Abstract
Objective:
To investigate whether paresthesia of the lower extremities following exposure to the World Trade Center (WTC) disaster was associated with signs of neuropathy, metabolic abnormalities or neurotoxin exposures.
Methods:
Case-control study comparing WTC-exposed paresthesia cases with “clinic controls” (WTC-exposed subjects without paresthesias), and “community controls” (WTC-unexposed persons).
Results:
Neurological histories and examination findings were significantly worse in cases than controls. Intraepidermal nerve fiber densities were below normal in 47% of cases and sural to radial sensory nerve amplitude ratios were <0.4 in 29.4%. Neurologic abnormalities were uncommon among WTC-unexposed community controls. Metabolic conditions and neurotoxin exposures did not differ among groups.
Conclusions:
Paresthesias among WTC-exposed individuals were associated with signs of neuropathy, small and large fiber disease. The data support WTC-related exposures as risk factors for neuropathy, and do not support non-WTC etiologies.
Keywords: Paresthesia, neuropathic symptoms, electrodiagnostic neurologic measurements, small nerve fiber density, peripheral neuropathy
INTRODUCTION
Lower extremity paresthesias have been reported among first-responders and community members exposed to the World Trade Center (WTC) disaster of September 11, 2001 (referred to herein as 9/11). We previously reported paresthesias among members of the WTC Environmental Health Center (EHC), a treatment program for community members (local workers, local residents, students and passersby) exposed to WTC dust and fumes on and after 9/11 who subsequently developed medical or mental health conditions thought to be due to this exposure.1, 2 The WTC EHC, now a federal program, provides services based upon exposure to the events of 9/11 and development of conditions certifiable as likely caused by WTC exposure.3 Risk factors for paresthesia included intensity of exposure to WTC dust on 9/11 and employment in a job that required cleaning of WTC dust.2 Time to paresthesia was associated with these same variables.4 Furthermore, electromyographic studies of 16 WTC-exposed first-responders found 1 case of possible demyelinating neuropathy and 3 cases of motor neuron disease without evidence of compression of the corticospinal tracts.5 Rodent nerves exposed ex vivo to WTC dust had lower conduction velocities than control nerves.6
Heavy metals and complex hydrocarbons (e.g., polycyclic aromatic hydrocarbons, polybrominated diphenyl ethers, polychlorinated biphenyls, and polychlorinated naphthalenes) in WTC dust and fumes have potential neurotoxicity and provide biologic plausibility for the hypothesis that WTC exposures were a risk for neuropathy.7-9 Additionally, cleaning of WTC dust may have exposed workers to neurotoxic cleaning agents. Repetitive motions during cleaning activities may have increased the risk of carpal tunnel syndrome.
We performed a case-control study of lower extremity paresthesia among WTC-exposed individuals to explore whether this subjective symptom was associated with clinical signs, including markers of small or large fiber nerve abnormalities, and metabolic and toxic blood markers for possible non-WTC neuropathic etiologies.
MATERIALS AND METHODS
Subjects and Study Design:
We enrolled patients primarily from the Bellevue Hospital WTC EHC. Study enrollment occurred during June 28, 2017 - August 22, 2018. WTC EHC patients completed questionnaires interrogating demographics, health and WTC exposures at their enrollment visits and at recall, or “monitoring” visits. Potential volunteers for the present study were identified among WTC EHC enrollees who consented to the use of their information for research, and who separately consented to future contact to discuss participation in related research projects. Inclusion criteria included age 18-75 years and enrollment in the WTC EHC between August 22, 2005 and March 30, 2018.
We employed a case-control study design to contrast symptoms, signs and exposures among 4 groups:
1. Cases:
WTC EHC patients who reported paresthesias of the lower extremities at their most recent WTC EHC visit and in the screening questionnaire with symptom frequency of “often” or “almost continuous.” We excluded isolated upper-extremity paresthesias to minimize cases of carpal tunnel syndrome.
2. Clinic Controls:
WTC EHC patients who did not report paresthesia of the upper or lower extremities at any study visit, and who continued to be free of paresthesias at the times of screening and enrollment into the present study. This group was enrolled primarily to estimate the prevalence of signs of neuropathy among paresthesia-free WTC survivors.
3. Community Controls:
Individuals without WTC exposure who did not live or work south of Canal Street, Manhattan, New York on 9/11. These subjects included: (1) friends or relatives of cases or clinic controls; (2) participants in other research studies being conducted at Bellevue Hospital Center; or (3) participants in the ResearchMatch, a secure registry at NYU Langone Medical Center open to individuals interested in participating in research studies. This group was enrolled primarily to estimate the prevalences of paresthesia and signs of neuropathy in the general population without severe WTC exposure.
4. Historical Controls for Small Nerve Fiber Density Measurements:
Normative data on small nerve fiber densities were obtained from Therapath Partners, LLC. Therapath provided a de-identified data set of epidermal small nerve fiber densities, sweat gland nerve fiber densities, sex and age for 128 individuals who had been classified as free of neuropathic symptoms on the basis of history and physical examination. This study had been conducted with oversight from The Copernicus Group Independent Review Board (study THE4-06-146). All subjects provided written informed consent. Study recruitment occurred during June 14, 2006 - March 28, 2014 (Arthur P. Hays, MD, principal investigator). Subjects were recruited at sites in Arizona, California, Idaho, Maryland, New Jersey, and New York. These proprietary data were used with permission by the owner, Therapath Partners, LLC, which also provided sex- and age-specific cut-points for abnormality.
Cases and clinic controls had to meet their respective group’s paresthesia requirements (see below) during the month prior to enrollment. Exclusion criteria applied to cases, clinic and community controls were: paresthesia before 9/11; cancer chemotherapy at any time; known vitamin B12 deficiency; diabetes mellitus; use of antiretroviral medications at any time; and conditions that would preclude elective skin punch biopsies, including bleeding disorders, use of anticoagulants, and history of keloid scarring.
We used frequency-matched sampling to recruit subjects into sex- and age (18-25, 25-34, 35-44, 45-54, 55-64, and 65-74 years)-defined strata. We planned to recruit 1 clinic control per case and 1 community control for every 2 cases.
Of 5,457 potential subjects aged 18-75 years enrolled in the Bellevue WTC EHC between August 22, 2005 - March 30, 2018, first monitoring visits were completed by 2,373 individuals (43.5% of all enrolled WTC EHC patients); second monitoring visits (conducted from December 5, 2012 on) were completed by 1,105 individuals (18.4% of enrollees); and third monitoring (conducted from April 1, 2015 on) were completed by 384 (5.6% of enrollees).
