Abstract
Background
Barrett's esophagus (BE), a complication of gastroesophageal reflux disease (GERD), predisposes patients to esophageal adenocarcinoma (EAC). Reliable biomarkers for early detection and discovery of potential drug targets are urgently needed for improved BE and EAC patient outcomes.
Methods
Patient biopsy samples were evaluated for COX1/2, and thromboxane A2 synthase (TBXAS) expression. Circulating prostaglandins biosynthesis was determined using enzyme immunoassay kits. Anchorage-independent cell growth assay, crystal violet staining assay, and xenograft experiments were conducted to assess BE and EAC cell growth. A surgical mouse model of reflux (i.e., esophagoduodenostomy) was established and samples were analyzed using an enzyme immunoassay kit, immunohistochemistry, immunoblotting, or RT-PCR. Esophageal biopsy samples (pre- and post-intervention) were obtained from a randomized clinical trial in which participants were administered esomeprazole (40 mg) twice daily in combination with an acetylsalicylic acid (ASA) placebo or 81 or 325 mg ASA for 28 days. Esophageal biopsy specimens before and after the intervention period were analyzed.
Findings
COX2 and TBXAS are highly expressed in BE and EAC patients accompanied by a pronounced elevation of circulating TXA2 levels. ASA suppressed BE and EAC growth by targeting the TXA2 pathway. Additionally, biopsies from 49 patients (with similar baseline characteristics) showed that ASA substantially decreased serum TXA2 levels, resulting in reduced inflammation.
Interpretation
This study establishes the importance of the COX1/2-driven TXA2 pathway in BE and EAC pathophysiology and lays the groundwork for introducing a TXA2-targeting strategy for EAC prevention and early detection.
Funding
Hormel Foundation, Exact Sciences, Pentax Medical, Intromedic and National Cancer.
Keywords: Thromboxane A2 pathway, Acetylsalicylic acid, Barrett's esophagus, Esophageal adenocarcinoma
1. Introduction
The incidence of esophageal adenocarcinoma (EAC) is increasing in Western populations and is a major cause of cancer-associated morbidity and mortality [1], [2], [3]. Barrett's esophagus (BE), a complication of gastroesophageal reflux disease (GERD), predisposes patients to EAC [4,5]. Approximately 10–15% of patients with GERD will develop BE [6]. Reliable biomarkers for early detection and discovery of potential drug targets are urgently needed for improved BE and EAC patient outcomes.
Research in context.
Evidence before this study
Barrett's esophagus (BE), a complication of gastroesophageal reflux disease (GERD), predisposes patients to esophageal adenocarcinoma (EAC). Reliable biomarkers for early detection and discovery of potential drug targets are urgently needed for improved BE and EAC patient outcomes. Although prostaglandin E2 (PGE2) is associated with BE and EAC and acetylsalicylic acid (ASA), a COX1/2 inhibitor, can decrease the level of PGE2 in BE patients, the profiles of circulating prostaglandins in BE and EAC have not been studied. In addition, studies also demonstrate that thromboxane A2 (TXA2) and its specific synthases TBXAS are involved in various signaling interactions and play an important role in some human cancers. However, the role of the TXA2 signaling pathway in BE and EAC is unclear.
Added value of this study
This study found TXA2 is a potential biomarker for detection of BE and EAC. TBXAS, a specific synthases for TXA2 production, regulated by COX1/2 and associated with BE and EAC cells growth in vitro and in a xenograft model. Importantly, ASA targets the COX1/2-driven TXA2 pathway to suppress BE and EAC development in surgical mouse model of reflux and a previously conducted phase II clinical trial. The combination of ASA and PPI effectively decreased the circulating levels of TXA2 and inflammation leading to a suppression of the development of BE.
Implications of all the available evidence
The clinical impact of this study is providing a potential biomarker for early detected BE and EAC, furthermore, providing a rationale for clinical evaluation of targeting TXA2 pathway for prevention and treatment of BE and EAC. Overall, this study established the importance of the COX1/2-driven TXA2 pathway in BE and EAC pathophysiology, and laid the groundwork for introducing a TXA2-targeting strategy to EAC prevention, early detection, and management.
CRediT authorship contribution statement
Tianshun Zhang: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing - original draft, Writing - review & editing. Qiushi Wang: Conceptualization, Data curation, Investigation, Methodology, Writing - original draft, Writing - review & editing. Wei-Ya Ma: Methodology, Project administration. Keke Wang: Data curation. Xiaoyu Chang: Formal analysis. Michele L. Johnson: Data curation. Ruihua Bai: Formal analysis. Ann M. Bode: Writing - original draft, Writing - review & editing. Nathan R. Foster: Data curation, Formal analysis. Gary W. Falk: Data curation. Paul J. Limburg: Funding acquisition, Writing - review & editing. Prasad G. Iyer: Funding acquisition, Writing - review & editing. Zigang Dong: Conceptualization, Funding acquisition, Project administration, Supervision, Writing - review & editing.
Alt-text: Unlabelled box
BE is closely associated with inflammation. Gastric acid and bile reflux to the esophagus causes injury to the esophageal mucosa and persistent injury might play a role in the development and progression of BE [7,8]. PG synthesis is driven by cyclooxygenases (COXs) [9] and COX enzymes convert arachidonic acid to the precursor molecule prostaglandin H2 (PGH2). PGH2 is then converted to one of five primary prostanoids, prostaglandin D2 (PGD2), prostaglandin E2 (PGE2), prostaglandin F2α (PGF2α), prostaglandin I2 (PGI2), and thromboxane A2 (TXA2), through specific synthases, PGDS, PGES, PGFS, PGIS, and TBXAS, respectively [10], [11], [12]. PGE2 and PGES are associated with BE and EAC [13,14] and acetylsalicylic acid (ASA), a COX1/2 inhibitor, can decrease the level of PGE2 in BE patients [15]. Studies also demonstrate that TXA2 and TBXAS are involved in various signaling interactions and play an important role in human cancers including colorectal cancer, lung cancer and breast cancer [16], [17], [18].
Extensive preclinical and clinical data suggest that nonsteroidal anti-inflammatory drugs (NSAIDs), particularly ASA, are protective against the development of BE and esophageal cancer by inhibiting COX1 and COX2 [19], [20], [21]. Although ASA decreased the level of PGE2 in BE patients in one clinical study [15], the profiles of circulating PGs in BE and EAC have not been studied.
In this study, we examined the function of the COX1/2-driven TXA2 signaling pathway in BE and EAC development. We also aimed to ascertain the mechanisms of TXA2 signaling related to BE and EAC development. A surgical mouse model of esophagoduodenostomy was used to study the effect of ASA on BE and EAC development. Finally, we studied samples from a previously reported, randomized clinical trial [15] to examine the effect of ASA on the COX1/2- driven TXA2 signaling pathway in BE patients.
2. Materials and methods
2.1. Reagents and antibodies
Cell culture media, gentamicin, penicillin, and L-glutamine were all obtained from Invitrogen (Grand Island, NY). Fetal bovine serum (FBS) was from Gemini Bio-Products (West Sacramento, CA). Tris, NaCl, and SDS for molecular biology and buffer preparation were purchased from Sigma-Aldrich (St. Louis, MO). Antibodies to detect COX2 (#D5H5), NFκB (p65) (#D14E12), p-ERKs (#9101), ERKs (#9102), and PCNA (#D3H8P) were from Cell Signaling Technology (Danvers, MA). The thromboxane synthase polyclonal antibody (#160715) and COX1 monoclonal antibody (#160110) were obtained from Cayman Chemical Company (Ann Arbor, MI). The β-actin (sc-47778), lamin B (sc-6216), and GAPDH (sc-25778) antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
2.2. Cell culture and transfection
Barrett's esophagus (BE) cell lines CP-B (also identified as CP-52731 or ChTERT, batch number #58599546) and CP-C (also identified as CP-94251, batch number #58597824) were purchased from American Type Culture Collection (ATCC; Manassas, VA). CP-B and CP-C were derived from endoscopic biopsy specimens obtained from regions of high-grade dysplasia patients and transduced with the retroviral expression vector, pLXSN-hTERT, to create an immortalized cell lines. The esophageal adenocarcinoma cell lines SKGT-4 and OE33 were kindly provided by Dr. Navtej (Nav) S. Buttar (Mayo Clinic, Rochester, MN) and authenticated by Genetica (York Court, NC). The cells were routinely screened to confirm mycoplasma-negative status and to verify their identity by short tandem repeat (STR) profiling before being frozen. Each vial was thawed and maintained in culture for a maximum of 2 months. Enough frozen vials of each cell line were available to ensure that all cell-based experiments were conducted on cells that had been tested and in culture for 8 weeks or less. All cells were cultured at 37 °C in a 5% CO2 humidified incubator. The CP-B and CP-C cells were cultured in keratinocyte serum free medium supplemented with human recombinant epidermal growth factor and bovine pituitary extract (Life technologies, Thermo Fisher Scientific, MA) [22]. SKGT-4 and OE33 BE-associated EAC cells were grown in RPMI-1640 medium supplemented with 10% FBS and 1% antibiotics penicillin-streptomycin solution (GenDEPOT, Barker, TX).
