Abstract
An epidemiological study of Greenlandic Inuit suggested that fish oil, or omega-3 polyunsaturated fatty acids (PUFA), was important in preventing atherosclerotic disease. After this landmark study, many large-scale epidemiological studies and meta-analyses have examined the health benefits of omega-3 PUFA as part of a fatty acid-rich diet to demonstrate its beneficial roles in the prevention of cardiovascular diseases. Recent research has also focused attention on the anti-inflammatory effects of omega-3 PUFA and on specialized pro-resolving mediators. Findings of these studies have led to the development of omega-3 PUFA preparations for the treatment of dyslipidemia, including a highly purified eicosapentaenoic acid (EPA)-ethyl ester product (Epadel®) in Japan and an EPA/docosahexaenoic acid (DHA) preparation (Lotriga®) in the United States and Europe. Although various large-scale clinical trials on the cardiovascular preventive effect of omega-3 PUFA were conducted and reported, the results were not always consistent. The issues of not targeting subjects with hypertriglyceridemia and using low dose of omega-3 PUFA have been suggested to contribute to the failure of demonstrating the preventive effect of omega-3 PUFA in these clinical trials. Taking into account the above issues, the REDUCE-IT trial evaluated a highly purified EPA preparation at a high dose of 4 g/day in patients with hypertriglyceridemia and high cardiovascular risk, and demonstrated an extraordinary outcome of 25% relative reduction in cardiovascular events. This article reviews studies on omega-3 fatty acids during the last 50 years, including the progress in elucidating molecular mechanisms and recent large-scale clinical studies.
Keywords: Omega-3 polyunsaturated fatty acids, Cardiovascular disease, Fatty acid metabolism, Large-scale clinical trial
Introduction
Fatty acids are classified into saturated fatty acids that have no double bonds and unsaturated fatty acids possessing double bonds. Fatty acids with multiple double bonds are called polyunsaturated fatty acids (PUFA). PUFA with double bonds starting from the sixth position from the methyl end of the fatty acid are termed as omega-6 series, and those from the third position as omega-3 series (Fig. 1). These PUFA are incorporated into the cell membrane. Arachidonic acid (AA), an omega-6 series PUFA, is a major component of phospholipids in the cell membrane and is important for maintaining the production function of eicosanoids. Linoleic acid (LA), an omega-6 series PUFA, and α-linolenic acid (ALA), an omega-3 series PUFA, are considered essential fatty acids because they cannot be synthesized by humans. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are representatives of omega-3 PUFA, and they can be converted from ALA in vivo. EPA and DHA are attracting attention due to their anti-arteriosclerotic effect. This article overviews the knowledge on the prevention of cardiovascular (CV) events with omega-3 PUFA and the mechanisms involved, focusing on the history of research on the anti-arteriosclerotic effect of fish oil.
Fig. 1.
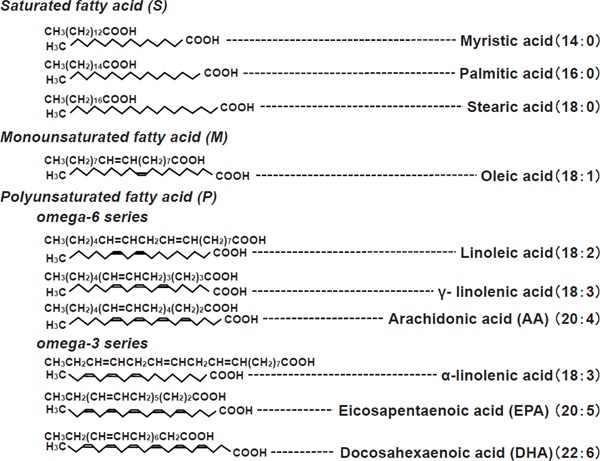
Saturated and unsaturated polyunsaturated fatty acids
History of Fish Oil and Lifestyle-Related Diseases
Mortality rate from atherosclerotic cardiovascular diseases (CVD), particularly myocardial infarction (MI), is high in Western countries. Epidemiological studies have thus focused on the differences in lifestyle, in particular dietary habit, among countries that differ in the incidence of atherosclerosis-associated MI. A study conducted in seven countries1) reported that the mortality from ischemic heart disease is lower in Japan and Mediterranean countries than that in the United States and Northern European countries and highlighted the role of unsaturated fatty acids that are abundant in Japanese and Mediterranean diets. In this context, an epidemiological study of the Greenlandic Inuit suggests that fish oil (omega-3 fatty acids) is important in preventing atherosclerotic diseases2). After that landmark study, the health benefits of omega-3 fatty acids as part of a fatty acid-rich diet have been extensively researched in large-scale epidemiological studies, clinical outcome trials, and metaanalyses, the results of which show a statistically significant reduction in the relative risk of CVD in persons consuming omega-3 fatty acids3, 4). In Japan, a study of fishermen at Kawazu, a village in Katsuura city in Chiba Prefecture, also demonstrated the contribution of an omega-3-rich diet in the prevention of CVD5). As a result, the first highly purified EPA preparation for human use was developed in Japan6). Omega-3 fatty acids are now widely recognized to have an important role in preventing atherosclerotic CVD, carcinogenesis, and a wide range of other diseases and conditions, including those of the central nervous system (such as dementia), CV system (such as arrhythmia and chronic heart failure [CHF]), and immune system (including rheumatoid arthritis and psoriasis), and in the defense against infections7–13).
Absorption of Omega-3 PUFAs and Metabolism in vivo
In the body, omega-3 fatty acids are primarily available as EPA and DHA and less abundantly available as docosapentaenoic acids (DPA)14). Omega-3 fatty acids are incorporated into chylomicron triglycerides in the gastrointestinal tract and transported to the liver, where EPA and DHA are incorporated into triglycerides as very-low-density lipoprotein cholesterol (VLDL-C) and released into the blood stream. Only a small proportion of omega-3 fatty acids are available as free fatty acids, most of which are bound to albumin15).
In the liver, ALA and EPA, which are omega-3 PUFA, are converted to DPA and DHA through the action of desaturase and fatty acid chain elongase. Conversely, the administration of DHA increases DPA and EPA. Changes of omega-3 PUFA in platelet membranes have been reported after fish oil administration16). The administration of highly purified EPA increases EPA and DPA but does not change DHA in the platelet membrane. On the contrary, the administration of highly purified DHA increases DHA, DPA, and EPA in the platelet membrane. These results suggest that the conversion from DPA to DHA is tightly controlled by the content of DHA in the platelet membrane. Thus, the incorporation of DHA into the membrane may be necessary for the increment of DHA in the membrane and DHA may be converted to DPA and EPA. The above findings in the human body have also been found in the arteries of arteriosclerosis model mice and the kidneys of metabolic syndrome model rats17, 18). These findings confirm that the administration of DHA is effective in increasing the amount of DHA in membrane phospholipids.
A recent study using a mouse model demonstrated in vivo changes in fatty acid synthesis and organ distribution in omega-3 PUFA deficiency19). In the study, mice were fed diets with different LA (as omega-6 fatty acid) to ALA (as omega-3 fatty acid) ratios. An omega-3-deficient condition resulting from feeding with an ALA-poor and LA-rich diet stimulated the expression of desaturase and fatty acid chain elongase and increased AA but decreased EPA and DHA in the liver. Interestingly, big differences in the reduction rate among omega-3 fatty acids were observed in the liver, with markedly greater reduction of EPA than DHA. In the brain, which is known to be rich in DHA, DHA reduction was much smaller and AA content slightly increased compared with those in the liver. On the contrary, EPA was markedly reduced in the brain, which is known to be poor in EPA. These results suggest that in omega-3 PUFA deficiency, desaturase and fatty acid elongase are activated in the liver to produce omega-3 PUFA, compensating DHA preferentially over EPA despite an excess supply of AA, and DHA is supplied to the brain to maintain a DHA-rich state in the brain tissues (Fig. 2). The preferential synthesis of DHA instead of EPA under an omega-3-deficient condition in the liver suggests the existence of an in vivo autonomic regulation to maintain DHA content, especially in the brain19).
Fig. 2.
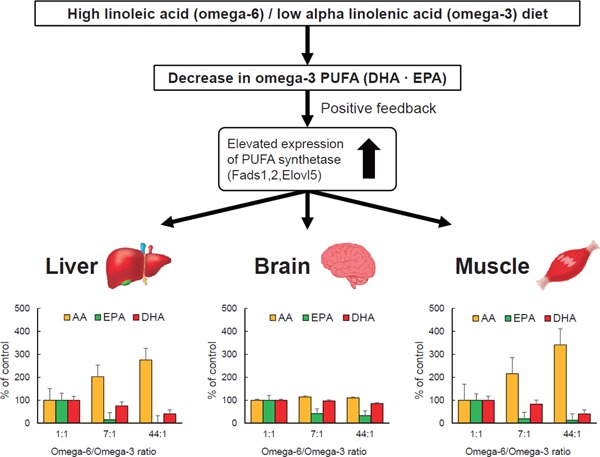
Effect of omega-3-deficient diet in the body
This figure was drawn based on Su et al.19).
History of Fish Oil Preparation Development: Highly Purified EPA and EPA/DHA Combination
In Japan, an epidemiological survey of the anti-arteriosclerotic effect of fish oil was conducted for the first time by Chiba University in 1980 5). In this study, fishermen who had high fish intake were compared with rural residents who had a relatively low fish intake. Despite the high calorie intake and high degree of obesity in the fishermen, the mortality rate due to ischemic heart disease and cerebrovascular disease was significantly lower than that in rural residents. The mean intake of EPA was 2600 mg/day in fishermen, approximately three times that of rural residents. This diet was associated with low platelet aggregation capacity and low blood consistency, and the triglyceride level of the fishermen was approximately 40% lower than that of the rural residents20).
