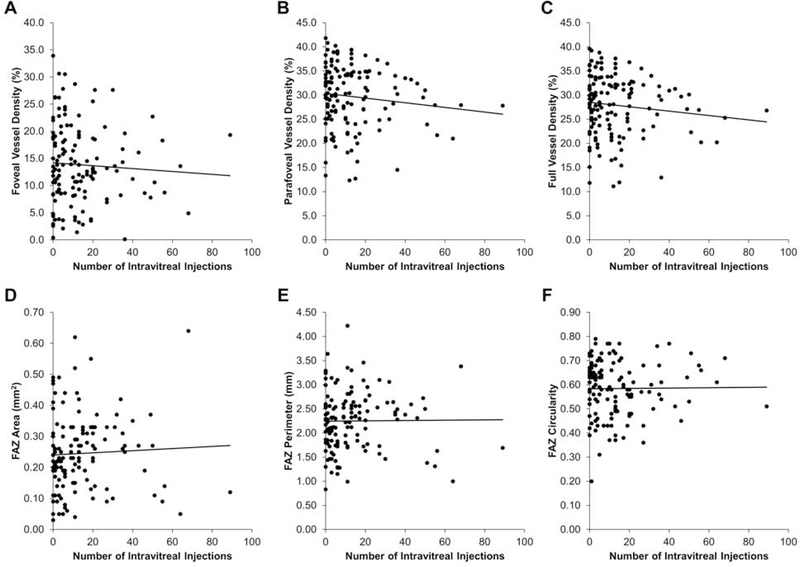
Based on their mechanisms of action, intravitreal anti-VEGF treatments have been shown to impact choroidal thickness33, 34 and reduce choriocapillaris and deep capillary plexus VD in eyes with exudative AMD.35 Among the 134 eyes in our cohort with exudative AMD and documented number of anti-VEGF treatments, the median number of prior anti-VEGF injections was 9 (range 0 to 89). To determine if multiple anti-VEGF treatments negatively impacts the superficial capillary plexus, we performed multivariate regression analyses adjusting for age, and found no adverse impact across all VD and FAZ measurements (Figures 3A–3F). Thus, the number of intravitreal anti-VEGF treatments did not appear to impact the integrity of the superficial retinal vasculature.
Figure 3. Retinal Vascular Measurements after Anti-Vascular Endothelial Growth Factor Treatments.
Scatterplots with trendlines showing the relationship between the number of intravitreal anti-vascular endothelial growth factor (anti-VEGF) injections and foveal (A), parafoveal (B), and full (C) VD, as well as FAZ area (D), perimeter (E), and circularity (F) in eyes with exudative AMD.
Retinal Vascular Measurements in Non-Exudative Age-Related Macular Degeneration
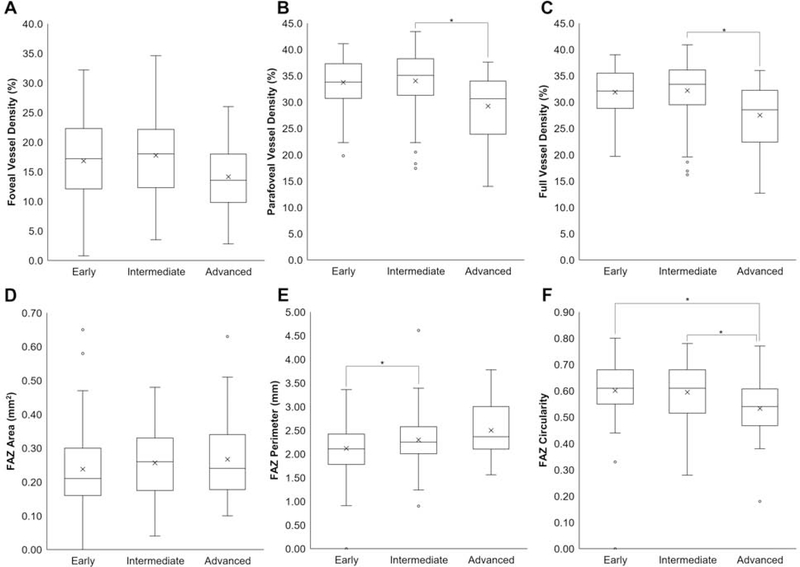
To examine if the severity of non-exudative AMD impacted superficial retinal VD and FAZ parameters, we performed subgroup analyses on eyes with early, intermediate, or advanced AMD. Adjusting for age and CST, we found that eyes in the advanced group exhibited lower parafoveal VD (29.2 ± 6.3% vs. 34.0 ± 5.5%, P = 0.030) and full VD (27.5 ± 6.0% vs. 32.2 ± 5.4%, P = 0.042) compared with eyes with intermediate AMD (Figures 4A–4C). There were no statistical differences in retinal VD between eyes with early and intermediate or advanced AMD at any location (P = 0.055 – 0.841). FAZ area was similar in all 3 subgroups (P = 0.102 – 0.926, Figure 4D), FAZ perimeter was slightly reduced in early versus intermediate AMD (2.1 ± 0.6 mm vs. 2.3 ± 0.5 mm, P = 0.015, Figure 4E), and FAZ circularity was reduced in advanced AMD compared with either early or intermediate AMD (0.5 ± 0.1 vs. 0.6 ± 0.1 vs. 0.6 ± 0.1, P = 0.039 – 0.047, Figure 4F).