2.3. Esophageal biopsy and peripheral blood samples
In the previously reported clinical trial (clinical trial registration number NCT00474903) [15], willing, consented participants underwent esophagogastroduodenoscopy (EGD) with biopsies and a peripheral blood draw prior to randomly assigned, 28-day intervention in one of three: Arm A (ASA placebo + PPI), ASA 81 mg placebo every day + ASA 325 mg placebo every day + esomeprazole 40 mg twice daily (n = 30); Arm B (low-dose ASA + PPI), ASA 81 mg every day + ASA 325 mg placebo every day + esomeprazole 40 mg twice daily (n = 47); and Arm C (high-dose ASA + PPI), ASA 81 mg placebo every day + ASA 325 mg every day + esomeprazole 40 mg twice daily (n = 45). EGD and venipuncture were repeated post-intervention; however, the post-intervention blood draw was not mandatory. Following collection, esophageal biopsies were formalin-fixed and paraffin-embedded per standard clinical protocols. Plasma samples were collected in EDTA tubes, processed and frozen (−20 °C or colder) in cryovials onsite, and shipped to Mayo Clinic for subsequent storage. For this study, a random sample of 20 patients from each treatment arm (60 patients total) was selected from a previously reported randomized clinical trial [15], where the 60 patients had the required pre- and post-treatment serum that was used for analysis. Tissue biopsy samples were analyzed for 49 of the original 60 patients, because only 49 participants had paraffin blocks with sufficient pre- and post-tissue samples from which we were able to cut slides for analysis. These additional tissue samples were analyzed because the results of the blood analyses were promising. Samples were obtained after informed consent was obtained as per Mayo IRB protocol #16–001,992 and 06–003,697.
2.4. Measurement of PGs
The measurement of plasma PGs was performed using enzyme immunoassay kits from Cayman Chemical Company (Ann Arbor, MI) following the manufacturer's instructions. Because PGD2, PGF2α, PGI2, and TXA2 are unstable in vivo, we measured their corresponding primary metabolites in patient serum samples as follows: 11-beta-PGF2α, 13,14-dihydro-15-keto-PGF2α, 6-keto-PGF1α, and TXB2, respectively. To perform principal component analysis (PCA), we first log2-transformed the sample matrix. Next, we utilized the R package "devtools", "factoextra" and "ggfortify" to perform PCA and create the two-dimensional PCA plot.
For the mouse study, blood was collected from the cheek (pre-treatment) or heart (post-treatment). The blood was drawn into a plasma separation tube containing heparin (10 μL, 50 mg/mL). Blood samples were then centrifuged at 2000 × g for 15 min. The measurement of TXA2 was performed using enzyme immunoassay kits from Cayman Chemical Company following the manufacturer's instructions.
2.5. Animals and treatment
All animal studies were approved by the University of Minnesota Institutional Animal Care and Use Committee (IACUC). The animals were housed in climate-controlled quarters with a 12-h light/12-h dark cycle. The mice were maintained and bred under virus- and antigen-free conditions.
The gastroesophageal reflux disease mouse model [23] (Protocol ID: 1501–32258A) was established to study the effects of ASA on BE and EAC development. Male C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Each mouse (7–9 weeks old) was anesthetized by inhalation of isoflurane. The midline abdominal cavity was opened with an incision of 4 mm at the esophagogastric junction, and a loop of duodenum was anastomosed to the esophagogastric junction. All sutures were interrupted 8–0 sutures and before closure of the abdominal wall, 1 mL of 0.9% NaCl was infused into the peritoneal cavity. The celiotomy was closed using 5–0 polypropylene sutures. A sham group was created as a control in which the mouse was anesthetized by inhalation of isoflurane. The midline abdominal cavity was opened and before closure of the abdominal wall, 1 mL of 0.9% NaCl was also infused into the peritoneal cavity. The celiotomy was closed using 5–0 polypropylene sutures. The level of anesthesia was monitored using toe pinch reflexes every 10–15 min during surgery. The analgesic agent buprenorphine SR (1 mg/kg B.W., Zoopharm, Windsor, CO) was administered by intraperitoneal injection prior to surgery and was continued for 72 h. At 36 weeks after the surgical procedure, blood was taken from the cheek of the mouse. The mice were then divided into 3 groups: 1) surgery-vehicle-treated; 2) surgery-ASA-treated; and 3) sham-vehicle-treated. The mice were administered ASA (100 mg/kg B.W) in PBS with 2.5% dimethyl sulfoxide (DMSO), 5% polyethyleneglycol 400 (PGE 400), and 5% Tween 80 or vehicle once a week for 16 weeks. The dose used in this study (100 mg/kg/day) can be translated to a clinical dose of 486 mg (60 kg person) for average body surface area or approximately one ASA tablet taken for analgesic purposes in humans [24]. Mice were monitored every day, weighed once a week, and euthanized by CO2 asphyxiation at 52 weeks after surgery. The blood and esophageal tissues were harvested for further analysis. Tissue lysates were prepared from pooled esophageal tumor nodules or normal esophageal tissue from each mouse of each group. Three sets were prepared for each group and each lane shows 1 set of pooled samples after Western blotting or RT-PCR.
For the xenograft mouse model (Protocol ID: 1803–35739A), female (6 weeks old) athymic nude mice (Jackson Laboratory) were divided into 6 groups (n = 7 per group). SKGT-4 or OE33 (shCon, shTBXAS#1, or shTBXAS#2) human BE-associated EAC cells (4 × 106/0.1 mL) were injected subcutaneously into the right flank of each mouse. Tumor and body weight measurements were obtained every week. For the SKGT-4 cells xenograft model, the tumor grown 22 days. For the OE33 cells xenograft model, the tumor grown 41 days.
2.6. Additional methods
Lentiviral infection, Western blotting, RT-PCR, Kinase phosphorylation profiling, immunofluorescence, and immunohistochemical analysis were performed using standard techniques. Proliferation was estimated by crystal violet assay and anchorage-independent growth was measured by soft agar cell growth assay. Details of the re-intervention endoscopy and post-intervention evaluation in the clinical study are included in Supplementary Materials and Methods.
2.7. Statistical analysis
All quantitative data are expressed as mean values ± standard deviation (S.D.) or standard error (S.E.) of at least 3 independent experiments. Significant differences were determined by one-way ANOVA, Kruskal Wallis test, Fisher Exact test, Wilcoxon signed rank test, or Wilcoxon rank sum test using SPSS software. A probability value of p < 0.05 was used as the criterion for statistical significance.
For the power analysis, R package “pwr” was utilized to calculate the sample size in xenograft animal study. The test type was one-way ANOVA test, significant level was 0.05, power was 0.8, and the estimated effect size was calculated by Cohen's d = |V2 - V1| ⁄ SDpooled. R package “samplesize” was utilized to calculate the sample size in surgery animal study. Significant level was 0.05, power was 0.8, sample size fraction was n/N = 1/2, where n was sample size of ASA group and N was the total sample size.