On the basis of these studies, Chiba University and Nippon Suisan Co., Ltd. developed a high-purity EPA preparation from fish oil and administered it to healthy people for the first time in the world, reporting a decrease in platelet aggregation ability and an improvement in erythrocyte deformability6). This highly purified EPA (Epadel®) was clinically used with an indication for obstructive arteriosclerosis in 1990. Thereafter, the indication for hyperlipidemia, especially for the improvement of hypertriglyceridemia, has been added.
While the highly purified EPA preparation has been used in Japan as Epadel® and the United States as Vascepa®, a different fish oil preparation containing a combination of EPA and DHA has been used in Europe and the United States for more than 20 years and has been evaluated in large-scale clinical trials3, 4, 21, 22). This EPA/DHA preparation (Lotriga®) was launched in Japan in January 2013. A randomized study comparing the highly purified EPA preparation developed in Japan with the EPA and DHA product developed in Europe and America indicated higher triglyceride-lowering efficacy of the EPA and DHA product when used at a high dose23, 24).
Vascular Effect and Anti-Arteriosclerotic Effect of Omega-3 PUFA: Hypertriglyceridemia as a Residual Risk in a Statin Treatment and Deterioration of EPA/AA Ratio
A meta-analysis of randomized studies25) has reported that fish oil supplementation lowers blood pressure modestly, possibly caused by reduced systemic vascular resistance, but does not lower cardiac output. Increased production of nitric oxide through the consumption of omega-3 PUFA may increase the expression of endothelial nitric oxide synthase. Indeed, several randomized studies have found that the intake of omega-3 PUFA improves the serum markers of endothelial dysfunction, such as E-selectin, VCAM-1, and ICAM-1 26, 27). A meta-analysis reveals that the intake of omega-3 PUFA improves flow-mediated vasodilation, among other parameters of endothelial function28). Tousoulis et al.29) reported that omega-3 PUFA improves endothelial function evaluated via flow-mediated dilation and arterial stiffness by carotid-femoral pulse wave velocity (PWV), with a parallel anti-inflammatory effect in adults with metabolic syndrome. Merino et al.30) also documented that omega-3 PUFA consumption improves the small peripheral artery function in patients with intermediate to high CVD risk, as evaluated by small artery reactive hyperemia index. Moreover, Chan et al.31) reported that omega-3 PUFA supplementation improves arterial elasticity measured by pulse contour analysis of the radial artery in patients on statin therapy for familial hypercholesterolemia.
Hypercholesterolemia is widely recognized as an important CVD risk factor, and large-scale clinical trials, such as 4S, WOSCOPS, MEGA study, and metaanalysis, have validated the importance of statins as the first choice for the primary and secondary prevention of CVD32–37). However, to reduce the risk of CVD, the reduction of low-density lipoprotein cholesterol (LDL-C) with statins alone is insufficient and the residual risk is evidently a problem38). Some studies have indicated hypertriglyceridemia as an additional CVD risk factor39, 40), and the importance of hypertriglyceridemia as a residual risk was proposed some two decades ago39). A subanalysis of the Japan Diabetes Complications Study examined the complications of Japanese patients with type 2 diabetes and showed that CVD risk markedly increases when hypertriglyceridemia overlaps with elevated HbA1c or LDL-C in patients with type 2 diabetes41). Elevated triglyceride concentrations are reported to be associated with, and may contribute to, the presence of highly atherogenic, small dense LDL particles and decrease high-density lipoprotein cholesterol (HDL-C) level, both being factors associated with increased CVD risk42). At present, guidelines for the prevention of CVD have been established, and cholesterol and triglycerides should be properly managed43–45).
Omega-3 PUFA have been reported to reduce serum triglycerides in patients with hypertriglyceridemia, and an increase in LDL-C and HDL-C can accompany a reduction in triglycerides. The increase in LDL-C is less than the reduction in VLDL-C, resulting in a net decrease in non-HDL-C (VLDL-C plus LDL-C)46). Regarding the triglyceride-lowering effects of the available fish oil preparations, a randomized clinical study conducted in Japanese subjects with hypertriglyceridemia showed similar triglyceride-lowering rates of approximately 11% with 1.8 g/day of highly purified EPA and 2 g/day of EPA/DHA preparation (Lotriga®) but a significantly higher rate of approximately 25% with 4 g/day of EPA/DHA preparation24). These findings suggest that the triglyceride-lowering effect of fish oil preparations depends on the dose rather than the type of omega-3 PUFA. Strong evidence show that reduction in triglyceride concentration is caused by mechanisms, such as reduced hepatic VLDL–triglyceride synthesis and secretion and increased triglyceride clearance from chylomicrons and VLDL particles47).
The Japan EPA Lipid Intervention Study (JELIS) is a large-scale clinical trial that evaluated the effect of highly purified EPA aiming on the reduction of the residual risk of CVD events in patients taking statins. This trial reveals that the administration of high-purity EPA reduces CVD risk by approximately 19%48). The effect of this highly purified EPA has been reported to be particularly effective in patients with metabolic syndrome manifesting hyperlipidemia and low HDL-C level49). EPA/DHA preparation has also been reported to have secondary preventive effect in post-MI patients in Italy, significantly reducing total death, sudden death, and CVD-related death16). The JELIS has reported that adherence to medication is directly linked to the prevention of CVD events49), indicating that regular dosage of fish oil preparations is undoubtedly important.
The health benefits of omega-3 PUFA have been extensively researched, and the balance between EPA or DHA and AA in the human body is likely to be important for regulating the production of mediators and subsequently vascular function. Indeed, serum EPA to AA ratio (EPA/AA) has been found to be a good biomarker for CVD risk not only in the general population50) but also in a post hoc analysis of the results of a clinical trial51). Intima–media thickness in carotid arteries, PWV, and cardio-ankle vascular index are surrogate markers for atherosclerotic diseases, and EPA/AA and DHA to AA ratio (DHA/AA) were found to be associated with these markers52–55). Albuminuria is also recognized as an independent risk factor for CVD morbidity and mortality in the general population56, 57). Fukami et al.58) reported a strong association between EPA/AA ratio and microalbuminuria.
The efficacy of statins for the primary and secondary prevention of CVD has been established59), and LDL-C-lowering therapy with statins has been used as the first-line treatment as mentioned above. Despite significant LDL-C lowering with statins, substantial residual CVD risk remains60), and several risk factors, such as low HDL-C level and high triglyceride level, have attracted attention. A particularly interesting finding is that increase in plasma AA concentration and decrease in plasma omega-3 PUFA concentration and/or plasma omega-3/AA ratio have been observed in patients treated with statins61, 62). These results may be associated with the residual risk after initiation of a statin treatment. A recent report has indicated that statin therapy decreases serum DHA level with a parallel reduction in the LDL-C level in patients with acute coronary syndrome (ACS) and that decreased DHA level after statin therapy and low EPA level on admission are risk factors for in-stent restenosis in patients with ACS63). These findings suggest that statin regulates the endogenous metabolism of PUFAs. PUFAs are endogenously metabolized from omega-6 and omega-3 PUFA precursors by position-specific desaturation and carbon-chain elongation reactions64).
Our recent studies using 3T3L1 adipocytes and HepG2 hepatocytes have demonstrated that statin-induced suppression of isoprenoid production, especially geranylgeranyl pyrophosphate (GGPP) synthesis, causes the inhibition of GGPP-dependent Rho kinase pathway to increase the expression of desaturase and fatty acid chain-lengthening enzyme (Fig. 3)65, 66). This mechanism leads to an increase in dominant conversion from LA to AA in patients who are deficient in omega-3 PUFA. The AA-dominant endogenous synthesis of PUFAs resulting in decreased plasma omega-3/AA ratio during a statin treatment may be clinically important because the serum EPA/AA ratio has been reported to be a good biomarker for CVD risk not only in the general population50) but also in clinical trial subjects51) as mentioned above. Therefore, recommending omega-3 PUFA supplementation in patients on a statin treatment, especially in those with an omega-3-deficient condition, to maintain adequate plasma omega-3 concentration and omega-3/AA ratio seems rational. Recently, the addition of EPA to high-dose pitavastatin has been reported to be effective for the reduction of coronary plaque volume in patients after undergoing percutaneous coronary intervention (PCI)67). Early initiation of treatment with EPA combined with statin after successful primary PCI reduced CVD events after ACS68). On the contrary, standard treatment with EPA did not reduce the progression of coronary artery calcification compared with standard pitavastatin treatment69).
Fig. 3.
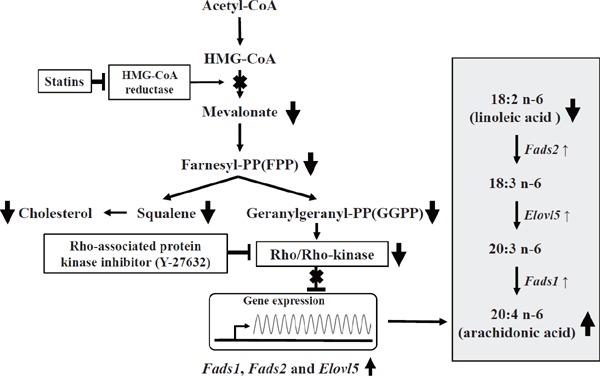
Proposed mechanism of statin-induced increment of Fads1, Fads2, and Elovl5 gene expression via geranylgeranyl pyrophosphate-dependent Rho kinase pathway
This figure was drawn based on Tanaka et al.66).
