Abstract
Radiotherapy (RT) has been used for decades as one of the main treatment modalities for cancer patients. The therapeutic effect of RT has been primarily ascribed to DNA damage leading to tumor cell death. Besides direct tumoricidal effect, RT affects antitumor responses through immune-mediated mechanism, which provides a rationale for combining RT and immunotherapy for cancer treatment. Thus far, for the combined treatment with RT, numerous studies have focused on the immune checkpoint inhibitors and have shown promising results. However, treatment resistance is still common, and one of the main resistance mechanisms is thought to be due to the immunosuppressive tumor microenvironment where myeloid-derived suppressor cells (MDSCs) play a crucial role. MDSCs are immature myeloid cells with a strong immunosuppressive activity. MDSC frequency is correlated with tumor progression, recurrence, negative clinical outcome, and reduced efficacy of immunotherapy. Therefore, increasing efforts to target MDSCs have been made to overcome the resistance in cancer treatments. In this review, we focus on the role of MDSCs in RT and highlight growing evidence for targeting MDSCs in combination with RT to improve cancer treatment.
Keywords: Radiotherapy, Immune checkpoint inhibitors, Tumor microenvironment, Myeloid-derived suppressor cells
Introduction
Radiotherapy (RT) is a major treatment modality for cancer patients. It is applied for approximately 50% of all cancer patients as curative or palliative treatment. RT is often used in combination with surgery, chemotherapy, or targeted therapy. Ionizing radiation delivered by RT induces DNA damage [1], which leads to tumor cell death through senescence, apoptosis, and necrosis [2]. Historically, the direct killing of tumor cells is considered the major effect of RT.
The ionizing radiation also affects lymphocytes (T cells, B cells, and natural killer [NK] cells), which are the most radiosensitive cells in the tumor microenvironment (TME) [3]. Moreover, systemic lymphopenia after localized RT has been observed in patients with solid tumors, such as high-grade glioma, lung cancer, head and neck cancer, esophageal cancer, pancreatic cancer, and cervical cancer [4-6]. Therefore, RT traditionally has been considered to have a suppressive effect on the immune system.
However, mounting evidence suggests that RT can augment immune responses against tumors. Radiation-induced DNA damage results in cytosolic DNA accumulation in tumor cells, which in turn triggers type I interferon (IFN) production via cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway [7]. Type I IFNs activate dendritic cells (DCs), thereby promoting T cell priming [8]. During cell death, danger-associated molecular patterns (DAMPs) are released, thereby activating DCs through Toll-like receptors [9]. After the phagocytosis of tumor cells, DCs present tumor antigens to T cells through major histocompatibility complex (MHC) molecules, which results in the priming and activation of T cells in the draining lymph nodes [10]. Then, tumor-reactive T cells migrate not only to the irradiated tumor sites but also to the non-irradiated sites, leading to a systemic antitumor response (termed abscopal effect) [11]. RT also induces secretion of inflammatory chemokines and cytokines that recruit immune cells to the TME, promoting antitumor responses [12].
The antitumor effect of RT can be hampered by the activation of immunosuppressive pathways. Radiation-induced DNA damage activates ataxia telangiectasia mutated (ATM)/ataxia telangiectasia and Rad3-related (ATR)/checkpoint kinase 1 (Chk1) pathway, which results in programmed death ligand-1 (PD-L1) upregulation via signal transducer and activator of transcription (STAT)/IFN regulatory factor (IRF) pathway [13]. Type I IFNs produced by DNA damage also activate STAT/IRF pathway, contributing to PD-L1 upregulation [14]. The programmed death-1 (PD-1)/PD-L1 pathway plays a key role in tumor immune escape [15]. Immune checkpoint inhibitors (ICIs) targeting PD-1/PD-L1 pathway protect T cells from anergy and apoptosis [16]. Thus, the combination of RT and ICIs could enhance antitumor responses more potently than either treatment alone. The combined effect of RT and ICIs have been evaluated and have shown promising results in preclinical studies [17-20] and clinical trials [21-25]. Furthermore, abscopal effects which seldom occur after RT alone have been increasingly reported in patients treated with the combination of RT and immunotherapy [26].
ICIs provide durable antitumor responses in various types of cancer, but the beneficial outcomes are limited to a minority of patients. The therapeutic resistance of ICIs is associated with immunosuppressive TME where MDSCs play a role as key drivers of immunosuppression [27]. MDSCs suppress antitumor responses of T and NK cells and expand regulatory T cells (Treg), promoting cancer progression [27]. Importantly, MDSC frequency is negatively correlated with therapeutic efficacy of existing anti-cancer therapies, including chemotherapy and RT as well as ICIs [28-31]. In addition, MDSCs are associated with the clinical stage, tumor burden, and overall survival [32]. Therefore, increasing efforts have been made for targeting MDSCs combined with various cancer therapies [32]. This review focuses on the role of MDSCs in RT and introduces the rationale for the combination strategies of RT and MDSC targeting to improve cancer treatment.
Myeloid-Derived Suppressor Cells
Myeloid cells are a highly heterogenous population derived from bone marrow. They include granulocytes (neutrophil, eosinophils, and basophils) and mononuclear cells (monocytes, macrophages, and DCs). The name MDSCs was first coined for myeloid cells with immunosuppressive function in 2007 [33]. These cells are phenotypically and morphologically similar to, but functionally distinct from neutrophils and monocytes. The original intent to introduce the name MDSCs was not to define a novel population of myeloid cells, but to unify different descriptions of these cells [34].