3. Results
3.1. The COX1/2-driven TXA2 signaling pathway is associated with BE and EAC development
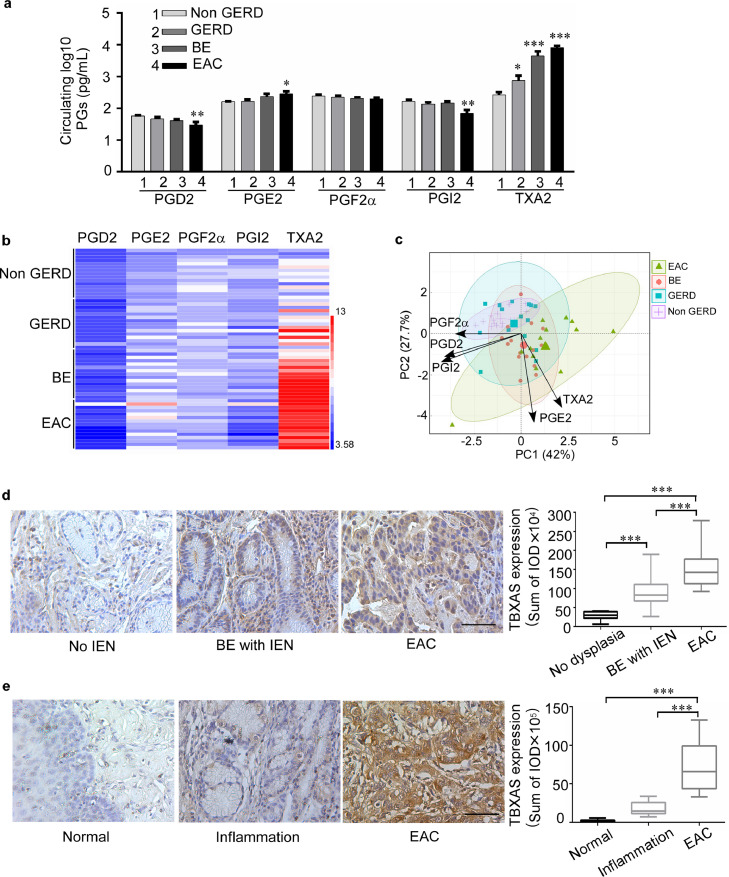
We first determined the protein expression level of COX1 and COX2 in non-(intraepithelial neoplasia) IEN, BE with IEN, and EAC patient esophageal biopsy samples (Supplementary Fig. 1a, b). Results indicated that increased the expression of COX2 is seen in EAC and BE patients with IEN than the patients with no evidence of IEN. The IOD value was analyzed from 3 different fields of each tumor tissue using the Image-Pro-PLUS (v.6) computer software program followed its protocol. In contrast, COX1 did not exhibit a difference during the histological progression. In addition, we analyzed the profiles of circulating PGs in patients with GERD, BE, and EAC. The baseline demographics of participants are shown in Supplementary Table 1. Among the five major bioactive PGs examined, TXA2 and not PGE2, was the most abundant PG in plasma from GERD, BE, and EAC patients (Fig. 1a, b). Compared with the non-GERD group, PGE2 and TXA2 were significantly elevated in the GERD, BE, and EAC groups, whereas PGD2, PGF2α, and PGI2 levels showed little change. Intriguingly, circulating PGE2 was moderately elevated at the BE and EAC stages, whereas circulating TXA2 was elevated through all stages of GERD, BE, and EAC. Notably, circulating TXA2 levels were markedly increased by 23.4-fold in BE patients and 27.9-fold in EAC patients compared to the level in non-GERD subjects. In contrast, the circulating PGE2 levels were only enhanced by 2.1-fold in BE and 2.5-fold in EAC. In addition, the principal component analysis (PCA) of PG levels shown that GERD, BE and EAC development was closely associated with TXA2 levels. Meanwhile PGE2 also shown the association with the disease, but the correlation is obvious less than TXA2 (Fig. 1c). To confirm the importance of the TXA2 pathway in BE and EAC development, we then examined the expression of TBXAS (a key enzyme for TXA2 biosynthesis) in biopsy samples (Fig. 1d). Our immunohistochemical staining results clearly showed that TBXAS was highly expressed in esophageal samples from patients with IEN, or EAC. Similar results were also observed in a human esophageal tissue array (Fig. 1e; Supplementary Fig. 1c), in which TBXAS was highly expressed in inflammation and EAC. The results showed that TBXAS mainly expressed in mucous gland, EAC cells and immune cells. Overall, these results indicate that the COX1/2-driven TXA2 pathway is associated with BE and EAC development.
Fig. 1.
The COX1/2-driven TXA2 pathway is associated with BE and EAC development. a, circulating PG biosynthesis in GERD, BE, and EAC; (1 = non-GERD, n = 15; 2 = GERD, n = 15; 3 = BE, n = 15; and 4 = EAC, n = 15). Plasma samples were collected for measurement of circulating PG levels by using an enzyme immunoassay kit. summary data are presented as means ± S.E. b, Heat map across all the samples showed the PG levels (log2 scale). c, Principal component analysis (PCA) of PG levels. The first two components shown explain the largest part of the variation in PG levels (log2). Individual insets are color-coded by Non GERD (purple), GERD (blue), BE (red) and EAC (Green). d, expression of TBXAS in non-IEN (n = 10), BE with IEN (n = 20), and EAC (n = 10; scale bar 50 μm) patient esophageal samples. e, expression of TBXAS in normal and EAC esophageal tissues (ES809), which include normal adjust esophageal tissues (n = 10), inflamed esophageal tissues (n = 10), and EAC tissues (n = 14). TBXAS protein detection was accomplished using immunohistochemistry. Density scores were obtained for each sample. The asterisks indicate a significant difference compared to the group of control subjects (*, p < 0.05; **, p < 0.01 and ***, p < 0.001, one-way ANOVA).
3.2. The COX1/2-driven TXA2 pathway mediates BE and EAC cell growth through ERKs and STAT3 pathways
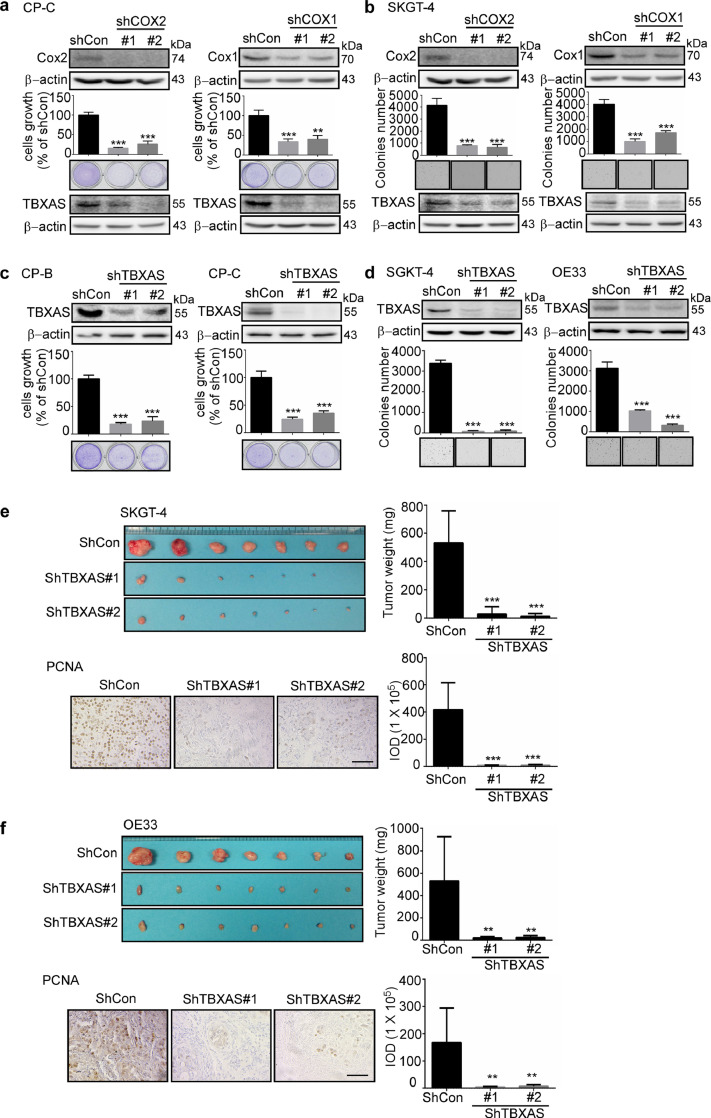
Uncontrolled cell growth and abnormalities in differentiation and survival are hallmarks of cancer. We conducted experiments to clarify the importance of the COX1/2-driven TXA2 pathway in BE and EAC cell growth. We first used two different small hairpin (sh)RNA sequences to generate COX1 or COX2 knockdown BE and EAC cells, respectively (Fig. 2a, b; Supplementary Fig. 2). Crystal violet and anchorage-independent cell growth assays were performed to evaluate the effect of knocking down COX1 or COX2 expression on cell growth. The results showed that knockdown of COX1 or COX2 expression in human BE and EAC cells resulted in decreased growth compared with mock control (shCon) cells. Interestingly, knocking down expression of COX1 or COX2 decreased the expression level of TBXAS in BE and EAC cells. We next determined whether TBXAS is directly associated with BE or EAC cell growth. We generated TBXAS knockdown in BE and EAC cells and the results showed that deficient TBXAS expression also results in reduction of BE and EAC cell growth (Fig. 2c, d). Furthermore, we used a xenograft mouse model to study the function of TBXAS in the growth of 2 EAC (SKGT-4 and OE33) cell lines. The results showed that knocking down TBXAS expression significantly decreased tumor size and weight (Fig. 2e, f). In addition, compared with control (shCon) cells, knocking down TBXAS levels decreased the expression of proliferating cell nuclear antigen (PCNA), which is a cell cycle–related antigen and has been used for the evaluation of the proliferation ability of tumors [25]. Collectively, these results suggested that blocking TBXAS significantly reduced the malignant potential of EAC.
Fig. 2.
