Abstract
PURPOSE
The pilot-phase report of the Joven & Fuerte prospective cohort broadly characterizes and assesses the needs of Mexican young women with breast cancer (YWBC).
PATIENTS AND METHODS
Women age ≤ 40 years with nonmetastatic primary breast cancer were consecutively accrued from 2 hospitals. Data were collected at the first/baseline oncology visit and 2 years later using a sociodemographic survey, European Organisation for Research and Treatment of Cancer Quality-of-Life (QOL) Questionnaire Core 30 (QLQ-C30) and Breast Cancer–Specific QOL Questionnaire (QLQ-BR23), Hospital Anxiety and Depression Scale (HADS), Female Sexual Functioning Index (FSFI), Sexual Satisfaction Inventory, and patients’ medical records. Pearson χ2 and 2-sided t tests were used for statistical analysis. An unadjusted P value < .05 was considered significant.
RESULTS
Ninety patients were included, all with government health care coverage. Most had low monthly household incomes (98%) and at least a high school education (59%). There was a considerable prevalence of unpartnered patients (36%) and unmet parity (25%). Patients’ most common initial symptom was a palpable mass (84%), and they were most frequently diagnosed with stage III disease (48%), with 51% having had a physician visit ≤ 3 months since detection but 39% receiving diagnosis > 12 months later. At baseline, 66% of patients were overweight/obese, and this proportion had significantly increased by 2 years (P < .001). Compared with baseline, global QLQ-C30 had improved significantly by 2 years (P = .004), as had HADS-Anxiety (P < .001). However, both at baseline and at 2 years, nearly half of patients exhibited FSFI sexual dysfunction.
CONCLUSION
These preliminary findings demonstrate that YWBC in Mexico have particular sociodemographic and clinicopathologic characteristics, reinforcing the necessity to further describe and explore the needs of these young patients, because they may better represent the understudied and economically vulnerable population of YWBC in limited-resource settings.
INTRODUCTION
Breast cancer (BC) is the leading cause of cancer-related death and disability among young Mexican women,1,2 with up to 15% of cases involving women age ≤ 40 years.2 Young women with BC (YWBC) have challenging age-related health needs, facing diagnostic delays, presenting with late-stage disease at diagnosis and aggressive clinicopathologic tumor characteristics, and experiencing worse clinical outcomes.3,4 Additionally, they are especially vulnerable to psychosocial distress and age-related concerns like the potential risks of infertility, premature menopause, and harboring a genetic mutation. Therefore, they require specific supportive interventions such as emotional guidance, social support, timely fertility referral, and genetic counseling.5 For this matter, specialized programs to address the unmet needs of YWBC have been created in several developed countries, like Canada and the United States.6,7
However, in limited-resource settings, management of YWBC is mainly focused on the medical aspects of the disease, bypassing supportive and survivorship care.1,8,9 Until recently, Latin America lacked formal supportive programs dedicated to YWBC. Furthermore, most of the existing evidence regarding this group has been obtained from research studies in higher-resource settings, with limited representation of the Latin American population and its unique socioeconomic and cultural backgrounds.5
To our knowledge, Joven & Fuerte: Program for YWBC in Mexico (J&F) is the first Latin American program designed to address this gap through the provision of supportive care services and the creation of a prospective 5-year cohort of YWBC.9 This pilot-phase description explores patient-reported and provider-collected data. Patients’ sociocultural contexts and specific needs were assessed through tailored questionnaires and internationally validated instruments evaluating quality of life (QOL), psychological health, and sexual function. Providers registered clinical features, treatment, and patient outcomes using the US National Institutes of Health breast oncology and local disease common data elements. This study seeks to increase knowledge regarding the characteristics and issues of Mexican YWBC and to aid in identifying areas for targeted interventions to ultimately improve patients’ clinical and psychosocial outcomes.
PATIENTS AND METHODS
Study Design
The J&F prospective cohort study was designed to describe the clinicopathologic features and outcomes of YWBC, evaluate QOL, psychosocial and sexual needs, and treatment-related issues across a 5-year period, and collect tumor and blood samples for future investigations. Enrollment began in November 2014, after approval by institutional health regulatory authorities. Patients provided written informed consent for collection and research use of survey responses and clinical information. Data collection, management, analysis, and manuscript writing were performed exclusively by the authors.
Study Population
Women age ≤ 40 years with newly diagnosed nonmetastatic primary BC were accrued at 2 cancer centers with government health care coverage: Instituto Nacional de Cancerologia in Mexico City and Hospital San Jose in Nuevo Leon. All patients were consecutively invited to participate during their first/baseline visit to the oncology department before cancer treatment.
End Points
This pilot-phase description explores patient-reported and provider-collected data in the 2-year period after study registration. Study follow-up comprised time since registration, time to clinical events, and time since primary surgery.
Assessments
Patients completed in-person baseline and 2-year sociodemographic surveys based on the Young and Strong and PYNK program questionnaires (Data Supplement). Questions were selected, translated, and adapted by BC oncologists and psycho-oncologists, a patient navigator, and a young-patient advocate. Questions were added to address culturally specific issues of Mexican YWBC. Questionnaires were piloted in 10 patients, revised, and completed by 10 more patients.
Patient-reported issues were assessed with the European Organisation for Research and Treatment of Cancer QOL Questionnaire Core 30 (QLQ-C30) and Breast Cancer–Specific QOL Questionnaire (QLQ-BR23),10 Hospital Anxiety and Depression Scale (HADS),11 Female Sexual Functioning Index (FSFI),12 and Sexual Satisfaction Inventory (SSI).13 Providers collected clinical data at baseline and annually thereafter (Data Supplement).
Baseline Clinical and Demographic Characteristics
Qualitative descriptive statistics are provided for education level, parity, number of children, desire for (more) biologic offspring, breast self-examination (BSE), detection method, time between symptom and physician visit, time from symptom to diagnosis, concomitant serious illness, and family history of cancer. Providers registered patients’ body mass index (BMI), Eastern Cooperative Oncology Group performance status, menopausal status, and tumor clinical stage and subtype as defined by immunohistochemistry hormone receptor (HR) stain positivity (estrogen and/or progesterone receptor positivity) in ≥ 1% of cells and human epidermal growth factor receptor 2 (HER2) positivity based on 2010 American Society of Clinical Oncology/College of American Pathologists guidelines.
Follow-Up Data
At baseline and 2 years, patients reported their occupation, medical affiliation, living arrangements, monthly household income in Mexican pesos, household financial contribution, marital status, and current relationship status. Providers recorded (neo)adjuvant therapy and breast surgery at 6 months; reconstruction, at 1 year; and BMI, recurrence, and death at 2 years. Changes in BMI between baseline and 2 years were assessed with a 1-sided Pearson χ2 test (considered significant at P < .05).
