Abstract
Objective:
Four autoantigens have been described recently in IgG4-related disease (IgG4-RD): prohibitin, annexin A11, laminin 511-E8, and galectin-3. However, no external validation has been performed and the possibility that some individuals break tolerance to more than one autoantigen has not been explored.
Methods:
Autoantibody responses against prohibitin, annexin A11 and laminin 511-E8 were measured by ELISA among a clinically diverse cohort of IgG4-RD patients (n=100). Autoantibody responses were correlated with disease severity and organ distribution.
Results:
The frequencies of IgG4 autoantibody responses against prohibitin (10%), annexin A11 (12%), and laminin 511-E8 (7%) were not significantly different from those of controls. A portion of the cohort (n = 86) had been analyzed previously at our center for anti-galectin-3 antibody responses with 25 (29%) having IgG4 anti-galectin-3 antibodies. Among these 86 subjects, 32 (37%) had IgG4 antibodies to at least one of the 4 auto-antigens and 12 (14%) showed reactivity to ≥2 of the tested antigens. The subset of patients with ≥2 autoantibodies had higher total IgG1, IgG2, IgG4, and C-reactive protein levels; were more commonly hypocomplementemic; and were more likely to have visceral organ involvement.
Conclusion:
Antibodies against prohibitin, annexin A11, and laminin 511-E8 were found in only a small portion of patients with IgG4-RD. A subset of IgG4-RD patients, however, had IgG4 antibodies against ≥2 autoantigens. Patients with antibodies against ≥2 autoantigens present with robust IgG subclass elevations, complement consumption, and visceral organ involvement. This broader break in immunological tolerance in IgG4-RD was associated with more severe disease.
Keywords: IgG4-related disease, IgG4-RD, auto-antigen, laminin 511-E8, annexin A11, prohibitin, galectin-3
Introduction:
IgG4-related disease (IgG4-RD) is an insidiously progressive autoimmune fibrotic disease of unknown etiology that often presents with tumor-like masses involving multiple organs (1). The seemingly disparate organ manifestations are unified by the pathological findings of a dense lymphoplasmacytic infiltrate, fibrosis in a “storiform” (storea = Latin for woven mat) pattern, and obliterative phlebitis, with prominent IgG4+ plasma cells noted on immunostaining (2). Characteristics of this disease include the oligoclonal expansion of both plasmablasts and tissue-infiltrating CD4+ cytotoxic T lymphocytes, the identification of specific auto-antigens as B cell targets, and consistent clinical responsiveness to B cell depletion (3–11). These observations support the possibility that IgG4-RD is an autoimmune disease, with an important role for adaptive immune responses in the associated tissue fibrosis.
Since 2015, four different auto-antigens have been described as potential triggers for IgG4-RD: prohibitin, annexin A11, laminin 511-E8 and galectin-3 (5–8). However, validation of these findings using external patient cohorts and characterization of the relationship between these specific auto-antigens has yet to be achieved. Furthermore, annexin A11 and laminin 511-E8 have been reported only in the organ-specific contexts of IgG4-related autoimmune pancreatitis (AIP) and IgG4-related autoimmune cholangitis (AIC) for the former, and only AIP for the latter (6, 8). In order to explore the role of the adaptive immune response in IgG4-RD, we examined the relative frequencies of antibody responses against prohibitin, annexin A11, laminin 511-E8, and galectin-3 using a large, clinically-diverse cohort of patients with IgG4-RD. We analyzed these 4 autoantibodies for correlation with organ distribution, disease severity and co-occurrence with each other.
Methods:
Patient Cohorts:
One hundred patients with IgG4-RD were recruited between January 10, 2012, and June 25, 2018 through the Division of Rheumatology, Allergy and Immunology of the Massachusetts General Hospital. IgG4-RD was defined by either the fulfillment of established histopathologic or comprehensive diagnostic criteria (2,12). The 100 IgG4-RD subjects included patients with involvement of the major salivary glands (55%, n = 55), pancreas (32%, n = 32), lacrimal glands (31%, n = 31), kidneys (21%, n = 21), retroperitoneum (20%, n = 20), lungs (18%, n = 18), and biliary tract (14%, n = 14) (Table 1). Information regarding demographics, treatment, disease activity, laboratory parameters, and disease manifestations was extracted from the medical records. Disease activity was quantified using the IgG4-RD-Responder Index, with active disease defined as an IgG4-RD Responder Index ≥1 (13). All patients had active disease at the time of sample collection despite the fact that 14% were on some form of immunosuppression. Seventy-six patients (76%) had an elevated serum IgG4 level and 30 (30%) were hypocomplementemic at the time of sampling. The present cohort consisted primarily of Caucasian patients (69%, n = 69) with only 13% (n = 13) of the patient’s being of Asian descent (Table 1). Visceral organ involvement was defined by the presence of lung, pancreas, bile duct, or kidney involvement. We clustered our cohort into subsets of patients according to their number of autoantibody responses (no responses, 1 response, and ≥2 responses) and compared clinical parameters among these groups.
