Abstract
Biosimilar medicines have shown similarity with the originator biologic and offer a similar clinical outcome generally at a lower cost. This paper identifies benefits of off-patent biologics and biosimilars, and illustrates these benefits with empirical data from Europe. We provide a narrative review of published literature on values and benefits of biosimilars in Europe. The results describe cost savings as the key driver stemming from the lower price of biosimilars, than that of originator products, and from price competition between biosimilar(s), originator, and next-generation products. Cost savings may then translate into a number of other associated benefits. The lower price of biosimilars and similar effectiveness to the originator biologics improve cost effectiveness, implying that reimbursement can be granted or extended to other patient groups, or that the biologic therapy can be moved to an earlier line of treatment. Cost savings from biosimilars can be used to increase patient access to therapy or to increase the number of healthcare professionals. Finally, competition between off-patent biologics and biosimilars may stimulate an innovation in the formulation and development of next-generation biologics. Our paper illustrates that the benefit of off-patent biologics and biosimilars is not restricted to cost savings, but that these medicines may contribute to an expansion of medical treatment options for patients, hence concomitantly contributing to the long-term sustainability of the healthcare system. This review provides a broader view for clinical and economic decision makers and healthcare professionals on the added benefits of off-patent biologics and their use in clinical practice.
Key Points
| Biosimilars are generally cheaper than originator biologics and may also incite price reductions of originator biologics; however, the benefit of biosimilars is not limited to cost savings. |
| Competition in European off-patent biologics and biosimilar markets may expand access to the treatment, improve cost effectiveness of the treatment, increase the number of healthcare professionals, and stimulate an incremental therapeutic innovation. |
Introduction
Biologics are being used in the treatment of the most serious, life-threatening, and chronic diseases such as cancers [1], immune-mediated inflammatory conditions [2], diabetes mellitus [3], and fertility [4] and are likely to be of use in treating other diseases in the future. However, the clinical benefits of biologic therapy are offset by challenges related to affordability of and accessibility to biologic medicines [5].
Biosimilars are highly similar and clinically equivalent forms of originator biologics. Development of biosimilars is complex because biologics are large and complex molecules derived from living cells by a complex manufacturing process. However, once assessed and licensed by an advanced regulatory agency, no meaningful difference between originator biologics and biosimilars is expected with respect to quality, safety and efficacy [5, 6]. Biosimilars are marketed following expiration of patent and exclusivities of the originator biologics. These medicines are generally less costly than the originator biologics, which may be due to an abbreviated clinical trial program, and possibly also to a more advanced and efficient production process [7–10].
By 2018, 34 biologics have become off-patent and 15 more biologics are expected to reach the patent cliff in the next 5 years in Europe [11, 12]. Hence, there is an opportunity for market access to biosimilars. As of May 2019, 59 biosimilars have been approved in Europe, six authorizations have been withdrawn after approval, and there are six applications under evaluation for marketing approval [13]. These include growth factors (epoetins, filgrastim), hormones (follitropin-α, insulin glargine, somatropin, teriparatide), monoclonal antibodies and fusion proteins (adalimumab, infliximab, rituximab, etanercept), and low-molecular weight heparins (enoxaparin sodium).
European countries have implemented a variety of incentives and policies to promote market access and uptake of biosimilars. The principle reason behind this favorable market environment is that countries wish to capture the savings arising from the lower price of biosimilars in an era of restricted healthcare budgets, an increase in the burden of life-threatening diseases, early detection of these diseases, and an increase in the ageing population. Biosimilars are at least 15–45% less expensive than the originator biologics [14], although prices of biosimilars vary across European countries [15]. However, price evolution of off-patent biologics and biosimilars is rapid across European countries and discounts on selected biosimilars can reach up to 80% [16]. Cumulative savings due to competition between originator biologics and biosimilars on eight key products (adalimumab, insulin glargine, etanercept, infliximab, rituximab, pegfilgrastim, trastuzumab, follitropin-α) are expected to reach €98 billion by 2020 in the EU group of 5 (G5: Germany, France, Italy, Spain, and UK) and the USA [17]. In Europe, the Top-10 biologics sales are €16.5 billion based on 2017 sales figures [18]. Most of these biologics are off-patent in Europe and biosimilars to these biologics are available for clinical use. Assuming that the discount on off-patent biologics and biosimilars will be at least 50%, annual savings could be as large as €8–10 billion by 2020.