In the circulation under healthy conditions, the frequency of granulocytes and monocytes is maintained by the coordinated cytokine expression, and MDSCs are almost absent. In pathological conditions such as cancer, infection, autoimmune disease, and graft versus host disease, MDSCs are generated as result of an altered myelopoiesis [35]. Chronic inflammatory conditions such as cancer induce the production of a variety of inflammatory mediators including granulocyte colony-stimulating factor (G-CSF), macrophage colony-stimulating factor (M-CSF), granulocyte/macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-6, IL-10, IL-1β, vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α), CC chemokine ligand 2 (CCL2), CCL5, and prostaglandin E2 (PGE2) [36]. These inflammatory mediators change normal myelopoiesis and skew myeloid differentiation toward MDSCs [35] (Fig. 1).
Fig. 1.
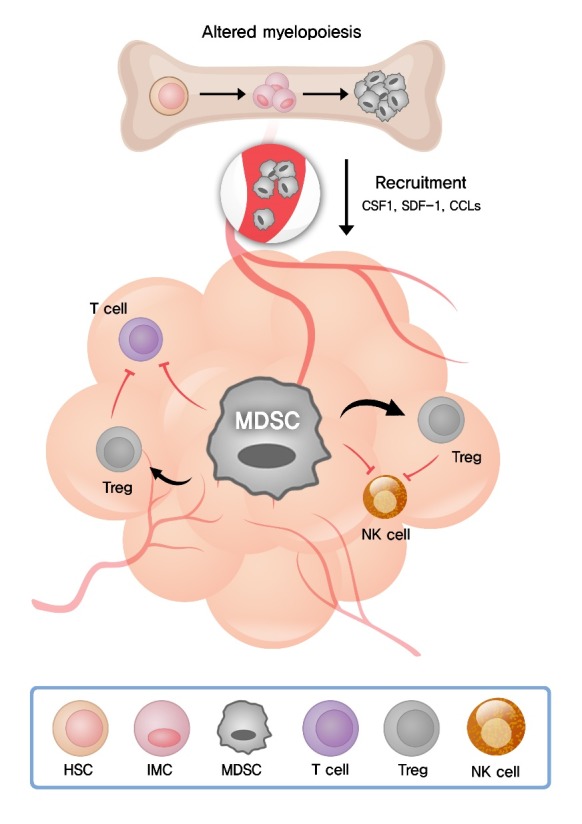
Schematic roles of myeloid-derived suppressor cells (MDSCs) during tumor progression. Tumor and immune cells release a variety of inflammatory mediators, leading to the altered myelopoiesis and the generation of MDSCs in the bone marrow. MDSCs are recruited to the tumor site by various cytokines and chemokines secreted in the tumor microenvironment (TME). Radiation is known to induce the expression of CSF1, SDF-1 and CCLs facilitating MDSC recruitment to the TME. MDSCs suppress antitumor immune responses of T and natural killer (NK) cells and expand regulatory T cells (Treg) via various mechanisms. CSF1, colony stimulating factor 1; SDF-1, stromal cell-derived factor-1; CCLs, CC chemokine ligands; HSC, hematopoietic stem cell; IMC, immature myeloid cell.
MDSCs are discriminated from other myeloid cells in which they possess potent immunosuppressive activity. Using a wide range of suppressive molecules, MDSCs suppress the functions of T and NK cells and promote the differentiation of Treg [32] (Fig. 1).
One of the main immunosuppressive molecules is arginase 1 (Arg1), which converts L-arginine into L-ornithine and urea. As a result of the enzymatic reaction, L-arginine is depleted from the TME. The lack of L-arginine causes downregulation of the T cell receptor (TCR) ζ-chain and G0/G1 phase cell cycle arrest in infiltrating T cells [37]. The induction of MDSCs in hepatitis C infection suppresses IFN-γ production of NK cells via L-arginine depletion [38].
MDSCs also overexpress indoleamine 2,3-dioxygenase (IDO), which correlates with increased infiltration of Treg in tumors and metastatic lymph nodes [39]. IDO converts L-tryptophan into N-formylkynurenine. The lack of tryptophan and production of kynurenine result in down-regulation of TCR ζ-chain in CD8+ T cells. Additionally, kynurenine produced by IDO activity induces regulatory phenotype in naïve CD4+ T cells [40].
Another important factor produced by MDSCs is suppressive reactive oxygen species (ROS). Different subsets of MDSCs employ different types of oxidative stress to regulate effector T cells. Polymorphonuclear (PMN)-MDSCs exert their function through NADPH oxidase expression and ROS generation, while monocytic (M)-MDSCs express inducible nitric oxide synthase (iNOS) and generate nitric oxide (NO). High levels of ROS induce T cell apoptosis and TCR ζ-chain downregulation. Reacting with NO, ROS nitrosylates the TCR, resulting in T cell anergy defined as a lack of responsiveness to antigen [41].
MDSCs secrete immunosuppressive cytokines such as TGF-β and IL-10 and reduce antitumor activity of effector T cells [36]. Furthermore, MDSCs exerts their immunosuppressive effects via upregulation of PD-L1. The binding of PD-L1 to PD-1 receptor on T cells leads to the anergic state in T cells [42].
