Introduction
Nicotinic receptors are ionotropic, ligand-gated ion channels that were originally defined by activation with nicotine, an alkaloid found in tobacco plants, and products made with tobacco. Nicotinic receptors are composed of five membrane spanning subunits (Cooper, 1991). As their full name implies, nAChR also are receptors for the endogenous neurotransmitter acetylcholine (ACh). Multiple neuronal nAChR subunits have been identified so far, named α2 to α10 and β2 to β4. Although many different possible combinations of subunits could come together to form an ion channel, certain α subunits are required for a functional binding site. In mammalian brain, homomeric receptors, which include five of the same α subunit, are thought to be limited to those containing five α7 subunits. In contrast, multiple heteromeric nAChR subtypes containing both α and β subunits have been identified. Each heteromeric nAChR subtype has a distinct biophysical property, physiological role, and pharmacology (Gotti et al., 2009).
The most prevalent heteromeric nAChR subtype in the mammalian brain are those made up of α4 and β2 subunits assembled with other nAChR subunits (Flores et al., 1997; Whiting and Lindstrom, 1987; Zoli et al., 2002), and is generally denoted as α4β2* nAChR with * added to denote the possible involvement of additional subunits. When assembled alone, the α4 and β2 subunits form two distinct functional isoforms: low-sensitivity (α4)3(β2)2 and high-sensitivity (α4)2(β2)3 nAChRs with the low ACh sensitivity (α4)3(β2)2 representing the majority of nAChRs in the cortex (DeDominicis et al., 2017). The designation of low- or high- affinity reflects the effect of subunit ratio on potency. The combination of (3) α4 plus (2) β2 subunit ratios has been found to possess low sensitivity to ACh (i.e., EC50 = 100 μM), and is in contrast to the combination of (2) α4 plus (3) β2 subunit ratios, which has been found to have high sensitivity to ACh (i.e., EC50 = 1 μM), (Moroni et al., 2006; Nelson et al., 2003). This effect is further reviewed in (Bertrand and Terry, 2018). The X-ray structure of human (α4)2(β2)3 nAChR has recently been published (Morales-Perez et al., 2016). The α4 and β2 subunits are highly homologous and share similar secondary structure, which consists of a 10-strand β sandwich N-terminal extracellular domain and a 4-helix bundle (TM1-TM4) transmembrane domain; however, they differ remarkably regarding their respective intracellular domains.
There is abundant evidence that the α4β2* subtype is of particular importance to the abuse potential of nicotine (Hurst et al., 2013; Besson et al., 2006; Picciotto et al., 1998). Anatomically, α4β2* nAChR are found on midbrain (i.e., ventral tegmental area and substantia nigra) dopaminergic neuronal projections, which are well-characterized to be involved in the development and maintenance of drug dependence (Klink et al., 2001). Chronic (i.e., 2 mg/kg twice daily for 10 days) nicotine treatment in rats has been documented to lead to substantial and selective upregulation of the α4 and β2 nAChR subtypes (Flores et al., 1992). Varenicline, a currently approved smoking cessation aid, is believed to exert at least some of its therapeutic effects via its actions as a α4β2 nAChR partial agonist (Reus et al., 2007). In rats varenicline decreases the acquisition, expression and reinstatement of nicotine’s effects in the place preference assay (Biala et al., 2010), and reduces the potency of nicotine in nicotine-induced intracranial self-stimulation (Vann et al., 2010). Additionally, in rats both varenicline and cytisine, another compound that has actions as a α4β2 nAChR partial agonist, decrease nicotine withdrawal-induced elevations in nicotine-induced intracranial self-stimulation thresholds, suggesting a decrease in nicotine withdrawal-induced dysphoria (Igari et al., 2014). The importance of the β2 subunit in the development and maintenance of nicotine dependence is supported by studies of β2 knockout mice, which do not self-administer nicotine. However, when β2 subunit functionality is restored, these mice self-administer nicotine (Picciotto et al., 1998). Additionally, when compared to wild-type mice, α4 knockout mice express fewer nicotine binding sites in the brain and have reduced neuronal activity and antinociceptive effects of nicotine (Marubio et al., 1999). Thus, pharmacotherapies that selectively target the α4β2* subtype of nAChR may be useful as smoking cessation therapeutics (Mohamed et al., 2015).
Both acute nicotine exposure and nicotine withdrawal in dependent subjects are well-documented to impact cognitive function (Loughead et al., 2010; Potter and Newhouse, 2008; Maskos et al., 2005). Likewise, nAChR, including the α4β2* subtype, play a role in cognitive disorders, and nAChR agonists can produce improvement in neurological diseases that impact cognitive function (Potter and Newhouse, 2008; Hurst et al., 2013). Specifically, the α4β2 partial agonist ispronicline (TC-1734, AZD3480) improves memory tasks in both rats and mice, as well as humans (Dunbar et al., 2011; Gatto et al., 2004). Meanwhile, the α4 knockout mouse exhibits a significant decrease of nicotine binding in the brain and an increase in anxiety-like behaviors, such as a decrease in time spent in the open arm of the elevated plus maze (Ross et al., 2000). Meanwhile, deletion of the β2 nAChR subunit in mice leads to improved responses in the passive avoidance task, which measures associative memory (Picciotto et al., 1995). However, these mice exhibit loss of sensitivity to nicotine’s ability to increase performance in the same paradigm (Picciotto et al., 1995).
Alzheimer’s disease is one neurological disease that may benefit from pharmacological interventions targeting the α4β2 nAChR. One hallmark of Alzheimer’s Disease is the deterioration of the cholinergic system, including nAChR function (Kasa et al., 1997). It is believed that basal cholinergic tone in the forebrain is critical for normal cognition, and deterioration of this tone may be responsible for some of the cognitive impairments associated with Alzheimer’s Disease (Auld et al., 2002). Further, a study of human post-mortem brain tissue found a significant decrease in choline acetyltransferase, the enzyme responsible for ACh synthesis, but not nAChR density or function (Flynn and Mash, 1986). However, in human post-mortem tissue, significant decreases in α4 and α7 subunits are correlated with increased hyper-phosporylated tau protein (Wevers et al., 1999). Additional evidence shows that the α4β2 nAChR located in the temporal cortex are particularly susceptible in the pathology of Alzheimer’s Disease (Warpman and Nordberg, 1995). In rats, the α4β2 nAChR is expressed within the motor cortex (DeDominicis et al., 2017), and in humans, it is believed that the motor cortex is significantly involved in the pathology of impaired motor function towards the later stages of Alzheimer’s Disease (Suva et al., 1999). In rat cell culture experiments nicotine protects neurons from the cytotoxic effects of beta amyloid peptide, well known to be associated with the pathobiology of Alzheimer’s Disease (Kihara et al., 1998). Further, these protective effects are mediated by the α4β2 nAChR, and cytisine, a α4β2 nAChR selective agonist, also produces similar protective effects (Kihara et al., 1998). Taken together, pharmacotherapies that selectively target the α4β2* subtype of nAChR may be useful as therapeutics to treat neurological diseases that impact cognitive function.
There is substantial evidence that nAChR, including the α4β2* subtype, play a role in mediating pathological pain, and that nAChR agonists can produce analgesia (Hurst et al., 2013; Jain, 2004). Systemic delivery of nicotine both reverses and prevents mechanical allodynia in a mouse model of chemotherapy-induced peripheral neuropathy (Kyte et al., 2018). Anatomically, nAChR, including the α4β2* subtype, are found on neurons within peripheral nociceptive nerve fibers, dorsal root ganglia, the dorsal horn of the spinal cord, rostral ventromedial medulla in the brainstem, and the periaqueductal grey – all sites that mediate pain and pain processing (Hone et al., 2011; Umana et al., 2013). Additionally, astrocytes within the spinal cord and pain-processing regions in the brain also express α4β2 nAChR (Gotti and Clementi, 2004). Further, the α4β2 nAChR is upregulated in the rat ventralposterolateral thalamic nucleus starting at two weeks after a nerve injury model of neuropathic pain (Ueda et al., 2010). In rats, administration of 5-iodo-3-(2(S)-azetidinylmethoxy)pyridine (5-iodo-A-85380), a selective and potent α4β2 nAChR agonist, administered into the ventralposterolateral thalamic nucleus dose-dependently reverses mechanical allodynia (Ueda et al., 2010). Additionally, the selective α4β2 nAChR agonist A-366833 dose-dependently reverses mechanical allodynia in a multitude of rat neuropathic pain models (i.e., chronic constriction injury of the sciatic nerve (CCI), partial sciatic nerve ligation, spinal nerve ligation, as well as diabetic- and chemotherapeutic-induced peripheral neuropathy), and in the Complete Freund’s adjuvant-induced inflammatory pain model (Nirogi et al., 2011). Recent evidence suggests that systemically administered α4β2 nAChR agonist TC-2559 decreases peripheral macrophage and spinal cord microglial activation concomitant with reversal of mechanical allodynia in a mouse partial sciatic nerve ligation model of neuropathic pain (Kiguchi et al., 2018). Thus, α4β2 nAChR orthosteric agonists may hold therapeutic promise for the treatment of pathological pain (Nirogi et al., 2013). In addition to nAChR that possess α4β2 subunits, both the α5 and α7 nAChR subunits have also shown preclinical promise as targets for treating both inflammatory and neuropathic pathological pain (Freitas et al., 2013; Bagdas et al., 2015).