Table 1.
Clinical and Demographic data of IgG4-RD cohort.
| IgG4-RD Cohort (n = 100) | |
|---|---|
| Demographic Data | |
| Age at Onset (median, IQR) | 55 (4–83) |
| Age at Diagnosis (median, IQR) | 61 (4–84) |
| Sex (n, % male) | 68, 68% |
| Ethnicity | |
| Caucasian (n, %) | 69, 69% |
| Asian (n, %) | 12, 12% |
| Hispanic (n, %) | 5, 5% |
| African American (n, %) | 4, 4% |
| Other(n, %) | 10, 10% |
| Clinical Data | |
| Clinically Active (n, %) | 100, 100% |
| IgG4-RD-RI (median, IQR) | 4 (0–20) |
| On Treatment (n, %) | 14, 14% |
| Organ Distribution | |
| Multi-organ Involvement (n, %) | 68, 68% |
| Number of Organs Involved (median, IQR) | 3 (1–10) |
| Lymph Nodes (n, %) | 41, 41% |
| Submandibular Gland (n, %) | 48, 48% |
| Pancreas (n, %) | 32, 32% |
| Parotid Gland (n, %) | 26, 26% |
| Lacrimal Gland (n, %) | 31, 31% |
| Lungs (n, %) | 18, 18% |
| Retroperitoneum (n, %) | 20, 20% |
| Kidney (n, %) | 21, 21% |
| Bile Ducts (n, %) | 14, 14% |
| Laboratory Parameters | |
| Elevated Total IgG, >1,295 mg/dL (n, %) | 63, 63% |
| Elevated serum IgG1, >928.6 mg/dL (n, %) | 43, 43% |
| Elevated serum IgG2, >700.3 mg/dL (n, %) | 39, 39% |
| Elevated serum IgG3, >176.1 mg/dL (n, %) | 36, 36% |
| Elevated serum IgG4, >86.4 mg/dL (n, %) | 76, 76% |
| Elevated serum IgE, >114 kU/L (n, %) | 54, 54% |
| Low serum IgM, <53 mg/dL (n, %) | 39, 39% |
| Hypocomplementemia, C3 < 81 mg/dL or C4 < 12 mg/dL (n, %) | 30, 30% |
| Elevated C-reactive protein, >8.0 mg/L (n, %) | 35, 35% |
| Elevated erythrocyte sedimentation rate, >13 mm/hr (n, %) | 63, 63% |
To control for non-specific observations that may arise in the setting of any chronic fibro-inflammatory disease, we used idiopathic pulmonary fibrosis (IPF) plasma samples as a disease control. Samples from IPF patients (n = 50) were obtained through the Massachusetts General Hospital Division of Pulmonary & Critical Care Medicine’s Biorepository of Interstitial Lung Diseases, as previously described (14). Fifty age- and sex-matched healthy donors were collected through the Division of Rheumatology, Allergy and Immunology of the Massachusetts General Hospital as well as the Partners HealthCare Biobank, an enterprise-wide repository of samples from consented subjects. For healthy controls, we excluded patients with any history of malignancy, autoimmune disease, or recurrent/chronic infections by review of the medical records. All studies were approved by the Partners Institutional Review Board and informed written consent was obtained prior to sample collection. Plasma was collected from these subjects, separated by centrifugation, and stored at −80°C until the time of use. Autoantibody diversity was defined by antibody responses to ≥2 self-antigens from an individual subject.
Enzyme-linked Immunosorbent Assay (ELISA):
ELISA assays for antibodies against prohibitin, annexin A11 and laminin 511-E8 were performed as we previously described in the identification of galectin-3 autoantibody responses in the context of IgG4-RD (7). Briefly, alternating rows of 96-well plates were coated with antigen to allow for negative control wells (BSA only) specific for each individual subject tested. Plate coating concentrations and plasma dilutions used for each antigen were consistent with previous publications: prohibitin (Abcam, 178465; coating concentration 0.5 ug/mL, plasma dilution 1:100), annexin A11 (Abcam, 101050; coating concentration 1 ug/mL; plasma dilution 1:100), and laminin 511-E8 (Nacalai USA, 892011; coating concentration 2 ug/mL, plasma dilution 1:20). Each plasma sample was tested in triplicate with corresponding triplicate BSA-only wells without antigen-coating to control for non-specific protein-protein and protein-plate interactions. Secondary antibodies included a rabbit anti-human IgG (Abcam, 6759), mouse anti-human IgG1 (Abcam, 99774), and mouse anti-human IgG4 (Abcam, 99817). The same mouse anti-human IgG4 secondary antibody that was used previously in our identification of anti-galectin 3 responses in IgG4-RD was employed in the present experiments, as well. If any individual well deviated by more than 50% from its respective replicates, that well was not included in calculating the average absorbance. A cut-off value of 2 standard deviations above the healthy donor mean was used to categorically define a positive antibody response.
