Abstract
Aim: β-actin (ACTB) participates in the vascular remodeling and contributes to the cardiovascular diseases. Herein, we investigated the associations of ACTB with hypertension and stroke.
Methods: Three single-nucleotide polymorphisms in ACTB were selected for genotyping in 2,012 hypertension cases and 2,210 controls. The associations of ACTB with hypertension and stroke were examined in another follow-up study. Logistic and Cox regression were performed in a case-control study and a follow-up study, respectively. Additive scale interaction was examined by calculating the relative excess risk due to interaction (RERI), attributable proportion due to interaction (AP) and synergy index (SI). The multiplicative interaction hazard ratio was calculated by fitting the Cox regression model. ACTB mRNA in peripheral blood mononuclear cells was measured in ischemic stroke (IS) cases and in controls.
Results: The associations of rs852426 with hypertension and stroke had statistical significance in drinkers but not after Bonferroni correction. An additive interaction of rs852426 and drinking was observed for stroke incidence, the adjusted RERI was −0.907 (p = 4.108×10−4), and the multiplicative interaction was still sound (HR = 0.541, p = 0.048). Furthermore, the significant interaction was further replicated in a nested case-control study. In the drinking population, the relative expression of ACTB mRNA in IS was lower (0.99 ± 0.26) than that in controls (1.13 ± 0.20), with a p value of 0.026.
Conclusions: ACTB rs852426 was significantly associated with alcohol consumption on stroke risk, and the expression of ACTB mRNA in IS who had a drinking habit was significantly down-regulated. This finding will provide a novel insight into the prevention of stroke.
Keywords: ACTB, Alcohol drinking, Variant, Ischemic stroke, Interaction
Introduction
Hypertension is a global public-health challenge because of its high prevalence and driving role in stroke. Stroke has been a leading cause of death in recent years in China1). The cerebrovascular remodeling induced by chronic hypertension promotes the development of stroke incidence. Although numerous established risk factors for hypertension and stroke have been highlighted, a particular genetic alteration or genotype combination and its interaction with environmental factors is also involved in the occurrence of cardiovascular diseases (CVDs)2, 3).
Abnormal vascular remodeling is a well-known risk factor for CVDs. The rho-associated kinase (ROCK) pathway regulates the intracellular actin cytoskeleton and initiates the remodeling of the vascular wall4). β-actin (encoded by ACTB , a downstream effector of ROCK) is widely distributed in all eukaryotic cells and underlies cell migration5, 6). As an abundant and highly conserved cytoskeleton structural protein, β-actin helps organize and maintain the cellular morphology by virtue of facilitating the processes of migration, division, growth, signaling and shaping the cytoskeleton7, 8). Marina Karakozova, et al.9) found that arginylation of β-actin regulated actin filament properties and lamella formation in motile cells. Animal experiments have confirmed that increased actin polymerization and stress fiber formation would generate mechanical force to trigger vascular hypertrophy and hypertension10). ACTB gene hypomorphic mice died of uncharacterized development defects11, 12), reflecting the essential role of ACTB in maintaining routine intracellular biological functions. However, there is no population-based study investigates the association of ACTB with hypertension.
The actin cytoskeleton has been validated its pivotal position in neuronal development and activity13). β-actin is also a signaling molecule. Studies have demonstrated that β-actin regulates endothelial nitric oxide synthase type 3 (eNOS-3) in platelets and vascular endothelial cells14, 15); these alterations probably contribute to vascular complications, atherosclerosis, and thrombotic diseases (such as ischemic stroke)16). Furthermore, a whole-exome sequencing study in syndromic brain malformations identified a novel de novo mutation (p.Gly268Arg) in ACTB. Determining whether ACTB variations were associated with stroke incidence would warrant a population-based study.
Given the abovementioned evidences, we speculate that β-actin might participate in the mechanism of vascular remodeling and thrombogenesis and subsequently mediate the pathogenesis of hypertension and stroke. Notably, Rothenfluh et al. and Offenhäuser et al. found that the dynamic regulation of the actin cytoskeleton in flies and mice, respectively, affects cellular and behavioral sensitivity to alcohol17, 18). Further research would be required to explore whether alcohol exposure in the nervous system would modify the biological activities of actin cytoskeleton.
The present study aimed to evaluate whether the variants of ACTB contribute to the genetic susceptibility to hypertension and stroke and to evaluate the light of gene-environment interactions with mRNA transcriptional analysis. These would provide a novel insight into our improved understanding of the genetic role of ACTB in hypertension and stroke.
Methods
Study Participants
In the case-control study, a total of 4,222 participants consisting of 2,012 hypertensive cases and 2,210 controls were drawn from the community hypertension survey (Yixing, Jiangsu Province, 2009)19). Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg or associated with patients who were then receiving anti-hypertensive medication, and patients who had a clinical history of secondary hypertension were excluded.
A total of 4,128 participants from the community hypertension survey in 2009 were enrolled. Flow charts of the case-control and follow-up studies are outlined in Supplementary Fig. 1. During a median follow-up time of 5.01 years, 613 incident cases with hypertension and 183 incident cases with stroke including 171 ischemic stroke (IS) cases and 12 hemorrhagic stroke (HS) cases were recorded on a disease register and a report system. The demographic characteristics for follow-up study are displayed in Supplementary Table 1.
Supplementary Fig. 1.
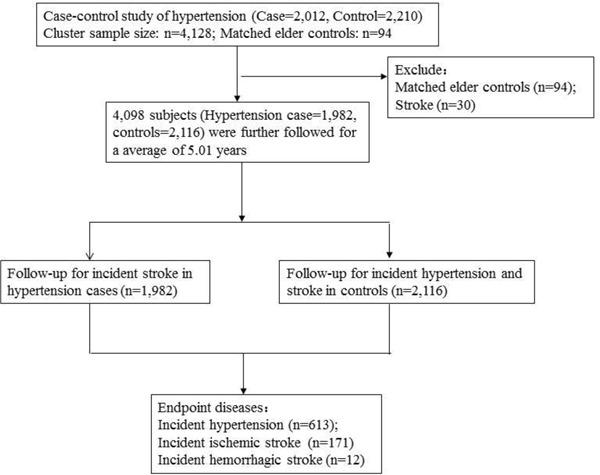
The flow chart of the case-control and follow-up studies
Inclusion of participants for evaluating the associations between ACTB and hypertension in the case-control study, and participants for the follow-up study. Matched elderly controls (n = 94) and 30 hypertension cases with history of stroke were excluded in the follow-up study.
Supplementary Table 1. Demographic characteristics the follow-up study.
| Characteristics | Group | Follow-up study for hypertension n = 2116 |
Follow-up study for stroke n = 4098 |
|---|---|---|---|
| Age (year) | 58.41 ± 10.28 | 60.27 ± 10.67 | |
| Gender (%) | Male | 853 (40.31%) | 1660 (40.51%) |
| Female | 1263 (59.69%) | 2438 (59.49%) | |
| BMI (kg/m2) | 23.7 ± 3.21 | 22.21 ± 3.38 | |
| TC (mmol/L) | 4.78 ± 1.01 | 4.85 ± 1.03 | |
| TG (mmol/L) | 1.54 ± 1.21 | 1.69 ± 1.41 | |
| HDL-C (mmol/L) | 1.37 ± 0.33 | 1.37 ± 0.33 | |
| LDL-C (mmol/L) | 2.65 ± 0.72 | 2.72 ± 0.81 | |
| GLU (mmol/L) | 5.47 ± 1.62 | 5.65 ± 1.86 | |
| Smokers (%) | Yes | 525 (24.81%) | 995 (24.28%) |
| No | 1591 (75.19%) | 3103 (75.72%) | |
| Drinkers (%) | Yes | 468 (22.12%) | 883 (21.55%) |
| No | 1648 (77.88%) | 3215 (78.45%) | |
| Hypertension (%) | Yes | - | 1985 (48.43%) |
| No | - | 2113 (51.56%) | |
| Diabetes (%) | T2DM | 193 (9.12%) | 461 (11.98%) |
| IFG | 418 (19.75%) | 891 (21.74%) | |
| NGT | 1505 (71.13%) | 2746 (66.28%) |
T2DM: type 2 diabetes mellitus; IFG: impaired fasting glucose; NGT: normal glucose tolerance
To access the disease status of subjects in the follow- up study, both concentrated and household surveys were conducted. Data collected from the local hospitals, centers for disease control and prevention (CDCs), community health service centers and a social security center were further inspected to reduce the information bias.
All participants were interviewed and underwent physical examinations and laboratory tests. The demographic characteristics of the participants were obtained by trained research staffs though a standard questionnaire, including data such as age, gender, smoking and drinking status. The weight, height and BP of the participants were measured as previously described19).
Participants with a drinking habit were defined as those who currently or previously consumed alcoholic beverages at least 2 times per week for at least 6 months per year. A total of 901 subjects were identified with a drinking habit. The amount of alcoholic beverages that they consumed on a daily or weekly basis was divided into three categories: white spirits, beer and wine (assuming that the content of ethanol was 42% in white spirits, 3% in beer and 10% in wine). The amount of alcohol consumed was calculated in grams per day. According to the standard drink of 15 g of ethanol, participators were divided into the following categories: never, light-to-moderate (women: ≤ 1.0 drink/day; men: ≤ 2.0 drink/day) and heavy drinking group (women: > 1.0 drink/day; men: > 2.0 drink/day). Besides, daily or weekly alcohol consumption data for 104 individuals with a drinking habit were missing. We grouped these subjects into the light-to-moderate drinking group out of conservative considerations. Finally, 3,321, 369 and 532 subjects were categorized as non-drinking, light-to-moderate drinking and heavy drinking, respectively.