Positive allosteric modulators (PAMs) of α4β2 nAChRs
As previously mentioned, the α4β2 nAChR is an attractive therapeutic target for nicotine dependence, neurological diseases that impair cognitive function, and pathological pain (Hurst et al., 2013). However, these therapeutic indications, especially with the use of orthosteric agonists of α4β2 nAChR, come with the possibility of producing addiction and dependence with prolonged use. Positive allosteric modulators (PAMs) can increase the binding affinity and/or efficacy of an orthosteric agonist (Pandya and Yakel, 2013), and may be a more advantageous long-term therapeutic strategy over othosteric α4β2 nAChR agonists (Wang and Lindstrom, 2018). Furthermore, numerous nAChR allosteric sites are thought to exist, with the exact number dependent on the subunit types that comprise the receptor (Wang and Lindstrom, 2018; Taly et al., 2009). Thus, PAMs represent a ‘tunable’ and highly selective method to influence nAChR pore activity. PAMs may produce increased receptor binding in several different ways. The prevailing consensus is that nAChRs are comprised of dynamic proteins, capable of multiple different states. Here we limit discussion to three possible states: closed, open and desensitized. At baseline equilibrium, when exogenous and endogenous signaling does not occur, nAChRs remain preferentially in the closed state. However, with an extremely low probability of shifting to the open state, the receptor may shift to the desensitized state. If a high concentration of agonist, sufficient to saturate all the possible binding sites is rapidly applied, α4β2 nAChRs have an 80% probability of simultaneous shifting transiently into the open state before reaching a new equilibrium in the desensitized state (Li and Steinbach, 2010). Furthermore, it is known that once receptors are in the desensitized state, agonists bind with much greater affinity (Gielen and Corringer, 2018; Papke et al., 2011).
One way that PAMs can exert their effects is by increasing the agonist binding to the closed state of the receptor, which is experimentally represented by an increase in the potency of the agonist. This type of modulation might be most advantageous under a condition where there is a low concentration of agonist which would not otherwise produce a maximal response. This lower concentration of an agonist is thought to limit potential off-target, or unwanted side-effects. Meanwhile, by increasing the potency of the agonist, lower concentrations would be able to produce the maximum intended target therapeutic response of the agonist (Grupe et al., 2015). However, it would remain impossible to exceed the maximum response if the modulator only changes the potency of the orthosteric ligand.
PAMs are often observed to increase the efficacy of an agonist. One way a PAM might accomplish this is via shifting the equilibrium between the open and closed states, making it easier to move from closed to open state. This would result in not only more receptors moving to the open state, but also a greater likelihood that they might move from closed to open more than once before shifting to the desensitized state. Thus, this would yield a concurrent decrease in rate of desensitization. Functionally, this could be observed as an overall increase in the time spent in the open state. Having this effect, a PAM can produce a transient increase in efficacy (Uteshev, 2014). Finally, a PAM might exert its effect by making the desensitized state less favorable. This would not be manifest as an increase in efficacy, or the maximum effect. Instead, it would be most apparent under conditions less affected by receptor desensitization.
The above is a simplified explanation of a theoretical model. Although there is experimental evidence that is consistent with these scenarios, it is not possible to prove or disprove them. These scenarios simply do not violate the current scientific knowledge. Furthermore, it has been reported that, at least under some conditions, only a small percentage of available nAChRs are capable of being activated at once (Li and Steinbach, 2010; McNerney et al., 2000). Thus, the ability of a modulator to change receptors from the inactive to the active state is yet another possible mechanism by which it might enhance agonist activity. Most commonly PAMs of nAChR are simplify classified into Type I or Type II PAMs based on above mentioned effects on nAChR gating kinetics and ACh-mediate responses. Type I generally refers to compound that enhances ACh sensitivity and peak ion current with no alteration of channel gating kinetics. Type II refers to compounds that have additional effects on channel gating kinetics (e.g. increase open channel duration, decrease desensitization) (Bertrand and Gopalakrishnan, 2007; Williams et al., 2011). Furthermore, a distinguishing feature of type II PAMs is that they can transiently reactivate desensitized nAChRs. Regardless of their exact mechanism, positive allosteric modulation of α4β2 nAChRs represents a potentially attractive therapeutic strategy which may circumvent the limitations inherent in targeting an orthosteric site. Importantly, PAMs possess the ability to amplify the amplitude of response patterns without necessarily altering the response pattern itself, such as what is observed with an agonist, which would result in sustained activation or enhanced desensitization pattern of responses. Examples of compounds that potentiate α4β2 nAChRs include physostigmine, galantamine, desformylflustrabromine (dFBr; N-(2-[6-bromo-2(1,1-dimethyl-2-propyl)-1H-indol-3-yl]ethyl-N-methylamine), NS9283 (3-[3-(pyridin-3-yl)-1,2,4-oxadiazol-5-yl]benzonitrile, CMPI (3-(2-chlorophenyl)-5-(5-methyl-1-(piperidin-4-yl)-1H-pyrrazol-4-yl)isoxazole), and LY2087101 ([2-[(4-Fluorophenyl)amino]-4-methyl-5-thiazolyl]-3-thienylmethanone). Figure 1 shows the structures of these compounds.
Figure 1.
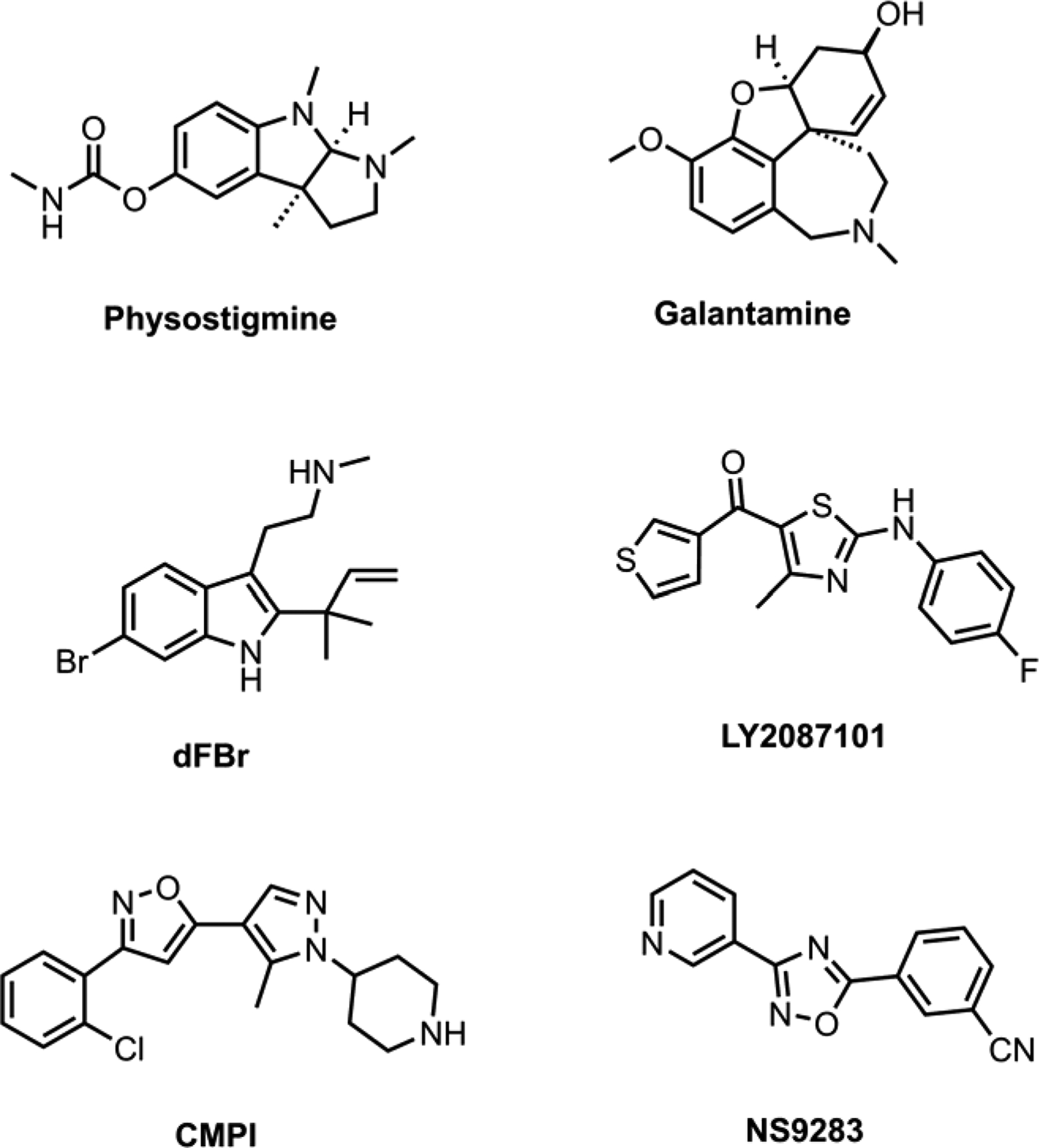
Structures of positive allosteric modulators of α4β2 nAChR.