Statistical Analysis:
Descriptive measures (such as median, interquartile range, frequencies, and percentages) were used to summarize the data. The Mann-Whitney test was used to compare continuous variables between two groups. For comparisons between more than 2 groups, the Kruskal-Wallis test with Dunn’s post-hoc analysis was used. Regression analyses using logistic regression models were utilized to assess the predictors of having diverse autoantibody responses. The maximum likelihood method was used to estimate model parameters and significance was tested using Wald’s test statistic. All p-values were 2-sided and considered statistically significant if < 0.05. Statistical analysis was performed using SAS 9.4 and GraphPad Prism 8.0.
Results:
IgG4 antibodies against annexin A11, laminin 511-E8 and prohibitin are observed at a low frequency in systemic IgG4-RD.
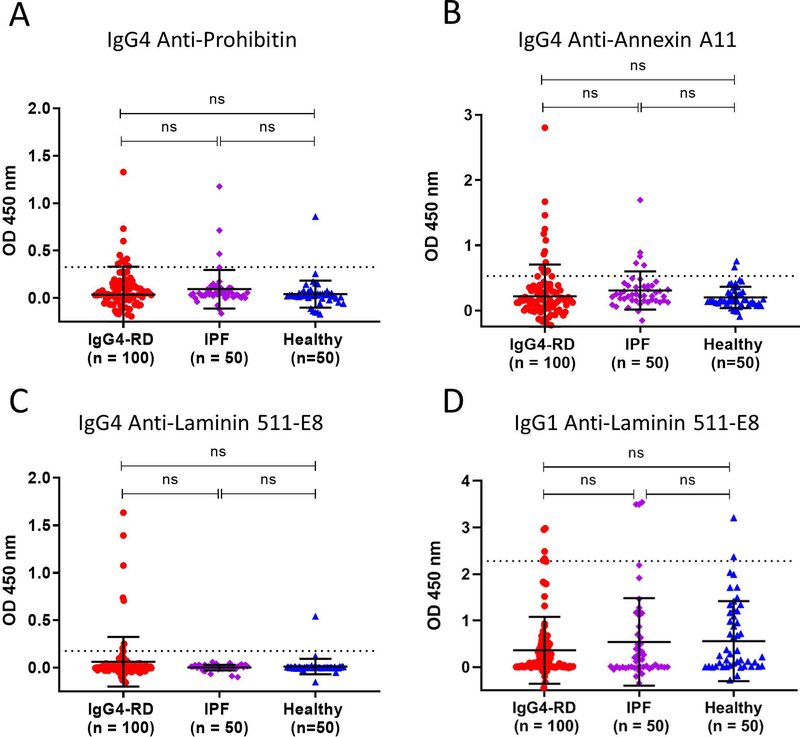
The frequencies of IgG4 antibodies were similar across all three auto-antigens, with 12% for annexin A11, 10% for prohibitin, and 7% for laminin 511-E8 (Figures 1A–C). None of these frequencies among the IgG4-RD patients differed significantly from those of controls.
Figure 1: Autoantibody responses against prohibitin, annexin A11 and laminin 511-E8 occur at a low frequency in systemic IgG4-RD.
Dot plots displaying means, standard deviations (error bars) and positivity cut-offs (dashed line representing the healthy donor mean + 2 standard deviations) show the IgG4 antibody frequencies for each studied auto-antigen among our cohort (A-C). Dot plot displaying IgG1 anti-laminin 511-E8 antibodies among our cohort (D).
For consistency with previously published data related to laminin 511-E8, we additionally examined total IgG and IgG1 specific responses against this antigen among our cohort but observed only a low frequency of reactivity using either of these specific secondary antibodies (1% for IgG, 6% for IgG1) (Figures 1D and S1A). For IgG anti-laminin 511-E8 responses, we actually observed higher frequencies among both control groups compared to IgG4-RD subjects (Figure S1A). To understand the discrepancy in IgG4 and total IgG antibody response frequencies against laminin 511-E8, we studied the relative sensitivity of each of these secondary antibodies in detecting purified human IgG4. We observed the ability of the anti-human IgG4 secondary antibody used in these studies to detect plate-bound IgG4 at a concentration of 1 ng/well. In contrast, the anti-human IgG secondary antibody required a coating concentration 10-fold greater in order to generate a comparable colorimetric signal on ELISA (Figure S1B).