The key driver for uptake of biosimilars is cost reduction relative to the originator biologics; however, in this paper we argue that there are other associated benefits stemming from this key driver. Some of these value propositions of biosimilars have already been outlined in a recent opinion paper [19]; however, it did not describe all the benefits of biosimilars supported by empirical evidence. The aim of this study is to provide an in-depth and structured review of the key driver and associated benefits of off-patent biologics and biosimilars and to illustrate these benefits with empirical data from Europe. Based on these results, a broader view is presented to policy and decision makers, the pharmaceutical industry, and other stakeholders of different benefits of off-patent biologics and biosimilars, thereby supporting their optimal use in society.
Methods
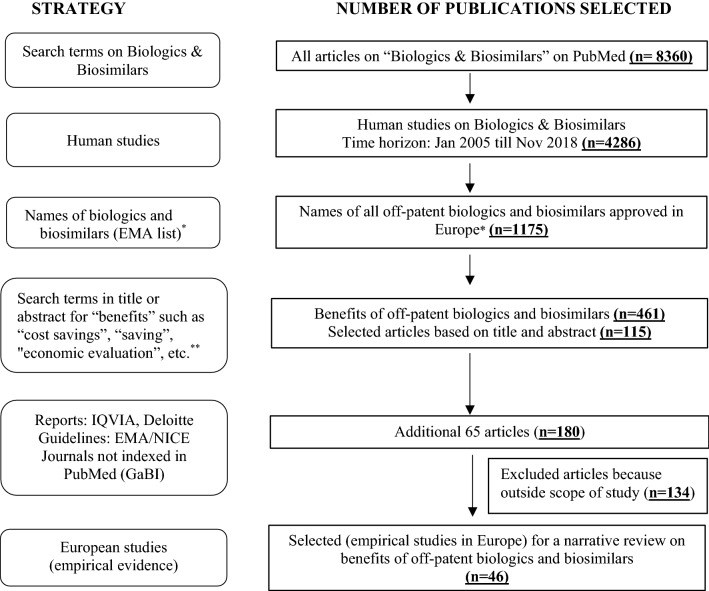
A comprehensive literature search was performed for a narrative review on the benefits of off-patent originator biologics and biosimilars, and of competition in the off-patent biologics and biosimilars market. MEDLINE and EMBASE were searched for studies published between January 2005 and November 2018, which encompassed the period during which biosimilars were first approved for use and launched in Europe [20]. Search terms were built on the concept of cost savings, economic evaluation, and other benefits of off-patent biologics and biosimilars beyond cost savings from payer, physician, patient, and market viewpoints. The search strings consisted of Medical Subject Heading (MeSH) and text terms for “biosimilar”, “reference, originator and off-patent medicines” and text terms for “value”, “lower price”, “price competition”, “supply chain benefit”, “access to treatment”, “competition”, “awarding reimbursement”, “extending reimbursement”, “earlier line of treatment”, “wrap around services”, “economic evaluation”, “off-patent biologics”, “cost effectiveness”, and “second generation reference product”. The PubMed MeSH terms were appropriately modified in accordance with the EMBASE database. Given a paucity of published literature on benefits of off-patent biologics and biosimilars as this is an emerging field of research, the bibliographic reference lists of eligible studies were also searched for other relevant sources such as the Generics and Biosimilar Initiative Journal (GaBI), which is not indexed in PubMed or EMBASE, and gray literature such as consultancy reports (IQVIA) and websites for the UK National Health Service (NHS), National Institute for Health and Care Excellence (NICE), and European Medicines Agency (EMA). Articles were selected for inclusion if they reported empirical data on benefits in the off-patent biologics and biosimilars markets in European countries. The search results from each database were limited to published references in the English language. Articles that reported on pharmaceutical or clinical aspects of off-patent biologics and biosimilars (such as bio-equivalence, immunogenicity, pharmacokinetics, or pharmacodynamic modelling) were excluded as such articles fell outside the scope of our literature review. A broad overview of the search strategy is schematically presented in Fig. 1.