Physostigmine and Galantamine
Physostigmine and galantamine are acetylcholinesterase (AChE) inhibitors and among the first nAChR allosteric potentiators to be identified (Maelicke et al., 2000). They are thought to potentiate muscle and neuronal type nAChRs but not muscarinic acetylcholine receptors (Samochocki et al., 2003; Farlow, 2003; Maelicke et al., 2001). Binding sites for galantamine and physostigmine on nAChRs have been identified using photoaffinity labeling, mutational analyses and computational docking (Luttmann et al., 2009; Hamouda et al., 2013). Physostigmine is thought to bind at both the agonist-binding and non-agonist binding subunit extracellular interfaces with the latter being equivalent to the positive allosteric binding site of benzodiazepines at γ-aminobutyric acid (GABA) type A receptors. Additional mutational analysis revealed physostigmine selectivity to the low-sensitivity (α4)3(β2)2 nAChRs and physostigmine’s ability to potentiate (α4)2α5(β2)2 nAChR (Jin et al., 2014). Galantamine is one of the most widely prescribed drugs for Alzheimer’s disease, and is also under examination as a smoking cessation pharmacotherapy (Schilström et al., 2007). Galantamine has been found to enhance dopaminergic neurotransmission through potentiation of both α7 and α4β2 nAChRs (Schilstrom et al., 2007). Altered dopamine signaling in the brain reward pathway (i.e., ventral tegmental area, nucleus accumbens, prefrontal cortex) is one of the major mechanisms that is critical for the development and maintenance of nicotine addiction and dependence (Mansvelder et al., 2002). Galantamine has been shown to attenuate nicotine self-administration in rats (Hopkins et al., 2012; Liu, 2013), and to reduce cigarette smoking among alcohol-dependent patients (Diehl et al., 2006). However, recent studies suggest that galantamine does not functionally act at human α4β2 or α7 nAChRs as a PAM (Kowal et al., 2018). Further, if galantamine or physostigmine produce PAM-like effects via increasing acetylcholine tone, and thus sufficient to produce nicotine-like effects, then one might predict other acetylcholinesterase inhibitors may produce similar results when applied to smoking cessation. Thus, it is unclear which mechanism of galantamine, AChE inhibition or allosteric modulation of nAChRs, might be responsible for its capacity to promote smoking cessation. Regardless of the exact mechanism, the pharmacology of galantamine, and possibly physostigmine, represents a potential repurposed target that may have potential as an aid for smoking cessation.
dFBr (desformylflustrabromine)
Desformylflustrabromine (dFBr) is one of the most studied nAChR PAMs and was discovered by serendipity. dFBr was first isolated, along with many other alkaloid metabolites, from the marine bryozoan Flustra foliacea and identified as a novel bromotryptamine derivative with pharmacological effects on nAChR (Peter et al., 2002; Sala et al., 2005). Following this discovery, syntheses of dFBr (in base form, water-soluble salts, and radioactive [3H]dFBr form) a large library of dFBr analogues has been described and used to investigate the in vitro nAChR effects, identify binding sites, and determine the structure-activity relationship of this novel nAChR pharmacophore (Lindel et al., 2006; Kim et al., 2007; German et al., 2011; Weltzin and Schulte, 2010; Pandya and Yakel, 2011; Hamouda et al., 2015, 2016; Deba et al., 2018; Dukat et al., 2018).
The effects of dFBr at muscle and neuronal nAChRs heterologously expressed in Xenpous laves oocytes or mammalian cell lines have been characterized using two-electrode voltage-clamp and patch-clamp electrophysiological recordings, respectively. dFBr by itself did not elicit nAChR-mediated current (i.e. did not activate nAChR). However, it enhanced peak ACh-induced current responses of α4β2 nAChR and α2β2 at submicromolar concentrations (Sala et al., 2005; Kim et al., 2007; Weltzin and Schulte, 2010; Pandya and Yakel, 2011). Further, it was found that dFBr potentiates both (α4)2(β2)3 and (α4)3(β2)2 nAChR isoforms with potentiation EC50s of ~0.4 and ~1.6 μM and potentiation Imax, of ~400 and ~300%, respectively, suggesting a stronger potentiation of (α4)3(β2)2 than (α4)2(β2)3 nAChR (Hamouda et al. 2016). The effect of dFBr on the ACh concentration-response curves of (α4)3(β2)2 and (α4)2(β2)3 nAChRs was characterized by ~4-fold increase in ACh efficacy with minimal effects on ACh potency (Hamouda et al., 2016). Single channel recordings of α4β2 nAChR current in the presence of ACh in the absence or presence of dFBr suggested an increase in ion channel open probability, which could be a result of an increase in the opening rate constant and/or a decrease the closing rate constant (Sala et al., 2005). Therefore, dFBr is considered a Type II PAM of the α4β2 nAChR as it enhances peak current of low and saturated concentrations of ACh and alters channel gating (Wang and Lindstrom, 2017).
Interestingly, dFBr did not potentiate ACh-induced responses of muscle-type, α3β2, α3β4, α4β4, or α7 nAChRs. Instead, dFBr inhibited muscle-type and α7 nAChRs with an IC50 of ~1μM and at higher concentration (>10 μM). dFBr inhibited ACh responses of most neuronal nAChRs including the α4β2 nAChRs (Sala et al., 2005; Kim et al., 2007; Weltzen and Schulte, 2010; Hamouda et al., 2015). Reversable binding analyses and [3H]dFBr photolabeling identified a higher affinity binding site for dFBr within the ion channel of muscle type nAChR (Hamouda et al., 2015), consistent with the notion that dFBr, at higher concentrations, inhibits nAChR responses by acting as an open channel blocker (Weltzen and Schulte, 2010; Hamouda et al., 2015). In addition, dFBr was found to bind at sites identified for physostigmine at the agonist-binding and non-agonist binding subunit extracellular interfaces in muscle-type nAChR (Hamouda et al., 2013; Hamouda et al., 2015).
There have been three recent publications using mutational analyses to identify dFBr binding sites in α4β2 nAChRs (Weltzin and Schulte, 2015; Alcaino et al., 2017; Deba et al., 2018). The first study (Weltzin and Schulte, 2015) reported that amino acid mutations in the β2 subunit significantly reduce dFBr potency in (α4)3(β2)2 and (α4)2(β2)3 nAChRs, suggesting an important role of the β2 subunit extracellular domain in dFBr modulation, especially the amino acid chain projecting from the β2 to the α4 subunit. However, a subsequent study (Alcaino et al., 2017) reported no effect of these mutations within the β2 subunit on dFBr modulation and using alanine substitution and substituted cysteine accessibility assay predicted a dFBr binding site within the the α4 subunit. The latter results were supported by mutational and computational docking analyses (Deba et al., 2018) which identified two district dFBr binding sites within the transmembrane domain of α4β2 nAChRs; an intrasubunit binding site within the α4 subunit helix bundle (equivalent to site identified in Alcaino et al., 2017) and an intersubunit site at the α4:α4 subunit interface. Deba et al. 2018 also modeled the binding mode for dFBr within these sites as well as possible interactions between dFBr and amino acids within these binding sites which were found consistent with experimental values obtained with a panel of dFBr analogues (Dukat et al., 2018).
So far, all published dFBr structure activity data come from the Richard Glennon’s group (German et al., 2011; Dukat et al., 2018). While dozens of dFBr analogues have been reported in these systematic structure activity studies, the naturally occurring structure of dFBr was found nearly optimal and none of these analogues were found to be substantially superior to dFBr in both efficacy and potency at α4β2 nAChR. Furthermore, when compared to dFBr, none of these dFBr analogues have exhibited significant improvement of a nAChR subtype selectivity profile (potentiation of α4β2 nAChR without inhibiting the α7 nAChR).
The first published in vivo use of dFBr was a study of rats trained to self-administer nicotine (0.03 mg/kg/infusion) (Liu, 2013). In the study, lower doses of dFBr (0.1 and 1 mg/kg) had no effect on nicotine self-administration, but larger doses (3 and 6 mg/kg) reduced the number of nicotine infusions earned. By itself, dFBr did not produce general depression of behavior. The elimination half-life of dFBr was estimated to be 8.6 hours and was present in the cerebrospinal fluid at about 30% of the concentration seen in plasma, indicating that dFBr crossed the blood-brain barrier. The observation that dFBr reduces nicotine self-administration has been attributed to the production of aversive behaviors resulting from increased potency of nicotine. Specifically, α4β2α5 nAChR in the medial habenula have been reported contribute to aversive behaviors in response to high nicotine doses (Fowler and Kenny, 2013). Additionally, in mice, by itself dFBr produces dose-dependent hypothermia (Moerke et al., 2016) and when paired with nicotine dFBr produced synergistic effects to produce a nicotine discriminative stimulus (Moerke et al., 2016). Recent in vivo studies demonstrate that dFBr can reverse behavioral signs of nicotine withdrawal in nicotine-dependent mice (Hamouda et al., 2018).