Patients with pancreatobiliary disease do not enrich for annexin A11 or laminin 511-E8 autoantibodies.
Because annexin A11 and laminin 511-E8 were described in smaller IgG4-RD cohorts enriched with pancreatobiliary (annexin A11) or pancreatic involvement (laminin 511-E8), we evaluated the frequency of those antibody responses within the corresponding subset of organ involvement in our cohort (6, 8). We found that 22% of our AIP or AIC patients had IgG4 antibody responses against annexin A11. For laminin 511-E8, we observed IgG4 antibodies among 12.5% of our AIP patients.
In contrast to annexin A11 and laminin 511-E8, only anti-prohibitin antibodies had been reported in the context of multi-organ, systemic IgG4-RD, thereby permitting a direct comparison with the present cohort (5). The frequency of anti-prohibitin responses observed among our systemic and clinically-diverse cohort was 10%.
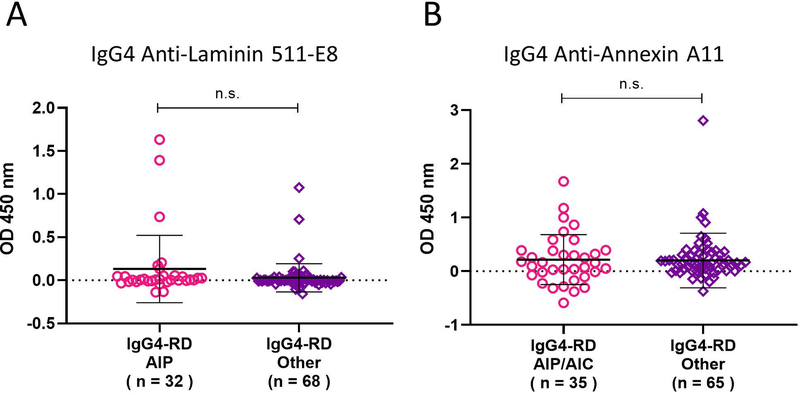
We also assessed whether antibody responses against annexin A11 or laminin 511-E8 could be predicted by stratifying our cohort based on specific organ involvement, with the stratifications designed to reflect the patient populations from the studies in which antibodies to annexin A11 and laminin 511-E8 were identified originally. We therefore compared annexin A11 autoantibody responses among IgG4-RD patients with AIP or AIC to those without such involvement and compared laminin 511-E8 responses among IgG4-RD patients with and without pancreatic involvement. No significant differences were seen between the groups, suggesting that these auto-antigen responses are not predicted by such organ-specific involvement. (Figures 2A–B).
Figure 2: Pancreatobiliary involvement does not enrich for annexin A11 or laminin 511-E8 autoantibodies.
Dot plots display IgG4 anti-annexin A11 and anti-laminin 511-E8 antibody responses between patients with pancreatic or biliary involvement to those without such involvement among our cohort (A-B).
Reactivity against multiple autoantigens is seen in a subset of IgG4-RD patients.
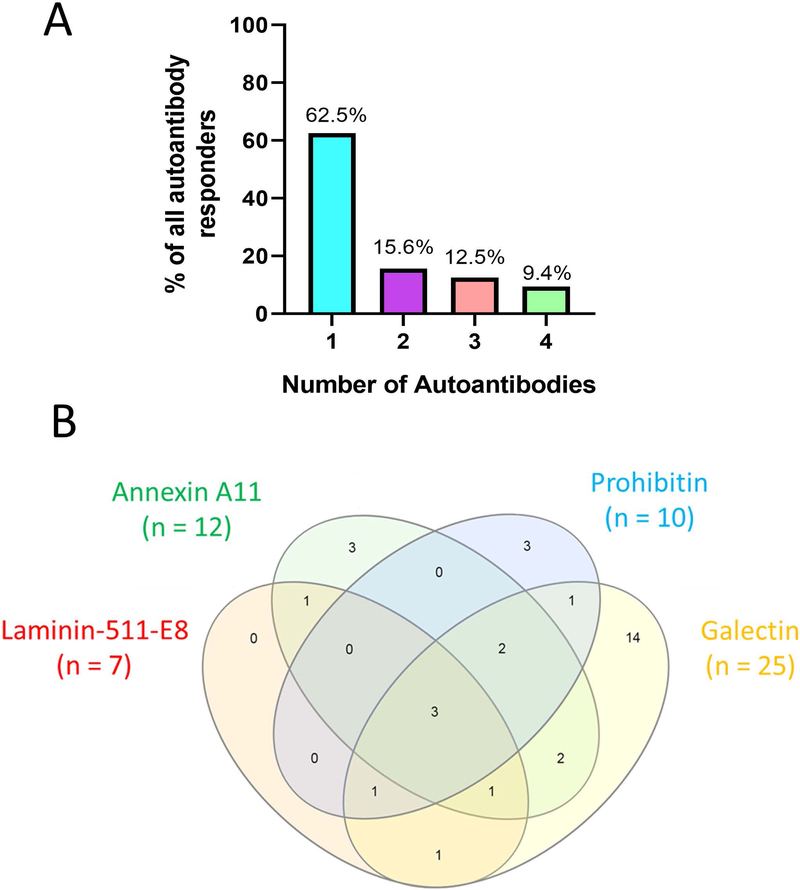
We reported previously that 28% of subjects from a cohort of 121 IgG4-RD patients had autoantibodies directed against galectin-3 (7). We examined the proportion of patients within the present cohort with data available regarding galectin-3 antibody responses (86% of the present cohort, n = 86). Among these patients, 32 (37%) had IgG4 reactivity to at least one of the 4 auto-antigens studied. We also explored the number of specific autoantibody responses for each patient among these 32 with at least one autoantibody response. The majority of these patients (n = 20; 62.5%) showed reactivity to only one of the 4 auto-antigens studied but 12 (37.5%) reacted to 2 or more auto-antigens. Five (15.6%), 4 (12.5%) and 3 (9.4%) responded to 2, 3 or all 4 autoantigens, respectively (Figure 3A).