The COX1/2-driven TXA2 pathway mediates BE and EAC cell growth. a, b knocking down expression of COX2 or COX1 suppresses BE and EAC cell growth and the expression level of TBXAS. CP-C (a) and SKGT-4 (b) cells with stable knockdown of COX2 and COX1 were established. The expression of COX2 and COX1 was determined by Western blotting. The CP-C cell growth was determined using crystal violet staining and SKGT-4 cell growth was determined using the anchorage-independent growth assay. Colonies were counted using a microscope and the Image-Pro-Plus (v.6) computer software program. The expression of TBXAS and β-actin was determined by Western blotting. c, d, knocking down TBXAS suppresses BE and EAC cell growth. Barrett's esophagus cells CP-B and CP-C (c), and esophageal adenocarcinoma cells SKGT-4, and OE33 (d) with stable knockdown of TBXAS were established. The expression of TBXAS was determined by Western blotting. The BE cell growth was determined using crystal violet staining and EAC cell growth was determined using the anchorage-independent growth assay. e, f, knocking down TBXAS decreases tumor growth in a xenograft mouse model. SKGT-4 (e) or OE33 (f) cells with stable knockdown of TBXAS or control (4 × 106/0.1 mL) were injected subcutaneously into the right flank of each mouse. After 24 and 49 days, respectively, tumors and tumor weight were measured and the expression of PCNA was detected by using immunohistochemistry staining (scale bar = 100 μm). Density scores were obtained for each sample. Data are presented as mean values ± S.D. from triplicate experiments. The asterisks indicate a significant difference compared to the shCon group (*, p < 0.05; **, p < 0.01 and ***, p < 0.001, one-way ANOVA).
Kinase phosphorylation profiling results showed that knocking down expression of TBXAS suppressed ERKs, MSK1/2, CREB, c-Jun and STAT3 activation (Supplementary Fig. 3). ERKs play an important role in BE and EAC development and are associated with the regulation of inflammation by NFκB. Bile acids are often refluxed into the lower esophagus and are candidate carcinogens in the development of EAC. We utilized one of the commonly refluxed bile acids, deoxycholic acid (DCA; 250 μM), to mimic bile reflux. Our results showed that DCA enhanced the activation of ERKs and NFκB in BE (CP-C) and EAC (SKGT-4) cells. Importantly, knocking down the expression of TBXAS decreased ERKs activation (Supplementary Fig. 4a, b) and NFκB p65 nuclear translocation (Supplementary Fig. 4c, d). Overall, we conclude that the COX1/2-driven TXA2 pathway mediates BE and EAC cell growth by suppressing ERKs and NFκB activation.
3.3. ASA targets the COX1/2-driven TXA2 pathway to influence BE and EAC development in mice
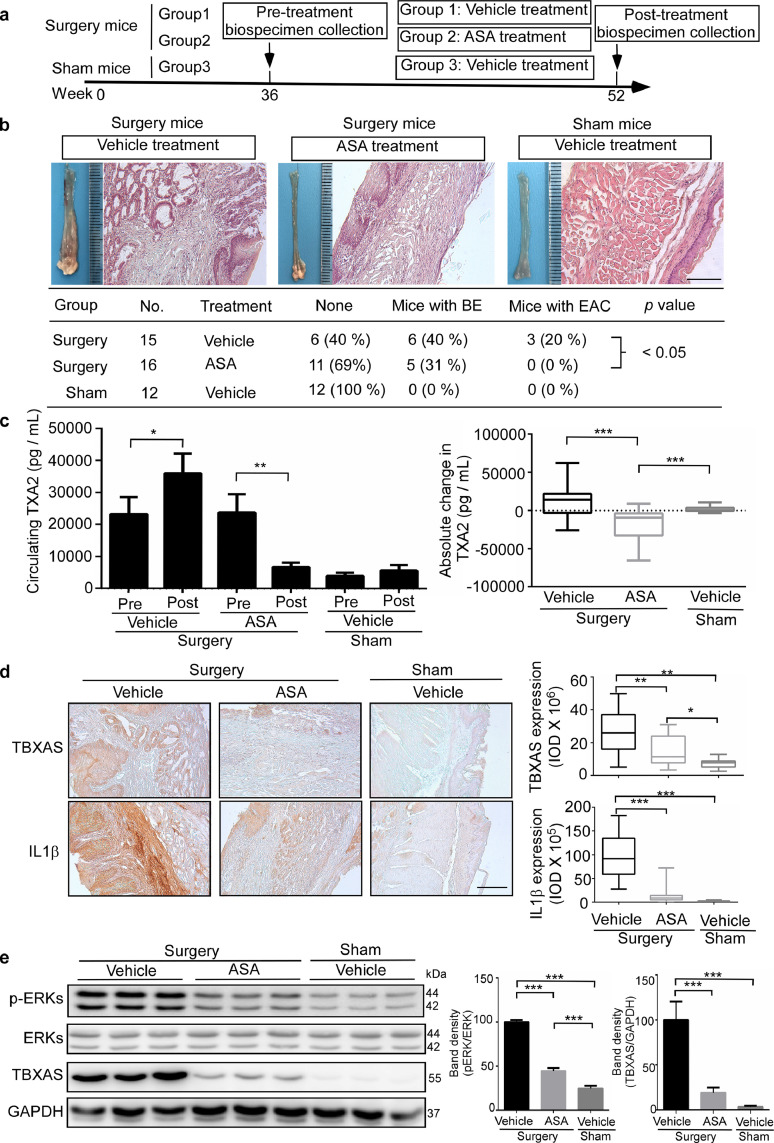
ASA is a well known COX1 and COX2 enzyme inhibitor and might play a protective role against the development of BE and EAC. In the present study, the surgical mouse model of esophagoduodenostomy was established to study the effect of ASA on BE and EAC development. At 36 weeks after surgery, blood was taken from the cheek of each mouse. The mice were randomized and administered ASA (100 mg/kg, B.W.) or vehicle daily for 16 weeks. Mice were euthanized by CO2 asphyxiation at 52 weeks after surgery (Fig. 3a). Analysis of pathology showed that ASA significantly decreased the incidence of BE and EAC compared with the vehicle-treated group (Fig. 3b). In the surgical-vehicle-treated group (n = 15), the number of mice developing BE was 6/15 or 40%, whereas the number developing EAC was 3/15 or 20%. In the surgery-ASA-treated group (n = 16), the number of mice developing BE was 5/16 or 31% and no mice developed EAC (0/16). All mice in the sham-vehicle-treated group (n = 12) were disease-free. We then measured the TXA2 level in mouse plasma. ASA significantly decreased the TXA2 level in plasma compared with the surgery-vehicle-treated group (Fig. 3c). Additionally, immunohistochemistry results showed that in the surgery groups, ASA significantly decreased TBXAS and IL1β expression (Fig. 3d). Furthermore, Western blotting showed that ASA inhibits TBXAS expression and ERKs activation (Fig. 3e). RT-PCR results indicated that ASA significantly decreased mRNA levels of IL1β, TNFα, and IL6 compared to the surgery-vehicle-treated group (Supplementary Fig. 5). Collectively, these results suggested that ASA inhibits the COX1/2-driven TXA2 pathway and reduced the development of BE and EAC.
Fig. 3.
ASA targets the COX1/2-driven TXA2 pathway and mediates BE and EAC development in a mouse model. a, esophagoduodenostomy was conducted as an established GERD mouse model to study the effect of ASA on BE and EAC development in C57BL/6 mice. At 36 weeks after surgery, blood was taken from the cheek of each mouse. The mice were administered ASA (100 mg/kg B.W.) in PBS with 2.5% dimethyl sulfoxide (DMSO), 5% polyethyleneglycol 400 (PGE 400), and 5% Tween 80 or vehicle daily for 16 weeks. Mice were euthanized by CO2 asphyxiation at 52 weeks after surgery. b, hematoxylin and eosin (H&E) staining was used to visualize and analyze pathology (scale bar = 100 μm). Significant differences between the vehicle group and ASA-treated group were determined by Wilcoxon rank sum test. c, circulating levels of TXA2 in pre-treated and post-treated mouse plasma was detected by using an enzyme immunoassay kit. Significant differences were determined by Wilcoxon signed rank test and Wilcoxon rank sum test. d, the expression of TBXAS and IL1β was determined by using immunohistochemistry staining. Density scores were obtained for each sample and significant differences were determined by one-way ANOVA. e, ERKs phosphorylation and TBXAS levels were determined by Western blotting. Density scores were obtained for each sample and data are presented as mean values ± S.D. The asterisks indicate a significant difference compared to the control group (*, p < 0.05; **, p < 0.01 and ***, p < 0.001, b, Wilcoxon rank sum test; c, Wilcoxon signed rank test and Wilcoxon rank sum test; d and e, one-way ANOVA).