In mice, MDSCs were first phenotypically identified by the expression of CD11b (a classical myeloid lineage marker) and Gr-1. The Gr-1 is a common marker of Ly6G and Ly6C molecules. MDSCs were initially defined as CD11b+Gr1+ cells, but this criterion is not sufficient to identify different subsets of MDSCs. According to the relative expression of Ly6G and Ly6C, MDSCs are classified into two subsets, PMN-MDSC (CD11b+Ly6G+Ly6Clo) and M-MDSC (CD11b+Ly6G-Ly6Chigh). Human MDSCs are generally identified based on the expression of the myeloid marker CD11b and low or absent HLA-DR. The equivalent to PMN-MDSC is further defined as CD14–CD15+, and M-MDSC is defined as CD14+CD15– [34].
However, theses phenotypic evaluations cannot discriminate PMN-MDSCs from neutrophil, and M-MDSCs from monocytes. Presently, there are no unique phenotypic marker for MDSCs. Therefore, an algorithm was proposed to identify cells as MDSCs. First, pathological conditions such as chronic inflammation and cancer should be associated with an expansion of cells with an MDSC phenotype. Then, isolated cells with MDSC phenotype must have immune suppressive activity [34].
MDSC Targeting for Cancer Treatments
It is important to prevent T cell inhibition to boost the existing antitumor responses as proven by the immune checkpoint targeting, but it is also important to regulate the TME for effective cancer treatment. In recent years, the accumulation of MDSCs have been highlighted as a major contributing factor in the immunosuppressive TME. The efficacy of MDSC inhibition have been evaluated in numerous preclinical and clinical studies [32]. There exist different approaches to target MDSCs: inhibition of MDSC accumulation, blocking of MDSC recruitment, and inhibition of MDSC function [32] (Table 1). Recently, MDSC inhibition in combination with immunotherapy have shown promising results in humans [43]. Clinical trials targeting MDSCs are summarized in Table 2.
Table 1.
Strategies for MDSC targeting
| Targeted process | Known mechanism of action | Agent |
|---|---|---|
| MDSC accumulation | Induction of MDSC differentiation | ATRA |
| Induction of MDSC apoptosis Inhibit DNA synthesis | Gemcitabine, 5-fluorouracil | |
| Inhibition of MDSC generation VEGFR and c-KIT inhibitor | Sunitinib | |
| MDSC recruitment | CCR2 antagonist | PF-04136309 |
| CCR5 antagonist | Maraviroc, vicriviroc | |
| Inhibition of CXCR2 | SX-682 | |
| MDSC function | Inhibition of PDE-5 | Sildenafil, tadalafil |
| Class I HDAC inhibitor | Entinostat | |
| IDO inhibitor | Indoximod |
MDSC, myeloid-derived suppressor cell; ATRA, all-trans retinoic acid; VEGFR, vascular endothelial growth factor receptor; CCL, CC chemokine ligand; CXCR2, CXC chemokine receptor 2; PDE-5, phosphodiesterase-5; HDAC, histone deacetylase; IDO, indoleamine 2,3-dioxygenase.
Table 2.
Clinical trials targeting MDSCs in cancer patients from ClinicalTrials.gov
| Trial number | Title | Conditions | Interventions |
|---|---|---|---|
| NCT03214718 | MDSCs and chronic myeloid leukemia | Chronic myeloid leukemia | Imatinib, nilotinib |
| NCT03302247 | Depletion of MDSCs to enhance anti-PD-1 therapy | Non-small lung cancer (stage IIIB) | Nivolumab, gemcitabine |
| NCT04022616 | MDSC function in breast cancer patients | Breast cancer | Specimen collection |
| NCT02868255 | MDSC control by signal regulatory protein-alpha: investigation in hepatocellular carcinoma | Hepatocellular carcinoma | Collection of human samples |
| NCT02916979 | MDSCs and checkpoint immune regulators’ expression in allogeneic SCT using FluBuATG | Leukemia, lymphoma, multiple myeloma | Fludarabine, busulfan, methotrexate, rabbit ATG |
| NCT02664883 | MDSC clinical assay in finding kidney cancer | Metastatic renal cell cancer, recurrent renal cell carcinoma, renal cell cancer (stage I, II, III) | - |
| NCT02669173 | Capecitabine + bevacizumab in patients with recurrent glioblastoma | Glioblastoma | Capecitabine, bevacizumab |
| NCT01803152 | Dendritic cell vaccine with or without gemcitabine pretreatment for adults and children with sarcoma | Sarcoma, soft tissue sarcoma, bone sarcoma | DC vaccine, gemcitabine, imiquimod |
| NCT03525925 | Ibrutinib and nivolumab in treating participants with metastatic solid tumors | Metastatic malignant solid neoplasm | Ibrutinib, nivolumab |
| NCT02637531 | A dose-escalation study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of IPI-549 | Advanced solid tumors, non-small cell lung cancer, melanoma, squamous cell cancer of the head and neck, triple negative breast cancer, adrenocortical carcinoma, mesothelioma, high-circulating MDSCs | IPI-549, nivolumab |
| NCT03161431 | SX-682 treatment in subjects with metastatic melanoma concurrently treated with pembrolizumab | Melanoma (stage III), melanoma (stage IV) | SX-682, pembrolizumab |
| NCT03848182 | Analyzing childhood recall antigens in patients with pancreatic cancer | Pancreatic cancer | Gemcitabine, TT vaccine booster |
| NCT03961698 | Evaluation of IPI-549 combined with front-line treatments in patients with triple-negative breast cancer or renal cell carcinoma | Breast cancer, renal cell carcinoma | IPI-549, atezolizumab, nab-paclitaxel, bevacizumab |
| NCT04105335 | A study of MTL-CEBPA in combination with a PD-1 inhibitor in patients with advanced solid tumors | Solid tumor | MTL-CEBPA, pembrolizumab |
| NCT02259231 | RTA 408 capsules in patients with melanoma | Melanoma, unresectable melanoma, metastatic melanoma | Omaveloxolone, ipilimumab, nivolumab |
| NCT03301636 | A study of indoximod or placebo plus pembrolizumab or nivolumab for subjects with unresectable or metastatic melanoma | Melanoma | Pembrolizumab, nivolumab, indoximod |
MDSC, myeloid-derived suppressor cell; PD-1, programmed death-1; SCT, stem cell transplant; ATG, anti-thymocyte globulin; DC, dendritic cell; TT, tetanus toxoid.
