Abstract
Background
Synovial membrane inflammation is common in osteoarthritis (OA) and increases cartilage injury. However, synovial fluid and histology studies suggest that OA inflammatory responses are not homogeneous. Greater understanding of these responses may provide new insights into OA disease mechanisms. Our objective was to develop a novel, multi-parameter approach to phenotype synovial responses in knee OA.
Methods
Cell composition and soluble protein production was measured by flow cytometry and multiplex ELISA in synovium collected from OA patients undergoing knee replacement surgery (n=35).
Results
Testing disaggregation conditions showed that aggressive digestion improved synovial cell yield and mesenchymal staining by flow cytometry, but negatively impacted CD4+ T cell and CD56+ natural killer (NK) cell staining. Less aggressive digestion preserved these markers and showed highly variable T cell infiltration (range 0–43%, n=32). Correlation analysis identified mesenchymal subpopulations associated with different non-mesenchymal populations, including macrophages and T cells (CD45+CD11b+HLA-DR+ myeloid cells with podoplanin (PDPN)+CD73+CD90−CD34−mesenchymal cells, r=0.65 p<0.0001; CD45+CD3+T cells withPDPN+CD73+CD90+CD34+ mesenchymal cells, r=0.50 p=0.003). IL-6 measured by flow cytometry correlated strongly with IL-6 released by ex vivo culture of synovial tissue (r=0.59 p=0.0012) and was highest in mesenchymal cells co-expressing CD90 and CD34. IL-6, IL-8, complement factor D (CFD), and IL-10 release positively correlated with tissue cellularity (p=0.0042, 0.018, 0.0012, and 0.038, respectively). Additionally, increased CD8+ T cell numbers also correlated with retinol binding protein 4 (RBP4) (p=0.033). Finally, combining flow cytometry and multiplex data identified patient clusters with different types of inflammatory responses.
Conclusions
We used a novel approach to analyze OA synovium, identifying patient-specific inflammatory clusters. This study argues that phenotyping synovial inflammation may provide new insights into OA patient heterogeneity and biomarker development.
INTRODUCTION
Osteoarthritis (OA) is a disabling disease of progressive mechanical joint failure, and no approved pharmacologic agents halt this progression. Although OA patients share similar radiographic findings, OA is a heterogeneous disease with diverse epidemiologic, structural, genetic, clinical, and pathologic risk factors/phenotypes(1–6). One consistent phenotype associated with worse clinical outcomes, including increased pain sensitization and accelerated joint damage, is joint inflammation, characterized by increased synovial tissue volume, vascularity, and pro-inflammatory signaling(7–12).
Although inflammation increases with OA progression, its true prevalence is difficult to estimate. Macroscopic visualization by arthroscopy, ultrasound, or magnetic resonance imaging suggests that up to half of early OA patients have at least intermittent synovitis(7, 8, 13, 14). However, microscopic examination demonstrates that synovial abnormalities are also frequent in early OA(4, 15). Synovial changes are thought to arise from cellular activation by damage signals released during joint injury, resulting in areas of synovial lining hyperplasia and sublining immune infiltration. This activation increases production of pro-inflammatory mediators and matrix-degrading proteinases, accelerating cartilage damage(11, 12).
Synovial histology and pro-inflammatory mediator expression suggests considerable patient-to-patient variability in tissue injury response(4, 15–20). Our objective was to use multi-parameter analysis to explore synovial response diversity across a late-stage knee OA cohort, with the hope that this would provide further insights into the cellular and molecular drivers of OA pathology. To achieve this objective, matched OA synovial samples were either disaggregated for cellular analysis by flow cytometry or cultured intact to measure soluble mediator release by multiplex array. We show that correlations between these datasets provide a rich platform to investigate OA synovial responses, identifying potential tissue cell-to-cell interactions and clustering patients by their response networks. We also demonstrate that optimizing tissue disaggregation protocols is critical, an important reminder as synovial tissue research moves into highly detailed cellular analyses. These results confirm that OA inflammation is far from homogeneous and indicates that further inflammatory phenotyping may provide new insights into OA pathology and patient heterogeneity.
MATERIALS AND METHODS
Patient Recruitment
Thirty-five OA patients referred for knee replacement surgery at Virginia Mason Medical Center (Seattle, WA) were recruited with approval from the Institutional Review Board, Benaroya Research Institute at Virginia Mason. Patient demographics (Suppl. Table 1): Age 65.39±8.33 (mean±SD) years, Gender 66% Female, BMI 32.47±7.72 (mean±SD) kg/m2, White/African American/Asian 32/1/2.
Synovial Tissue Processing
Knee synovium was collected during replacement surgery at surgical discretion, with patients divided equally between three surgeons (Suppl. Table 1). Synovial tissue was dissected into ~100 mg samples and randomly distributed between assays. Samples were weighed (average 106 mg ± 31.5 mg) and then cultured overnight in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 1% fetal bovine serum (FBS, Gemini), L-glutamine, antibiotics, 2-mercaptoethanol (50 μM) and amino acids (6-well plates, 1 sample/well) (Fig. 1A). Samples conditioning media for ELISA were cultured for 24 hours in media alone. Independent samples for flow cytometry were cultured in 10 μM monensin (Sigma Aldrich) for 14 to 16 hours to block cytokine release for intracellular staining.
Figure 1. Influence of digestion conditions on synovial cell yield.
(A) Experimental approach summarized. Briefly, fresh OA synovial samples (~100 mg) were cultured overnight. Media from three samples were analyzed for soluble mediator release using a multi-analyte, flow-based ELISA. Independent samples were cultured with the protein transport inhibitor monensin prior to disaggregation for surface marker and intracellular IL-6 analysis by flow cytometry (12 samples/digestion). (B) Summary of digestion conditions tested for synovial disaggregation. (C) Mean cell yield/g tissue for each digestion condition was determined by manual counting. (D) Mean hematopoietic immune (CD45+), endothelial (CD45−CD31+) and mesenchymal (CD45−CD31−) cell number was calculated for each digestion condition by multiplying total cell yield by the cell percentage measured by flow cytometry. (E) The percent of CD45+, CD45−CD31+, and CD31−CD45− cells released by digestion condition is shown. (C-E) Statistics: Mean with standard deviation shown (n=4–5). Statistically significant differences across digestion conditions were assessed by parametric repeated measures ANOVA. P values not shown are greater than 0.2.
Synovial Tissue Disaggregation
Unless otherwise indicated, twelve samples/donor (~1200 mg tissue) were minced and enzymatically digested at 37°C with gentle tube inversion every five minutes. Base digestion medium was DMEM with 50 μg/ml DNase (Roche). Volume, composition and time varied by digestion condition (Figure 1A and Suppl. Methods). Released cells were collected in cold recovery buffer (20 ml DMEM, 10% FBS, 5 mM EDTA, 10 μM monensin), debris removed with 70 μm mesh, and erythrocytes lysed (Alfa Aesar Red Blood Cell Lysis Buffer, Thermo Fisher Scientific). Live synovial cells were manually counted using trypan blue exclusion.
Flow Cytometry
Human cell surface marker and intracellular IL-6 expression was analyzed by flow cytometry using the listed reagents (Suppl. Table 2). Data was analyzed with Kaluza™ software (Beckman Coulter), with positive populations identified using isotype (IL-6) or fluorescence minus one (FMO) controls. Detailed staining protocols described in Supplemental Methods.