3.4. ASA targets the COX1/2-driven TXA2 pathway to reduce inflammation in BE patients
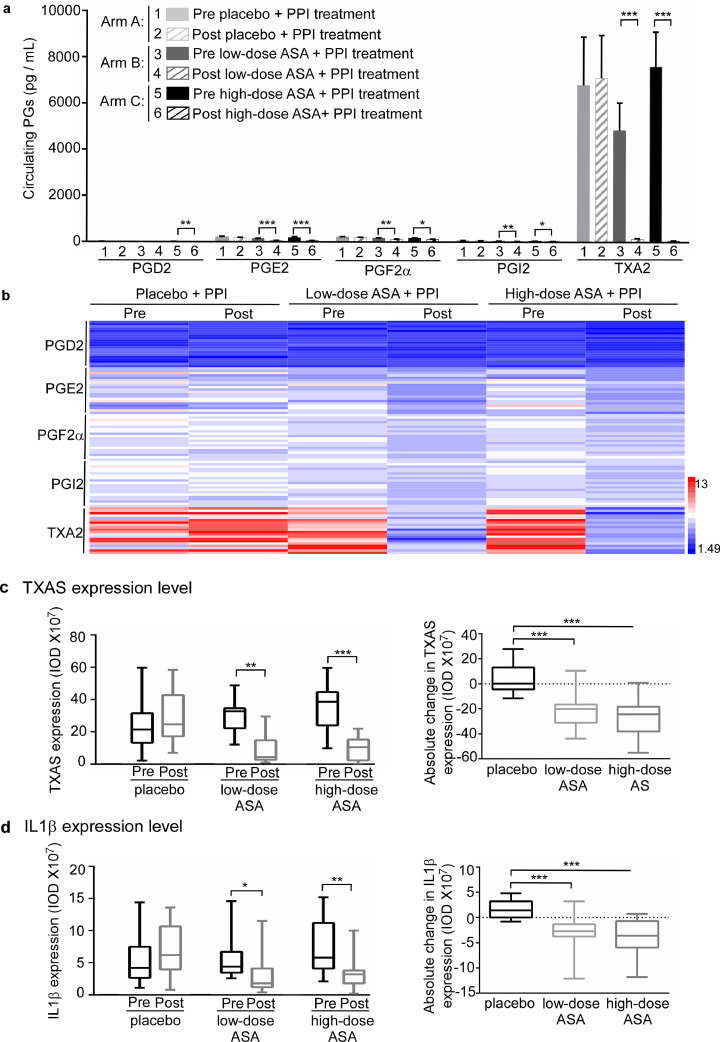
A random 60 patients samples obtained from the previously conducted clinical trial [15], Intervention arms were evenly balanced with respect to age (p = 0.7183, Kruskal Wallis), sex (p = 0.8998, Fisher Exact), and length of BE (p = 0.6822, Fisher Exact). Other baseline variables were similar between the intervention arms (Table 1). The circulating TXA2 levels were substantially higher than any of the other 4 PGs (Fig. 4a, b). Importantly, both lower-dose (81 mg per day) + PPI and higher-dose ASA (325 mg per day) + PPI significantly decreased the average level of TXA2 from 4835.1 pg/mL to 142.4 pg/mL (88.9 ± 23.2% decrease, p < 0.001, Wilcoxon rank sum test) and from 7602.9 pg/mL to 66.7 pg/mL (96.3 ± 5.9% decrease, p < 0.001, Wilcoxon rank sum test), respectively. In addition, both lower- and higher-dose ASA + PPI significantly decreased the absolute change between post-treatment and pre-treatment TXA2 by 4692.7 ± 5400.5 pg/mL (p = 0.001, vs placebo + PPI group) and 7536.2 ± 6722.2 pg/mL (p < 0.001, vs placebo + PPI group, Wilcoxon rank sum test), respectively. However, the ASA placebo + PPI did not significantly affect the TXA2 level between pre- and post-treatment (Supplementary Fig. 6a, b; Table 2). In the ASA-treated groups, the level of the other 4 PGs also decreased. However, the changes are obviously less than the changes in the TXA2 level (Supplementary Tables 2–5). Additionally, we conducted an immunohistochemistry assay to compare pre- and post-treatment biopsy specimen expression levels of TBXAS and IL1β in BE patients. ASA significantly decreased the expression level of TBXAS and IL1β, (Fig. 4c, d; Supplementary Fig. 6c, d) in BE esophageal tissues. Overall, our findings indicated that inhibition of COX1/2-TBXAS signaling limited inflammation and suppressed BE and EAC development.
Table 1.
Baseline demographics, intervention adherence, and adverse events by intervention arm.
| Arm A Placebo (n = 20) | Arm B Low-dose aspirin (n = 20) | Arm C High-dose aspirin (n = 20) | Total (n = 60) | p value | |
|---|---|---|---|---|---|
| Age (y) | 0.7185a | ||||
| Mean (S.D.) | 61.7 (12.0) | 58.1 (12.6) | 60.2 (11.1) | 60.0 (11.8) | |
| Median | 61.0 | 59.5 | 62.0 | 61.0 | |
| Range | (32.0–86.0) | (34.0–79.0) | (35.0–80.0) | (32.0–86.0) | |
| Sex, n (%) | 0.8998b | ||||
| Female | 2 (10.0%) | 4 (20.0%) | 3 (15.0%) | 9 (15.0%) | |
| Male | 18 (90.0%) | 16 (80.0%) | 17 (85.0%) | 51 (85.0%) | |
| ECOG performance score, n (%) | 1.0000b | ||||
| 0 | 19 (95.0%) | 20 (100.0%) | 20 (100.0%) | 59 (98.3%) | |
| 1 | 1 (5.0%) | 0 (0.0%) | 0 (0.0%) | 1 (1.7%) | |
| Length of Barrett's segment, n (%) | 0.6822b | ||||
| < 5 cm if circumferential involvement | 9 (45.0%) | 9 (45.0%) | 12 (60.0%) | 30 (50.0%) | |
| > = 5 cm of circumferential involvement | 11 (55.0%) | 11 (55.0%) | 8 (40.0%) | 30 (50.0%) | |
| BMI | 0.1715a | ||||
| Mean (S.D.) | 28.0 (2.7) | 29.0 (4.5) | 30.3 (5.1) | 29.1 (4.3) | |
| Median | 27.5 | 28.6 | 29.1 | 28.1 | |
| Range | (25.3–36.5) | (22.4–40.4) | (23.4–43.8) | (22.4–43.8) | |
| Prior history of NSAID use, n (%) | 0.3103b | ||||
| No | 20 (100.0%) | 19 (95.0%) | 17 (85.0%) | 56 (93.3%) | |
| Yes | 0 (0.0%) | 1 (5.0%) | 3 (15.0%) | 4 (6.7%) | |
| Intervention Adherence: | |||||
| No. of Esomoprazole run-in pills | 0.5812a | ||||
| Mean (S.D.) | 55.7 (3.5) | 56.8 (3.8) | 56.4 (4.5) | 56.3 (3.9) | |
| Median | 55.5 | 57.0 | 56.0 | 56.0 | |
| Range | (49.0–62.0) | (49.0–66.0) | (46.0–64.0) | (46.0–66.0) | |
| Esomeprazole run-in compliance | 0.8022a | ||||
| Mean (S.D.) | 99.9 (0.4) | 99.8 (0.5) | 99.3 (2.6) | 99.7 (1.6) | |
| Median | 100.0 | 100.0 | 100.0 | 100.0 | |
| Range | (98.1–100.0) | (98.2–100.0) | (88.5–100.0) | (88.5–100.0) | |
| No. of Esomoprazole intervention pills | 0.9126a | ||||
| Mean (S.D.) | 55.9 (3.9) | 56.3 (5.6) | 56.0 (6.7) | 56.1 (5.4) | |
| Median | 56.0 | 56.0 | 56.5 | 56.0 | |
| Range | (49.0–64.0) | (48.0–68.0) | (37.0–68.0) | (37.0–68.0) | |
| Esomeprazole intervention compliance | 0.4615a | ||||
| Mean (S.D.) | 99.4 (1.9) | 99.8 (0.9) | 98.6 (6.5) | 99.3 (3.9) | |
| Median | 100.0 | 100.0 | 100.0 | 100.0 | |
| Range | (91.7–100.0) | (96.2–100.0) | (71.2–100.0) | (71.2–100.0) | |
| No. of Aspirin 81 mg/placebo intervention pills | 0.8260a | ||||
| Mean (S.D.) | 27.8 (1.7) | 28.1 (2.7) | 28.2 (2.3) | 28.0 (2.2) | |
| Median | 28.0 | 28.0 | 28.0 | 28.0 | |
| Range | (25.0–32.0) | (24.0–34.0) | (25.0–33.0) | (24.0–34.0) | |
| Aspirin 81 mg/placebo compliance | 0.3555a | ||||
| Mean (S.D.) | 99.0 (3.7) | 99.8 (0.9) | 100.0 (0.0) | 99.6 (2.2) | |
| Median | 100.0 | 100.0 | 100.0 | 100.0 | |
| Range | (83.9–100.0) | (96.0–100.0) | (100.0–100.0) | (83.9–100.0) | |
| No. of Aspirin 325 mg/placebo intervention pills | 0.9346a | ||||
| Mean (S.D.) | 27.8 (1.8) | 28.2 (2.7) | 28.1 (2.2) | 28.0 (2.2) | |
| Median | 28.0 | 28.0 | 28.0 | 28.0 | |
| Range | (25.0–32.0) | (24.0–34.0) | (25.0–33.0) | (24.0–34.0) | |
| Aspirin 325 mg/placebo Compliance | 0.9989a | ||||
| Mean (S.D.) | 99.8 (0.8) | 99.8 (0.9) | 99.8 (0.8) | 99.8 (0.8) | |
| Median | 100.0 | 100.0 | 100.0 | 100.0 | |
| Range | (96.4–100.0) | (96.0–100.0) | (96.6–100.0) | (96.0–100.0) |
Kruskal Wallis.