Other in vivo studies have shown that dFBr decreases anxiety-like behavior in the mouse marble burying assay as well as in the open-field assay (Mitra et al., 2017). Further, dFBr has been found to block the inhibitory effect of β Amyloid (Aβ1–42) peptide and to restore the function of both α2β2 and α4β2 nAChR expressed in Xenopus oocytes (Pandya and Yakel, 2011). However, more studies are needed to determine the in vivo therapeutic implications of dFBr for the treatment of neurological diseases that impact cognitive function.
The nAChR PAM dFBr may also hold promise as a treatment for pathological pain. Specifically, dFBr increases the anti-allodynic effects of nicotine in a mouse model of CCI (Bagdas et al., 2017). Meanwhile, administration of dFBr alone appears to produce analgesic effects in both early and late phases of the formalin assay, as well as in the acetic acid writhing assay (Weggel and Pandya, 2019). The effects of the nAChR PAM dFBr are summarized in Table 1.
Table 1.
Positive allosteric modulators of α4β2 nAChR and the work done thus far investigating them as novel therapeutics.
| Compound | nAChR subtype activity in vitro | In vivo findings |
|---|---|---|
| Physostigmine | Full activity at α4β2, α7 | Clinically used acetylcholinesterase inhibitor and antidot for anticholinergic toxicities. |
| Galantamine | Full activity at α4β2, α7 | |
| - reduces cigarette smoking among alcohol-dependent patients | ||
| (dFBr) | Full activity at α4β2, partial activity at α7 | |
| - analgesic effects in formalin assay, and in the acetic acid writhing assay | ||
| NS9283 | Selective activity at α4β2 | |
| - Enhances analgesic efficacy of nAChR agonist in neuropathic pain model. | ||
| CMPI | Selective activity at α4β2 | N.A. |
| LY 2087101 | Full activity at α4β2, α4β4, α7 subtypes | - does not potentiate the effect of nicotine in the mouse drug discrimination assay |
See article text under the corresponding compound for supporting references.
Overall, several lines of research, both in vitro and in vivo, have established the preclinical efficacy and tolerability of dFBr as a potent nAChR PAM that preferentially potentiates the α4β2* nAChRs. Nevertheless, major limitations still face the preclinical development of dFBr into a clinically useful drug including: 1) dFBr inhibits the α7 nAChRs and muscle-type nAChR at concentrations that are equivalent or only slightly higher than that required for α4β2 nAChR potentiation.; 2) following subcutaneous administration, only one third of a dFBr dose crossed the blood-brain barrier; and 3) variability of dFBr effects, alone or co-administered with agonist, in different animal models of pain; and 4) the fact that α4 and β2 nAChR subunits assemble in two stoichiometries ((α4)3(β2)2 and (α4)3(β2)2 nAChRs) and can assemble with other nAChR subunits (e.g. α6, α5) forming a number of functionally and pharmacological unique nAChR subtypes that can be affected by dFBr. Therefore, the overall extent of dFBr’s therapeutic effect is predicted to depend on the expression level of each isoform in the affected brain area, the relative contributions of these subtypes to a specific disease state, and the extent to which dFBr can potentiate each α4β2* nAChR isoform. However, the extent to which these potential issues might be relevant to the clinical use of dFBr is currently unknown.
LY 2087101 ([2-[(4-Fluorophenyl)amino]-4-methyl-5-thiazolyl]-3-thienylmethanone)
Drug development and high-throughput screening of the chemical library at Eli Lilly and Company has identified three 2-amino-2-keto-thiazole derivatives (LY2087101, LY1078733, and LY2087133) with nAChR PAM activity (Broad et al., 2006). In vitro, LY2087101 potentiated current responses of the α4β2, α4β4, and α7 nAChR but not in α3β2 or α3β4 nAChRs establishing LY2087101 as a nAChR PAM that interacts mainly with the α4 and α7 subunits (Broad et al., 2006). LY2087101 is considered a Type I nAChR PAM because it produced an increase in both potency and magnitude of nicotine-induced currents (Broad et al., 2006) with little effect on the rate of receptor desensitization (Young et al., 2008). Specifically regarding α4β2 nAChRs, LY2087101 was an equipotent potentiator (potentiation EC50 ~1 μM) of both the low-sensitivity (α4)3(β2)2 nAChR and high-sensitivity (α4)2(β2)3 nAChR isoform but 2-fold more efficacious at the (α4)3(β2)2 than (α4)2(β2)3 nAChR (Deba et al., 2018). LY2087101 does not appear to alter ACh potency and its effect on ACh efficacy was higher in the (α4)3(β2)2 than (α4)2(β2)3 nAChRs. The more profound effect of LY2087101 at the (α4)3(β2)2 nAChR is thought to be consistent with the number of α4 subunits which confer LY2087101 binding to nAChRs. Subsequent mutational and computational analyses have identified two LY2087101 binding sites within the transmembrane domain of the (α4)3(β2)2 nAChRs: one at the interface between two adjacent α4 subunits (intersubunit binding site) and the other within the α4 subunit transmembrane helix bundle (intrasubunit binding site), (Deba et al., 2018). The intrasubunit binding site within the α4 subunit was found to be equivalent to the binding site identified for LY2087101 in the helix bundle of α7 nAChR (Young et al., 2008). Molecular docking studies predicted that LY2087101 binding within the α4 subunit helix bundle (intrasubunit site) is governed by hydrogen bond interactions with amino acids in the third transmembrane helix (TM3) whereas LY2087101 binding within the intersubunit site is governed by multiple non-bond hydrophobic interactions with amino acids at the interface of the adjacent α4 subunits (Deba et al., 2018).
In vivo, LY2087101 failed to produce substitution for 1.0 mg/kg nicotine at any dose tested in the mouse drug discrimination assay, even at doses that produce significant reduction of schedule-controlled responding (Moerke et al., 2016). Further, when LY 2087101 is paired with doses of nicotine that do not produce significant substitution for 1 mg/kg nicotine, no potentiation is observed (Moerke et al., 2016). Thus, there appears to be a disconnect between the in vitro and in vivo literature regarding whether LY 2087101 is a true functional nAChR PAM. The effects of LY 2087101 are summarized in Table 1.
CMPI (3-(2-chlorophenyl)-5-(5-methyl-1-(piperidin-4-yl)-1H-pyrrazol-4-yl)isoxazole)
CMPI was developed at Amgen Inc. via chemical modification of substituted piperidine structure with nAChR PAM activity (Albrecht et al., 2008; Springer et al., 2008). At submicromolar concentrations, CPMI potentiated α4β2 nAChR but not any other major nAChR subtype (Albrecht et al., 2008). Subsequent studies have established CMPI as a nAChR PAM that potentiates the low-sensitivity (α4)3(β2)2 nAChR but not the high-sensitivity (α4)2(β2)3 nAChR (Hamouda et al., 2016) and identified the binding site for CMPI within the extracellular domain at the α4:α4 subunit interface which exists only in the (α4)3(β2)2 nAChR subtype (Wang et al., 2017). The effect of CMPI on ACh responses of (α4)3(β2)2 nAChR was characterized by enhancement of ACh potency by ~100 fold with no significant effect on the efficacy of ACh (Wang et al., 2017). While the selectively of CMPI to (α4)3(β2)2 nAChRs, the major subpopulation of nAChRs in the cortex (DeDominicis et al., 2017), is viewed as pharmacologically favorable, it has yet to be determined if CMPI has in vivo biological activity.
NS9283 (3-[3-(pyridin-3-yl)-1,2,4-oxadiazol-5-yl]benzonitrile
NS9283 is an α2- and α4-selective nAChR PAM that was discovered via research efforts at Neurosearch A/S (Timmerman et al., 2012). It was found that NS9283 increased the potency of currents evoked with ACh in HEK293 cells transfected with human α4β2 nAChR. Further, it was found that NS9283 did not alter the rate of desensitization of currents evoked with ACh (Grupe et al., 2013). It has been found that NS9283 selectively and preferentially acts on nAChR with the combination of (3) α4 plus (2) β2 subunit ratios (Grupe et al., 2013; Timmermann et al., 2012). Single channel recordings have shown that (α4)3(β2)2 nAChRs show high single channel conductance, brief mean open lifetime, and high potentiation by NS9283, whereas (α4)3(β2)2 nAChRs display low single channel conductance, long mean open lifetime, and are not potentiated by NS9283 (Mazzaferro et al., 2017). It has been thought that NS9283 is a PAM which is only able to potentiate (α4)3(β2)2 nAChRs by binding to a site at the α4:α4 subunit extracellular interface (Timmerman et al., 2012; Mazzaferro et al., 2011; Harpose et al. 2011; Eaton et al., 2014). However, studies suggest that NS9283 not actually a PAM, but is rather an agonist selective for the α4:α4 ACh binding site in the (α4)3 stoichiometry (Wang et al., 2015). Further, in a recent single channel recording study, mutations of amino acid in the β2 subunit that contribute to the β2:α4 subunit extracellular interface decreased NS9283 effect, indicating that other subunit interfaces play essential role in NS9283 binding and/or potentiation of (α4)3(β2)2 nAChR (Mazzaferro et al., 2019).