Figure 4. Retinal Vascular Measurements in Non-exudative Age-Related Macular Degeneration.
Box-and-whisker plots comparing foveal (A), parafoveal (B), and full (C) VD, as well as FAZ area (D), perimeter (E), and circularity (F) in eyes with early, intermediate, or advanced non-exudative AMD. *, P<0.05
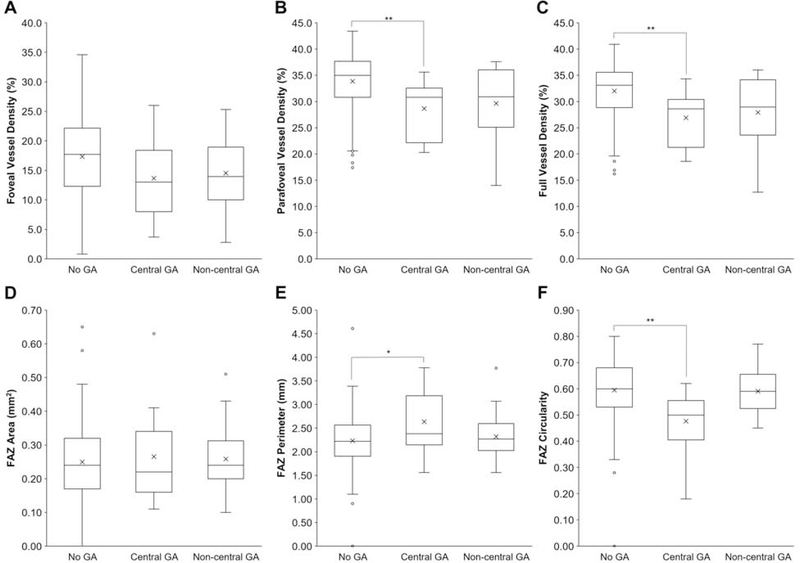
To further assess if the presence and location of GA affected retinal VD or FAZ parameters, we compared non-exudative AMD eyes with no GA, center-involving GA, and non-central GA. Adjusting for age, we found that eyes with central GA demonstrated lower parafoveal VD (28.7 ± 5.3% vs. 33.9 ± 5.2%, P = 0.007) and full VD (26.9 ± 4.9% vs. 32.0 ± 5.1%, P = 0.005) compared with eyes without any GA, while the foveal VD showed no difference between the 3 groups (P = 0.205 – 0.300), likely due to the lower VD and variable FAZ in this central foveal region (Figures 5A–5C). We also found that eyes with central GA showed slightly greater FAZ perimeter (2.6 ± 0.7 mm vs. 2.2 ± 0.6 mm, P = 0.047) and reduced FAZ circularity (0.5 ± 0.1 vs. 0.6 ± 0.1, P = 0.002) compared with eyes without any GA, but no clear difference in FAZ area between the groups (P = 0.861, Figures 5D–5F). Eyes with non-central GA did not differ significantly with eyes without GA across all retinal vascular measurements (Figures 5A–5F). Together, our data suggests that the presence of central GA may also reduce retinal VD and FAZ morphology.
Figure 5. Retinal Vascular Measurements in Geographic Atrophy.