Fig. 1.
A broad search strategy and different stages of narrative literature search. *IQVIA Report [20]; **see Sect. 2. EMA European Medicines Agency, GaBI Generics and Biosimilar Initiative Journal, NICE National Institute for Health and Care Excellence
Results
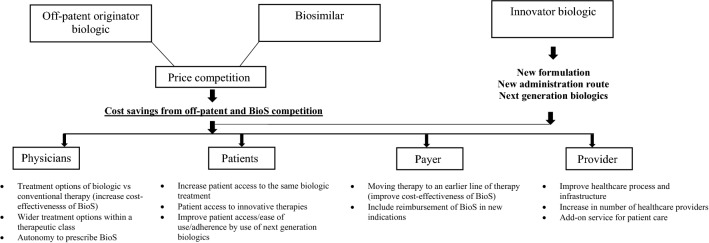
Figure 2 schematically presents different benefits of off-patent biologics and biosimilars for several stakeholders. This section defines and distinguishes between these benefits, and includes an aggregate report on a number of empirical studies illustrating each benefit in the European setting. The price of biosimilars in these studies are the ex-manufacturing price or an average price weighted cost across EU G5 countries (France, Germany, Italy, Spain, and the UK). Results of empirical studies are outlined in Table 1.
Fig. 2.
Benefits of off-patent biologics and biosimilars for different stakeholders. BioS biosimilars
Table 1.
Summary of studies illustrating benefits of off-patent biologics and biosimilars
| Benefit | Study no. | Year/setting | Scope of the study relevant to benefit of BioS | Study design | Result |
|---|---|---|---|---|---|
| Cost savings from BioS | 1 |
Year: 2015 Setting: EU G5 countriesa |
Estimate the economic impact by converting originator (Neupogen®) to BioS (Zarzio®) filgrastim for the treatment of chemotherapy-induced febrile neutropenia [21] | Simulation study on hypothetical panel of 10,000 patients/14-day treatment regimen | Cost saving from €3.9 million on day – 4 to €13 million on day – 14, assuming 100% conversion of patients from originator to BioS filgrastim. Other scenarios in this study showed a similar outcome |
| 2 |
Year: 2017 Setting: 28 European countries |
Estimate cost savings by introducing rituximab BioS (CT-P10) for patients with rheumatoid arthritis and cancer diagnoses [22] | Budget impact analysis (1–3 years) | Cost saving calculated on drug ranged from €90 million to €570 million. The study explored 3 scenarios | |
| 3 |
Year: 2012 Setting: EU G5 countriesa |
Estimate cost savings by converting originator (Neupogen®) to BioS (Zarzio®) and pegylated (Neulasta®) filgrastim for the treatment of chemotherapy-induced febrile neutropenia in patients across all tumor types. Cumulative costs of Neupogen® and Zarzio® were compared with the cost of a single dose of Neulasta® [23] | Simulation study/14-day treatment regimen | Cost saving calculated for treatment with Zarzio® over Neupogen® and Neulasta® was €457.84 and €78.50 | |
| 4 |
Year: 2015 Setting: 5 European countries (Germany, UK, Italy, Netherlands, and Belgium) |
Estimate cost savings by converting originator infliximab (Remicade®) to BioS (Remsima®), assuming BioS discount ranges between 10% and 30% of originator price [24] | Budget impact analysis (1 year) | Cumulative cost savings between €25.79 million and €77.37 million across 6 licensed indications. Both naive (50%) and switch (25%) patients were included in the analysis | |
| 5 |
Year: 2014 Setting: EU G5 countriesa |
Estimate cost saving by replacing originator (Eprex®) ESA with BioS (Binocrit®) [25] | Simulation study on hypothetical panel of 100,000 cancer patients with chemotherapy-induced anemia | Total estimated saving was €110.5 million and €146.1 million in 100% conversion from originator to BioS, under fixed and weight-based dosing scenarios, respectively. Variations in dosing schemes and treatment scenarios show similar cost savings | |
| 6 |
Year: 2015 Setting: UK, teaching hospital |
Estimate cost saving by introducing BioS infliximab for treating patients with IBD [26] | Case study (1 year) | Cost saving of approximately €516,000 in 1 year | |
| 7 |
Year: 2017 Setting: EU G5 countriesa |
Study factors influencing the adoption of BioS infliximab in rheumatology and IBD in 6 different scenarios [28] | Budget impact analysis (5 years) | In scenario analyses, the market entry of BioS etanercept and BioS rituximab was also considered. Over a time horizon of 5 years, the rheumatology budget decreased in Germany, Italy, Spain, and the UK. The budget for IBD fell in Italy and Spain but increased in Germany and the UK due to the availability of a new biologic, vedolizumab. Savings increased when BioS infliximab discounts grew to 75% and when BioS etanercept and BioS rituximab were introduced | |