Anti-Inflammatory and Cardioprotective Effect of Fish Oil: AA Cascade and Inflammation
Omega-3 PUFA also have anti-inflammatory properties. An epidemiological study of Greenland Inuit70) found that autoimmune diseases, such as bronchial asthma and psoriasis, are extremely rare among Inuit primarily subsisting on fish. Several subsequent animal and human studies provided evidence that omega-3 PUFA, particularly EPA, have anti-inflammatory and immunomodulatory properties71, 72).
The omega-6 PUFA AA is stored within cell membranes. It is released in response to cell stimulation and metabolized by proinflammatory lipid mediators, such as prostaglandin and leukotriene in the AA cascade, thereby aggravating pre-existing inflammation. Omega-3 PUFA are also stored within cell membranes, where they replace and thus reduce the storage of AA. Furthermore, while omega-3 PUFA are also metabolized by proinflammatory lipid mediators in the AA cascade, their active metabolites are assumed to be less potent than those of AA, thus tipping the balance toward the inhibition of inflammation73). In particular, atherosclerosis is suppressed in leukotriene receptor B-knockout mice74) and the administration of EPA75).
DHA has not received as much attention as EPA, but recent report indicates that DHA-rich fish oil is more potent than EPA-rich fish oil in suppressing inflammation76). In addition, DHA-rich fish oil prolongs survival in a mouse model of systemic lupus erythematosus, a typical autoimmune disease77). The factors involved have been identified to be resolvins and neuroprotectins produced from omega-3 fatty acids, particularly DHA, at the end of an inflammatory process78). These metabolites are potent anti-inflammatory lipid mediators (specialized pro-resolving mediators, SPMs) (Fig. 4)79).
Fig. 4.
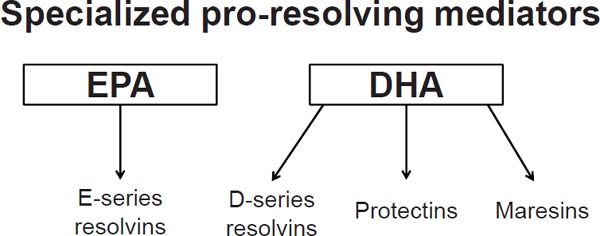
Anti-inflammatory lipid mediators (specialized pro-resolving mediators) derived from EPA and DHA
Among the SPMs, the resolvin E (RvE) series are synthesized from EPA through the conversion of 18-hydroxyeicosapentaenoic acid by aspirin-acetylated COX2 or CYP450 monooxygenase. RvE1 actively switches off leukocyte trafficking to the inflamed site, promotes the clearance of inflammatory cells and debris, and suppresses cytokine production, thereby leading to the resolution of acute inflammation80). DHA-derived mediators, such as protectins, resolvin D series, and maresins, are generated by 15-lipoxygenase (15-LOX) in humans or by 12/15-LOX in mice.
Evidence accumulated in recent years has shown that SPMs have direct cardioprotective action in vivo. Keyes et al.81) reported that the administration of RvE1 attenuates the infarct size in rats subjected to ischemia/reperfusion injury. The results of their study suggest that RvE1 directly affects cardiomyocytes and protects against cardiac injury. Another study has shown that during acute inflammation following MI in mice, RvD1 promotes the production of SPMs in the spleen and induces a switch to anti-inflammatory M2 macrophages in the left ventricle to prevent myocardial fibrosis and maintain cardiac function82). Indeed, according to the OMEGA-REMODEL study, the administration of EPA/DHA preparation at a high dose of 4 g per day for 6 months inhibits myocardial fibrosis after acute MI, thereby improving remodeling and protecting cardiac function83).
Recent studies using animal models have proven that SPMs also play an important role in preventing the onset and severity of avian influenza84). Furthermore, the effect of omega 3 PUFA on glomerular sclerosis was demonstrated in SHR-cp rat, a model of metabolic syndrome18). In this study, EPA/DHA preparation significantly inhibited glomerular sclerosis compared with highly purified EPA preparation. Mechanistic investigations showed that EPA and DPA were elevated, but DHA did not increase in the kidney of rats administered with the highly purified EPA preparation, whereas DHA increased in rats given with the EPA/DHA preparation. These results suggest that the increase in production of SPMs derived from DHA may have led to the suppression of glomerular sclerosis.
Cell Membrane Stabilization and Antiarrhythmic Effect of Fish Oil
As mentioned above, omega-3 PUFA absorbed into the body are eventually incorporated in the cell membrane, where they act on the AA cascade when the cells are activated to control inflammation. Another important function of the incorporated omega-3 fatty acids is the stabilization of cell membrane fluidity. Receptors and ion channels for various physiologically active substances are present on the cell membrane, and omega-3 PUFA incorporated into the membrane may regulate the functions of these receptors and ion channels. Fish oil has an antiarrhythmic effect, and the effect of fish oil in preventing sudden death caused by arrhythmia, which often happens in the case of MI, has been known for a long time85). As a mechanism of action, the inhibitory effect of omega-3 PUFA, particularly DHA, on Na channel in cardiomyocytes has been reported86).
Omega-3 PUFA prevent the induction of arrhythmia caused by persistent Na overflow in myocardial cells after MI. In connection with such membrane- stabilizing effects, the administration of omega-3 PUFA also decreases CVD events in patients with heart failure, reduces hospitalization due to heart failure, and lowers mortality4). The use of omega-3 PUFA has been incorporated in the American College of Cardiology (ACC)Foundation/American Heart Association (AHA) guidelines for the management of heart failure in 2013 87).
A few small-scale randomized studies on the effect of omega-3 PUFA in patients with defibrillators implanted for ventricular tachycardia88, 89) have yielded mixed results, and meta-analyses90, 91) also showed no significant benefit. As these studies varied in terms of the study design, it may be too early to arrive at definitive conclusions. Although a study on fish oil intake in patients with atrial fibrillation (AF)92) has reported a reduced risk of developing AF, a subsequent large-scale randomized study93) reveals no reduction in the incidence of postoperative AF. Moreover, meta-analyses of published studies93, 94) conclude that fish oil and omega-3 PUFA have no benefit against AF. Thus, the effects of fish oil intake in preventing postoperative AF and in the secondary prevention of AF in patients with existing AF remain unclear. Large-scale prospective intervention studies are required to determine whether fish oil protects against new-onset AF in non-AF patients.
Large-Scale Clinical Trials of Omega-3 PUFA for CVD Prevention and Issues
The dose of fish oil supplementation, the type of omega-3 PUFA preparation, the study period, and the endpoints to estimate clinical effects for primary or secondary CVD prevention depend on the population studied. A meta-analysis of large-scale prospective cohort studies and randomized studies reported that fish and fish oil consumption reduced coronary heart disease (CHD)-related mortality and sudden cardiac death, although these beneficial effects did not exhibit a linear dose-response relationship95). A subsequent meta-analysis of 13 randomized controlled trials found a significant reduction in cardiac death after fish oil supplementation, but this effect was nonsignificant after adjustment for multiple covariates96). In the Japanese general population, omega-3 PUFA intake was inversely and independently associated with the longterm risk of total CVD mortality97). De Oliveira Otto et al.98) reported that dietary and circulating EPA and DHA, but not ALA or omega-6 PUFA, are inversely associated with CVD incidence. These findings indicate that fish oil may reduce fatal MI or sudden cardiac death.
Although the secondary prevention analysis of JELIS trail did show a benefit for omega-3 PUFA99), another secondary prevention trials did not show a clear benefit100, 101), perhaps in part because many of the study participants were concomitantly treated with aspirin, angiotensin-converting enzyme inhibitors, beta-adrenergic antagonists, and statins during the studies. The large-scale Risk and Prevention Study102) showed that omega-3 PUFA did not clearly reduce CHD-related mortality in patients with multiple CVD risk factors. A possible reason for the failure of these secondary prevention studies to demonstrate significant benefits of fish oil is that the study participants had received aggressive pharmacotherapy, which may have reduced the effectiveness of omega-3 PUFA against cardiac death. The investigators noted that large sample sizes will be needed to yield statistically significant results. Regarding primary prevention, the intake of tuna and dark fish, ALA, and marine omega-3 PUFA is not associated with the risk of major CVD in a cohort of women without a history of CVD103). From a pooled analysis of 19 cohort studies, omega-3 biomarkers of ALA, DPA, and DHA were associated with a lower risk of fatal CHD104).
By contrast, several observational studies105–108) found that fish oil and omega-3 PUFA contributed to the prevention of non-fatal MI and ACS, although subsequent large-scale randomized studies reported mixed outcomes. Some reported benefits of omega-3 PUFA, most importantly protection against CVD death48, 109), whereas other studies failed to show such benefits21, 100, 101, 110). Indeed, a meta-analysis of randomized trials found that while the risk of non-fatal CVD was lower in persons receiving fish oil, the decrease was not significant after adjustment for confounders96). Thus, the benefit of fish oil for non-fatal CVD remains unclear.
An Italian cohort study (AGE-IM) that examined total PUFA levels (as the percentage of whole blood fatty acids) reported that total omega-3 PUFA and total omega-6 PUFA were lower in MI patients than in matched control subjects111). These data suggest an association of the total omega-3 and total omega-6 blood levels, with CVD risk. Another study revealed that high consumptions of marine (EPA/DHA) and plant (ALA) omega-3 PUFA were independently associated with the reduced risk of CVD mortality in a Chinese population112).