Box-and-whisker plots comparing foveal (A), parafoveal (B), and full (C) VD, as well as FAZ area (D), perimeter (E), and circularity (F) in eyes with non-exudative AMD and no geographic atrophy (GA), central GA, or non-central GA. *, P<0.05; **, P<0.01
DISCUSSION
While the hemodynamic contribution of the choroid to AMD pathogenesis has been well-established, the role of retinal vessels in AMD has been less clear. Retinal VD was previously shown to be decreased in eyes with intermediate AMD compared to age-matched normal subjects, but longitudinal changes in retinal capillary perfusion did not correlate with drusen volume growth over 12 months.36 Although the sensitivity for measuring drusen volume or VD changes using OCT-A over this time period may not be sufficiently high, it is also possible that eyes with lower VD are predisposed to drusen progression without necessarily exhibiting a correlative decline. In this study, we found that VD is reduced in eyes with exudative AMD as compared with non-exudative AMD, but was not impacted by the amount of anti-VEGF therapy. Due to the retrospective nature of our study, we cannot conclude whether the lower retinal VD may increase the risk of developing CNV, or occurs as a consequence of CNV development in eyes with AMD. It is also unclear if anti-VEGF therapy may reduce VD in eyes with exudative AMD, although the absence of a linear relationship with total intravitreal injections opposes this notion.
Despite the differences in VD, we noted no difference in FAZ area, circularity, or perimeter between exudative and non-exudative AMD. Our findings are consistent with prior studies showing decreased VD but no difference in FAZ measurements between eyes with early or intermediate AMD and normal eyes.11, 37 Similarly, although the presence of a cilioretinal artery is protective against CNV, whether the vessel traverses the foveal center did not impact this effect.38 These evidences suggest that while retinal perfusion in the macula may play a role in the pathogenesis of AMD or CNV, perfusion of the foveal center may not be as important. Unlike retinal vascular conditions such as diabetic retinopathy or retinal vein occlusions, where disease severity is associated with FAZ enlargement, the impact of retinal perfusion in AMD may be more widespread. Additional studies to explore the relationship between the cilioretinal artery and VD in AMD eyes may provide further insight.
In subgroup analyses, we found lower retinal VD and FAZ circularity in eyes with advanced AMD compared to those with intermediate AMD. We also found slightly lower VD, larger FAZ perimeter, and reduced FAZ circularity in eyes with center-involving GA when compared to eyes without GA, but not those with non-central GA. Although we excluded eyes with severe GA and inner retinal layer loss on structural OCT, we cannot exclude the possibility of inner retinal dysfunction without incorporation of functional imaging. Nevertheless, recent studies suggest that choriocapillary impairment at GA margins may predict progression.39 Thus, future studies to correlate GA areas with retinal VD defects may help determine if the retinal vasculature may also play a role in the pathogenesis of GA.
Our study focused only on the superficial, rather than the intermediate or deep retinal capillary plexuses, because the VD and FAZ parameters in the superficial layer has been well-validated using this OCT-A platform,25, 26, 40 and less likely to be impacted by CNV pathology in the outer retinal layers. Thus, we cannot exclude the possibility that vascular parameters from deeper retinal layers may have a stronger relationship with exudative or non-exudative AMD. Anti-VEGF treatments have been shown to impair the choriocapillaris and deep retinal capillary plexus, but not superficial capillary plexus in eyes with exudative AMD,35 indicating the need for more robust analyses of different retinal capillary layers. However, different OCT-A algorithms employ different boundaries for layer segmentation,41 and may contribute to differences in VD and FAZ measurements across different imaging platforms. Comparisons between different studies must therefore account for differences in OCT-A systems and measurement methodologies.
An advantage of focusing on the superficial retinal layers is the avoidance of signal attenuation and artifacts associated with drusen and other AMD pathologies and avoiding shadowing effects that can impact the deeper retinal layer VD on OCTA.42, 43 In our study, we also performed manual adjustments of OCT segmentation to ensure accuracy of OCT-A measurements. We excluded images with poor signal strength or uncorrectable segmentation, although the exclusion process may also have resulted in some selection bias. Additional strengths of our study include the large sample size, exclusion of eyes with other retinal pathologies, and independent determination of GA presence by image graders. Finally, because macular perfusion and VD decreases with age,29, 44, 45 we adjusted for age in all statistical analyses.
As mentioned previously, the retrospective design only demonstrates an inverse relationship between retinal vascular perfusion and exudative AMD. Future studies are necessary to determine if eyes with lower VD are more likely to develop CNV and/or GA in a prospective manner. Nevertheless, our findings support the intriguing notion that the superficial retinal vasculature may play a role in the pathogenesis of exudative AMD and provide prognostic information for risk for CNV development.