Figure 3: A subset of IgG4-RD patients demonstrate B cell responses to a variety of auto-antigens.
Bar chart displaying percentages of subjects with IgG4 antibodies among all responders (n = 32) (A). Venn diagram showing overlap in antigen reactivity between annexin A11, laminin 511-E8, prohibitin and galectin-3 among all subjects with at least one IgG4 autoantibody response (n = 32) (B).
Analyzing the overlapping reactivity between these four autoantigens, we did not observe any IgG4 anti-laminin 511-E8 responses in isolation (Figure 3B). All 7 patients with IgG4 anti-laminin 511-E8 responses also showed reactivity to at least one other autoantigen studied. Galectin-3, the autoantigen with the highest baseline frequency of reactivity in this cohort, had the highest proportion of isolated autoantibody responses. Fourteen (56%) of the 25 subjects with anti-galectin-3 antibodies had no responses to any of the other 3 autoantigens studied. Overall, we did not observe any specific clustering in co-occurrence of antibody responses among the 4 auto-antigens tested (Figure 3B).
Disease severity in individual patients with IgG4-RD correlates with T-dependent B cell responses to multiple auto-antigens.
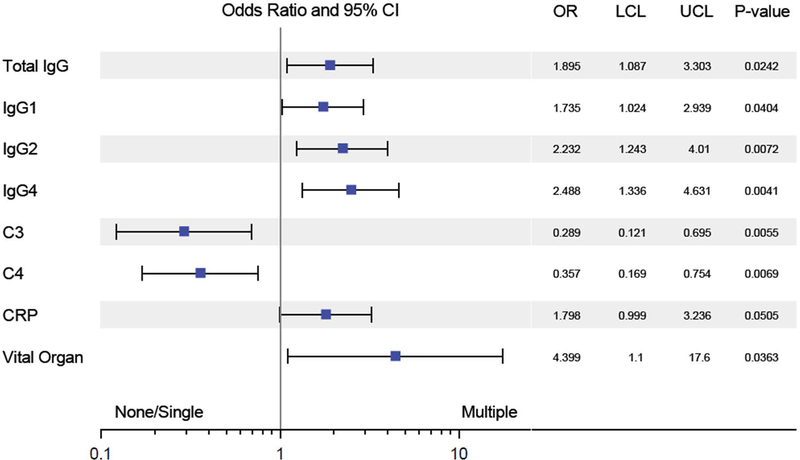
We examined the correlation between the number of specific autoantibody responses per patient and severity of the clinical phenotype. No clinical phenotypic differences were observed between the group with no autoantibody responses and those with a single autoantibody response (data not shown). Given the similarity between these groups, we then compared the subset of patients with ≥ 2 autoantibody responses, representing 14% of our cohort (n = 12 of 86), to all other patients and observed a distinctly more severe clinical phenotype among these subjects. Higher levels of IgG1, IgG2, IgG4 and C-reactive protein, greater complement consumption, and more frequent visceral organ involvement were associated with diverse autoantibody responses (Figure 4).
Figure 4: Response to multiple autoantibodies is linked to disease severity in IgG4-RD.
Forest plot showing odds ratio and 95% confidence intervals of having multiple auto-antigen responses for categorical and continuous variables. Variables favoring one or no response (left of midline) and multiple responses (right of midline) are shown. CRP = C-reactive protein.