A nested case-control study was conducted to validate the observed interactive associations of single-nucleotide polymorphisms (SNPs) and drinking with stroke. A total of 24,352 subjects from a community-based epidemiological survey were recruited in Nantong city, Jiangsu Province from 2007 to 2008. Over a course of 9 years of follow-up, 860 incident stroke cases including 821 IS cases and 39 HS cases were identified according to the records from the local hospitals and CDCs. And 1,960 age- (5-year-old group) and gender-matched controls were enrolled for the analysis (Supplementary Table 2).
Supplementary Table 2. Demographic characteristics of the nested case-control study.
| Characteristics | Group | Stroke n = 860 |
Controls n = 1960 |
t/χ2 | P |
|---|---|---|---|---|---|
| Age (year) | 63.24 ± 7.24 | 61.78 ± 4.94 | 6.341 | <0.001 | |
| Gender (%) | Male | 353 (41.05%) | 793 (40.46%) | 0.085 | 0.77 |
| Female | 507 (58.95%) | 1167 (59.54%) | |||
| BMI (kg/m2) | 24.06 ± 3.85 | 23.64 ± 4.42 | 2.363 | 0.018 | |
| TC (mmol/L) | 4.32 ± 1.64 | 4.24 ± 0.92 | 1.545 | 0.122 | |
| TG (mmol/L) | 1.63 ± 1.54 | 1.51 ± 1.38 | 2.076 | 0.038 | |
| HDL-C (mmol/L) | 1.57 ± 0.41 | 1.63 ± 0.43 | 3.534 | <0.001 | |
| LDL-C (mmol/L) | 2.08 ± 1.56 | 1.99 ± 0.72 | 2.057 | 0.04 | |
| GLU (mmol/L) | 5.01 ± 2.61 | 5.51 ± 3.10 | 3.941 | <0.001 | |
| Smokers (%) | Yes | 213 (24.77%) | 445 (22.70%) | 1.325 | 0.25 |
| No | 647 (75.23%) | 1515 (77.30%) | |||
| Drinkers (%) | Yes | 229 (26.63%) | 583 (29.74%) | 2.894 | 0.089 |
| No | 631 (73.37%) | 1377 (70.26%) | |||
| Hypertension (%) | Yes | 446 (51.86%) | 701 (35.76%) | 64.17 | <0.001 |
| No | 414 (48.14%) | 1259 (64.24%) | |||
| Diabetes (%) | T2DM | 99 (11.51%) | 466 (23.77%) | 70.727 | <0.001 |
| IFG | 26 (3.02%) | 110 (5.61%) | |||
| NGT | 735 (85.47%) | 1384 (70.62%) |
T2DM: type 2 diabetes mellitus; IFG: impaired fasting glucose; NGT: normal glucose tolerance
The study was approved by the ethics committee of Nanjing Medical University and conducted according to the principles of the Declaration of Helsinki. The written informed consent was obtained from all subjects during epidemiological interviews.
SNP Selection
The ACTB gene locates on chromosome 7p22.1 (Gene ID: 60; NC_000007.14), spans 3.4 kbps and contains 6 exons. We searched the SNP-covered ACTB gene from the upstream 5 kb to the downstream 2 kb and selected tagging SNPs (tagSNPs) from the database of the Chinese Han population in Beijing, China of the International Hap MAP Project (HapMap Data Rel 24/phase II Nov08, on NCBI B36 assembly, dbSNPb126). All tagSNPs were selected with a minor allele frequency (MAF) ≥ 0.05 and linkage disequilibrium (LD) r2 ≥ 0.8. We also applied a functional candidate strategy to select potential functional SNPs on the bioinformatics effect prediction website (SNPINFO, https://snpinfo.niehs.nih.gov/). Finally, rs852426 (T>C), rs852423 (A>G), and rs2966449 (T>C) were selected, and their specific biological information was summarized in Supplementary Table 3.
Supplementary Table 3. The biological information and function prediction of three tagSNPs of ACTB gene.
| No | SNP | Chromosome | Position | Allele | LDsnp | Pop/LD | TFBS | RegPotential | Conservation | Nearby Gene | Distance (bp) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | rs852426 | 7 | 5532879 | T/C | rs852426 | 1 | -- | 0 | 0.002 | MIRN589||ACTB | −30806||−433 |
| 2 | rs852423 | 7 | 5534892 | A/G | rs852423 | 1 | -- | 0.266 | 0 | ACTB | 1580||1855 |
| 3 | rs2966449 | 7 | 5538151 | T/C | rs2966449 | 1 | Msx-1 | 0.135 | 0 | ACTB||FSCN1 | −1404||−60829 |
TFBS, transcription factor binding site; RegPotential, regulatory potential score.
Blood Sampling and SNP Genotyping
Blood samples were donated by participants for genotyping with adding ethylenediamine tetraacetic acid (EDTA)-containing receptacles. After an over-night fasting (>10 h), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and glucose (GLU) were measured. DNA was extracted using a standard phenole-chloroform method and stored at −20°C. Genotyping was performed using the TaqMan allelic discrimination assay in 384-well plates on the platform of 7900HT realtime polymerase chain reaction (PCR) system (Applied Biosystems, Foster City, CA). The primers and probes were designed using Primer Express Oligo Design software ver. 2.0 (ABI PRISM). Genotyping results were determined using SDS 2.3 Allelic Discrimination Software (Applied Biosystems). Meanwhile, each plate included blank samples as negative controls for the genotyping quality confirmation. The successful call rates of SNPs rs852426, rs852423 and rs2966449 were 99.90%, 99.95% and 99.92%, respectively.
Determination of ACTB mRNA
The expression of ACTB mRNA (NCBI Reference Sequence: NM_001101.4) in peripheral blood mononuclear cells (PBMCs) was measured between IS cases and age- and gender- matched controls (72 vs. 72). All IS cases were enrolled from the People's Hospital of Yixing City, Department of Neurology.
Total RNA was isolated from PBMCs (100 µl) using an RNA blood kit (Cat#Yu-B02-1, Yuan Corp., Wuxi, China) according to the manual instructions. A total of 7.5 µl RNA was used for cDNA reversion. cDNA was synthesized using TAKARA reverse transcription kits (RR047A Takara PrimeScript™ RT reagent kit with gDNA Eraser, Japan). The primers used were designed by using Primer Premier 5.0 software, and the primer sequences were listed as follows: forward (5′-3′), TGACGTGGACATCCGCAAAG and reversed (5′-3′), CTGGAAGGTGGACAGCGAGG.
Quantitative real-time PCR (qPCR) was processed in a final volume of 10 µl (2 µl cDNA, 5 µl HieffTM qPCR SYBR® GEEN Master Mix, 0.2 µl of each primer and 5 µl RNase-free water) running in triplicate using the ABI RT-PCR 7900 system (Applied Biosystems; Thermo Fisher Scientific, Inc.). Using the glyceraldehyde phosphate dehydrogenase (GAPDH) gene as internal control, amplification of the ACTB gene was performed in the same tube under following thermal cycling conditions: initial denaturation at 95°C for 5 min, 95°C for 10 s, 60°C for 20 s and 72°C for 20 s, 95°C for 15 s, 60°C for 1 min and then 95°C for 15 s with 40 cycles. Melting curve includes one cycle with 95°C for 15 s, 60°C for 1 min and 95°C for 15 s. The standard deviation (SD) of cycle threshold (CT) values among each duplicate sample was less than 0.5. The expression of ACTB mRNA was calculated with the 2−ΔΔCT method.
Statistical Analysis
Unpaired Student's t-test was used to test the differences in all the quantitative variables among groups presented as mean ± SD. The Hardy−Weinberg equilibrium (HWE) for genotype frequencies was estimated with a Fisher's exact test in controls. Chi-square (χ2) test was performed to compare the allele and genotype frequency distributions. Multiple unconditional logistic regression analyses were applied to evaluate the genetic effects of screened SNPs on hypertension by calculating the odds ratio (OR) and its 95% confidence interval (CI). Cox regression was applied to estimate hazard ratios (HRs) and 95% CI in the follow-up study. The interaction of drinking and ACTB polymorphisms with stroke incidence was estimated. The additive interaction was displayed by calculating the relative excess risk due to interaction (RERI), attributable proportion (AP) due to interaction and synergy index (SI)20). The multiplicative interaction hazard ratio was calculated by fitting the Cox regression model. Stratification analyses by gender, age group, smoking and drinking status were further conducted in both case-control and follow-up studies. A logistic regression model was used to evaluate the genetic effects of SNPs on stroke for the nested case-control study. A two-tailed p value of 0.05 was defined as statistical significance. All statistical analyses were performed with SPSS version 25.0 (SPSS, Inc., Chicago, IL).
Results
Demographic Characteristics at Baseline
The demographic characteristics of participants were summarized in Table 1. The hypertensive cases were average 3.42 years older than the controls (p < 0.001), although an age-matched (5 years) method was used for the analysis. Subjects with hypertension had higher levels of BMI, TC, TG, LDL-C and GLU than those of controls (p < 0.001), whereas no significant differences in gender, HDL-C, smoking status and drinking status were observed (p > 0.05). Overall, these characteristics were adjusted as confounding factors to evaluate the association of ACTB with hypertension.