Synovial Tissue Culture Supernatant Analysis
Supernatants from three independent 24-hour synovial tissue cultures/donor were stored at −20 °C until testing. Thirteen soluble mediator concentrations were determined using BioLegend LEGENDplex™ Human Adipokine flow cytometry-based ELISA (full list Suppl. Methods) and normalized to tissue sample weight (pg/ml/mg tissue).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 7, with p≤0.05 considered statistically significant. Digestion condition comparisons (Fig. 1–2, Suppl. Fig 1–2., Suppl. Table 3) were summarized as mean ± standard deviation and differences assessed by parametric testing (paired t-test, one-way analysis of variance (ANOVA) with Dunnett’s test for multiple comparisons). Patient-to-patient comparisons (Fig. 3–6, Suppl. Table 4–5) were summarized by median(interquartile range 25th and 75th percentiles, IQR) and differences assessed by Kruskal-Wallis test with Dunn’s test for multiple comparisons) or Spearmann correlation analysis (rs). Hierarchical clustering by correlation was performed by the web-based tool ClustVis (biit.cs.ut.ee/clustvis/)(21).
Figure 2. Optimization of disaggregation for ex vivo tissue flow cytometry.
Representative (A) CD4/CD8 (pre-gate: CD45+CD3+CD11b−CD20−) or (B) NK (pre-gate CD45+CD3−CD11b−CD20−) cell staining after digested by different conditions (one donor): 1-collagenase+800 μg/ml (high) dispase; 3-collagenase+8 μg/ml (low) dispase; 4-Liberase™ 30 minutes; and 6-collagenase only. Recovery of (C) CD4+ T cells or (D) NK cell subsets was compared between digestion protocols by normalizing cell percentage or MFI to the highest expressing condition. CD4+ T cells normalized to condition 6 (%CD4+ left y-axis/grey bars; CD4 MFI right y-axis/patterned bars). NK cells normalized to condition 4 (%CD16+CD56dim left y-axis/grey bars; CD16dim/−CD56high right y-axis/striped bars). (E) Representative podoplanin (PDPN), CD146, CD34, CD73, and CD90 staining on mesenchymal (pre-gate CD45−CD31−) cells isolated from one donor synovium after high dispase (condition 1) or short Liberase™ (condition 4) digestion. (F) Relative recovery of CD146+ (upper), CD34+ (middle) and CD73+ (lower) mesenchymal cells was compared between digestion protocols by normalizing cell percentage for each condition to the condition 1. (C, D, F) Statistics: Mean and standard deviation are shown (n=3–5). Statistically significant differences from the indicated condition were calculated by repeated measures ANOVA with Dunnet’s test for multiple comparisons. P values as follows: * p≤0.05, **p≤0.01. ***p≤0.001, ****p≤0.0001.
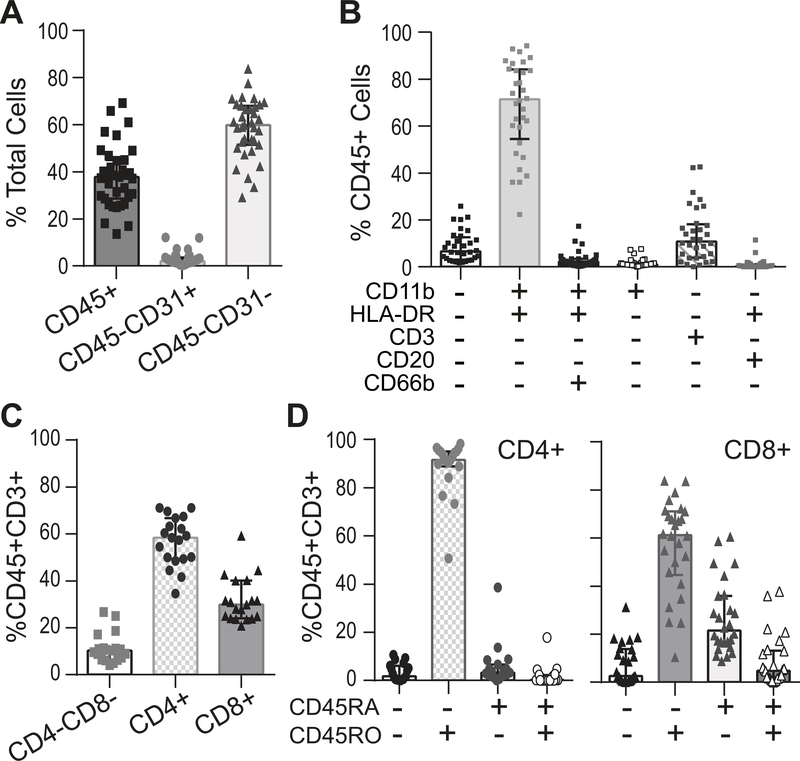
Figure 3. Hematopoietic immune cell analysis shows wide patient variability in T cell accumulation.
(A) Percentage of hematopoietic immune (CD45+), endothelial (CD45−CD31+) and mesenchymal (CD45−CD31−) cells in OA synovium were measured by flow cytometry (n=35. digestion condition 1-high dispase). (B) Percentage of different hematopoietic immune (CD45+) cell populations was determined by surface expression of myeloid (CD11b, HLA-DR), T cell (CD3), B cell (CD20), and neutrophil (CD66b) markers (n=32; digestion condition 1). (C) The percentage of CD4+ helper and CD8+ cytotoxic T cells is shown (n=19, gate CD45+CD3+, digestion condition 3-low dispase). (D) Expression of CD45RA+ (naïve) and CD45RO+ (effector/memory) expression on CD4+ (n=19) or CD8+ (n=26) cells was analyzed (digestion condition 3-low dispase). T cell analysis started using high dispase digestion (condition 1). Once it was determined that CD4 staining was not reliable, digestion was switched to low dispase (condition 3), accounting for the reduced number of donors for CD4+ cell analysis. Statistics: All plots show median and IQR.
Figure 6. Correlation-based clustering using synovial soluble mediator release and cell composition reveals different OA response patterns.
(A) Diagram demonstrates the positive correlations between soluble mediator release after synovial tissue culture across multiple donors (n=27, line thickness reflects p values). No positive correlations detected with adiponectin and leptin release (Suppl. Table 4). (B) Table showing strength of correlations between the indicated cell population numbers and soluble mediators (Spearman correlation co-efficient (top number), p value (bottom number), darker shading represents higher rs values, n=19–27). (C) The data obtained from analysis of OA synovium by cellular composition (flow cytometry, italics) and soluble mediator release (multi-analyte ELISA, bold) was combined with BMI in a correlation-based hierarchical clustering algorithm to separate different OA populations (ClustVis; clustering method columns and rows: correlation; clustering distance columns and rows: Ward). Limited clinical data separated by cluster provided below the heatmap (statistics: continuous variables Kruskal-Wallis test, categorical variables Chi-square test).
RESULTS
Developing a Multi-Parameter Approach to Analyze OA Synovium
Assay Approach
Many studies demonstrate heterogeneity in OA synovial responses(4, 15–18, 22–25), reflecting potential differences in disease pathology. To phenotype synovial response patterns in knee OA, we combined ex vivo flow cytometry and multi-analyte ELISA after limited culture of intact tissue (Fig. 1A). This approach allowed simultaneous measurement of soluble mediators and cellular composition for correlation analysis. Intracellular IL-6 staining was chosen for this investigation because IL-6 independently associates with joint damage and has highly variable expression in joint injury(17, 18, 20, 23, 25).
