1. Introduction
Glaucoma is the leading cause of irreversible blindness worldwide. 1 Early diagnosis and monitoring with appropriate treatment are necessary to prevent visual loss which is irreversible and usually becomes symptomatic only in its late stages. Current methods of assessing glaucoma and its progression have significant limitations. The visual field (VF) directly assesses function, but the testing is subjective, time consuming, and poorly repeatable.2–4 Quantitative imaging of the peripapillary retinal nerve fiber layer (NFL) with optical coherence tomography (OCT) provides a faster, more repeatable and objective assessment in the diagnosis and monitoring glaucoma.5–8 However, NFL thickness has only moderate correlation with 24–2 VF parameters, and the correlation further deteriorates in the later stages of glaucoma due to the “floor effect.”7–10 Therefore, structural OCT NFL measurements perform relatively poorly in the monitoring of moderate to severe glaucoma.11 To further improve glaucoma monitoring, we need a new objective measure of glaucoma severity that retains good correlation with VF in the later stages of glaucoma.
Optical coherence tomography angiography (OCTA) is a noninvasive imaging modality to evaluate blood flow. OCTA parameters such as capillary density and flow index of the optic nerve head, peripapillary retina, and macula are reduced in glaucoma compared to normal participants.12–15 OCTA-derived nerve fiber layer plexus (NFLP) capillary density measurements are highly reproducible and, compared to NFL thickness, are more tightly correlated with 24–2 VF parameters, even in the later stages of glaucoma.9, 12–14, 16–18 Thus, NFLP parameters may hold promise for improving glaucoma monitoring.
Although the correlation between NFLP capillary density (NFLP_CD) and VF parameters is high, it is not linear. One reason is that NFLP_CD is measured on the linear scale (% area), while VF parameters are typically represented in a logarithmic scale (i.e. dB change in retinal sensitivity). Another reason is that NFLP loss is weighted by peripapillary analytic area, while retinal sensitivity is weighted by VF area. Thus, the summary parameters for NFLP (average capillary density) and VF (mean deviation) are calculated on different scales with different weighting. We believe that the correlation between NFLP and VF could be further improved by bridging these methodological differences. To test this hypothesis, we developed a method to simulate 24–2 VF using OCTA measurements of NFLP on a sector basis. The simulated VF is then evaluated in terms of agreement and correlation with actual VF in the glaucoma patient. Its glaucoma diagnostic accuracy and reproducibility are also assessed and compared with actual VF and NFL thickness parameters. The overall aims are to develop an optimal OCTA NFLP-based 24–2 VF simulation and provide a preliminary evaluation of its potential as a new objective metric for the diagnosis, staging, and monitoring of glaucoma.
2. Methods
2.1. Study Population
This prospective cross-sectional study was performed from October 6, 2014 to June 21, 2017 at the Casey Eye Institute, Oregon Health & Science University (OHSU). The research protocols were approved by the Institutional Review Board at OHSU, and carried out in accordance with the tenets of the Declaration of Helsinki. Written informed consent was obtained from each participant.
All participants were part of the “Functional and Structural Optical Coherence Tomography for Glaucoma” study. The inclusion criteria for the perimetric glaucoma (PG) group were: (1) an optic disc rim defect (thinning or notching) or NFL defect visible on slit-lamp biomicroscopy; and (2) a consistent glaucomatous pattern, on both qualifying Humphrey SITA 24–2 VFs, meeting at least one of the following criteria: pattern standard deviation (PSD) outside normal limits (p < 0.05) or glaucoma hemifield test outside normal limits. The inclusion criteria for the pre-perimetric glaucoma (PPG) group were: (1) an optic disc rim defect (thinning or notching) or NFL defect visible on slit-lamp biomicroscopy; and (2) VF not meeting the criteria for the PG group.
For the normal group, the inclusion criteria were: (1) no evidence of retinal pathology or glaucoma; (2) a normal Humphrey 24–2 visual field; (3) intraocular pressure < 21 mm Hg; (4) central corneal pachymetry > 500 microns; (5) no chronic ocular or systemic corticosteroid use; (6) an open angle on gonioscopy; (7) a normal appearing optic nerve head (ONH) and NFL; and (8) symmetric ONH between left and right eyes.
The exclusion criteria for both groups were: (1) best-corrected visual acuity (BCVA) worse than 20/40; (2) age < 30 or > 80 years; (3) refractive error of > +3.00 D or < −7.00 D; (4) previous intraocular surgery except for an uncomplicated cataract extraction with posterior chamber intraocular lens implantation; (5) any diseases that may cause VF loss or optic disc abnormalities; or (6) inability to perform reliably on automated VF testing. One eye from each participant was scanned and analyzed. For normal eyes, the eye was randomly selected. For the PPG and PG group, the eye with the worse VF was selected.
2.2. Visual Field Testing
VF tests were performed with the Humphrey Field Analyzer II (Carl Zeiss, Inc.) set for the 24–2 threshold test, size III white stimulus, using the SITA standard algorithm. The 24–2 VF test was done at baseline for all participants and then every 6 months for glaucoma participants.
2.3. Nerve fiber layer plexus capillary density measurement and conversion to decibel scale
2.3.1. Optical Coherence Tomography
A 70-kHz, 840-nm wavelength spectral-domain OCT system (Avanti, Optovue Inc.) with the AngioVue OCTA software (Version 2016.2.0.35) was used.
2.3.2. Image Acquisition and Processing
Pharmacologic pupil dilation was used for OCTA scanning to optimize image quality. The peripapillary retinal region was scanned using a 4.5x4.5-mm volumetric angiography scan centered on the optic disc. Each volume was comprised of 304 line-scan locations at which 2 consecutive B-scans were obtained. Each B-scan contained 304 A-scans. The AngioVue software used the split-spectrum amplitude-decorrelation angiograph (SSADA) algorithm,19, 20 which compared the consecutive B-scans at the same location to detect flow using motion contrast. Each scan set was comprised of 2 volumetric scans: 1 vertical-priority raster and 1 horizontal-priority raster. The AngioVue software used an orthogonal registration algorithm to register the 2 raster volumes to produce a merged 3D OCT angiogram.19 Two sets of scans were performed within one visit. The OCT angiogram with higher signal strength index (SSI) was used in the following analysis. The OCTA scan was done at baseline for all participants and then every 6 months for glaucoma participants.
The merged volumetric angiograms were then exported for custom processing using the Center for Ophthalmic Optics & Lasers-Angiography Reading Toolkit (COOL-ART) software.21 The OCTA scans contained both volumetric flow (decorrelation) data as well as structural (reflectance) data. Segmentation of the retinal layers was performed by automated MATLAB programs that operate on the structural OCT data. Further manual correction of the segmentation was conducted by certified graders, if required. An en face angiogram of retinal nerve fiber layer plexus (NFLP) was obtained by maximum flow (decorrelation value) projection. The NFLP slab was bounded by the inner limiting membrane and the outer edge of the nerve fiber layer.