In the rat drug discrimination assay, NS9283 fails to produce substitution for 0.4 mg/kg nicotine at any dose tested (Mohler et al., 2014). When NS9283 is paired with doses of nicotine that do not produce significant substitution for 0.4 mg/kg nicotine, full substitution is observed. In the rat self-administration assay, NS9283 is not readily self-administered (Maskos et al., 2017). However, both acute and repeated administration of NS9283 dose-dependently reduces nicotine self-administration in rats (Maurer et al., 2017). The observation that NS9283 reduces nicotine self-administration in rats suggest that it might contribute to enhanced desensitization of the (α4)3 stoichiometry or that the (α4)3 stoichiometry contributes to responses that trigger aversion, such those observed after high nicotine doses.
NS9283 alone did not alter pain threshold for mechanical allodynia in neuropathic pain model; However, it enhanced the analgesic efficacy of nicotinic agonist in same model suggested that NS9283 can be used to improve the therapeutic index of nicotinic agonist in pain treatment (Lee et al., 2011). NS9283 has shown promise in improving cognitive function in rodents. Specifically, in rats NS9283 improves social memory, task acquisition in the hippocampal-dependent spatial memory Morris Water Maze task, as well as attention performance in the five-choice serial reaction time task (Timmerman et al., 2012). However, more studies are needed to determine the in vivo therapeutic implications of NS9283 for the treatment of neurological diseases that impact cognitive function. The effects of the nAChR PAM NS9283 are summarized in Table 1.
Conclusions
A major challenge in the development of novel nAChR therapeutics lies in the apparent probability that several different nAChR subtypes play important roles in the development and maintenance of nicotine dependence and addiction, cognitive function, and pathological pain. Further, these receptors can be dynamically regulated in different manners, which adds to the complexity of designing therapeutic interventions with limited side-effects. However, rightly so, the α4β2 nAChR remains a substantial focus of research for novel compounds to treat these diseases. The therapeutic utility of orthosteric α4β2 nAChR agonists leaves room for the development of better therapeutics for smoking cessation aids, cognitive and neurological diseases as well as pathological pain. PAMs at α4β2 nAChR are attractive alternatives, as lower concentration of an agonists is needed to produce maximal therapeutic effects, and this decrease in agonist dose is thought to limit potential off-target, or unwanted side-effects. Interestingly, it has recently been reported that the binding sites for dFBr, LY2087101, and Br-PBTC, a novel nAChR PAM at multiple subunits including α4β2, have been reported to be very close to one another (Norleans et al., 2019). In this study, derivatives of Br-PBTC have been found that interact with α5 subunits, which suggests that it might be possible to develop PAMs selective for α4α5β2 nAChRs. Further, one limitation to the experimental compounds listed in this review is that when administered in vivo, most were administered via a systemic injection, leaving other routes of administration unexplored thus far. It may be that varying the route of administration may enhance the therapeutic window of these compounds. Regardless, α4β2 nAChR PAMs may hold promise as smoking cessation aids, as well as treatments for neurological diseases and pathological pain.
Highlights.
Antimicrobial mechanism of carvacrol against uropathogenic E. coli
Carvacrol demonstrated membrane depolarization and oxidative burst in E. coli
Carvacrol induced the release of DNA, proteins and ions from E. coli cells
Carvacrol reduced the levels of inflammatory proteins COX-2 and iNOS.
Carvacrol inhibited cell mortality and β-lactamase enzyme activity
Nonstandard Abbreviations:
- ACh
acetylcholine
- CCI
chronic constriction injury
- nAChR
nicotinic acetylcholine receptor
- PAM
Positive Allosteric Modulator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- Albrecht BK, Berry V, Boezio AA, Cao L, Clarkin K, Guo W, Harmange JC, Hierl M, Huang L, Janosky B, Knop J, Malmberg A, McDermott JS, Nguyen HQ, Springer SK, Waldon D, Woodin K, and McDonough SI, 2008. Discovery and optimization of substituted piperidines as potent, selective, CNS-penetrant alpha4beta2 nicotinic acetylcholine receptor potentiators. Bioorg Med Chem Lett. 18, 5209–12. 10.1016/j.bmcl.2008.08.080. [DOI] [PubMed] [Google Scholar]
- Alcaino C, Musgaard M, Minguez T, Mazzaferro S, Faundez M, Iturriaga-Vasquez P, Biggin PC, Bermudez I, 2017. Role of the Cys Loop and Transmembrane Domain in the Allosteric Modulation of α4β2 Nicotinic Acetylcholine Receptors. J Biol Chem. 292, 551–562. 10.1074/jbc.M116.751206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auld DS, Kornecook TJ, Bastianetto S, Quirion R, 2002. Alzheimer’s disease and the basal forebrain cholinergic system: relations to beta-amyloid peptides, cognition, and treatment strategies. Prog Neurobiol. 68, 209–45. 10.1016/S0301-0082(02)00079-5. [DOI] [PubMed] [Google Scholar]
- Bagdas D, AlSharari SD, Freitas K, Tracy M, Damaj MI, 2015. The role of alpha5 nicotinic acetylcholine receptors in mouse models of chronic inflammatory and neuropathic pain. Biochem. Pharmacol 97, 590–600. 10.1016/j.bcp.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, Ergun D, Jackson A, Toma W, Schulte MK, Damaj MI, 2017. Allosteric modulation of α4β2* nicotinic acetylcholine receptors: Desformylflustrabromine potentiates antiallodynic response of nicotine in a mouse model of neuropathic pain. Eur J Pain. 1, 84–93. 10.1002/ejp.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D, Terry AV Jr., 2018. The wonderland of neuronal nicotinic acetylcholine receptors. Biochem Pharmacol 151, 214–225 10.1016/j.bcp.2017.12.008. [DOI] [PubMed] [Google Scholar]
- Besson M, David V, Suarez S, Cormier A, Cazala P, Changeux JP, Granon S, 2006. Genetic dissociation of two behaviors associated with nicotine addiction: beta-2 containing nicotinic receptors are involved in nicotine reinforcement but not in withdrawal syndrome. Psychopharmacology (Berl) 187, 189–199. 10.1007/s00213-006-0418-z. [DOI] [PubMed] [Google Scholar]
- Biala G, Staniak N, Budzynska B, 2010. Effects of varenicline and mecamylamine on the acquisition, expression, and reinstatement of nicotine-conditioned place preference by drug priming in rats. Naunyn Schmiedebergs Arch Pharmacol 381, 361–370. 10.1007/s00210-010-0498-5. [DOI] [PubMed] [Google Scholar]
- Broad LM, Zwart R, Pearson KH, Lee M, Wallace L, McPhie GI, Emkey R, Hollinshead SP, Dell CP, Baker SR, Sher E, 2006. Identification and pharmacological profile of a new class of selective nicotinic acetylcholine receptor potentiators. J Pharmacol Exp Ther. 318, 1108–1117. 10.1124/jpet.106.104505. [DOI] [PubMed] [Google Scholar]
- Cooper E, Couturier S, Ballivet M, 1991. Pentameric structure and subunit stoichiometry of a neuronal nicotinic acetylcholine receptor. Nature 350, 235–238. 10.1038/350235a0. [DOI] [PubMed] [Google Scholar]
- Deba F, Ali HI, Tairu A, Ramos K, Ali JH, Hamouda A, 2018. LY2087101 and dFBr share transmembrane binding sites in the (α4)3(β2)2 Nicotinic Acetylcholine Receptor. Scientific Reports 8, 1249 10.1038/s41598-018-19790-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeDominicis KE, Sahibzada N, Olson TT, Xiao Y, Wolfe BB, Kellar KJ, Yasuda RP, 2017. The (α4)3(β2)2 Stoichiometry of the Nicotinic Acetylcholine Receptor Predominates in the Rat Motor Cortex. Mol Pharmacol. 92, 327–337. 10.1124/mol.116.106880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl A, Nakovics H, Croissant B, Smolka MN, Batra A, Mann K, 2006. Galantamine reduces smoking in alcohol-dependent patients: a randomized, placebo-controlled trial. Int J Clin Pharmacol Ther 44, 614–622. 10.5414/cpp44614. [DOI] [PubMed] [Google Scholar]
- Dukat M, Jain A, German N, Ferrara-Pontoriero R, Huang Y, Ma Y, Schulte MK, Glennon RA, 2018. des-Formylflustrabromine (dFBr): A Structure-Activity Study on Its Ability To Potentiate the Action of Acetylcholine at α4β2 Nicotinic Acetylcholine Receptors. ACS Chem Neurosci. 12, 2984–2996. 10.1021/acschemneuro.8b00156. [DOI] [PubMed] [Google Scholar]