Establishing Culture Conditions for OA Tissue Analysis
We first determined the synovial tissue amount needed to accurately sample hematopoietic immune (CD45+), endothelial (CD45−CD31+), and mesenchymal (CD45−CD31−) populations (gating strategy, Suppl. Fig. 1A). Pooling randomly selected samples demonstrated that although cell yield increased linearly with tissue amount (Suppl. Fig. 1B), low variability in marker expression between technical replicates was seen with as little as ~400 mg tissue (4 samples) (Suppl. Fig. 1C–E). As variability trended lower with higher tissue amounts, twelve samples (~1200 mg) were pooled for each flow cytometry staining panel. As all flow cytometry samples were treated with the protein transport inhibitor monensin to prevent cytokine secretion (necessary for intracellular staining), we also compared the cell recovery between fresh surgical (day 0) and monensin-cultured (day 1) samples. Overnight culture with monensin decreased the cell yield by a third (Suppl. Fig. 2A), but this cell loss did not disproportionately affect the recovery of total or specific hematopoietic immune, mesenchymal, or endothelial cell populations (Suppl. Fig. 2B–D), validating this ex vivo approach for intracellular cytokine staining.
Optimizing Enzymatic Digestion Protocols for Ex Vivo Tissue Flow Cytometry
Enzymatic tissue disaggregation was chosen over mechanical disruption alone to facilitate joint cell release from their connective tissue matrix. Since enzymatic digestion may limit flow staining by surface molecule cleavage, a major technical objective was to optimize digestion conditions across diverse cell markers. We tested six protocols derived from prior synovial or mesenchymal cell studies (Fig. 1B)(26–30). All six enzyme cocktails included collagenase and DNase, but varied based on media change, digestion time and other added enzymes. The first three conditions (1 to 3), modified from lymph node fibroblastic reticular cell isolation(31), increased digestion efficiency by adding dispase and replacing enzymes every 15 minutes until tissue dissolved (~2 hours). Conditions 4 to 6 were more characteristic of prior synovial protocols(27, 30). Tissue was digested without enzyme replacement for a fixed time, using either commercially available Liberase™ TL (collagenase and thermolysin, conditions 4 and 5) or collagenase P (Col P) alone.
Dispase addition with enzyme replacement (conditions 1 to 3) increased average cell yields compared to fixed time digestions with Liberase™ TL or collagenase (Fig. 1C). Cell yield in high dispase (condition 1) averaged 1.8×106 cells/g tissue, more than twice that of other conditions. Differences in cell yields were not statistically different between other conditions, but trended higher with dispase (conditions 2 and 3). Not surprisingly, the absolute number of hematopoietic immune (CD45+) and mesenchymal (CD45−CD31−) cells recovered was also highest in condition 1, with endothelial cells (CD45−CD31+) also following this trend (Fig. 1D). However, no significant differences were detected in the proportion (relative percentage) of CD45+, CD45−CD31− and CD45−CD31+ cells isolated between conditions (Fig. 1E), indicating that all major cell types were adequately sampled by all protocols. Overall, mesenchymal cells were the most abundant, followed by hematopoietic immune cells (Fig. 1D, Fig. 3A), although there was wide patient variability. Endothelial cells were significantly less abundant (median(IQR): mesenchymal cells 59.9(16.6)%; hematopoietic cells 37.8(16)%; endothelial cells 2.11(2.23)%).
Fine-Tuning Digestion Condition by Cell Type
We next tested the stability of surface marker staining across different digestion conditions. For many markers, no differences were detected, including for CD8, CD45RA, CD45RO, CD11b, HLA-DR, CD14, and CD206 (Suppl. Table 3). However, digestion protocol did impact cell viability and detection of other cell populations, including CD4+ T helper, natural killer (NK), and mesenchymal cells (Fig. 2, Suppl. Table 3).
Reducing dispase significantly improved detection of CD4, the T helper cell co-receptor, as shown in example flow plots (Fig. 2A) and measured by average CD4+ cell percentage (grey bars) and mean fluorescence intensity (MFI, patterned bars) (Fig. 2C). CD4+ T cell percentage was similar in the low dispase (condition 3), short Liberase™ (condition 4) and collagenase alone (condition 6) conditions, while CD4 signal intensity (MFI) was strongest with collagenase alone, with either dispase (conditions 1–3) or thermolysin (Liberase™ component, conditions 4 and 5) addition reducing staining brightness.
Slightly different digestion conditions facilitated detection of the NK cell populations, CD3−CD16+CD56dim and CD3−CD16dim/−CD56high cells (Fig. 2B, D). CD16+CD56dim cells were detected in all conditions (grey bars, Fig. 2D), although it was best with short Liberase™ digestion (condition 4). CD16dim/−CD56high NK cell detection was more difficult (Fig. 2D, striped bars), with staining best using Liberase™ (conditions 4–5) and completely lost with dispase addition (conditions 1–3).
In contrast to T and NK cells, specific mesenchymal markers are less well-defined. CD146, podoplanin (PDPN), CD73, CD34, and CD90 were chosen based on prior histology studies (32–36) and recent synovial fibroblast flow cytometry and transcriptional analysis (29). Detection of these markers (Fig. 2E–F, Suppl. Table 3) was facilitated by more aggressive, high dispase digestion. Improved staining was not solely due to increased digestion time, as marker staining, particularly for CD146, increased with dose-dependent dispase addition (Fig. 2F). Indeed, most mesenchymal markers trended to better detection with increased dispase concentrations (Suppl. Table 3).
These results show that reliable ex vivo tissue flow cytometry requires careful optimization of tissue disaggregation protocols to ensure marker stability. For this study, two disaggregation protocols were chosen. High dispase digestion (condition 1) was used for mesenchymal and hematopoietic immune cell analysis. Low dispase digestion (condition 3) was used for T cell analysis, as it represented the best balance between CD4 discrimination, cost, and cell yield. Further NK cell analysis was not performed in this study.
OA Synovial Cell Analysis
Hematopoietic Immune Cells
Analysis showed that ~40% of synovial cells were hematopoietic (CD45+) in origin, with wide variability between patients (Fig. 3A). CD11b+HLA-DR+ cells (majority macrophages) were most abundant (71.4(29.7)%), followed by T cells (CD3+, 10.8(14.3)%), neutrophils (CD11b+HLA−DR+CD66b+, 2.22(2.49)%), another myeloid CD11b+HLA-DR− population (1.10(1.13)%) and B cells (CD20+HLA-DR+, 0.310(1.14)%) (Fig. 3B). A CD45+ lineage marker negative population (6.59(9.60)%) was also detected, likely representing a mixture of other hematopoietic cells (e.g., mast cells, NK cells) and cells with marker cleavage during digestion.