1. Inhibition of MDSC accumulation
The frequency of MDSCs can be reduced by all-trans retinoic acid (ATRA), an active metabolite of vitamin A. ATRA has been successfully used in the treatment of acute promyelocytic leukemia where it terminally differentiates immature myeloid cells into mature myeloid cells, resulting in leukemic cell death [44]. This concept of differentiation therapy provides a rationale for the use of ATRA in reducing MDSC accumulation. ATRA induced the differentiation MDSCs into DCs and macrophages, and thereby improved T cell function in mouse and human samples [45,46]. The antitumor effects of ATRA have been extensively studied in numerous studies in the past decades, but MDSCs were not almost evaluated in these studies [47]. In some clinical studies, ATRA has been reported to reduce the frequencies of circulating MDSCs [43,48,49]. ATRA treatment improved the immune response to cancer vaccine [48] and antigen-specific T-cell response [49].
Chemotherapeutic agents such as gemcitabine and 5-fluorouracil have been shown to reduce the number of MDSCs and to enhance antitumor immunity in mouse tumor models [50,51]. These agents had no significant effect on the frequencies of T cells and NK cells [50,51]. Similar to the preclinical observations, gemcitabine reduced MDSC frequency in the peripheral blood of patients with pancreatic cancer [52]. In a recent report, a liver X receptor (LXR) beta agonist, RGX-104 induced MDSC apoptosis in the periphery and TME, leading to enhanced T cell-mediated antitumor immunity in various mouse tumor models [53].
Sunitinib is a tyrosine kinase inhibitor acting toward VEGF receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), KIT and fetal liver tyrosine kinase receptor 3 (FLT3). It was approved for the treatment of patients with advanced renal cell carcinoma (RCC) and imatinib-resistant gastrointestinal stromal tumor (GIST) in 2006 [54]. Since VEGF was implicated in the accumulation of MDSCs, the effect of sunitinib on MDSCs was evaluated [55]. The elevated levels of circulating MDSCs were decreased after sunitinib treatment in patients with RCC [56] and oligometastases [28].
2. Blocking of MDSC recruitment
MDSC recruitment to the tumor is essential process for their immunosuppressive function. This process is mediated by various chemokines secreted in the TME. MDSCs express chemokine receptor CCR2, CCR5, and CXCR2, which mobilize them to the blood and the tumor sites.
CCR2 interacts with its ligands CCL2 and CCL5, which is required for the recruitment of M-MDSCs [57]. In a phase Ib clinical trial, a CCR2 antagonist (PF-04136309) was tested in combination with nab-paclitaxel plus gemcitabine in patients with pancreatic ductal adenocarcinoma. However, the results showed that PF-04136309 did not improve the efficacy compared to nab-paclitaxel plus gemcitabine [58].
CCR5 interacts with its ligand CCL3, CCL4 and CCL5, and the CCR5-CCL5 axis is required for the mobilization of PMN-MDSCs. Targeting CCR5-CCL5 axis has been reported to block MDSC recruitment and prevent tumor growth in preclinical studies [59,60]. Intriguingly, individual who carry CCR5 deletion mutation (CCR5Δ32) are physiologically normal, whereas tumor cells overexpress CCR5. With plausible rationale for CCR5 targeting, increasing number of studies have focused on targeting CCR5 in combination with immunotherapy [61].
CXCR2 is also overexpressed in PMN-MDSCs and required for recruitment of these MDSCs to the TME. Recent studies have shown that targeting CXCR2 inhibits recruitment of PMN-MDSCs and enhances immunotherapy in preclinical studies [62,63].
3. Inhibition of MDSC function
Phosphodiesterase-5 (PDE-5) inhibitors are widely used in the treatment of erectile dysfunction and pulmonary hypertension. It was reported that PDE5 inhibitors induced antitumor immune responses and substantially delayed tumor progression. PDE5 inhibitor sildenafil downregulated Arg1 and iNOS expression, and inhibited the suppressive function of MDSCs in mouse tumor models [64,65]. Recent clinical trials showed that PDE5 inhibitor tadalafil inhibited MDSC function and promoted antitumor immunity in patients with head and neck squamous cell carcinoma [66,67].
Histone deacetylase (HDAC) inhibitors have been reported to have potent immunomodulatory activity in mouse tumor models and cancer patients [68]. A class I HDAC inhibitor entinostat combined with ICIs has been evaluated in mouse tumor models [69,70]. Entinostat reduced Arg1 and iNOS expression and inhibited the immunosuppressive function of MDSCs, resulting in enhanced responsiveness to ICIs [69,70].