Discussion:
Considerable insight into the immunologic mechanisms of IgG4-RD has been achieved in the last decade, but few systematic efforts at identifying the antigens driving this immune response have been undertaken (17). Given the protean clinical nature of IgG4-RD and the possibility that a diverse array of proteins may be involved in driving the disease in different patients, external validation of any proposed auto-antigen in this disease is essential. We leveraged a large, clinically-diverse cohort of IgG4-RD patients to assess the frequency of antibody responses against three recently published antigens associated with IgG4-RD: annexin A11, laminin 511-E8, and prohibitin. We observed IgG4-specific autoantibody responses against each of these proteins, but the frequency of such responses was relatively low among our cohort compared to the results in the three separate cohorts in which these autoantibody responses were described initially (5,6,8). For these three auto-antibodies, the low frequency seen in IgG4-RD patients is similar to the frequency observed in controls. With antibody response frequencies of only 7%, 10%, 12% and 28% for laminin 511-E8, prohibitin, annexin A11, and galectin-3, respectively, it seems clear that none of these auto-antigens constitute a dominant antigen in the context of IgG4-RD. We also did not observe any organ-specific enrichment for pancreatic or biliary involvement among the patients with autoantibodies directed against annexin A11 and laminin 511-E8 (6,8). The frequencies of antibody responses against these two auto-antigens among our AIP or AIC subsets of patients were comparable with those previously reported: 22% vs 18% for annexin A11 in AIP/AIC patients (6) and 12.5% vs 10% for laminin 511-E8 in AIP patients (8).
Some of the antibody response frequencies observed among patients in this cohort were widely discrepant from those previously reported. Anti-laminin 511-E8 IgG was detected in only 1% of our IgG4-RD cohort, a figure comparable to the 1.6% response frequency observed among controls by Shiokawa et al, but markedly below the 51% frequency reported from their cohort of IgG4-related AIP patients (8). Similarly, although anti-prohibitin IgG4 was reported to occur in 73% of IgG4-RD patients with diverse organ manifestations, we found only 10% IgG4 reactivity among the patients in our similarly diverse cohort (5). One possible explanation for these discrepancies may be related to genetic differences between the studied cohorts. For example, HLA class II molecules that are more prevalent among East Asians compared to Caucasians may be much more efficient at presenting immune-dominant peptides from laminin 511-E8 or prohibitin to follicular helper T cells, thereby permitting such IgG or IgG4 antibody responses. This possibility highlights the importance of cross-validation studies in patients of both East Asian and Western descent. More exuberant serum IgG4 increases were recently demonstrated to occur among East Asian patients with IgG4-RD compared to other patients (18). Such ethnic comparisons will be important in understanding the generalizability of regional findings related to IgG4-RD.
Autoantibody responses are surrogate markers of autoreactive B cell clones that have evaded normal tolerance mechanisms. In IgG4-RD, the oligoclonal proliferation of antibody-producing cells has been tied to disease activity and the marked clinical responsiveness to B-cell depletion reinforces the importance of B cells in the pathophysiology of IgG4-RD (3,9,15,19,20). Whether this is related to cytokine secretion, the elaboration of pathogenic autoantibodies, the presentation of antigen to disease-driving CD4+ cytotoxic T cells, a direct cytotoxic effect of B cell subsets or a combination of these effector functions remains unknown. The adoptive transfer of purified immunoglobulin from IgG4-RD patients to mice results in disease within organs typical of IgG4-RD, suggesting the possibility that antibodies may have a direct pathogenic role in IgG4-RD (16). The finding of a distinct subset of IgG4-RD patients with multiple autoantibodies of differing specificity suggests that some patients have a more flagrant breach of B cell tolerance compared to others. This subset of patients was clinically marked by a robust humoral immune response (higher IgG, IgG1, IgG2, IgG4), greater complement consumption (low C3 or C4), more systemic inflammation (higher C-reactive protein levels), and more severe disease (higher likelihood of visceral organ involvement). These correlations suggest that patients with diverse B cell auto-antigen responses have a more severe clinical phenotype.
The correlation of hypocomplementemia with diverse autoantibody responses supports the possibility that immune-complexes are relevant to the disease process in a subset of patients with IgG4-RD (21,22). However, it is also possible that patients with more severe disease break immunological tolerance more efficiently, generate higher non-pathogenic antibody titers against self-antigens, and induce the transient formation of circulating immune complexes and thus hypocomplementemia. These findings could also therefore merely represent epiphenomena of limited relevance to the pathogenesis of IgG4-RD.