Study Size:
We planned to recruit 40 cases, 40 clinic controls and 20 community controls, a sample size with adequate power at the 2-tailed alpha significance level to show a difference between a 30% or greater prevalence of abnormal small nerve fiber densities in cases compared to an anticipated prevalence of 5% or fewer among historical controls with a power of 97%.10 For other parameters of interest, such as abnormal neurological examination results, the samples of 40 cases and 40 clinic controls would provide 88% power at a 5% 2-tailed alpha significance level if the risk factor were present in 30% or more of cases and 5% or fewer of clinic controls. For comparisons between the planned 40 cases and 20 community controls, the study would have power of 76% at an alpha-significance level of 0.05 to detect the difference between a factor present in 30% of cases and 5% of community controls.
Interview Measures:
At the single study visit required for the present study, subjects responded to a questionnaire regarding demographics, health history and WTC exposures. The questionnaire included a self-administered alcohol assessment (CAGE questionnaire11) modified to inquire into alcohol issues within the past year and the Post-Traumatic Checklist-17 (PCL), used to quantify subjects’ degree of post-traumatic stress disorder. We used the Occupational Integrated Database Exposure Assessment System (OccIDEAS) to elicit histories of exposure to potential neurotoxins during work or hobbies (see Appendix 1 for a list of these agents).12 Interview data from first monitoring visits were used to complete data on WTC exposures that were missing from the present study’s questionnaire responses for several subjects. We also mailed a 5-question supplemental questionnaire to 13 subjects to further supplement missing data on WTC exposures (returned by 8 [62%] of these subjects).
Body mass index (BMI) and vital signs were obtained using standard equipment.
Neurological Examination:
All subjects underwent neurologic examination with trained neurologists. Subjects completed additional self-administered questionnaires, including: the Utah Early Neuropathy Scale,13 the University of Michigan Neuropathy Screening Instrument – Patient Version,19 a modification of the neurological history and examination used by the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (personal communication, Brian Callaghan, February 2, 2017),14, 15 and the Norfolk Quality of Life – Diabetic Neuropathy (QOL-DN).16
Nerve conduction studies:
We evaluated large fiber neuropathy via nerve conduction studies using methods recommended for the early identification of diabetic symmetrical peripheral neuropathy.15 Controls were not subjected to nerve conduction studies out of concern that there would be no benefit to most controls and that anticipated discomfort associated with these studies would reduce participation rates. We measured distal latencies and amplitudes, and calculated conduction velocities of the peroneal, tibial and ulnar motor nerves; and of the sural sensory and radial sensory nerves. These nerves were chosen because they were included in the nerve impairment score (NIS) + 7 reported by Suanprasert, et al.17 and have previously been shown to be useful in describing diabetic neuropathy.18 Studies were performed whenever possible on the left side. We also analyzed the sural to radial sensory nerve amplitude ratio and categorized subjects as abnormal if this ratio was < 0.4, as has been suggested for diagnosis of axonal polyneuropathy.19 In a study of Sjorgren’s syndrome, 20.4% of subjects had sural to radial sensory nerve amplitude ratios < 0.4 compared to 6% of healthy controls.20 Nerve conduction studies were performed after subjects had been in temperature-controlled spaces maintained at 32° C for at least 1 hour. We did not conduct electromyography (EMG) out of concern that the required insertion of needles might reduce participation rates. Cut-points for abnormality of electrodiagnostic test results were provided by the Bellevue Hospital Center Neurology Laboratory where the testing was performed.
Epidermal nerve and sweat glands nerve fiber densities:
We evaluated small fiber neuropathy by measuring the density of small nerve fibers in skin biopsies.21, 22 Each case was asked to undergo a single, circular 3 mm skin punch biopsy of the distal left leg, approximately 10 cm above the lateral malleolus. A commercial laboratory (Therapath Partners, LLC) stained and evaluated the biopsy specimens for epidermal nerve fiber density. If the epidermal nerve fiber density was normal, sweat gland nerve fiber density was evaluated. This testing resulted in two measures: the quantitative epidermal nerve fiber density per mm of epidermal length, and a dichotomous characterization of the epidermal or sweat gland small nerve fiber density as “normal” or “abnormal” when compared with age- and gender-specific normative values. Some samples obtained did not have any visible sweat glands due to either sampling or compression of the glands during transfer to the specimen vial.
Study neurologists performed the skin punch biopsies and the nerve conduction velocity studies. Due to their involvement in these procedures and the scheduling thereof, we were unable to mask the neurologists to subjects’ case or control status.
Alternative metabolic, infectious, nutritional, or toxic causes of paresthesia and peripheral neuropathy:
Venous peripheral blood samples were evaluated by the NYU Langone Medical Center Clinical Laboratory to evaluate possible metabolic, infectious, nutritional, or toxic causes of peripheral neuropathy (Table 1).23 Syphilis testing followed the reverse algorithm.24 Blood lead concentrations were measured by a commercial laboratory (ARUP Laboratories, Salt Lake City, Utah). Blood test results were analyzed as continuous variables and also were dichotomized into normal vs. abnormal with abnormal defined by the participating laboratories as hemoglobin A1c ≥ 6%, C-reactive protein ≥ 3 units, methylmalonic acid > 0.4 μmol/L, vitamin B12 < 213 pg/mL, folate < 6 ng/mL, hemoglobin A1c ≥ 6%, fasting glucose > 100 mg/dL, ANA inverse titers ≥ 40, C-reactive protein ≥ 3 units, gliadin peptide IgA antibodies ≥ 19 units, gliadin peptide IgG antibodies ≥ 19 units, lead ≥ 4.9 μg/dL, and B. burgdorferi antibodies ≥ 0.91 units.
Table 1.
Blood tests performed to investigate known causes of paresthesia or neuropathic injury
| System | Blood test |
|---|---|
| Endocrine: | Fasting glucose and hemoglobin A1C |
| Infectious disorders: | Syphilis (Treponema pallidum IgG Ab), Lyme (Borrelia burgdorferi) |
| Nutritional: | B12, methylmalonic acid, folate |
| Autoimmune disorders: | Antinuclear antibody, antibodies to deamidated gliadin peptide (associated with celiac disease) |
| Hematologic: | Serum protein electrophoresis with immunofixation |
| Inflammatory reactions | C-reactive protein |
| Toxic exposures | Blood lead concentration |
Data management:
We managed study data using the REDCap (Research Electronic Data Capture) system hosted by NYU School of Medicine.25
Statistical Methods:
Categorical variables were summarized using counts and proportions, and statistically evaluated using the chi-squared or Fisher’s exact tests. Continuous variables were summarized using means and standard deviations and differences tested using the t-test, analysis of variance or linear regression; or for variables that were not normally distributed, medians, inter-quartile ranges (IQRs) and the Wilcoxon rank-sum or Kruskal-Wallis test. The Mantel-Haenszel technique was used to calculate summary odds ratios after stratifying on the potential confounders of sex and categorized age used in the frequency-matched enrollment process.26 Counts for some variables did not sum to the total numbers of subjects in each group due to missing data.
Ethical Oversight:
All study procedures were approved by the NYU School of Medicine Institutional Review Board (study number 016-1490 and WTC EHC research database NCT00404898). Written informed consent was provided by all study participants. Data on historical controls were provided by Therapath, Inc., in a HIPAA-compliant, de-identified manner.