OA T cells
Immune cell analysis showed high donor-to-donor variability in synovial T cell percentage (Fig. 3B, 0 – 42.6%). Although T cell function in OA is poorly understood, animal models suggest both CD4+ and CD8+ T cell promote pathology after injury(37, 38). Therefore, OA T cells were further characterized by flow cytometry (Fig. 3). As expected(39, 40), helper CD4+ T cells (58.4(4.83)%) were increased compared to cytotoxic CD8+ T cells (29.9(16.1)% (Fig. 3C). However, both T cell types accumulated with increasing total T cell numbers (Suppl. Table 4). Most CD4+ T cells expressed the effector/memory marker CD45RO (91.6(6.25)%, Fig. 3D). Most CD8+ T cells were also effector/memory cells (61.3(26.6)% CD8+CD45RO+), but there was a higher percentage of naïve CD45RA cells (21.5(20.0)%) and cells positive for both markers (4.85(11.4)%).
Mesenchymal Cells
Compared to hematopoietic immune cells, much less is known about OA mesenchymal (CD45−C31−) cell populations. The existence of functionally distinct synovial mesenchymal/fibroblast populations is supported by recent reports showing that CD34 and/or CD90 expression skews toward different gene transcription and functional profiles(29, 41). For this analysis, vascular-associated mesenchymal cells (e.g., pericytes) were first separated by expression of CD146, also known as melanoma cell adhesion molecule (MCAM) (Fig. 4A). CD45−CD31−CD146+ cells help regulate vascular tone and represent a small subset of total mesenchymal cells. The remaining CD146− cells, mainly synovial fibroblasts by transcriptional analysis(29), were separated into seven populations averaging at least 1% of total mesenchymal population based on PDPN, CD73, CD90 and CD34 surface expression (Fig. 4B). The majority of cells were PDPN+CD73+, with CD90−CD34− cells the largest subset (32.4(29.2)%), followed by CD90+CD34+ (20.0(17.6)%), CD90+CD34− (16.7(24.1)%), and CD90−CD34+ (2.68(5.42)%). Two PDPN+CD73− subsets were also identified: CD90−CD34− (5.23(15.4)%) and CD90+CD34− (1.48(2.28%) cells. There was also a significant marker negative population (8.28(6.93)%), representing either a unique population and/or cells whose surface markers were cleaved during digestion.
Figure 4. Correlations between synovial fibroblast and hematopoietic immune cell populations.
(A) Mean %CD146+ vascular and CD146− non-vascular mesenchymal cells was analyzed in OA synovium after high dispase digestion (n=28). (B) CD45−CD31−CD146− cells were further analyzed by PDPN, CD73, CD90, and CD34 expression using tree analysis (Kaluza). Seven CD146− subsets with mean percent expression greater than 1% of total CD146− cells were identified. The %CD45−CD31−CD146− CD90−CD34− (C, D) and %CD45−CD31−CD146− CD90+CD34+ (E, F) mesenchymal cells were inversely correlated with the %CD45+CD11b+CD66b- myeloid (C, E) and %CD45+CD3+ T cells (D, F) (n=28, Spearman (rs) correlation coefficient).
Clues to Mesenchymal Cell Function
To examine the possible function of different mesenchymal/fibroblast populations, associations between mesenchymal and non-mesenchymal cells were assessed. Significant correlations were found between the two largest mesenchymal and hematopoietic immune populations (Fig. 4C–F). PDPN+CD73+CD90−CD34− cells correlated positively with CD45+CD11b+HLA-DR+ cells (largely synovial macrophages) and negatively with CD45+CD3+ T cells. (Fig. 4C–D). This result suggests a synovial lining fibroblast population, as lining fibroblasts tightly co-compact with synovial macrophages. In contrast, PDPN+CD73+CD90+CD34+ cells correlated positively with T cells and negatively with synovial macrophages (Fig. 4E–F), suggesting a sublining fibroblast population. These correlations are consistent with prior histology(32, 36) and tissue immunofluorescence(29, 33–35, 41) staining that show strong CD34 and CD90 expression in rheumatoid arthritis (RA) and OA synovial sublining. Additional correlations suggest other possible mesenchymal cell interactions with endothelial cells, T cells, B cells, and lineage negative hematopoietic cells (Suppl. Table 5).
OA IL-6 Analysis in OA Synovium
Major Contribution by Mesenchymal Cells in OA IL-6 Response
Although IL-6 associates with increased OA pain and joint damage(9, 18, 19, 25), OA synovial fluid IL-6 levels are highly variable, ranging from barely detectable to levels similar to RA(17). To better understand this variation, synovial cell IL-6 responses were measured ex vivo by flow cytometry (Fig. 5A). Validating this approach, the calculated number of IL-6+ synovial cells/donor by flow cytometry strongly correlated with the IL-6 released by independent synovial tissue cultures from the same donor (Fig. 5B).
Figure 5. Diversity of IL-6 production in OA synovium with robust mesenchymal expression.
(A) Representative synovial hematopoietic (CD45+) and mesenchymal (CD45−CD31−) cell intracellular IL-6 flow cytometry staining with corresponding isotype controls. (B) IL-6 synovial tissue culture release (Adipokine Panel, LEGENDplex™, n=27, three independent samples averaged/donor) positively correlated with the total IL-6+ cell number independently calculated by flow cytometry (Spearman (rs) correlation co-efficient). (C, F, H) The percent IL-6+ cells were measured by flow cytometry in the major cell (C), CD146− mesenchymal (F), and CD45+ hematopoietic immune (H) populations. (D) IL-6+ cell number in major cell populations was calculated from the total donor cell yield. (E, G, I) IL-6 MFI was calculated by subtracting background signal (antibody isotype) from population IL-6 MFI for the major cell (E), CD146− mesenchymal (G), and CD45+ hematopoietic immune (I) populations. (J) Synovial tissue culture IL-6 release did not correlate with the adipocyte surrogates, body mass index (BMI) or leptin release. Statistics: (C-I) Kruskal-Wallis test with Dunn’s multiple comparisons test was used to assess for differences from the following reference populations: (C-E) CD45−CD31−, n=32; (F-G) PDPN+CD73+CD90+CD34+, n=28; and (H-I) CD11b+CD66b−CD3−CD20−HLA-DR+, n=28. P values as listed or *p≤ 0.0001, **p<0.0006, ***p=0.007, ****p=0.004. (J) Spearman correlation analysis (n=27).
This analysis showed that although all cell types produce some IL-6, the cell number and intensity of expression varied significantly between different donors and cell types (Fig. 5A, C–E). For most donors, the percentage of IL-6+ cells was higher in mesenchymal cells (13.6(19.6)%) compared to hematopoietic (4.44(17.4)%) or endothelial cells (3.74(19.6)%) (Fig. 5A, C). However, in a small number of donors (6 out of 32), this trend was reversed (example Fig. 5A), with a greater percentage of CD45+IL-6+ cells. There were also a greater number of IL-6+ mesenchymal cells (0.74×105(2.6×105) cells/g tissue) compared to hematopoietic (0.16×105(0.96×105) cells/g tissue) or endothelial (0.0094×105(0.023×105) cells/g tissue) cells (Fig. 5D). In addition, mesenchymal cells averaged three times more IL-6 per cell, as measured by MFI (Fig. 5E). Overall, this analysis shows considerable variability in IL-6 production by synovial cell type, with mesenchymal cells proving a major source in most patients.
IL-6 Production in Synovial Fibroblast Subsets
Although IL-6 was expressed by all mesenchymal populations, the percentage of IL-6+ cells was highest in the following PDPN+CD73+ populations: CD90−CD34−, CD90+CD34−, and CD90+CD34+ (Fig. 5F). These results mirror the relative abundance of these subsets in OA synovium (Fig. 4B). However, the IL-6 staining intensity (MFI) was highest in the PDPN+CD73+CD90+CD34+ (likely sublining) population, decreasing in the PDPN+CD73+CD90−CD34− (likely lining) population (Fig. 5G). These results suggest an IL-6 gradient across the OA synovium, pointing to further complexity in synovial IL-6 responses.