QOL, Depression, Anxiety, Sexual Function, and Satisfaction
For nonrecurrent patients, group and matched differences between baseline and 2-year data were assessed with a 2-sided t test for QLQ-C30 score (0-100), QLQ-BR23 domain (0-100), HADS-Anxiety, HADS-Depression, FSFI full scale, and SSI total score. Cut points for HADS (doubtful case, 8-10; probable case, ≥ 11), FSFI (morbidity < 26.55), and SSI (morbidity ≤ 111) were used to describe morbidity levels at baseline and 2 years. Statistical tests were nominally significant at unadjusted P < .05.
RESULTS
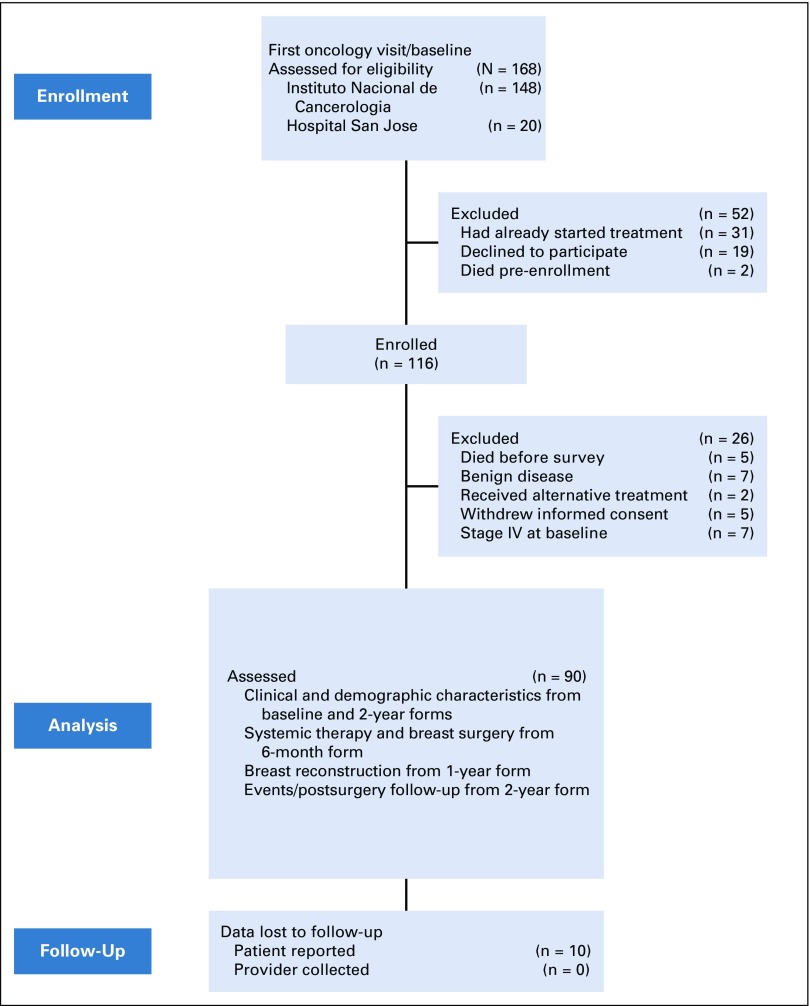
From November 2014 to April 2016, 186 patients were invited to participate, and 116 agreed to be enrolled. Twenty-six were excluded, leaving 90 patients for analysis (Fig 1). Participants’ baseline clinical and sociodemographic characteristics are listed in Tables 1 and 2. Median age was 35 years (range, 21-40 years); 59% had at least a high school education, 26% were childless, and 25% desired (more) children.
FIG 1.
Flow diagram of study.
TABLE 1.
Patient-Reported Clinical and Demographic Characteristics at Baseline (N = 90)
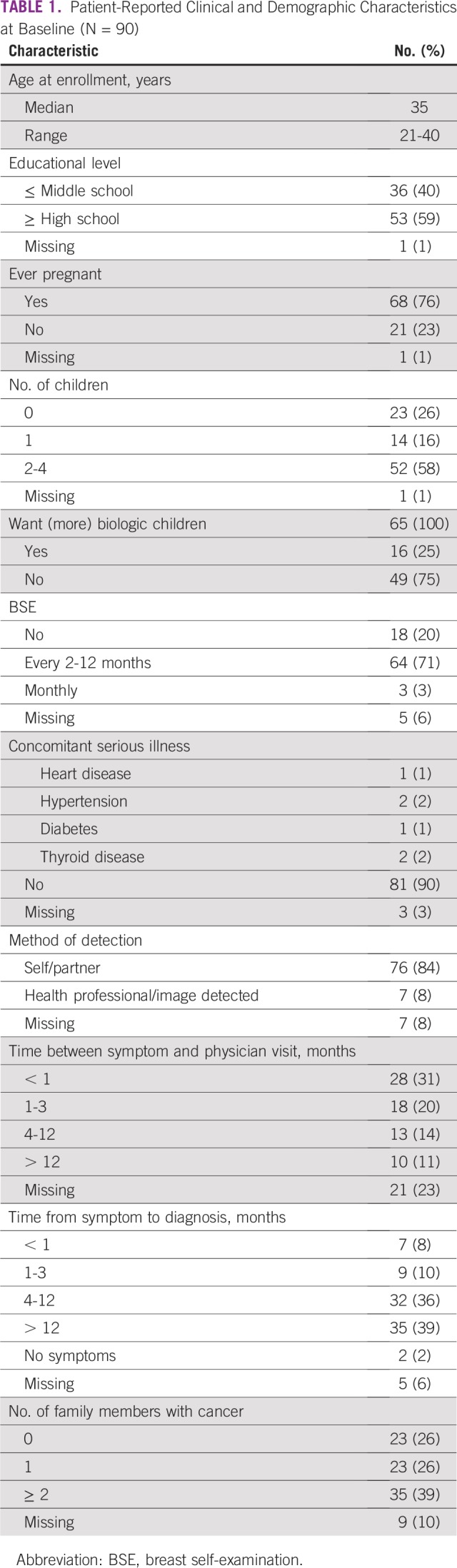
TABLE 2.
