Abstract
Peripheral neuropathy associated with chronic occupational and deliberate overexposure to neurotoxic organic solvents results from axonal degeneration in the central and peripheral nervous system. Human and experimental studies show that axonopathy is triggered by the action of neuroprotein-reactive γ-diketone metabolites formed from exposure to certain aliphatic solvents (n-hexane, 2-hexanone) and aromatic compounds (1,2-diethylbenzene, 1,2–4-triethylbenzene, 6-acetyl-1,1,4,4-tetramethyl-7-ethyl-1,2,3,4-tetralin). Neuroprotein susceptibility is related primarily to their differential content of lysine, the ε-amino group of which is targeted by γ-diketones. Specific neuroprotein targets have been identified, and the sequence of molecular mechanisms leading to axonal pathology has been illuminated. While occupational n-hexane neuropathy continues to be reported, lessons learned from its experimental study may have relevance to other causes of peripheral neuropathy, including those associated with aging and diabetes mellitus.
Keywords: n-Hexane; 1,2-Diacetylbenzene; Neuropathy; Axonopathy; Lysine; Neuroproteins; Diabetes mellitus
1. Introduction
Repeated workplace overexposure to n-hexane, an inexpensive organic solvent that has been widely used in industry, is an established cause of a self-limiting distal symmetrical peripheral neuropathy underpinned neuropathologically by central-peripheral distal axonal degeneration.1,2 Other neurological phenomena associated with workplace exposure to n-hexane and solvent mixtures containing n-hexane include: reduced balance control,3 impaired temperature sensation (cold allodynia),4 changes in color vision,5,6 maculopathy and optic neuropathy,7,8 and abnormal or reduced sense of smell.9 High-dose n-hexane overexposure, which alone or in a solvent mixture has been used to induce euphoria, can precipitate a subacute onset of neuropathy resulting in severe muscle weakness and wasting.10–15
Peripheral neuropathy arising from repeated exposure to n-hexane was demonstrated 50 years ago in printing plants, sandal and shoe-making shops, and furniture factories in Asia, Europe, and the United States.10,16–22 The problem has continued into the 21st century, with reports of n-hexane neuropathy among American automotive technicians,23 shoe factory workers in Turkey,24 screen printers in India,25,26 electronic industry workers in Japan and South Korea,27,28 and in a Chinese medicine plant in Taiwan.29–32 Additionally, between 1996–2004, there were 16 published reports of occupational n-hexane neuropathy in mainland China (Fujian, Guangdong, Helonjiang, Henan, Jiangsu, Liaoning) and, through 2009, 137 employees in a Suzhou electronic factory received hospital treatment for n-hexane neuropathy.33 Sixty patients were treated for n-hexane intoxication in Guangdong Province between January 2017 and January 2018.34 The main causes of chronic n-hexane poisoning (females > males) in an electronic enterprise of Guangdong’s Shenzhen City in 2017 were: long working hours, poor ventilation of workshops, unsatisfactory personal protective equipment, inadequate occupational health management, and an imperfect occupational health examination mechanism.35
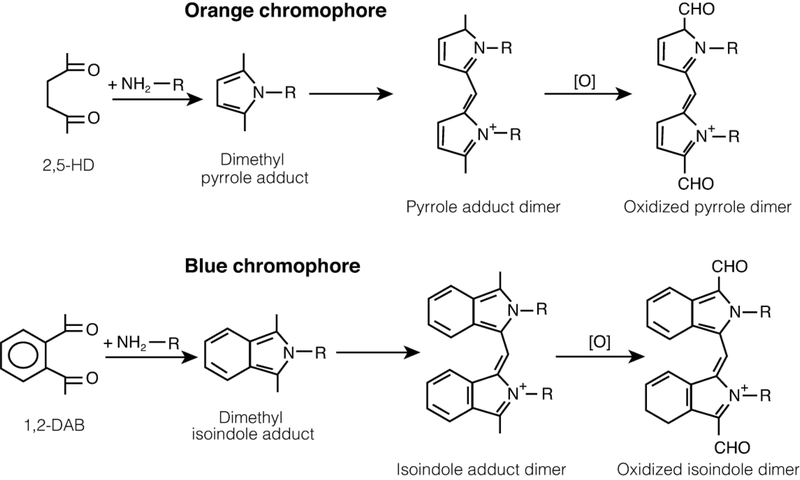
n-Hexane is metabolized by cytochrome P4502EI (CYP2E1) to compounds with progressively greater neurotoxic potency, including 2-hexanol, 2-hexanone and 2,5-hexanedione,36–38 a 1,4-diketone (γ-diketone) that binds directly with the ε-amino group of lysine to form a 2,5-dimethyl pyrrole adduct (Figure 1).39–41 Pyrrole formation is an absolute requirement for neurotoxicity resulting in peripheral neuropathy.42–44 Similarly, 1,2-diethylbenzene, a solvent used in ion exchange resins and in powderless etching, appears to be metabolized to 1,2-diacetylbenzene (1,2-DAB),45,46 which reacts with lysine to form a benzo-fused pyrrole or isoindole. The isoindole and pyrrole adducts form oxidized dimers and polymers that are seen as blue-violet and orange-colored pigments, respectively (Figure 1). Thus, rodents treated systemically with 1,2-DAB develop a bluish discoloration of skin, eyes and internal organs, including nerves, spinal cord and brain, which reflects the reaction between the γ-diketone and proteins throughout the body. Calculations based on density functional theory indicate the chromophore formed from the reaction of 1,2-DAB with proteins is likely to be composed of dimers of oxidized isoindoles.47 A yellow discoloration results from systemic treatment of rodents with the aliphatic γ-diketone, 2,5-hexanedione (2,5-HD), which produces the lowest number of adducts (rat) serum and the highest in liver, where it has potential toxicity. 41
Figure 1.
γ-Diketone reactivity with lysine. Reaction of 2,5-hexanedione (2,5-HD, upper) and 1,2-diacetylbenzene (1,2-DAB, lower) with lysine to form dimethyl-pyrrole and -isoindole adducts, respectively, which form dimers that oxidize and polymerize to cross-link targeted proteins. The colored pigments that result appear to be associated with the dimer formation. Antioxidants block 1,2-DAB-induced cytotoxicity of SHSY5Y cells.95
Whereas 2,5-HD and 1,2-DAB are protein-reactive, chromogenic and induce central-peripheral axonopathy, closely related isomers of the aromatic γ-diketone (e.g. 1,3-DAB) and the aliphatic γ-diketone (e.g. 2,4-HD) do not react with proteins to form chromogens and do not affect nerve fibers in the central and peripheral nervous system.48–52 It can therefore be inferred that protein (lysine) reactivity is directly related to γ-diketone neurotoxic potential, and chromogenicity is a biomarker of neurotoxic action. Unknown is whether the same molecular mechanism underlies the ability of 2,5-HD to inhibit proliferation of neuroprogenitor cells in primary neuronal cultures, disrupt murine hippocampal neurogenesis,53 and impair neurodevelopment of chick embryos.54
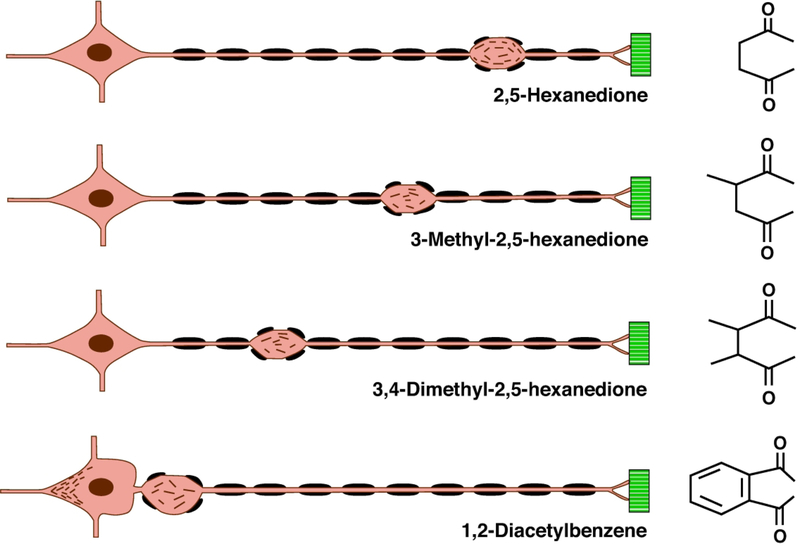
The neurotoxic potency of γ-diketones increases stepwise from 2,5-HD, 3-methyl-2,5-HD, 3,4-dimethyl-2,5-HD (DMHD) and 1,2-DAB (Figure 1), presumably because the molecular configuration stepwise increases access of the reactive 1,4-diketo group to the target ε-amino groups of protein lysines.49,55 Similarly, 1,2-diethylbenzene (but not 1,3-DEB) and 1,2,4-triethylbenzene (but not 1,3,5-TEB), the parent compounds of 1,2-DAB and 1,2,4-DAB, respectively, induce peripheral neuropathy in rodents.52 Other aromatic compounds, such as the polycyclic musk 6-acetyl-1,1,4,4-tetramethyl-7-ethyl-1,2,3,4-tetralin (AETT), formerly used as fragrance and food additive, yields a metabolite that is protein-reactive, forms a bluish chromogenic pigment, and induces nerve fiber damage in systemically treated rodents.56,57 By contrast, neither tetralin nor its α-tetralol metabolite form a chromogen in vitro or induce axonal degeneration in mice treated by repeated intraperitoneal injection with either compound.58 With the exception of tetralin, other aromatic hydrocarbons that have been reported in early studies to cause blue-green colored urine, have not been studied for chronic neurotoxic potential (Table 1).