Because we found OCTA measurements to be strongly correlated with signal strength index in previous studies, we developed a reflectance-adjustment method that corrected the artifactually lower flow signal in regions of reduced reflectance (e.g. due to media opacity or pupil vignetting).22 The method was experimentally calibrated by using optical filters to attenuated signal strength and then measuring the resulting statistical distribution of flow noise (decorrelation value) in nonvascular retinal in the foveal avascular zone (FAZ). The relationship between the reflectance in the FAZ and reflectance in the extrafoveal mid-retinal layers (inner nuclear layer, outer plexiform layer, and outer nuclear layer) was also calibrated experimentally. The calibration was then used to perform reflectance-based adjustment in the flow signal threshold for vessel versus nonvascular tissue classification. Clinical validation showed that this algorithm was able to remove the dependence of retinal OCTA measurements on the signal strength index and reduce population variation. 15, 18, 22
The capillary density, defined as the percentage area occupied by capillaries, was evaluated in the 4×4 mm analytic area excluding the central 2mm diameter circle, which was centered on the optic disc boundary as defined by the Bruch’s membrane opening. The disc boundary segmentation was first performed by automated software and then verified by certified graders based examination of the both the en face OCT (mean projection of whole volume) and cross-sectional OCT images. Arterioles and venules (larger vessels) were automatically identified by thresholding the en face mean projection of OCT reflectance within the all-plexus slab. The all-plexus slab was bounded by the inner limiting membrane and the outer edge of the outer plexiform layer. After these larger vessels were excluded, the remaining angiogram was used to compute capillary density. A scan is considered to be grossly decentered if the 4x4-mm analytic area was cropped by more than 5%. Grossly decentered scans were excluded. Smaller amount of cropping was tolerated and CD was calculated in the remaining analytic areas.
2.3.3. Nerve Fiber Layer Plexus Sector Division
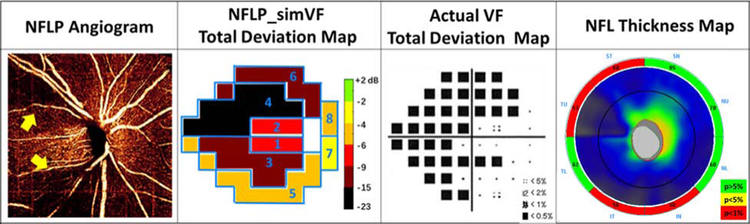
The en face OCTA of the NFLP and the 24–2 VF total deviation map were divided into 8 corresponding sectors according to a modified Garway-Heath scheme (Figure 1). The original Garway-Heath scheme divided the disc rim into 6 sectors.23, 24 We added horizontal dividing lines to the original nasal and temporal sectors to increase the total number of sectors to 8. The sector boundaries were extended outward along nerve fiber trajectories obtained from structural OCT nerve fiber flux analysis.25
FIGURE 1.
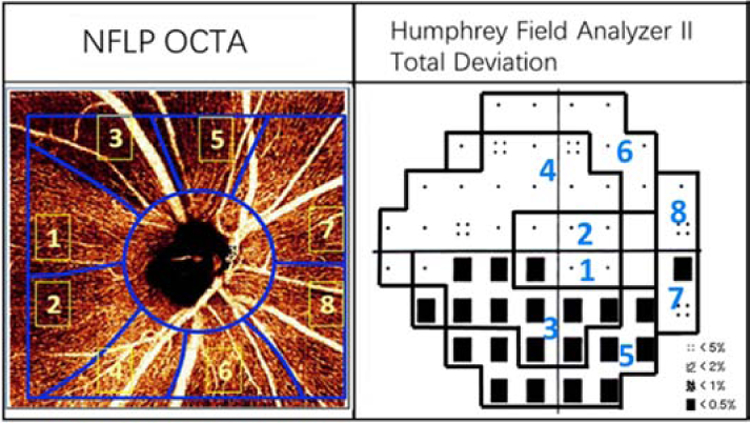
In a perimetric glaucomatous eye, the 4.5x4.5-mm en face OCT angiogram of the nerve fiber layer plexus (NFLP) and the 24–2 visual field (VF) map were divided into 8 corresponding sectors according to a modified Garway-Heath scheme.
2.3.4. Floor Value of Nerve Fiber Layer Plexus Capillary Density
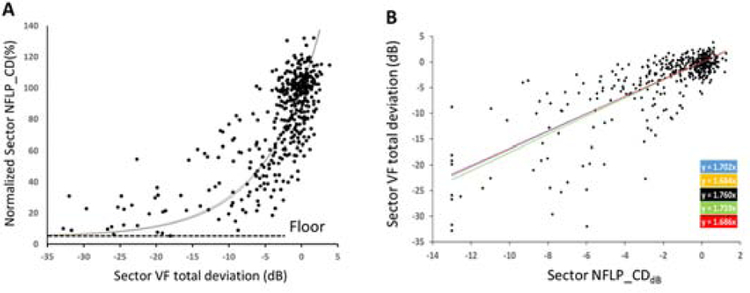
The NFLP_CD has a nonlinear relationship with VF – the slope is relatively steep in early stages of glaucoma and flattens in later stages.9 Our mathematical model linking NFLP_CD and VF includes an NFLP_CD floor value, which represents the residual NFLP capillaries in end-stage glaucoma. The floor value is proportional to the normal values for each sector. This common floor fraction is estimated using the normalized sector NFLP_CD, which is defined as the sector NFLP_CD divided by the reference NFLP_CD of the corresponding sector. The reference NFLP_CD was obtained by averaging measurements in the normal group. The normalized NFLP_CD values of all sector measurements in the glaucoma group were pooled (Figure 2A). The first percentile cut off point of the pooled distribution was used as the residual fraction. Finally, each sector’s floor value is defined as the floor fraction times the normal reference value.
FIGURE 2.
(A) Plot of normalized sector NFLP_CD (%) and sector visual field total deviation (dB) shows an exponential decay relationship with a floor value of 7.0%. (B) Plot of sector visual field total deviation and sector NFLP_CD on a dB scale, which shows a linear relationship. Five linear regression results are shown (color-coded regression formulas on right) according to the 5-fold cross-validation method. The linear fit was used for VF simulation after placing a lower limit of −13.0 dB on the NFLP_CDdB value.
2.3.5. Converting Sector Nerve Fiber Layer Plexus Capillary Density to a Decibel Scale
The following formula is used to transform NFLP_CD on a %area scale to NFLP_CD loss on a dB scale.
where f is the floor, N is the normal reference value (average value of healthy eyes in our normal group).
We limited the minimum value of sector NFLP_CDdB to −13 dB to avoid extremely negative dB values that could be obtained when NFLP_CD is near the floor. The −13 dB limit was based on the coefficient of variation of sector NFLP_CD of 5.0% for repeat measurements in normal eyes.