Provider-Collected Clinical and Demographic Characteristics at Baseline (N = 90)
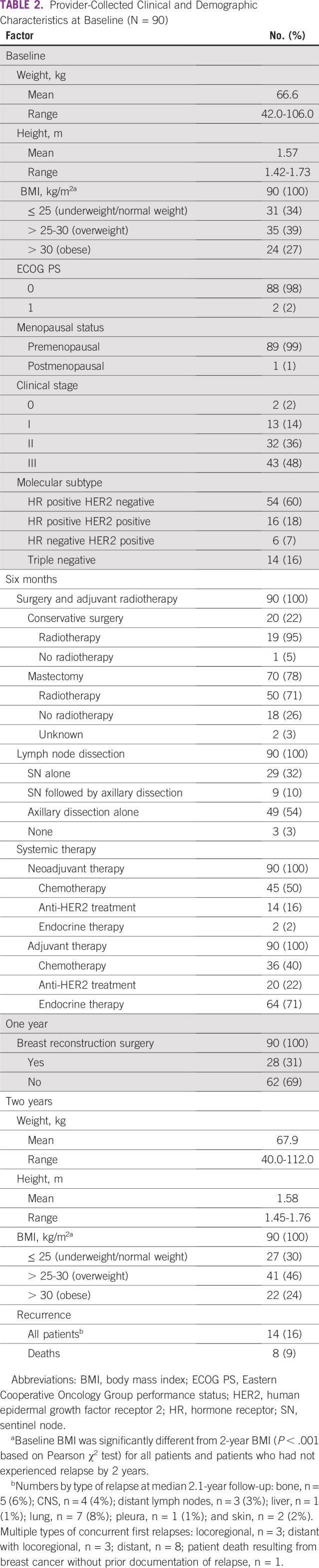
Although 74% reported performing BSE, only 3% practiced it monthly. Additionally, 84% reported self- or partner-detected tumor, and 51% reported having a physician visit within 3 months of the first symptom, whereas 39% received diagnosis > 12 months after the initial symptom.
Clinical stages were as follows: I, 14%; II, 36%; and III, 48%. Tumor subtypes were as follows: HR positive HER2 negative, 60%; HR positive HER2 positive, 18%; HR negative HER2 positive, 7%; and triple negative, 16%. Regarding locoregional therapy, 78% of patients underwent mastectomy and 67% axillary dissection, 74% received adjuvant radiotherapy, and 31% underwent breast reconstruction. Systemic treatment consisted of (neo)adjuvant chemotherapy for 50% of patients, adjuvant endocrine therapy for 71%, and anti-HER2 treatment for 22%.
By the 2-year form, median follow-up after primary surgery was 2.1 years. At that timepoint, 16% of patients had experienced recurrence and 1 patient had died without prior documented relapse. All 8 deaths (9%) were secondary to BC.
At baseline, mean patient height was 1.57 m and mean weight was 66.6 kg, which had increased to 67.9 kg at 2 years. BMI categories changed significantly (P < .001) between baseline and 2 years for all patients: normal or underweight, from 34% to 30%; overweight, from 39% to 46%; and obese, from 27% to 24%.
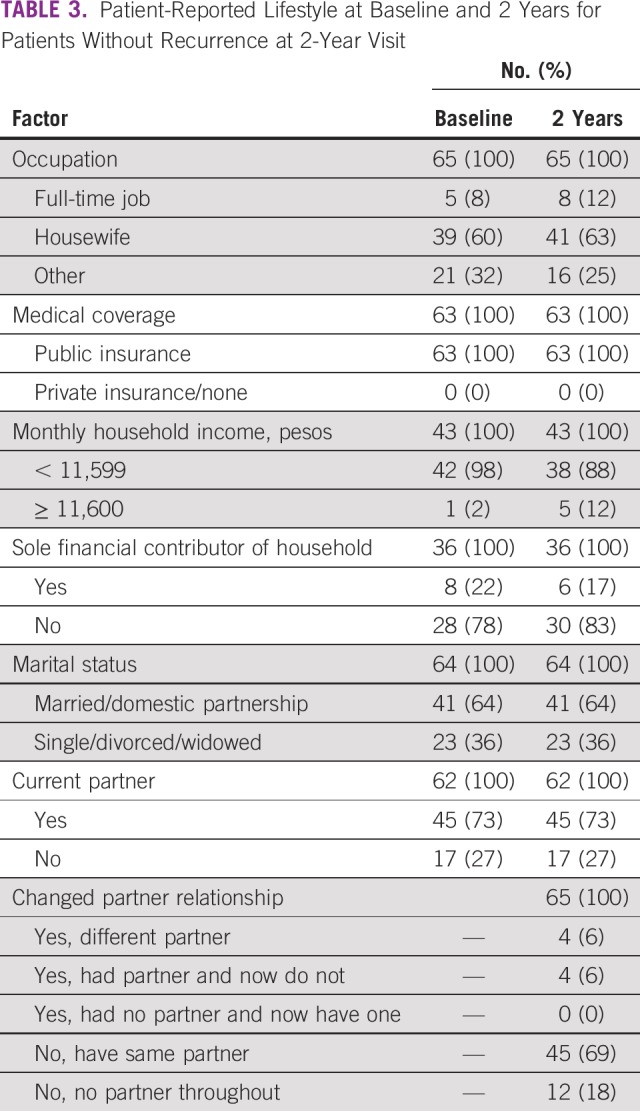
Nonrecurrent patients reported similar sociodemographic characteristics at baseline and 2 years (Table 3), with 60% versus 63% being housewives, 22% versus 17% being the sole financial contributor of the household, 36% being single, divorced, or widowed at both timepoints, and 69% having the same partner at follow-up. At both baseline and 2 years, most patients (98% and 88%, respectively) had monthly incomes < 11,600 Mexican pesos (US$610; minimum Mexican wage per day, US$5.25).14 Of these, > 50% had monthly incomes < 2,700 Mexican pesos (US$142).
TABLE 3.