IL-6 Production in Other Cells
Hematopoietic cells also contribute significantly to OA IL-6 (Fig 5A, C–E), with the greatest percentage of IL-6+ cells in synovial myeloid cells/macrophages (CD11b+CD66b−) and, surprisingly, neutrophils (CD66b+) (Fig. 5H). IL-6 response was significantly lower in T cells (CD3+) and B cells (CD20+). Interestingly, IL-6 production per cell (MFI) was highest and of comparable intensity in synovial macrophages and neutrophils, roughly five times that of lymphocytes (Fig. 5I). However, by cell number, neutrophils likely contribute little to the total IL-6 response as they are a minor cell population (Fig. 3B). Similarly, although IL-6 release was clearly detectable in endothelial cells, they also likely contribute only a small proportion to total synovial IL-6 based on cell number (Fig. 5C–E). Finally, adipocytes are another potential source of synovial IL-6. As adipocytes are damaged by tissue disaggregation, their IL-6 production cannot be measured by flow cytometry. However, IL-6 release did not correlate with the adipocyte surrogates, body mass index (BMI) or leptin release (Fig. 5J), suggesting adipocytes are not a major IL-6 source in these samples.
Understanding IL-6 Responses by Correlation Analysis
To determine if other soluble mediators also associate with OA IL-6 responses, correlations were assessed between IL-6 synovial culture release and a panel of twelve cytokines, chemokines, and adipokines (Fig. 6A, Suppl. Table 4). IL-6 most strongly correlated with IL-8, but also directly correlated with complement factor D (CFD), IL-10, CCL2, and CXCL10 release (Fig. 6A, Suppl. Fig. 3A, Suppl. Table 4). An additional strong expression network formed between TNF-α, IFN-γ, IL-1β, and resistin, linking to IL-6 through IL-10 and CCL2 expression.
Further analysis revealed additional positive correlations between IL-6, several mediators (gray shading, Fig. 6A), and the numbers of total and specific synovial cell populations (Fig. 6B, Suppl. Fig. 3). Increased total cell number correlated with IL-6, IL-8, IL-10, and CFD release, but not other soluble mediators including CCL2 and TNF-α (Fig.6, Suppl. Fig. 3, Suppl. Table 4). In general, these four soluble mediators correlated with increased cell numbers across synovial cell populations, although there were exceptions (Fig. 6B). IL-6 release correlated less strongly with the dominant CD4+ CD45RA−RO+ T cell population, but did correlate with mesenchymal cells, macrophages, CD8+ T cells, and naïve CD4+ T cells. IL-10 release also correlated best with total T cells numbers and specifically CD8+ T cell numbers. Finally, RBP4, and to less extent CCL2 release, emerged as a distinct marker of increased T cell and/or T-cell associated mesenchymal cell numbers. These data indicate specific groups of soluble mediators may be used to predict elements of synovial cell composition, such as increased cellularity or T cell accumulation.
Clustering of OA Patients Based on Synovial Responses
To move beyond single correlations, we used hierarchical correlation-based clustering of flow cytometry, soluble mediator, and clinical data to determine if there were specific response patterns associated with the observed patient variability. Indeed, three major patterns were identified (Fig. 6C). Cluster 1 was most strongly associated with increased T cells and T cell-associated cytokines (IL-10, IFN-γ, TNF-α), while clusters 2 and 3 were associated with increased macrophage expression (%CD45+CD11b+CD66b−CD3−CD20−). Cluster 2 showed the highest percentage of macrophages and potential lining (PDPN+CD73+CD90−CD34−) fibroblasts, including a subcluster with high adiponectin expression. In contrast, cluster 3 correlated best with high total cell yield and IL-6 and IL-8 responses, especially by mesenchymal cells.
Next, we examined if these clusters associate with any of the limited clinical data collected (Fig. 6C). There was a non-statistically significant trend for cluster two to associate with female sex (p=0.078) and cluster 3 to associate with prior arthroscopic or joint replacement surgery (p=0.052). These finding’s clinical relevance is uncertain, as this study does not correct for demographic and patient comorbidity confounders. However, these results raise the possibility that phenotyping of OA synovial responses may provide new insights into patient heterogeneity and disease pathology.
DISCUSSION
Synovial inflammation is associated with increasing OA pain and joint damage, although there is considerable patient-to-patient variability in inflammatory response(7, 9, 14, 16, 17, 19, 23, 25, 42). How this variability impacts disease development is hampered by limited understanding of the cellular and molecular mechanisms active in OA synovitis. In this study, we developed a multi-parameter flow cytometry and ELISA approach that showed several findings relevant to OA pathology and synovial tissue research generally.
Synovial tissue flow cytometry was chosen because it increases cell identification compared to standard immunohistochemistry. However, ex vivo tissue flow cytometry requires effective tissue disaggregation without significant loss of cell viability or surface markers. We found that optimal digestion protocols depends on cell type and marker (Fig. 2, Suppl. Table 3), with particular difficulty staining lymphocytes and mesenchymal cells with the same protocol. Similar challenges were recently reported by the Accelerating Medicines Partnership (AMP) Network, which found combining a commercial dissociator system and Liberase™ TL digestion optimal in their tested conditions(27). One study strength is to highlight how accurate synovial flow cytometry analysis requires clear data on how cell yield and surface maker stability is influenced by choice of disaggregation protocol.
Another study strength is that it demonstrates that correlation analysis can be used to suggest potential cellular interactions. For example, this study detected two prominent PDPN+CD73+ fibroblast populations: CD90−CD34− and CD90+CD34+ cells (Fig. 4). These populations inversely correlated with macrophage/myeloid (CD45+CD11b+CD3−CD20−CD66b−) and T (CD45+CD3+) cells, suggesting that CD90−CD34− cells are lining fibroblasts (positively correlated with myeloid cells) and CD90+CD34+ cells are sublining fibroblasts (positively correlated with T cells). These conclusions are independently supported by tissue staining studies showing enriched CD90 and CD34 expression in OA and RA synovial sublining(29, 32, 33, 35, 36, 41). Recent ex vivo studies also support that mesenchymal subpopulations may have unique functions in RA(29, 41), although their role in OA remains undefined. Given several potential cell interactions were detected using this small patient cohort (Fig. 4, Suppl. Table 5), we predict that an expanded analysis will provide further guidance about how cellular niches function in synovial pathology.
Cell and correlation analysis also points to potential differences in CD4+ and CD8+ T cell compartments. There was pronounced patient-to-patient variability in T cell percentage (Fig. 3), consistent with the variable lymphocytic sublining aggregates observed in OA(39). As reported(39, 40, 43, 44), CD4+ T cells were more prevalent than CD8+ cells. However, CD8+ cells showed increased variability in expression of CD45RA (naïve) and CD45RO (effector/memory) markers (Fig. 3D). In addition, CD8+ T cell numbers more strongly correlated with tissue release of several pro-inflammatory/regulatory molecules (IL-6, IL-8, CFD, IL-10, RBP4, CCL2; Fig. 6B). Prior studies suggest both CD4+ and CD8+ T cells contribute to OA pathology, with both subtypes having a Th1 phenotype (40, 43, 45, 46) and knockout of each reducing damage in mouse OA models(37, 38). This study broadens our perspective on OA T cells, suggesting more complex roles for specific T cell subpopulations.