As described above, IDO is a key enzyme required for immunosuppressive activity of MDSCs. IDO inhibition reversed tumor-associated immunosuppression and showed antitumor effect in mouse tumor models [71]. However, clinical trials with IDO-1 monotherapy have not produced satisfactory results as observed in preclinical studies. Consequently, clinical trials have been redesigned to test IDO inhibitors in combination with other therapies, such as ICIs, chemotherapy, and radiotherapy [72].
MDSC Targeting for RT
RT has two opposite effects on MDSCs, dependent on dose-fractionation schemes and tumor models. Although it seems difficult to draw a consistent conclusion from recent studies, some patterns emerge, that is, conventional fractionated radiotherapy (CFRT) increases MDSCs while ablative hypofractionated radiotherapy (ABHRT) decreases MDSCs [73]. However, regardless of RT scheme, targeting of MDSCs has been shown to increase the antitumor effect of RT in several studies.
Radiation induced colony stimulating factor 1 (CSF1) expression through ABL1-dependent transcription. In response to CSF1, MDSCs were recruited to tumor sites and expanded systematically in the tumor, spleen, lymph nodes, and peripheral blood. CSF1/CSF1 receptor (CSF1R) blockade inhibited MDSC infiltration and tumor growth after irradiation (3 Gy × 5). In accordance with mouse studies, serum CSF1 was elevated in prostate cancer patients after RT [74]. As mentioned above, several chemokines and their receptors promote MDSC recruitment into the TME. Stromal cell-derived factor-1 (SDF-1) is a chemokine up-regulated in tumor tissues after radiation [75,76]. SDF-1 receptor CXCR4 is expressed on immunosuppressive cells including Treg and MDSCs, which are attracted by SDF-1 produced within the tumor [77]. CXCR4 antagonist AMD3100 was shown to prevent tumor regrowth when combined with radiation [75,76]. These studies suggest that CD11b+F4/80+ myeloid cells are associated with tumor regrowth after radiation. However, M-MDSCs also express F4/80 marker and distinct phenotypic markers for M-MDSCs were not used in these studies. Therefore, it cannot be ruled out that CD11b+F4/80+ myeloid cells may be attributed to M-MDSCs. Radiation-induced STING activation also contributes to MDSC infiltration. This activation caused tumor cells to produce type I IFN which, in turn induced expression of CCL2, CCL7, and CCL12, chemoattractants for MDSCs. Chemokine receptor CCR2 knockout blocked MDSC accumulation and enhanced tumor regression following radiation (20 Gy) in MC38 and LLC tumor models. Treatment with anti-CCR2 antibody also enhanced antitumor effects of radiation [78]. More recently, it was reported that radiation (12 Gy × 3) increased infiltration of MDSCs in tumors, which was suppressed by IDO1 inhibitor. Radiation combined with IDO1 inhibitor enhanced tumor growth inhibition in LLC tumor model [79].
On the other hand, several recent studies have shown that high-dose irradiation decreases MDSC frequencies. A single high-dose irradiation (30 Gy) induced complete remissions, which was related to an increased CD8+ T cell infiltration and a reduced MDSC infiltration into the TME in CT26 and MC38 tumor models [80]. In hepatocellular carcinoma (HCC) patients, the frequency of MDSCs after RT was significantly decreased and inversely correlated with overall survival. These results suggest that patients with a high frequency of MDSCs should be monitored closely and the inhibition of MDSCs may improve treatment outcomes after RT [31]. In mouse studies, ABHRT inhibited MDSC recruitment into tumors and significantly inhibited the tumor growth compared with CFRT. VEGF expression, which mediated MDSC recruitment, was relatively lower after ABHRT than CFRT. VEGFR2 blocking antibody plus CFRT reduced infiltrating MDSCs in tumors and inhibited tumor growth more efficiently than CFRT alone [81]. These results indicate that via blockade of MDSC recruitment, the therapeutic efficacy of ABHRT could be achieved and the effect of CFRT enhanced. As described above, receptor tyrosine kinase inhibitor sunitinib inhibited MDSC accumulation, and thereby restored antitumor immunity. Concurrent sunitinib and stereotactic body radiotherapy (SBRT) reversed MDSC-mediated immunosuppression and resulted in favorable clinical outcomes in patients with oligometastases [82].
When combined with ICI treatment, RT have shown improved efficacy in preclinical studies [17-20] and clinical trials [21-25]. In TUBO and MC38 tumor models, high levels of radiation (12 Gy for TUBO and 20 Gy for MC38) combined with PD-L1 blockade synergistically amplified the antitumor effect, which was correlated with a reduction of MDSCs mediated by tumor infiltrating CD8+ T cells [17]. Similarly, in LLC tumor model, radiation (6 Gy in 3 fractions) and anti- PD-L1 antibody effectively inhibited tumor growth compared to either therapy alone [19]. A recent clinical study evaluated whether SBRT could enhance the effect of ICI treatment in patients with advanced NSCLC. Interestingly, PD-L1-negative patients had the largest benefit of improved overall survival and progression-free survival in the combined treatment of RT [24]. These results suggest that RT may convert the immunosuppressive TME to a more ICI-responsive one. One more important finding is no increase in treatment-related toxic effect.
The therapeutic effect of anti-PD-L1 antibody was initially assumed to result from blockade of PD-L1 expressed on the tumor cell itself. However, several recent studies highlighted the essential roles of PD-L1 expressed on host myeloid cells. These studies suggest that PD-L1 on DCs, macrophages and MDSCs rather than tumor cells is the relevant mechanistic target for PD-1/PD-L1 inhibitors [83,84].