A prospective cohort study of 2735 adults without CHF113) reported an inverse correlation between blood concentration of omega-3 PUFA and incidence of CHF in elderly subjects. Other cohort studies114, 115) reported that increased intake of boiled or grilled, but not fried fish, contributed to the prevention of CHF onset. However, very few studies have investigated the protective effects of omega-3 PUFA against new-onset CHF. Additional data from primary prevention settings are needed. With respect to secondary prevention, a large-scale randomized, double-blind, placebo-controlled trial of 7046 patients with existing CHF found a significant survival benefit in those given omega-3 PUFA (Lotriga®), with improvement in the left ventricular ejection rate after a mean treatment duration of 3.9 years116). On the basis of these findings, omega-3 PUFA are described as effective against CHF in the ACC/AHA guidelines117).
Meta-analyses of relatively large, prospective cohort studies118, 119) reported that fish oil intake did not correlate with the incidence of hemorrhagic stroke but inversely correlated with the incidence of ischemic stroke in subjects receiving a moderate dose of fish oil. However, prospective intervention studies yielded inconsistent results. A subanalysis of the JELIS trial on highly purified EPA (Epadel®) showed no benefit in the primary prevention but some benefits in the secondary prevention of stroke120). Other studies showed that omega-3 PUFA had no protective effect against stroke onset21, 100). Thus, the effects of fish oil and omrga-3 PUFA preparations on CVD widely vary depending on the endpoint used, which may be attributed to the differences in omega-3 PUFA dosage, duration of use, and patient characteristics (particularly disease severity and use of concomitant medications) among studies.
Table 1 summarizes the results of the five past representative large-scale intervention studies: GISSI-P, JELIS, GISSI-HF, ORIGIN, and GISSI-R & P3, 4, 21, 22, 48). Only the JELIS used a highly purified EPA preparation, whereas the others used EPA/DHA preparations. Regarding the dosage, clinical trials conducted in Europe and the United States used EPA/DHA preparations at a relatively low dose of 1 g/day, whereas JELIS evaluated a highly purified EPA preparation at a dose of 1.8 g/day.
Table 1. Past large-scale clinical studies of omega-3 PUFA.
| Study | GISSI-P | JELIS | GISSI-HF | ORIGIN | GISSI-R&P |
|---|---|---|---|---|---|
| CV event reduction | YES | YES | YES | NO | NO |
| Study period | 1993–1995 | 1994–2006 | 2002–2005 | 2003–2005 | 2004–2007 |
| Paper (year) | Lancet (1999)3) | Lancet (2007)48) | Lancet (2008)4) | NEJM (2012)21) | NEJM (2013)102) |
| Subject background | Prior MI (within 3 mo.) |
Hypercholesterole mia (> 250 mg/dl) (Primary 80.3%, secondary 19.7%) |
CHF | IGT/IFG/DM | Multiple CV risks |
| Baseline TG (mg/dL) | 162.1 | 154.2 | NA | ω: 142 c: 140 | ω: 150 c: 150 |
| Omega-3 preparation | EPA/DHA | EPA | EPA/DHA | EPA/DHA | EPA/DHA |
| Dosage (g/day) | 1 | 1.8 | 1 | 1 | 1 |
| No. of subjects | 11,324 | 18,645 | 7,046 | 12,612 | 12,513 |
| Follow-up (year) | 3.5 | 4.6 | 3.9 | 6.2 | 5 |
| Diabetes | NA | NA | NA | HbA1c (ω 6.4%: c 6.4%) |
NA |
| Statin use (%) | 29 | 100 | 23 | 54 | 62 |
| Use of ACE-I/ARB (%) | 41 | UN | 94 | 71 | 75 |
| Use of antiplatelets (%) | 88% | 14% | 87% | 79% | 60% |
| Event rate | 12.7% vs 14.1% | 2.8% vs 3.2% | 27% vs 29% | 9.1% vs 9.3% | 11.7% vs 11.9% |
| (ω vs c) | p < 0.05 | p = 0.011 | p = 0.041 | p = 0.72 | p = 0.58 |
CV: cardiovascular; MI: myocardial infarction; CHF: chronic heart failure; IGT: impaired glucose tolerance; IFG: impaired fasting glucose; DM: diabetes mellitus; EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid; ω: omega-3, c: control; NA: not available.
As mentioned before, a subanalysis of the JELIS data demonstrates marked reduction of CVD events by EPA treatment in patients with hypertriglyceridemia and low HDL-C level, indicating that hypertriglyceridemia and low HDL-C level are two good markers for omega-3 PUFA treatment for the prevention of CVD49). However, previous large intervention studies did not necessarily target patients with hypertriglyceridemia. Therefore, the selection of subjects with hypertriglyceridemia and using high-dose omega-3 PUFA are considered to be two important criteria when conducting new large-scale interventional studies. In recent years, five large-scale intervention trials (ASCEND, VITAL, REDUCE-IT, STRENGTH and RESPECT-EPA) have been initiated121–125), and the results in three of them were reported in 2018 (Table 2).
Table 2. Recently completed and ongoing large-scale clinical studies of omega-3 PUFA.
| Study | ASCEND | VITAL | REDUCE-IT | STRENGTH | RESPECT-EPA |
|---|---|---|---|---|---|
| CV event reduction | No | No | Yes | in progress | in progress |
| Study period | 2005–2011 | 2011–2014 | 2011–2016 | 2014–2019 | 2013–2021 |
| Paper (year) | NEJM 2018 121) | NEJM 2019 123) | NEJM 2019 122) | in progress124) | in progress125) |
| Subject background | Type 1 DM | Middle-aged | -Middle-aged, history of CVD or DM | -Age ≥ 18 years with CV risk | -Age 20–79 years with CAD |
| Type 2 DM (No history of CVD) | (No history of CVD and cancer) | -TG 150–499 mg/dL -LDL-C 40–100 mg/ dL with statin |
-LDL-C <100 mg/dL with statin -TG 180–499 mg/dL -low HDL-C level |
-Treated with statin | |
| Baseline TG (mg/dL) | NA | NA |
ω: 216.5 (last visit 170) c: 216 (202.0) |
in progress | in progress |
| Omega-3 preparation | EPA/DHA | EPA/DHA | EPA | EPA/DHA (carboxylic acid) | EPA |
| Dosage (g/day) | 1 | 1 | 4 | 4 | 1.8 |
| No. of subjects | 15,480 | 25,871 | 8,179 | in progress | in progress |
| Follow-up (year) | 7.4 | 5.3 | 4.9 | in progress | in progress |
| Diabetes (%) | 100 | 13.7 | ω: 58.5, c: 58.6 | in progress | in progress |
| Statin use (%) | ω: 74.8, c: 75.7 | 37.5 (Cholesterol-lowering medication) | ω: 99.7, c: 99.5 | in progress | in progress |
| Use of ACE-I/ARB (%) | ω: 59, c: 58 | 49.8% (treated with medication) | NA | in progress | in progress |
| Use of antiplatelets (%) | ω: 35.5, c: 35.7 | 45.4 | NA | in progress | in progress |
| Event rate (ω vs c) |
8.9% vs 9.2% p = 0.55 |
2.98% vs 3.24% p = 0.24 |
17.2% vs 22.0% p < 0.001 |
in progress | in progress |
CV: cardiovascular; CVD: cardiovascular disease; MI: myocardial infarction; CHF: chronic heart failure; CAD: coronary artery disease; IGT: impaired glucose tolerance; IFG: impaired fasting glucose; DM: diabetes mellitus; EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid; ω: omega-3, c: control; NA: not available.
The efficacy of omega-3 PUFA for primary prevention was not demonstrated in ASCEND trial conducted in diabetic patients98) and VITAL trial in the general population100). The two studies did not include hypertriglyceridemia in the selection criteria and used a low dose of 1 g/day. By contrast, in the REDUCE-IT trial, highly purified EPA preparation was administered at a high dose of 4 g/day to a group of patients with high CVD risk who had hypertriglyceridemia during the administration of statin. This trial reported a surprising result that CVD events were reduced by 25% relative to controls122). As expected, this study suggests that targeting subjects with hypertriglyceridemia and using high dose of omega-3 PUFA are two key points to achieve favorable outcome from the intervention. However, questions remain as to whether only highly purified EPA is effective or the same outcome can be obtained using EPA/DHA preparations and whether high-dose EPA/DHA preparation is important. To answer these questions, the results of the ongoing STRENGTH trial are eagerly awaited. In this study, statin-treated patients with high CVD risk and hypertriglyceridemia are given EPA/DHA preparation at a high dose of 4 g/day. In addition, this preparation is in a free fatty acid form instead of the conventional ester form. The free fatty acid form is used to increase the efficiency of absorption because the ester form requires postprandial administration and necessitates decomposition by lipase in the intestinal tract for absorption126). The results of the STRENGTH trial are expected to provide more definite evidence on the use of omega-3 PUFA to reduce CVD risk. In Japan, RESPECT-EPA trial is in progress. In this study, patients with chronic coronary artery disease receiving LDL-C lowering treatment by statin will be randomized to either a control group (standard treatment) or EPA group (standard treatment plus EPA) to examine the effects of EPA on the incidence of CVD. The results are awaited.
Conclusion
Although only five decades have passed since the beginning of research on omega-3 PUFA, omega-3 PUFA clearly play an important role in homeostasis of the living body and health maintenance. However, along with the Westernization of the lifestyle in Japan, the traditional fish-eating habit is being lost, especially in the younger generation. This is an aspect of great concern in the prospect of future Japanese health.
Declaration of Interest
Ichiro Tatsuno received lecture fees from Takeda Pharmaceutical Co., Ltd. and Novartis Pharma K.K., and received research grants from Takeda Pharmaceutical Co., Ltd., and Sunny Health Co., Ltd. The other authors have nothing to disclose.