Fisher Exact.
Fig. 4.
Effects of ASA on circulating PG levels and the expression of TBXAS and IL1β in a human clinical trial. a, effects of regular ASA use on circulating PG biosynthesis in pre- and post-treated BE patients were assessed by using an enzyme immunoassay kit: 1 = pre placebo + PPI treatment; 2 = post placebo + PPI treatment; 3 = pre low-dose (81 mg) + PPI treatment; 4 = post low-dose (81 mg) + PPI treatment; 5 = pre high-dose (325 mg) + PPI treatment; 6 = post high-dose (325 mg) + PPI treatment. The summary data are presented as mean values ± S.E. The asterisks indicate a significant difference compared to the pre-treated group. Significant differences were determined by Wilcoxon signed rank test. b, the heat map across all the samples showed the PG levels (log2 scale). c, d, ASA decreases the expression of TBXAS and IL1β in the biopsy specimens from BE patients. An immunohistochemistry assay was conducted to compare expression levels of TBXAS (c) and IL1β (d) in pre-treated and post-treated biopsy specimens from BE patients. Density scores were obtained from each sample and statistical significance was determined by Wilcoxon signed rank test and Wilcoxon rank sum test. Data are presented as mean values ± S.E. from triplicate experiments. The asterisks indicate a significant difference compared to the pre-treated group (*, p < 0.05; **, p < 0.01 and ***, p < 0.001, a, Wilcoxon signed rank test; c and d, Wilcoxon signed rank test and Wilcoxon rank sum test).
Table 2.
TXA2 summary in all 60 evaluable participants.
| TXA2 measurement | Arm AAspirin placebo + PPI(n = 20) | Arm BLow-dose aspirin + PPI(n = 20) | Arm CHigh-dose aspirin + PPI(n = 20) |
|---|---|---|---|
| Pre-treatment | |||
| Mean (S.D.) | 6800.7 (9307.0) | 4835.1 (5401.7) | 7602.9 (6724.6) |
| Median (range) | 2954.4 (104.8 to 33,277.1) | 2876.4 (144.5 to 20,813.6) | 5180.9 (219.5 to 19,871.5) |
| Post-treatment | |||
| Mean (S.D.) | 7111.0 (8286.7) | 142.4 (93.4) | 66.7 (24.0) |
| Median (range) | 4102.5 (179.0 to 30,121.6) | 117.5 (43.5 to 415.2) | 61.1 (31.5 to 119.7) |
| Absolute change | |||
| Mean (S.D.) | 310.3 (5469.6) | −4692.7 (5400.5) | −7536.2 (6722.2) |
| Median (range) | 211.7 (−10,078.9 to 16,905.9) | −2776.1 (−20,675.2 to 4.8) | −5071.3 (−19,813.9 to 168.8) |
| Within-arm signed rank p value | p = 0.765 | p < 0.001 | p < 0.001 |
| Wilcoxon rank sum p value | vs aspirin placebo | vs aspirin placebo | |
| p = 0.001 | p <0.001 | ||
| low-dose vs high-dose aspirin | |||
| p = 0.201 | |||
| Percent change | |||
| Mean (S.D.) | 182.4 (379.1) | −88.9 (23.2) | −96.3 (5.9) |
| Median (range) | −2.2 (−66.3 to 1343.1) | −96.4 (−99.3 to 3.3) | −98.8 (−99.7 to −76.9) |
| Within-arm signed rank p value | p = 0.179 | p < 0.001 | p < 0.001 |
| Wilcoxon rank sum p value | vs aspirin placebo | vs aspirin placebo | |
| p < 0.001 | p < 0.001 | ||
| Low dose vs high-dose aspirin | |||
| p = 0.015 | |||
Note: all values are expressed as picograms per milliliter.
4. Discussion
In the present study, we provide new evidence demonstrating that TXA2 may be a potential biomarker for the early detection of BE and EAC and that the COX1/2-driven TXA2 pathway is a possible drug target for improved outcomes of BE and EAC patients. Remarkably, over the past 40 years, the incidence of EAC has increased more than 6-fold in Western countries [26]. The strongest known risk factor for EAC is GERD, alongside its more severe manifestation, BE [4,5,27,28]. EAC is a lethal disease with a 5-year survival rate of less than 40% even combined chemotherapy and surgery [29]. Survival is stage-dependent and early spread before the onset of symptoms is common with this tumor. Currently, the best hope for improved survival of patients with EAC is detection at an early and potentially curable stage. Our findings might provide a strategy that will benefit patients with GERD, BE, and even EAC.
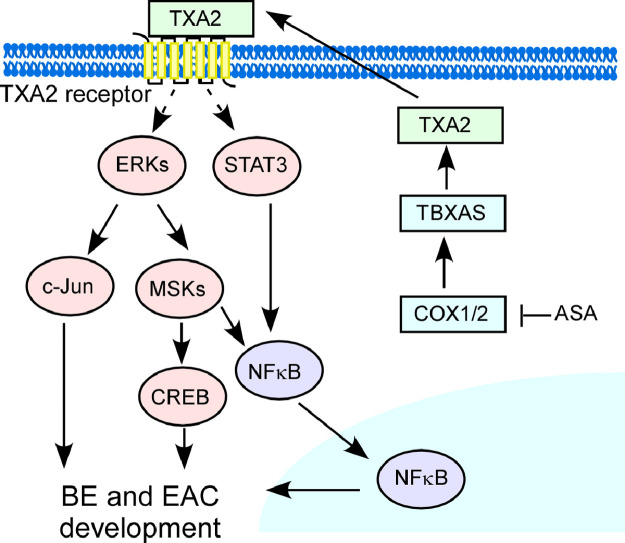
COX1/2, PGs, and PG-endoperoxide synthases are connected to various pathological processes [10,11]. Previous study showed that COX1/2 inhibitors and PGE2 receptor antagonist AH-23848B suppressed tumor growth in a xenograft derived from OE33 cells. However, the PGE2 receptor antagonist AH-23848B failed to significantly inhibit tumor growth in this model [30]. This result indicates that PGE2 pathway might not be the major way for mediating EAC development. Our findings provide new evidence showing that the COX1/2-driven TXA2 pathway is involved in BE and EAC pathophysiology (Fig. 1d, e; Supplementary Fig. 1). Notably, the development of BE and EAC is associated with higher circulating TXA2 levels (Fig. 1a, b, c), which merits further investigation as a predictor of EAC risk. PGE2 and PGES have been associated with BE and EAC [13,14]. Importantly, we found that the circulating PGE2 level was also increased in BE and EAC patients, but by only 2- to 3-fold compared with non-GERD subjects. In contrast, the circulating TXA2 level was markedly increased in GERD, BE, and EAC patients by 20- to 30-fold compared with non-GERD subjects. These results provide compelling evidence supporting TXA2 as a potential biomarker for the early detection of BE and EAC and that the TXA2 pathway might play a role in the development of BE and EAC. However, the current trial was not organized as a longitudinal study. The levels of TXA2 in those who will progress compared to those who do not progress to EAC, need to assess in another independent study cohort. COX1 is referred to as a “constitutive isoform”, and is expressed in most tissues under basal conditions. In contrast, COX2 is referred to as an “inducible isoform”, which is believed to be undetectable in most normal tissues, but can be up-regulated during various conditions, many of them pathological [31]. Our results suggest that either or both COX1 and COX2 mediate BE and EAC cell growth through the TXA2 pathway, and COX inhibition results in decreased BE and EAC cell growth and reduced EAC xenograft tumor growth (Fig. 2). Our kinase phosphorylation profiling results showed that knockdown expression of TBXAS markedly decreased the activation of ERKs, MSK1/2, CREB, c-Jun and STAT3. ERKs serve as a central intermediate in the mitogen-activated protein kinase (MAPK) pathways, participating in the regulation of cell proliferation, survival, and differentiation [32,33]. It is well known that ERKs-MSK1/2 protein kinase cascade induces CREB activation, which are involved in NFκB transactivation and c-Jun activation [34], [35], [36], [37]. Additionally, STAT3-NFκB signalling pathway is associated with BE [38]. Recent investigations suggest that the NFκB signaling cascade might be a central mediator of gastrointestinal malignancies, including esophageal cancer [39,40]. We suggest that the TXA2 pathway effectively mediates ERKs and STAT3 signaling pathways in BE and EAC development (Fig. 5;Supplementary Fig. 3, 4).