2.3.6. Simulating Sector Visual Field Deviation
A linear regression was used to fit the sector NFLP_CDdB to VF sector deviation (Figure 2B). Data from both normal and glaucomatous eyes were used. Measurements from sector 1 to sector 6 were pooled. We did not use data from sector 7 and 8 in the regression analysis because these sectors contained only 2 VF test points each and had relatively poor reproducibility. The intercept was fixed at zero with the a priori knowledge that an average normal NFLP_CDdB should correspond to an average normal VF. Using the regression formula, the sector NFLP_CDdB was converted to simulated sector visual field deviation (NFLP_simVF) with the unit in the decibel scale. A color-coded NFLP_simVF total deviation map with 8 sectors was generated by the sector NFLP_simVF.
2.4. Calculating the Mean Deviation of the Simulated Visual Field
The sector NFLP_simVF values were weighted and averaged to generate the NFLP simulated visual field mean deviation (NFLP_MD) with the unit in the decibel (dB) scale. The weight for each sector is proportional to the number of VF test points within the sector (Figure 1). The weights in the 8 sectors were set proportional to the number of VF test points in the corresponding VF sector.
The wi is the weight of sector i. The weights of sectors 1 to 8 are 0.06, 0.06, 0.19, 0.25, 0.21, 0.15, 0.04, and 0.04. They sum to 1.
In order to reduce measurement noise, we averaged OCT, OCTA, and VF parameters from 2 visits 6 months apart for the glaucoma eyes.
2.5. Structural OCT Analysis
Peripapillary NFL thickness was measured from the Avanti’s ONH structural OCT scan. The ONH scan pattern consists of concentric circular (1.3–4.9 mm diameter) scans centered on the optic disc. In post processing, the ONH scan was automatically registered with a baseline three-dimensional disc scan to provide the disc margin information. The NFL thickness profile at a diameter of 3.4 mm was resampled on the NFL thickness map, then re-centered to the detected optic disc center. The result for each participant was the average value of two sets of images obtained at one visit.
2.6. Five-fold Cross Validation
The participants were each randomly divided into 5 subsets with equal numbers in each disease severity classification. While one subset was taken as a test set, the other four subsets were used to calculate the regression formula for simulating the VF. This provided five sets of regression results that were used to convert sector NFLP_CDdB to NFLP_simVF.
2.7. Image Quality Control and Statistical Analysis
Image quality was assessed for all OCTA scans. Poor quality scans with SSI below 55, or registered image sets with residual motion artifacts (obvious break in the pattern of large vessels) were excluded from analysis. Between-visit reproducibility was assessed by the pooled standard deviation (Pooled SD) and the intraclass correlation coefficient (ICC). Agreement between NFLP_MD and VF_MD was also assessed by Bland-Altman analysis. The Student’s t test was used to compare normal and glaucoma groups. The area under the receiver operating characteristic curve (AROC), sensitivity, and specificity were used to evaluate diagnostic accuracy. The estimated sensitivities for fixed specificities were calculated by the method of Zhou et al.26 McNemar test was used to compare these sensitivities. To investigate the location correspondence of NFLP_simVF and actual VF, the worst sector was defined as the sector having the lowest average value and its location was compared between NLFP_simVF and actual VF. The statistical significance was assumed at P<0.05. All statistical analyses were performed with SPSS20.0 (SPSS Inc., Chicago, IL) and MedCalc 10.1.3.0 (MedCalc Software, Ostend, Belgium).
3. Results
3.1. Study Population
Thirty-one normal eyes from 31 normal participants and 39 glaucomatous eyes from 39 glaucoma participants were analyzed in this study. In the glaucoma group, 1 participant had pre-perimetric glaucoma, 23 had early glaucoma (MD > −6 dB), 10 had moderate glaucoma (MD between −6 and −12 dB), and 5 had severe glaucoma (MD between −12 and −20 dB), according to the modified Hodapp-Parrish-Anderson classification system.27 There were 31 primary open angle glaucoma, 7 normal tension glaucoma, and 1 pigmentary glaucoma eyes. There was no statistically significant difference between the normal and glaucoma groups for age, intraocular pressure, and systolic/diastolic blood pressures (Table 1). The glaucoma group had worse VF, thinner NFL, and lower NFLP_CD than the normal control group, as expected (Table 1). No scans were excluded for gross decentration. Among the decentered OCTA scans, the maximum cropped area percentage is 3.76% of the 4x4-mm analytic area. By using the reflectance-adjusted algorithm to measure CD, SSI was not correlated with NLFP_CD (P = 0.10) or NFLP_MD (P = 0.28) in the normal group. The SSI in glaucoma group was significantly lower than the SSI in the normal group (Table 1).
Table 1.
Participants’ Characteristics
| Parameter | Normal | Glaucoma | Difference (P value) | |
|---|---|---|---|---|
| Participants, n |
31 | 39 | ||
| Eyes, n |
31 | 39 | ||
| Age (Years) |
65 ± 9 | 65 ± 10 | 0 (0.853) | |
| Intraocular Pressure (mm Hg) |
14.6 ± 3.6 | 15.3 ± 2.7 | 0.7 (0.767) | |
| Diastolic Blood Pressure (mm Hg) |
76.0 ± 15.0 | 77.5 ± 11.2 | 1.5 (0.697) | |
| Systolic Blood Pressure (mm Hg) | 120.9 ± 23.2 | 124.9 ± 14.7 | 4.0 (0.385) | |
| Visual Field | MD (dB) | −0.1 ± 1.3 | −6.0 ± 4.4 | −5.9 (<0.001) |
| PSD (dB) | 1.0 ± 0.2 | 6.4± 3.9 | 5.4 (<0.001) | |
| Structural OCT Thickness Measurement | NFL (μm) | 98.8 ± 7.6 | 75.8 ± 12.3 | −23.0 (<0.001) |
| OCT Angiography Measurements | Signal Strength Index | 71 ± 7 | 65 ± 6 | −6 (<0.001) |
| Overall NFLP_CD (%area) | 69.2 ± 4.9 | 45.3 ± 12.6 | −23.9 (<0.001) | |
| NFLP_MD (dB) | −0.1 ± 0.5 | −5.6 ± 3.2 | −5.5 (<0.001) | |
Group mean ± standard deviation are shown. VF_MD = visual field mean deviation; NFLP_MD = retina nerve fiber layer plexus mean deviation (simulated visual field mean deviation); NFLP_CD = retina nerve fiber layer plexus capillary density; NFL = nerve fiber layer
The normal population SD for NFLP_MD was 0.5 dB, which was significantly (P< 0.001) tighter than 1.3 dB for the normal population SD for VF_MD (Table 1). The differences between glaucomatous eyes and normal eyes were similar for the two parameters. Thus, the tighter normative distribution for NFLP_MD may provide an advantage for distinguishing glaucomatous eyes from normal eyes.