Supplementary Material
ACKNOWLEDGMENT
a. Funding/Support: GY is supported by NIH K08 EY026101, NIH R21 EY031108, E. Matilda Ziegler Foundation for the Blind, Barr Foundation for Retinal Research, and the Macula Society. The funding organizations did not play any role in the design or conduct of this retrospective study; the collection, management, analysis, or interpretation of data; or the preparation, review, approval, submission decision of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
b. Financial Disclosures: Dr. Yiu report grants and personal fees from Alcon, Alimera, Allergan, Carl Zeiss Meditec, Genentech, and Iridex, all outside of the submitted work. No other disclosures were reported.
c. Other Acknowledgments: The authors thank Dr. Alexander Vu, Dr. Jeffrey Caspar, and UC Davis Eye Center staff for providing or volunteering to be normal control subjects.
Biography
Dr. Glenn Yiu is an Associate Professor of Ophthalmology at UC Davis. He earned his MD-PhD at Harvard Medical School, residency at Massachusetts Eye & Ear Infirmary, and vitreoretinal fellowship at Duke. He joined UC Davis in 2014, where he now leads a translational research program studying age-related macular degeneration (AMD) and other retinal diseases. His focuses include ocular imaging technologies, gene editing, drug and gene delivery, and primate models of retinal disease.
Sophie C. Lee recently graduated Phi Beta Kappa from UCLA with a Bachelor of Science in Psychobiology. Since then, she has been working with Dr. Yiu in the Department of Ophthalmology at UC Davis. Her research efforts include implementing a telemedicine program for diabetic retinopathy screenings and studying the pathogenesis of age-related macular degeneration, and she is primarily interested in global vision health disparities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Al-Zamil WM, Yassin SA. Recent developments in age-related macular degeneration: a review. Clin Interv Aging 2017;12:1313–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw PX, Stiles T, Douglas C, et al. Oxidative stress, innate immunity, and age-related macular degeneration. AIMS Mol Sci 2016;3(2):196–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarrett SG, Boulton ME. Consequences of oxidative stress in age-related macular degeneration. Mol Aspects Med 2012;33(4):399–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrington DA, Sinha D, Kaarniranta K. Defects in retinal pigment epithelial cell proteolysis and the pathology associated with age-related macular degeneration. Prog Retin Eye Res 2016;51:69–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Handa JT, Cano M, Wang L, Datta S, Liu T. Lipids, oxidized lipids, oxidation-specific epitopes, and Age-related Macular Degeneration. Biochim Biophys Acta Mol Cell Biol Lipids 2017;1862(4):430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman E A hemodynamic model of the pathogenesis of age-related macular degeneration. Am J Ophthalmol 1997;124(5):677–82. [DOI] [PubMed] [Google Scholar]
- 7.Lutty G, Grunwald J, Majji AB, Uyama M, Yoneya S. Changes in choriocapillaris and retinal pigment epithelium in age-related macular degeneration. Mol Vis 1999;5:35. [PubMed] [Google Scholar]
- 8.Mullins RF, Johnson MN, Faidley EA, Skeie JM, Huang J. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci 2011;52(3):1606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Böker T, Fang T, Steinmetz R. Refractive error and choroidal perfusion characteristics in patients with choroidal neovascularization and age-related macular degeneration. Ger J Ophthalmol 1993;2(1):10–3. [PubMed] [Google Scholar]
- 10.Pauleikhoff D, Chen JC, Chisholm IH, Bird AC. Choroidal perfusion abnormality with age-related Bruch’s membrane change. Am J Ophthalmol 1990;109(2):211–7. [DOI] [PubMed] [Google Scholar]
- 11.Lee B, Ahn J, Yun C, Kim SW, Oh J. Variation of Retinal and Choroidal Vasculatures in Patients With Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci 2018;59(12):5246–5255. [DOI] [PubMed] [Google Scholar]