- Dunbar GC, Kuchibhatla RV, Lee G, 2011. A randomized double-blind study comparing 25 and 50 mg TC-1734 (AZD3480) with placebo, in older subjects with age-associated memory Impairment. J Psychopharmacol 25, 1020–1029. 10.1111/j.1527-3458.2004.tb00010.x [DOI] [PubMed] [Google Scholar]
- Farlow MR, 2003. Clinical pharmacokinetics of galantamine. Clin Pharmacokinet 42, 1383–1392. 10.2165/00003088-200342150-00005. [DOI] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ, 1992. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol 41, 31–7. [PubMed] [Google Scholar]
- Flores CM, Davila-Garcia MI, Ulrich YM, Kellar KJ, 1997. Differential regulation of neuronal nicotinic receptor binding sites following chronic nicotine administration. J Neurochem 69, 2216–2219. 10.1046/j.1471-4159.1997.69052216.x. [DOI] [PubMed] [Google Scholar]
- Flynn DD, Mash DC, 1986. Characterization of L-[3H] nicotine binding in human cerebral cortex: comparison between Alzheimer’s disease and the normal. J Neurochem. 6, 1948–54. 10.1111/j.1471-4159.1986.tb13113.x. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ, 2013. Nicotine aversion: Neurobiological mechanisms and relevance to tobacco dependence vulnerability. Neuropharmacology 76 Pt B, 533–544. DOI: 10.1016/j.neuropharm.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas K, Carroll FI, Damaj MI, 2013. The antinociceptive effects of nicotinic receptors α7-positive allosteric modulators in murine acute and tonic pain models. J Pharmacol Exp Ther. 11, 264–75. https://doi.org/0.1124/jpet.112.197871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto GJ, Bohme GA, Caldwell WS, Letchworth SR, Traina VM, Obinu MC, Laville M, Reibaud M, Pradier L, Dunbar G, Bencherif M, 2004. TC-1734: an orally active neuronal nicotinic acetylcholine receptor modulator with antidepressant, neuroprotective and long-lasting cognitive effects. CNS Drug Rev 10, 147–166. 10.1111/j.1527-3458.2004.tb00010.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- German N, Kim JS, Jain A, Dukat M, Pandya A, Ma Y, Weltzin M, Schulte MK, Glennon RA, 2011. Deconstruction of the α4β2 nicotinic acetylcholine receptor positive allosteric modulator desformylflustrabromine. J Med Chem. 54, 7259–67. 10.1021/jm200834x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielen M, Corringer PJ, 2018. The dual-gate model for pentameric ligand-gated ion channels activation and desensitization. J Physiol 596, 1873–1902. 10.1113/JP275100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Clementi F, 2004. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 74, 363–96. 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Grupe M, Grunnet M, Bastlund JF, Jensen AA, 2015. Targeting α4β2 nicotinic acetylcholine receptors in central nervous system disorders: perspectives on positive allosteric modulation as a therapeutic approach. Basic Clin. Pharmacol. Toxicol 116, 187–200. 10.1111/bcpt.12361. [DOI] [PubMed] [Google Scholar]
- Grupe M, Jensen AA, Ahring PK, Christensen JK, Grunnet M, 2013. Unravelling the mechanism of action of NS9283, a positive allosteric modulator of (alpha4)3(beta2)2 nicotinic ACh receptors. Br J Pharmacol 168, 2000–2010. 10.1111/bph.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamouda AK, Deba F, Wang ZJ, Cohen JB, 2016. Photolabeling a Nicotinic Acetylcholine Receptor (nAChR) with an (α4)3(β2)2 nAChR-Selective Positive Allosteric Modulator. Mol Pharmacol. 89, 575–84. 10.1124/mol.116.103341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamouda AK, Jayakar SS, Chiara DC, Cohen JB, 2014. Photoaffinity labeling of nicotinic receptors: diversity of drug binding sites! J Mol Neurosci. 53, 480–6. 10.1007/s12031-013-0150-1. [DOI] [PubMed] [Google Scholar]
- Hamouda AK, Kimm T, Cohen JB, 2013. Physostigmine and galanthamine bind in the presence of agonist at the canonical and noncanonical subunit interfaces of a nicotinic acetylcholine receptor. J Neurosci 33, 485–494. 10.1523/JNEUROSCI.3483-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamouda AK, Wang ZJ, Stewart DS, Jain AD, Glennon RA, Cohen JB, 2015. Desformylflustrabromine (dFBr) and [3H]dFBr-Labeled Binding Sites in a Nicotinic Acetylcholine Receptor. Mol Pharmacol. 88, 1–11. 10.1124/mol.115.098913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamouda AK, Jackson A, Bagdas D, Damaj MI, 2018. Reversal of Nicotine Withdrawal Signs Through Positive Allosteric Modulation of α4β2 Nicotinic Acetylcholine Receptors in Male Mice. Nicotine Tob Res. 20, 903–907. 10.1093/ntr/ntx183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpsøe K, Ahring PK, Christensen JK, Jensen ML, Peters D, Balle T, 2011. Unraveling the high- and low-sensitivity agonist responses of nicotinic acetylcholine receptors. J. Neurosci 31, 10759–10766. 10.1523/JNEUROSCI.1509-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hone AJ, Meyer EL, McIntyre M, McIntosh JM, 2011. Nicotinic acetylcholine receptors in dorsal root ganglion neurons include the α6β4* subtype. FASEB J. 26, 91–26. 10.1096/fj.11-195883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins TJ, Rupprecht LE, Hayes MR, Blendy JA, Schmidt HD, 2012. Galantamine, an acetylcholinesterase inhibitor and positive allosteric modulator of nicotinic acetylcholine receptors, attenuates nicotine taking and seeking in rats. Neuropsychopharmacology 37, 2310–2321. 10.1038/npp.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst R, Rollema H, Bertrand D, 2013. Nicotinic acetylcholine receptors: From basic science to therapeutics. Pharmacol. Ther 137, 22–54. 10.1016/j.pharmthera.08.012. [DOI] [PubMed] [Google Scholar]
- Igari M, Alexander JC, Ji Y, Qi X, Papke RL, Bruijnzeel AW, 2014. Varenicline and cytisine diminish the dysphoric-like state associated with spontaneous nicotine withdrawal in rats. Neuropsychopharmacology 39, 455–465. 10.1038/npp.2013.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain KK, 2004. Modulators of nicotinic acetylcholine receptors as analgesics. Curr Opin Investig Drugs. 5, 76–81. [PubMed] [Google Scholar]
- Jensen AA, Frølund B, Liljefors T, Krogsgaard-Larsen P, 2005. Neuronal nicotinic acetylcholine receptors: structural revelations, target identifications, and therapeutic inspirations. J Med Chem 48, 4705–4745. 10.1021/jm040219e. [DOI] [PubMed] [Google Scholar]
- Jin X, Bermudez I, Steinbach JH, 2014. The Nicotinic α5 Subunit Can Replace Either an Acetylcholine-Binding or Nonbinding Subunit in the α4β2* Neuronal Nicotinic Receptor. Mol Pharmacol. 85, 11–17. 10.1124/mol.113.089979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalamida D, Poulas K, Avramopoulou V, Fostieri E, Lagoumintzis G, Lazaridis K, Sideri A, Zouridakis M, Tzartos SJ, 2007. Muscle and neuronal nicotinic acetylcholine receptors Structure, function and pathogenicity. FEBS 274, 3799–3845. 10.1111/j.1742-4658.2007.05935.x. [DOI] [PubMed] [Google Scholar]
- Kasa P, Rakonczay Z, Gulya K, 1997. The cholinergic system in Alzheimer’s disease. Prog. Neurobiol 52, 511–535. 10.1016/S0301-0082(97)00028-2 [DOI] [PubMed] [Google Scholar]
- Kiguchi N, Kobayashi D, Saika F, Matsuzaki S, Kishioka S, 2018. Inhibition of peripheral macrophages by nicotinic acetylcholine receptor agonists suppresses spinal microglial activation and neuropathic pain in mice with peripheral nerve injury. J Neuroinflam 15, 96 10.1186/s12974-018-1133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara T, Shimohama S, Urushitani M, Sawada H, Kimura J, Kume T, Maeda T, Akaike A, 1998. Stimulation of alpha4beta2 nicotinic acetylcholine receptors inhibits beta-amyloid toxicity. Brain Research 792, 331–334. 10.1016/s0006-8993(98)00138-3 [DOI] [PubMed] [Google Scholar]