RESULTS
Study Enrollment:
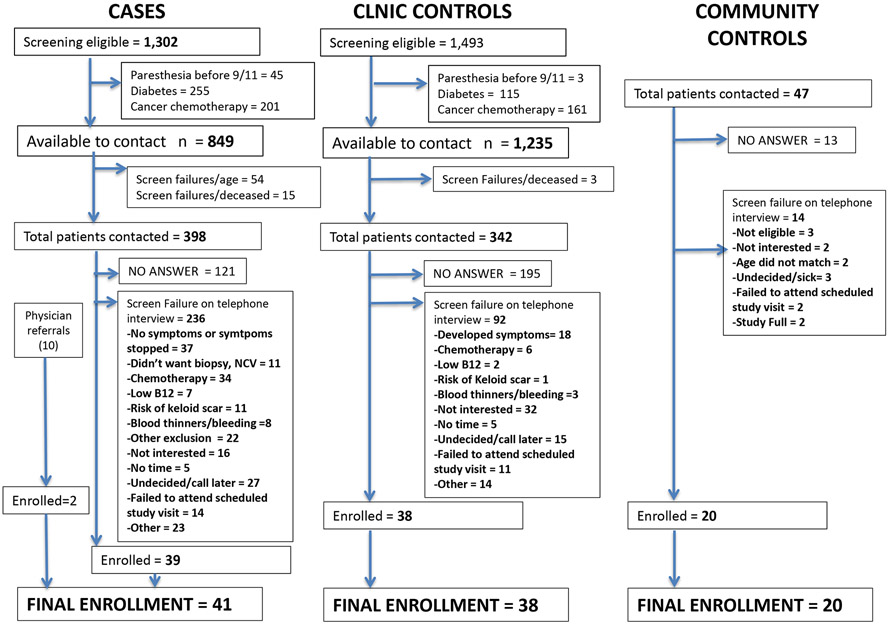
We identified 1,302 patients who met the paresthesia case definition, 1,493 who met the clinic control definition, and 2,586 who met neither definition. Patients were excluded based on paresthesias prior to 9/11 (n = 47), diabetes (n = 370) or cancer chemotherapy (n = 711). There resulted 849 potential cases and 1,235 potential controls (Figure 1). Two enrolled cases were referrals from a collaborating hospital to the WTC EHC for certification of illnesses. Participation rates were 11% among cases and clinic controls and 43% among community controls. We enrolled 41 cases, 38 clinic controls and 20 community controls.
Figure 1.
Flow chart of recruitment for case-control study of paresthesia.
Enrollment Demographics:
Due to frequency-matching during enrollment, the age and gender distributions were similar across groups (Table 2). There were no significant differences in race or ethnicity. Cases reported lower annual household incomes compared with clinic or community controls. The distributions of BMI did not differ among groups. CAGE scores did not differ significantly among groups.
Table 2.
Demographic description of subjects enrolled in a case-control study of paresthesia and World Trade Center exposures.a
| Variable | Value | Cases | Clinic Controls |
Community Controls |
p-value* |
|---|---|---|---|---|---|
| Number enrolled | 41 | 38 | 20 | ||
| Gender | |||||
| Male | 15 (37) | 13 (34) | 8 (40) | 0.9 | |
| Female | 26 (63) | 25 (66) | 12 (60) | ||
| Age | |||||
| 25-34 | 2 (5) | 1 (3) | 1 (5) | 1.0 | |
| 35-44 | 4 (10) | 4 (11) | 2 (10) | ||
| 45-54 | 8 (20) | 7 (18) | 4 (20) | ||
| 55-64 | 16 (39) | 15 (39) | 7 (35) | ||
| ≥ 65 | 11 (27) | 11 (30) | 6 (30) | ||
| Race | |||||
| Caucasian | 15 (37) | 21 (55) | 8 (40) | 0.16 | |
| African-American | 11 (37) | 8 (21) | 9 (45) | ||
| American Indian, Asian and other | 3 (7) | 4 (11) | 0 (0) | ||
| Don't know/Refused to answer | 12 (29) | 5 (13) | 3 (15) | ||
| Ethnicity | |||||
| Not Hispanic | 27 (66) | 29 (76) | 14 (70) | 0.8 | |
| Hispanic | 13 (32) | 8 (21) | 6 (30) | ||
| Refused to answer | 1(2) | 1 (3) | 0 (0) | ||
| Annual household income (USD) | |||||
| <$25,000 | 15 (37) | 6 (16) | 7 (35) | 0.04¶ | |
| $25,001 - $75,000 | 17 (41) | 15 (39) | 7 (35) | ||
| > $75,000 | 3 (12) | 16 (42) | 6 (30) | ||
| Refused to answer | 4 (10) | 1 (3) | 0 (0) | ||
| Body mass index BMI (kg/m2) | < 25 | 6 (15) | 12 (32) | 7 (35) | 0.2 |
| 25 to < 30 | 15 (37) | 12 (32) | 7 (35) | ||
| 30 to < 35 | 10 (24) | 6 (16) | 5 (25) | ||
| ≥ 35 | 10 (24) | 8 (21) | 1 (5) | ||
| CAGE alcoholism score | < 2 | 39 (98) | 31 (86) | 18 (95) | 0.3 |
| ≥ 2 | 1 (3) | 4 (11) | 1 (5) | ||
| Weekday alcohol consumption outside mealtimes | No | 37 (95) | 31 (89) | 14 (74) | 0.06 |
| Yes | 2 (5) | 4 (11) | 5 (26) | ||
| Days per week consumed alcohol | 0 – 1 | 31 (82) | 23 (66) | 11 (61) | 0.3 |
| 2 | 4 (11) | 4 (11) | 4 (22) | ||
| ≥ 3 | 3 (8) | 8 (22) | 4 (17) | ||
| Caught in WTC dust cloud on 9/11 | No | 17 (41) | 17 (45) | 0.8 | |
| Yes | 24 (59) | 21 (55) | |||
| Heavily covered with dust on 9/11 | No | 30 (73) | 33 (87) | 0.17¶ | |
| Yes | 11 (27) | 5 (13) | |||
| Worked in a job requiring cleaning of WTC dust | No | 32 (78) | 34 (89) | 0.23¶ | |
| Yes | 9 (22) | 4 (11) |
Counts not summing to the total number of subjects in each group reflect missing data.
p-values from the chi-squared test unless otherwise indicated.
p-value from Fisher's exact test
WTC Exposure Histories:
27% (11/41) of cases reported being “heavily covered and unable to dust most of the dust off with their hands before returning home on 9/11” compared to 13% (5/38) of clinic controls (p =0.17, Fisher’s exact test). The Mantel-Haenszel age- and sex-adjusted summary odds ratio (OR) for paresthesia associated with being heavily exposed to WTC dust on 9/11 was 2.3 (95% CI = 0.7-7.8, p=0.18). There also was a suggestion that paresthesia was associated with having worked in a job requiring cleaning of WTC dust (22%, 9/41) compared to controls (11%, 4/38, p=0.23, Fisher’s exact test; age- and sex-adjusted OR = 2.4, 95% confidence interval = 0.7-8.3, p=0.16).