This study also provides the most detailed examination of synovial IL-6 production to date. Although a pathologic role for IL-6 is well-recognized in RA, joint IL-6 also independently associates with OA pain and radiographic progression(9, 25) and its blockade attenuates joint damage in a mouse OA model(47). Furthermore, IL-6 is one of the most variable pro-inflammatory cytokines in joint injury, varying 10,000-fold between patients(17, 20). This study suggests that the considerable diversity in cellular IL-6 source (Fig. 5) may help explain part of this patient variability. On average, IL-6 expression was highest in mesenchymal cells, although in a handful of patients, hematopoietic immune cell (mainly macrophage) expression was more dominant. This complexity suggests that defining IL-6 levels and source may be important in predicting how this cytokine affects patient outcomes.
This study also shows the potential power of combining different data types to analyze OA patient responses (Fig. 6C). By clustering, patients broadly separated into T cell/lymphocyte (1) and myeloid (2 and 3) clusters, with cluster 3, in particular, associated with high tissue and mesenchymal cell IL-6 and IL-8 release. There are early suggestions that these clusters reflect with different patient phenotypes, with cluster 2 trending with female sex and cluster 3 with a history of prior joint surgery (arthroscopy/arthroplasty) (Fig. 6C). It remains to be seen if these clusters can be better defined and how they related to disease progression and clinical phenotypes.
There are several study limitations, including the need to dissect and culture synovial tissue for flow and ELISA analysis, which may potentially change cellular phenotypes. In addition, tissue sampling bias may have influenced study results. Prior RA and OA studies suggest good histologic or RNA sequencing reproducibility with three to four ultrasound-guided synovial biopsy samples(15, 48) or ~150 mg tissue(27). We found low variability with four ~100 mg OA samples, but generally used higher tissue amounts to reduce bias. A larger confounder is that we did not control for anatomic location in sampling, an issue due to patchiness in OA inflammation and regional variations in synovial anatomy. How anatomy effects synovial responses remains an open issue in the field and requires additional comparative studies to define how synovial responses vary by distinct regional locations within each patient. Finally, it is known that synovial findings change in OA as disease progresses(4, 15), so a similar analysis comparing early and late disease tissue, along with analyzing radiographic damage and additional clinical variables, are important subjects for future investigations. Despite these limitations, this study demonstrates that multi-parameter investigations of OA synovial responses may shed insights into OA patient heterogeneity and supports the possibility that the synovial sampling may be a future tool in OA patient care.
Supplementary Material
Supplemental Table 1.Study Demographics. Basic demographic information about the OA patients whose synovial samples were used in this study.
Supplemental Table 2. Flow Cytometry Reagents. List of reagents used for flow cytometry staining.
Supplemental Table 3.Influence of digestion condition on surface marker detection by flow cytometry. Synovial tissue from each donor (n=3–5) was digested by six different conditions (see Fig. 1B). Released cells were subsequently stained for the listed viability (Zombie Aqua), mesenchymal, and hematopoietic cell surface molecules. Statistically significant differences (bold underlined font) in cell percentages (non-normalized) across digestion conditions were determined using repeated measures one-way ANOVA with Dunnet’s test for multiple comparisons, selecting the condition with the overall highest percentage of positive staining as the reference condition (italic font).
Supplemental Table 4.Correlation matrix summarizing soluble mediator data. Matrix summarizing the Spearman correlations between all soluble mediators.
Supplemental Table 5.Correlations of synovial mesenchymal subsets with non-mesenchymal cell populations. Shown are the statistically significant correlations (p<0.05) between the percentage of synovial mesenchymal (pre-gate CD45−CD31−CD146−) and non-mesenchymal populations in OA synovium (n=32, Spearman correlation coefficient (rs) (top), p values (bottom), n.s. not significant).
Supplemental Figure 1.Influence of tissue amount on synovial tissue flow cytometry reproducibility. (A) Example of the flow cytometry gating strategy that defines hematopoietic immune (CD45+), endothelial (CD45-CD31+) and mesenchymal (CD45-CD31−) cell populations in disaggregated OA synovium. Also shown is the range of synovial cell viability post-digestion (n=35, digestion condition 1, median with IQR). (B-E) Four, eight, or twelve OA synovial tissue samples/donor (~100 mg/samples) were pooled, enzymatically digested, and analyzed by flow cytometry. For each donor, pooled digestions were done in duplicate (technical replicates). (B) The total cell yield was determined by manual counting (n=4, technical replicates averaged). Cell yield differences were statistically significant by one-way ANOVA (p=0.044). (C-E) Variability introduced by pooling tissue samples (four, eight or twelve samples) was estimated by calculating the average difference between technical replicates for the percentage of (C) major, (D) mesenchymal (pre-gate CD45−CD31−), and (E) hematopoietic immune (CD45+) cell populations (n=4, differences between digestions not statistically significant by parametric repeated measures ANOVA).
Supplemental Figure 2.Influence of overnight culture with monensin on synovial cell yield and composition. Synovial tissue from three donors was divided to compare cell yield and percentages from freshly digested tissue (day 0) with tissue digested after overnight culture with monensin (day 1) (digestion condition 1). The cell count (A) and percentage (B) of hematopoietic (CD45+), endothelial (CD45-CD31+) and mesenchymal (CD45-CD31−) cells in disaggregated synovium was determined by manual counting and flow cytometry, respectively (paired Student’s t-test (day 0 vs 1) with p value for (A) 0.02 and (B) non-significant). The percentage of mesenchymal (C) or hematopoietic immune (D) cell populations on day 1 was expressed as a fraction of day 0 (differences not statistically significant by repeated measures one-way ANOVA).
Suppl. Fig. 3. Correlations shown between the synovial tissue release of (A) IL-6 and IL-8 or between total cell number and the synovial tissue release of (B) IL-6, (C) CFD, (D) IL-10, (E) CCL2, and (G) TNF-α (Spearman analysis).
Acknowledgements
This paper contains parts of the doctoral thesis of Hannah Labinsky.