The IgG4-RD-RI is designed to measure disease activity longitudinally in an individual basis but is not suited to comparisons of disease severity across subjects (13). For instance, a patient with asymptomatic disease confined to the lacrimal, parotid and submandibular glands would be designated a score of 6 while a patient admitted to the hospital with obstructive jaundice secondary to a pancreatic mass would be given a score of 4. Similarly, the raw number of organs involved, or multiple organ involvement vs single organ involvement are susceptible to such limitations in broadly gauging disease severity. Without a validated tool for gauging disease severity, we used the likelihood of having visceral organ involvement (lung, pancreas, bile ducts, kidney) as a surrogate indicator of disease severity. In contrast to patients with disease limited to the lacrimal or salivary glands, those with visceral organ involvement generally require more urgent therapeutic intervention. This clinical phenotype of visceral organ involvement did correlate with the number of different autoantibody responses per individual patient. Our group has recently described the clustering of IgG4-RD patients based on the clinical phenotypes of organ distribution with certain clusters being distinguished by visceral organ involvement (23). The positive correlation of more severe disease involvement with IgG4 autoantibody diversity supports the possibility that such antibodies may be pathogenic in IgG4-RD. Additional studies of IgG4 autoantibodies in the pathophysiology and IgG4-RD and their relationship to disease severity are indicated.
A potential limitation of the present study is the use of our previously reported IgG4 galectin-3 autoantibody responses observed among our cohort of IgG4-RD subjects (7). The plasma samples utilized here to validate anti-annexin A11, anti-laminin 511-E8 and anti-prohibitin autoantibody responses were chosen based on sample availability. Among the 86 subjects used for the validation studies here that also had available data regarding galectin-3 responses, 25 (29%) had known IgG4 anti-galectin-3 autoantibody responses. This response rate is quite comparable to the 28% anti-galectin-3 response frequency we previously reported among the larger cohort of 121 IgG4-RD subjects, which suggests that the subjects used in the current analysis were reflective of the greater cohort. Moreover, as a means of eliminating detection bias, the same exact healthy subject and idiopathic pulmonary fibrosis plasma samples were used for the present studies that we had employed in our previous work on galectin-3 (7).
In summary, we have evaluated prohibitin, annexin A11 and laminin 511-E8 as auto-antigens in IgG4-RD using a large and clinically-diverse cohort of IgG4-RD patients. Antibodies to these auto-antigens were observed only at a low frequency among the cohort studied here, were equally frequent in control subjects and did not enrich for specific organ involvement. We identified the presence of a subset of IgG4-RD patients with multiple auto-antigen B-cell responses that was clinically-characterized by more severe disease with higher IgG4 levels, more complement consumption, higher C-reactive protein levels and more visceral organ involvement. While these findings suggest an underlying importance for B cell tolerance and the potential pathogenicity of autoantibodies in IgG4-RD, they also highlight the unmet need in identifying a dominant and specific antigen that may drive the pathogenesis of IgG4-RD. Such a discovery would create a novel diagnostic option and broaden our understanding of this immune-mediated fibrotic disease.
Supplementary Material
Dot plot displays means, standard deviations (error bars) and positivity cut-offs (dashed line representing the healthy donor mean + 2 standard deviations) showing the IgG antibody frequencies against laminin 511-E8 (A). Points with connecting lines and error bars display a 10-fold greater sensitivity of the anti-IgG4 secondary antibody used in detecting plate coated IgG4 compared to that of the anti-IgG secondary antibody used (B).
Acknowledgements:
This work was primarily supported by the NIH Autoimmune Centers of Excellence funding to SP and JHS including U19AI110495 and UM1 AI144295. CAP was supported by a Rheumatology Research Foundation Scientist Development Award and a Sponsored Research Agreement with UCB. MG was supported by grants from the National Institutes of Health NIH/NIAID P30 AI060354. ZSW was supported by a Scientist Development Award from the Rheumatology Research Foundation. SBM receives grant funding from the Parker B. Francis Foundation and the Scleroderma Foundation.