Table 1.
Chromogenic organic solvents as reported by Gerarde126 from his experimental animal studies using a single subcutaneous injection of the listed substances of unknown purity in a dose of approximately 5 ml/kg. Gerarde reported that treatment of male and female rats and hamsters with 1,2-diethylbenzene (1,2-DEB) or 1,3-diethylbenzene (1,3-DEB), but not 1,4-diethylbenzene (1,4-DEB), caused the excretion of a blue dye in the urine. The sclera, blood, plasma and tissues were stained a deep blue that persisted for many days. Later work using purified compounds found that 1,2-DEB (95%) and DEB mixtures,127 but not 1,3-DEB (99%) or 1,4-DEB (96%) alone, were chromogenic in rats given repeated oral doses of 500 and 750 mg/kg/day, 5 days/week for 10 weeks.46,49 Humans may also excrete colored (green) urine following exposure to tetralin,127 and a blue discoloration of the skin and internal organs develops in rodents repeatedly treated with acetylethyltetramethyltetralin.56,57
| Monocyclic | Dicyclic |
|---|---|
| 1,2-Xylene | Indene |
| 1,2-Ethyltoluene | Tetralin |
| 1,2-Diethylbenzene | Diphenyl |
| 1,3-Diethylbenzene | Diphenylmethane |
| 1,2-Diisopropylbenzene | 1-Methylnaphthalene |
| Triethylbenzene (mixture) | 2-Methylnaphthalene |
| Diethyldisopropylbenzene | 1-Ethylnaphthalene |
| Indane | 2-Ethylnaphthalene |
The neuropathologic lesion induced by repeated systemic treatment with γ-diketones is one in which large-diameter and elongate nerve fibers in the central nervous system (CNS, mostly ascending and descending spinal cord tracts), as well as the peripheral nervous system (PNS), initially develop axonal changes.59 Small-diameter and unmyelinated fibers are less susceptible but also exhibit degenerative changes. Experimental studies with rodents show that n-hexane, 2-hexanone and 2,5-HD each induces degenerative changes in the distal regions of limb nerves and ascending and descending spinal cord tracts,21,59 with comparable changes in the optic nuclei and hypothalamus.60,61 Characteristic nerve fiber changes consist of focal axonal swellings filled with 10 nm neurofilaments that commonly develop on the proximal sides of nodes of Ranvier, thereby resulting in retraction of paranodal myelin followed by localized demyelination and remyelination and distal, retrograde (“dying-back”) axonal degeneration.21
Neurofilaments are synthesized in nerve cell bodies and transported distally (anterograde) along the axon at a rate of approximately 1 mm/day during which they would be continuously exposed to the blood-borne neurotoxic agent. The spatio-temporal evolution of these pathological changes has been observed directly in organotypic cultures composed of structurally and functionally coupled explants of spinal cord, spinal ganglia and striated muscle during continuous treatment (2.8 mM) with n-hexane or metabolites (2-hexanol, 2,5-hexanediol, 2-hexanone, 5-hydroxy-2-hexanone, 2,5-hexanedione) and 2,4-HD as a negative control.62 Comparable treatment with the most potent lysine-reacting aliphatic γ-diketone, DMHD,63 induced giant axonal swellings filled with neurofilaments in more proximal regions of peripheral nerve fibers in culture, followed later by distal, retrograde axonal degeneration.62
The neuropathological picture is modified by the rate of systemic intoxication (dose, frequency, duration) and the timing of examination. For example, axonal atrophy is prominent in peripheral nerves and ascending (gracile fasciculus and spinocerebellar tract) and descending (corticospinal and rubrospinal tracts) spinal tracts of rats treated by gavage with very high (up to 400 mg/kg/d) doses of 2,5-HD,64,65 probably because the chemical assault rapidly arrests the anterograde transport of neurofilaments, the density and subunit composition of which regulate axon diameter.66 This is particularly evident when animals are treated with 1,2-DAB, a compound with markedly greater neurotoxic potency than 2,5-HD.49,50 Animals treated with 1,2-DAB display massive proximal neurofilament swellings, some of which develop intraspinally just distal to the initial axonal segment of the motor neuron.49 When proximal axonal accumulation occurs in spinal roots, marked localized demyelination and remyelination results as a secondary response to axonal swelling and then attenuation, respectively. Indeed, the extent of demyelination and remyelination was so extensive in the spinal roots of AETT-treated animals that it was erroneously interpreted as a primary demyelinating disease.51
The molecular basis of these compound-dependent patterns of γ-diketone-induced nerve fiber pathology appears to lie in their differential neurotoxic potency and, by extension, degree of protein adduction and polymerization. Highly potent neurotoxic γ-diketones, such as 1,2-DAB and DMHD, rapidly degrade one or more of the three proteins (NF-heavy, NF-M, NF-light) that make up the formed neurofilament (NF) and/or the transport system on which anterograde neurofilament transport depends. By contrast, lower-potency γ-diketones, such as 2,5-HD, appears to complex with and degrade axonal proteins more slowly, such that the neurofilaments are able to move anterograde along the axon until further transport is impeded in distal regions of the nerve fiber where they accumulate proximal to nodes of Ranvier. The rapid segregation of axonal microtubules from neurofilaments induced by intrafascicular injection of 2,5-HD into the sciatic nerve in vivo may perturb the transport of neurofilaments,67 which then become disorganized and later accumulate in focal axonal swellings. The proposed relationship between the chemical structure of γ-diketones, relative potency, and resulting location of focal axonal swellings with amassed 10nm neurofilaments, is shown in Figure 2.
Figure 2.
Nerve fiber response to γ-diketones. Diagram of the location of initial focal axon (orange) swellings and associated secondary myelin (black) retraction in an elongate, large-diameter peripheral nerve fiber originating from the neuron soma (left) and terminating at sites of muscle (green) innervation. γ-Diketone potency and associated neuroprotein reactivity increase from 2,5-hexanedione (2,5-HD, upper) to 1,2-diacetylbenzene (1,2-DAB, lower), where neurofilaments accumulate both in the neuronal soma, distal to the initial segment, and in proximal spinal roots (as shown). The differential spatial “rigidity” (ability to rotate in space) of the 1,4-diketo groups may dictate the protein reactivity and, hence, neurotoxic potency of the illustrated γ-diketones. This diagram illustrates the principle that the toxic potential of the γ -diketone dictates how far neurofilaments are transported from the neuron cell body along the nerve fiber before they accumulate distally in focal axonal swellings; the greatest transport distance representing the least potent (2,5-HD) and, hence, the slowest developing effect. The most potent γ -diketone, 1,2-DAB, rapidly reacts with axonal proteins, such that anterograde transport of neurofilaments is arrested before or shortly after their departure from the neuron cell body. This figure is constructed from the author’s examination of single teased nerve fibers, and light and electron micrographs of cross-sectioned nerves, from peripheral nerve fibers undergoing γ -diketone axonopathy [2,51,52,59,60,62,108,113,114].
Assessment in vitro of the chromogenic reaction of amino acids with DAB isomers demonstrates that 1,3-DAB fails to react with lysine, ornithine, glycine, γ-aminobutyric acid (GABA) or L-cysteine. By contrast, with the notable exception of cysteine, 1,2-DAB reacts rapidly and progressively with these compounds, most notably with L-lysine (Table 2). Since γ-diketones are particularly reactive in vitro with the exposed ε-amino group of lysine, the total lysine content of axonal proteins may be important in regard to their susceptibility to γ-diketone-induced adduct formation and subsequent cross-linkage of proteins, including neurofilament and microtubule proteins.68–70 With regard to 1,2-DAB, the total lysine content of axonal proteins seems to correlate with their susceptibility in vitro, those rich in lysine (NF-H, NF-M, kinesin, dynein, tau) showing greater vulnerability relative to those with low lysine content (NF-L, β-tubulin)49 (Table 3). Kinesin and dynein are motor proteins responsible for anterograde and retrograde axonal transport of cargoes, respectively, and the microtubule-associated protein tau appears to spatially regulate the balance of microtubule-dependent axonal transport in neurons.71 Rat spinal cord slices incubated with 1,2-DAB showed a concentration-dependent decrease of motor and cytoskeletal proteins not seen with 1,3-DAB. While dynein and tau appeared similarly affected by 1,2-DAB, kinesin was most impacted by the neurotoxicant. Repeated systemic treatment of rats with 1,2-DAB, but not 1,3-DAB, affected motor and cytoskeletal proteins of sciatic nerves to a greater degree than those in the spinal cord. Motor proteins that drive axonal transport anterogradely (kinesin) and retrogradely (dynein), cytoskeletal protein NF-M, which is slowly transported in the anterograde direction, and microtubule-associated protein, tau, were differentially impacted by 1,2-DAB.72
Table 2.