For the combined treatment with RT, numerous studies so far have focused on ICIs, which target PD-1/PD-L1 interactions. The available data provide evidence that the therapeutic efficacy of RT could be enhanced when combined with MDSC targeting therapy.
Conclusion
RT-mediated immune responses support both tumor immune recognition and tumor immune evasion. Thus, combining RT and immunotherapy can be a rational strategy to improve cancer treatment. Numerous studies have shown promising results in the combination of RT and immunotherapy. However, translational research behind this approach is still needed to maximize the therapeutic efficacy and increase the response rate in cancer patients. In the light of therapeutic resistance, recent studies highlight the immunosuppressive TME which promote tumor immune evasion. MDSCs are increasingly recognized as important contributors to immunosuppression in the TME and are also closely associated with resistance to RT. Therefore, MDSC targeting could be a successful complementary strategy for RT, and also RT combined with other therapies. Further work is needed to identify specific markers for MDSCs, which would enable the development of methods to selectively target these cells. Improving specificity in targeting MDSCs could help to find the strategy to maximize the efficacy of antitumor therapies.
Acknowledgments
This study was supported by a grant from the Korean Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry for Health & Welfare, Republic of Korea (No. HI15C0972) and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Republic of Korea (No. NRF-2019R1A2C2009183).
Footnotes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Toulany M. Targeting DNA double-strand break repair pathways to improve radiotherapy response. Genes (Basel) 2019;10:25. doi: 10.3390/genes10010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lauber K, Ernst A, Orth M, Herrmann M, Belka C. Dying cell clearance and its impact on the outcome of tumor radiotherapy. Front Oncol. 2012;2:116. doi: 10.3389/fonc.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diegeler S, Hellweg CE. Intercellular communication of tumor cells and immune cells after exposure to different ionizing radiation qualities. Front Immunol. 2017;8:664. doi: 10.3389/fimmu.2017.00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campian JL, Ye X, Brock M, Grossman SA. Treatment-related lymphopenia in patients with stage III non-small-cell lung cancer. Cancer Invest. 2013;31:183–8. doi: 10.3109/07357907.2013.767342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellsworth SG. Field size effects on the risk and severity of treatment-induced lymphopenia in patients undergoing radiation therapy for solid tumors. Adv Radiat Oncol. 2018;3:512–9. doi: 10.1016/j.adro.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venkatesulu BP, Mallick S, Lin SH, Krishnan S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol. 2018;123:42–51. doi: 10.1016/j.critrevonc.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Deng L, Liang H, Xu M, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41:843–52. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnette BC, Liang H, Lee Y, et al. The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71:2488–96. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 10.Roberts EW, Broz ML, Binnewies M, et al. Critical role for CD103(+)/CD141(+) dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell. 2016;30:324–36. doi: 10.1016/j.ccell.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–70. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol. 2017;14:365–79. doi: 10.1038/nrclinonc.2016.211. [DOI] [PubMed] [Google Scholar]
- 13.Sato H, Niimi A, Yasuhara T, et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat Commun. 2017;8:1751. doi: 10.1038/s41467-017-01883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shevtsov M, Sato H, Multhoff G, Shibata A. Novel approaches to improve the efficacy of immuno-radiotherapy. Front Oncol. 2019;9:156. doi: 10.3389/fonc.2019.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–77. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 16.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–95. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–68. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 19.Gong X, Li X, Jiang T, et al. Combined radiotherapy and anti-PD-L1 antibody synergistically enhances antitumor effect in non-small cell lung cancer. J Thorac Oncol. 2017;12:1085–97. doi: 10.1016/j.jtho.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–7. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauml JM, Mick R, Ciunci C, et al. Pembrolizumab after completion of locally ablative therapy for oligometastatic non-small cell lung cancer: a phase 2 trial. JAMA Oncol. 2019;5:1283–90. doi: 10.1001/jamaoncol.2019.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bozorgmehr F, Hommertgen A, Krisam J, et al. Fostering efficacy of anti-PD-1-treatment: nivolumab plus radiotherapy in advanced non-small cell lung cancer - study protocol of the FORCE trial. BMC Cancer. 2019;19:1074. doi: 10.1186/s12885-019-6205-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HJ, Chang JS, Roh MR, et al. Effect of radiotherapy combined with pembrolizumab on local tumor control in mucosal melanoma patients. Front Oncol. 2019;9:835. doi: 10.3389/fonc.2019.00835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theelen WS, Peulen HM, Lalezari F, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 2019;5:1276–82. doi: 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu JI, Lee SJ, Lee J, et al. Clinical significance of radiotherapy before and/or during nivolumab treatment in hepatocellular carcinoma. Cancer Med. 2019;8:6986–94. doi: 10.1002/cam4.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yilmaz MT, Elmali A, Yazici G. Abscopal effect, from myth to reality: from radiation oncologists' perspective. Cureus. 2019;11:e3860. doi: 10.7759/cureus.