References
- 1). Kimura N, Keys A: Coronary heart disease in seven countries. X. Rural southern Japan. Circulation, 1970; 41: I101-112 [PubMed] [Google Scholar]
- 2). Bang HO, Dyerberg J, Nielsen AB: Plasma lipid and lipoprotein pattern in Greenlandic West-coast Eskimos. Lancet, 1971; 1: 1143-1145 [DOI] [PubMed] [Google Scholar]
- 3). Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet, 1999; 354: 447-455 [PubMed] [Google Scholar]
- 4). Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G: Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet, 2008; 372: 1223-1230 [DOI] [PubMed] [Google Scholar]
- 5). Hirai A, Hamazaki T, Terano T, Nishikawa T, Tamura Y, Kamugai A, Jajiki J: Eicosapentaenoic acid and platelet function in Japanese. Lancet, 1980; 2: 1132-1133 [DOI] [PubMed] [Google Scholar]
- 6). Terano T, Hirai A, Hamazaki T, Kobayashi S, Fujita T, Tamura Y, Kumagai A: Effect of oral administration of highly purified eicosapentaenoic acid on platelet function, blood viscosity and red cell deformability in healthy human subjects. Atherosclerosis, 1983; 46: 321-331 [DOI] [PubMed] [Google Scholar]
- 7). Canhada S, Castro K, Perry IS, Luft VC: Omega-3 fatty acids' supplementation in Alzheimer's disease: A systematic review. Nutr Neurosci, 2018; 21: 529-538 [DOI] [PubMed] [Google Scholar]
- 8). Yagi S, Fukuda D, Aihara KI, Akaike M, Shimabukuro M, Sata M: n-3 Polyunsaturated Fatty Acids: Promising Nutrients for Preventing Cardiovascular Disease. J Atherosclero Thromb, 2017; 24: 999-1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Siscovick DS, Barringer TA, Fretts AM, Wu JH, Lichtenstein AH, Costello RB, Kris-Etherton PM, Jacobson TA, Engler MB, Alger HM, Appel LJ, Mozaffarian D: Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation, 2017; 135: e867-e884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Gioxari A, Kaliora AC, Marantidou F, Panagiotakos DP: Intake of omega-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: A systematic review and meta-analysis. Nutrition, 2018; 45: 114-124. e114 [DOI] [PubMed] [Google Scholar]
- 11). Upala S, Yong WC, Theparee T, Sanguankeo A: Effect of omega-3 fatty acids on disease severity in patients with psoriasis: A systematic review. Int J Rheum Dis, 2017; 20: 442-450 [DOI] [PubMed] [Google Scholar]
- 12). Gerber M: Omega-3 fatty acids and cancers: a systematic update review of epidemiological studies. Br J Nutr, 2012; 107 Suppl 2: S228-239 [DOI] [PubMed] [Google Scholar]
- 13). Husson MO, Ley D, Portal C, Gottrand M, Hueso T, Desseyn JL, Gottrand F: Modulation of host defence against bacterial and viral infections by omega-3 polyunsaturated fatty acids. J Infect, 2016; 73: 523-535 [DOI] [PubMed] [Google Scholar]
- 14). Masson S, Latini R, Tacconi M, Bernasconi R: Incorporation and washout of n-3 polyunsaturated fatty acids after diet supplementation in clinical studies. J Cardiovasc Med, 2007; 8 Suppl 1: S4-10 [DOI] [PubMed] [Google Scholar]
- 15). Nordoy A, Barstad L, Connor WE, Hatcher L: Absorption of the n-3 eicosapentaenoic and docosahexaenoic acids as ethyl esters and triglycerides by humans. Am J Clin Nutr, 1991; 53: 1185-1190 [DOI] [PubMed] [Google Scholar]
- 16). Terano T: Polyunsaturated fatty acids: Interpretation of EPA, DHA and AA in human body (in Japanese). Anti-Aging Medicine, 2010; 6: 540-547 [Google Scholar]
- 17). Takashima A, Fukuda D, Tanaka K, Higashikuni Y, Hirata Y, Nishimoto S, Yagi S, Yamada H, Soeki T, Wakatsuki T, Taketani Y, Shimabukuro M, Sata M: Combination of n-3 polyunsaturated fatty acids reduces atherogenesis in apolipoprotein E-deficient mice by inhibiting macrophage activation. Atherosclerosis, 2016; 254: 142-150 [DOI] [PubMed] [Google Scholar]
- 18). Katakura M, Hashimoto M, Inoue T, Al Mamun A, Tanabe Y, Iwamoto R, Arita M, Tsuchikura S, Shido O: Omega-3 fatty acids protect renal functions by increasing docosahexaenoic acid-derived metabolite levels in SHR.Cg-Lepr(cp)/NDmcr rats, a metabolic syndrome model. Molecules, 2014; 19: 3247-3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Su H, Zhou D, Pan YX, Wang X, Nakamura MT: Compensatory induction of Fads1 gene expression in heterozygous Fads2-null mice and by diet with a high n-6/n-3 PUFA ratio. J Lipid Res, 2016; 57: 1995-2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Hirai A: An epidemiological study on the dietary ingestion of eicosapentaenoic acid (EPA) and platelet function in Japanese. Intern Med, 1985; 74: 13-20 [DOI] [PubMed] [Google Scholar]
- 21). Investigators OT, Bosch J, Gerstein HC, Dagenais GR, Diaz R, Dyal L, Jung H, Maggiono AP, Probstfield J, Ramachandran A, Riddle MC, Ryden LE, Yusuf S: n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med, 2012; 367: 309-318 [DOI] [PubMed] [Google Scholar]
- 22). Roncaglioni MC, Tombesi M, Avanzini F, Barlera S, Caimi V, Longoni P, Marzona I, Milani V, Silletta MG, Tognoni G, Marchioli R: n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med, 2013; 368: 1800-1808 [DOI] [PubMed] [Google Scholar]
- 23). Tatsuno I, Saito Y, Kudou K, Ootake J: Long-term safety and efficacy of TAK-085 in Japanese subjects with hypertriglyceridemia undergoing lifestyle modification: the omega-3 fatty acids randomized long-term (ORL) study. J Clin Lipidol, 2013; 7: 615-625 [DOI] [PubMed] [Google Scholar]
- 24). Tatsuno I, Saito Y, Kudou K, Ootake J: Efficacy and safety of TAK-085 compared with eicosapentaenoic acid in Japanese subjects with hypertriglyceridemia undergoing lifestyle modification: the omega-3 fatty acids randomized double-blind (ORD) study. J Clin Lipidol, 2013; 7: 199-207 [DOI] [PubMed] [Google Scholar]
- 25). Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ: Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens, 2002; 20: 1493-1499 [DOI] [PubMed] [Google Scholar]
- 26). Kris-Etherton PM, Harris WS, Appel LJ: Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation, 2002; 106: 2747-2757 [DOI] [PubMed] [Google Scholar]
- 27). Robinson JG, Stone NJ: Antiatherosclerotic and antithrombotic effects of omega-3 fatty acids. Am J Cardiol, 2006; 98: 39i-49i [DOI] [PubMed] [Google Scholar]
- 28). Wang Q, Liang X, Wang L, Lu X, Huang J, Cao J, Li H, Gu D: Effect of omega-3 fatty acids supplementation on endothelial function: a meta-analysis of randomized controlled trials. Atherosclerosis, 2012; 221: 536-543 [DOI] [PubMed] [Google Scholar]
- 29). Tousoulis D, Plastiras A, Siasos G, Oikonomou E, Verveniotis A, Kokkou E, Maniatis K, Gouliopoulos N, Miliou A, Paraskevopoulos T, Stefanadis C: Omega-3 PUFAs improved endothelial function and arterial stiffness with a parallel antiinflammatory effect in adults with metabolic syndrome. Atherosclerosis, 2014; 232: 10-16 [DOI] [PubMed] [Google Scholar]
- 30). Merino J, Sala-Vila A, Kones R, Ferre R, Plana N, Girona J, Ibarretxe D, Heras M, Ros E, Masana L: Increasing long-chain n-3PUFA consumption improves small peripheral artery function in patients at intermediate-high cardiovascular risk. J Nutr Biochem, 2014; 25: 642-646 [DOI] [PubMed] [Google Scholar]
- 31). Chan DC, Pang J, Barrett PHR, Sullivan DR, Mori TA, Burnett JR, van Bockxmeer FM, Watts GF: Effect of Omega-3 Fatty Acid Supplementation on Arterial Elasticity in Patients with Familial Hypercholesterolaemia on Statin Therapy. Nutr Metab Cardiovasc Dis, 2016; 26: 6-11 [DOI] [PubMed] [Google Scholar]
- 32). Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet, 1994; 344: 1383-1389 [PubMed] [Google Scholar]
- 33). Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ: Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med, 1995; 333: 1301-1307 [DOI] [PubMed] [Google Scholar]
- 34). Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, Nakaya N, Nishimoto S, Muranaka M, Yamamoto A, Mizuno K, Ohashi Y: Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet, 2006; 368: 1155-1163 [DOI] [PubMed] [Google Scholar]
- 35). Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R: Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet, 2010; 376: 1670-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, Baigent C: The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: metaanalysis of individual data from 27 randomised trials. Lancet, 2012; 380: 581-590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet, 2019; 393: 407-415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Lim S, Park YM, Sakuma I, Koh KK: How to control residual cardiovascular risk despite statin treatment: focusing on HDL-cholesterol. Int J Cardiol, 2013; 166: 8-14 [DOI] [PubMed] [Google Scholar]
- 39). Assmann G, Schulte H, von Eckardstein A: Hypertriglyceridemia and elevated lipoprotein(a) are risk factors for major coronary events in middle-aged men. Am J Cardiol, 1996; 77: 1179-1184 [DOI] [PubMed] [Google Scholar]