3.2. Floor Values of Nerve Fiber Layer Plexus Capillary Density and Visual Field Simulation
The normative reference values of NFLP_CD in sectors 1 to 8 were 67.5, 61.7, 87.2, 88.7, 81.2, 74.8, 66.6 and 54.8 % area, respectively. The floor fraction was 7.0%, representing the residual capillary density in sectors with the most significant perfusion loss (1 percentile cutoff). The average VF sector total deviation in the sectors below the cutoff was −22.8 dB, confirming that these sectors have end-stage glaucoma damage. This floor fraction (Figure 2A) was used in converting NFLP_CD to a dB scale as described in the methods section.
For the purpose of 5-fold cross-validation, the participants were each randomly divided into 5 subsets with equal numbers in each disease severity classification. This provided 5 sets of regression formulas that were used to convert sector NFLP_CDdB to NFLP_simVF. Sector NFLP_simVF = slope*sector NFLP_CDdB, slope =1.718 ± 0.038 (Mean ± Standard Deviation); and R2 ranged from 0.626 to 0.659 for formulas 1 to 5 (Figure 2B). There was no significant difference in regression fit parameters if the sectors were analyzed separately, thus the pooled regression approach was permissible. A lower limit of −13.0 dB was placed on NFLP_CDdB value, corresponding to the coefficient of variation of sector NFLP_CD of 5.0%, for repeat measurements in normal eyes. The equivalent lower limits on NFLP_simVF ranged from −21.9 dB to −22.9 dB for formula 1 to 5. This limit prevents capillary density measurement noise from being excessively amplified when NFLP_CD values are close to the floor.
The sector NFLP_simVF values were averaged with weights proportional to VF area to generate the global simulated visual field mean deviation (NFLP_MD) as mentioned in Methods Section 2.4.
3.3. Agreement and Reproducibility of OCTA and VF parameters
The NFLP-based VF simulation had a better between-visit reproducibility than actual visual fields on both sector-wise (Table 2) and global (Table 3) analyses. These differences were statistically significant for sector and mean deviation values for the pooled SD (P<0.02). But, the differences in ICC were not statistically significant. Since the reproducibility was calculated from visits 6 months apart, we examined whether disease progression in the glaucoma group could have biased the pooled SD values. In the glaucoma group, the average difference between the baseline NFLP_MD and repeat measurement 6 months later was 0.08 dB, which was small compared to the within-visit repeatability of 0.69 dB and the between-visit reproducibility of 0.63 dB (Pooled SD). The average difference between the baseline VF_MD and repeat measurement 6 months later was 0.16 dB, which is small compared to the between-visit reproducibility of 1.03 dB (Pooled SD). Therefore, we concluded that disease progression did not significantly affect the calculation of the reproducibility of NFLP_MD and VF_MD.
Table 2.
Sectorwise Agreement and Reproducibility for Visual Field & Optical Coherence Tomographic Angiography
| NFLP_simVF v. VF‡ | VF v. VF* | NFLP_simVF v. NFLP_simVF * | |
|---|---|---|---|
| Intraclass Correlation Coefficient (95% Confidence Interval) | 0.745 (0.706 to 0.780) | 0.931 (0.916 to 0.944) | 0.945 (0.937 to 0.960) |
| Pooled Standard Deviation (dB) | 2.63 | 1.69 | 1.20 |
The 8 Garway-Heath sectors were pooled for this analysis.
The agreement between sector visual field (VF) deviation (average total deviation value of test points within each sector) and retina nerve fiber layer plexus-based simulated visual field sector values (NFLP_simVF) measured on the same visit were assessed. Both normal and glaucoma groups were used in this analysis.
The reproducibility of VF and NFLP_simVF sector values were measured in the glaucoma group using data from 2 visits 6 months apart.
Table 3.
Global Agreement and Reproducibility for Visual Field & Optical Coherence Tomographic Angiography
| NFLP_MD v. VF_MD‡ | VF_MD v. VF_MD* | NFLP_MD v. NFLP_MD* | |
|---|---|---|---|
| Intraclass Correlation Coefficient (95% Confidence Interval) | 0.808 (0.709 to 0.876) | 0.946 (0.900 to 0.971) | 0.954 (0.910 to 0.977) |
| Pooled Standard Deviation (dB) | 1.78 | 1.03 | 0.63 |
The agreement between visual field mean deviation (VF_MD) and retina nerve fiber layer plexus mean deviation (NFLP_MD) measured on the same visit were assessed. Both normal and glaucoma groups were used in this analysis.
The between-visit reproducibility of VF_MD and NFLP_MD were measured in the glaucoma group using data from 2 visits 6 months apart.
The sector NFLP-based VF simulation and actual VF had fair agreement on a sector basis (Table 2) and good agreement on a mean deviation basis (Table 3).
The difference analysis (Table 4) and Bland-Altman analysis (Figure 3) showed that the agreement between NFLP_MD and VF_MD was good in early and moderate glaucoma stages. However, in the advanced glaucoma stage, NFLP_MD tended to under-estimate the severity of actual visual field. Although the difference between NFLP_MD and VF_MD in the severe glaucoma group was not statistically significant (p=0.06) due to the small sample size, the magnitude of the difference (5.8 dB) was clinically significant. The spread between NFLP_MD and VF_MD increased with increasing glaucoma severity. One possible explanation for the discrepancy between NFLP_MD and VF_MD is cataract severity. Therefore, we examined cataract severity and BCVA in the different stages of glaucoma (Table 4). No significant differences in cataract severity or BCVA were found between glaucoma stages.
Table 4.
Visual Field, Optical Coherence Tomographic Angiography, and Clinical Parameters Stratified by Glaucoma Severity
| Parameter | Normal | PPG + Early PG | Moderate PG | Severe PG |
|---|---|---|---|---|
|
# of Subjects in Group |
31 | 24 | 10 | 5 |
|
VF_MD (dB) |
−0.1 ± 1.3 | −3.1 ± 1.6 | −8.9 ± 1.7 | −14.3 ± 2.4 |
|
NFLP_MD (dB) |
−0.1 ± 0.5 | −4.0 ± 2.5 | −8.0 ± 2.1 | −8.4 ± 3.0 |
|
NFLP_MD – VF_MD (dB) |
0.1 ± 1.2 | −0.9 ± 2.0 | 0.9 ± 2.9 | 5.8 ± 3.2 |
|
Cataract (0–4) |
0.8 ± 0.9 | 1.1 ± 1.1 | 1.9 ± 0.7 | 1.4 ± 0.7 |
| BCVA (LogMAR) | 0.0 ± 0.10 | 0.0 ± 0.10 | 0.06 ± 0.16 | 0.04 ± 0.09 |
Group mean ± standard deviation are shown. VF_MD = visual field mean deviation; NFLP_MD = retina nerve fiber layer plexus mean deviation (simulated visual field mean deviation); BCVA = best corrected visual acuity; logMAR = logarithm of minimum angle of resolution; PPG = pre-perimetric glaucoma; PG = perimetric glaucoma.