Table 1. Demographic and clinical characteristics of the hypertension case-control study.
| Characteristics | Group | Hypertension n = 2,012 |
Normotension n = 2,210 |
t/χ2 | p |
|---|---|---|---|---|---|
| Age (year) | 62.35 ± 10.73 | 58.93 ± 10.45 | 10.484 | <0.001 | |
| Gender (%) | Male | 829 (41.20) | 884 (40.00) | 0.632 | 0.427 |
| Female | 1,183 (58.80) | 1,326 (60.00) | |||
| SBP (mm Hg) | 142.86 ± 14.30 | 124.24 ± 11.36 | 47.018 | <0.001 | |
| DBP (mm Hg) | 87.53 ± 8.54 | 79.08 ± 6.51 | 36.369 | <0.001 | |
| BMI (kg/m2) | 24.76 ± 3.51 | 23.64 ± 3.20 | 10.844 | <0.001 | |
| TC (mmol/L) | 4.94 ± 1.05 | 4.78 ± 1.01 | 4.574 | <0.001 | |
| TG (mmol/L) | 1.86 ± 1.58 | 1.53 ± 1.21 | 7.618 | <0.001 | |
| HDL-C (mmol/L) | 1.36 ± 0.33 | 1.36 ± 0.33 | 0.175 | 0.751 | |
| LDL-C (mmol/L) | 2.80 ± 0.89 | 2.64 ± 0.73 | 6.227 | <0.001 | |
| GLU (mmol/L) | 5.83 ± 2.05 | 5.46 ± 1.61 | 6.684 | <0.001 | |
| Smokers (%) | Yes | 480 (23.86) | 533 (24.12) | 0.039 | 0.843 |
| No | 1,532 (76.14) | 1,677 (75.88) | |||
| Drinkers (%) | Yes | 424 (21.07) | 477 (21.58) | 0.163 | 0.686 |
| No | 1,588 (78.93) | 1,733 (78.42) |
BMI, body mass index; DBP, diastolic blood pressure; GLU, glucose; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride.
Association Analysis for the Case-Control Study of Hypertension
The distributions of rs852426, rs852423 and rs2966449 in controls were consistent with HWE (p >0.05). No significant associations were observed between each of the three SNPs and hypertension in the whole population (Supplementary Table 4). Stratification analysis by drinking status indicated that drinkers with the rs852426 CC genotype had a higher risk of hypertension than that of TT/TC carriers; after adjustment for age, gender, BMI, GLU, HDL-C, LDL-C, TC, TG and smoking status, the OR (95% CI) was 1.971 (1.053−3.691) with a p value of 0.034 (Supplementary Table 5). However, the associations mentioned above were not statistically significant after Bonferroni correction.
Supplementary Table 4. Association analyses of ACTB with hypertension in the case-control study.
| SNP | Group | WT/HT/MT |
OR (95% CI)a |
Allele gene |
||||
|---|---|---|---|---|---|---|---|---|
| Additive model | Dominant model | Recessive model | Major/minor | OR (95% CI)/Pb | Pc | |||
| rs852426 | TT/TC/CC | T/C | ||||||
| Case | 1217/696/99 | 1.036 (0.929–1.156) | 1.006 (0.884–1.144) | 1.282 (0.944–1.740) | 3130/894 | 0.963 (0.869–1.068) | 0.478 | |
| Control | 1342/776/88 | P = 0.521 | P = 0.930 | P = 0.112 | 3460/952 | P = 0.239 | ||
| rs852423 | AA/AG/GG | A/G | ||||||
| Case | 993/809/209 | 1.040 (0.946–1.144) | 1.049 (0.925–1.190) | 1.063 (0.863–1.309) | 2795/1227 | 0.961 (0.874–1.056) | 0.406 | |
| Control | 1119/869/221 | P = 0.413 | P = 0.457 | P = 0.568 | 3107/1311 | P = 0.235 | ||
| rs2966449 | TT/TC/CC | T/C | ||||||
| Case | 1135/763/114 | 0.973 (0.877–1.080) | 0.975 (0.858–1.107) | 0.933 (0.713–1.220) | 3033/991 | 1.005 (0.910–1.110) | 0.924 | |
| Control | 1250/823/134 | P = 0.606 | P = 0.696 | P = 0.612 | 3323/1091 | P = 0.462 | ||
WT, wild type, HT, heterozygote, MT, mutant type.
Adjusted for age, gender, body mass index (BMI), glucose (GLU), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), triglyceride (TG), smoking and drinking status.
P value of χ2 test for comparison of allele frequencies between hypertension case and control groups.
P value of χ2 test for comparison of genotype between hypertension case and control groups.
The unsuccessful genotyped number of rs852426, rs852423 and rs2966449 was 4, 2 and 3, respectively.
Supplementary Table 5. Stratification analysis by age, gender, smoking and drinking status for the association of ACTB with hypertension in the case-control study.
| SNP | Stratum | Group | WT/HT/MT |
OR (95% CI) |
||
|---|---|---|---|---|---|---|
| Additive model | Dominant model | Recessive model | ||||
| rs852426 | TT/TC/CC | |||||
| Male | Case | 509/273/47 | 0.957 (0.810–1.129)a | 0.892 (0.729–1.090)a | 1.275 (0.818–1.986)a | |
| Control | 527/316/41 | P = 0.601 | P = 0.263 | P = 0.284 | ||
| Female | Case | 708/423/52 | 1.115 (0.964–1.289)a | 1.110 (0.937–1.315)a | 1.316 (0.862–2.009)a | |
| Control | 815/460/47 | P = 0.141 | P = 0.227 | P = 0.204 | ||
| <55 years | Case | 339/172/23 | 0.931 (0.765–1.135)b | 0.897 (0.711–1.130)b | 1.067 (0.610–1.868)b | |
| Control | 521/302/34 | P = 0.481 | P = 0.356 | P = 0.820 | ||
| ≥ 55 years | Case | 878/524/76 | 1.089 (0.955–1.243)b | 1.063 (0.910–1.243)b | 1.387 (0.959–2.008)b | |
| Control | 821/474/54 | P = 0.202 | P = 0.441 | P = 0.083 | ||
| Smoking | Case | 295/161/24 | 1.010 (0.805–1.267)c | 0.931 (0.713–1.217)c | 1.633 (0.854–3.123)c | |
| Control | 324/191/18 | P = 0.932 | P = 0.603 | P = 0.138 | ||
| Non-smoking | Case | 922/535/75 | 1.046 (0.924–1.185)c | 1.030 (0.888–1.194)c | 1.209 (0.854–1.711)c | |
| Control | 1018/585/70 | P = 0.475 | P = 0.695 | P = 0.285 | ||
| Drinking | Case | 256/141/27 | 1.098 (0.871–1.385)d | 0.997 (0.753–1.322)d | 1.971 (1.053–3.691)d | |
| Control | 287/171/19 | P = 0.429 | P = 0.985 | P = 0.034 | ||
| Non-drinking | Case | 961/555/72 | 1.026 (0.906–1.161)d | 1.014 (0.877–1.174)d | 1.132 (0.796–1.612)d | |
| Control | 1055/605/69 | P = 0.685 | P = 0.847 | P = 0.490 | ||
| rs852423 | AA/AG/GG | |||||
| Male | Case | 410/330/88 | 0.999 (0.863–1.156)a | 0.974 (0.800–1.185)a | 1.066 (0.776–1.465)a | |
| Control | 439/351/94 | P = 0.986 | P = 0.790 | P = 0.694 | ||
| Female | Case | 583/479/121 | 1.079 (0.952–1.223)a | 1.116 (0.946–1.316)a | 1.069 (0.810–1.412)a | |
| Control | 680/518/127 | P = 0.233 | P = 0.195 | P = 0.636 | ||
| <55 years | Case | 266/219/48 | 1.000 (0.842–1.187)b | 1.022 (0.816–1.280)b | 0.936 (0.635–1.380)b | |
| Control | 434/339/84 | P = 0.998 | P = 0.849 | P = 0.740 | ||
| ≥ 55 years | Case | 727/590/161 | 1.062 (0.948–1.191)b | 1.066 (0.915–1.242)b | 1.127 (0.878–1.447)b | |
| Control | 685/530/137 | P = 0.298 | P = 0.413 | P = 0.349 | ||
| Smoking | Case | 237/192/50 | 1.057 (0.869–1.285)c | 1.012 (0.780–1.312)c | 1.271 (0.824–1.960)c | |
| Control | 272/211/50 | P = 0.579 | P = 0.930 | P = 0.278 | ||
| Non-smoking | Case | 756/617/159 | 1.037 (0.930–1.156)c | 1.059 (0.916–1.223)c | 1.020 (0.804–1.295)c | |
| Control | 847/658/171 | P = 0.514 | P = 0.441 | P = 0.870 | ||
| Drinking | Case | 201/174/48 | 1.112 (0.906–1.364)d | 1.102 (0.837–1.452)d | 1.276 (0.822–1.981)d | |
| Control | 235/192/50 | P = 0.309 | P = 0.489 | P = 0.277 | ||
| Non-drinking | Case | 792/635/161 | 1.027 (0.923–1.144)d | 1.043 (0.904–1.202)d | 1.017 (0.801–1.290)d | |
| Control | 884/677/171 | P = 0.622 | P = 0.567 | P = 0.892 | ||
| rs2966449 | TT/TC/CC | |||||
| Male | Case | 471/313/45 | 0.916 (0.779–1.076)a | 0.921 (0.755–1.122)a | 0.805 (0.531–1.220)a | |
| Control | 489/336/58 | P = 0.285 | P = 0.412 | P = 0.306 | ||
| Female | Case | 664/450/69 | 1.018 (0.888–1.167)a | 1.017 (0.861–1.202)a | 1.042 (0.731–1.484)a | |
| Control | 761/487/76 | P = 0.800 | P = 0.840 | P = 0.820 | ||
| <55 years | Case | 318/186/30 | 0.917 (0.760–1.106)b | 0.871 (0.694–1.094)b | 1.046 (0.644–1.697)b | |
| Control | 483/324/48 | P = 0.364 | P = 0.234 | P = 0.856 | ||
| ≥ 55 years | Case | 817/577/84 | 0.988 (0.870–1.121)b | 1.031 (0.883–1.203)b | 0.894 (0.648–1.235)b | |
| Control | 767/499/86 | P = 0.846 | P = 0.701 | P = 0.498 | ||
| Smoking | Case | 269/188/23 | 1.010 (0.811–1.257)c | 1.016 (0.782–1.320)c | 0.987 (0.544–1.791)c | |
| Control | 298/207/27 | P = 0.931 | P = 0.903 | P = 0.966 | ||
| Non-smoking | Case | 866/575/91 | 0.964 (0.856–1.085)c | 0.962 (0.831–1.113)c | 0.930 (0.688–1.257)c | |
| Control | 952/616/107 | P = 0.544 | P = 0.603 | P = 0.639 | ||
| Drinking | Case | 231/167/26 | 1.022 (0.817–1.279)d | 1.027 (0.780–1.354)d | 1.027 (0.584–1.807)d | |
| Control | 263/183/31 | P = 0.847 | P = 0.848 | P = 0.925 | ||
| Non-drinking | Case | 904/596/88 | 0.966 (0.858–1.087)d | 0.963 (0.834–1.112)d | 0.938 (0.690–1.275)d | |
| Control | 987/640/103 | P = 0.561 | P = 0.605 | P = 0.683 | ||
Adjusted for age, BMI, glucose, HDL-C, LDL-C, TC, TG, smoking and drinking status.