- 40). Nordestgaard BG, Varbo A: Triglycerides and cardiovascular disease. Lancet, 2014; 384: 626-635 [DOI] [PubMed] [Google Scholar]
- 41). Sone H, Tanaka S, Tanaka S, Iimuro S, Oida K, Yamasaki Y, Oikawa S, Ishibashi S, Katayama S, Ohashi Y, Akanuma Y, Yamada N, Study JDC : Serum Level of Triglycerides Is a Potent Risk Factor Comparable to LDL Cholesterol for Coronary Heart Disease in Japanese Patients with Type 2 Diabetes: Subanalysis of the Japan Diabetes Complications Study (JDCS). J Clin Endocrinol Metab, 2011; 96: 3448-3456 [DOI] [PubMed] [Google Scholar]
- 42). Bays HE, Tighe AP, Sadovsky R, Davidson MH: Prescription omega-3 fatty acids and their lipid effects: physiologic mechanisms of action and clinical implications. Expert Rev Cardiovasc Ther, 2008; 6: 391-409 [DOI] [PubMed] [Google Scholar]
- 43). Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, Ferranti Sd, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC, Sperling L, Virani SS, Yeboah J: 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation, 0: CIR. 0000000000000625 [Google Scholar]
- 44). Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC, Virani SS, Williams KA, Yeboah J, Ziaeian B: 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. Circulation, 0: CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45). Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, Umemoto S, Egusa G, Ohmura H, Okamura T, Kihara S, Koba S, Saito I, Shoji T, Daida H, Tsukamoto K, Deguchi J, Dohi S, Dobashi K, Hamaguchi H, Hara M, Hiro T, Biro S, Fujioka Y, Maruyama C, Miyamoto Y, Murakami Y, Yokode M, Yoshida H, Rakugi H, Wakatsuki A, Yamashita S: Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. J Atheroscler Thromb, 2018; 25: 846-984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46). McKenney JM, Sica D: Prescription omega-3 fatty acids for the treatment of hypertriglyceridemia. Am J Health Syst Pharm, 2007; 64: 595-605 [DOI] [PubMed] [Google Scholar]
- 47). Harris WS, Bulchandani D: Why do omega-3 fatty acids lower serum triglycerides? Curr Opin Lipidol, 2006; 17: 387-393 [DOI] [PubMed] [Google Scholar]
- 48). Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K: Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded end-point analysis. Lancet, 2007; 369: 1090-1098 [DOI] [PubMed] [Google Scholar]
- 49). Saito Y, Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K: Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: sub-analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis, 2008; 200: 135-140 [DOI] [PubMed] [Google Scholar]
- 50). Ninomiya T, Nagata M, Hata J, Hirakawa Y, Ozawa M, Yoshida D, Ohara T, Kishimoto H, Mukai N, Fukuhara M, Kitazono T, Kiyohara Y: Association between ratio of serum eicosapentaenoic acid to arachidonic acid and risk of cardiovascular disease: The Hisayama Study. Atherosclerosis, 2013; 231: 261-267 [DOI] [PubMed] [Google Scholar]
- 51). Itakura H, Yokoyama M, Matsuzaki M, Saito Y, Origasa H, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K, Matsuzawa Y: Relationships between plasma fatty acid composition and coronary artery disease. J Atheroscler Thromb, 2011; 18: 99-107 [DOI] [PubMed] [Google Scholar]
- 52). Umemoto N, Ishii H, Kamoi D, Aoyama T, Sakakibara T, Takahashi H, Tanaka A, Yasuda Y, Suzuki S, Matsubara T, Murohara T: Reverse association of omega-3/omega-6 polyunsaturated fatty acids ratios with carotid atherosclerosis in patients on hemodialysis. Atherosclerosis, 2016; 249: 65-69 [DOI] [PubMed] [Google Scholar]
- 53). Tomiyama H, Takazawa K, Osa S-i, Hirose K-i, Hirai A, Iketani T, Monden M, Sanoyama K, Yamashina A: Do Eicosapentaenoic Acid Supplements Attenuate Age- Related Increases in Arterial Stiffness in Patients with Dyslipidemia?: A Preliminary Study. Hypertens Res, 2005; 28: 651-655 [DOI] [PubMed] [Google Scholar]
- 54). Yoshida H, Ito K, Sato R, Kurosawa H, Tomono Y, Hirowatari Y, Shimizu M, Tada N: Clinical relevance of decreased ratios of serum eicosapentaenoic acid/arachidonic acid (AA) and docosahexaenoic acid/AA to impaired arterial stiffness. Int J Cardiol, 2014; 177: 517-519 [DOI] [PubMed] [Google Scholar]
- 55). Ito R, Satoh-Asahara N, Yamakage H, Sasaki Y, Odori S, Kono S, Wada H, Suganami T, Ogawa Y, Hasegawa K, Shimatsu A: An Increase in the EPA/AA Ratio is Associated with Improved Arterial Stiffness in Obese Patients with Dyslipidemia. J Atheroscler Thromb, 2014; 21: 248-260 [DOI] [PubMed] [Google Scholar]
- 56). Hillege HL, Fidler V, Diercks GFH, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, Gans ROB, Janssen WMT, Grobbee DE, de Jong PE, Prevention of R and Vascular End Stage Disease Study G : Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation, 2002; 106: 1777-1782 [DOI] [PubMed] [Google Scholar]
- 57). Yuyun MF, Khaw K-T, Luben R, Welch A, Bingham S, Day NE, Wareham NJ: Microalbuminuria independently predicts all-cause and cardiovascular mortality in a British population: The European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) population study. Int J Epidemiol, 2004; 33: 189-198 [DOI] [PubMed] [Google Scholar]
- 58). Fukami A, Adachi H, Hirai Y, Enomoto M, Otsuka M, Kumagai E, Nakamura S, Yoshimura A, Obuchi A, Nohara Y, Nakao E, Hori K, Fukumoto Y: Association of serum eicosapentaenoic acid to arachidonic acid ratio with microalbuminuria in a population of community-dwelling Japanese. Atherosclerosis, 2015; 239: 577-582 [DOI] [PubMed] [Google Scholar]
- 59). Fulcher J, O'Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, Simes J, Collins R, Kirby A, Colhoun H, Braunwald E, La Rosa J, Pedersen TR, Tonkin A, Davis B, Sleight P, Franzosi MG, Baigent C, Keech A, De Lemos J, Braunwald E, Blazing M, Murphy S, Downs JR, Gotto A, Clearfield M, Holdaas H, Gordon D, Davis B, Koren M, Dahlöf B, Poulter N, Sever P, Knopp RH, Fellström B, Holdaas H, Jardine A, Schmieder R, Zannad F, Goldbourt U, Kaplinsky E, Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Fuller J, Neil A, Wanner C, Krane V, Sacks F, Moy L, Pfeffer M, Hawkins CM, Braunwald E, Kjekshus J, Wedel H, Wikstrand J, Barter P, Keech A, Tavazzi L, Maggioni A, Marchioli R, Tognoni G, Franzosi MG, Maggioni A, Bloomfield H, Robins S, Collins R, Armitage J, Keech A, Parish S, Peto R, Sleight P, Pedersen TR, Ridker PM, Holman R, Meade T, Simes J, Keech A, MacMahon S, Marschner I, Tonkin A, Shaw J, Serruys PW, Nakamura H, Knatterud G, Furberg C, Byington R, Macfarlane P, Cobbe S, Ford I, Murphy M, Blauw GJ, Packard C, Shepherd J, Kjekshus J, Pedersen T, Wilhelmsen L, Braunwald E, Cannon C, Murphy S, Collins R, Armitage J, Bowman L, Parish S, Peto R, Sleight P, Baigent C, Landray M, Collins R, La Rosa J, Rossouw J, Probstfield J, Shepherd J, Cobbe S, Macfarlane P, Ford I, Flather M, Kastelein J, Newman C, Shear C, Tobert J, Varigos J, White H, Yusuf S, Barnes EH, Keech AC, Kirby A, Marschner IC, Simes J, Baigent C, Blackwell L, Collins R, Emberson J, Herrington WG, Holland LE, Reith C: Efficacy and safety of LDL-lowering therapy among men and women: Meta-analysis of individual data from 174 000 participants in 27 randomised trials. The Lancet, 2015; 385: 1397-1405 [DOI] [PubMed] [Google Scholar]
- 60). Sampson UK, Fazio S, Linton MRF: Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: The evidence, etiology, and therapeutic challenges. Curr Atheroscler Rep, 2012; 14: 1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61). Nozue T, Yamamoto S, Tohyama S, Fukui K, Umezawa S, Onishi Y, Kunishima T, Sato A, Nozato T, Miyake S, Takeyama Y, Morino Y, Yamauchi T, Muramatsu T, Hibi K, Terashima M, Michishita I: Comparison of Effects of serum n-3 to n-6 polyunsaturated fatty acids ratios on coronary atherosclerosis in statin-treated patients with coronary artery disease. Am J Cardiol, 2013; 111: 6-11 [DOI] [PubMed] [Google Scholar]
- 62). De Lorgeril M, Salen P, Guiraud A, Zeghichi S, Boucher F, De Leiris J: Lipid-lowering drugs and essential omega-6 and omega-3 fatty acids in patients with coronary heart disease. Nutr Metab Cardiovasc Dis, 2005; 15: 36-41 [DOI] [PubMed] [Google Scholar]