Chromogenic reaction of selected amino acids with neurotoxic (1,2-DAB) and non-neurotoxic (1,3-DAB) isomers of diacetylbenzene. Values represent mean absorbance ± standard deviation of the purple/blue color read at 540 nm on a scanning spectrophotometer. In triplicate, 0.5 ml aliquots of amino acid solution (0.1M phosphate buffer, pH 7.4) were incubated with 0.5 ml of 1,2-DAB or 1,3-DAB (1 mM) at 37°C for 30 or 60 minutes. L-Lysine, glycine and γ-aminobutyric acid and ornithine, a non-protein amino acid and higher homologue of L-lysine, were the most reactive of 22 tested amino acids. L-Cysteine was among the least reactive of the amino acids tested. Modified from Kim et al.48
| Diketone | 1,2-Diacetylbenzene (1,2-DAB) | 1,3-DAB | |
|---|---|---|---|
| Amino Acid | 30 min | 60 min | 60 min |
| L-Lysine | 3.22 ± 0.02 | 3.51 ± 0.02 | 0.00 |
| Ornithine | 2.80 + 0.01 | 3.56 ± 0.01 | 0.00 |
| Glycine | 2.21 + 0.02 | 3.32 + 0.04 | 0.00 |
| GABA | 1.93 + 0.03 | 3.02 + 0.02 | 0.00 |
| L-Cysteine | 0.00 | 0.02 | 0.00 |
Table 3.
Total lysine content of axonal proteins. Protein reactivity of 1,2-DAB and formation of high molecular weight adducts correlated with lysine content. Both in vivo and in vitro, rat neurofilament (NF) heavy chain (NF-H) and NF-M formed polymers more readily than either NF-L or tubulin. Other 1,2-DAB protein targets involved in anterograde axonal transport included rat microtubule-associated tau, the rat motor proteins kinesin and dynein, and the rabbit glycolytic enzymes glyceraldehyde phosphate dehydrogenase (GAPDH: 8% lysine) and lactate dehydrogenase (LDH: 7–8% lysine). Stathmin, which contains a very high lysine content, was not tested. Data from Kim et al.48
| Axonal Protein | Percentage Lysine Content |
|---|---|
| (Stathmin | 15.5) |
| Neurofilament Heavy Chain (NF-H) | 13–17 |
| Neurofilament Medium Chain (NF-M) | 11–15 |
| Neurofilament Light Chain (NF-L) | 6–7 |
| Beta-Tubulin | 3–4 |
| Kinesin | 8–12 |
| Dynein | 8 |
| Tau | 7–8 |
2. γ-Diketone-induced changes in brain gene expression
Toxicogenomic studies have determined changes in brain gene expression following single intraperitoneal treatment of male C57BL/6 mice with 1,2-DAB (50 mg/kg) vs. 1,3-DAB (50 mg/kg) or Vehicle (2% acetone in saline). One-hour post-treatment, significant (i.e. <1.5 fold, q<0.1) 1,2-DAB-upregulated genes included: transcription factors/regulator (Zic1, Fus, Junb, Klf4, Mapk8ip); those involved in cell cycle progression/proliferation (Rif1 homologue, Junb, Btg2, Mapk8ip); transmitter-related function (Gabt4, BQ746452 [similar to Gabbr1], Cplx2); protein degradation (Ube2s, AK034214 [similar to Dirc2]); lipid metabolism (BB32327894 [similar to Gpam]), and microtubule binding (Eml2, which negatively regulates microtubule polymerization, and AK038316 (similar to Dock9, a member of a family of genes coding for proteins that regulate the actin cytoskeleton). At six hours post-treatment, 1,2-DAB-upregulated genes included: transcription factors/regulator (Dsip1, Mafk), those involved in cell cycle progression/proliferation (Pmaip1, Nfkbia, Igfbp1, Dsipl, Mafk, Cdkn1a [P21]), apoptosis regulating (Pmaip1, Angpt14, Dsip1, Cdkn1a (P21)), lipid metabolism (Tts-2.2 homologue), inhibitor of protein degradation (Ct1a2b) and neuronal antioxidant (Mt2). These findings demonstrate a complex and temporally evolving pattern of brain transcriptional perturbation in response to systemic diketone treatment.
There is sparse information on changes in gene expression associated with longer-term treatment with n-hexane and 2,5-HD, its protein-binding neurotoxic metabolite. Neurofilament subunit gene expression was modestly reduced (approximately 20%) in the dorsal root ganglia of rats with 2,5-HD neuropathy.73 Chronic treatment of rats with 2,5-HD reportedly increased cellular apoptosis in the spinal cord of rats by downregulating Nerve Growth Factor expression and subsequently repressing the PI3K/Akt signaling pathway.74 However, long-term systemic treatment of rats with 2,5-HD reportedly increased the percentage of proliferating neural stem and progenitor cells in the granule cell layer of the hippocampus and the subgranular zone.75 Neuropathological changes were found in the mammillary body and visual nuclei of cats treated with low levels of 2,5-HD for prolonged periods. 60
n-Hexane inhalation altered the methylation status of promoters of genes associated with ovarian cell apoptosis and steroid hormone biosynthesis,76 and treatment with 2,5-HD increased apoptosis in human ovarian granulosa cells.77,78
3. γ-Diketone-induced changes in neuroprotein abundance
3.1. Early CNS protein changes
The brain proteome was examined in mice recovering from a single intraperitoneal injection of 1,2-DAB, 1,3-DAB or DAB vehicle (Table 4). Marked 1,2-DAB-associated changes were noted in the relative abundance of brain proteins involved in microtubule interaction, other structural proteins and cell energy metabolism. The greatest change was seen for actin α−2. Association of actin filaments with microtubules is important for various cellular processes, such as cell division, migration, vesicle and organelle transport, and axonal growth.79 Actin α−2 has at least 10 lysine-acetylation sites, and lysine-acetylated actin can interact with cyclase-associated protein to form an inhibitor of inverted formin 2 (INF2). Significantly, dominant missense mutations in INF2 are linked to Charcot–Marie–Tooth disease, an inherited human axonal neuropathy. 80,81
Table 4.
DAB Neuroproteome Relative abundance of brain proteins of young adult C57/BL6 mice 168 hours after a single intraperitoneal injection of 1,2-DAB (50 mg/kg body weight), 1,3-DAB (50 mg/kg), or DAB Vehicle (2% acetone in saline). Half brains were pooled from two animals from each group. Each sample was homogenized in 1 ml 100 mM ammonium bicarbonate, 5 mM dithiothreitol, pH 7.8, centrifuged at 80K for 1h in a TL-100 rotor, the supernatant collected and assayed for protein at a 1:100 dilution. 100 mcg of each sample was digested with 1:50 trypsin to protein (w/w), for 1h at 37oC (20% ACN). Peptide clean-up was performed on a SP-C18 column and eluted in 80% acetonitrile (CAN), 0.1% acetic acid, and 0.01% trifluoroacetic acid. Each sample was run in duplicate on a 9-tesla Fourier-transform ion cyclotron resonance-mass spectrometer. The mouse brain voxel PMT database was used for peak matching of proteins of differing abundance. A total of 1643 redundant proteins was identified, of which 548 proteins were modulated by 1,2-DAB, with 196 mapped to cell networks. Performed in collaboration with J. Pounds and J. Adkins, Pacific Northwest National Laboratory, U.S. Department of Energy Richland, Washington.
| PROTEIN ABUNDANCE | Change in Abundance | Relative Abundance | |||
|---|---|---|---|---|---|
| PROTEIN NAME/FUNCTION | 1,2-DAB/1,3-DAB | 1,2-DAB/Vehicle | 1,2-DAB | 1,3-DAB | Vehicle |
| MICROTUBULE-INTERACTING | |||||
| Stathmin | 6.98 | 8.85 | 108.54 | 15.55 | 12.27 |
| oral-facial-digital syndrome 1 | 4.68 | ∞ | 46.46 | 9.96 | 0.00 |
| restin (clip-170, clip-50) | 3.38 | 5.71 | 8.62 | 2.55 | 1.51 |
| microtubule-associated protein 2 | 5.47 | 3.79 | 3.94 | 0.72 | 1.04 |
| Synuclein (alpha + beta) | 4.12 | 2.09 | 2.72 | 0.66 | 1.30 |
| OTHER STRUCTURAL PROTEINS | |||||
| neurofilament medium (NF-M) | 4.09 | 7.37 | 2.21 | 0.54 | 0.30 |
| neurofilament light (NF-L) | 1.64 | 1.24 | 0.46 | 0.28 | 0.37 |
| actin alpha 1 | 2.30 | 1.60 | 18.77 | 8.15 | 11.73 |
| actin alpha 2 | 14.85 | 50.77 | 15.74 | 1.06 | 0.31 |
| procollagen type V1 alpha 2 | 3.29 | 5.38 | 5.16 | 1.57 | 0.96 |
| Peripherin | 3.84 | 2.32 | 1.23 | 0.32 | 0.53 |
| CELLULAR ENERGY METABOLISM | |||||
| malate dehydrogenase, soluble | 23.59 | 75.19 | 24.06 | 1.02 | 0.32 |
| triosephosphate isomerase | 2.20 | 3.97 | 18.10 | 8.22 | 4.56 |
| enolase-2 (neuronal) | 1.73 | ∞ | 14.92 | 8.64 | 0.00 |
| NADH dehydrogenase Fe-S protein 3 | 0.31 | 0.45 | 10.93 | 35.83 | 24.13 |
| phosphoglycerate kinase 2 | 5.34 | 10.69 | 7.48 | 1.40 | 0.70 |
| ATP synthase beta-chain | 3.17 | 5.07 | 0.76 | 0.24 | 0.15 |
| VESICULAR TRACKING | |||||
| synapsin 1 | ∞ | 2.65 | 11.46 | 0.00 | 4.33 |
| kinesin family member 20A | 38.13 | ∞ | 6.10 | 0.16 | 0.00 |
It seems reasonable to posit that 1,2-DAB attacks actin’s lysine sites, thereby blocking formation of INF2 and contributing to the genesis of γ-diketone axonopathy. There was also a markedly increased abundance in 1,2-DAB vs.1,3-DAB or Vehicle of CLIP-1 (CAP-GLY domain containing linker protein), a microtubule plus-end-associated protein that interacts with dynein and binds tightly to formins to accelerate actin filament elongation.82,83 1,2-DAB vs. 1,3-DAB also showed some increased abundance of microtubule-associated protein 2 and α-synuclein, which facilitates formation of short, mobile transportable microtubules that play an important role in axonal transport.84 These changes in protein expression should be confirmed with additional proteomic studies.