Standardized Neurological Questionnaires and Objective Tests:
Scores on the Norfolk Diabetic Neuropathy Quality of Life (QoL) – Diabetic Neuropathy instrument were significantly higher (worse) in paresthesia cases than in both control groups (Table 3). High total QoL scores as well high large- and small-fiber nerves scores indicated impairment.
Table 3.
Findings from the Norfolk Quality of Life – Diabetic Neuropathy (QOL-DN) instrument, the Utah Early Neuropathy Scale, and the Michigan Neuropathy Screening Instrument among paresthesia cases, clinic controls and community controls (higher scores indicate greater neuropathic impairment).
| Scale: | Cases [median, IQR] (n=41) |
Clinic Controls [median, IQR] (n=38) |
Community Controls [median, IQR] (n=20) |
p- value* |
|---|---|---|---|---|
| Norfolk Physical Functioning/Large Fiber Score | 31 (16,41) | 0 (−1,9) | 0 (−1,9) | < 0.0001 |
| Norfolk Small Fiber Score | 2 (1,4) | 0 (0,0) | 0 (0,0) | < 0.0001 |
| Norfolk Total Score | 53 (32,70) | 2 (−1,9) | 0 (−1.5,3.5) | < 0.0001 |
| Utah Early Neuropathy Scale | 6 (4,10) | 0 (0,2) | 0 (0,2) | < 0.0001 |
| Michigan Neuropathy Screening Instrument | 5 (4,7) | 1 (0,2) | 0 (0,1) | < 0.0001 |
p from the Kruskall-Wallis test
The Utah Early Neuropathy Scale indicated significant impairments among cases (median score = 6, IQR = 4 - 10) compared to both clinic controls (median score = 0, IQR = 0 - 2) and community controls (median score = 0, IQR = 0 - 2), p < 0.0001) (Table 3). The greatest impairments of cases compared to controls among the components of the Utah Early Neuropathy Scale were in pin sensation and deep tendon reflex, and great toe joint position (Supplemental Table 1).
The Michigan Neuropathy Screening Instrument indicated significantly higher scores for neuropathic symptoms among paresthesia cases than controls (Table 3). The neurological history and examination also indicated significantly higher (worse) scores among paresthesia cases compared to controls (Supplemental Table 2). The physician-administered neurological examinations showed that 39% of cases had multiple examination findings consistent with distal symmetric polyneuropathy vs. 8% of clinic controls and 0% of community controls. Considering examination findings and symptoms, the study’s neurologists rated 58% of cases as “definitely yes” and 34% as “possible yes” for having clinically evident distal symmetric polyneuropathy, which was significantly greater than the 3% and 21%, respectively, among clinic controls, and the 0% and 0% among community controls.
Total PCL total scores were higher among paresthesia cases (median = 44, IQR = 32 - 62) than clinic controls (median = 25, IQR = 21 - 34) or community controls (median = 18.5, IQR = 17 - 20; p < 0.0001).
Nerve Conduction studies:
Peroneal and ulnar motor nerve conduction velocities were below the cutoff for normality in 3% of paresthesia cases, a prevalence that did not differ from expected (Table 4).14 However, delayed latencies were present in 40% of subjects for the tibial motor nerve and low amplitude nerve action potentials were present in 0 – 36% of nerves tested. The sural to radial sensory nerve amplitude ratio was abnormal (< 0.4) in 29.4% (10/34) of subjects. Of the 10 paresthesia cases with abnormal sural to radial sensory nerve amplitude ratios, 60% (6/10) had reduced epidermal skin or sweat gland fiber densities compared to 52% (11/23) of those with normal sural to radial sensory nerve amplitude ratios. Quantitative sural to radial sensory nerve amplitude ratios also were not correlated with epidermal nerve fiber densities (Spearman r = 0.07, p = 0.7). The Wilcoxon rank-sum test revealed no significant differences in the blood measures shown in Table 6 (below) between subjects with reduced (<0.4) sural to radial sensory nerve amplitude ratios and those with normal ratios. None of the subjects had clinical or nerve conduction evidence of chronic inflammatory demyelinating polyneuropathy.
Table 4.
Frequencies of abnormal nerve conduction velocity findings among paresthesia cases enrolled in the World Trade Center Environmental Health Center.
| Nerve | Latency (% [n abnormal/ n tested]) |
Amplitude (% [n abnormal/ n tested]) |
Conduction velocity (% [n abnormal/ n tested]) |
|---|---|---|---|
| Motor nerves: | |||
| Peroneal Motor Nerve (Left) | |||
| Ankle – extensor digitorum brevis | 8.3 (3/36)a | 13.9 (5/36)b | |
| Fibular head – ankle | 13.9 (5/36)b | 3.0 (1/33)c | |
| Tibial Motor Nerve (Left) | |||
| Ankle - Abductor hallucies brevis muscle | 40.0 (14/35)d | 8.8 (3/34)e | |
| Ulnar Motor Nerve (Left) | |||
| Wrist - Abductor digiti minimi muscle | 0 (0/36)f | 25.0 (9/36)g | |
| Below elbow – Wrist | 36.1 (13/36)g | 2.9 (1/35)h | |
| Sensory Nerves: | |||
| Radial Sensory Nerve (Left) | |||
| Forearm - extensor pollicis longus tendon | 22.9 (8/35)i | 20.0 (7/35)j | 0 (0/35)h |
| Sural Sensory Nerve (Left) | |||
| Calf - Lateral Malleolus | 2.9 (1/34)k | 0 (0/34)l | 0 (0/34)c |
Abnormal if > 6.0 ms;
Abnormal if < 2.0 mV;
Abnormal if < 40 m/s;
Abnormal if > 5.6 ms;
Abnormal if < 3 mV;
Abnormal if > 3.5 ms;
Abnormal if < 6 mV;
Abnormal if < 48 m/s;
Abnormal if > 2.7 ms;
Abnormal if < 20 μV;
Abnormal if > 4.2 ms;
Abnormal if < 5 μV
Table 6.