- 12.Vujosevic S, Toma C, Villani E, et al. Quantitative choriocapillaris evaluation in intermediate age-related macular degeneration by swept-source optical coherence tomography angiography. Acta Ophthalmol 2019;97(6):e919–e926. [DOI] [PubMed] [Google Scholar]
- 13.Alten F, Heiduschka P, Clemens CR, Eter N. Exploring choriocapillaris under reticular pseudodrusen using OCT-Angiography. Graefes Arch Clin Exp Ophthalmol 2016;254(11):2165–2173. [DOI] [PubMed] [Google Scholar]
- 14.Borrelli E, Shi Y, Uji A, et al. Topographic Analysis of the Choriocapillaris in Intermediate Age-related Macular Degeneration. Am J Ophthalmol 2018;196:34–43. [DOI] [PubMed] [Google Scholar]
- 15.Borrelli E, Souied EH, Freund KB, et al. Reduced Choriocapillaris Flow in Eyes with Type 3 Neovascularization and Age-Related Macular Degeneration. Retina 2018;38(10):1968–1976. [DOI] [PubMed] [Google Scholar]
- 16.Treister AD, Nesper PL, Fayed AE, Gill MK, Mirza RG, Fawzi AA. Prevalence of Subclinical CNV and Choriocapillaris Nonperfusion in Fellow Eyes of Unilateral Exudative AMD on OCT Angiography. Transl Vis Sci Technol 2018;7(5):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moult EM, Alibhai AY, Rebhun C, et al. SPATIAL DISTRIBUTION OF CHORIOCAPILLARIS IMPAIRMENT IN EYES WITH CHOROIDAL NEOVASCULARIZATION SECONDARY TO AGE-RELATED MACULAR DEGENERATION: A Quantitative OCT Angiography Study. Retina 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nassisi M, Tepelus T, Nittala MG, Sadda SR. Choriocapillaris flow impairment predicts the development and enlargement of drusen. Graefes Arch Clin Exp Ophthalmol 2019. [DOI] [PubMed] [Google Scholar]
- 19.Nassisi M, Baghdasaryan E, Borrelli E, Ip M, Sadda SR. Choriocapillaris flow impairment surrounding geographic atrophy correlates with disease progression. PLoS One 2019;14(2):e0212563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thulliez M, Zhang Q, Shi Y, et al. Correlations between Choriocapillaris Flow Deficits around Geographic Atrophy and Enlargement Rates Based on Swept-Source OCT Imaging. Ophthalmol Retina 2019;3(6):478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yiu G, Chiu SJ, Petrou PA, et al. Relationship of central choroidal thickness with age-related macular degeneration status. Am J Ophthalmol 2015;159(4):617–26. [DOI] [PubMed] [Google Scholar]
- 22.Yiu G, Vuong VS, Tran S, et al. Vascular Response to Sildenafil Citrate in Aging and Age-Related Macular Degeneration. Sci Rep 2019;9(1):5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snyder K, Yazdanyar A, Mahajan A, Yiu G. Association Between the Cilioretinal Artery and Choroidal Neovascularization in Age-Related Macular Degeneration: A Secondary Analysis From the Age-Related Eye Disease Study. JAMA Ophthalmol 2018;136(9):1008–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toto L, Borrelli E, Di Antonio L, Carpineto P, Mastropasqua R. RETINAL VASCULAR PLEXUSES’ CHANGES IN DRY AGE-RELATED MACULAR DEGENERATION, EVALUATED BY MEANS OF OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY. Retina 2016;36(8):1566–72. [DOI] [PubMed] [Google Scholar]
- 25.Durbin MK, An L, Shemonski ND, et al. Quantification of Retinal Microvascular Density in Optical Coherence Tomographic Angiography Images in Diabetic Retinopathy. JAMA Ophthalmol 2017;135(4):370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee MW, Kim KM, Lim HB, Jo YJ, Kim JY. Repeatability of vessel density measurements using optical coherence tomography angiography in retinal diseases. Br J Ophthalmol 2018. [DOI] [PubMed] [Google Scholar]
- 27.Ho J, Dans K, You Q, Nudleman ED, Freeman WR. COMPARISON OF 3 MM x 3 MM VERSUS 6 MM x 6 MM OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY SCAN SIZES IN THE EVALUATION OF NON-PROLIFERATIVE DIABETIC RETINOPATHY. Retina 2019;39(2):259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadda SR, Guymer R, Holz FG, et al. Consensus Definition for Atrophy Associated with Age-Related Macular Degeneration on OCT: Classification of Atrophy Report 3. Ophthalmology 2018;125(4):537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iafe NA, Phasukkijwatana N, Chen X, Sarraf D. Retinal Capillary Density and Foveal Avascular Zone Area Are Age-Dependent: Quantitative Analysis Using Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci 2016;57(13):5780–5787. [DOI] [PubMed] [Google Scholar]