Adjusted for gender, BMI, glucose, HDL-C, LDL-C, TC, TG, smoking and drinking status.
Adjusted for age, gender, BMI, glucose, HDL-C, LDL-C, TC, TG and drinking status.
Adjusted for age, gender, BMI, glucose, HDL-C, LDL-C, TC, TG and smoking status.
Association Analysis for the Follow-Up Study
The incidences density for hypertension and stroke were 681.85/104 person-years and 86.55/104 person-years, respectively. No significant associations were observed between the SNPs and hypertension, nor was stroke incidence in the whole population (Supplementary Table 6). A further stratification analysis by gender, age, smoking status and drinking status was conducted (Supplementary Table 7). Males with rs852423 AG/GG genotypes had a higher incidence of hypertension compared to AA genotype carriers; after being adjusted for covariates, HR (95% CI) was 1.334 (1.044−1.705), with p = 0.021. In females, individuals with the rs852423 GG genotype presented a higher risk of hypertension when compared with AA/AG carriers, the HR (95% CI) was 1.466 (1.038−2.068), p = 0.030. In the <55 years age group, the rs852423 variation increased the risk of hypertension (p = 0.043), and the adjusted HR (95% CI) was 1.261 (1.008−1.578). In the drinking subpopulation, rs852426 T>C variation had a significant protective effect on stroke, and the adjusted HR (95% CI) was 0.470 (0.227−0.975), with a p value of 0.042 (Table 2). These results were not valid after Bonferroni correction.
Supplementary Table 6. Association analyses of ACTB with hypertension and stroke in the follow-up study.
| End Point | SNP | Genotype | N | Person-years | Incidence density (/104 Person-years) |
HR (95% CI) |
||
|---|---|---|---|---|---|---|---|---|
| Additive model | Dominant model | Recessive model | ||||||
| Hypertension | rs852426 | TT | 357 | 5471.94 | 652.42 | 1.129 (0.984–1.297)a | 1.167 (0.992–1.372)a | 1.073 (0.712–1.619)a |
| TC | 232 | 3170.40 | 731.77 | P = 0.085 | P = 0.063 | P = 0.735 | ||
| CC | 24 | 346.59 | 692.46 | |||||
| rs852423 | AA | 290 | 4526.55 | 640.66 | 1.120 (0.993–1.265)a | 1.157 (0.986–1.357)a | 1.154 (0.880–1.513)a | |
| AG | 262 | 3587.49 | 730.32 | P = 0.066 | P = 0.073 | P = 0.299 | ||
| GG | 59 | 874.90 | 674.36 | |||||
| rs2966449 | TT | 338 | 5043.27 | 670.20 | 1.039 (0.911–1.186)a | 1.075 (0.916–1.262)a | 0.923 (0.642–1.327)a | |
| TC | 244 | 3396.07 | 718.48 | P = 0.568 | P = 0.374 | P = 0.665 | ||
| CC | 31 | 546.53 | 567.21 | |||||
| Stroke | rs852426 | TT | 106 | 12895.59 | 82.20 | 1.059 (0.821–1.366)b | 1.074 (0.795–1.450)b | 1.052 (0.514–2.151)b |
| TC | 69 | 7341.14 | 93.99 | P = 0.658 | P = 0.644 | P = 0.890 | ||
| CC | 8 | 905.11 | 88.39 | |||||
| rs852423 | AA | 83 | 106 | 63.54 | 77.841.170 (0.941–1.455)b | 1.289 (0.958–1.735)b | 1.080 (0.662–1.764)b | |
| AG | 82 | 8366.72 | 98.01 | P = 0.157 | P = 0.093 | P = 0.757 | ||
| GG | 18 | 2105.38 | 85.50 | |||||
| rs2966449 | TT | 98 | 11952.30 | 81.99 | 1.014 (0.793–1.298)b | 1.064 (0.791–1.432)b | 0.801 (0.391–1.640)b | |
| TC | 77 | 7943.78 | 96.93 | P = 0.910 | P = 0.680 | P = 0.544 | ||
| CC | 8 | 1239.61 | 64.54 | |||||
Adjusted for age, gender, BMI, HDL-C, LDL-C, TC, TG, smoking status, drinking status and T2DM.
Adjusted for age, gender, BMI, HDL-C, LDL-C, TC, TG, smoking status, drinking status, T2DM and hypertension.
Supplementary Table 7. Stratification analysis by age, gender, smoking and drinking status for the association of ACTB with hypertension and stroke in the follow-up study.
| End Point | SNP | Stratum | Genotype | N | Person-years | Incidence density (/104 Person-years) |
HR (95% CI) |
||
|---|---|---|---|---|---|---|---|---|---|
| Additive model | Dominant model | Recessive model | |||||||
| Hypertension | rs852426 | Male | TT | 150 | 2154.16 | 696.33 | 1.106 (0.899–1.361)a | 1.204 (0.940–1.542)a | 0.754 (0.384–1.481)a |
| TC | 107 | 1269.52 | 842.84 | P = 0.341 | P = 0.142 | P = 0.413 | |||
| CC | 9 | 159.13 | 565.58 | ||||||
| Female | TT | 207 | 3317.79 | 623.91 | 1.172 (0.969–1.418)a | 1.148 (0.924–1.426)a | 1.599 (0.942–2.714)a | ||
| TC | 125 | 1900.87 | 657.59 | P = 0.101 | P = 0.214 | P = 0.082 | |||
| CC | 15 | 187.45 | 800.21 | ||||||
| <55 years | TT | 105 | 2225.96 | 471.71 | 1.120 (0.872–1.438)a | 1.169 (0.868–1.575)a | 1.011 (0.491–2.085)a | ||
| TC | 70 | 1331.86 | 525.58 | P = 0.376 | P = 0.304 | P = 0.975 | |||
| CC | 8 | 157.06 | 509.36 | ||||||
| ≥ 55 years | TT | 252 | 3245.98 | 776.34 | 1.166 (0.986–1.378)a | 1.201 (0.989–1.459)b | 1.158 (0.700–1.914)b | ||
| TC | 162 | 1838.53 | 881.14 | P = 0.072 | P = 0.065 | P = 0.568 | |||
| CC | 16 | 189.53 | 844.19 | ||||||
| Smoking | TT | 85 | 1364.35 | 623.01 | 1.127 (0.856–1.485)c | 1.248 (0.902–1.726)c | 0.636 (0.231–1.751)c | ||
| TC | 70 | 787.22 | 889.21 | P = 0.394 | P = 0.181 | P = 0.381 | |||
| CC | 4 | 83.65 | 478.18 | ||||||
| Non-smoking | TT | 272 | 4107.6 | 662.19 | 1.102 (0.935–1.299)c | 1.090 (0.901–1.319)c | 1.312 (0.833–2.065)c | ||
| TC | 162 | 2383.18 | 679.76 | P = 0.245 | P = 0.373 | P = 0.241 | |||
| CC | 20 | 262.94 | 760.63 | ||||||
| Drinking | TT | 86 | 1193.82 | 720.38 | 1.130 (0.854–1.496)d | 1.214 (0.870–1.693)d | 0.872 (0.375–2.025)d | ||
| TC | 63 | 709.91 | 887.44 | P = 0.392 | P = 0.254 | P = 0.749 | |||
| CC | 6 | 86.02 | 697.51 | ||||||
| Non-drinking | TT | 271 | 4284.35 | 632.53 | 1.144 (0.972–1.347)d | 1.159 (0.960–1.399)d | 1.233 (0.764–1.990)d | ||
| TC | 169 | 2460.48 | 686.86 | P = 0.105 | P = 0.126 | P = 0.390 | |||
| CC | 18 | 260.57 | 690.79 | ||||||
| rs852423 | Male | AA | 119 | 1768.20 | 673.00 | 1.141 (0.952–1.368)a | 1.334 (1.044–1.705)a | 0.841 (0.536–1.320)a | |
| AG | 126 | 1448.11 | 870.10 | P = 0.153 | P = 0.021 | P = 0.451 | |||
| GG | 21 | 366.50 | 572.99 | ||||||
| Female | AA | 171 | 2758.35 | 619.94 | 1.116 (0.948–1.315)a | 1.056 (0.854–1.306)a | 1.466 (1.038–2.068)a | ||
| AG | 137 | 2139.39 | 640.37 | P = 0.188 | P = 0.612 | P = 0.030 | |||
| GG | 38 | 508.40 | 747.44 | ||||||
| <55 years | AA | 82 | 1859.30 | 441.03 | 1.261 (1.008–1.578)b | 1.265 (0.941–1.701)b | 1.581 (0.985–2.539)b | ||
| AG | 81 | 1503.42 | 538.77 | P = 0.043 | P = 0.120 | P = 0.058 | |||
| GG | 20 | 352.17 | 567.91 | ||||||