FIGURE 3.
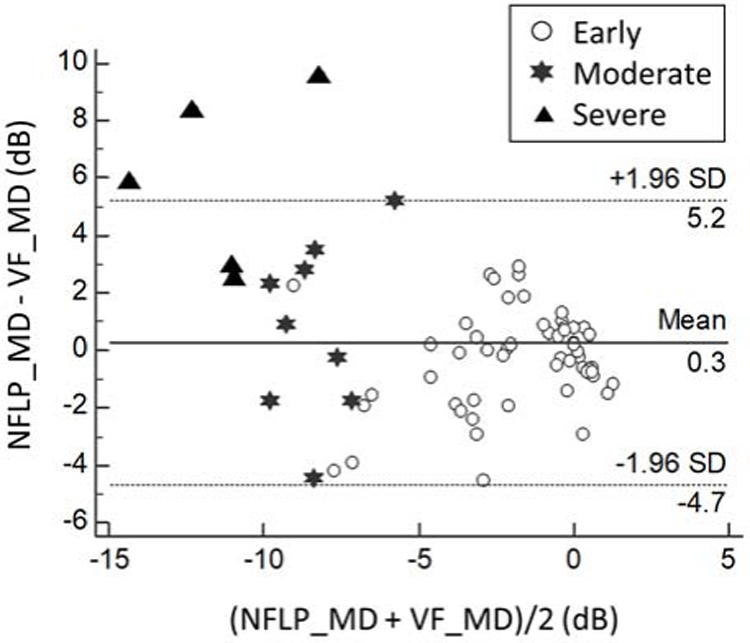
Bland-Altman analysis of the agreement between nerve fiber layer plexus mean deviation (NFLP_MD) and visual field mean deviation (VF_MD). Data from the normal and glaucoma groups were combined.
The Pearson correlation between NFLP_MD and VF_MD was significantly (p=0.001) higher than that between NFLP_CD (in % area scale) and VF_MD (Figure 4). This shows that our method of VF simulation improved correlation with actual VF. The correlation appears to be linear in all stages of the disease. However, in the moderate to severe stage eyes, there were several outliers in which VF_MD indicated more severe damage than NFLP_MD.
FIGURE 4.
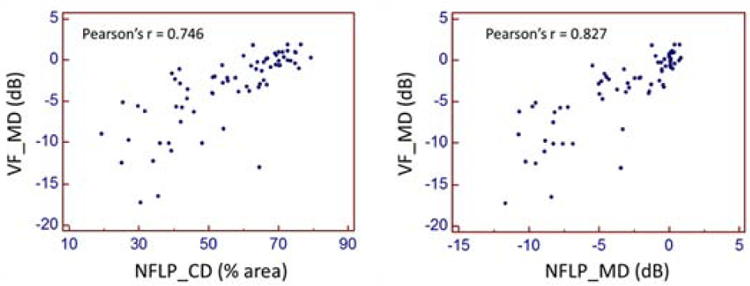
Pearson correlation of optical coherence tomographic angiography parameters and visual field mean deviation (VF-MD). NFLP_MD correlated with VF_MD better than NFLP_CD (P=0.001). NFLP_MD = nerve fiber layer plexus mean deviation; (simulated visual field mean deviation); NFLP_CD = nerve fiber layer plexus capillary density.
3.4. Diagnostic Accuracy of OCTA, Simulated VF and actual VF parameters
The diagnostic accuracies of NFLP_MD, NFLP_CD, VF_MD and NFL thickness were compared (Table 5). For the discrimination between normal and glaucoma groups, NFLP_MD had the highest AROC. But the AROC advantage was not statistically significant. When the specificity was fixed at 99%, NFLP_MD had a significantly better sensitivity than VF_MD (P<0.001) and overall NFL thickness (P=0.03).
Table 5.
Diagnostic Accuracy of Optical Coherence Tomographic Angiography and Visual Field Parameters
| NFLP_MD | NFLP_CD | VF_MD | NFL thickness | |
|---|---|---|---|---|
| AROC | 0.975 | 0.964 | 0.954 | 0.921 |
| Sensitivity at 99% Specificity | 97.4% | 87.2% | 66.7% | 82.1% |
AROC = The area under the receiver operating characteristic curve; VF_MD = visual field mean deviation; NFLP_MD = retina nerve fiber layer plexus mean deviation (simulated visual field mean deviation); NFLP_CD = nerve fiber layer plexus capillary density; NFL = retinal nerve fiber layer.
3.5. Location Correspondence of Nerve Fiber Layer Plexus Simulated VF and Actual VF
Comparing NFLP_simVF total deviation map and actual VF map (average sector total deviation) in 38 perimetric glaucomatous eyes, the worst sector was in the same or neighbor location in the same hemisphere 97% of the time. In 22 (58%) of these eyes, the worst sector of NFLP_simVF and actual VF were in the same location. In 15 (39%) perimetric glaucomatous eyes, the worst sectors were in adjacent locations in the same hemisphere.
3.6. Examples of Simulated and Actual Visual Fields
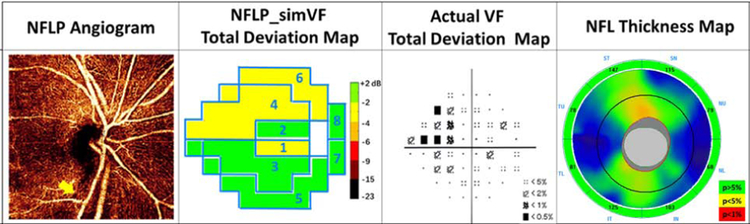
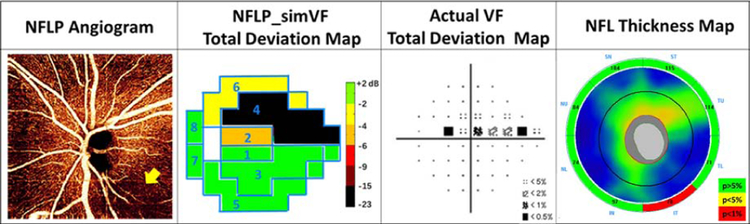
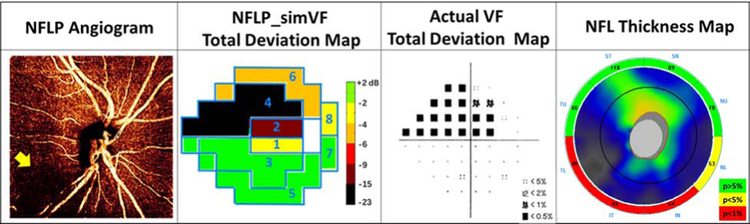
Several examples are shown to give insight on the performance NFLP_simVF. Figure 5 demonstrates that NFLP_simVF based on OCTA could detect glaucoma earlier than structural OCT NFL thickness measurement. Figure 6 shows a case of early glaucoma in which NFLP_simVF agreed with VF in the locations of glaucoma damage, but demonstrated greater severity. Figure 7 shows a moderate glaucoma case in which NFLP_simVF, VF, and NFL thickness all agreed well on the locations and severity of glaucoma damage. Figure 8 shows an advanced glaucoma case where the NFLP_simVF pattern agreed well with the VF map, but the NFLP_MD underestimated glaucoma severity compared to VF_MD. Generally, the color-coded NFLP_simVF maps identified glaucoma damage in the same sectors as VF, but the severity of damage did not always correspond.