- 30.Shahlaee A, Samara WA, Hsu J, et al. In Vivo Assessment of Macular Vascular Density in Healthy Human Eyes Using Optical Coherence Tomography Angiography. Am J Ophthalmol 2016;165:39–46. [DOI] [PubMed] [Google Scholar]
- 31.Wang Q, Chan S, Yang JY, et al. Vascular Density in Retina and Choriocapillaris as Measured by Optical Coherence Tomography Angiography. Am J Ophthalmol 2016;168:95–109. [DOI] [PubMed] [Google Scholar]
- 32.Garrity ST, Iafe NA, Phasukkijwatana N, Chen X, Sarraf D. Quantitative Analysis of Three Distinct Retinal Capillary Plexuses in Healthy Eyes Using Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci 2017;58(12):5548–5555. [DOI] [PubMed] [Google Scholar]
- 33.Yiu G, Manjunath V, Chiu SJ, Farsiu S, Mahmoud TH. Effect of anti-vascular endothelial growth factor therapy on choroidal thickness in diabetic macular edema. Am J Ophthalmol 2014;158(4):745–751 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Todorich B, Yiu G, Hahn P. Current and investigational pharmacotherapeutic approaches for modulating retinal angiogenesis. Expert Rev Clin Pharmacol 2014;7(3):375–91. [DOI] [PubMed] [Google Scholar]
- 35.Hikichi T, Agarie M. Reduced Vessel Density of the Choriocapillaris during Anti-Vascular Endothelial Growth Factor Therapy for Neovascular Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci 2019;60(4):1088–1095. [DOI] [PubMed] [Google Scholar]
- 36.Reiter GS, Told R, Schlanitz FG, Baumann L, Schmidt-Erfurth U, Sacu S. Longitudinal Association Between Drusen Volume and Retinal Capillary Perfusion in Intermediate Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci 2019;60(7):2503–2508. [DOI] [PubMed] [Google Scholar]
- 37.Stavrev V, Sivkova N, Koleva-Georgieva D. Quantitative Assessment of Foveal Avascular Zone in Patients with Early and Intermediate Nonexudative Age-Related Macular Degeneration Using Optical Coherence Tomography-Angiography. Open Journal of Ophthalmology 2018;8(3):133–139. [Google Scholar]
- 38.Snyder K, Yiu GC. Statistical Issues on Evaluating Association Between the Cilioretinal Artery and Age-Related Macular Degeneration-Reply. JAMA Ophthalmol 2019;137(7):856. [DOI] [PubMed] [Google Scholar]
- 39.Sacconi R, Corbelli E, Carnevali A, Querques L, Bandello F, Querques G. Optical Coherence Tomography Angiography in Geographic Atrophy. Retina 2018;38(12):2350–2355. [DOI] [PubMed] [Google Scholar]
- 40.Lei J, Durbin MK, Shi Y, et al. Repeatability and Reproducibility of Superficial Macular Retinal Vessel Density Measurements Using Optical Coherence Tomography Angiography En Face Images. JAMA Ophthalmol 2017;135(10):1092–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spaide RF, Curcio CA. Evaluation of Segmentation of the Superficial and Deep Vascular Layers of the Retina by Optical Coherence Tomography Angiography Instruments in Normal Eyes. JAMA Ophthalmol 2017;135(3):259–262. [DOI] [PubMed] [Google Scholar]
- 42.Alten F, Lauermann JL, Clemens CR, Heiduschka P, Eter N. Signal reduction in choriocapillaris and segmentation errors in spectral domain OCT angiography caused by soft drusen. Graefes Arch Clin Exp Ophthalmol 2017;255(12):2347–2355. [DOI] [PubMed] [Google Scholar]
- 43.Wong SS, Vuong VS, Cunefare D, Farsiu S, Moshiri A, Yiu G. Macular Fluid Reduces Reproducibility of Choroidal Thickness Measurements on Enhanced Depth Optical Coherence Tomography. Am J Ophthalmol 2017;184:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu J, Jiang C, Wang X, et al. Macular perfusion in healthy Chinese: an optical coherence tomography angiogram study. Invest Ophthalmol Vis Sci 2015;56(5):3212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei Y, Jiang H, Shi Y, et al. Age-Related Alterations in the Retinal Microvasculature, Microcirculation, and Microstructure. Invest Ophthalmol Vis Sci 2017;58(9):3804–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.