| GG | 20 | 352.17 | 567.91 | ||||||
| ≥ 55 years | AA | 208 | 2667.25 | 779.83 | 1.072 (0.929–1.238)b | 1.135 (0.938–1.373)b | 0.984 (0.706–1.371)b | ||
| AG | 181 | 2084.08 | 868.49 | P = 0.340 | P = 1.135 | P = 0.924 | |||
| GG | 39 | 522.74 | 746.07 | ||||||
| Smoking | AA | 69 | 1138.23 | 606.20 | 1.155 (0.906–1.473)c | 1.267 (0.919–1.746)c | 1.212 (0.890–1.650)c | ||
| AG | 77 | 886.57 | 868.51 | P = 0.246 | P = 0.148 | P = 0.222 | |||
| GG | 13 | 210.41 | 617.84 | ||||||
| Non-smoking | AA | 221 | 3388.32 | 652.24 | 1.092 (0.948–1.259)c | 1.088 (0.904–1.310)c | 1.018 (0.572–1.814)c | ||
| AG | 186 | 2700.92 | 688.65 | P = 0.222 | P = 0.373 | P = 0.951 | |||
| GG | 46 | 664.49 | 692.26 | ||||||
| Drinking | AA | 65 | 973.00 | 668.04 | 1.208 (0.950–1.535)d | 1.392 (0.999–1.940)d | 1.036 (0.600–1.789)d | ||
| AG | 75 | 811.04 | 924.74 | P = 0.124 | P = 0.051 | P = 0.898 | |||
| AG | 75 | 811.04 | 924.74 | P = 0.124 | P = 0.051 | P = 0.898 | |||
| Non-drinking | AA | 225 | 3559.53 | 632.11 | 1.099 (0.953–1.267)d | 1.099 (0.914–1.322)d | 1.216 (0.887–1.663)d | ||
| AG | 188 | 2776.46 | 677.12 | P = 0.192 | P = 0.316 | P = 0.225 | |||
| GG | 44 | 669.43 | 657.28 | ||||||
| rs2966449 | Male | TT | 143 | 2002.44 | 714.13 | 1.072 (0.882–1.303)a | 1.179 (0.923–1.506)a | 0.769 (0.439–1.346)a | |
| TC | 110 | 1343.78 | 818.59 | P = 0.486 | P = 0.187 | P = 0.357 | |||
| CC | 13 | 233.50 | 556.75 | ||||||
| Female | TT | 195 | 3040.83 | 641.27 | 1.000 (0.835–1.199)a | 0.981 (0.792–1.215)a | 1.110 (0.686–1.795)a | ||
| TC | 134 | 2052.30 | 652.93 | P = 0.997 | P = 0.861 | P = 0.671 | |||
| CC | 18 | 313.03 | 575.02 | ||||||
| <55 years | TT | 103 | 2070.92 | 497.36 | 1.020 (0.797–1.307)b | 1.010 (0.750–1.359)b | 1.100 (0.576–2.102)b | ||
| TC | 70 | 1426.06 | 490.86 | P = 0.873 | P = 0.950 | P = 0.772 | |||
| CC | 10 | 209.84 | 476.55 | ||||||
| ≥ 55 years | TT | 235 | 2972.36 | 790.62 | 1.055 (0.903–1.233)b | 1.122 (0.927–1.358)b | 0.842 (0.542–1.308)b | ||
| TC | 174 | 1970.02 | 883.24 | P = 0.501 | P = 0.238 | P = 0.444 | |||
| CC | 21 | 336.69 | 623.72 | ||||||
| Smoking | TT | 85 | 1271.34 | 668.59 | 1.073 (0.825–1.395)c | 1.152 (0.836–1.587)c | 0.819 (0.378–1.775)c | ||
| TC | 67 | 837.37 | 800.12 | P = 0.598 | P = 0.387 | P = 0.613 | |||
| CC | 7 | 123.41 | 567.21 | ||||||
| Non-smoking | TT | 253 | 3771.93 | 670.74 | 0.999 (0.855–1.168)c | 1.001 (0.831–1.207)c | 0.988 (0.652–1.495)c | ||
| TC | 177 | 2558.71 | 691.75 | P = 0.993 | P = 0.988 | P = 0.955 | |||
| CC | 24 | 423.12 | 567.21 | ||||||
| Drinking | TT | 82 | 1094.57 | 749.15 | 1.080 (0.831–1.402)d | 1.092 (0.786–1.516)d | 1.128 (0.602–2.117)d | ||
| TC | 62 | 757.97 | 817.97 | P = 0.566 | P = 0.601 | P = 0.707 | |||
| CC | 11 | 137.20 | 801.75 | ||||||
| Non-drinking | TT | 256 | 3954.92 | 647.30 | 1.013 (0.867–1.184)d | 1.049 (0.868–1.260)d | 0.865 (0.551–1.358)d | ||
| TC | 182 | 2638.10 | 689.89 | P = 0.870 | P = 0.636 | P = 0.529 | |||
| CC | 20 | 409.32 | 488.62 | ||||||
| Stroke | rs852426 | Male | TT | 54 | 5271.40 | 102.44 | 0.908 (0.622–1.325)a | 0.900 (0.583–1.387)a | 0.860 (0.268–2.756)a |
| TC | 36 | 2981.01 | 120.76 | P = 0.617 | P = 0.632 | P = 0.799 | |||
| CC | 3 | 420.76 | 71.30 | ||||||
| Female | TT | 52 | 7670.07 | 67.80 | 1.287 (0.902–1.836)a | 1.340 (0.870–2.064)a | 1.442 (0.561–3.705)a | ||
| TC | 33 | 4393.60 | 75.11 | P = 0.164 | P = 0.184 | P = 0.447 | |||
| CC | 5 | 484.35 | 103.23 | ||||||
| <55 years | TT | 7 | 4766.93 | 14.68 | 1.308 (0.406–4.214)b | 1.603 (0.417–6.156)b | - | ||
| TC | 5 | 2630.37 | 19.01 | P = 0.653 | P = 0.492 | - | |||
| CC | 0 | 313.26 | - | ||||||
| ≥ 55 years | TT | 99 | 8174.54 | 121.11 | 1.059 (0.815–1.376)b | 1.068 (0.783–1.456)b | 1.086 (0.529–2.229)b | ||
| TC | 64 | 4744.24 | 134.90 | P = 0.666 | P = 0.679 | P = 0.823 | |||
| CC | 8 | 591.85 | 135.17 | ||||||
| Smoking | TT | 29 | 3202.08 | 90.57 | 0.734 (0.408–1.322)c | 0.711 (0.369–1.372)c | 0.627 (0.085–4.652)c | ||
| TC | 16 | 1824.74 | 87.68 | P = 0.303 | P = 0.309 | P = 0.648 | |||
| CC | 1 | 212.43 | 47.07 | ||||||
| Non-smoking | TT | 77 | 9739.39 | 79.06 | 1.167 (0.873–1.558)c | 1.193 (0.843–1.689)c 1.241 | (0.573–2.686)c | ||
| TC | 53 | 5549.87 | 95.50 | P = 0.296 | P = 0.319 | P = 0.584 | |||
| CC | 7 | 692.68 | 101.06 | ||||||
| Drinking | TT | 29 | 2799.11 | 103.60 | 0.470 (0.227–0.975)d | 0.473 (0.219–1.020)d | - | ||
| TC | 11 | 1643.04 | 66.95 | P = 0.042 | P = 0.056 | - | |||
| CC | 0 | 233.57 | - | ||||||
| Non-drinking | TT | 77 | 10142.37 | 75.92 | 1.278 (0.969–1.686)d | 1.319 (0.943–1.847)d | 1.448 (0.704–2.979)d | ||
| TC | 58 | 5731.56 | 101.19 | P = 0.083 | P = 0.106 | P = 0.314 | |||
| CC | 8 | 671.54 | 119.13 | ||||||
| CC | 8 | 671.54 | 119.13 | ||||||
| rs852423 | Male | AA | 41 | 4335.75 | 94.56 | 1.179 (0.856–1.624)a | 1.304 (0.851–2.000)a | 1.055 (0.505–2.203)a | |
| AG | 44 | 3432.82 | 128.17 | P = 0.313 | P = 0.223 | P = 0.884 | |||
| GG | 8 | 898.40 | 89.05 | ||||||
| Female | AA | 42 | 6368.86 | 65.95 | 1.200 (0.884–1.628)a | 1.314 (0.862–2.202)a | 1.175 (0.600–2.300)a | ||
| AG | 38 | 4972.19 | 76.43 | P = 0.243 | P = 0.204 | P = 0.638 | |||
| GG | 10 | 1206.99 | 82.85 | ||||||
| <55 years | AA | 6 | 3894.35 | 15.41 | 1.055 (0.364–3.057)b | 1.543 (0.384–6.209)b | - | ||
| AG | 6 | 3088.90 | 19.42 | P = 0.921 | P = 0.541 | - | |||
| GG | 0 | 721.09 | - | ||||||
| ≥ 55 years | AA | 77 | 6810.25 | 113.06 | 1.182 (0.946–1.478)b | 1.298 (0.956–1.763)b | 1.120 (0.685–1.833)b | ||
| AG | 76 | 5316.10 | 142.96 | P = 0.142 | P = 0.095 | P = 0.651 | |||
| GG | 18 | 1384.29 | 130.03 | ||||||
| Smoking | AA | 22 | 2649.69 | 83.03 | 0.893 (0.549–1.454)c | 0.977 (0.528–1.809)c | 0.522 (0.125–2.177)c | ||
| AG | 22 | 2068.29 | 106.37 | P = 0.650 | P = 0.941 | P = 0.372 | |||
| GG | 2 | 515.05 | 38.83 | ||||||
| Non-smoking | AA | 61 | 8054.91 | 75.73 | 1.254 (0.978–1.607)c | 1.370 (0.971–1.934)c | 1.290 (0.762–2.184)c | ||
| AG | 60 | 6336.72 | 94.69 | P = 0.074 | P = 0.073 | P = 0.342 | |||
| GG | 16 | 1590.34 | 100.61 | ||||||
| Drinking | AA | 19 | 2269.49 | 83.72 | 1.192 (0.718–1.979)d | 1.499 (0.760–2.955)d | 0.669 (0.159–2.814)d | ||
| AG | 19 | 1893.11 | 100.36 | P = 0.496 | P = 0.242 | P = 0.583 | |||