Patient-Reported Lifestyle at Baseline and 2 Years for Patients Without Recurrence at 2-Year Visit
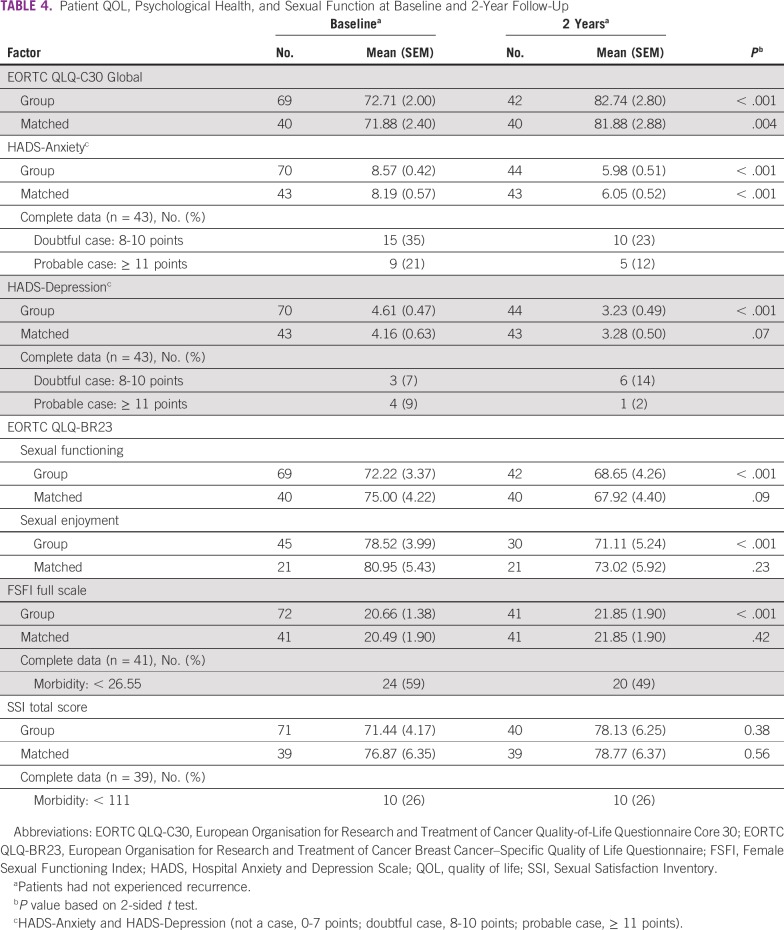
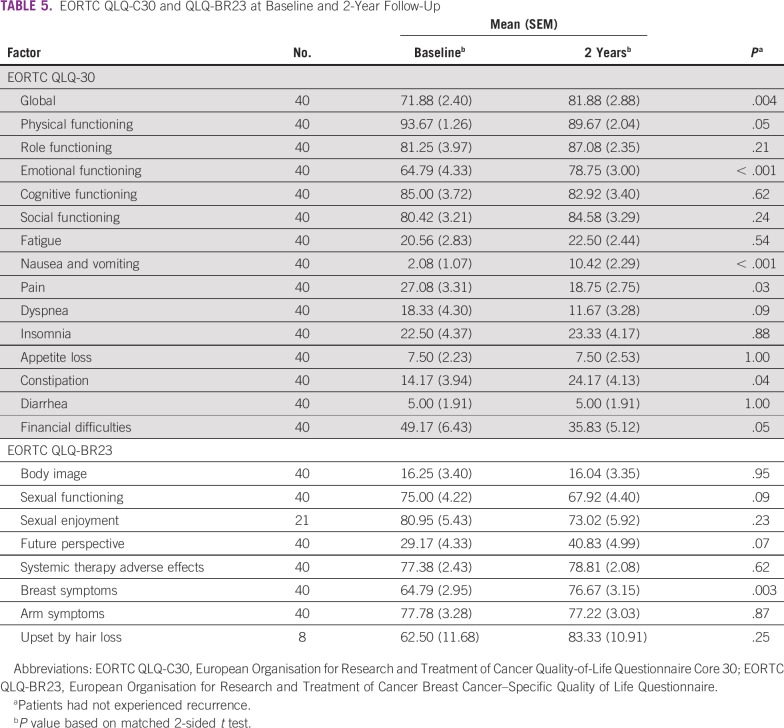
Changes between baseline and 2 years regarding QOL, depression, anxiety, sexual function, and sexual satisfaction are summarized in Tables 4 and 5. Statistically, QOL scores had improved significantly by 2 years in matched tests for mean QLQ-C30 global QOL (P = .004), emotional functioning (P < .001), pain (P = .03), and financial difficulties (P = .05). However, scores were statistically worse for physical functioning (P = .05), nausea/vomiting (P < .001), and constipation (P = .04). Likewise, QLQ-BR23 indicated significantly more breast symptoms (P = .003) at 2 years. HADS-Anxiety was significantly reduced (P < .001), from 21% at baseline to 12% at 2 years; HADS-Depression had a borderline reduction (P = .07), from 9% at baseline to 2% at 2 years. FSFI scores revealed sexual dysfunction rates of 59% at baseline and 49% at 2 years, with no significant change. SSI morbidity was present in 26% of patients at both timepoints.
TABLE 4.
Patient QOL, Psychological Health, and Sexual Function at Baseline and 2-Year Follow-Up
TABLE 5.
EORTC QLQ-C30 and QLQ-BR23 at Baseline and 2-Year Follow-Up
DISCUSSION
Pilot-phase baseline and 2-year data are reported for the first prospective Mexican YWBC cohort. Patients were characterized by sociodemographic, clinical, and psychosocial features, as well as QoL domains.
All patients were covered by Seguro Popular (active from 2003 to 2020), a Mexican government health care insurance that enabled diagnosis and management of BC and some other cancer types among the otherwise nonentitled population. This insurance covered almost half of the Mexican population15-17; by this means, practically all Mexican women with BC had access to anticancer treatment, and the proportion of patients lost to follow-up because of an inability to pay for treatment has been minimized, from 30% to < 6%.18
Notably, 22% of patients were the sole financial contributor of their household, and 98% had a low monthly household income, which is in line with recent National Institute of Statistics and Geography data indicating that 96% of Mexicans have monthly incomes < 11,600 Mexican pesos (US$610), equivalent to the minimum wage for 10 days of work in the United States.14,19 Therefore, BC diagnosis could represent a significant economic burden to this young group. Regarding educational background, 59% of patients had completed at least high school, which is consistent with Mexican education levels and other Mexican and Brazilian BC cohorts, with younger generations exceeding the literacy levels of older ones.4,20,21
At diagnosis, 36% of patients were single, divorced, or widowed, consistent with previous reports of YWBC that have indicated rates of 30% to 45%.4,22,23 At 2 years, 69% of patients had the same partner, whereas 6% had changed partners and 6% no longer had one. Similarly, others have concluded that most BC patient-partner relationships were stable in the short term, with 5.5% being divorced or separated at 18-month follow-up.24
Regarding parity, a considerable 26% of the cohort had never been pregnant, and 25% wanted (more) children, raising concern about treatment-related risks of premature ovarian failure and fertility impairment. Considering that the average age at first birth in the general Mexican population is 23.7 years (lower than the world average of 26.3 years),25 it is not surprising that other authors have reported even higher nulliparous rates of 35% among patients in the United States.26 In a previous study, only 31% of Mexican YWBC recalled receiving information about infertility risks related to BC treatment, and just 1 patient underwent embryo preservation.22 Therefore, it is crucial that all young patients receive fertility counseling and appropriate management. Unfortunately, these measures are usually unaffordable, given that neither private nor Mexican government health insurance covers them.