| 8 |
Year: 2018 Setting: Spain, tertiary hospital |
Study cost savings from the uptake of new drugs, anti-TNF-α BioS, and therapeutic optimization for treating patients with spondyloarthritis [27] | Retrospective, observational study (2009–2016) | Cumulative cost saving of €798,614 in 2016. Original biologics, new biologics, and BioS incited economic competition such as a lower list price, official discounts, and negotiated rebates. Discounts varied for each drug, reaching a maximum of 31.9% for BioS infliximab. These factors effectively reduced the annual cost per drug per patient | |
| Improvement in cost effectiveness of treatment | 9 |
Year: 2018 Setting: 9 European countries |
Cost effectiveness of biologic therapy compared with standard care [30] | Cost-effectiveness study (5 years) | Use of BioS infliximab therapy was most cost effective, with the highest level of clinical evidence. Cost-effectiveness ratio calculation was repeated by decreasing the price of infliximab by 30%, which resulted in an ICER decrease in the range of 19–30% |
| 10 |
Year: 2015 Setting: EU countries |
Cost effectiveness of originator biologic trastuzumab was reviewed for a first-line innovative therapy for metastatic breast cancer in the EU [31] | Cost-effectiveness study of originator biologic trastuzumab | 30% decrease in price of trastuzumab originator and uptake of BioS at this price can make this therapy cost effective by decreasing the ICER value from €63,137 to €147,320 (per QALY, per life-year gained) to €29,520 to €68,880 | |
| 11 |
Year: 2016 Setting: NICE guidelines |
Update of treatment guideline in UK following introduction of BioS infliximab [32] | Clinical guidelines in the UK | Adult patients with non-radiographic axial spondyloarthritis can be treated with infliximab. Earlier this indication was restricted for originator infliximab (Remicade®) due to high cost | |
| 12 |
Year: 2014 Setting: NICE guidelines |
Update of treatment guideline for the treatment of anemia with ESA [33] | Clinical guidelines in UK | Use of BioS epoetin alfa for cancer patients with chemotherapy-induced anemia was most cost effective (£19,429 per QALY gained) | |
| 13 |
Year: 2016 Setting: EU, IMS Institute report |
Strategic health authorities in the UK reassessed the existing guidelines relating to the use of G-CSF medicines [34] | Clinical guidelines updated | Improvement in the cost effectiveness of BioS filgrastim vs. alternative treatment. G-CSF was moved to first-line cancer treatment | |
| Increase in patient access to pharmacological treatment | 4 |
Year: 2015 Setting: 5 European countries (Germany, UK, Italy, Netherlands, and Belgium) |
Estimate additional number of patients treated by cost savings incurred by the use of infliximab BioS (Remsima®) across 6 licensed indications [24] | Budget impact analysis (1 year) | Average budget saving of €43.13 million, which could be used to treat 3900 additional patients with BioS, thereby increasing patient access to TNF-α therapy |
| 2 |
Year: 2017 Setting: 28 European countries |
Estimate additional number of patients with rheumatoid arthritis and cancer diagnosis who could be treated by the cost savings from rituximab BioS (CT-P10) under different scenarios [22] | Budget impact analysis (1–3 years) | Savings in drug costs associated with uptake of the rituximab BioS would allow treatment of an additional 7531–47,695 patients, depending on the scenario | |
| 1 |
Year: 2015 Setting: EU G5 countriesa |
Estimate number of patients with malignant tumors accessing therapeutic innovations (rituximab and trastuzumab therapies) [21] | Simulation study on hypothetical panel of 10,000 patients/14-day treatment regimen | Total conversion of patients from originator to BioS filgrastim can generate cost savings of €3.9–13 million (day 4 to day 14), which could be reallocated to treat an additional 347–1213 B cell NHL patients with rituximab therapy or an additional 132–461 breast cancer patients with trastuzumab therapy | |