| GG | 2 | 506.90 | 39.46 | ||||||
| Non-drinking | AA | 64 | 8435.11 | 75.87 | 1.207 (0.946–1.541)d | 1.307 (0.934–1.829)d | 1.218 (0.721–2.057)d | ||
| AG | 63 | 6511.89 | 96.75 | P = 0.130 | P = 0.119 | P = 0.460 | |||
| GG | 16 | 1598.48 | 100.10 | ||||||
| rs2966449 | Male | TT | 46 | 4881.39 | 94.24 | 1.325 (0.945–1.859)a | 1.436 (0.940–2.194)a | 1.308 (0.561–3.051)a | |
| TC | 41 | 3256.39 | 125.91 | P = 0.103 | P = 0.094 | P = 0.534 | |||
| CC | 6 | 529.21 | 113.38 | ||||||
| Female | TT | 52 | 7127.86 | 72.95 | 0.759 (0.521–1.104)a | 0.794 (0.516–1.222)a | 0.348 (0.083–1.464)a | ||
| TC | 36 | 4709.80 | 76.44 | P = 0.149 | P = 0.294 | P = 0.150 | |||
| CC | 2 | 710.40 | 28.15 | ||||||
| <55 years | TT | 8 | 4438.70 | 18.02 | 0.706 (0.194–2.570)b | 0.754 (0.186–3.050)b | - | ||
| TC | 4 | 2828.25 | 14.14 | P = 0.597 | P = 0.692 | - | |||
| CC | 0 | 432.45 | - | ||||||
| ≥ 55 years | TT | 90 | 7570.55 | 118.88 | 1.066 (0.828–1.371)b | 1.131 (0.833–1.535)b | 0.855 (0.417–1.755)b | ||
| TC | 73 | 5137.94 | 142.08 | P = 0.620 | P = 0.429 | P = 0.670 | |||
| CC | 8 | 807.16 | 99.11 | ||||||
| Smoking | TT | 27 | 2950.55 | 91.51 | 0.804 (0.461–1.400)c | 0.810 (0.432–1.516)c | 0.548 (0.074–4.076)c | ||
| TC | 18 | 2014.60 | 89.35 | P = 0.441 | P = 0.509 | P = 0.557 | |||
| CC | 1 | 267.90 | 37.33 | ||||||
| Non-smoking | TT | 71 | 9058.70 | 78.38 | 1.077 (0.813–1.428)c | 1.143 (0.811–1.612)c | 0.888 (0.408–1.933)c | ||
| TC | 59 | 5951.58 | 99.13 | P = 0.605 | P = 0.445 | P = 0.765 | |||
| CC | 7 | 971.71 | 72.04 | ||||||
| Drinking | TT | 26 | 2558.70 | 101.61 | 0.768 (0.419–1.408)d | 0.853 (0.434–1.675)d | - | ||
| TC | 14 | 1806.60 | 77.49 | P = 0.394 | P = 0.644 | - | |||
| CC | 0 | 310.42 | - | ||||||
| Non-drinking | TT | 72 | 9450.55 | 76.19 | 1.096 (0.832–1.442)d | 1.152 (0.823–1.612)d | 0.969 (0.466–2.015)d | ||
| TC | 63 | 6159.58 | 102.28 | P = 0.515 | P = 0.410 | P = 0.933 | |||
| CC | 8 | 929.19 | 86.10 | ||||||
Adjusted for age, BMI, HDL-C, LDL-C, TC, TG, smoking status, drinking status and T2DM.
Adjusted for gender, BMI, HDL-C, LDL-C, TC, TG, smoking status, drinking status and T2DM.
Adjusted for age, gender, BMI, HDL-C, LDL-C, TC, TG, drinking status and T2DM.
Adjusted for age, gender, BMI, HDL-C, LDL-C, TC, TG, smoking status and T2DM.
Adjusted for age, BMI, HDL-C, LDL-C, TC, TG, smoking status, drinking status, T2DM and hypertension.
Adjusted for gender, BMI, HDL-C, LDL-C, TC, TG, smoking status, drinking status, T2DM and hypertension.
Adjusted for age, gender, BMI, HDL-C, LDL-C, TC, TG, drinking status, T2DM and hypertension.
Adjusted for age, gender, BMI, HDL-C, LDL-C, TC, TG, smoking status, T2DM and hypertension
Table 2. Stratification analyses of rs852426 with hypertension and stroke in the follow-up study.
| End point | Stratum | Genotype | N | Person-years | Incidence density (/104 Person-years) | HR (95% CI) |
||
|---|---|---|---|---|---|---|---|---|
| Additive model | Dominant model | Recessive model | ||||||
| Hypertension | Drinking | TT | 86 | 1193.82 | 720.38 | 1.123 (0.848–1.487)* | 1.205 (0.863.1.683)* | 0.863 (0.371.2.007)* |
| TC | 63 | 709.91 | 887.44 | p = 0.418 | p = 0.272 | p = 0.733 | ||
| CC | 6 | 86.02 | 697.51 | |||||
| Non-drinking | TT | 271 | 4284.35 | 632.53 | 1.147 (0.974.1.349)* | 1.162 (0.962.1.403)* | 1.236 (0.766.1.995)* | |
| TC | 169 | 2460.48 | 686.86 | p = 0.100 | p = 0.119 | p = 0.385 | ||
| CC | 18 | 260.57 | 690.79 | |||||
| Stroke | Drinking | TT | 29 | 2799.11 | 103.60 | 0.470 (0.227.0.975)# | 0.473 (0.219.1.021)# | - |
| TC | 11 | 1643.04 | 66.95 | p = 0.042 | p = 0.057 | - | ||
| CC | 0 | 233.57 | - | |||||
| Non-drinking | TT | 77 | 10142.37 | 75.92 | 1.278 (0.969.1.687)# | 1.320 (0.943.1.847)# | 1.449 (0.705.2.980)# | |
| TC | 58 | 5731.56 | 101.19 | p = 0.083 | p = 0.106 | p = 0.313 | ||
| CC | 8 | 671.54 | 119.13 | |||||
HR, hazard ratio; CI, confidence interval
Adjusted for age, gender, BMI, HDL-C, LDL-C, TC, TG, smoking status and T2DM.
Adjusted for age, gender, BMI, HDL-C, LDL-C, TC, TG, smoking status, T2DM and hypertension.
The Interaction Analysis between ACTB rs852426 and Drinking
The analysis stratified by never, light-to-moderate drinking and heavy drinking status indicated that the rs852426 variation had a protective effect on stroke in subjects with the light-to-moderate or heavy drinking habit, the HRs (95% CIs) were 0.523 (0.174−1.571) and 0.485 (0.176−1.336), although the p values did not reach statistical significance (p values were 0.248 and 0.162). The association of rs852426 with stroke incidence mentioned above had no heterogeneity between light-to-moderate drinking and heavy drinking groups (Q = 0.010, p = 0.935). Thus, we combined light-to-moderate drinking and heavy drinking groups when we assessed the interaction between rs852426 and drinking.
It is observed that rs852426 significantly interacted with drinking in stroke incidence. The additive interaction analysis indicated that the rs852426 and drinking had a negative interaction; the RERI (95% CI) was −0.705(−1.377, −0.237), and the p value was 3.164×10−3. After being adjusted for covariates, the interaction was still sound; the RERI (95% CI) was −0.907 (−1.411, −0.404) with a p value of 4.108×10−4. The multiplicative interaction was still sound, the HR (95% CI) for the multiplicative interaction term was 0.541 (0.291, 0.988), with p = 0.048. The detailed results are displayed in Table 3.
Table 3. Association of rs852426 and drinking with stroke in the follow-up study.
| Interaction | Model 1 | Model 2 | |
|---|---|---|---|
| Drinking* rs852426 | |||
| HR (95% CI) | 0.618 (0.349, 1.093) | 0.541 (0.291, 0.994) | |
| p | 0.098 | 0.048 | |
| Drinking+rs852426 | |||
| RERI | HR (95% CI) | −0.705 (−1.377, −0.237) | −0.907 (−1.411, −0.404) |
| p | 3.164×10−3 | 4.108×10−4 | |
| AP | −0.994 | −1.398 | |
| SI | 0.704 | −0.630 |
HR, hazard ratio; CI, confidence interval; RERI, relative excess risk due to interaction; AP, attributable proportion due to interaction; SI, synergy index
Model 1 did not adjust any covariates.
Model 2 adjusted for age, gender, BMI, HDL-C, LDL-C, TC, TG, smoking status, T2DM and hypertension.