Brains of mice one week after a single 1,2-DAB treatment showed increased abundance of stathmin (Table 4) and proteins involved in stathmin regulation: cyclin-dependent kinase 5 (cdk5), RAS-related C3 botulinum substrate 1 (rac1), heat shock protein conjugate 70 (hsv70), and protein phosphatase 2a (pp2a). These brains also showed changes in stathmin-regulated genes including: cyclin-dependent kinase inhibitor 1a (Cdkn1a, P21), epidermal growth factor (Egf), and Krüppel-like factor 4 (Klf4). Stathmin is a ubiquitous, brain-enriched phosphoprotein with a very high lysine content that binds to tubulin and inhibits microtubule assembly, an activity regulated by phosphorylation.85.86 Stathmin is of special interest in relation to γ-diketone axonopathy because aged stathmin knock-out mice also develop axonal degeneration and secondary demyelination. 87 Additionally, incubation of mouse brain and testes homogenates with 1,2-DAB (1–10 mM) reduced the intensity of the native stathmin band in a concentration-dependent manner, with corresponding increases in high-molecular-weight protein adducts. By contrast, under similar conditions,1,3-DAB had no effect on stathmin, and adducts were not observed. Stathmin is therefore of potential central mechanistic relevance to γ-diketone axonopathy.88
3.2. Later CNS Protein Changes
Neuroprotein studies have been performed on the lumbo-sacral spinal cord of rats with severe γ-diketone neuropathy. 1,2-DAB significantly altered the expression of protein disulfide isomerase, an enzyme involved in protein folding, and gelsolin, an actin-capping and -severing protein. Thirty-four proteins were markedly modified by 2,5-HD, of which NF-L, gelsolin, protein disulfide isomerase, glutathione S-transferase, nicotinamide adenine dinucleotide (reduced) dehydrogenase 1α, pyruvate kinase, and fatty acid synthase were also modified by 1,2-DAB. 2,5-HD induced a 1,2-DAB–like proteomic signature by altering the expression levels of proteins involved in maintaining the physical integrity of axons (reduced), in controlling redox and protein-folding mechanisms (reduced), and in supporting energy metabolism (increased).50 While the spinal cord proteome also suggested a reduction of α-II spectrin (Spna2), a key protein in the maintenance of axonal integrity, degradation of Spna2 by calpain- and/or caspase is reportedly not central to the pathogenesis of 1,2-DAB axonopathy.89
Perturbation of energy metabolism resulting in reduced ATP has been postulated to be linked to the etiology of central-peripheral axonopathy.90 2,5-HD reduced the rate of ATP synthesis in isolated brain mitochondria,91 and pyruvate restored ATP deficits in cat nerves treated with 2,5-HD.92 Consistent with this hypothesis, recovery from systemic treatment with 1,2-DAB was associated with a very marked increase in brain abundance of soluble malate dehydrogenase and, to a lesser extent, other proteins involved in energy metabolism, including glycolysis (triosephosphate isomerase, phosphoglycerate kinase 2, enolase 2) and the electron transport chain (NADH dehydrogenase Fe-S protein 3; ATP synthase beta-chain) (Table 4). Oxidative stress and resulting tissue damage have been attributed to γ-diketone toxicity of liver, kidney, brain and SY5Y neuroblastoma cells.93–95
4. Normal aging and diabetes mellitus
Are neurotoxic γ-diketones physiological metabolites and might they participate in other types of peripheral neuropathy, including those associated with the aging process and diabetes mellitus? One study of 31 normal subjects with no known n-hexane exposure found low levels (<0.006 mg/L) of free 2,5-HD in urine.96 A second reported that healthy subjects without occupational exposure to n-hexane had detectable levels of 2,5-HD in blood (6–30 microgram/L) and urine (0.17 and 0.98 mg/L), only a minimal part of which was considered to have derived from exposure to hydrocarbon-polluted air.97 However, a third study found that 1.3% of 1200 normal subjects with no known occupational exposure to n-hexane had blood levels of the neurotoxic alkane above the method detection limit.98 A fourth investigation of urine samples from 123 healthy Italian subjects recorded a 2,5-HD reference value of 0.795 mg/L for men and 0.627 mg/L for women.99 A fifth very large study of healthy Chinese subjects (n= 8235) with no occupational exposure to n-hexane or 2-hexanone showed a median urine 2,5-HD concentration of 0.171 mg/L for males and 0.147 mg/L for females, with increasing 2,5-HD excretion with the advance of age.100 In a sixth study, investigation of 208 male and female subjects revealed a median level of urinary pyrrole adducts of unstated origin of 0.91 nmol/ml.101 Finally, with respect to diabetes mellitus, a small study of serum samples from normal individuals, and from subjects with type-2 diabetic neuropathy, revealed similar qualitative profiles of volatile metabolites (low nanogram concentration), including relatively high and low concentrations respectively of 2-hexanone and 3-heptanone, both of which can undergo ω-oxidation to form γ-diketones.102 Also present in similar concentrations in normal and diabetic sera was 2-butanone (methyl ethyl ketone), a compound that potentiates the neurotoxic potency of n-hexane and 2-hexanone.103–106 Since degenerative nerve fiber changes with the advance of aging resemble those seen in the early stages of distal axonopathies,107 and central-peripheral axonopathy underpins type-2 diabetic neuropathy,108 it will be important to determine the origin of γ-diketones and precursors with neurotoxic potential in normal subjects and whether they contribute to nerve fiber changes in aged subjects and those with diabetic neuropathy.
5. Conclusion
Structure-activity studies51,109 show that γ-diketone metabolites of certain aliphatic and aromatic solvents selectively react with proteins throughout the body to form chromogenic polymers. Despite this global assault and widespread formation of protein adducts (in serum, liver, kidney, brain),110 other than the nervous system, few tissues (testicle, liver, kidney) undergo degenerative/atrophic changes in γ-diketone-treated animals.93,111 The special susceptibility of CNS and PNS neurons to γ-diketones appears to be related to their requirement for transport of proteins over very long distances (i.e. from cell soma via the axon to the nerve terminal). Although protected by blood-brain/nerve barriers, γ-diketones not only cross the regulatory interfaces but can also increase their permeability.112 Elongate, large-diameter nerve fibers are affected first, probably because of the large volume of neurofilament and other proteins undergoing anterograde transport. However, increasing the γ-diketone potency and dosage results in more rapid protein adduction, such that neurofilaments are arrested shortly after commencing their transport from sites of synthesis in neuronal somata. This results in the formation of focal giant axonal swellings containing masses of disoriented neurofilaments, while more distal regions of axons undergo atrophy because they fail to receive a normal complement of neurofilaments. Distal, retrograde axonal degeneration then ensues by a proposed mechanism in which trapped mitochondria release stored calcium and trigger calcium-activated proteases that effectively sever the axon distal to the site of neurofilament accumulation.113
The exact targets of γ-diketones are not clear. The structure and function of microtubules are unequivocally impacted by γ-diketones, either directly or indirectly, or both. In vitro, 2,5-HD-treated tubulin had a decreased lag time and an increased maximal velocity of microtubule assembly.111 Systemic 2,5-HD treatment causes longitudinal aggregation of axonal microtubules, sometimes in isolated clusters and sometimes decorating the perimeter of mitochondria, an event that occurs within minutes of intraneural γ-diketone injection67 and followed by the development of neurofilament-laden axonal swellings days later.114 This seems to be associated with loss of the 3- to 5-nm thick cross bridges formed by MAP 2 or tau in axonal microtubules115 or from the carboxy-terminal tail domains of phosphorylated NF-H and NF-M that laterally interconnect intermediate neurofilaments.116–118 NF-M (and NF-H?) contains a variable lysine–serine–proline (KSP) repeat sub-domain flanked by two highly conserved sub-domains, the number of tri-tetra- and pentapeptide KSP repeats (KSP, KSD, KXSP, KXXSP) dispersed along the sub-domain increasing in proportion to axon length and caliber.119 Given the high affinity of γ-diketones for lysine residues, their reactivity with high-lysine-content NF-H and NF-M, the exceptional vulnerability of long and large-diameter axons in systemically treated rodents, and the earlier and greater involvement of lower relative to upper extremities in γ-diketone neuropathy, suggests the neurofilament sub-units NF-H and NF-M are intimately involved in the pathogenesis of γ-diketone axonopathy. Additionally, NF-H knock-out mice are refractory to the γ-diketone-like giant neurofilament swellings induced by systemic treatment with β, β’-iminodipropionitrile, which suggests that NF-H is a key mediator of this type of axonopathy.120
Normal anterograde axonal transport of neurofilament subunits appears to be dependent on myosin-mediated interactions within the actin cortex that lines the inner axolemmal surface.121 Actin is also involved in microtubule-dependent vimentin transport.122 Actin contains 19 lysines of variable reactivity, of which K325 and K327 are most reactive with acetic anhydride.123 Given the very large increased production of brain actin following systemic treatment with 1,2-DAB, actin lysines are also likely to be important γ-diketone targets. Stathmin, which binds to and sequesters monomeric tubulin, thereby preventing microtubule polymerization, has a very high lysine content and is thus a prominent target for γ-diketones, a position supported by the marked increase in brain abundance of stathmin after systemic treatment with 1,2-DAB. Serine 63, a key phosphorylation site for stathmin-induced destabilization of microtubules, is next to lysine 62,86,124 which might be an important site for γ-diketone attack. Stathmin binds directly along the microtubule wall, such that a pool of this protein is immediately available to participate in protofilament dissociation when the moving plus end of a depolymerizing microtubule reaches stathmin molecules.125 Other axonal proteins with significant lysine components are the motor proteins dynein and kinesin, although it is noteworthy that increased brain abundance of these neuroproteins was not apparent after systemic treatment with 1,2-DAB.