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber R, Fleming V, Hu X, et al. Myeloid-derived suppressor cells hinder the anti-cancer activity of immune checkpoint inhibitors. Front Immunol. 2018;9:1310. doi: 10.3389/fimmu.2018.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen HM, Ma G, Gildener-Leapman N, et al. Myeloid-derived suppressor cells as an immune parameter in patients with concurrent sunitinib and stereotactic body radiotherapy. Clin Cancer Res. 2015;21:4073–85. doi: 10.1158/1078-0432.CCR-14-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang A, Zhang B, Wang B, Zhang F, Fan KX, Guo YJ. Increased CD14(+)HLA-DR (-/low) myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer Immunol Immunother. 2013;62:1439–51. doi: 10.1007/s00262-013-1450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mengos AE, Gastineau DA, Gustafson MP. The CD14(+)HLA-DR(lo/neg) monocyte: an immunosuppressive phenotype that restrains responses to cancer immunotherapy. Front Immunol. 2019;10:1147. doi: 10.3389/fimmu.2019.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D, An G, Xie S, Yao Y, Feng G. The clinical and prognostic significance of CD14(+)HLA-DR(-/low) myeloid-derived suppressor cells in hepatocellular carcinoma patients receiving radiotherapy. Tumour Biol. 2016;37:10427–33. doi: 10.1007/s13277-016-4916-2. [DOI] [PubMed] [Google Scholar]
- 32.Fleming V, Hu X, Weber R, et al. Targeting myeloid-derived suppressor cells to bypass tumor-induced immunosuppression. Front Immunol. 2018;9:398. doi: 10.3389/fimmu.2018.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabrilovich DI, Bronte V, Chen SH, et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bronte V, Brandau S, Chen SH, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19:108–19. doi: 10.1038/s41590-017-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Groth C, Hu X, Weber R, et al. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br J Cancer. 2019;120:16–25. doi: 10.1038/s41416-018-0333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109:1568–73. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goh CC, Roggerson KM, Lee HC, Golden-Mason L, Rosen HR, Hahn YS. Hepatitis C virus-induced myeloid-derived suppressor cells suppress NK cell IFN-γ production by altering cellular metabolism via arginase-1. J Immunol. 2016;196:2283–92. doi: 10.4049/jimmunol.1501881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu J, Du W, Yan F, et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. 2013;190:3783–97. doi: 10.4049/jimmunol.1201449. [DOI] [PubMed] [Google Scholar]
- 40.Fallarino F, Grohmann U, You S, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–61. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 41.Ohl K, Tenbrock K. Reactive oxygen species as regulators of MDSC-mediated immune suppression. Front Immunol. 2018;9:2499. doi: 10.3389/fimmu.2018.02499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noman MZ, Desantis G, Janji B, et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–90. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tobin RP, Jordan KR, Robinson WA, et al. Targeting myeloid-derived suppressor cells using all-trans retinoic acid in melanoma patients treated with Ipilimumab. Int Immunopharmacol. 2018;63:282–91. doi: 10.1016/j.intimp.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang ME, Ye YC, Chen SR, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72:567–72. doi: 10.1182/blood-2016-11-750182. [DOI] [PubMed] [Google Scholar]
- 45.Kusmartsev S, Su Z, Heiser A, et al. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2008;14:8270–8. doi: 10.1158/1078-0432.CCR-08-0165. [DOI] [PubMed] [Google Scholar]
- 46.Nefedova Y, Fishman M, Sherman S, Wang X, Beg AA, Gabrilovich DI. Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res. 2007;67:11021–8. doi: 10.1158/0008-5472.CAN-07-2593. [DOI] [PubMed] [Google Scholar]
- 47.Schenk T, Stengel S, Zelent A. Unlocking the potential of retinoic acid in anticancer therapy. Br J Cancer. 2014;111:2039–45. doi: 10.1038/bjc.2014.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iclozan C, Antonia S, Chiappori A, Chen DT, Gabrilovich D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother. 2013;62:909–18. doi: 10.1007/s00262-013-1396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mirza N, Fishman M, Fricke I, et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66:9299–307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–21. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 51.Vincent J, Mignot G, Chalmin F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–61. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 52.Eriksson E, Wenthe J, Irenaeus S, Loskog A, Ullenhag G. Gemcitabine reduces MDSCs, tregs and TGFβ-1 while restoring the teff/treg ratio in patients with pancreatic cancer. J Transl Med. 2016;14:282. doi: 10.1186/s12967-016-1037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tavazoie MF, Pollack I, Tanqueco R, et al. LXR/ApoE activation restricts innate immune suppression in cancer. Cell. 2018;172:825–40. doi: 10.1016/j.cell.2017.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le Tourneau C, Raymond E, Faivre S. Sunitinib: a novel tyrosine kinase inhibitor. A brief review of its therapeutic potential in the treatment of renal carcinoma and gastrointestinal stromal tumors (GIST) Ther Clin Risk Manag. 2007;3:341–8. doi: 10.2147/tcrm.2007.3.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol. 2018;9:978. doi: 10.3389/fimmu.2018.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ko JS, Zea AH, Rini BI, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–57. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 57.Karin N, Razon H. The role of CCR5 in directing the mobilization and biological function of CD11b(+)Gr1(+)Ly6C(low) polymorphonuclear myeloid cells in cancer. Cancer Immunol Immunother. 2018;67:1949–53. doi: 10.1007/s00262-018-2245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noel M, O'Reilly EM, Wolpin BM, et al. Phase 1b study of a small molecule antagonist of human chemokine (C-C motif) receptor 2 (PF-04136309) in combination with nab-paclitaxel/gemcitabine in first-line treatment of metastatic pancreatic ductal adenocarcinoma. Invest New Drugs. 2019 Jul 12; doi: 10.1007/s10637-019-00830-3. [Epub]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blattner C, Fleming V, Weber R, et al. CCR5(+) myeloid-derived suppressor cells are enriched and activated in melanoma lesions. Cancer Res. 2018;78:157–67. doi: 10.1158/0008-5472.CAN-17-0348. [DOI] [PubMed] [Google Scholar]