- 63). Yagi S, Kondo D, Ise T, Fukuda D, Yamaguchi K, Wakatsuki T, Kawabata Y, Ito H, Saijo Y, Seno H, Sutou K, Ueno R, Todoroki T, Kusunose K, Matsuura T, Tobiume T, Yamada H, Soeki T, Shimabukuro M, Aihara KI, Akaike M, Sata M: Association of Decreased Docosahexaenoic Acid Level After Statin Therapy and Low Eicosapentaenoic Acid Level with In-Stent Restenosis in Patients with Acute Coronary Syndrome. J Atheroscler Thromb, 2019; 26: 272-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64). Abedi E, Sahari MA: Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci Nutr, 2014; 2: 443-463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65). Ishihara N, Suzuki S, Tanaka S, Watanabe Y, Nagayama D, Saiki A, Tanaka T, Tatsuno I: Atorvastatin increases Fads1, Fads2 and Elovl5 gene expression via the geranylgeranyl pyrophosphate-dependent Rho kinase pathway in 3T3-L1 cells. Mol Med Rep, 2017; 16: 4756-4762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66). Tanaka S, Ishihara N, Suzuki S, Watanabe Y, Nagayama D, Yamaguchi T, Ohira M, Saiki A, Tanaka T, Tatsuno I: Fatty acid desaturase 2 is up-regulated by the treatment with statin through geranylgeranyl pyrophosphate- dependent Rho kinase pathway in HepG2 cells. Sci Rep, 2019; 9: 10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67). Watanabe T, Ando K, Daidoji H, Otaki Y, Sugawara S, Matsui M, Ikeno E, Hirono O, Miyawaki H, Yashiro Y, Nishiyama S, Arimoto T, Takahashi H, Shishido T, Miyashita T, Miyamoto T, Kubota I: A randomized controlled trial of eicosapentaenoic acid in patients with coronary heart disease on statins. J Cardiol, 2017; 70: 537-544 [DOI] [PubMed] [Google Scholar]
- 68). Nosaka K, Miyoshi T, Iwamoto M, Kajiya M, Okawa K, Tsukuda S, Yokohama F, Sogo M, Nishibe T, Matsuo N, Hirohata S, Ito H, Doi M: Early initiation of eicosapentaenoic acid and statin treatment is associated with better clinical outcomes than statin alone in patients with acute coronary syndromes: 1-year outcomes of a randomized controlled study. Int J Cardiol, 2017; 228: 173-179 [DOI] [PubMed] [Google Scholar]
- 69). Miyoshi T, Kohno K, Asonuma H, Sakuragi S, Nakahama M, Kawai Y, Uesugi T, Oka T, Munemasa M, Takahashi N, Mukohara N, Habara S, Koyama Y, Nakamura K, Ito H: Effect of Intensive and Standard Pitavastatin Treatment With or Without Eicosapentaenoic Acid on Progression of Coronary Artery Calcification Over 12 Months- Prospective Multicenter Study. Circ J, 2018; 82: 532-540 [DOI] [PubMed] [Google Scholar]
- 70). Kromann N, Green A: Epidemiological studies in the Upernavik district, Greenland. Incidence of some chronic diseases 1950–1974. Acta Med Scand, 1980; 208: 401-406 [PubMed] [Google Scholar]
- 71). Prickett JD, Robinson DR, Steinberg AD: Dietary enrichment with the polyunsaturated fatty acid eicosapentaenoic acid prevents proteinuria and prolongs survival in NZB × NZW F1 mice. J Clin Invest, 1981; 68: 556-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72). Chang KJ, Saito H, Tatsuno I, Tamura Y, Yoshida S: Role of 5-lipoxygenase products of arachidonic acid in cell-to-cell interaction between macrophages and natural killer cells in rat spleen. J Leukoc Biol, 1991; 50: 273-278 [DOI] [PubMed] [Google Scholar]
- 73). Tatsuno I, Saito H, Chang KJ, Tamura Y, Yoshida S: Comparison of the effect between leukotriene B4 and leukotriene B5 on the induction of interleukin 1-like activity and calcium mobilizing activity in human blood monocytes. Agents Actions, 1990; 29: 324-327 [DOI] [PubMed] [Google Scholar]
- 74). Cao RY, St Amand T, Grabner R, Habenicht AJ, Funk CD: Genetic and pharmacological inhibition of the 5-lipoxygenase/leukotriene pathway in atherosclerotic lesion development in ApoE deficient mice. Atherosclerosis, 2009; 203: 395-400 [DOI] [PubMed] [Google Scholar]
- 75). Matsumoto M, Sata M, Fukuda D, Tanaka K, Soma M, Hirata Y, Nagai R: Orally administered eicosapentaenoic acid reduces and stabilizes atherosclerotic lesions in ApoE-deficient mice. Atherosclerosis, 2008; 197: 524-533 [DOI] [PubMed] [Google Scholar]
- 76). Allaire J, Couture P, Leclerc M, Charest A, Marin J, Lepine MC, Talbot D, Tchernof A, Lamarche B: A randomized, crossover, head-to-head comparison of eicosapentaenoic acid and docosahexaenoic acid supplementation to reduce inflammation markers in men and women: the Comparing EPA to DHA (ComparED) Study. Am J Clin Nutr, 2016; 104: 280-287 [DOI] [PubMed] [Google Scholar]
- 77). Halade GV, Rahman MM, Bhattacharya A, Barnes JL, Chandrasekar B, Fernandes G: Docosahexaenoic acid-enriched fish oil attenuates kidney disease and prolongs median and maximal life span of autoimmune lupus-prone mice. J Immunol, 2010; 184: 5280-5286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78). Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL: Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med, 2002; 196: 1025-1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79). Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN: Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature, 2009; 461: 1287-1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80). Serhan CN: Pro-resolving lipid mediators are leads for resolution physiology. Nature, 2014; 510: 92-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81). Keyes KT, Ye Y, Lin Y, Zhang C, Perez-Polo JR, Gjorstrup P, Birnbaum Y: Resolvin E1 protects the rat heart against reperfusion injury. Am J Physiol Heart Circ Physiol, 2010; 299: H153-H164 [DOI] [PubMed] [Google Scholar]
- 82). Kain V, Ingle KA, Colas RA, Dalli J, Prabhu SD, Serhan CN, Joshi M, Halade GV: Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J Mol Cell Cardiol, 2015; 84: 24-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83). Heydari B, Abdullah S, Pottala JV, Shah R, Abbasi S, Mandry D, Francis SA, Lumish H, Ghoshhajra BB, Hoffmann U, Appelbaum E, Feng JH, Blankstein R, Steigner M, McConnell JP, Harris W, Antman EM, Jerosch-Herold M, Kwong RY: Effect of Omega-3 Acid Ethyl Esters on Left Ventricular Remodeling After Acute Myocardial Infarction: The OMEGA-REMODEL Randomized Clinical Trial. Circulation, 2016; 134: 378-391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84). Morita M, Kuba K, Ichikawa A, Nakayama M, Katahira J, Iwamoto R, Watanebe T, Sakabe S, Daidoji T, Nakamura S, Kadowaki A, Ohto T, Nakanishi H, Taguchi R, Nakaya T, Murakami M, Yoneda Y, Arai H, Kawaoka Y, Penninger JM, Arita M, Imai Y: The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell, 2013; 153: 112-125 [DOI] [PubMed] [Google Scholar]
- 85). Mozaffarian D, Rimm EB: Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA, 2006; 296: 1885-1899 [DOI] [PubMed] [Google Scholar]
- 86). Pignier C, Revenaz C, Rauly-Lestienne I, Cussac D, Delhon A, Gardette J, Le Grand B: Direct protective effects of poly-unsaturated fatty acids, DHA and EPA, against activation of cardiac late sodium current: a mechanism for ischemia selectivity. Basic Res Cardiol, 2007; 102: 553-564 [DOI] [PubMed] [Google Scholar]
- 87). Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL: 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation, 2013; 128: e240-327 [DOI] [PubMed] [Google Scholar]
- 88). Leaf A, Albert CM, Josephson M, Steinhaus D, Kluger J, Kang JX, Cox B, Zhang H, Schoenfeld D: Prevention of fatal arrhythmias in high-risk subjects by fish oil n-3 fatty acid intake. Circulation, 2005; 112: 2762-2768 [DOI] [PubMed] [Google Scholar]
- 89). Brouwer IA, Zock PL, Camm AJ, Bocker D, Hauer RN, Wever EF, Dullemeijer C, Ronden JE, Katan MB, Lubinski A. Buschler H and Schouten EG: Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators: the Study on Omega-3 Fatty Acids and Ventricular Arrhythmia (SOFA) randomized trial. JAMA, 2006; 295: 2613-2619 [DOI] [PubMed] [Google Scholar]
- 90). Jenkins DJ, Josse AR, Beyene J, Dorian P, Burr ML, LaBelle R, Kendall CW, Cunnane SC: Fish-oil supplementation in patients with implantable cardioverter defibrillators: a meta-analysis. CMAJ, 2008; 178: 157-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91). Brouwer IA, Raitt MH, Dullemeijer C, Kraemer DF, Zock PL, Morris C, Katan MB, Connor WE, Camm JA, Schouten EG, McAnulty J: Effect of fish oil on ventricular tachyarrhythmia in three studies in patients with implantable cardioverter defibrillators. Eur Heart J, 2009; 30: 820-826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92). Mozaffarian D, Psaty BM, Rimm EB, Lemaitre RN, Burke GL, Lyles MF, Lefkowitz D, Siscovick DS: Fish intake and risk of incident atrial fibrillation. Circulation, 2004; 110: 368-373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93). Mozaffarian D, Wu JH, de Oliveira Otto MC, Sandesara CM, Metcalf RG, Latini R, Libby P, Lombardi F, O'Gara PT, Page RL, Silletta MG, Tavazzi L, Marchioli R: Fish oil and post-operative atrial fibrillation: a metaanalysis of randomized controlled trials. J Am Coll Cardiol, 2013; 61: 2194-2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94). Mariani J, Doval HC, Nul D, Varini S, Grancelli H, Ferrante D, Tognoni G, Macchia A: N-3 polyunsaturated fatty acids to prevent atrial fibrillation: updated systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc, 2013; 2: e005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95). Mozaffarian D, Rimm EB: Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA, 2006; 296: 1885-1899 [DOI] [PubMed] [Google Scholar]