The foregoing description of n-hexane neuropathy represents one of the most detailed analyses of the molecular mechanisms underlying a central-peripheral distal axonopathy, a pattern of nervous system damage seen in a number of genetic, metabolic, nutritional and toxic neuropathies. The molecular mechanisms involved in the neurotoxic actions of γ-diketones may therefore have relevance in comparable neuropathologic states, such as those associated with aging and diabetes mellitus.
Acknowledgments
The experimental studies reviewed here were variously performed by students, technicians, post-doctoral fellows and faculty colleagues at Albert Einstein College of Medicine, Oregon Health & Science University, Oregon State University, Pacific Northwest National Laboratory, and University of Houston, including in surname alphabetical order: Joshua Adkins (proteomics), Monica Bischoff (animal studies), Alan Cranson (toxicogenomics), Max Deinzer (mass spectrometry), David Dixon (computation), Dickran Houropian (neuropathology), Seyed Hashemii (protein studies), Robert Kayton (electron microscopy) Min-Sun Kim (neuropathology), Michael Lasarev (biostatistics), Valerie Palmer (animal studies and toxicogenomics), the late Edith Peterson (tissue culture), Michael Politis (animal studies), Colin Poole (chromatography), Joel Pounds (proteomics), Mohammad Sabri (protein studies), Herbert Schaumburg (neuropathology), Mary Seelig (tissue culture), Dean Sproles (toxicogenomics), Arnold Sterman (neuropathology), Sarah Trimpkin (mass spectrometry), Desire Tshala-Katumbay (neuropathology and protein studies), Bellina Veronesi (tissue culture), Joy Zagoren (animal studies), Chang-Guo Zhan (computation studies), and the late Albert Zlatkis (chromatography). I thank Chen Xiao for discussion. Dr. Ann Hubbs kindly reviewed the manuscript.
The toxicogenomic and proteomic studies reported here for the first time were supported by grant U19ES011384 from the National Institute of Environmental Health Sciences.
Footnotes
Declaration of Conflicting Interests Statement
The author(s) declared no potential, real, or perceived conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Spencer PS, Schaumburg HH. Sabri MI, Veronesi B The enlarging view of hexacarbon neurotoxicity. Crit Rev Toxicol. 1980;7:279–356. [DOI] [PubMed] [Google Scholar]
- 2.Spencer PS, Schaumburg H. Ultrastructural studies of the dying-back process. IV. Differential vulnerability of PNS and CNS fibers in experimental central-peripheral distal axonopathies. J Neuropathol Exp Neurol. 1977b;36:300–320. [DOI] [PubMed] [Google Scholar]
- 3.Herpin G, Gargouri I, Gauchard GC, et al. Effect of chronic and subchronic organic solvents exposure on balance control of workers in plant manufacturing adhesive materials. Neurotox Res. 200915:179–186. doi: 10.1007/s12640-009-9018-0 [DOI] [PubMed] [Google Scholar]
- 4.Pradhan S, Tandon R. N-hexane neuropathy with vertigo and cold allodynia in a silk screen printer: A case study. Int J Occup Med Environ Hlth. 2015;28:915–919. doi: 10.13075/ijomeh.1896.00327 [DOI] [PubMed] [Google Scholar]
- 5.Chang YC. Patients with n-hexane induced polyneuropathy: a clinical follow up. Br J Ind Med. 1990;47:485–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beckman S, Eisen EA, Bates MN, et al. Acquired color vision defects and hexane exposure: A study of San Francisco Bay Area automotive mechanics. Am J Epidemiol. 2016;183:969–976. doi: 10.1093/aje/kwv328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sobue I, Yamamura Y, Ando K, Iida M, Takayanagi T. n-Hexane polyneuropathy. Outbreak among vinyl sandal manufacturers. Clin Neurol. (Tokyo). 1968;8:393–403. [Google Scholar]
- 8.Chang CM, Yu CW, Fong KY, et al. N-Hexane neuropathy in offset printers. J Neurol Neurosurg Psychiat. 1993;56:538–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu IT, Lee NL, Zhang XH, Chen WQ, Lam YT, Wong TW. Occupational exposure to mixtures of organic solvents increases the risk of neurological symptoms among printing workers in Hong Kong. J Occup Env Med. 2004;46:323–330. [DOI] [PubMed] [Google Scholar]
- 10.Goto I, Matsumura M, Inoue N, Murai Y, Shida K. Toxic polyneuropathy due to glue sniffing. J Neurol Neurosurg Psychiat. 1974;37:848–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korobkin R, Asbury AK, Sumner AJ, Nielsen SL. Glue-sniffing neuropathy. Arch Neurol. 1975;32:158–162. [DOI] [PubMed] [Google Scholar]
- 12.Altenkirch H, Mager J. [Toxic polyneuropathy after sniffing contact glue thinner]. Dtsch Med Wochenschr. 1976;101:95–99. [German] [DOI] [PubMed] [Google Scholar]
- 13.Smith AG, Alpers JW. N-Hexane neuropathy due to rubber cement sniffing. Muscle Nerve. 1997;20:1445–1450. [DOI] [PubMed] [Google Scholar]
- 14.Becker CE, Lee DE, Troost BT. Glue sniffing polyneuropathy: an under recognized aspect of a public health hazard. J Adolesc Hlth. 2004;34:94–96. [DOI] [PubMed] [Google Scholar]
- 15.Huang CC. Polyneuropathy induced by n-hexane intoxication in Taiwan. Acta Neurol Taiwan 2008;17:3–10. [PubMed] [Google Scholar]
- 16.Yamamura Y. n-Hexane polyneuropathy. Folia Psychiat Neurol Jpn. 1969);23:45–57. [DOI] [PubMed] [Google Scholar]
- 17.Herskowitz A, Ishii N, Schaumburg H. N-hexane neuropathy. A syndrome occurring as a result of industrial exposure. N Engl J Med. 1971;285:82–85. [DOI] [PubMed] [Google Scholar]
- 18.Abbritti G, Siracusa A, Cianchetti C, et al. Shoe-makers’ polyneuropathy in Italy: the aetiological problem. Br J Ind Med. 1976;33:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cianchetti C, Abbritti G, Perticoni G, Siracusa A, Curradi F. Toxic polyneuropathy of shoe-industry workers. A study of 122 cases. J Neurol Neurosurg Psychiat. 1976;39:1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paulson GW, Waylonis GW. Polyneuropathy due to n-hexane. Arch Intern Med. 1976;136:880–882. [PubMed] [Google Scholar]
- 21.Buiatti E, Cecchini S, Ronchi O, Dolara P, Bulgarelli G. Relationship between clinical and electromyographic findings and exposure to solvents, in shoe and leather workers. Br J Ind Med. 1978;35:168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang CC, Chu NS, Shih TS, Wu TN. Occupational neurotoxic diseases in Taiwan: a review of the outbreaks and clinical features. Changgeng Yi Xue Za Zhi. 1997;20:71–78. [PubMed] [Google Scholar]
- 23.Harrison R, Larabee P, Con J, et al. n-Hexane--related peripheral neuropathy among automotive technicians -- California, 1999—2000. Mort Morb Wkly Rep. 2001;50:1011–1013. [PubMed] [Google Scholar]
- 24.Kutlu G, Gomceli YB, Sonmez T, Inan LE. Peripheral neuropathy and visual evoked potential changes in workers exposed to n-hexane. J Clin Neurosci. 2009;16:296–299. [DOI] [PubMed] [Google Scholar]
- 25.Puri V, Chaudry N, Tatk M. N-hexane neuropathy in screen printers. Electromyogr Clin Neurophysiol. 2007;47:145–152. [PubMed] [Google Scholar]
- 26.Misra UK, Kalita J. Toxic neuropathies. Neurol India. 2009;57:697–705. [DOI] [PubMed] [Google Scholar]
- 27.Kim EA, Kang SK. Occupational neurological disorders in Korea. J Korean Med Sci. 2010;25 (Suppl): S26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeuchi Y. n-Hexane polyneuropathy in Japan: a review of n-hexane poisoning and its preventive measures. Environ Res. 1993;62:76–80. [DOI] [PubMed] [Google Scholar]
- 29.Zhang JB, Zhang QY, Wang Y, Sun DY. [Pathology and neurophysiology analysis for peripheral neuropathy of four patients with chemicals poisoning]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2012;30:126–129; discussion 129–30. [Chinese]. [PubMed] [Google Scholar]