Fig. 5.
Schematic diagram illustrating the COX1/2-driven TBXA2 pathway mediating BE and EAC development.
ASA is a clinically used inhibitor to irreversibly inactivate COX1 or COX2. It could be a suitable drug to suppress BE and EAC development by influencing the TXA2 signaling pathway. Indeed, we first successfully used a surgery mouse model of esophagoduodenostomy to demonstrate that ASA could efficiently reduce BE and EAC development. Importantly, ASA decreased the circulating TXA2 level and TBXAS expression level in tissues resulting in decreased ERKs activation and inflammation (Fig. 3; Supplementary Fig. 5). Several observations and bench study results suggested that ASA and other NSAIDs protect against the development of BE and EAC [20,21,[41], [42], [43], [44]. Overall, we suggest that ASA targeting of the COX1/2-driven TXA2 pathway might be a potential strategy for inhibiting BE and EAC development. Whether this impact occurs directly or indirectly (i.e., through platelet-derived signaling) [45], requires further investigation. However, while ASA is effective in suppressing the COX1/2 pathway, more targeted distal inhibition of this pathway focusing on TXA2 synthesis or implementation of function (i.e., using specific inhibitors such as seratrodast) might be more effective, since long-term use of ASA can have serious adverse effects, such as intracerebral hemorrhage and gastrointestinal ulceration.
In the clinic, proton pump inhibitors (PPIs) reduce acid reflux, which is thought to be one of the main drivers of BE. Observational data suggested that patients with BE who are taking PPIs have reduced neoplastic progression [46], but evidence is not conclusive [47]. ASA, in combination with PPI therapy is a potential esophageal cancer chemopreventive strategy [48]. In this study, we analyzed samples from a clinical trial to study the effects of a combination of ASA and the PPI esomeprazole on the circulating levels of PG in patients with BE. Our results indicate that ASA strongly decreased TXA2 levels and reduced the expression level of TBXAS and IL1β in BE esophageal tissues (Fig. 4; Table 2). In spite of these promising results, this study still has limitations such as small sample size with only 20 participants in each arm. Additionally, we obtained BE tissues from only 49 participants to conduct further analysis. ASA and NSAIDs are available over the counter, thus participants might have taken these drugs without reporting their consumption to the investigators. However, this would have biased the results towards the null hypothesis and therefore would only underestimate the efficacy of ASA. However, we clearly observed that ASA strongly decreased TXA2 pathway signaling.
Additionally, the combination of ASA and esomeprazole chemoprevention in a clinical trial showed that high-dose ASA combined with high-dose PPI significantly reduced the risk of progression to EAC [48]. We suggest that the inhibitory effects might occur mainly through the COX1/2-driven TXA2 pathway. Inhibition of COX1/2-driven TXA2 pathway in the GERD or BE stage should be a promising approach to preventing EAC. Our results also indicated that low-dose ASA also significantly decreased TXA2 levels and thus, low-dose ASA combined with PPI might also be beneficial in BE patients. Further study will be needed to support this idea.
Based on our findings and a recent report [48], ASA should be a suitable drug for targeting the COX1/2-driven TXA2 pathway to inhibit BE and EAC development. Another recent clinical report also showed that 1% of participants exhibited long-term study-treatment-related serious adverse events [48]. These observations caused us to think about novel drug targets that are necessary to reduce or overcome the adverse events associated with ASA alone or a combination of ASA and PPI. Based on our findings, we suggest that enzymes and receptors in the TXA2 pathway might be potential targets for prevention and treatment of BE and EAC. Currently, our group is using a genetic mouse model to determine the function of the TXA2 receptor in BE and EAC development. We are also using patient-derived tumor xenografts (PDX) to study the effects of TXA2 receptor antagonists on EAC development. We hope these efforts will be beneficial in the future to patients with BE and EAC but future experiments will need to be conducted to support these ideas.
In summary, this study established the importance of the COX1/2-driven TXA2 pathway in BE and EAC pathophysiology, and laid the groundwork for introducing a TXA2-targeting strategy to EAC prevention, early detection, and management. ASA targets the COX1/2-driven TXA2 pathway to suppress BE and EAC development. The combination of ASA and PPI effectively decreased the circulating levels of TXA2 and inflammation leading to a suppression of the development of BE. Overall, targeting the COX1/2-driven TXA2 pathway is a potential strategy to suppress BE and EAC development.
Funding sources
This work was supported by the Hormel Foundation (Z. Dong). This work was supported by the grants from Exact Sciences, Pentax Medical, and Intromedic (CP-0016-1 P.G. Iyer). This work was also supported by the National Cancer Institute, Division of Cancer Prevention (contracts HHSN261200421 and N01CN35000 to P.J. Limburg). The funding bodies played no role in study design, data collection, data analysis, interpretation, writing of the report.
Declaration of Competing Interest
Dr. Dong reports grants from Hormel Foundation, during the conduct of the study; Dr. Iyer reports grants from Exact Sciences, grants from Pentax Medical, grants from Intromedic, non-financial support from Medtronic, other from Medtronic, other from CSA Medical, during the conduct of the study; Dr. Limburg reports other from National Cancer Institute, during the conduct of the study; other from Exact Sciences, outside the submitted work.
Acknowledgments
The authors thank Tara Adams for the supporting animal experiments; Becky Earl for assistance in submitting our manuscript; and Dr. Rebecca Morris for providing the Zeiss Axioskop microscope system (The Hormel Institute, University of Minnesota).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2019.10.038.
Appendix. Supplementary materials
References
- 1.Pennathur A., Gibson M.K., Jobe B.A., Luketich J.D. Oesophageal carcinoma. Lancet. 2013;381(9864):400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 2.Lepage C., Rachet B., Jooste V., Faivre J., Coleman M.P. Continuing rapid increase in esophageal adenocarcinoma in England and Wales. Am J Gastroenterol. 2008;103(11):2694. doi: 10.1111/j.1572-0241.2008.02191.x. [DOI] [PubMed] [Google Scholar]
- 3.Reid B.J., Li X., Galipeau P.C., Vaughan T.L. Barrett's oesophagus and oesophageal adenocarcinoma: time for a new synthesis. Nature Rev Cancer. 2010;10(2):87. doi: 10.1038/nrc2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thrift A.P., Kramer J.R., Qureshi Z., Richardson P.A., El-Serag H.B. Age at onset of Gerd symptoms predicts risk of Barrett's esophagus. Am J Gastroenterol. 2013;108(6):915. doi: 10.1038/ajg.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaheen N.J., Richter J.E. Barrett's oesophagus. Lancet. 2009;373(9666):850–861. doi: 10.1016/S0140-6736(09)60487-6. [DOI] [PubMed] [Google Scholar]
- 6.Schlottmann F., Patti M.G. Esophageal adenocarcinoma: pathogenesis and epidemiology. Esophageal Cancer: Springer. 2018:21–28. [Google Scholar]
- 7.Fitzgerald R., Omary M.B., Triadafilopoulos G. Altered sodium-hydrogen exchange activity is a mechanism for acid-induced hyperproliferation in Barrett's esophagus. Am J Physiol. 1998;275(1):G47–G55. doi: 10.1152/ajpgi.1998.275.1.G47. [DOI] [PubMed] [Google Scholar]
- 8.Shirvani V.N., Ouatu-Lascar R., Kaur B.S., Omary M.B., Triadafilopoulos G. Cyclooxygenase 2 expression in Barrett's esophagus and adenocarcinoma: ex vivo induction by bile salts and acid exposure. Gastroenterology. 2000;118(3):487–496. doi: 10.1016/s0016-5085(00)70254-x. [DOI] [PubMed] [Google Scholar]
- 9.Vane J., Bakhle Y., Botting R. CYCLOOXYGENASES 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38(1):97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 10.Wang D., DuBois R.N. Eicosanoids and cancer. Nature Rev Cancer. 2010;10(3):181. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M.-.T., Honn K.V., Nie D. Cyclooxygenases, prostanoids, and tumor progression. Cancer Metastasis Rev. 2007;26(3–4):525. doi: 10.1007/s10555-007-9096-5. [DOI] [PubMed] [Google Scholar]