References:
- 1.Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet 2015;385:1460–1471. [DOI] [PubMed] [Google Scholar]
- 2.Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol 2012;25:1181–1192. [DOI] [PubMed] [Google Scholar]
- 3.Mattoo H, Mahajan VS, Della-Torre E, Sekigami Y, Carruthers M, Wallace ZS, et al. De novo oligoclonal expansions of circulating plasmablasts in active and relapsing IgG4-related disease. J Allergy Clin Immunol 2014;134:679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattoo H, Mahajan VS, Maehara T, Deshpande V, Della-Torre E, Wallace ZS, et al. Clonal expansion of CD4+ cytotoxic T lymphocytes in patients with IgG4-related disease. J Allergy Clin Immunol 2016;138:825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du H, Shi L, Chen P, Yang W, Xun Y, Yang C, et al. Prohibitin Is Involved in Patients with IgG4 Related Disease. PLoS One 2015;10:e0125331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hubers LM, Vos H, Schuurman AR, Erken R, Oude Elferink RP, Burgering B, et al. Annexin A11 is targeted by IgG4 and IgG1 autoantibodies in IgG4-related disease. Gut 2018;67:728–735. [DOI] [PubMed] [Google Scholar]
- 7.Perugino CA, AlSalem SB, Mattoo H, Della-Torre E, Mahajan V, Ganesh G, et al. Identification of galectin-3 as an autoantigen in patients with IgG4-related disease. J Allergy Clin Immunol 2019;143:736–745.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiokawa M, Kodama Y, Sekiguchi K, Kuwada T, Tomono T, Kuriyama K, et al. Laminin 511 is a target antigen in autoimmune pancreatitis. Sci Transl Med 2018;10 Available at: 10.1126/scitranslmed.aaq0997. [DOI] [PubMed] [Google Scholar]
- 9.Carruthers MN, Topazian MD, Khosroshahi A, Witzig TE, Wallace ZS, Hart PA, et al. Rituximab for IgG4-related disease: a prospective, open-label trial. Ann Rheum Dis 2015;74:1171–1177. [DOI] [PubMed] [Google Scholar]
- 10.Majumder S, Mohapatra S, Lennon RJ, Piovezani Ramos G, Postier N, Gleeson FC, et al. Rituximab Maintenance Therapy Reduces Rate of Relapse of Pancreaticobiliary Immunoglobulin G4-related Disease. Clin Gastroenterol Hepatol 2018;16:1947–1953. [DOI] [PubMed] [Google Scholar]
- 11.Ebbo M, Grados A, Samson M, Groh M, Loundou A, Rigolet A, et al. Long-term efficacy and safety of rituximab in IgG4-related disease: Data from a French nationwide study of thirty-three patients. PLoS One 2017;12:e0183844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umehara H, Okazaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD). Mod Rheumatol 2012;22(1):21–30. [DOI] [PubMed] [Google Scholar]
- 13.Wallace ZS, Khosroshahi A, Carruthers MD, Perugino CA, Choi H, Campochiaro C, et al. An International, Multi-Specialty Validation Study of the IgG4-Related Disease Responder Index. Arthritis Care Res 2018. Available at: 10.1002/acr.23543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lagares D, Ghassemi-Kakroodi P, Tremblay C, Santos A, Probst CK, Franklin A, et al. ADAM10-mediated ephrin-B2 shedding promotes myofibroblast activation and organ fibrosis. Nat Med 2017;23:1405–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace ZS, Mattoo H, Carruthers M, Mahajan VS, Della Torre E, Lee H, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis 2015;74:190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiokawa M, Kodama Y, Kuriyama K, Yoshimura K, Tomono T, Morita T, et al. Pathogenicity of IgG in patients with IgG4-related disease. Gut 2016;65:1322–1332. [DOI] [PubMed] [Google Scholar]
- 17.Perugino CA, Mattoo H, Mahajan VS, Maehara T, Wallace ZS, Pillai S, et al. Emerging Treatment Models in Rheumatology: IgG4-Related Disease: Insights Into Human Immunology and Targeted Therapies. Arthritis Rheumatol 2017;69:1722–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi R, Chen LYC, Park S, Irvine R, Seidman MA, Kelsall JT, et al. Utility of Serum IgG4 Levels in a Multiethnic Population. Am J Med Sci 2018;355:61–66. [DOI] [PubMed] [Google Scholar]
- 19.Doorenspleet ME, Hubers LM, Culver EL, Maillette de Buy Wenniger LJ, Klarenbeek PL, Chapman RW, et al. Immunoglobulin G4(+) B-cell receptor clones distinguish immunoglobulin G 4-related disease from primary sclerosing cholangitis and biliary/pancreatic malignancies. Hepatology 2016;64:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Della-Torre E, Feeney E, Deshpande V, Mattoo H, Mahajan V, Kulikova M, et al. B-cell depletion attenuates serological biomarkers of fibrosis and myofibroblast activation in IgG4-related disease. Ann Rheum Dis 2015;74:2236–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornell LD. IgG4-related kidney disease. Semin Diagn Pathol 2012;29:245–250. [DOI] [PubMed] [Google Scholar]
- 22.Zhang P, Cornell LD. IgG4-Related Tubulointerstitial Nephritis. Adv Chronic Kidney Dis 2017;24:94–100. [DOI] [PubMed] [Google Scholar]
- 23.Wallace ZS, Zhang Y, Perugino CA, Naden R, Choi HK, Stone JH, et al. Clinical phenotypes of IgG4-related disease: an analysis of two international cross-sectional cohorts. Ann Rheum Dis 2019;78:406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dot plot displays means, standard deviations (error bars) and positivity cut-offs (dashed line representing the healthy donor mean + 2 standard deviations) showing the IgG antibody frequencies against laminin 511-E8 (A). Points with connecting lines and error bars display a 10-fold greater sensitivity of the anti-IgG4 secondary antibody used in detecting plate coated IgG4 compared to that of the anti-IgG secondary antibody used (B).