- 30.Zhang J, Li Z, Wang J, et al. [Misdiagnosis of occupational chronic n-hexane poisoning: an analysis of 16 cases]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2014;32:930–931. [Chinese] [PubMed] [Google Scholar]
- 31.Pan JH, Peng CY, Lo CT, Dai CY, Wang CL, Chuang HY. n-Hexane intoxication in a Chinese medicine pharmaceutical plant: a case report. J Med Case Rep. 2017;11:120. doi: 10.1186/s13256-017-1280-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang C, Chen S, Wang Z. Electrophysiological follow-up of patients with chronic peripheral neuropathy induced by occupational intoxication with n-hexane. Cell Biochem Biophys. 2014;70:579–585. doi: 10.1007/s12013-014-9959-7 [DOI] [PubMed] [Google Scholar]
- 33.Wade S. Apple releases new supplier responsibility report. 2001. https://www.businesshumanrights.org/en/apple-releases-supply-chain-report-details-findings-on-child-labourworker-illness-from-n-hexane-remedial-steps-pledges-greater-cooperation-with-chinese-ngos See also: https://www.pbs.org/newshour/show/in-china-workers-detail-health-effects-ofproducing-i-phones
- 34.Cheng S, Shunchun M, Wang Y. Effect of integrated Chinese and western medicine in the treatment of peripheral neuropathy caused by n-hexane poisoning. Clin J Chin Med. 2018;24 ISSN:1674–7860 [Chinese] http://new.oversea.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFDLAST2018&filename=ZYLY201824014&v=MDAwNDFyQ1VSTE9mWXVSbUZ5amdVYjNJUHpUSGQ3RzRIOW5PcTQ5RVlJUjhlWDFMdXhZUzdEaDFUM3FUcldNMUY= [Accessed 6.2.19] [Google Scholar]
- 35.Investigation of a chronic n-hexane poisoning accident in an electronic enterprise of Shenzhen City in 2017. Occup Health. 2019; 5. doi: 10.13329/j.cnki.zyyjk.2019.0187 [Chinese] http://new.oversea.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFDLAST2019&filename=ZYJK201905029&v=MDAwNDFyQ1VSTE9mWXVSbUZ5amdWcjdBUHpUQlpiRzRIOWpNcW85SGJZUjhlWDFMdXhZUzdEaDFUM3FUcldNMUY= [Accessed 6.2.19] [DOI] [Google Scholar]
- 36.Krasavage WJ, O’Donoghue JL, DiVincenzo GD, Terhaar CJ. The relative neurotoxicity of methyl-n-butyl ketone, n-hexane and their metabolites. Toxicol Appl Pharmacol. 1980; 52:433–441. [DOI] [PubMed] [Google Scholar]
- 37.Couri D, Milks M. Toxicity and metabolism of the neurotoxic hexacarbons n-hexane, 2-hexanone, and 2,5-hexanedione. Annu Rev Pharmacol Toxicol. 1982;22:145–166. [DOI] [PubMed] [Google Scholar]
- 38.Iba MM, Fung J, Gonzalez FJ. Functional Cyp2e1 is required for substantial in vivo formation of 2,5-hexanedione from n-hexane in the mouse. Arch Toxicol. 2000;74:582–586. [DOI] [PubMed] [Google Scholar]
- 39.Genter St Clair MB, Amarnath V, Moody MA, Anthony DC Anderson CW, Graham DG. Pyrrole oxidation and protein cross-linking as necessary steps in the development of γ-diketone neuropathy. Chem Res Toxicol. 1988;1:179–185. [DOI] [PubMed] [Google Scholar]
- 40.DeCaprio AP, Kinney EA, LoPachin RM. Comparative covalent protein binding of 2,5-hexanedione and 3-acetyl-2,5-hexanedione in the rat. Toxicol Environ Health A 2009;72:861–869. [DOI] [PubMed] [Google Scholar]
- 41.Yin H, Guo Y, Zeng T, Zhao X, Xie K. Correlation between levels of 2,5-hexanedione and pyrrole adducts in tissues of rats exposure to n-hexane for 5-days. PLoS One. 2013;8(9):e76011. doi: 10.1371/journal.pone.0076011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sayre LM, Shearson CM, Wongmongkolrit T, Medori R, Gambetti P. Structural basis of gamma-diketone neurotoxicity: non-neurotoxicity of 3,3-dimethyl-2,5-hexanedione, a gamma-diketone incapable of pyrrole formation. Toxicol Appl Pharmacol. 1986;84:36–44. [DOI] [PubMed] [Google Scholar]
- 43.Genter MB, Szakál-Quin G, Anderson CW, Anthony DC, Graham DG. Evidence that pyrrole formation is a pathogenetic step in gamma-diketone neuropathy. Toxicol Appl Pharmacol. 1987;87:351–362. [DOI] [PubMed] [Google Scholar]
- 44.DeCaprio AP, Briggs G, Jackowski SJ, Kim JC. Comparative neurotoxicity and pyrrole-forming potential of 2,5-hexanedione and perdeuterio-2,5-hexanedione in the rat. Toxicol Appl Pharmacol. 1988;92:75–85. [DOI] [PubMed] [Google Scholar]
- 45.Gagnaire F, Ensminger A, Marignac B, De Ceaurri J. Possible involvement of 1,2-diacetylbenzene in diethylbenzene-induced neuropathy in rats. J Appl Toxicol. 1991;11:261–268. [DOI] [PubMed] [Google Scholar]
- 46.Gagnaire F, Marignac B, De Ceaurriz J. Diethylbenzene-induced sensorimotor neuropathy in rats. J Appl Toxicol. 1990;10:105–112. [DOI] [PubMed] [Google Scholar]
- 47.Zhan C-G, Spencer PS, Dixon DA. Computational insights into the chemical structures and mechanisms of the chromogenic and neurotoxic effects of aromatic γ-diketones. J Phys Chem B. 2003;107:2853–2861. [Google Scholar]
- 48.Kim M-S, Hashemi S, Spencer PS, Sabri I. Amino acid and protein targets of 1,2-diacetylbenzene, a potent aromatic γ-diketone that induces proximal neurofilamentous axonopathy. Toxicol Appl Pharmacol. 2002;18355–18365. [DOI] [PubMed] [Google Scholar]
- 49.Kim MS, Sabri MI, Miller VH, Kayton RJ, Dixon DA, Spencer PS. 1,2-Diacetylbenzene, the neurotoxic metabolite of a chromogenic aromatic solvent, induces proximal axonopathy. Toxicol Appl Pharmacol. 2001;177:121–31. [DOI] [PubMed] [Google Scholar]
- 50.Tshala-Katumbay D, Monterroso V, Kayton R, Lasarev M, Sabri M, Spencer P. Probing mechanisms of axonopathy. Part I: Protein targets of 1,2-diacetylbenzene, the neurotoxic metabolite of aromatic solvent 1,2-diethylbenzene. Toxicol Sci. 2008;105:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tshala-Katumbay DD, Palmer VS, Kayton RJ, Sabri MI, Spencer PS. A new murine model of giant proximal axonopathy. Acta Neuropathol. (Berl.) 2005;109:405–410. [DOI] [PubMed] [Google Scholar]
- 52.Tshala-Katumbay DD, Palmer VS, Lasarev MR, Kayton RJ, Sabri M, Spencer PS. Monocyclic and dicyclic hydrocarbons: Structural requirements for proximal giant axonopathy. Acta Neuropathol. (Berl.) 2006;112:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim M, Park HR, Park M, et al. Neurotoxic effect of 2,5-hexanedione on neural progenitor cells and hippocampal neurogenesis. Toxicology. 2009;260:97–103. [DOI] [PubMed] [Google Scholar]
- 54.Cheng X, Wang G, Ma ZL, et al. Exposure to 2,5-hexanedione can induce neural malformations in chick embryos. Neurotoxicology. 2012;33:1239–1247. [DOI] [PubMed] [Google Scholar]
- 55.Anthony DC, Boejelheide K, Graham DG. The effect of 3,4-dimethyl substitution on the neurotoxicity of 2,5-hexanedione. I. Accelerated clinical neuropathy is accompanied by more proximal axonal swellings. Toxicol Appl Pharmacol. 1983; 71:362–372. [DOI] [PubMed] [Google Scholar]
- 56.Spencer PS, Sterman AB, Horoupian DS, Foulds MM. Neurotoxic fragrance produces ceroid and myelin disease. Science. 1979;204:633–635. [DOI] [PubMed] [Google Scholar]
- 57.Spencer PS, Sterman AB, Horoupian D, Bischoff M, Foster G. Neurotoxic changes in rats exposed to the fragrance compound acetyl ethyl tetramethyl tetralin. Neurotoxicology. 1979;1:221–237. [Google Scholar]
- 58.Palmer VS, Tshala-Katumbay DD, Hashemi SB, Sabri MI, Spencer PS. Tetralin and metabolites: protein reactivity, chromogenicity and neurotoxicity. Toxicol Sci. 2004;78: S-1. [Abstract] [Google Scholar]
- 59.Spencer PS, Schaumburg HH. Ultrastructural studies of the dying-back process. III. The evolution of experimental peripheral giant axonal degeneration. J Neuropathol Exp Neurol. 1977;36:2762–99. [DOI] [PubMed] [Google Scholar]
- 60.Schaumburg HH, Spencer PS. Environmental hydrocarbons produce degeneration in cat hypothalamus and optic tract. Science. 1978;199:199–200. [DOI] [PubMed] [Google Scholar]