One of the most relevant findings in this report was the high proportion of advanced-stage diagnoses (48%), comparable to the 40% reported in previous retrospective Mexican series,27,28 although higher than in Chile (31%), Brazil (32%), and Peru (38%).29-31 In contrast, stage III diagnoses comprised 17% and 27% of YWBC cases in large US and New Zealand cohorts, respectively.32,33
Advanced stage at diagnosis in YWBC may occur because screening recommendations exclude patients age ≤ 40 years34; consequently, young women only seek medical advice when symptomatic. In this cohort, 84% presented with a self- or partner-detected mass, a rate similar to the 92% observed among young Egyptian women35 and in line with a Mexican retrospective study in which the most common presenting symptom was a self-detected breast mass.36 Remarkably, only 3% of patients performed Mexican guideline–recommended monthly BSE.37 This low BSE practice may reflect young women’s limited BC awareness,38 which could contribute to delays in seeking medical care.39
Moreover, 51% of patients sought medical care within 3 months of the initial symptom, but a striking 39% reported receiving a BC diagnosis after 12 months. This suggests that YWBC may also have diagnostic delays arising from health care providers lacking suspicion of malignancy or misinterpreting symptoms,28 which could contribute to advanced disease at presentation.40
Additionally, tumor biology in young women (more frequently triple negative, HER2 positive, or luminal B)27,36 might also explain advanced stage at diagnosis.4,28,29 It should be noted that the high proportion (25%) of HER2-positive tumors in this cohort is consistent with previous studies in YWBC.27,41
By a median 2.1 years of follow-up, 16% of patients had experienced relapse, similar to the 15% recurrence rate reported in a Mexican retrospective study27 and higher than the 11.7% recurrence rate reported in a retrospective young US cohort at the same timepoint.42 Likewise, a substantive 9% of patients had died at 2 years, comparable to the 10% mortality rate reported in the previously mentioned Mexican cohort.27
Regarding weight and BMI, patients had high rates of overweight/obesity (66%), consistent with the 73% overweight/obesity proportion in the Mexican general female population.43 Moreover, the percentage of overweight patients increased from baseline (39%) to 2 years (46%). This is worrisome, given that overweight and obesity may be risk factors for BC recurrence and systemic therapy failure, including in premenopausal women.44,45 Therefore, cancer care teams should emphasize the importance of weight management among patients and help them initiate weight-loss programs.46
Regarding QOL, the baseline mean global QLQ-C30 score was 71.9 in this cohort. Global QOL scores in other studies focused on young women have ranged between 56.8 and 66.7.47-49 The better QOL in this cohort may arise from differences between studies regarding sociocultural factors, adaptation and resilience skills, timing of assessments, and advanced-disease case proportions.
At 2 years, matched patient mean global QOL scores indicated significant improvement, to 81.9 (P = .004), representing an increase in ≥ 10 points as compared with baseline, which, without normative levels, has been recognized by others as clinically significant.50 The international SUPREMO trial including both young and older BC patients also found a 10-point improvement, from 60.9 at baseline to 70.2 at 2 years.51 It is reassuring that, regardless of baseline score, global QOL in YWBC can improve over time to be comparable to that of age-matched healthy women.47,52 Emotional functioning, pain, and financial difficulties also significantly improved over time in this cohort; similarly, a Moroccan study reported better QOL in these domains at 1-year follow-up.48
Nevertheless, our results, like others, indicate that not all QOL domains improve over time.49,51 Patients’ physical functioning, nausea/vomiting, constipation, and breast symptom scores were statistically worse at 2 years, although only the last 2 could be considered clinically relevant because of their ≥ 10-point change.50 Notably, decreased physical functioning was also found in a prospective study evaluating QOL in Malaysian patients with BC at baseline and 1-year follow-up.53 The increased intensity of adverse effects such as nausea/vomiting and constipation in this cohort may have resulted from patients being treatment naïve at baseline, whereas at 2 years, they had undergone management, including chemotherapy (90%) and endocrine therapy (71%). Interestingly, patients’ breast symptom scores at both assessments were considerably worse than those of other young groups,49,54 possibly because relatively few of our patients (31%) underwent breast reconstruction.
As for HADS-Anxiety and HADS-Depression, patients’ scores had improved at 2 years. Others have also reported that the prevalence of anxiety and depression in BC survivors decreased with time.55,56 Higher baseline anxiety and depression levels might be related to the psychological distress imposed by the recent BC diagnosis and the overwhelming feeling induced by treatment options, possible adverse effects, and decision-making processes.57,58
Patients’ QLQ-BR23 scores indicated moderately high baseline sexual functioning and enjoyment, with mean scores of 72.2 and 78.5, respectively. Other age-independent BC populations have reported similar baseline sexual functioning scores of 76.7 to 77.3, but much lower sexual enjoyment scores of 50.0 to 55.6.48,59 Diversity across studies could be the result of differences in age and ethnic, social, or cultural circumstances.
At 2 years, matched analysis indicated patients experienced numerically worsened sexual QOL, with sexual functioning and enjoyment scores declining to 68.5 and 71.1, respectively. Similarly, previous studies have reported sexual functioning deterioration and higher rates of sexual dysfunction in YWBC compared with older BC survivors and healthy women their age.60-63
As for sexual function measured by FSFI, the baseline scores of this cohort showed a lower sexual dysfunction (FSFI morbidity) rate (59%), compared with a meta-analysis of patients with BC (73%).64 However, matched analysis indicated that a substantial proportion of the cohort (49%) remained sexually dysfunctional at 2 years.
Overall, this cohort can be presumed to be representative of YWBC in Mexico because it consists of patients who received care at two of the most important referral cancer centers for both the northern and southern parts of the country. Moreover, our findings can be generalized to other young Mexican patients with BC, given that most of the population in Mexico receives care in public health care institutions, as did patients in this cohort.
Additionally, the QOL, emotional, and sexuality findings may serve as references for the development of targeted interventions especially designed for the needs of young Mexican patients. Systematically asking for patients’ most relevant care needs and symptoms will make it possible for their multidisciplinary health care teams to address them and offer directed, timely solutions.
Over these years of follow-up, the J&F cohort has encountered some sustainability barriers, mainly related to financial constraints and limited personnel resources. Because it is a nonprofit, nongovernmental program, J&F must continuously seek short- and long-term sustainability options, mainly through governmental funds, donations from nongovernmental organizations, and grants, to maintain its operations, research, and support services. These resource struggles have resulted in a considerable attrition in patient survey completion over time.65 Therefore, now a fewer number of follow-up surveys are applied, and patient outreach occurs via social media and telephone.