FIGURE 5.
An early perimetric glaucoma eye with −3.4 dB visual field mean deviation (VF_MD) showing how the OCTA parameter can detect glaucoma earlier than structural OCT. The NFLP angiogram showed an infratemporal bundle of capillary dropout (arrow). Both the NFLP_CD (51.1%) and NFLP_MD (−1.5 dB) were abnormally low (below 99% specificity cutoff). The simulated VF (NFLP_simVF) shows defects in sectors 1, 4, and 6 in agreement with the actual VF. The NFL thickness were within normal limits (95% specificity cutoff) for overall (106 µm) and sector values.
FIGURE 6.
An early perimetric glaucoma with −0.7 dB visual field mean deviation (VF_MD) where the nerve fiber layer plexus simulated visual field (NFLP_simVF) showed more severe glaucoma damage relative to the actual VF. The NFLP angiogram showed an inferotemporal area of capillary dropout (arrow). In the NFLP_simVF map, sector 2 showed moderate defect in agreement with the actual VF, whereas in sector 4 the NFLP_simVF defect was more advanced than that on the actual VF. The retinal nerve fiber layer (NFL) thickness was thinnest in the inferotemporal (IT) sector, matching the location of the worst NFLP defect. The NFLP_MD of this case was −5.4 dB, significantly worse than the VF_MD.
FIGURE 7.
A moderate glaucoma eye with −7.5 dB visual field mean deviation (VF_MD) where the nerve fiber layer plexus simulated visual field (NFLP_simVF) agreed well with the actual VF. The NFLP angiogram showed an inferior and temporal capillary dropout (arrow). In the NFLP_simVF total deviation map, sector 2, 4, and 6 had moderate to advanced defects matching the actual visual field (VF). The retinal nerve fiber layer (NFL) thickness was abnormally thin in all inferior sectors, matching the location of the NFLP defects. The NFLP_MD of this case was –8.2 dB, agreeing well with the actual VF_MD.
FIGURE 8.
An advanced glaucoma with −17.3 dB visual field mean deviation (VF_MD) where the nerve fiber layer plexus simulated visual field (NFLP_simVF) showed less severe glaucoma damage relative to the actual VF. The NFLP angiogram showed inferior and superotemporal areas of capillary dropout (arrow). In the NFLP_simVF map, sector 3, 4 and 6 showed advanced defects in agreement with the actual VF, whereas in sector 5 the NFLP_simVF defect was less severe than that on the actual VF. The retinal nerve fiber layer (NFL) thickness was abnormally thin in the inferior and superotemporal sectors, matching the location of the NFLP defects. The NFLP_MD of this case was −11.7 dB, significantly better than the VF_MD.
3.7. The linear to linear scale comparison between OCTA and actual VF parameters
As opposed to converting OCTA parameters from a linear scale to a decibel scale, VF parameters can be converted from a decibel scale to a linear scale. We investigated whether the linear scale of OCTA parameters and VF parameters can be used for VF simulation. We averaged the VF total deviation in 1/Lambert linear scale (VF_AVG in 1/Lambert unit). Compared with VF_MD, the AROC of VF_AVG significantly dropped from 0.954 to 0.871 (P<0.01), the sensitivity at 99% specificity significantly dropped from 66.7% to 30.8% (P<0.01). The correlation between VF_AVG and NFLP_CD (Pearson’s r = 0.702) was significantly lower (P = 0.01) than that between VF_MD and NFLP_MD (Pearson’s r = 0.827). The reproducibility of VF_MD (ICC = 0.946) was better than VF_AVG (ICC = 0.754). We also found the dB scale NFLP_MD had a higher sensitivity of 97.4% at 99% specificity, while the linear scale had a sensitivity of 87.2%. The reproducibility of NFLP_MD (ICC = 0.954) was not significantly different from NFLP_CD (ICC = 0.972). Therefore, we believe the dB scale is better suited for clinical practice and VF simulation.
4. Discussion
Visual field test is the standard clinical test to evaluate glaucoma progression. However, the poor reproducibility of visual fields means that many tests over long periods of time are needed to reliably detect significant progression and determine its rate. According to one analysis, a glaucomatous eye experiencing −1 dB/year of rapid VF_MD loss would require 9 tests over 4 years to have a 90% chance of confirming statistically significant (p<0.05) trend of worsening.28 An objectively measured surrogate for VF that has better reproducibility could allow earlier detection of rapid glaucoma progression and more timely modification of treatment that could prevent vision loss. Structural OCT measurements have been used to predict 24–2 VF test results.29, 30 But to the best of our knowledge, this is the first investigation into the use of OCTA measurement for VF simulation. The correlation between OCTA and VF parameters tends to be better than the correlation between structural OCT and 24–2 VF, especially in the moderate and severe stages of glaucoma.9, 13–15, 18 Therefore OCTA could potentially be a better input for VF simulation for the purpose of monitoring glaucoma progression in the later stages.
Converting OCTA perfusion measurements as a functional equivalent serves several purposes in terms of clinical interpretation. First, information on the location and severity of visual loss is more closely related to the impact on the patient’s visual function and quality of life. Second, measuring the rate of glaucoma progression in a dB scale VF equivalent is more familiar to the clinician and better reflects the impact of glaucoma on visual function. On the linear scale customary for OCT and OCTA measurements (e.g. NFL thickness in μm or capillary density in % area), the progression of glaucoma appears very rapid in the early stages and very slow in the later stages.11 This distorted perspective makes it difficult to assess the speed of disease progression in a clinically appropriate fashion. As illustrated by this hypothetical comparison: An early glaucoma eye with 1 million retinal nerve fibers could afford to lose 50,000 fibers per year, which constitutes a −5% or −0.22 dB change; but a severe glaucoma eye with 100,000 nerve fibers left could not afford to lose the same number of fibers, which would constitute a −50% or −3 dB change. The logarithmic dB scale offers a more appropriate perspective to compare the rate of disease progression across all stages.31 Therefore converting all types of glaucoma assessment, whether structure, perfusion, or retinal sensitivity to a common dB scale would facilitate a better illustration of the speed of glaucoma disease progression.