| 5 |
Year: 2014 Setting: EU G5 countriesa |
Estimate number of additional patients who could access therapeutic innovation (rituximab for B cell NHL, bevacizumab for metastatic colorectal cancer, and trastuzumab for metastatic HER2-positive breast cancer) by cost savings gained by converting the use of originator with BioS ESA (Binocrit®) in anemic cancer patients [25] | Simulation study on hypothetical panel of 100,000 cancer patients with chemotherapy-induced anemia | Estimated cost savings of €146.1 million could be translated into an additional 12,913 rituximab, 5171 bevacizumab, or 4908 trastuzumab treatments for patients with B cell NHL, metastatic colorectal cancer, and metastatic HER2-positive breast cancer, respectively | |
| 14 |
Year: 2014 Setting: Sweden, southern healthcare region |
Change in prescribing requirements/agreement to initiate biologic treatment for patients with febrile neutropenia [35, 42] | Change in prescribing guideline | Requirement to obtain an agreement of 3 physicians to initiate biologic treatment for febrile neutropenia was no longer required and individual physician has right to start treatment with the filgrastim BioS | |
| Increase in number of healthcare professionals | 6 |
Year: 2018 Setting: Belgium, hospital |
Estimate increase in nursing staff by reallocating funds as a result of cost savings generated by the use of BioS infliximab [26] | Case study (1 year) | Cost saving by switching originator to BioS infliximab for treating patients with IBD was diverted to employ a part-time nurse and a full-time nurse to support patients in the hospital |
| 15 |
Year: 2017 Setting: UK, university hospital |
Undertake a “managed switching programme” in 143 patients with IBD. Patients treated with originator infliximab were switched to BioS infliximab CT-P13, which generated cost savings used to hire extra healthcare staff [37] | “Managed switching programme” using a gain-share agreement between the NHS hospital trust and clinician group in UK | By investing 12% of the gross savings, 7 IBD specialist nurses, 0.5 WTE clerical support staff, 0.2 WTE pharmacists, and 0.2 WTE dieticians were employed to undertake patient education relating to the switch to BioS drug, risk management plan (e.g., robust pharmacovigilance and drug traceability procedures) to address potential risk due to switching drug, and secondary patient support services. Gain-sharing and incentivizing healthcare professionals from the cost saving as a result of the reduced drug acquisition cost improved health care resource allocation, resulting in a better service and higher quality of patient care | |
| Incremental innovation of biologics | 16 |
Year: 2018 Setting: Netherlands, hospital pharmacy and oncology day care unit |
Enrol 126 patients from 6 hospitals and compare societal costs between the IV and SC formulations of originator trastuzumab (Herceptin®) and rituximab (MabThera®) [38] | Innovation in formulation of off-patent biologics between IV and SC administration | Results indicated that total costs of one administration of SC trastuzumab were 5% lower than those of IV trastuzumab. One administration of SC rituximab was 8% less expensive than IV rituximab, both at list prices |
BioS biosimilars, ESA erythropoiesis-stimulating agent, G5 EU-Group of 5, G-CSF granulocyte colony-stimulating factor, IBD inflammatory bowel disease, ICER incremental cost-effectiveness ratio, IV intravenous, NHL non-Hodgkin’s lymphoma, NICE National Institute for Health and Care Excellence, QALY quality-adjusted life-year, SC subcutaneous, TNF tumor necrosis factor, WTE whole time equivalent
aEU G5 countries: France, Germany, Italy, Spain, and the UK
Cost Savings from Biosimilars