In conclusion, this report provides a broad pilot-phase description of the J&F Mexican YWBC cohort. Subsequent analyses and reports will serially document the characteristics and needs of the complete cohort throughout the planned 5-year follow-up of the program, enabling a comprehensive description and evaluating each studied domain and its association with other clinical and therapeutic aspects.
The most relevant findings were that most patients from the 2 public hospitals had low household incomes, high levels of education, and a high prevalence of unmet parity. Furthermore, they were frequently married, although the proportion of unpartnered patients was not negligible. Patients’ most frequent initial symptom was a palpable mass, and most were diagnosed with stage III disease. Moreover, they were often overweight/obese, and this proportion had increased at follow-up. Finally, although patients experienced improvement in some QOL domains, others were significantly worse at follow-up, with a high rate of sexual dysfunction.
Analysis of these preliminary data suggests that select sociodemographic and clinicopathologic characteristics of Mexican YWBC might be different from what has been described in more developed contexts, reinforcing the need to further characterize this young group of patients, because they may better represent the understudied and economically vulnerable population of YWBC in limited-resource settings. These results serve as an initial characterization and provide the foundation for additional lines of research. Ultimately, these findings will facilitate the development and implementation of targeted strategies to better support this unique group and improve patient-centered care.
ACKNOWLEDGMENT
We thank Ann Partridge (Young and Strong Program for Young Women With Breast Cancer) and Ellen Warner (PYNK: Breast Cancer Program for Young Women); Abelardo Meneses, Alexandra Bukowski, Cesar J. Marquez-Perez, Christian Aguila, Janeth Castro, Jessica St. Louis, Juan Matus-Santos, Pier Ramos-Elias, Yoatzin Vega, and the breast cancer teams of the Departamento de Tumores Mamarios y Departmeno de Investigacion, Instituto Nacional de Cancerologia and the Centro de Cancer de Mama, Tecnologico de Monterrey; and Richard Zellars of the US National Institutes of Health Breast Oncology Local Disease Task Force.
Footnotes
Presented as an abstract at the 39th Annual San Antonio Breast Cancer Symposium, San Antonio, TX, December 6-10, 2016.
AUTHOR CONTRIBUTIONS
Conception and design: Cynthia Villarreal-Garza, Alejandra Platas, Judy-Anne Chapman, Alejandro Mohar, Paul Goss
Administrative support: Cynthia Villarreal-Garza, Alejandra Platas, Juan E. Bargallo-Rocha
Provision of study material or patients: Cynthia Villarreal-Garza, Alejandra Platas, Carmen L. Galvez-Hernandez, Juan E. Bargallo-Rocha, Dione Aguilar, Mauricio Canavati
Collection and assembly of data: Cynthia Villarreal-Garza, Alejandra Platas, Melina Miaja, Alan Fonseca, Fernanda Mesa-Chavez, Marisol Garcia-Garcia, Judy-Anne Chapman, Edna A. Lopez-Martinez, Claudia Pineda, Carmen L. Galvez-Hernandez, Andrea Castro-Sanchez, Bertha-Alejandra Martinez-Cannon, Regina Barragan-Carrillo, Jose F. Muñoz-Lozano, Paul Goss, Juan E. Bargallo-Rocha, Dione Aguilar, Mauricio Canavati
Data analysis and interpretation: Cynthia Villarreal-Garza, Fernanda Mesa-Chavez, Marisol Garcia-Garcia, Judy-Anne Chapman, Edna A. Lopez-Martinez, Alejandro Mohar, Carmen L. Galvez-Hernandez, Regina Barragan-Carrillo, Jose F. Muñoz-Lozano, Paul Goss, Juan E. Bargallo-Rocha, Servando Cardona
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Cynthia Villarreal-Garza
Consulting or Advisory Role: Roche, Novartis, Pfizer, Eli Lilly
Speakers’ Bureau: Roche, Myriad Genetics, Novartis
Research Funding: AstraZeneca (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Roche, MSD Oncology, Pfizer
No other potential conflicts of interest were reported.
REFERENCES
- 1. Anderson BO, Yip CH, Smith RA, et al: Guideline implementation for breast healthcare in low-income and middle-income countries: Overview of the breast health global initiative Global Summit 2007. Cancer 113:2221-2243, 2008 (suppl) [DOI] [PubMed] [Google Scholar]
- 2.Villarreal-Garza C, Aguila C, Magallanes-Hoyos MC, et al. Breast cancer in young women in Latin America: An unmet, growing burden. Oncologist. 2013;18:1298–1306. doi: 10.1634/theoncologist.2013-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han JG, Jiang YD, Zhang CH, et al. Clinicopathologic characteristics and prognosis of young patients with breast cancer. Breast. 2011;20:370–372. doi: 10.1016/j.breast.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 4. Franzoi MA, Rosa DD, Zaffaroni F, et al: Advanced stage at diagnosis and worse clinicopathologic features in young woman with breast cancer: A sub- analysis of Brazilian population through the AMAZONA III study. J Glob Oncol 10.1200/JGO.19.00263. [DOI] [PMC free article] [PubMed]
- 5. Gálvez-Hernández CL, González-Robledo MC, Barragán-Carrillo R, et al: Special needs of young women with breast cancer in limited resource settings. Rev Invest Clin 69:210-222, 2017. [PubMed]
- 6.Partridge AH, Ruddy KJ, Kennedy J, et al. Model program to improve care for a unique cancer population: Young women with breast cancer. J Oncol Pract. 2012;8:e105–e110. doi: 10.1200/JOP.2011.000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali A, Warner E. PYNK: Breast cancer program for young women. Curr Oncol. 2013;20:e34–e39. doi: 10.3747/co.20.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villarreal-Garza C, Martinez-Cannon BA, Platas A, et al. Specialized programs to support young women with breast cancer. Curr Opin Support Palliat Care. 2015;9:308–316. doi: 10.1097/SPC.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 9.Villarreal-Garza C, Castro-Sánchez A, Platas A, et al. “Joven & Fuerte”: Program for young women with breast cancer in Mexico—Initial results. Rev Invest Clin. 2017;69:223–228. doi: 10.24875/ric.17002280. [DOI] [PubMed] [Google Scholar]
- 10.Cerezo O, Oñate-Ocaña LF, Arrieta-Joffe P, et al. Validation of the Mexican-Spanish version of the EORTC QLQ-C30 and BR23 questionnaires to assess health-related quality of life in Mexican women with breast cancer. Eur J Cancer Care (Engl) 2012;21:684–691. doi: 10.1111/j.1365-2354.2012.01336.x. [DOI] [PubMed] [Google Scholar]
- 11.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 12.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 13. Alvarez-Gayou Jurgenson JL, Honold Espinosa JA, Millán Alvarez P: ¿Qué hace una relación sexual?: Percepción de un grupo de mujeres y hombres mexicanas y diseño de una escala autoaplicable para la evaluación de la satisfacción sexual [in Spanish]. Arch Hispanoam Sexol 11:91-110, 2005. [Google Scholar]
- 14. Secretaría del Trabajo y Previsión Social México: Salarios mínimos 2019. https://www.gob.mx/cms/uploads/attachment/file/426395/2019_Salarios_Minimos.pdf.