- 12.Menter D.G., Schilsky R.L., DuBois R.N. Cyclooxygenase-2 and cancer treatment: understanding the risk should be worth the reward. Clinical Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-09-0788. 1078-0432. CCR-09-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang T., Min S., Bae J., Jung K., Lee J., Kim J. Expression of cyclooxygenase 2, microsomal prostaglandin e synthase 1, and EP receptors is increased in rat oesophageal squamous cell dysplasia and Barrett's metaplasia induced by duodenal contents reflux. Gut. 2004;53(1):27–33. doi: 10.1136/gut.53.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaur B.S., Triadafilopoulos G. Acid-and bile-induced PGE2 release and hyperproliferation in Barrett's esophagus are COX-2 and PKC-ε dependent. Am J Physiol. 2002;283(2):G327–GG34. doi: 10.1152/ajpgi.00543.2001. [DOI] [PubMed] [Google Scholar]
- 15.Falk G.W., Buttar N.S., Foster N.R., Ziegler K.L., Demars C.J., Romero Y. A combination of esomeprazole and aspirin reduces tissue concentrations of prostaglandin E(2) in patients with Barrett's esophagus. Gastroenterology. 2012;143(4):917–926. doi: 10.1053/j.gastro.2012.06.044. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H., Liu K., Boardman L.A., Zhao Y., Wang L., Sheng Y. Circulating prostaglandin biosynthesis in colorectal cancer and potential clinical significance. EBioMedicine. 2015;2(2):165–171. doi: 10.1016/j.ebiom.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang R., Li M., Hsin M., Underwood M., Ma L., Mok T. 4-Methylnitrosamino-1-3-pyridyl-1-butanone (NNK) promotes lung cancer cell survival by stimulating thromboxane a 2 and its receptor. Oncogene. 2011;30(1):106. doi: 10.1038/onc.2010.390. [DOI] [PubMed] [Google Scholar]
- 18.Li H., Lee M.-.H., Liu K., Wang T., Song M., Han Y. Inhibiting breast cancer by targeting the thromboxane a 2 pathway. NPJ Precis Oncol. 2017;1(1):8. doi: 10.1038/s41698-017-0011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corley D.A., Kerlikowske K., Verma R., Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology. 2003;124(1):47–56. doi: 10.1053/gast.2003.50008. [DOI] [PubMed] [Google Scholar]
- 20.Omer Z.B., Ananthakrishnan A.N., Nattinger K.J., Cole E.B., Lin J.J., Kong C.Y. Aspirin protects against Barrett's esophagus in a multivariate logistic regression analysis. Clinical Gastroenterol Hepatol. 2012;10(7):722–727. doi: 10.1016/j.cgh.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huo X., Zhang X., Yu C., Cheng E., Zhang Q., Dunbar K.B. Aspirin prevents nf-κb activation and CDX2 expression stimulated by acid and bile salts in oesophageal squamous cells of patients with Barrett's oesophagus. Gut. 2017 doi: 10.1136/gutjnl-2016-313584. gutjnl-2016-313584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suchorolski M.T., Paulson T.G., Sanchez C.A., Hockenbery D., Reid B.J. Warburg and Crabtree effects in premalignant Barrett's esophagus cell lines with active mitochondria. PLoS ONE. 2013;8(2) doi: 10.1371/journal.pone.0056884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He J., Fang Y., Chen X. Surgical models of gastroesophageal reflux with mice. JoVE (Journal of Visualized Experiments). 2015(102):e53012. [DOI] [PMC free article] [PubMed]
- 24.Nair A.B., Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall P., Levison D., Woods A., Yu C.W., Kellock D., Watkins J. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some, neoplasms. J Pathol. 1990;162(4):285–294. doi: 10.1002/path.1711620403. [DOI] [PubMed] [Google Scholar]
- 26.Hur C., Miller M., Kong C.Y., Dowling E.C., Nattinger K.J., Dunn M. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. 2013;119(6):1149–1158. doi: 10.1002/cncr.27834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lagergren J., Bergström R., Lindgren A., Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. New Engl J Med. 1999;340(11):825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 28.Hvid-Jensen F., Pedersen L., Drewes A.M., Sørensen H.T., Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett's esophagus. New Engl J Med. 2011;365(15):1375–1383. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 29.Al-Batran S.-.E., Homann N., Pauligk C., Goetze T.O., Meiler J., Kasper S. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948–1957. doi: 10.1016/S0140-6736(18)32557-1. [DOI] [PubMed] [Google Scholar]
- 30.Santander S., Cebrián C., Esquivias P., Conde B., Esteva F., Jiménez P. Cyclooxygenase inhibitors decrease the growth and induce regression of human esophageal adenocarcinoma xenografts in nude mice. Int J Oncol. 2012;40(2):527–534. doi: 10.3892/ijo.2011.1219. [DOI] [PubMed] [Google Scholar]
- 31.Zidar N., Odar K., Glavac D., Jerse M., Zupanc T., Stajer D. Cyclooxygenase in normal human tissues–is COX‐1 really a constitutive isoform, and COX2 an inducible isoform? J Cell Mol Med. 2009;13(9b):3753–3763. doi: 10.1111/j.1582-4934.2008.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts P.J., Der C.J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26(22):3291. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y., Liu W.-.Z., Liu T., Feng X., Yang N., Zhou H.-.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Receptors Signal Transduction. 2015;35(6):600–604. doi: 10.3109/10799893.2015.1030412. [DOI] [PubMed] [Google Scholar]
- 34.Deak M., Clifton A.D., Lucocq J.M., Alessi D.R. Mitogen and stress activated protein kinase‐1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 1998;17(15):4426–4441. doi: 10.1093/emboj/17.15.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kefaloyianni E., Gaitanaki C., Beis I. ERK1/2 and p38-MAPK signalling pathways, through MSK1, are involved in NF-κB transactivation during oxidative stress in skeletal myoblasts. Cell Signal. 2006;18(12):2238–2251. doi: 10.1016/j.cellsig.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Wiggin G.R., Soloaga A., Foster J.M., Murray-Tait V., Cohen P., Arthur J.S.C. MSK1 and MSK2 are required for the mitogen-and stress-induced phosphorylation of CREB and ATF1 in fibroblasts. Mol Cell Biol. 2002;22(8):2871–2881. doi: 10.1128/MCB.22.8.2871-2881.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez-Bergami P., Huang C., Goydos J.S., Yip D., Bar-Eli M., Herlyn M. Rewired ERK-JNK signaling pathways in melanoma. Cancer Cell. 2007;11(5):447–460. doi: 10.1016/j.ccr.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gibson M.K., Dhaliwal A.S., Clemons N.J., Phillips W.A., Dvorak K., Tong D. Barrett's esophagus: cancer and molecular biology. Ann N Y Acad. Sci. 2013;1300(1):296–314. doi: 10.1111/nyas.12252. [DOI] [PubMed] [Google Scholar]
- 39.Baud V., Karin M. Is NF-κB a good target for cancer therapy? Hopes and pitfalls. Nature Rev Drug Discov. 2009;8(1):33. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gambhir S., Vyas D., Hollis M., Aekka A., Vyas A. Nuclear factor kappa B role in inflammation associated gastrointestinal malignancies. World J Gastroenterol. 2015;21(11):3174. doi: 10.3748/wjg.v21.i11.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liao L.M., Vaughan T.L., Corley D.A., Cook M.B., Casson A.G., Kamangar F. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology. 2012;142(3):442–452. doi: 10.1053/j.gastro.2011.11.019. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jovani M., Cao Y., Feskanich D., Drew D.A., Hur C., Fuchs C.S. Aspirin use is associated with lower risk of Barrett's esophagus in women. Clin Transl Gastroenterol. 2017;8(12):e131. [Google Scholar]
- 43.Rothwell P.M., Fowkes F.G.R., Belch J.F., Ogawa H., Warlow C.P., Meade T.W. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377(9759):31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 44.Cuzick J., Thorat M., Bosetti C., Brown P., Burn J., Cook N. Estimates of benefits and harms of prophylactic use of aspirin in the general population. Ann Oncol. 2014;26(1):47–57. doi: 10.1093/annonc/mdu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zacharias-Millward N., Menter D.G., Davis J.S., Lichtenberger L., Hawke D., Hawk E. Beyond COX-1: the effects of aspirin on platelet biology and potential mechanisms of chemoprevention. Cancer Metastasis Rev. 2017;36(2):289–303. doi: 10.1007/s10555-017-9675-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solanky D., Krishnamoorthi R., Crews N., Johnson M., Wang K., Wolfsen H. Barrett esophagus length, nodularity, and low-grade dysplasia are predictive of progression to esophageal adenocarcinoma. J Clin Gastroenterol. 2019;53(5):361–365. doi: 10.1097/MCG.0000000000001027. [DOI] [PubMed] [Google Scholar]
- 47.Hu Q., Sun T.-.T., Hong J., Fang J.-.Y., Xiong H., Meltzer S.J. Proton pump inhibitors do not reduce the risk of esophageal adenocarcinoma in patients with Barrett's esophagus: a systematic review and meta-analysis. PLoS ONE. 2017;12(1) doi: 10.1371/journal.pone.0169691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jankowski J.A., de Caestecker J., Love S.B., Reilly G., Watson P., Sanders S. Esomeprazole and aspirin in Barrett's oesophagus (AspECT): a randomised factorial trial. Lancet. 2018 doi: 10.1016/S0140-6736(18)31388-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.