Cost savings may accrue from the lower price of biosimilars, than that of their originator products and from the price competition between biosimilars, originator, and next-generation products. With respect to the former, our literature search identified eight empirical studies. Six of these studies carried out a hypothetical budget impact analysis of biosimilars and used it in a number of scenarios relating to the price difference between the originator product and biosimilars, and to other parameters such as uptake, conversion rate, and time horizon [21–26]. One study calculated actual savings arising from the introduction of biosimilar infliximab in a UK hospital [26]. A Spanish study computed actual savings accruing from competition between the reference, biosimilar, and new anti-tumor necrosis factor (TNF)-α products, and from therapeutic optimization [27]. All of these studies pointed to substantial savings in pharmaceutical costs, except for one study in which the budget increased when a new, alternative biologic entered the market during the time horizon of the budget impact analysis [28].
Improvement in Cost Effectiveness of Treatment
The lower price and similar effectiveness of biosimilars compared with the originator biologics improve the cost effectiveness of the biologic therapy [29–33], implying that reimbursement can be granted or extended to other patient groups.
Improved cost effectiveness of biosimilars may also allow biologic therapy to be used in an earlier line of treatment and enable patients to access the biologic therapy at an early stage of disease. For instance, biosimilar filgrastim was moved to first-line cancer treatment in the UK as a result of its improved cost effectiveness when compared to alternative treatments [34]. This suggests that the cost effectiveness of originator biologics should be re-visited with new cost data on biosimilars.
Increase in Patient Access to Pharmacological Treatment
Cost savings from biosimilars can be used to increase patient access to biologic therapy. Several hypothetical budget impact studies have computed how many additional patients with the same disease or how many patients with a different disease can be treated with the money saved from biosimilars [22, 24]. A Swedish study showed that there was an increase in patient access to filgrastim treatment when restrictions to prescribe filgrastim for febrile neutropenia were relaxed following the market entry of biosimilar filgrastim in a specific region [17, 35].
Cost savings from biosimilars may also serve to support patient access to other innovative treatments. Two simulation studies showed how access to targeted antineoplastic treatments could be expanded by drawing on savings generated by treating chemotherapy-induced febrile neutropenia with biosimilar filgrastim and by treating anemia with biosimilar epoetin alfa [21, 25].
Increase in the Number of Healthcare Professionals
Cost savings from biosimilars can be divided among stakeholders (such as payers, hospitals, and physicians) through so-called ‘gain-sharing arrangements’ with a view to promoting uptake of biosimilars [36], and could be reinvested in employing a greater number of healthcare professionals. This efficient reallocation of savings can reduce the waiting time for patients and improve utilization of healthcare resources in a capacity-constrained hospital [26, 37].
Incremental Innovation of Biologics
Competition between off-patent biologics and biosimilars may stimulate innovations in formulation, route of administration (e.g., intravenous vs. subcutaneous), new approaches to promote patient adherence to the treatment, and development of next-generation biologics (e.g., filgrastim, pegfilgrastim, and lipegfilgrastim). There are both tangible and intangible benefits from innovation in the formulation, which could be extended to patient flexibility, patient care, and productivity, hence resulting in an overall societal gain [38, 39]. However, reimbursement of incremental innovation varies across European countries, which might be detrimental relating to the uptake of improved off-patent biologics [39]. Moreover, incremental innovation of the originator biologic compared with the biosimilar may be expensive; hence, such innovations warrant an economic evaluation to demonstrate their cost effectiveness. These features may not be clinically superior or cost effective, and therefore it may not be a preferred choice for physicians to treat their patients at a higher cost [40, 41].