Blood tests investigating factors potentially associated with paresthesias among cases, clinic controls, and community controls
| Cases | Clinic Controls | Community Controls | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood measure | N | Median | Min | Max | N | Median | Min | Max | N | Median | Min | Max | p- value* |
p- value** |
| Vitamin B12 (pg/mL) | 41 | 546 | 230 | 2500 | 38 | 439 | 222 | 2500 | 19 | 493 | 209 | 1150 | 0.8 | 0.5 |
| Methylmalonic acid (umol/L) | 40 | 0.145 | 0.05 | 0.33 | 38 | 0.15 | 0.05 | 0.29 | 20 | 0.155 | 0.11 | 0.47 | 0.4 | 0.4 |
| Folate (ng/mL) | 40 | 12.8 | 4.6 | 60 | 38 | 12.1 | 5 | 60 | 19 | 13.5 | 9.2 | 60 | 0.2 | 0.8 |
| Hemoglobin A1c (%) | 41 | 5.4 | 4.8 | 6.7 | 38 | 5.45 | 4.3 | 6.4 | 18 | 5.35 | 5 | 9.4 | 0.7 | 0.9 |
| Glucose, fasting (mg/dL) | 40 | 86.5 | 70 | 107 | 38 | 85 | 64 | 111 | 18 | 83 | 59 | 196 | 0.5 | 0.4 |
| ANA (inverse titers) | 32 | 20 | 20 | 1280 | 25 | 20 | 20 | 640 | 15 | 20 | 20 | 160 | 0.5 | 0.2 |
| C-reactive protein (mg/L) | 41 | 2.3 | 0.1 | 11 | 38 | 2.05 | 0.1 | 10 | 19 | 1.8 | 0.4 | 9.8 | 0.6 | 1 |
| Gliadin peptide antibody IgA (units) | 39 | 5 | 2 | 37 | 36 | 5 | 2 | 15 | 20 | 5 | 3 | 15 | 0.4 | 0.3 |
| Gliadin peptide antibody IgG (units) | 39 | 3 | 2 | 28 | 36 | 2 | 2 | 16 | 20 | 3 | 1 | 5 | 0.4 | 0.2 |
| Lead concentration (μg/dL) | 39 | 1 | 1 | 2.9 | 37 | 1 | 1 | 2.4 | 18 | 1 | 1 | 3 | 0.4 | 0.2 |
| Lyme antibody IgG/IgM (Western blot [normal < 0.91]) | 40 | 0.32 | 0.11 | 1 | 37 | 0.34 | 0.08 | 1.1 | 20 | 0.31 | 0.11 | 2.14 | 0.9 | 0.8 |
p from the Kruskall-Wallis tests comparing cases, clinic controls and community controls
p from the Wilcoxon rank-sum test comparing cases and clinic controls
Lead levels shown by the laboratory as < 2.0 were assigned a value of 1.0.
Skin Biopsy Examination for Epidermal Nerve and Sweat Gland Nerve Fiber Density:
Epidermal or sweat gland nerve fiber densities were significantly reduced among cases compared to historical controls (Table 5, Part A). This difference remained significant after adjusting for age and gender by linear regression. The composite measure of epidermal or sweat gland small nerve fiber density was abnormally low in 57% of cases compared to an expected prevalence of abnormal findings of 4-5%, as estimated from the age- and gender-similar historical controls (Table 5, Part B, p < 0.0001). Heights of cases with reduced intraepidermal nerve fiber densities were greater (median = 166.5 cm, min = 149.3, max = 202) than heights of cases with normal intraepidermal nerve fiber densities (median = 159.3 cm, min = 146, max = 170.5; Wilcoxon rank-sum p=0.01). The difference in heights remained significant after adjustment for age and gender.
Table 5.
Epidermal or sweat gland nerve fiber densities in cases and historical controls.
| Part A. Quantitative comparison of epidermal nerve fiber densities | |||
|---|---|---|---|
| Group | Mean density (fibers/mm of epidermal length) |
95% Confidence interval |
p‐value* |
| Paresthesia cases (n=37) | 4.70 | 3.95 ‐ 5.45 | < 0.0001 |
| Historical controls (n=128) | 8.32 | 7.86 ‐ 8.77 | |
| * From generalized linear models regression analysis adjusted for age and gender. | |||
| Part B. Categorical comparison of the
prevalence of abnormally reduced nerve fiber densities based on a composite evaluation of epidermal and sweat gland nerve fiber densities. | ||||
|---|---|---|---|---|
| Composite nerve fiber density measure | ||||
| Group | Abnormal (n, (%)) | Normal | Total | p-value, Fisher’s exact test |
| Paresthesia cases | 21 (57%) | 16 (43%) | 37 | < 0.0001 |
| Historical controls (n=128) | 5 (3.9%) | 123 (96%) | 128 | |
When categorized by frequency of paresthesia symptoms, there was a suggestion (p = 0.10, Fisher’s exact test) of a trend of increasing proportion of subjects with abnormal epidermal nerve fiber density with increasing frequency of paresthesia (0% [0/2] of those with frequency of “occasional” vs. 50% [8/16] of those with frequency of “often” vs. 68% [13/19] of those with frequency of “almost continuous;” p = 0.10, Cochran-Armitage exact trend test). Scores on the Utah Early Neuropathy scale also were higher (worse) among persons with abnormally reduced small nerve densities compared to those with normal epidermal nerve fiber densities; this difference remained significant after adjustment for age (p=0.01).
Association of Paresthesias with Known Causes of Neuropathy:
None of the blood tests for factors commonly associated with peripheral neuropathy differed significantly across groups (Table 6). Syphilis testing revealed 1 positive individual who was referred for treatment and 5 individuals (all cases) whose syphilis tests indicated either false-positive screening tests or syphilis that had been treated and resolved. When blood tests were dichotomized into abnormal vs. normal, the only blood test that showed significant heterogeneity across groups was methylmalonic acid, with community controls having a higher prevalence of abnormally high levels (suggestive of vitamin B12 deficiency, found in 2/20, 10%) compared to cases (0/40, 0%) or clinic controls (0/38, 0%) (p = 0.04, Fisher’s exact test). There were no significant differences among groups in the prevalence of high hemoglobin A1c (p=0.5), high fasting glucose (p=0.9), or other dichotomized blood values. When blood lead concentrations were dichotomized at < 2 μg/dL vs. 2-4.9 μg/dL, there was a suggestion of an excess of higher lead concentrations in cases (15.4%, 6/39, in the range of 2-4.9 μg/dL) compared to clinic controls (5.4%, 2/37, in the range of 2-4.9 μg/dL, p = 0.26, Fisher’s exact test). Community controls had an intermediate prevalence (11.1%, 2/18) resulting in an p-value comparing all 3 groups of 0.4 (Fisher’s exact test).
ANA titers did not differ among groups, and ANA patterns among ANA-positivity also did not differ significantly across the 3 groups. Among the 14 ANA-positive cases, however, 2 (14%) had nucleolar ANA patterns and 1 (7%) had nuclear dots, whereas these patterns were not found in the 9 ANA-positive clinic controls or 6 ANA-positive community controls.
Serum protein electrophoresis (SPEP) results did not reveal important differences across groups: 67% (26/39) of cases, 71% (27/38) of clinic controls and 85% of community controls had normal SPEP findings. Immunofixation electrophoresis results also did not differ among groups; no monoclonal bands were detected in 90% (36/40) of cases, 84% (32/38) of clinic controls, and 85% (17/20) of community controls.