- Kim JS, Padnya A, Weltzin M, Edmonds BW, Schulte MK, Glennon RA, 2007. Synthesis of desformylflustrabromine and its evaluation as an alpha4beta2 and alpha7 nACh receptor modulator. Bioorg Med Chem Lett 17, 4855–4860. 10.1016/j.bmcl.2007.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP, 2001. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci 21,1452–1463. 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal NM, Ahring PK, Liao VWY, Indurti DC, Harvey BS, O’Connor SM, Chebib M, Olafsdottir ES, Balle T, 2018. Galantamine is not a positive allosteric modulator of human alpha4beta2 or alpha7 nicotinic acetylcholine receptors. Br J Pharmacol 175, 2911–2925. 10.1111/bph.14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte SL, Toma W, Bagdas D, Meade JA, Schurman LD, Lichtman AH, Chen ZJ, Del Fabbro E, Fang X, Bigbee JW, Damaj MI, Gewirtz DA., 2018. Nicotine Prevents and Reverses Paclitaxel-Induced Mechanical Allodynia in a Mouse Model of CIPN. J Pharmacol Exp Ther. 364,110–119. 10.1124/jpet.117.243972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Zhu C, Malysz J, Campbell T, Shaughnessy T, Honore P, Polakowski J, Gopalakrishnan M, 2011. α4β2 neuronal nicotinic receptor positive allosteric modulation. An approach for improving the therapeutic index of α4β2 nAChR agonists in pain. Biochem. Pharmacol 82, 959–966. 10.1016/j.bcp.2011.06.044. [DOI] [PubMed] [Google Scholar]
- Li P, Steinbach JH, 2010. The neuronal nicotinic alpha4beta2 receptor has a high maximal probability of being open. Br J Pharmacol 160, 1906–1915. 10.1111/j.1476-5381.2010.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindel T, Bräuchle L, Golz G, Böhrer P, 2007. Total synthesis of flustramine C via dimethylallyl rearrangement. Org Lett 9, 283–6. 10.1021/ol0627348. [DOI] [PubMed] [Google Scholar]
- Liu X, 2013. Positive allosteric modulation of alpha4beta2 nicotinic acetylcholine receptors as a new approach to smoking reduction: evidence from a rat model of nicotine self-administration. Psychopharmacology (Berl) 230, 203–213. 10.1007/s00213-013-3145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J, Ray R, Wileyto EP, Ruparel K, Sanborn P, Siegel S, Gur RC, Lerman C, 2010. Effects of the alpha4beta2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biol Psychiatry. 8, 715–21. 10.1016/j.biopsych.2010.01.016 [DOI] [PubMed] [Google Scholar]
- Luttmann E, Ludwig J, Höffle-Maas A, Samochocki M, Maelicke A, Fels G, 2009. Structural model for the binding sites of allosterically potentiating ligands on nicotinic acetylcholine receptors. Chem Med Chem 4, 1874–1882. 10.1002/cmdc.200900320 [DOI] [PubMed] [Google Scholar]
- Maelicke A, Samochocki M, Jostock R, Fehrenbacher A, Ludwig J, Albuquerque EX, Zerlin M, 2001. Allosteric sensitization of nicotinic receptors by galantamine, a new treatment strategy for Alzheimer’s disease. Biol Psychiatry 49, 279–288. 10.1016/s0006-3223(00)01109-4. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS, 2002. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron 33, 905–919. 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Marubio LM, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Léna C, Le Novère N, de Kerchove d’Exaerde A, Huchet M, Damaj MI, Changeux JP 1999. Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature 398, 805–810. 10.1038/19756. [DOI] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, Dufour N, Tayarani-Cloëz I, Bemelmans AP, Mallet J, Gardier AM, David V, Faure P, Garnon S, Changeux JP, 2005. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436, 103–107. 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- Maurer JJ, Sandager-Nielsen K, Schmidt HD, 2017. Attenuation of nicotine taking and seeking in rats by the stoichiometry-selective alpha4beta2 nicotinic acetylcholine receptor positive allosteric modulator NS9283. Psychopharmacology (Berl). 234, 475–484. 10.1007/s00213-016-4475-7 [DOI] [PubMed] [Google Scholar]
- Mazzaferro S, Bermudez I, Sine SM, 2017. Α4β2 nicotinic acetylcholine receptors: relationships between subunit stoichiometry and function at the single channel level. J Biol Chem. 292, 2729–2740. 10.1074/jbc.M116.764183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzaferro S, Bermudez I, Sine SM, 2019. Potentiation of a neuronal nicotinic receptor via pseudo-agonist site. Cell Mol Life Sci. 76, 1151–1167. 10.1007/s00018-018-2993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzaferro S, Benallegue N, Carbone A, Gasparri F, Vijayan R, Biggin PC, Moroni M, Bermudez I, 2011. Additional acetylcholine (ACh) binding site at alpha4/alpha4 interface of (alpha4beta2)2alpha4 nicotinic receptor influences agonist sensitivity. J Biol Chem. 286, 31043–54. 10.1074/jbc.M111.262014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNerney ME, Pardi D, Pugh PC, Nai Q, Margiotta JF, 2000. Expression and channel properties of alpha-bungarotoxin-sensitive acetylcholine receptors on chick ciliary and choroid neurons. J Neurophysiol 84, 1314–1329. 10.1152/jn.2000.84.3.1314. [DOI] [PubMed] [Google Scholar]
- Mitra S, Mucha M, Khatri SN, Glenon R, Schulte MK, Bult-Ito A, 2017. Attenuation of Compulsive-Like Behavior Through Positive Allosteric Modulation of α4β2 Nicotinic Acetylcholine Receptors in Non-Induced Compulsive-Like Mice. Front Behav Neurosci. 10, 244 10.3389/fnbeh.2016.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerke MJ, de Moura FB, Koek W, McMahon LR, 2016. Effects of nicotine in combination with drugs described as positive allosteric nicotinic acetylcholine receptor modulators in vitro: discriminative stimulus and hypothermic effects in mice. Eur J Pharmacol. 786, 169–178. 10.1016/j.ejphar.2016.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler EG, Franklin SR, Rueter LE, 2014. Discriminative-stimulus effects of NS9283, a nicotinic α4β2* positive allosteric modulator, in nicotine-discriminating rats. Psychopharmacology (Berl). 231, 67–74. 10.1007/s00213-013-3207-5. [DOI] [PubMed] [Google Scholar]
- Morales-Perez CL, Noviello CM, Hibbs RE, 2016. X-ray structure of the human alpha4beta2 nicotinic receptor. Nature 538, 411–415. 10.1038/nature19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni M, Zwart R, Sher E, Cassels BK and Bermudez I, 2006. alpha4beta2 nicotinic receptors with high and low acetylcholine sensitivity: pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Mol Pharmacol 70, 755–768. 10.1124/mol.106.023044. [DOI] [PubMed] [Google Scholar]
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J, 2003. Alternate stoichiometries of alpha4beta2 nicotinic acetylcholine receptors. Mol Pharmacol 63, 332–341. 10.1124/mol.63.2.332. [DOI] [PubMed] [Google Scholar]
- Nirogi R, Goura V, Abraham R, Jayarajan P, 2013. α4β2* neuronal nicotinic receptor ligands (agonist, partial agonist and positive allosteric modulators) as therapeutic prospects for pain. Eur J Pharmacol. 712, 22–9. 10.1016/j.ejphar.2013.04.021. [DOI] [PubMed] [Google Scholar]
- Nirogi R, Jabaris SL, Jayarajan P, Abraham R, Shanmuganathan D, Rasheed MA, Royapalley PK, Goura V, 2011. Antinociceptive activity of α4β2* neuronal nicotinic receptor agonist A-366833 in experimental models of neuropathic and inflammatory pain. Eur J Pharmacol. 668, 155–62. 10.1016/j.ejphar.2011.06.032. [DOI] [PubMed] [Google Scholar]
- Norleans J, Wang J, Kuryatov A, Leffler A, Doebelin C, Kamenecka TM, Lindstrom J, 2019. Discovery of an intrasubunit nicotinic acetylcholine receptor-binding site for the positive allosteric modulator Br-PBTC. J Biol Chem 294, 12132–12145. doi: 10.1074/jbc.RA118.006253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JA, Kastrup JS, Peters D, Gajhede M, Balle T, Ahring PK, 2013. Two distinct allosteric binding sites at α4β2 nicotinic acetylcholine receptors revealed by NS206 and NS9283 give unique insights to binding activity-associated linkage at Cys-loop receptors. J Biol Chem. 288, 35997–6006. 10.1074/jbc.M113.498618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JA, Ahring PK, Kastrup JS, Gajhede M, Balle T, 2014. Structural and Functional Studies of the Modulator NS9283 Reveal Agonist-like Mechanism of Action at α4β2 Nicotinic Acetylcholine Receptors. J Biol Chem. 289, 24911–21. 10.1074/jbc.M114.568097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya A, Yakel JL, 2011. Allosteric modulator Desformylflustrabromine relieves the inhibition of α2β2 and α4β2 nicotinic acetylcholine receptors by β-amyloid(1–42) peptide. J Mol Neurosci 45, 42–47. 10.1007/s12031-011-9509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya AA, Yakel JL, 2013. Effects of neuronal nicotinic acetylcholine receptor allosteric modulators in animal behavior studies. Biochem Pharmacol 86, 1054–1062. 10.1016/j.bcp.