Discussion
Although the impetus for biosimilars development has been largely a reduction in the cost of biologics, there are other benefits emerging from the main argument of cost saving, which have been illustrated in this narrative review and supported by empirical data within Europe such as cost savings related to the use of biosimilars could be reallocated to increase access to biologic therapy for patients who could not previously be treated, the biologic therapy could be moved to an earlier line of treatment, demand would be reallocated within a broader class of medicines, and there could be an increase in the number of healthcare staff. The benefits arising from the cost containment have different impacts on various healthcare stakeholders and are schematically represented in Fig. 2. These benefits are inter-related; for example, improving the cost effectiveness of the biotherapeutic treatment may move therapy to an earlier line of treatment, widening patient access and thus resulting in a better health outcome for more patients. Cost savings by the use of lower-priced off-patent biologics and biosimilars could be reallocated to increase patient access to innovative therapies, thus fostering headroom for innovations and supporting holistic benefit of biotherapeutics. Hence, benefits associated with the use of off-patent medicines and biosimilars are integrated and not additive.
Competition between off-patent biologics and biosimilars stimulates incremental innovation by pharmaceutical companies. However, incremental innovation by the originator manufacturing company could also be related to a strategic pricing policy put in place by the manufacturing company for the originator medicine before the launch of biosimilars, and developing next-generation products with better formulations and other add-on features could be a defense mechanism to save the market share of the originator medicines. These innovations may not be clinically superior to the biosimilar or originator [40].
Our study has illustrated the key benefits in the European off-patent biologics and biosimilars market with practical examples as derived from budget impact analyses, economic evaluations, and other studies. The shortcomings of these studies, such as a limited range of cost parameters, assumptions used to populate the budget impact model regarding hypothetical drug pricing and biosimilars uptake, the limited range of sensitivity analysis, lack of local adaptation and validation of economic studies, have been analyzed in the literature [5] and fell outside the scope of this study. The value of biosimilars may also be different across various regions of Europe. For Central and Eastern European countries, access to the originator biologics and biosimilars is a major challenge, whereas in Western European countries, the high price of these medicines is a major issue, which poses a financial burden to the healthcare system. Empirical studies illustrating benefits of biosimilars (Table 1) are based on hypothetical or descriptive studies from one or a few countries. The results cannot be generalized on the same parameters across all European countries.
The reimbursement system is different in each country and patients’ access to the biologic treatments may still be challenging, especially if there is a co-payment or limited insurance coverage for these novel therapies. As a result, patients are less likely to have access to these expensive biologic treatments [43, 44]. The purchasing price of off-patent originators and biosimilars, which is regulated by the national authority, can also vary in different European countries, and within the same country prices may also be different if dispensed via ambulatory care or a retailer [15]. Additionally, there could be other factors such as a negotiated price between the manufacturers and hospitals, local tenders, existing contractual arrangements, and the price sensitivity of payers for biosimilars. Sometimes the price of the off-patent originator may also be lower than that of biosimilars. Moreover, healthcare resources in Central and Eastern European countries are lower than in Western European countries [44]. An increase in patient access to pharmacological treatment is, therefore, likely to be a more relevant benefit in Central and Eastern European countries, where equity of access to the treatment is more of an issue than in other European countries. The current body of evidence illustrated in Table 1 is derived from hypothetical and descriptive studies in specific countries, thus inhibiting the generalizability of results. Due to the heterogeneity of the reimbursement systems and biosimilar policies between countries, benefits of off-patent biologics and biosimilars markets observed in one country cannot be extrapolated to another country. Therefore, there is a need to investigate and conduct a comparative analysis of benefits in off-patent originator biologics and biosimilars markets at a national, regional, and local level. Reimbursement assistance and innovative financial agreements may add to the overall benefits of the biologic therapy. Additionally, access to these biologic medicines can be improved by including them in the list of World Health Organization (WHO) essential medicines, based on their cost and clinical effectiveness data. This could be a supportive argument for off-patent originator biologics and biosimilars being included in the national reimbursement lists of many countries. Furthermore, in an integrated health system, gain-sharing arrangements may be a potential solution to distributing cost savings from biosimilars, such as increasing the number of specialized nurses and support staff for a high-quality, cost-effective patient service, resulting in a favorable patient outcome [37]. These initiatives could also promote team-based approaches for a continuous process improvement in hospital settings.