The first step in converting NFLP_CD to the dB-scale VF equivalent is determining the floor value representing the residual perfusion that remains after nearly complete loss of retinal sensitivity. The second step is a regression operation to establish the relationship between dB-scale NFLP_CD and VF retinal sensitivity (total deviation). We had to pool all sectors together (except sector 7 and 8) to obtain a large enough sample to perform these steps. The pooling was also necessary because almost all severe glaucoma damage occurred in sectors 3 and 4 (arcuate sectors), so that there is insufficient information in the other sectors for floor and regression calculations. The results justified this approach - the correlation between pooled normalized logarithmic sector NFLP_CD and VF was high (r2 = 0.622) and highly linear. In addition, there was no significant difference in fit parameters if the sectors were separately analyzed. The floor fraction of 7% for NFLP_CD was low, much lower than the floor value of 38 um to 45 um for NFL thickness.32–34 These results indicate that NFLP_CD was a good basis for predicting and simulating visual fields, and that our simulation approach is workable.
An important metric for the performance of a simulation is the level of agreement with the actual quantity. We found that the agreement between NFLP_MD and VF_MD were good in the early and moderate PG stages. However, NFLP_MD tended to underestimate VF damage in the advanced PG stage. One reason for this discrepancy is the −23 dB limit we placed on the minimum value of sector NFLP_simVF to avoid extremely negative dB values that could be obtained when NFLP_CD is near the floor. This is 10 dB higher on the lower limit for actual VF total deviation of −33 dB on the Humphrey Field Analyzer. This limit was unavoidable – without it, the reproducibility of the simulated VF would be very poor in severe glaucoma eyes. Near the end stage of glaucoma damage, any small error in the NFLP_CD measurement or deviation in the simulation model (i.e. reference and floor fraction values) becomes magnified in the dB conversion calculation. Thus, the use of the NFLP-based VF simulation in disease staging needs to be done with caution – eyes suspected of having severe glaucoma need an actual VF for accurate staging.
In terms of the location of glaucoma damage, the agreement between the simulated and actual VF sector maps were good; as shown in the analysis of worst sector identification and the patterns of loss in the various stages of glaucoma severity. Locating VF loss is clinically important because damage in the paracentral sectors (1 & 2) are much more important to patient function than damage in the peripheral sectors (5, 6, 7, 8).
Looking at the structural aspect, we found that NFLP_MD had a better diagnostic accuracy than NFL thickness in detecting glaucoma. One possible reason is that OCTA might be detecting reduced perfusion associated with lower metabolism in dysfunctional retinal ganglion cells before they undergo apoptosis and cause structural thinning. Previous studies also indicated that OCTA may be able to detect glaucoma at an earlier stage. Akii et al. showed the disc and peripapillary retinal vessel density had a better diagnostic accuracy (AROC = 0.956) than NFL thickness (AROC = 0.772) in differentiating between PPG and normal groups.35 Yarmohammadi et al found disc and peripapillary retinal vessel density had a better AROC (0.84) than NFL thickness (0.77) in differentiating fellow eyes of unilateral glaucoma from normal eyes.36
We were surprised to further find that NFLP_MD had higher diagnostic sensitivity than NFLP_CD. Although the difference was not statistically significant, it was impressive that NFLP_MD had almost perfect diagnostic sensitivity. One possible explanation is that averaging the sector NFLP_CD values on a dB scale accentuates focal defect. Averaged on a linear scale, an isolated 90% loss in 1 out of 8 sectors (ignoring weighting) would average to −0.52 dB loss, versus on a dB scale, where the focal −10 dB loss (equal to 90% loss) in 1 out 8 sectors would average a −1.25 dB loss. Furthermore, weighting by VF area emphasizes the inferior and superior arcuate areas most often affected by early glaucoma. These possible advantages of NFLP_MD in early glaucoma detection deserve further study with a larger sample of very early glaucoma (PPG) participants.
The primary motivation of this simulation project was to improve the monitoring of glaucoma disease progression. The results here show that the simulated VF has the potential for achieving that goal. There was a high correlation between NFLP_MD and VF_MD and the correlation appeared to exist through all stages of glaucoma severity. The reproducibility of NFLP_MD was better than VF_MD, indicating a potential for earlier detection of statistically significant progression trends. What has been achieved here represents a first step – method development and preliminary validation. A large longitudinal study will be needed to determine if the simulated VF could identify patients with rapid glaucoma progression earlier than actual VF, and whether the rate of progression measured by the simulated VF agree well with actual VF.
There are several limitations of this study. First, the study only used the 24–2 VF test. The wider 30–2 VF better approximates the total VF and could correlate even better with NFLP capillary density, while the 10–2 could better detect central defects missed by the sparser wide-field tests.37 The use of the 24–2 VF was a compromise necessary to keep testing time within reasonable bounds for the sake of cost control and patient retention. Second, the sample size is small. Third, the majority of glaucoma patients in this study were in the early stage. Future studies with larger sample size are needed to validate the applicability of our new method in the more advanced stages of glaucoma. Fourth, the sectorwise approach does not provide fine-grained information on individual VF points. Future studies using OCTA of the macular could provide more detailed simulation of the central VF.
In conclusion, we have developed a method to simulate 24–2 VF based on OCTA NFLP measurements. The simulated VF correlated well with actual VF and is more reproducible than actual VF, thus holding promise for improving the monitoring of glaucoma progression. The VF simulation may also be useful in diagnosing or detecting early glaucoma, and in assessing the location and severity of glaucoma damage in patients who cannot be reliably tested with traditional perimetry methods. This is a first paper on this topic, and is based on a relatively small sample size and a simple simulation model. Refinement of the methodology and further evaluation of the proposed clinical applications in larger clinical studies are warranted.
Supplementary Material
Acknowledgments
Grant Support: NIH grants R01 EY023285, P30 EY010572, R01 EY010145, R21 EY027007, ARVO Dr. David L. Epstein Award, Oregon Health & Science University (OHSU) foundation, and an unrestricted grant from Research to Prevent Blindness.
Biography
Liang Liu, MD, is currently a research associate at Casey Eye Institute, Oregon Health & Science University, Portland. He earned MD at Peking University, Beijing in 2007. He had ophthalmic training and practice at Peking Union Medical College Hospital. Since he joined Dr. David Huang’s lab in 2014, he authored several highly cited papers in OCT angiography clinical application. His research interests include the development of new biomarkers to assess glaucoma and other optic neuropathies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: OHSU, Yali Jia, and David Huang have financial interest in Optovue, Inc., a company that may have a commercial interest in the results of this research and technology. These potential conflicts of interest have been reviewed and are managed by OHSU. The other authors do not report any potential financial conflicts of interest.