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke D, Gonzalez-Gutierrez G, Grosman C, 2011. Desensitization of neurotransmitter-gated ion channels during high-frequency stimulation: a comparative study of Cys-loop, AMPA and purinergic receptors. J Physiol 589, 1571–1585. 10.1113/jphysiol.2010.203315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters L, König GM, Terlau H, Wright AD, 2002. Four new bromotryptamine derivatives from the marine bryozoan Flustra foliacea. J Nat Prod 65, 1633–1637. 10.1021/np0105984. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Léna C, Bessis A, Lallemand Y, Le Novère N, Vincent P, Pich EM, Brûlet P, Changeux JP, 1995. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature 374, 65–67. 10.1038/374065a0 [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, Changeux JP, 1998. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391, 173–177. 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA, 2008. Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharmacol Biochem Behav 88, 407–417. 10.1016/j.pbb.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Reus VI, Obach RS, Coe JW, Faessel H, Rollema H, Watsky E, Reeves K, 2007. Varenicline: new treatment with efficacy in smoking cessation. Drugs Today (Barc). 43, 65–75. 10.1358/dot.2007.43.2.1069956 [DOI] [PubMed] [Google Scholar]
- Ross SA, Wong JY, Clifford JJ, Kinsella A, Massalas JS, Horne MK, Scheffer IE, Kola I, Waddington JL, Berkovic SF, Drago J, 2000. Phenotypic characterization of an alpha 4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J Neurosci 20, 6431–6441. 10.1523/JNEUROSCI.20-17-06431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala F, Mulet J, Reddy KP, Bernal JA, Wikman P, Valor LM, Peters L, König GM, Criado M, Sala S, 2005. Potentiation of human alpha4beta2 neuronal nicotinic receptors by a Flustra foliacea metabolite. Neurosci Lett. 373, 144–9. 10.1016/j.neulet.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Samochocki M, Höffle A, Fehrenbacher A, Jostock R, Ludwig J, Christner C, Radina M, Zerlin M, Ullmer C, Pereira EF, Lübbert H, Albuquerque EX, Maelicke A, 2003. Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J Pharmacol Exp Ther 305, 1024–36. 10.1124/jpet.102.045773. [DOI] [PubMed] [Google Scholar]
- Schilström B, Ivanov VB, Wiker C, Svensson TH, 2007. Galantamine enhances dopaminergic neurotransmission in vivo via allosteric potentiation of nicotinic acetylcholine receptors. Neuropsychopharmacology 32, 43–53. 10.1038/sj.npp.1301087. [DOI] [PubMed] [Google Scholar]
- Springer SK, Woodin KS, Berry V, Boezio AA, Cao L, Clarkin K, Harmange JC, Hierl M, Knop J, Malmberg AB, McDermott JS, Nguyen HQ, Waldon D, Albrecht BK, McDonough SI, 2008. Synthesis and activity of substituted carbamates as potentiators of the alpha4beta2 nicotinic acetylcholine receptor. Bioorg Med Chem Lett. 18, 5643–7. 10.1016/j.bmcl.2008.08.092. [DOI] [PubMed] [Google Scholar]
- Suva D, Favre I, Kraftsik R, Esteban M, Lobrinus A, Miklossy J, 1999. Primary motor cortex involvement in Alzheimer disease. J Neuropathol Exp Neurol 58, 1125–1134. 10.1097/00005072-199911000-00002. [DOI] [PubMed] [Google Scholar]
- Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP, 2009. Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Disc 8, 733–750. 10.1038/nrd2927. [DOI] [PubMed] [Google Scholar]
- Timmermann DB, Sandager-Nielsen K, Dyhring T, Smith M, Jacobsen AM, Nielsen EØ, Grunnet M, Christensen JK, Peters D, Kohlhaas K, Olsen GM, Ahring PK, 2012. Augmentation of cognitive function by NS9283, a stoichiometry-dependent positive allosteric modulator of α2- and α4-containing nicotinic acetylcholine receptors. Br J Pharmacol. 167, 164–82. 10.1111/j.1476-5381.2012.01989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M, Iida Y, Tominaga A, Yoneyama T, Ogawa M, Magata Y, Nishimura H, Kuge Y, Saji H, 2010. Nicotinic acetylcholine receptors expressed in the ventralposterolateral thalamic nucleus play an important role in anti-allodynic effects. Br J Pharmacol. 6, 1201–10. 10.1111/j.1476-5381.2009.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umana IC, Daniele CA, McGehee DS, 2013. Neuronal nicotinic receptors as analgesic targets: it’s a winding road. Biochem Pharmacol. 8, 1208–14. 10.1016/j.bcp.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uteshev VV, 2014. The therapeutic promise of positive allosteric modulation of nicotinic receptors. Eur. J. Pharmacol 727, 181–185. 10.1016/j.ejphar.2014.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann R, Tobey K, Lobe S, Kipps B, Kwilasz A, Aceto M, Harris L, 2011. Varenicline does not alter brain stimulation reward thresholds and reverses nicotine-facilitated thresholds in rats. Drug Dev. Res 72, 310–314. 10.1007/s00213-012-2703-3. [DOI] [Google Scholar]
- Walsh RM, Roh SH, Gharpure A, Morales-Perez CL, Teng J, Hibbs RE, 2018. Structural principles of distinct assemblies of the human α4β2 nicotinic receptor. Nature 557, 261–265. 10.1038/s41586-018-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Kuryatov A, Sriram A, Jin Z, Kamenecka TM, Kenny PJ, Lindstrom J, 2015. An Accessory Agonist Binding Site Promotes Activation of alpha4beta2* Nicotinic Acetylcholine Receptors. J Biol Chem 290, 13907–13918. doi: 10.1074/jbc.M115.646786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lindstrom J, 2018. Orthosteric and allosteric potentiation of heteromeric neuronal nicotinic acetylcholine receptors. Br J Pharmacol. 11, 1805–1821. 10.1111/bph.13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZJ, Deba F, Mohamed TS, Chiara DC, Ramos K, Hamouda AK, 2017. Unraveling amino acid residues critical for allosteric potentiation of (alpha4)3(beta2)2-type nicotinic acetylcholine receptor responses. J Biol Chem. 292, 9988–10001. 10.1074/jbc.M116.771246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warpman U, Nordberg A, 1995. Epibatidine and ABT 418 reveal selective losses of α4β2 nicotinic receptors in Alzheimer brains. Neuroreport. 6, 2419–2423. 10.1097/00001756-199511270-00033 [DOI] [PubMed] [Google Scholar]
- Weggel LA, Pandya AA, 2019. Acute Administration of Desformylflustrabromine Relieves Chemically Induced Pain in CD-1 Mice. Molecules. 24, 944 10.3390/molecules24050944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltzin MM, Schulte MK, 2015. Desformylflustrabromine Modulates α4β2 Neuronal Nicotinic Acetylcholine Receptor High- and Low-Sensitivity Isoforms at Allosteric Clefts Containing the β2 Subunit. J Pharmacol Exp Ther. 354, 184–194. https://doi.org/0.1124/jpet.115.223933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltzin MM, Huang Y, Schulte MK, 2014. Allosteric modulation of alpha4beta2 nicotinic acetylcholine receptors by HEPES. Eur J Pharmacol 732, 159–168. 10.1016/j.ejphar.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wevers A, Monteggia L, Nowacki S, Bloch W, Schütz U, Lindstrom J, Pereira EF, Eisenberg H, Giacobini E, de Vos RA, Steur EN, Maelicke A, Albuquerque EX, Schröder H, 1999. Expression of nicotinic acetylcholine receptor subunits in the cerebral cortex in Alzheimer’s disease: histotopographical correlation with amyloid plaques and hyperphosphorylated-tau protein. Eur J Neurosci. 11, 2551–65. 10.4103/1673-5374.147943. [DOI] [PubMed] [Google Scholar]
- Whiting P, Lindstrom J, 1987. Affinity labelling of neuronal acetylcholine receptors localizes acetylcholine-binding sites to their beta-subunits. FEBS Lett 213, 55–60. 10.1016/0014-5793(87)81464-3 [DOI] [PubMed] [Google Scholar]
- Williams DK, Wang J, Papke RL, 2011. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: Advantages and limitations. Biochem. Pharmacol 82, 915–930. 10.1016/j.bcp.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GT, Zwart R, Walker AS, Sher E, Millar NS, 2008. Potentiation of a7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc. Natl. Acad. Sci. U.S.A 105, 14686–14691. 10.1073/pnas.0804372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C, 2002. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci 22, 8785–8789. 10.1523/JNEUROSCI.22-20-08785.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart R, Vijverberg HP, 1998. Four pharmacologically distinct subtypes of alpha4beta2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Mol Pharmacol 54, 1124–1131. 10.1124/mol.54.6.1124. [DOI] [PubMed] [Google Scholar]