Financial Support:
HL: Studienstiftung des Deutschen Volkes Scholarship
BM: NIH T32 AR07198
EHN: NIH NIAMS K08 AR063696, Rheumatology Research
Foundation Bridge Funding K Supplement
JHB: NIH 1R01AI132774
Footnotes
Financial Disclosures
HL: None
PMP: None
KAL: None
DKK: None
BM: None
VM: None
JC: None
KMM: None
PJV: None
JHB: None
EHN: None
REFERENCES
- 1.Cibrian Uhalte E, Wilkinson JM, Southam L, Zeggini E. Pathways to understanding the genomic aetiology of osteoarthritis. Hum Mol Genet. 2017;26(R2):R193–R201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beekhuizen M, Gierman LM, van Spil WE, Van Osch GJ, Huizinga TW, Saris DB, et al. An explorative study comparing levels of soluble mediators in control and osteoarthritic synovial fluid. Osteoarthritis Cartilage. 2013;21(7):918–22. [DOI] [PubMed] [Google Scholar]
- 3.Knoop J, van der Leeden M, Thorstensson CA, Roorda LD, Lems WF, Knol DL, et al. Identification of phenotypes with different clinical outcomes in knee osteoarthritis: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2011;63(11):1535–42. [DOI] [PubMed] [Google Scholar]
- 4.Oehler S, Neureiter D, Meyer-Scholten C, Aigner T. Subtyping of osteoarthritic synoviopathy. Clin Exp Rheumatol. 2002;20(5):633–40. [PubMed] [Google Scholar]
- 5.van Spil WE, Jansen NW, Bijlsma JW, Reijman M, DeGroot J, Welsing PM, et al. Clusters within a wide spectrum of biochemical markers for osteoarthritis: data from CHECK, a large cohort of individuals with very early symptomatic osteoarthritis. Osteoarthritis Cartilage. 2012;20(7):745–54. [DOI] [PubMed] [Google Scholar]
- 6.Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30(2):160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis -- results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 2005;13(5):361–7. [DOI] [PubMed] [Google Scholar]
- 8.Krasnokutsky S, Belitskaya-Levy I, Bencardino J, Samuels J, Attur M, Regatte R, et al. Quantitative magnetic resonance imaging evidence of synovial proliferation is associated with radiographic severity of knee osteoarthritis. Arthritis Rheum. 2011;63(10):2983–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neogi T, Guermazi A, Roemer F, Nevitt MC, Scholz J, Arendt-Nielsen L, et al. Association of Joint Inflammation With Pain Sensitization in Knee Osteoarthritis: The Multicenter Osteoarthritis Study. Arthritis Rheumatol. 2016;68(3):654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Philp AM, Davis ET, Jones SW. Developing anti-inflammatory therapeutics for patients with osteoarthritis. Rheumatology (Oxford). 2017;56(6):869–81. [DOI] [PubMed] [Google Scholar]
- 11.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51(2):249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6(11):625–35. [DOI] [PubMed] [Google Scholar]
- 13.Bevers K, Bijlsma JW, Vriezekolk JE, van den Ende CH, den Broeder AA. The course of ultrasonographic abnormalities in knee osteoarthritis: 1 year follow up. Osteoarthritis Cartilage. 2014;22(10):1651–6. [DOI] [PubMed] [Google Scholar]
- 14.Guermazi A, Hayashi D, Roemer FW, Zhu Y, Niu J, Crema MD, et al. Synovitis in knee osteoarthritis assessed by contrast-enhanced magnetic resonance imaging (MRI) is associated with radiographic tibiofemoral osteoarthritis and MRI-detected widespread cartilage damage: the MOST study. J Rheumatol. 2014;41(3):501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minten MJM, Blom A, Snijders GF, Kloppenburg M, van den Hoogen FHJ, den Broeder AA, et al. Exploring longitudinal associations of histologically assessed inflammation with symptoms and radiographic damage in knee osteoarthritis: combined results of three prospective cohort studies. Osteoarthritis Cartilage. 2018. [DOI] [PubMed] [Google Scholar]
- 16.Cuellar VG, Cuellar JM, Kirsch T, Strauss EJ. Correlation of Synovial Fluid Biomarkers With Cartilage Pathology and Associated Outcomes in Knee Arthroscopy. Arthroscopy. 2016;32(3):475–85. [DOI] [PubMed] [Google Scholar]
- 17.Kaneko S, Satoh T, Chiba J, Ju C, Inoue K, Kagawa J. Interleukin-6 and interleukin-8 levels in serum and synovial fluid of patients with osteoarthritis. Cytokines Cell Mol Ther. 2000;6(2):71–9. [DOI] [PubMed] [Google Scholar]
- 18.Larsson S, Englund M, Struglics A, Lohmander LS. Interleukin-6 and tumor necrosis factor alpha in synovial fluid are associated with progression of radiographic knee osteoarthritis in subjects with previous meniscectomy. Osteoarthritis Cartilage. 2015;23(11):1906–14. [DOI] [PubMed] [Google Scholar]
- 19.Stannus OP, Jones G, Blizzard L, Cicuttini FM, Ding C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: a prospective cohort study. Ann Rheum Dis. 2013;72(4):535–40. [DOI] [PubMed] [Google Scholar]
- 20.Watt FE, Paterson E, Freidin A, Kenny M, Judge A, Saklatvala J, et al. Acute Molecular Changes in Synovial Fluid Following Human Knee Injury: Association With Early Clinical Outcomes. Arthritis Rheumatol. 2016;68(9):2129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metsalu T, Vilo J. ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015;43(W1):W566–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambert C, Dubuc JE, Montell E, Verges J, Munaut C, Noel A, et al. Gene expression pattern of cells from inflamed and normal areas of osteoarthritis synovial membrane. Arthritis Rheumatol. 2014;66(4):960–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livshits G, Zhai G, Hart DJ, Kato BS, Wang H, Williams FM, et al. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: The Chingford Study. Arthritis Rheum. 2009;60(7):2037–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma CH, Lv Q, Cao Y, Wang Q, Zhou XK, Ye BW, et al. Genes relevant with osteoarthritis by comparison gene expression profiles of synovial membrane of osteoarthritis patients at different stages. Eur Rev Med Pharmacol Sci. 2014;18(3):431–9. [PubMed] [Google Scholar]
- 25.Stannus O, Jones G, Cicuttini F, Parameswaran V, Quinn S, Burgess J, et al. Circulating levels of IL-6 and TNF-alpha are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage. 2010;18(11):1441–7. [DOI] [PubMed] [Google Scholar]
- 26.Autengruber A, Gereke M, Hansen G, Hennig C, Bruder D. Impact of enzymatic tissue disintegration on the level of surface molecule expression and immune cell function. Eur J Microbiol Immunol (Bp). 2012;2(2):112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donlin LT, Rao DA, Wei K, Slowikowski K, McGeachy MJ, Turner JD, et al. Methods for high-dimensonal analysis of cells dissociated from cyropreserved synovial tissue. Arthritis Res Ther. 2018;20(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagman DK, Kuzma JN, Larson I, Foster-Schubert KE, Kuan LY, Cignarella A, et al. Characterizing and quantifying leukocyte populations in human adipose tissue: impact of enzymatic tissue processing. J Immunol Methods. 2012;386(1–2):50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizoguchi F, Slowikowski K, Wei K, Marshall JL, Rao DA, Chang SK, et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat Commun. 2018;9(1):789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogata Y, Mabuchi Y, Yoshida M, Suto EG, Suzuki N, Muneta T, et al. Purified Human Synovium Mesenchymal Stem Cells as a Good Resource for Cartilage Regeneration. PLoS One. 2015;10(6):e0129096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fletcher AL, Lukacs-Kornek V, Reynoso ED, Pinner SE, Bellemare-Pelletier A, Curry MS, et al. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med. 2010;207(4):689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bauer S, Jendro MC, Wadle A, Kleber S, Stenner F, Dinser R, et al. Fibroblast activation protein is expressed by rheumatoid myofibroblast-like synoviocytes. Arthritis Res Ther. 2006;8(6):R171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ekwall AK, Eisler T, Anderberg C, Jin C, Karlsson N, Brisslert M, et al. The tumour-associated glycoprotein podoplanin is expressed in fibroblast-like synoviocytes of the hyperplastic synovial lining layer in rheumatoid arthritis. Arthritis Res Ther. 2011;13(2):R40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurth TB, Dell’accio F, Crouch V, Augello A, Sharpe PT, De Bari C. Functional mesenchymal stem cell niches in adult mouse knee joint synovium in vivo. Arthritis Rheum. 2011;63(5):1289–300. [DOI] [PubMed] [Google Scholar]