On interview, lead exposure since 9/11 was more often reported by cases (11/41, 26.8%) than clinic controls (2/38, 5.3%) or community controls (0/20, 0%) (Fisher’s exact p = 0.003). A majority (64%; 7/11) of the cases who said they had been exposed to lead since 9/11 reported these exposures occurred in the home, while 27% (3/11) reported WTC-related lead exposures, and 9% (1/11) reported hobby-related lead exposures. There also were suggestions of differences in post-9/11 exposures to mercury, with 9.8% (4/41) of cases reporting such exposure compared to 0% (0/38) of clinic controls, and 0% (0/20) of community controls) (p = 0.07, Fisher’s exact test); and exposure to solvents, with 14.6% (6/41) of cases reporting such exposure compared to 5.3% (2/38) of clinic controls and 0% (0/20) of community controls (p = 0.12, Fisher’s exact test).
DISCUSSION
This study shows that the high prevalences of paresthesia we and others have observed among WTC-exposed individuals were associated with abnormal neurological examination findings. Importantly, pathologic evaluation revealed reduced epidermal or sweat gland nerve fiber densities among 57% of cases. Nerve conduction studies showed reduced sural to radial sensory nerve amplitude ratios in 29.4% of cases. Clinical examinations and histories, conducted with standardized neuropathy scales revealed findings consistent with both small and large fiber neuropathy more often in cases than in either of the two control groups. Blood tests did not reveal any other clear associations with non-WTC-related disease, metabolic, or toxic etiologies of peripheral neuropathy.27 Paresthesia and abnormal neurologic findings were infrequent among the community control group. These findings, including objective measures of disease, support an association between WTC exposures and neurological injury or disease in a substantial proportion of WTC-exposed members of the general community.
WTC-associated dust and fumes contained heavy metals, including lead, mercury and arsenic, and complex hydrocarbons.7-9 Exposure to these materials occurred both at the time of the disaster when massive dust clouds were released upon collapse of the towers, and subsequently when materials were re-suspended. Workers involved in WTC clean-up activities also may have been exposed to neurotoxic cleaning agents or other substances after 9/11 at work, during hobbies or at home. These exposures provide biologic plausibility for the findings and are consistent with reports of small fiber neuropathies associated with exposure to environmental toxins, including lead, solvents and chemical warfare agents.28-37
The high prevalence of small fiber abnormalities in our sample of paresthesia cases suggests that a small nerve fiber condition may be operative. Intraepidermal nerve fiber density measurement has been recommended as a sensitive and specific tool for diagnosis of small nerve fiber disease.38 Nerve conduction velocities, which evaluate large fiber nerve function, were abnormal in 29.4% of paresthesia cases and reduced nerve action potentials, were observed in up to 36% of subjects depending on the nerve tested, suggesting axonal injury in some subjects.39
The mechanisms by which toxic exposures contribute to the development of paresthesias are unclear. Small fiber neuropathies have been associated with exposure to environmental toxins. Prior studies have suggested autoimmune factors may play a role in chemically-mediated neuropathy.40 We observed no significant difference in ANA positivity or serum protein electrophoresis findings among groups. ANA nucleolar and nuclear dot patterns, present in several cases but not in controls, suggests a possible role of autoimmune disorders in a small fraction of WTC-associated paresthesia cases.
The possibility exists that post-9/11 exposure to environmental toxins contributed to the neurologic symptoms. More cases than controls reported exposures to lead since 9/11, and there was a suggestion in the data that blood lead concentrations in the range of 2 – 4.99 μg/dL were present more often among cases than clinic controls. Lead levels below 5 μg/dL, however, have not been reported to be associated with neuropathy. Furthermore, it is not possible to know whether blood lead concentrations measured during the study might be due to exposures that occurred on or after September 11, 2001, rather than previous or substantially after that date. An alternative explanation, might be recall bias, with cases more likely to remember and report exposures to neurotoxins than controls, perhaps because of concern for their symptoms. Participation bias also might have been present if persons with post-9/11 neurotoxin exposures were more likely to enroll due to fears of health consequences and out of a desire to obtain related testing and medical advice.
Heights were greater among cases with reduced epidermal or sweat gland nerve fiber densities compared to cases with normal densities, even after adjustment for sex and age. This suggests the possibility of a length-dependent effect. Greater height is a known risk factor for diabetic peripheral neuropathy.41, 42
There are potential limitations to our study. We did not conduct skin punch biopsies in some subjects due to extended blood pressure or heart rate excursions well beyond normal. Some subjects refused the procedure despite initial consent. Nerve conduction studies were not conducted in a few subjects due to equipment problems or refusal because the subjects had previously undergone nerve conduction studies elsewhere. Our dependence on historical controls as a comparison group for the intraepidermal nerve fiber measurements in cases could have led to bias if there had been drift in the evaluating pathologists’ methods over time. Observer bias may have occurred due to the lack of masking of the study neurologists,43 a situation necessitated by the neurologists’ conduct of the skin punch biopsies and nerve conduction studies that were performed only among cases. Inclusion criteria for the study were based on a screening questionnaire that may have caused under- or over-reporting of paresthesia and the frequency thereof. Two subjects changed their responses to the question on the frequency of paresthesia, saying during screening that they “often” experienced paresthesia while during the main study interview they reported experiencing this symptom “occasionally.” It was of interest, however, that neither of these subjects had reduced intraepidermal small nerve density, and that there was an increasing prevalence of abnormal intraepidermal nerve fiber densities as the reported frequency of paresthesia increased from “occasionally” to “often” to “almost continuous.”
We enlisted paresthesia cases and clinic controls from the WTC EHC, meaning that subjects in each of those groups were exposed to the WTC disaster. In an ideal case-control study design, cases and controls would be drawn at random from pools of subjects with and without disease, and not from subject pools in which exposure has been in any way pre-determined. The WTC EHC patient population is heterogeneous, however, and includes individuals with very different severities of WTC exposures, as well as widely different exposures to clean-up activities following the events of 9/11. This heterogeneity allowed us to ask whether there was any evidence for differential exposures among those with and without paresthesia, although this was not the primary aim of the study. We thus found non-significant, but suggestive exposure differences indicating that more paresthesia cases than clinic controls had jobs that required cleaning of WTC dust, and more paresthesia cases than controls were heavily covered in dust on 9/11. The study’s sampling scheme would be expected to bias the associations with WTC exposure variables towards the null. The lack of significance in these findings may reflect in part the fact that the study size was derived without consideration of analyzing associations between signs of neuropathy and WTC exposures. We have previously demonstrated significant associations between the symptom of paresthesia and WTC exposure variables.2, 4
In sum, this study demonstrates increased prevalence of clinical and laboratory-test abnormalities indicative of neuropathy among individuals with WTC exposure and paresthesia of the lower extremities. These abnormalities were observed more often among paresthesia cases than among members of the control groups. Our data are consistent with the hypothesis that WTC exposures or clean-up activities contributed to the development of peripheral neuropathy with paresthesia. Our findings reinforce the need for neurologic evaluation after toxic exposures in WTC-exposed as well as other populations. Future research should seek to identify possible mechanisms and longitudinal course of symptoms. Moreover, the impact of these symptoms on mental health and functional status should be investigated.