Validation of the Association of rs852426 and Alcohol Consumption with Stroke
In the nested case-control study, the interaction between rs852426 additive model and alcohol consumption on stroke was further validated in Nantong population (Supplementary Table 8). Although the association of rs852426 and stroke did not reach statistical significance, significant additive interaction between drinking and rs852426 was observed in the nested case-control study. The RERI (95% CI) was −0.370 (−0.622, −0.118), with a p value of 3.945×10−3, and the OR (95% CI) was 0.749 (0.575−0.976), with a p value of 0.033 for the multiplicative interaction (Table 4).
Supplementary Table 8. Association analyses of rs852426 with stroke in the nested case-control study.
| Stratification | Group | WT/HT/MT |
OR (95% CI) |
||
|---|---|---|---|---|---|
| Additive model | Dominant model | Recessive model | |||
| Total Population | TT/TC/CC | ||||
| Case | 537/280/43 | 0.996 (0.859–1.154)a | 1.040 (0.865–1.250)a | 0.821 (0.555–1.215)a | |
| Control | 1232/598/130 | P = 0.958a | P = 0.678 | P = 0.324 | |
| Drinking | TT/TC/CC | ||||
| Case | 155/65/9 | 0.801 (0.591–1.087)b | 0.807 (0.557–1.168)b | 0.560 (0.236–1.327)b | |
| Control | 352/195/36 | P = 0.154b | P = 0.255 | P = 0.188 | |
| Non-drinking | TT/TC/CC | ||||
| Case | 383/214/34 | 1.080 (0.910–1.281)b | 1.150 (0.927–1.427)b | 0.926 (0.593–1.446)b | |
| Control | 880/403/94 | P = 0.380b | P = 0.204 | P = 0.735 | |
WT, wild type; HT, heterozygote; MT, mutant type; OR, odds ratio; CI, interval confidence.
Adjusted for age, gender, BMI, HDL-C, LDL-C, TC, TG, smoking status, drinking status, T2DM and hypertension.
Adjusted for age, gender, BMI, HDL-C, LDL-C, TC, TG, smoking status, T2DM and hypertension.
Table 4. Association of rs852426 and drinking with stroke in the nested case-control study.
| Interaction | Model 1 | Model 2 | |
|---|---|---|---|
| Drinking* rs852426 | |||
| HR (95% CI) | 0.721 (0.568, 0.915) | 0.749 (0.575, 0.976) | |
| p | 0.007 | 0.033 | |
| Drinking+rs852426 | |||
| RERI | HR (95% CI) | −0.435 (−0.803, −0.066) | −0.370 (−0.622, −0.118) |
| p | 0.021 | 3.945×10−3 | |
| AP | −0.589 | −0.509 | |
| SI | −1.520 | −2.815 |
HR, hazard ratio; CI, interval confidence; RERI, relative excess risk due to interaction; AP, attributable proportion due to interaction; SI, synergy index
Model 1 did not adjust any covariates.
Model 2 adjusted for age, gender, BMI, HDL-C, LDL-C, TC, TG, smoking status, T2DM and hypertension.
Comparison of ACTB mRNA Relative Expression Level between IS Cases and Controls
No significant difference of the ACTB mRNA relative expression level was observed between IS and controls (1.03 ± 0.28 vs. 1.1 ± 0.18, Supplementary Fig. 2). A further stratified analysis by drinking status indicated that in the drinking population, the expression of ACTB in IS was significantly lower (0.99 ± 0.26) than that in the controls (1.13 ± 0.20), with a p value of 0.026 (Fig. 1). However, there is no significant difference of the ACTB mRNA level among rs852426 genotypes in IS or in controls (data not provided).
Supplementary Fig. 2.
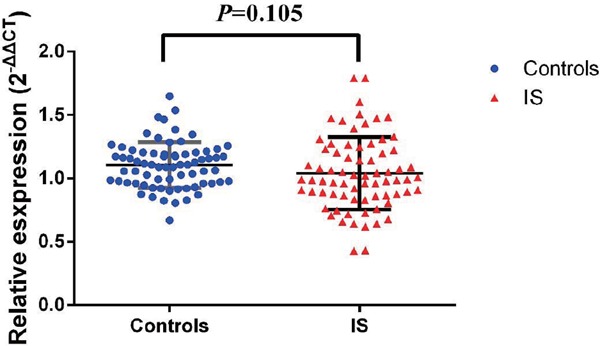
The comparison of ACTB mRNA expression between IS and controls
No significant difference of ACTB mRNA was observed between IS and controls (1.03 ± 0.28 vs 1.1 ± 0.18, P = 0.105)
Fig. 1.
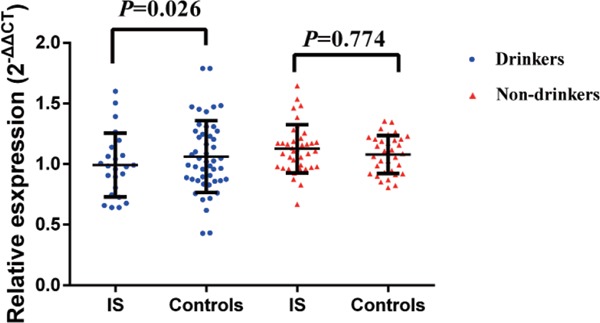
The comparison of ACTB mRNA relative expression between IS cases and controls stratified by drinking status
The expression of ACTB in IS with a drinking habit was significantly lower (0.99 ± 0.26) than that in the controls (1.13 ± 0.20), with p = 0.026.
Discussion
The current study evaluated the associations of ACTB polymorphisms with hypertension and stroke. The key findings indicated that rs852426 polymorphisms were significantly associated with the effect of alcohol consumption on stroke incidence. In the drinking population, it was first observed that the expression of ACTB in IS was significantly down-regulated.
Alcohol consumption is mostly taken as one of the covariates in numerous genetic association studies; thus, the molecular mechanism behind the alcoholic effect on stroke is easily neglected. Our novel finding indicated that the expression of ACTB was significantly down-regulated in IS who had a drinking habit. This might speculate that a drug that can up-regulate of ACTB expression will be a novel treatment method for the drinking population.
As generally regarded as a constitutive house-keeping gene, ACTB is an optimal reference gene for the quantitative reverse transcription polymerase chain reaction (RT-PCR) studies21). The obvious impact of alcohol on ACTB mRNA expression in IS dramatically indicates that selection of ACTB mRNA as a reference of a RT-PCR analysis for CVD may not be favorable.
Ongoing large GWAS have identified novel SNPs and pathogenic pathways22), which could partly elucidate the mechanisms involved in the progression of stroke. The pathogenesis of stroke is affected by not only genetic factors but also interactions between genetics and environment risk factors (such as alcohol drinking, smoking, or physical activities). Light-to-moderate alcohol consumption has generally been accepted as a potential protective factor for stroke, whereas heavy alcohol consumption was recognized as a risk factor23, 24). Alcohol reduces thrombus formation by inhibiting platelet aggregation25), antioxidant capacity and insulin sensitivity26); nonetheless, the biochemical mechanisms behind the cardiovascular beneficial effects of alcohol are still not fully elucidated.
The mutual protective effect between ACTB rs852426 polymorphisms and alcohol consumption on stroke provides a novel explanation for alcohol consumption attenuating the risk of stroke. Meanwhile, this interaction was further validated in a nested case-control study. To our knowledge, this study firstly observed the significant interaction of ACTB rs852426 and alcohol consumption on stroke. However, no significant difference of the ACTB mRNA level among rs852426 genotypes was observed; thus, elucidating the underlying modification of alcohol on ACTB mRNA expression in IS would warrant further biological function research.
The protective effect of ACTB rs852426 T>C variation on stroke incidence was observed both in the light-to-moderate and heavy drinking sub-population, although the p values did not reach statistical significance after multiple corrections. This could be explained by the limited stroke incidence in the follow- up study. The association of rs852426 with stroke has no heterogeneity between light-to-moderate and drinking heavy drinking groups. Given the universality of drinking in Chinese daily life, we may speculate that drinking is deprecated for the patients with the rs852426 TT genotype predisposed to stroke in clinic management.
A regional LD plot (http://www.broadinstitute.org/mpg/snap/ldplot.php) was depicted for rs852426 (Supplementary Fig. 3). Four loci (rs2537620, rs852432, rs2966450 and rs852446) were estimated near rs852426 with high LD (r2>0.8), thus suggesting that a fine mapping for this region would be warranted.
Supplementary Fig. 3.
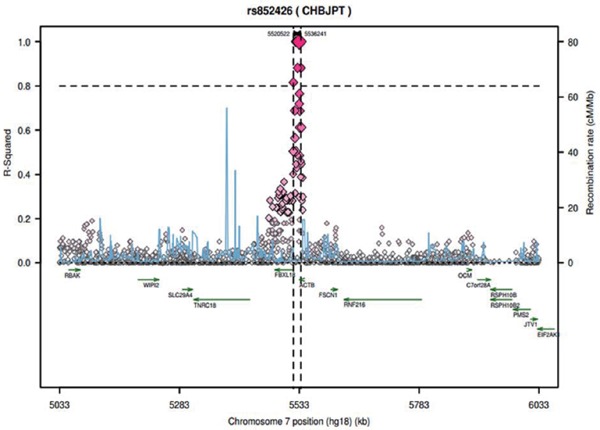
The regional LD Plot for rs852426, rs2537620, rs852432, rs2966450, and rs852446 were estimated loci near rs852426 with high LD (r2>0.8)
Several limitations were presented in the current study. First, we selected candidate SNPs with the criteria of MAF ≥ 0.05 and could have missed the chance of evaluating rare variants at ACTB for hypertension and stroke. Second, all participants were from the south China Han population; thus, the subject diversity of varied cultures and lifestyles was limited. Third, the significance in results obtained was not consistent in follow-up studies. This may be attributed to the fact that the case-control studies have a larger sample size than the number of incident events in the follow-up studies. Fourth, the median follow-up time of this study is 5.01 years, and only 613 hypertensions and 183 stroke incidences were recorded. The cumulative effect of ACTB on hypertension and stroke incidence should to be further confirmed by large-scale studies.