- 35.Middleton J, Americh L, Gayon R, Julien D, Mansat M, Mansat P, et al. A comparative study of endothelial cell markers expressed in chronically inflamed human tissues: MECA-79, Duffy antigen receptor for chemokines, von Willebrand factor, CD31, CD34, CD105 and CD146. J Pathol. 2005;206(3):260–8. [DOI] [PubMed] [Google Scholar]
- 36.Miyake K, Nishida K, Kadota Y, Yamasaki H, Nasu T, Saitou D, et al. Inflammatory cytokine-induced expression of vasohibin-1 by rheumatoid synovial fibroblasts. Acta Med Okayama. 2009;63(6):349–58. [DOI] [PubMed] [Google Scholar]
- 37.Hsieh JL, Shiau AL, Lee CH, Yang SJ, Lee BO, Jou IM, et al. CD8+ T cell-induced expression of tissue inhibitor of metalloproteinses-1 exacerbated osteoarthritis. Int J Mol Sci. 2013;14(10):19951–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen PC, Wu CL, Jou IM, Lee CH, Juan HY, Lee PJ, et al. T helper cells promote disease progression of osteoarthritis by inducing macrophage inflammatory protein-1gamma. Osteoarthritis Cartilage. 2011;19(6):728–36. [DOI] [PubMed] [Google Scholar]
- 39.de Lange-Brokaar BJ, Ioan-Facsinay A, van Osch GJ, Zuurmond AM, Schoones J, Toes RE, et al. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage. 2012;20(12):1484–99. [DOI] [PubMed] [Google Scholar]
- 40.Li YS, Luo W, Zhu SA, Lei GH. T Cells in Osteoarthritis: Alterations and Beyond. Front Immunol. 2017;8:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Croft AP, Campos J, Jansen K, Turner JD, Marshall J, Attar M, et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature. 2019;570(7760):246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roemer FW, Guermazi A, Felson DT, Niu J, Nevitt MC, Crema MD, et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis. 2011;70(10):1804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haynes MK, Hume EL, Smith JB. Phenotypic characterization of inflammatory cells from osteoarthritic synovium and synovial fluids. Clin Immunol. 2002;105(3):315–25. [DOI] [PubMed] [Google Scholar]
- 44.Moradi B, Rosshirt N, Tripel E, Kirsch J, Barie A, Zeifang F, et al. Unicompartmental and bicompartmental knee osteoarthritis show different patterns of mononuclear cell infiltration and cytokine release in the affected joints. Clinical and experimental immunology. 2015;180(1):143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berner B, Akca D, Jung T, Muller GA, Reuss-Borst MA. Analysis of Th1 and Th2 cytokines expressing CD4+ and CD8+ T cells in rheumatoid arthritis by flow cytometry. J Rheumatol. 2000;27(5):1128–35. [PubMed] [Google Scholar]
- 46.Ishii H, Tanaka H, Katoh K, Nakamura H, Nagashima M, Yoshino S. Characterization of infiltrating T cells and Th1/Th2-type cytokines in the synovium of patients with osteoarthritis. Osteoarthritis Cartilage. 2002;10(4):277–81. [DOI] [PubMed] [Google Scholar]
- 47.Latourte A, Cherifi C, Maillet J, Ea HK, Bouaziz W, Funck-Brentano T, et al. Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann Rheum Dis. 2017;76(4):748–55. [DOI] [PubMed] [Google Scholar]
- 48.Humby F, Kelly S, Hands R, Rocher V, DiCicco M, Ng N, et al. Use of ultrasound-guided small joint biopsy to evaluate the histopathologic response to rheumatoid arthritis therapy: recommendations for application to clinical trials. Arthritis Rheumatol. 2015;67(10):2601–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1.Study Demographics. Basic demographic information about the OA patients whose synovial samples were used in this study.
Supplemental Table 2. Flow Cytometry Reagents. List of reagents used for flow cytometry staining.
Supplemental Table 3.Influence of digestion condition on surface marker detection by flow cytometry. Synovial tissue from each donor (n=3–5) was digested by six different conditions (see Fig. 1B). Released cells were subsequently stained for the listed viability (Zombie Aqua), mesenchymal, and hematopoietic cell surface molecules. Statistically significant differences (bold underlined font) in cell percentages (non-normalized) across digestion conditions were determined using repeated measures one-way ANOVA with Dunnet’s test for multiple comparisons, selecting the condition with the overall highest percentage of positive staining as the reference condition (italic font).
Supplemental Table 4.Correlation matrix summarizing soluble mediator data. Matrix summarizing the Spearman correlations between all soluble mediators.
Supplemental Table 5.Correlations of synovial mesenchymal subsets with non-mesenchymal cell populations. Shown are the statistically significant correlations (p<0.05) between the percentage of synovial mesenchymal (pre-gate CD45−CD31−CD146−) and non-mesenchymal populations in OA synovium (n=32, Spearman correlation coefficient (rs) (top), p values (bottom), n.s. not significant).
Supplemental Figure 1.Influence of tissue amount on synovial tissue flow cytometry reproducibility. (A) Example of the flow cytometry gating strategy that defines hematopoietic immune (CD45+), endothelial (CD45-CD31+) and mesenchymal (CD45-CD31−) cell populations in disaggregated OA synovium. Also shown is the range of synovial cell viability post-digestion (n=35, digestion condition 1, median with IQR). (B-E) Four, eight, or twelve OA synovial tissue samples/donor (~100 mg/samples) were pooled, enzymatically digested, and analyzed by flow cytometry. For each donor, pooled digestions were done in duplicate (technical replicates). (B) The total cell yield was determined by manual counting (n=4, technical replicates averaged). Cell yield differences were statistically significant by one-way ANOVA (p=0.044). (C-E) Variability introduced by pooling tissue samples (four, eight or twelve samples) was estimated by calculating the average difference between technical replicates for the percentage of (C) major, (D) mesenchymal (pre-gate CD45−CD31−), and (E) hematopoietic immune (CD45+) cell populations (n=4, differences between digestions not statistically significant by parametric repeated measures ANOVA).
Supplemental Figure 2.Influence of overnight culture with monensin on synovial cell yield and composition. Synovial tissue from three donors was divided to compare cell yield and percentages from freshly digested tissue (day 0) with tissue digested after overnight culture with monensin (day 1) (digestion condition 1). The cell count (A) and percentage (B) of hematopoietic (CD45+), endothelial (CD45-CD31+) and mesenchymal (CD45-CD31−) cells in disaggregated synovium was determined by manual counting and flow cytometry, respectively (paired Student’s t-test (day 0 vs 1) with p value for (A) 0.02 and (B) non-significant). The percentage of mesenchymal (C) or hematopoietic immune (D) cell populations on day 1 was expressed as a fraction of day 0 (differences not statistically significant by repeated measures one-way ANOVA).
Suppl. Fig. 3. Correlations shown between the synovial tissue release of (A) IL-6 and IL-8 or between total cell number and the synovial tissue release of (B) IL-6, (C) CFD, (D) IL-10, (E) CCL2, and (G) TNF-α (Spearman analysis).