Supplementary Material
Acknowledgements:
The authors thank the research participants for their generosity, time and interest; and the staff and administration of Bellevue Hospital Center, NYC Health + Hospitals for their assistance and oversight.
Study funding: This study was supported by the cooperative agreement U01 OH010395 from the National Institute for Occupational Health and Safety (NIOSH), Centers for Disease Control and Prevention to the NYU School of Medicine; NYU Clinical and Translational Science Award (CTSA) number UL1TR001445 from the National Center for Advancing Translational Sciences (NCATS), National Institute of Health (NIH); NIOSH contracts 200-2017-93327 (Data Center) and 200-2017-93427 (Clinical Center of Excellence) to NYC Health + Hospitals, Bellevue Hospital Center; and Center Grant 5P30ES000260 from the National Institute of Environmental Health Sciences to NYU School of Medicine.
Disclosures:
This study was sponsored by the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
Dr. Marmor has been funded by CDC/NIOSH cooperative agreement 1U01-OH011305-01 and U01OH010394-01A1, and NIH grant R01EY015520. He has no other disclosures.
Dr. Thawani has been funded by has been funded by CDC/NIOSH cooperative agreement 1U01-OH011305-01. She has no other disclosures.
Dr. Cotrina has been funded by cooperative agreement 1U01-OH011305-01 She has no other disclosures.
Dr. Shao has been funded by the cooperative agreement 1U01-OH011305-01 and U01OH010394-01A1, U01 OH010395 from the National Institute for Occupational Health and Safety (NIOSH), Centers for Disease Control and Prevention to the NYU School of Medicine; NYU Clinical and Translational Science Award (CTSA) number UL1TR001445 from the National Center for Advancing Translational Sciences (NCATS), National Institute of Health (NIH); NIOSH contracts 200-2017-93327 (Data Center) to NYC Health + Hospitals, Bellevue Hospital Center; and Center Grant 5P30ES000260 from the National Institute of Environmental Health Sciences to NYU School of Medicine. He has no other disclosures.
Dr. Wong has been funded by has been funded by CDC/NIOSH cooperative agreement 1U01-OH011305-01. She has no other disclosures.
Dr. Stecker has receives royalties from UPTo Date and research support (not related to the present project) from the Campos Family Farms. He has no other disclosures.
Ms. Wang has been funded by cooperative agreement 1U01-OH011305-01. She has no other disclosures.
Dr. Alexander has no disclosures.
Dr. Wilkenfeld has no disclosures.
Ms. E. Vinik has not received study funding in the past two years. She and Dr. Aaron Vinik hold a licensing agreement with Eastern Virginia Medical School for the use of the Norfolk QOL-DN to commercial companies. No license or fee is required for its use by academic or non-profit institutions. She has no other disclosures or conflict of interest.
Dr. A. Vinik has been funded by National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke (NINOS), NeuroNEXT Clinical Trial (U01) - Topiramate as a disease altering therapy for CSPN; ViroMed Laboratories: A Phase II, Double-Blind, Randomized, Placebo-Controlled, Multicenter Study to Assess the Safety and Efficacy of VM202 in Subjects with Painful Diabetic Peripheral Neuropathy. Veroscience LLC: Impact of Timed Bromocriptine-QR Therapy upon Measures of Sympathetic Tone and Vascular Biology in Type 2 Diabetes Subjects; ; Novo Nordisk A/S: The Effects of Liraglutide on Sudomotor Function and Inflammation in Type 2 Diabetes; Impeto: Medical, Inc. “Autonomic Neuropathy in Patients in Patients with Psoriasis” Aptinyx Inc.: “A Randomized, Double-Blind, Parallel-Group, Placebo-Controlled, Multiple-Dose Study to Assess the Efficacy and Safety of NYX-2925 in Subjects with Neuropathic Pain Associated with Diabetic Peripheral Neuropathy’. Dr. Vinik also has been a Consultant/Speaker for: Merck, Pfizer, Alnylam and Astellas. He has a patent on Norfolk QOL-DN. He has no other disclosures or conflict of interest.
Dr. Reibman has been funded National Institute of Occupational Safety and Health (NIOSH) contracts 200–2017–93327 and 200–2017–93427. She has no other disclosures.
Appendix 1. Neurotoxins queried as part of the OccIDEAS instrument.
Chemical exposures we asked subjects about included arsenic, lead, manganese, mercury, tin, acrylamide, carbon disulfide, ethylene oxide, methyltertiarybutyletone, methylchloride, n-hexane, perchlorethylene, toluene, trichloroethylene, pesticides, carbon monoxide, gasoline, and “other chemical exposures.”
Footnotes
Data Availability Statement: De-identified data on the cases, clinic controls and community controls can be obtained by contacting Yongzhao Shao, Ph.D. (Yongzhao.Shao@nyulangone.org).