- 61.Jones HB, Cavanagh JB. (1982) Recovery from 2,5-hexanediol intoxication of the retinotectal tract of the rat. An ultrastructural study. Acta Neuropathol. (Berl.) 1982;58:286–290. ` [DOI] [PubMed] [Google Scholar]
- 62.Veronesi B, Peterson ER, Bornstein MB, Spencer PS. Ultrastructural studies of the dying-back process. VI. Examination of nerve fibers undergoing giant axonal degeneration in organotypic culture. J Neuropathol Exp Neurol. 1983;42:153–165. [PubMed] [Google Scholar]
- 63.Anthony DC, Giangaspero F, Graham DG. The spatio-temporal pattern of the axonopathy associated with the neurotoxicity of 3,4-dimethyl-2,5-hexanedione in the rat. J Neuropathol Exp Neurol. 1983;42:548–660. [DOI] [PubMed] [Google Scholar]
- 64.Lehning EJ, Jortner BS, Fox JH, Arezzo JC, Kitano T, LoPachin RM. Gamma-diketone peripheral neuropathy. I. Quality morphometric analyses of axonal atrophy and swelling. Toxicol Appl Pharmacol. 2000;165:127–140. [DOI] [PubMed] [Google Scholar]
- 65.LoPachin RM, Jortner S, Reid ML, Das S. gamma-diketone central neuropathy: quantitative morphometric analysis of axons in rat spinal cord white matter regions and nerve roots. Toxicol Appl Pharmacol. 2003;193:29–46. [DOI] [PubMed] [Google Scholar]
- 66.Xu Z, Marszalek JR, Lee MK, et al. Subunit composition of neurofilaments specifies axonal diameter. J Cell Biol. 1996;133:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zagoren JC, Politis MJ, Spencer PS. Rapid reorganization of the axonal cytoskeleton induced by a γ-diketone. Brain Res. 1983;270:162–164. [DOI] [PubMed] [Google Scholar]
- 68.Graham DG, Anthony DC, Boekelheide K, et al. Studies of the molecular pathogenesis of hexane neuropathy. II. Evidence that pyrrole derivatization of lysyl residues leads to protein crosslinking. Toxicol Appl Pharmacol. 1982;64:415–422. [DOI] [PubMed] [Google Scholar]
- 69.Lapadula DM, Irwin RD, Suwita E, Abou-Donia MB. Cross-linking of neurofilament proteins of rat spinal cord in vivo after administration of 2,5-hexanedione. J Neurochem. 1986;46:1843–1850. [DOI] [PubMed] [Google Scholar]
- 70.Boekelheide K. 2,5-Hexanedione alters microtubule assembly. II. Enhanced polymerization of crosslinked tubulin. Toxicol Appl Pharmacol. 1987;88:383–396. [DOI] [PubMed] [Google Scholar]
- 71.Dixit R, Ross JL, Goldman YE, Holzbaur ELF. Differential regulation of dynein and kinesin motor proteins by tau. Science. 2008;319:108–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sabri MI, Hashemi SB, Lasarev MR, Spencer PS. Axonopathy-inducing 1,2-diacetylbenzene forms adducts with motor and cytoskeletal proteins required for axonal transport. Neurochem Res. 2007;32:2152–2159. [DOI] [PubMed] [Google Scholar]
- 73.Opanashuk LA, He DK, Lehning EJ, LoPachin RM. Gamma-diketone peripheral neuropathy III. Neurofilament gene expression. Neurotoxicology. 2001;22:215–220. [DOI] [PubMed] [Google Scholar]
- 74.Wang Z, Qiu Z, Gao C, et al. 2,5-hexanedione downregulates nerve growth factor and induces neuron apoptosis in the spinal cord of rats via inhibition of the PI3K/Akt signaling pathway. PLoS One. 2017;2(6):e0179388. doi: 10.1371/journal.pone.0179388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang L, Liu S, Su D, et al. 2,5-Hexanedione increases the percentage of proliferative Sox2+ cells in rat hippocampus. Toxicol Ind Hlth. 2018;34:589–595. doi: 10.1177/0748233718772767. [DOI] [PubMed] [Google Scholar]
- 76.Li H, Liu J, Sun Y, et al. N-hexane inhalation during pregnancy alters DNA promoter methylation in the ovarian granulosa cells of rat offspring. J Appl Toxicol. 2014;34:841–856. [DOI] [PubMed] [Google Scholar]
- 77.Sun Y, Lin Y, Li H, Liu J, Sheng X, Zheng W. 2,5-Hexanedione induces human ovarian granulosa cell apoptosis through BCL-2, BAX, and CASPASE-3 signaling pathways. Arch Toxicol. 2012;86:205–215. doi: 10.1007/s00204-011-0745-7 [DOI] [PubMed] [Google Scholar]
- 78.Liu J, Huang HL, Pang F, Zhang WC. The effect of n-hexane on the gonad toxicity of female mice. Biomed Environ Sci. 2012;25:189–196. [DOI] [PubMed] [Google Scholar]
- 79.Mohan R, John A. Microtubule-associated proteins as direct crosslinkers of actin filaments and microtubules. UBMB Life. 2015;67:395–403. [DOI] [PubMed] [Google Scholar]
- 80.Mu A, Fung TS, Kettenbach AN, Chakrabarti R, Higgs HN. A complex containing lysine-acetylated actin inhibits the formin INF2. Nat Cell Biol. 2019;21:592–602. doi: 10.1038/s41556-019-0307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bis-Brewer DM, Danzi MC, Wuchty S, Züchner S. A network biology approach to unraveling inherited axonopathies. Sci Rep. 2019;9:1692. doi: 10.1038/s41598-018-37119-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Henty-Ridilla JL, Rankova A, Eskin JA, Kenny K, Goode BL. Accelerated actin filament polymerization from microtubule plus ends. Science. 2016;352:1004–1009. doi: 10.1126/science.aaf1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lim WM, Ito Y, Sakata-Sogawa K, Tokunaga M. CLIP-170 is essential for MTOC repositioning during T cell activation by regulating dynein localisation on the cell surface. Sci Rep. 2018;8:17447. doi: 10.1038/s41598-018-35593-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Toba S, Jin M, Yamada M, et al. Alpha-synuclein facilitates to form short unconventional microtubules that have a unique function in the axonal transport. Sci Rep. 2017;7:16386. doi: 10.1038/s41598-017-15575-3. Correction: doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ozon S, Maucuer A, Sobel A. The stathmin family -- molecular and biological characterization of novel mammalian proteins expressed in the nervous system. Eur J Biochem. 1997;248:794–806. [DOI] [PubMed] [Google Scholar]
- 86.Horwitz SB, Shen HJ, He L, et al. The microtubule-destabilizing activity of metablastin (p19) is controlled by phosphorylation. J Biol Chem. 1997;272:8129–8132. [DOI] [PubMed] [Google Scholar]
- 87.Liedtke W, Leman EE, Fyff RE, Raine CS, Schubart UK. Stathmin-deficient mice develop an age-dependent axonopathy of the central and peripheral nervous systems. Am J Pathol. 2002;160,:469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sabri MI, Hashemi SB, Chohan S, et al. γ-Diketone toxicity: A role for stathmin in nerve and testes damage? Pacific Northwest Association of Toxicologists, 21st Annual Meeting, Bend, OR;2004. [Google Scholar]
- 89.Kassa R,. Monterroso V, Wentzell J, et al. Proximal giant neurofilamentous axonopathy in mice genetically engineered to resist calpain and caspase cleavage of α-II spectrin. J Mol Neurosci. 2012;47:631–638. doi: 10.1007/s12031-011-9699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spencer PS, Sabri MI, Schaumburg HH, Moore CL. Does a defect of energy metabolism in the nerve fiber underlie axonal degeneration in polyneuropathies? Ann Neurol. 1979;5:501–507. [DOI] [PubMed] [Google Scholar]
- 91.Sickles DW, Fowler SR, Testino AR. Effects of neurofilamentous axonopathy-producing neurotoxicants on in vitro production of ATP by brain mitochondria. Brain Res. 1990; 528:25–31. [DOI] [PubMed] [Google Scholar]
- 92.Sabri MI. In vitro effect of n-hexane and its metabolites on selected enzymes in glycolysis, pentose phosphate pathway and citric acid cycle. Brain Res. 1984;297:145–150. [DOI] [PubMed] [Google Scholar]
- 93.Adedara IA, Abolaji AO, Odion BE, et al. Impairment of hepatic and renal functions by 2,5-hexanedione is accompanied by oxidative stress in rats. J Toxicol. 2014;2014:239240. doi: 10.1155/2014/239240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kang SW, Kim SJ, Kim MS. Oxidative stress with tau hyperphosphorylation in memory impaired 1,2-diacetylbenzene-treated mice. Tox Lett. 2017;279:53–59. doi: 10.1016/j.toxlet.2017.07.892 [DOI] [PubMed] [Google Scholar]