References
- 1.Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 2014;121:2081–2090. [DOI] [PubMed] [Google Scholar]
- 2.Katz J, Quigley HA, Sommer A. Repeatability of the Glaucoma Hemifield Test in automated perimetry. Invest Ophthalmol Vis Sci 1995;36:1658–1664. [PubMed] [Google Scholar]
- 3.Lee AC, Sample PA, Blumenthal EZ, et al. Infrequent confirmation of visual field progression. Ophthalmology 2002;109:1059–1065. [DOI] [PubMed] [Google Scholar]
- 4.Artes PH, Iwase A, Ohno Y, et al. Properties of perimetric threshold estimates from Full Threshold, SITA Standard, and SITA Fast strategies. Invest Ophthalmol Vis Sci 2002;43:2654–2659. [PubMed] [Google Scholar]
- 5.Mwanza JC, Oakley JD, Budenz DL, et al. Cirrus Optical Coherence Tomography Normative Database Study G. Ability of cirrus HD-OCT optic nerve head parameters to discriminate normal from glaucomatous eyes. Ophthalmology 2011;118:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hood DC, Raza AS. Method for comparing visual field defects to local RNFL and RGC damage seen on frequency domain OCT in patients with glaucoma. Biomed Opt Express 2011;2:1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Beltagi TA, Bowd C, Boden C, et al. Retinal nerve fiber layer thickness measured with optical coherence tomography is related to visual function in glaucomatous eyes. Ophthalmology 2003;110:2185–2191. [DOI] [PubMed] [Google Scholar]
- 8.Williams ZY, Schuman JS, Gamell L, et al. Optical coherence tomography measurement of nerve fiber layer thickness and the likelihood of a visual field defect. Am J Ophthalmol 2002;134:538–546. [DOI] [PubMed] [Google Scholar]
- 9.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Relationship between Optical Coherence Tomography Angiography Vessel Density and Severity of Visual Field Loss in Glaucoma. Ophthalmology 2016;123:2498–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wollstein G, Kagemann L, Bilonick RA, et al. Retinal nerve fibre layer and visual function loss in glaucoma: the tipping point. Br J Ophthalmol 2012;96:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Dastiridou A, Francis BA, et al. Comparison of Glaucoma Progression Detection by Optical Coherence Tomography and Visual Field. Am J Ophthalmol 2017;184:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L, Jia Y, Takusagawa HL, et al. Optical Coherence Tomography Angiography of the Peripapillary Retina in Glaucoma. JAMA Ophthalmol 2015;133:1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mammo Z, Heisler M, Balaratnasingam C, et al. Quantitative Optical Coherence Tomography Angiography of Radial Peripapillary Capillaries in Glaucoma, Glaucoma Suspect, and Normal Eyes. Am J Ophthalmol 2016;170:41–49. [DOI] [PubMed] [Google Scholar]
- 14.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Optical Coherence Tomography Angiography Vessel Density in Healthy, Glaucoma Suspect, and Glaucoma Eyes. Invest Ophthalmol Vis Sci 2016;57:October451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L, Edmunds B, Takusagawa H, et al. Projection-Resolved Optical Coherence Tomography Angiography of the Peripapillary Retina in Glaucoma. Am J Ophthalmol 2019;207:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia Y, Wei E, Wang X, et al. Optical Coherence Tomography Angiography of Optic Disc Perfusion in Glaucoma. Ophthalmology 2014;121:1322–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moghimi S, Bowd C, Zangwill LM, et al. Measurement Floors and Dynamic Ranges of OCT and OCT Angiography in Glaucoma. Ophthalmology 2019;126:980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takusagawa HL, Liu L, Ma KN, et al. Projection-Resolved Optical Coherence Tomography Angiography of Macular Retinal Circulation in Glaucoma. Ophthalmology 2017;124:1589–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express 2012;20:4710–4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao SS, Liu G, Huang D, et al. Optimization of the split-spectrum amplitude-decorrelation angiography algorithm on a spectral optical coherence tomography system. Optics letters 2015;40:2305–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang M, Wang J, Pechauer AD, et al. Advanced image processing for optical coherence tomographic angiography of macular diseases. Biomed Opt Express 2015;6:4661–4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao SS, Jia Y, Liu L, et al. Compensation for Reflectance Variation in Vessel Density Quantification by Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci 2016;57:4485–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le PV, Tan O, Chopra V, et al. Regional correlation among ganglion cell complex, nerve fiber layer, and visual field loss in glaucoma. Invest Ophthalmol Vis Sci 2013;54:4287–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garway-Heath DF, Poinoosawmy D, Fitzke FW, et al. Mapping the visual field to the optic disc in normal tension glaucoma eyes. Ophthalmology 2000;107:1809–1815. [DOI] [PubMed] [Google Scholar]
- 25.Tan O, Liu L, Liu L, et al. Nerve Fiber Flux Analysis Using Wide-Field Swept-Source Optical Coherence Tomography. Translational Vision Science & Technology 2018;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou X-H, McClish DK, Obuchowski NA. Statistical methods in diagnostic medicine. New York: John Wiley & Sons; 2009. [Google Scholar]
- 27.Hodapp EPRI, Anderson DR. Clinical decisions in glaucoma. St Louis: CV Mosby Co; 1993:52–61. [Google Scholar]
- 28.Wu Z, Saunders LJ, Daga FB, et al. Frequency of Testing to Detect Visual Field Progression Derived Using a Longitudinal Cohort of Glaucoma Patients. Ophthalmology 2017;124:786–792. [DOI] [PubMed] [Google Scholar]
- 29.Guo Z, Kwon YH, Lee K, et al. Optical Coherence Tomography Analysis Based Prediction of Humphrey 24–2 Visual Field Thresholds in Patients With Glaucoma. Invest Ophthalmol Vis Sci 2017;58:3975–3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bogunovic H, Kwon YH, Rashid A, et al. Relationships of retinal structure and humphrey 24–2 visual field thresholds in patients with glaucoma. Invest Ophthalmol Vis Sci 2014;56:259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caprioli J, Mock D, Bitrian E, et al. A method to measure and predict rates of regional visual field decay in glaucoma. Invest Ophthalmol Vis Sci 2011;52:4765–4773. [DOI] [PubMed] [Google Scholar]
- 32.Bowd C, Zangwill LM, Weinreb RN, et al. Estimating Optical Coherence Tomography Structural Measurement Floors to Improve Detection of Progression in Advanced Glaucoma. Am J Ophthalmol 2017;175:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sihota R, Sony P, Gupta V, et al. Diagnostic capability of optical coherence tomography in evaluating the degree of glaucomatous retinal nerve fiber damage. Invest Ophthalmol Vis Sci 2006;47:2006–2010. [DOI] [PubMed] [Google Scholar]
- 34.Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res 2007;26:688–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akil H, Huang AS, Francis BA, et al. Retinal vessel density from optical coherence tomography angiography to differentiate early glaucoma, pre-perimetric glaucoma and normal eyes. PLoS One 2017;12:e0170476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yarmohammadi A, Zangwill LM, Manalastas PIC, et al. Peripapillary and Macular Vessel Density in Patients with Primary Open-Angle Glaucoma and Unilateral Visual Field Loss. Ophthalmology 2018;125:578–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Traynis I, De Moraes CG, Raza AS, et al. Prevalence and nature of early glaucomatous defects in the central 10 degrees of the visual field. JAMA Ophthalmol 2014;132:291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.