- 96). Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS: Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA, 2012; 308: 1024-1033 [DOI] [PubMed] [Google Scholar]
- 97). Miyagawa N, Miura K, Okuda N, Kadowaki T, Takashima N, Nagasawa Sy, Nakamura Y, Matsumura Y, Hozawa A, Fujiyoshi A, Hisamatsu T, Yoshita K, Sekikawa A, Ohkubo T, Abbott RD, Okamura T, Okayama A, Ueshima H: Long-chain n-3 polyunsaturated fatty acids intake and cardiovascular disease mortality risk in Japanese: A 24-year follow-up of NIPPON DATA80. Atherosclerosis, 2014; 232: 384-389 [DOI] [PubMed] [Google Scholar]
- 98). de Oliveira Otto MC, Wu JHY, Baylin A, Vaidya D, Rich SS, Tsai MY, Jacobs DR, Mozaffarian D: Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi- Ethnic Study of Atherosclerosis. J Am Heart Assoc, 2013; 2: e000506-e000506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99). Matsuzaki M, Yokoyama M, Saito Y, Origasa H, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K, Matsuzawa Y: Incremental effects of eicosapentaenoic acid on cardiovascular events in statin-treated patients with coronary artery disease. Circ J, 2009; 73: 1283-1290 [DOI] [PubMed] [Google Scholar]
- 100). Galan P, Kesse-Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S: Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ, 2010; 341: c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101). Kromhout D, Giltay EJ, Geleijnse JM: n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med, 2010; 363: 2015-2026 [DOI] [PubMed] [Google Scholar]
- 102). Roncaglioni MC, Tombesi M, Avanzini F, Barlera S, Caimi V, Longoni P, Marzona I, Milani V, Silletta MG, Tognoni G, Marchioli R: n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med, 2013; 368: 1800-1808 [DOI] [PubMed] [Google Scholar]
- 103). Rhee JJ, Kim E, Buring JE, Kurth T: Fish Consumption, Omega-3 Fatty Acids, and Risk of Cardiovascular Disease. Am J Prev Med, 2016; 52: 10-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104). Gobbo LCD, Imamura F, Aslibekyan S, Marklund M, Virtanen JK, Wennberg M, Yakoob MY, Chiuve SE, Cruz L, Frazier-wood AC, Fretts AM, Guallar E, Matsumoto C, Prem K, Tanaka T, Wu JHY, Zhou X, Helmer C, Jansson J-h, Johansson I, Khaw K-t, Koh W-p, Lemaitre RN, Lind L, Luben RN, Rimm EB, Risérus U, Samieri C, Franks PW, Siscovick DS, Stampfer M, Steffen LM, Steffen BT, Tsai MY, Dam RMV, Voutilainen S: ω-3 Polyunsaturated Fatty Acid Biomarkers and Coronary Heart Disease Pooling Project of 19 Cohort Studies. JAMA Intern Med, 2016; 94305: 1-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105). Morris MC, Manson JE, Rosner B, Buring JE, Willett WC, Hennekens CH: Fish consumption and cardiovascular disease in the physicians' health study: a prospective study. Am J Epidemiol, 1995; 142: 166-175 [DOI] [PubMed] [Google Scholar]
- 106). Mozaffarian D, Lemaitre RN, Kuller LH, Burke GL, Tracy RP, Siscovick DS: Cardiac benefits of fish consumption may depend on the type of fish meal consumed: the Cardiovascular Health Study. Circulation, 2003; 107: 1372-1377 [DOI] [PubMed] [Google Scholar]
- 107). Iso H, Kobayashi M, Ishihara J, Sasaki S, Okada K, Kita Y, Kokubo Y, Tsugane S: Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: the Japan Public Health Center-Based (JPHC) Study Cohort I. Circulation, 2006; 113: 195-202 [DOI] [PubMed] [Google Scholar]
- 108). Block RC, Harris WS, Reid KJ, Sands SA, Spertus JA: EPA and DHA in blood cell membranes from acute coronary syndrome patients and controls. Atherosclerosis, 2008; 197: 821-828 [DOI] [PubMed] [Google Scholar]
- 109). Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, Mantini L, Marfisi RM, Mastrogiuseppe G, Mininni N, Nicolosi GL, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C, Valagussa F: Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation, 2002; 105: 1897-1903 [DOI] [PubMed] [Google Scholar]
- 110). Rauch B, Schiele R, Schneider S, Diller F, Victor N, Gohlke H, Gottwik M, Steinbeck G, Del Castillo U, Sack R, Worth H, Katus H, Spitzer W, Sabin G, Senges J: OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation, 2010; 122: 2152-2159 [DOI] [PubMed] [Google Scholar]
- 111). Marangoni F, Novo G, Perna G, Perrone Filardi P, Pirelli S, Ceroti M, Querci A, Poli A: Omega-6 and omega-3 polyunsaturated fatty acid levels are reduced in whole blood of Italian patients with a recent myocardial infarction: The AGE-IM study. Atherosclerosis, 2014; 232: 334-338 [DOI] [PubMed] [Google Scholar]
- 112). Koh AS, Pan A, Wang R, Odegaard AO, Pereira Ma, Yuan J-M, Koh W-P: The association between dietary omega-3 fatty acids and cardiovascular death: the Singapore Chinese Health Study. Eur J Prev Cardiol, 2015; 22: 364-372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113). Mozaffarian D, Bryson CL, Lemaitre RN, Burke GL, Siscovick DS: Fish intake and risk of incident heart failure. J Am Coll Cardiol, 2005; 45: 2015-2021 [DOI] [PubMed] [Google Scholar]
- 114). Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB: Effect of fish oil on heart rate in humans: a meta-analysis of randomized controlled trials. Circulation, 2005; 112: 1945-1952 [DOI] [PubMed] [Google Scholar]
- 115). Belin RJ, Greenland P, Martin L, Oberman A, Tinker L, Robinson J, Larson J, Van Horn L, Lloyd-Jones D: Fish intake and the risk of incident heart failure: the Women's Health Initiative. Circ Heart Fail, 2011; 4: 404-413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116). Ghio S, Scelsi L, Latini R, Masson S, Eleuteri E, Palvarini M, Vriz O, Pasotti M, Gorini M, Marchioli R, Maggioni A, Tavazzi L: Effects of n-3 polyunsaturated fatty acids and of rosuvastatin on left ventricular function in chronic heart failure: a substudy of GISSI-HF trial. Eur J Heart Fail, 2010; 12: 1345-1353 [DOI] [PubMed] [Google Scholar]
- 117). Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL: 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation, 2013; 128: e240-319 [DOI] [PubMed] [Google Scholar]
- 118). He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, Goldbourt U, Greenland P: Fish consumption and incidence of stroke: a meta-analysis of cohort studies. Stroke, 2004; 35: 1538-1542 [DOI] [PubMed] [Google Scholar]
- 119). Xun P, Qin B, Song Y, Nakamura Y, Kurth T, Yaemsiri S, Djousse L, He K: Fish consumption and risk of stroke and its subtypes: accumulative evidence from a meta-analysis of prospective cohort studies. Eur J Clin Nutr, 2012; 66: 1199-1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120). Tanaka K, Ishikawa Y, Yokoyama M, Origasa H, Matsuzaki M, Saito Y, Matsuzawa Y, Sasaki J, Oikawa S, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K: Reduction in the recurrence of stroke by eicosapentaenoic acid for hypercholesterolemic patients: subanalysis of the JELIS trial. Stroke, 2008; 39: 2052-2058 [DOI] [PubMed] [Google Scholar]
- 121). Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, Murawska A, Young A, Lay M, Chen F, Sammons E, Waters E, Adler A, Bodansky J, Farmer A, McPherson R, Neil A, Simpson D, Peto R, Baigent C, Collins R, Parish S, Armitage J: Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus. N Engl J Med, 2018; 379: 1540-1550 [DOI] [PubMed] [Google Scholar]
- 122). Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Jr., Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM: Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med, 2019; 380: 11-22 [DOI] [PubMed] [Google Scholar]
- 123). Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Albert CM, Gordon D, Copeland T, D'Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE: Marine n-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N Engl J Med, 2019; 380: 23-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124). Nicholls SJ, Lincoff AM, Bash D, Ballantyne CM, Barter PJ, Davidson MH, Kastelein JJP, Koenig W, McGuire DK, Mozaffarian D, Pedersen TR, Ridker PM, Ray K, Karlson BW, Lundstrom T, Wolski K, Nissen SE: Assessment of omega-3 carboxylic acids in statin-treated patients with high levels of triglycerides and low levels of high-density lipoprotein cholesterol: Rationale and design of the STRENGTH trial. Clin Cardiol, 2018; 41: 1281-1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125). UMIN-CTR Clinical Trials Registry. https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000014051 (accessed June 10, 2019).
- 126). Endo J, Arita M: Cardioprotective mechanism of omega-3 polyunsaturated fatty acids. J Cardiol, 2016; 67: 22-27 [DOI] [PubMed] [Google Scholar]