- 15. Secretaría de Salud México: El seguro popular 2019. http://www.documentos.seguro-popular.gob.mx/dgss/CAUSES_2019_Publicación.pdf.
- 16. Secretaría de Salud México: Beneficiarios de protección social en salud de seguro popular 2019. https://datos.gob.mx/busca/dataset/beneficiarios-de-proteccion-social-en-salud-de-seguro-popular.
- 17.Knaul FM, González-Pier E, Gómez-Dantés O, et al. The quest for universal health coverage: Achieving social protection for all in Mexico. Lancet. 2012;380:1259–1279. doi: 10.1016/S0140-6736(12)61068-X. [DOI] [PubMed] [Google Scholar]
- 18. Arce-Salinas C, Lara-Medina FU, Alvarado-Miranda A, et al: Evaluation of breast cancer treatment at a tertiary-level institution with popular health insurance in Mexico [in Spanish]. Rev Invest Clin 64:9-16, 2012. [PubMed]
- 19. INEGI: Población ocupada con ingresos de hasta un salario mínimo 2019. https://www.inegi.org.mx/app/tabulados/default.html?nc=602.
- 20. Parker SW, Pederzini C: Gender differences in education in Mexico. http://documents.worldbank.org/curated/en/659741468752986916/pdf/multi-page.pdf.
- 21.Villarreal-Garza C, Platas A, Martinez-Cannon BA, et al. Information needs and Internet use of breast cancer survivors in Mexico. Breast J. 2017;23:373–375. doi: 10.1111/tbj.12747. [DOI] [PubMed] [Google Scholar]
- 22.Villarreal-Garza C, Martinez-Cannon BA, Platas A, et al. Fertility concerns among breast cancer patients in Mexico. Breast. 2017;33:71–75. doi: 10.1016/j.breast.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Partridge AH, Gelber S, Peppercorn J, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22:4174–4183. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- 24.Dorval M, Maunsell E, Taylor-Brown J, et al. Marital stability after breast cancer. J Natl Cancer Inst. 1999;91:54–59. doi: 10.1093/jnci/91.1.54. [DOI] [PubMed] [Google Scholar]
- 25.Mathews TJ, Hamilton BE. Mean age of mothers is on the rise: United States, 2000-2014. NCHS Data Brief. 2016;232:1–8. [PubMed] [Google Scholar]
- 26.Ruddy KJ, Gelber SI, Tamimi RM, et al. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J Clin Oncol. 2014;32:1151–1156. doi: 10.1200/JCO.2013.52.8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villarreal-Garza C, Mohar A, Bargallo-Rocha JE, et al. Molecular subtypes and prognosis in young Mexican women with breast cancer. Clin Breast Cancer. 2017;17:e95–e102. doi: 10.1016/j.clbc.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 28. doi: 10.1200/JGO.19.00093. Unger-Saldaña K, Fitch-Picos K, Villarreal-Garza C: Breast cancer diagnostic delays in young Mexican patients are associated with lack of suspicion by healthcare providers at first presentation. J Glob Oncol 10.1200/JGO.19.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Lima Vazquez F, Silva TB, Da Costa Vieira RA, et al. Retrospective analysis of breast cancer prognosis among young and older women in a Brazilian cohort of 738 patients, 1985-2002. Oncol Lett. 2016;12:4911–4924. doi: 10.3892/ol.2016.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acevedo F, Camus M, Sanchez C. Breast cancer at extreme ages: A comparative analysis in Chile. Asian Pac J Cancer Prev. 2015;16:1455–1461. doi: 10.7314/apjcp.2015.16.4.1455. [DOI] [PubMed] [Google Scholar]
- 31. Vallejos Sologuren C, Gomez HL, Abugattas JE, et al: Clinicopathologic, molecular subtype, and survival prognostic features in premenopausal breast cancer patients by age at diagnosis. J Clin Oncol 28, 2010 (suppl; abstr 653) [Google Scholar]
- 32.Zimmer A. S., Zhu K, Steeg PS, et al. Analysis of breast cancer in young women in the Department of Defense (DOD) database. Breast Cancer Res Treat. 2018;168:501–511. doi: 10.1007/s10549-017-4615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seneviratne S, Lawrenson R, Harvey V, et al. Stage of breast cancer at diagnosis in New Zealand: Impacts of socio-demographic factors, breast cancer screening and biology. BMC Cancer. 2016;16:129. doi: 10.1186/s12885-016-2177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Breast Cancer (version 3.2018). Plymouth Meeting, PA, National Comprehensive Cancer Network, 2018.
- 35.Darwish AD, Helal AM, Aly El-Din NH, et al. Breast cancer in women aging 35 years old and younger: The Egyptian National Cancer Institute (NCI) experience. Breast. 2017;31:1–8. doi: 10.1016/j.breast.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Robles-Castillo J, Ruvalcaba-Limón E, Maffuz A, et al. Cancer de mama en mujeres mexicanas menores de 40 años [in Spanish] Ginecol Obstet Mex. 2011;79:482–488. [PubMed] [Google Scholar]
- 37. Dávalos Rodríguez ML, Hernández San Román E (eds): Prevención, tamizaje y referencia oportuna de casos sospechosos de cáncer de Mama. http://www.cenetec-difusion.com/CMGPC/S-001-08/ER.pdf.