- 60.Hawila E, Razon H, Wildbaum G, et al. CCR5 directs the mobilization of CD11b(+)Gr1(+)Ly6C(low) polymorphonuclear myeloid cells from the bone marrow to the blood to support tumor development. Cell Rep. 2017;21:2212–22. doi: 10.1016/j.celrep.2017.10.104. [DOI] [PubMed] [Google Scholar]
- 61.Jiao X, Nawab O, Patel T, et al. Recent advances targeting CCR5 for cancer and its role in immuno-oncology. Cancer Res. 2019;79:4801–7. doi: 10.1158/0008-5472.CAN-19-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Highfill SL, Cui Y, Giles AJ, et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med. 2014;6:237ra67. doi: 10.1126/scitranslmed.3007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun L, Clavijo PE, Robbins Y, et al. Inhibiting myeloid-derived suppressor cell trafficking enhances T cell immunotherapy. JCI Insight. 2019;4:e126853. doi: 10.1172/jci.insight.126853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meyer C, Sevko A, Ramacher M, et al. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc Natl Acad Sci U S A. 2011;108:17111–6. doi: 10.1073/pnas.1108121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Serafini P, Meckel K, Kelso M, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Califano JA, Khan Z, Noonan KA, et al. Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:30–8. doi: 10.1158/1078-0432.CCR-14-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weed DT, Vella JL, Reis IM, et al. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:39–48. doi: 10.1158/1078-0432.CCR-14-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen L, Orillion A, Pili R. Histone deacetylase inhibitors as immunomodulators in cancer therapeutics. Epigenomics. 2016;8:415–28. doi: 10.2217/epi.15.118. [DOI] [PubMed] [Google Scholar]
- 69.Christmas BJ, Rafie CI, Hopkins AC, et al. Entinostat converts immune-resistant breast and pancreatic cancers into checkpoint-responsive tumors by reprogramming tumor-infiltrating MDSCs. Cancer Immunol Res. 2018;6:1561–77. doi: 10.1158/2326-6066.CIR-18-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Orillion A, Hashimoto A, Damayanti N, et al. Entinostat neutralizes myeloid-derived suppressor cells and enhances the antitumor effect of PD-1 inhibition in murine models of lung and renal cell carcinoma. Clin Cancer Res. 2017;23:5187–201. doi: 10.1158/1078-0432.CCR-17-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holmgaard RB, Zamarin D, Li Y, et al. Tumor-expressed IDO recruits and activates MDSCs in a treg-dependent manner. Cell Rep. 2015;13:412–24. doi: 10.1016/j.celrep.2015.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu MMT, Dancsok AR, Nielsen TO. Indoleamine dioxygenase inhibitors: clinical rationale and current development. Curr Oncol Rep. 2019;21:2. doi: 10.1007/s11912-019-0750-1. [DOI] [PubMed] [Google Scholar]
- 73.Ostrand-Rosenberg S, Horn LA, Ciavattone NG. Radiotherapy both promotes and inhibits myeloid-derived suppressor cell function: novel strategies for preventing the tumor-protective effects of radiotherapy. Front Oncol. 2019;9:215. doi: 10.3389/fonc.2019.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu J, Escamilla J, Mok S, et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–94. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kozin SV, Kamoun WS, Huang Y, Dawson MR, Jain RK, Duda DG. Recruitment of myeloid but not endothelial precursor cells facilitates tumor regrowth after local irradiation. Cancer Res. 2010;70:5679–85. doi: 10.1158/0008-5472.CAN-09-4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Susek KH, Karvouni M, Alici E, Lundqvist A. The role of CXC chemokine receptors 1-4 on immune cells in the tumor microenvironment. Front Immunol. 2018;9:2159. doi: 10.3389/fimmu.2018.02159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liang H, Deng L, Hou Y, et al. Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nat Commun. 2017;8:1736. doi: 10.1038/s41467-017-01566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li A, Barsoumian HB, Schoenhals JE, et al. IDO1 inhibition overcomes radiation-induced "rebound immune suppression" by reducing numbers of IDO1-expressing myeloid-derived suppressor cells in the tumor microenvironment. Int J Radiat Oncol Biol Phys. 2019;104:903–12. doi: 10.1016/j.ijrobp.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 80.Filatenkov A, Baker J, Mueller AM, et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res. 2015;21:3727–39. doi: 10.1158/1078-0432.CCR-14-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lan J, Li R, Yin LM, et al. Targeting myeloid-derived suppressor cells and programmed death ligand 1 confers therapeutic advantage of ablative hypofractionated radiation therapy compared with conventional fractionated radiation therapy. Int J Radiat Oncol Biol Phys. 2018;101:74–87. doi: 10.1016/j.ijrobp.2018.01.071. [DOI] [PubMed] [Google Scholar]
- 82.Kao J, Chen CT, Tong CC, et al. Concurrent sunitinib and stereotactic body radiotherapy for patients with oligometastases: final report of a prospective clinical trial. Target Oncol. 2014;9:145–53. doi: 10.1007/s11523-013-0280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin H, Wei S, Hurt EM, et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J Clin Invest. 2018;128:805–15. doi: 10.1172/JCI96113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tang H, Liang Y, Anders RA, et al. PD-L1 on host cells is essential for PD-L1 blockade-mediated tumor regression. J Clin Invest. 2018;128:580–8. doi: 10.1172/JCI96061. [DOI] [PMC free article] [PubMed] [Google Scholar]