References
- 1.Reibman J, Liu M, Cheng Q, et al. Characteristics of a residential and working community with diverse exposure to World Trade Center dust, gas, and fumes. J Occup Environ Med 2009;51:534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marmor M, Shao Y, Bhatt DH, et al. Paresthesias Among Community Members Exposed to the World Trade Center Disaster. J Occup Environ Med 2017;59:389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reibman J, Levy-Carrick N, Miles T, et al. Destruction of the World Trade Center Towers. Lessons Learned from an Environmental Health Disaster. Annals of the American Thoracic Society 2016;13:577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thawani S, Wang B, Shao Y, Reibman J, Marmor M. Time to Onset of Paresthesia Among Community Members Exposed to the World Trade Center Disaster. Int J Environ Res Public Health 2019;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stecker MM, Yu H, Barlev R, Marmor M, Wilkenfeld M. Neurologic Evaluations of Patients Exposed to the World Trade Center Disaster. J Occup Environ Med 2016;58:1150–1154. [DOI] [PubMed] [Google Scholar]
- 6.Stecker M, Segelnick J, Wilkenfeld M. Analysis of short-term effects of World Trade Center dust on rat sciatic nerve. J Occup Environ Med 2014;56:1024–1028. [DOI] [PubMed] [Google Scholar]
- 7.Lioy PJ, Weisel CP, Millette JR, et al. Characterization of the dust/smoke aerosol that settled east of the World Trade Center (WTC) in lower Manhattan after the collapse of the WTC 11 September 2001. Environ Health Perspect 2002;110:703–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butt CM, Diamond ML, Truong J, Ikonomou MG, Helm PA, Stern GA. Semivolatile organic compounds in window films from lower Manhattan after the September 11th World Trade Center attacks. Environ Sci Technol 2004;38:3514–3524. [DOI] [PubMed] [Google Scholar]
- 9.Rayne S, Ikonomou MG, Butt CM, Diamond ML, Truong J. Polychlorinated dioxins and furans from the World Trade Center attacks in exterior window films from lower Manhattan in New York City. Environ Sci Technol 2005;39:1995–2003. [DOI] [PubMed] [Google Scholar]
- 10.Hintze J PASS 2008 Kaysville, UT: NCSS, LLC, 2011. [Google Scholar]
- 11.Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA 1984;252:1905–1907. [DOI] [PubMed] [Google Scholar]
- 12.Fritschi L OccIDEAS - occupational exposure assessment in community-based studies. Occup Med (Lond) 2019;69:156–157. [DOI] [PubMed] [Google Scholar]
- 13.Singleton JR, Bixby B, Russell JW, et al. The Utah Early Neuropathy Scale: a sensitive clinical scale for early sensory predominant neuropathy. J Peripher Nerv Syst 2008;13:218–227. [DOI] [PubMed] [Google Scholar]
- 14.Hoffken O, Maier C, Richter H, Tegenthoff M, Schwenkreis P. A simplified screening protocol predicts pathological electroneurographic results in patients with suspected polyneuropathy. Int J Neurosci 2010;120:28–35. [DOI] [PubMed] [Google Scholar]
- 15.Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vinik EJ, Hayes RP, Oglesby A, et al. The development and validation of the Norfolk QOL-DN, a new measure of patients' perception of the effects of diabetes and diabetic neuropathy. Diabetes Technol Ther 2005;7:497–508. [DOI] [PubMed] [Google Scholar]
- 17.Suanprasert N, Berk JL, Benson MD, et al. Retrospective study of a TTR FAP cohort to modify NIS+7 for therapeutic trials. J Neurol Sci 2014;344:121–128. [DOI] [PubMed] [Google Scholar]
- 18.Dyck PJ, Carter RE, Litchy WJ. Modeling nerve conduction criteria for diagnosis of diabetic polyneuropathy. Muscle Nerve 2011;44:340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutkove SB, Kothari MJ, Raynor EM, Levy ML, Fadic R, Nardin RA. Sural/radial amplitude ratio in the diagnosis of mild axonal polyneuropathy. Muscle Nerve 1997;20:1236–1241. [DOI] [PubMed] [Google Scholar]
- 20.Yasemin E, Gongor YN, Ozisler C. Polyneuropathy and the sural/radial sensory nerve action potential ratio in primary Sjogren's syndrome. Neurol Res 2019. [DOI] [PubMed]
- 21.Chan AC, Wilder-Smith EP. Small fiber neuropathy: Getting bigger! Muscle Nerve 2016. [DOI] [PubMed]
- 22.Lauria G, Hsieh ST, Johansson O, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol 2010;17:903–912, e944–909. [DOI] [PubMed] [Google Scholar]
- 23.England JD, Gronseth GS, Franklin G, et al. Practice Parameter: evaluation of distal symmetric polyneuropathy: role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review). Report of the American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation. Neurology 2009;72:177–184. [DOI] [PubMed] [Google Scholar]
- 24.Binnicker MJ, Jespersen DJ, Rollins LO. Direct comparison of the traditional and reverse syphilis screening algorithms in a population with a low prevalence of syphilis. J Clin Microbiol 2012;50:148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–748. [PubMed] [Google Scholar]
- 27.Callaghan BC, Price RS, Chen KS, Feldman EL. The Importance of Rare Subtypes in Diagnosis and Treatment of Peripheral Neuropathy: A Review. JAMA Neurol 2015;72:1510–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pradhan S, Tandon R. N-hexane neuropathy with vertigo and cold allodynia in a silk screen printer: A case study. Int J Occup Med Environ Health 2015;28:915–919. [DOI] [PubMed] [Google Scholar]
- 29.Guimaraes-Costa R, Schoindre Y, Metlaine A, et al. N-hexane exposure: a cause of small fiber neuropathy. J Peripher Nerv Syst 2018;23:143–146. [DOI] [PubMed] [Google Scholar]
- 30.Terkelsen AJ, Karlsson P, Lauria G, Freeman R, Finnerup NB, Jensen TS. The diagnostic challenge of small fibre neuropathy: clinical presentations, evaluations, and causes. Lancet Neurol 2017;16:934–944. [DOI] [PubMed] [Google Scholar]
- 31.Majersik JJ, Caravati EM, Steffens JD. Severe neurotoxicity associated with exposure to the solvent 1-bromopropane (n-propyl bromide). Clin Toxicol (Phila) 2007;45:270–276. [DOI] [PubMed] [Google Scholar]
- 32.Sclar G Encephalomyeloradiculoneuropathy following exposure to an industrial solvent. Clin Neurol Neurosurg 1999;101:199–202. [DOI] [PubMed] [Google Scholar]
- 33.Samukawa M, Ichihara G, Oka N, Kusunoki S. A case of severe neurotoxicity associated with exposure to 1-bromopropane, an alternative to ozone-depleting or global-warming solvents. Arch Intern Med 2012;172:1257–1260. [DOI] [PubMed] [Google Scholar]
- 34.Karam C, Dyck PJ. Toxic Neuropathies. Semin Neurol 2015;35:448–457. [DOI] [PubMed] [Google Scholar]
- 35.Kushlaf HA. Emerging toxic neuropathies and myopathies. Psychiatr Clin North Am 2013;36:209–218. [DOI] [PubMed] [Google Scholar]
- 36.Thomson RM, Parry GJ. Neuropathies associated with excessive exposure to lead. Muscle Nerve 2006;33:732–741. [DOI] [PubMed] [Google Scholar]
- 37.Holisaz MT, Rayegani SM, Hafezy R, Khedmat H, Motamedi MH. Screening for peripheral neuropathy in chemical warfare victims. Int J Rehabil Res 2007;30:71–74. [DOI] [PubMed] [Google Scholar]
- 38.Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 2008;131:1912–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCorquodale D, Smith AG. Clinical electrophysiology of axonal polyneuropathies. Handb Clin Neurol 2019;161:217–240. [DOI] [PubMed] [Google Scholar]
- 40.Abou-Donia MB, Lieberman A, Curtis L. Neural autoantibodies in patients with neurological symptoms and histories of chemical/mold exposures. Toxicol Ind Health 2018;34:44–53. [DOI] [PubMed] [Google Scholar]
- 41.Adler AI, Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Smith DG. Risk factors for diabetic peripheral sensory neuropathy. Results of the Seattle Prospective Diabetic Foot Study. Diabetes Care 1997;20:1162–1167. [DOI] [PubMed] [Google Scholar]
- 42.Unmar Y, Zafar MI, Gao F. Factors associated with peripheral neuropathy in type 2 diabetes: Subclinical versus confirmed neuropathy. J Huazhong Univ Sci Technolog Med Sci 2017;37:337–342. [DOI] [PubMed] [Google Scholar]
- 43.Delgado-Rodriguez M, Llorca J. Bias. J Epidemiol Community Health 2004;58:635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.