- 38.Üçüncü MZ, Üçüncü MM, Toprak D. Evaluation knowledge, attitude, and behaviour for breast cancer among young women living in two different habitats of Turkey. Asian Pac J Cancer Prev. 2018;19:3179–3185. doi: 10.31557/APJCP.2018.19.11.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. doi: 10.1093/eurpub/ckt131. Jassem J, Ozmen V, Bacanu F, et al: Delays in diagnosis and treatment of breast cancer: A multinational analysis. Eur J Public Health 24:761-767, 2014. [DOI] [PubMed] [Google Scholar]
- 40.Ruddy KJ, Gelber S, Tamimi RM, et al. Breast cancer presentation and diagnostic delays in young women. Cancer. 2014;120:20–25. doi: 10.1002/cncr.28287. [DOI] [PubMed] [Google Scholar]
- 41.Collins LC, Marotti JD, Gelber S, et al. Pathologic features and molecular phenotype by patient age in a large cohort of young women with breast cancer. Breast Cancer Res Treat. 2012;131:1061–1066. doi: 10.1007/s10549-011-1872-9. [DOI] [PubMed] [Google Scholar]
- 42.Alabdulkareem H, Pinchinat T, Khan S, et al. The impact of molecular subtype on breast cancer recurrence in young women treated with contemporary adjuvant therapy. Breast J. 2018;24:148–153. doi: 10.1111/tbj.12853. [DOI] [PubMed] [Google Scholar]
- 43. Dávila-Torres J, Gonzalez-Izquierdo JJ, Barrera-Cruz A: Panorama de la obesidad en México [in Spanish]. Rev Med Inst Mex Seguro Soc 53:1-12, 2015. [PubMed] [Google Scholar]
- 44.Loi S, Milne RL, Friedlander ML, et al. Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1686–1691. doi: 10.1158/1055-9965.EPI-05-0042. [DOI] [PubMed] [Google Scholar]
- 45.Lee K, Kruper L, Dieli-Conwright CM, et al. The impact of obesity on breast cancer diagnosis and treatment. Curr Oncol Rep. 2019;21:41. doi: 10.1007/s11912-019-0787-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ligibel JA, Alfano CM, Courneya KS, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32:3568–3574. doi: 10.1200/JCO.2014.58.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.King MT, Kenny P, Shiell A, et al. Quality of life three months and one year after first treatment for early stage breast cancer: Influence of treatment and patient characteristics. Qual Life Res. 2000;9:789–800. doi: 10.1023/a:1008936830764. [DOI] [PubMed] [Google Scholar]
- 48.Traore BM, El Fakir S, Charaka H, et al. Evolution of quality of life in patients with breast cancer during the first year of follow-up in Morocco. BMC Cancer. 2018;18:109. doi: 10.1186/s12885-018-4008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee ES, Lee MK, Kim SH, et al. Health-related quality of life in survivors with breast cancer 1 year after diagnosis compared with the general population: A prospective cohort study. Ann Surg. 2011;253:101–108. doi: 10.1097/sla.0b013e3181f662ce. [DOI] [PubMed] [Google Scholar]
- 50.King MT. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual Life Res. 1996;5:555–567. doi: 10.1007/BF00439229. [DOI] [PubMed] [Google Scholar]
- 51.Velikova G, Williams LJ, Willis S, et al. Quality of life after postmastectomy radiotherapy in patients with intermediate-risk breast cancer (SUPREMO): 2-year follow-up results of a randomised controlled trial. Lancet Oncol. 2018;19:1516–1529. doi: 10.1016/S1470-2045(18)30515-1. [DOI] [PubMed] [Google Scholar]
- 52.Bantema-Joppe EJ, de Bock GH, Woltman-van Iersel M, et al. The impact of age on changes in quality of life among breast cancer survivors treated with breast-conserving surgery and radiotherapy. Br J Cancer. 2015;112:636–643. doi: 10.1038/bjc.2014.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ng CG, Mohamed S, See MH, et al. Anxiety, depression, perceived social support and quality of life in Malaysian breast cancer patients: A 1-year prospective study. Health Qual Life Outcomes. 2015;13:205. doi: 10.1186/s12955-015-0401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. doi: 10.22034/APJCP.2016.17.12.5063. El Fakir S, El Rhazi K, Zidouh A, et al: Health-related quality of life among breast cancer patients and influencing factors in Morocco. Asian Pac J Cancer Prev 17:5063-5069, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taira N, Shimozuma K, Shiroiwa T, et al. Associations among baseline variables, treatment-related factors and health-related quality of life 2 years after breast cancer surgery. Breast Cancer Res Treat. 2011;128:735–747. doi: 10.1007/s10549-011-1631-y. [DOI] [PubMed] [Google Scholar]
- 56.Hopwood P, Sumo G, Mills J, et al. The course of anxiety and depression over 5 years of follow-up and risk factors in women with early breast cancer: Results from the UK Standardisation of Radiotherapy Trials (START) Breast. 2010;19:84–91. doi: 10.1016/j.breast.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 57.Deshields T, Tibbs T, Fan MY, et al. Ending treatment: The course of emotional adjustment and quality of life among breast cancer survivors immediately following radiation therapy. Support Care Cancer. 2005;13:1018–1026. doi: 10.1007/s00520-005-0801-z. [DOI] [PubMed] [Google Scholar]
- 58.Schwarz R, Krauss O, Höckel M, et al. The course of anxiety and depression in patients with breast cancer and gynaecological cancer. Breast Care (Basel) 2008;3:417–422. doi: 10.1159/000177654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yusuf A, Ahmad Z, Keng SL. Quality of life in Malay and Chinese women newly diagnosed with breast cancer in Kelantan, Malaysia. Asian Pac J Cancer Prev. 2013;14:435–440. doi: 10.7314/apjcp.2013.14.1.435. [DOI] [PubMed] [Google Scholar]
- 60.Kedde H, van de Wiel HBM, Weijmar Schultz WCM, et al. Sexual dysfunction in young women with breast cancer. Support Care Cancer. 2013;21:271–280. doi: 10.1007/s00520-012-1521-9. [DOI] [PubMed] [Google Scholar]
- 61.Harirchi I, Montazeri A, Zamani Bidokhti F, et al. Sexual function in breast cancer patients: A prospective study from Iran. J Exp Clin Cancer Res. 2012;31:20. doi: 10.1186/1756-9966-31-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jankowska M. Sexual functioning in young women in the context of breast cancer treatment. Rep Pract Oncol Radiother. 2013;18:193–200. doi: 10.1016/j.rpor.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fobair P, Stewart SL, Chang S, et al. Body image and sexual problems in young women with breast cancer. Psychooncology. 2006;15:579–594. doi: 10.1002/pon.991. [DOI] [PubMed] [Google Scholar]
- 64.Jing L, Zhang C, Li W, et al. Incidence and severity of sexual dysfunction among women with breast cancer: A meta-analysis based on female sexual function index. Support Care Cancer. 2019;27:1171–1180. doi: 10.1007/s00520-019-04667-7. [DOI] [PubMed] [Google Scholar]
- 65. Villarreal Garza CM, Valdez-Rojas L, Fonseca A, et al: Follow-up status, patient retention and data collection of the “Joven & Fuerte” cohort for young women with breast cancer in Mexico. The Breast 41:S29-S30, 2018. [Google Scholar]