- 95.Kim MS, Kim MK, Kim KS, et al. Cytotoxicity of 1,2-diacetylbenzene in human neuroblastoma SHSY5Y cells is mediated by oxidative stress. Toxicology. 2008;243:216–223. [DOI] [PubMed] [Google Scholar]
- 96.Sakai T, Araki T, Ushio K, Takeuchi Y, Ikeya Y. [Effect of hydrolysis conditions on the determination of urinary 2,5-hexanedione in workers exposed or not exposed to N-hexane]. Sangyo Igaku. 1992;34:440–447. [Japanese] [DOI] [PubMed] [Google Scholar]
- 97.Perbellini L, Pezzoli G, Brugnone F, Canesi M. Biochemical and physiological aspects of 2,5-hexanedione: endogenous or exogenous product? Int Arch Occup Environ Hlth. 1993;65:49–52. [DOI] [PubMed] [Google Scholar]
- 98.Chambers DM, Blount BC, McElprang DO, Waterhouse MG, Morrow JC. Picogram measurement of volatile n-alkanes (n-hexane through n-dodecane) in blood using solid-phase microextraction to assess nonoccupational petroleum-based fuel exposure. Anal Chem. 2008;80:4666–4674. [DOI] [PubMed] [Google Scholar]
- 99.Bavazzano P, Apostoli P, Balducci C, et al. Determination of urinary 2,5-hexanedione in the general Italian population. Int Arch Occup Environ Hlth. 1988;71:284–288. [DOI] [PubMed] [Google Scholar]
- 100.Xing-Fu P, Ya-Ling Q, Wei Z, et al. Determination of total urinary 2,5-hexanedione in the Chinese general population. Environ Res. 2016;150:645–650. doi: 10.1016/j.envres.2016.05.030 [DOI] [PubMed] [Google Scholar]
- 101.Wang H, Wang Y, Zhou Z, Wang S, Yin H, Xie K. [Determination of normal reference value of pyrrole adducts in urine in young people in a university in Shandong, China]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2015;33:435–437. [Chinese] [PubMed] [Google Scholar]
- 102.Zlatkis A, Poole CF, Brazeli R, Bafus DA, Spencer PS. Volatile metabolites in sera of normal and diabetic subjects. J Chromatogr. 1980;182:137–145. [DOI] [PubMed] [Google Scholar]
- 103.Altenkirch H, Stoltenburg G, Wagner HM. Experimental studies on hydrocarbon neuropathies induced by methyl-ethyl-ketone (MEK). J Neurol. 1978; 219:159–170. [DOI] [PubMed] [Google Scholar]
- 104.Veronesi B, Lington AW, Spencer PS. A tissue culture model of methyl ethyl ketone’s potentiation of n-hexane neurotoxicity. Neurotoxicology. 1984;5:43–52. [PubMed] [Google Scholar]
- 105.Misumi J, Nagano M. Experimental study on the enhancement of the neurotoxicity of methyl n-butyl ketone by non-neurotoxic aliphatic monoketones. Br J Ind Med. 1985;42:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu RC, Hattis D, Landaw EM, Froines JR. Toxicokinetic interaction of 2,5-hexanedione and methyl ethyl ketone. Arch Toxicol. 2002;75:643–652. [DOI] [PubMed] [Google Scholar]
- 107.Spencer PS, Ochoa J. The mammalian peripheral nervous system in old age In: Johnson JE Jr., ed Aging and Cell Structure, Vol. I, New York, Plenum, 1981:35–103. [Google Scholar]
- 108.Spencer PS, Schaumburg HH. Central-peripheral distal axonopathy - the pathology of dying-back polyneuropathies In: Zimmerman HM, ed, Progress in Neuropathology, Vol. III, New York, Grune and Stratton, 1976:253–295. [Google Scholar]
- 109.Spencer PS, Bischoff MC, Schaumburg HH. On the specific molecular configuration of neurotoxic aliphatic hexacarbon compounds causing central-peripheral distal axonopathy. Toxicol Appl Pharmacol. 1976;44:17–28. [DOI] [PubMed] [Google Scholar]
- 110.DeCaprio AP, Strominger NL, Weber P. Neurotoxicity and protein binding of 2,5-hexanedione in the hen. Toxicol Appl Pharmacol. 1983;68:297–307. [DOI] [PubMed] [Google Scholar]
- 111.Markelewicz R Jr., Hall SJ, Boekelheide K. 2,5-Hexanedione and carbendazim coexposure synergistically disrupts rat spermatogenesis despite opposing molecular effects on microtubules. Toxicol Sci. 2004;80:92–100. [DOI] [PubMed] [Google Scholar]
- 112.Liu Q, Duan H, Dai Y, et al. The effect of 2,5-hexanedione on permeability of blood-nerve barrier in rat. Hum Exp Toxicol. 2010;29:497–506. [DOI] [PubMed] [Google Scholar]
- 113.Spencer PS, Thomas PK. Ultrastructural studies of the dying-back process. II. The sequestration and removal by Schwann cells and oligodendrocytes of organelles from normal and diseases axons. J Neurocytol. 1974;3:763–783. [DOI] [PubMed] [Google Scholar]
- 114.Politis MJ, Pellegrino RG, Spencer PS. Ultrastructural studies of the dying-back process. V. Axonal neurofilaments accumulate at sites of 2,5-hexanedione application: evidence for nerve fibre dysfunction in experimental hexacarbon neuropathy. J Neurocytol. 1980;9:505–516. [DOI] [PubMed] [Google Scholar]
- 115.Chen J, Kanai Y, Cowan NJ, Hirokawa N. Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons. Nature. 1992;360:674–677. [DOI] [PubMed] [Google Scholar]
- 116.Gotow T. Neurofilament cross-bridge – A structure associated specifically with the neurofilament among the intermediate filament family. In: Nixon A, Yuan A, eds, Advances in Neurobiology 3, Cytoskeleton of the Nervous System; 2010:225–247. [Google Scholar]
- 117.Gotow T, Tanaka J. Phosphorylation of neurofilament H subunit as related to arrangement of neurofilaments. J Neurosci Res. 1994;37:691–713. [DOI] [PubMed] [Google Scholar]
- 118.Chen J, Nakata T, Zhang Z, Hirokawa N. The C-terminal tail domain of neurofilament protein-H (NF-H) forms the crossbridges and regulates neurofilament bundle formation. J Cell Biol. 2000:113:3861–3869. [DOI] [PubMed] [Google Scholar]
- 119.Barry DM, Carpenter C, Yager C, et al. Variation of the neurofilament medium KSP repeat sub-domain across mammalian species: implications for altering axonal structure. J Exp Biol. 2010;213:128–136. doi: 10.1242/jeb.033787 [DOI] [PubMed] [Google Scholar]
- 120.Zhu Q, Lindenbaum M, Levavasseur F, Jacomy H, Julien JP. Disruption of the NF-H gene increases axonal microtubule content and velocity of neurofilament transport: relief of axonopathy resulting from the toxin beta, beta’-iminodipropionitrile. J Cell Biol. 1988;143:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jung C, Chylinski TM, Pimenta A, Ortiz D, Shea TB. Neurofilament transport is dependent on actin and myosin. J Neurosci. 2004;24:9486–9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Robert A, Herrmann H, Davidson MW, Gelfand VI. Microtubule-dependent transport of vimentin filament precursors is regulated by actin and by the concerted action of Rho- and p21-activated kinases. FASEB J. 2014;28:2879–2890. doi: 10.1096/fj.14-250019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hitchcock-De Gregori SE, Mandala S, Sachs A. Changes in actin lysine reactivities during polymerization detected using a competitive labeling method. J Biol Chem. 1982;257:12573–12580. [PubMed] [Google Scholar]
- 124.Charbaut E, Curmi PA, Ozon S, Lachkar S, Redeker V, Sobel A. Stathmin family proteins display specific molecular and tubulin binding properties. J Biol Chem. 2001;276:16146–16154. [DOI] [PubMed] [Google Scholar]
- 125.Nouar R, Breuzard G, Bastonero S, et al. Direct evidence for the interaction of stathmin along the length and the plus end of microtubules in cells. FASEB J. 2016;30:3202–3215. doi: 10.1096/fj.201500125R [DOI] [PubMed] [Google Scholar]
- 126.Gerarde H. Toxicology and biochemistry of aromatic hydrocarbons In: Browning E, ed, Elsevier Monographs on Toxic Agents. Amsterdam, Elsevier, p. 73 1960. [Google Scholar]
- 127.American Chemistry Council, Ethyl Benzene Panel HPV Task Group, Diethylbenzene Subteam. Report for the Diethylbenzene-rich Streams Category. High Production Volume (HPV) Chemical Challenge Program; April 26, 2004. [Google Scholar]
- 128.Browning E. Toxicity and Metabolism of Industrial Solvents. New York, American Elsevier; 1965. [Google Scholar]