Conclusion
Our findings suggest that significant negative interaction between rs852426 and alcohol consumption could attenuate the stroke risk. The expression of ACTB in IS who had a drinking habit was significantly down-regulated compared to that in controls. It will be interesting to determine the potentially important role of alcohol consumption and ACTB polymorphisms in the prevention and treatment of stroke.
Conflict of Interests
There are no conflicts of interests.
Sources of Funding
This work was supported by the National Natural Science Foundation of China (grant nos. 81573232, 81541071 and 81573227), the Priority Academic Program Development of Jiangsu Higher Education Institutions (Public Health and Preventive Medicine) and the Flagship Major Development of Jiangsu Higher Education Institutions. The funders had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
References
- 1). ZC: The Third Nationwide Survey on Causes of Death. Beijing: The Peking Union Medical College Press, 2008; [Google Scholar]
- 2). Meschia JF, Worrall BB, Rich SS: Genetic susceptibility to ischemic stroke. Nat Rev Neurol, 2011; 7: 369-378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Waken RJ, de las Fuentes L, Rao DC: A Review of the Genetics of Hypertension with a Focus on Gene-Environment Interactions. Current Hypertension Reports, 2017; 19: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Chapados R, Abe K, Ihida-Stansbury K, McKean D, Gates AT, Kern M, Merklinger S, Elliott J, Plant A, Shimokawa H, Jones PL: ROCK controls matrix synthesis in vascular smooth muscle cells - Coupling vasoconstriction to vascular remodeling. Circulation Research, 2006; 99: 837-844 [DOI] [PubMed] [Google Scholar]
- 5). Artman L, Dormoy-Raclet V, von Roretz C, Gallouzi IE: Planning your every move: the role of beta-actin and its post-transcriptional regulation in cell motility. Seminars in cell & developmental biology, 2014; 34: 33-43 [DOI] [PubMed] [Google Scholar]
- 6). Rubenstein PA: The functional importance of multiple actin isoforms. BioEssays : news and reviews in molecular, cellular and developmental biology, 1990; 12: 309-315 [DOI] [PubMed] [Google Scholar]
- 7). Chen G, Zou Y, Zhang X, Xu L, Hu Q, Li T, Yao C, Yu S, Wang X, Wang C: beta-Actin protein expression differs in the submandibular glands of male and female mice. Cell Biol Int, 2016; 40: 779-786 [DOI] [PubMed] [Google Scholar]
- 8). Herman IM: Actin isoforms. Current opinion in cell biology, 1993; 5: 48-55 [DOI] [PubMed] [Google Scholar]
- 9). Karakozova M, Kozak M, Wong CC, Bailey AO, Yates JR, 3rd, Mogilner A, Zebroski H, Kashina A: Arginylation of beta-actin regulates actin cytoskeleton and cell motility. Science, 2006; 313: 192-196 [DOI] [PubMed] [Google Scholar]
- 10). Moustafa-Bayoumi M, Alhaj MA, El-Sayed O, Wisel S, Chotani MA, Abouelnaga ZA, Hassona MD, Rigatto K, Morris M, Nuovo G, Zweier JL, Goldschmidt-Clermont P, Hassanain H: Vascular hypertrophy and hypertension caused by transgenic overexpression of profilin 1. J Biol Chem, 2007; 282: 37632-37639 [DOI] [PubMed] [Google Scholar]
- 11). Shawlot W, Deng JM, Fohn LE, Behringer RR: Restricted beta-galactosidase expression of a hygromycin-lacZ gene targeted to the beta-actin locus and embryonic lethality of beta-actin mutant mice. Transgenic research, 1998; 7: 95-103 [DOI] [PubMed] [Google Scholar]
- 12). Shmerling D, Danzer CP, Mao X, Boisclair J, Haffner M, Lemaistre M, Schuler V, Kaeslin E, Korn R, Ledermann B, Kinzel B, Muller M: Strong and ubiquitous expression of transgenes targeted into the beta-actin locus by Cre/lox cassette replacement. Genesis, 2005; 42: 229-235 [DOI] [PubMed] [Google Scholar]
- 13). Nelson JC, Stavoe AK, Colon-Ramos DA: The actin cytoskeleton in presynaptic assembly. Cell Adh Migr, 2013; 7: 379-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Ji Y, Ferracci G, Warley A, Ward M, Leung KY, Samsuddin S, Leveque C, Queen L, Reebye V, Pal P, Gkaliagkousi E, Seager M, Ferro A: beta-Actin regulates platelet nitric oxide synthase 3 activity through interaction with heat shock protein 90. Proc Natl Acad Sci U S A, 2007; 104: 8839-8844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Searles CD, Ide L, Davis ME, Cai H, Weber M: Actin cytoskeleton organization and posttranscriptional regulation of endothelial nitric oxide synthase during cell growth. Circ Res, 2004; 95: 488-495 [DOI] [PubMed] [Google Scholar]
- 16). Tsutsui M: Neuronal nitric oxide synthase as a novel antiatherogenic factor. J Atheroscler Thromb, 2004; 11: 41-48 [DOI] [PubMed] [Google Scholar]
- 17). Rothenfluh A, Threlkeld RJ, Bainton RJ, Tsai LT, Lasek AW, Heberlein U: Distinct behavioral responses to ethanol are regulated by alternate RhoGAP18B isoforms. Cell, 2006; 127: 199-211 [DOI] [PubMed] [Google Scholar]
- 18). Offenhauser N, Castelletti D, Mapelli L, Soppo BE, Regondi MC, Rossi P, D'Angelo E, Frassoni C, Amadeo A, Tocchetti A, Pozzi B, Disanza A, Guarnieri D, Betsholtz C, Scita G, Heberlein U, Di Fiore PP: Increased ethanol resistance and consumption in Eps8 knockout mice correlates with altered actin dynamics. Cell, 2006; 127: 213-226 [DOI] [PubMed] [Google Scholar]
- 19). Chen J, Zhao X, Wang H, Chen Y, Wang W, Zhou W, Wang X, Tang J, Zhao Y, Lu X, Chen S, Wang L, Shen C, Yang S: Common variants in TGFBR2 and miR-518 genes are associated with hypertension in the Chinese population. Am J Hypertens, 2014; 27: 1268-1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Andersson T, Alfredsson L, Kallberg H, Zdravkovic S, Ahlbom A: Calculating measures of biological interaction. Eur J Epidemiol, 2005; 20: 575-579 [DOI] [PubMed] [Google Scholar]
- 21). Perez-Rico A, Crespo F, Sanmartin ML, De Santiago A, Vega-Pla JL: Determining ACTB, ATP5B and RPL32 as optimal reference genes for quantitative RT-PCR studies of cryopreserved stallion semen. Anim Reprod Sci, 2014; 149: 204-211 [DOI] [PubMed] [Google Scholar]
- 22). Traylor M, Farrall M, Holliday EG, Sudlow C, Hopewell JC, Cheng YC, Fornage M, Ikram MA, Malik R, Bevan S, Thorsteinsdottir U, Nalls MA, Longstreth W, Wiggins KL, Yadav S, Parati EA, Destefano AL, Worrall BB, Kittner SJ, Khan MS, Reiner AP, Helgadottir A, Achterberg S, Fernandez-Cadenas I, Abboud S, Schmidt R, Walters M, Chen WM, Ringelstein EB, O'Donnell M, Ho WK, Pera J, Lemmens R, Norrving B, Higgins P, Benn M, Sale M, Kuhlenbaumer G, Doney AS, Vicente AM, Delavaran H, Algra A, Davies G, Oliveira SA, Palmer CN, Deary I, Schmidt H, Pandolfo M, Montaner J, Carty C, de Bakker PI, Kostulas K, Ferro JM, van Zuydam NR, Valdimarsson E, Nordestgaard BG, Lindgren A, Thijs V, Slowik A, Saleheen D, Pare G, Berger K, Thorleifsson G, Australian Stroke Genetics Collaborative WTCCC. Hofman A, Mosley TH, Mitchell BD, Furie K, Clarke R, Levi C, Seshadri S, Gschwendtner A, Boncoraglio GB, Sharma P, Bis JC, Gretarsdottir S, Psaty BM, Rothwell PM, Rosand J, Meschia JF, Stefansson K, Dichgans M, Markus HS, International Stroke Genetics C : Genetic risk factors for ischaemic stroke and its subtypes (the META-STROKE collaboration): a meta-analysis of genome-wide association studies. Lancet Neurol, 2012; 11: 951-962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Cunningham SA, Mosher A, Judd SE, Matz LM, Kabagambe EK, Moy CS, Howard VJ: Incident Stroke and Alcohol Consumption among Older Adults. Stroke, 2015; 46: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Larsson SC, Wallin A, Wolk A, Markus HS: Differing association of alcohol consumption with different stroke types: a systematic review and meta-analysis. BMC Med, 2016; 14: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Booyse FM, Pan WS, Grenett HE, Parks DA, Darley-Usmar VM, Bradley KM, Tabengwa EM: Mechanism by which alcohol and wine polyphenols affect coronary heart disease risk. Annals of Epidemiology, 2007; 17: S24-S31 [DOI] [PubMed] [Google Scholar]
- 26). Vasdev S, Gill V, Singal PK: Beneficial effect of low ethanol intake on the cardiovascular system: possible biochemical mechanisms. Vasc Health Risk Manag, 2006; 2: 263-276 [DOI] [PMC free article] [PubMed] [Google Scholar]