Our literature search included benefits of the off-patent originator biologics and biosimilars market for which European empirical data were available. Although additional drivers such as innovation in manufacturing technology, new branding, and marketing strategies exist, we did not find publicly available empirical data supporting these benefits.
Although our study has focused on those factors that contribute to the benefit of competition in the off-patent biologics and biosimilars markets, other factors exist that undermine the attainment of benefits in this market. For instance, there has been a low uptake of biosimilars due to lack of confidence among physicians prescribing these medicines [45] and a ‘nocebo’ effect experienced by patients when switching originator medicines to biosimilars [46] in which patients anticipate negative consequences after switching to biosimilars from the originator biologic, which may lead to a negative implication on their health outcome. These barriers can be addressed by providing professional education with scientific evidence to prescribers, and implementing an awareness program regarding the use and potential benefits of biosimilars for patients and other healthcare professionals.
Future studies are required to analyze and illustrate other benefits of competition in the off-patent biologics and biosimilars markets, such as a robust supply chain to avoid drug shortages, professional education for healthcare professionals, and patient care pathways. Also, empirical research illustrating the various benefits of off-patent biologic medicines and biosimilars needs to move away from hypothetical studies to evidence generated from real-world data.
Conclusion
In this article we have reviewed benefits offered by off-patent biologic medicines and biosimilars, beyond cost containment. These benefits may include improving patient access and affordability, moving biologic treatment to an earlier line of therapy, and provision of budget flexibility to fund novel therapies. Off-patent biologics and biosimilars may also create market competition and stimulate incremental innovation by the manufacturers. These benefits when executed in real-life scenarios could result in wider use of biologic treatments than the standard of care in inflammatory diseases and oncology.
Compliance with Ethical Standards
Funding
This manuscript is supported by the KU Leuven Fund on Market Analysis of Biologics and Biosimilars following Loss of Exclusivity (MABEL).
Conflict of interest
SS, IH, and AGV have conducted biosimilar research sponsored by Hospira (now Pfizer). SS was involved in a stakeholder roundtable on biosimilars sponsored by Amgen, Pfizer, and MSD, and has participated in an advisory board meeting for Pfizer. SS currently works with Pfizer, and works with Mundipharma and Celltrion as a consultant, to carry out biosimilar research. AGV is involved in consulting, advisory work, and speaking engagements for a number of companies, including AbbVie, Accord, Amgen, Biogen, EGA/Medicines for Europe, Fresenius-Kabi, Pfizer/Hospira, Mundipharma, Roche, and Sandoz.
References
- 1.Henry D, Taylor C. Pharmacoeconomics of cancer therapies: considerations with the introduction of biosimilars. Semin Oncol. 2014;41(Suppl 3):S13–S20. doi: 10.1053/j.seminoncol.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Landewe R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–977. doi: 10.1136/annrheumdis-2016-210715. [DOI] [PubMed] [Google Scholar]
- 3.Heinemann L. Biosimilar insulin and costs: what can we expect? J Diabetes Sci Technol. 2015;10(2):457–462. doi: 10.1177/1932296815605337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Medicines Agency GONAL-f. https://www.ema.europa.eu/en/medicines/human/EPAR/gonal-f. Accessed 14 Mar 2019.
- 5.Simoens S, Jacobs I, Popovian R, Isakov L, Shane LG. Assessing the value of biosimilars: a review of the role of budget impact analysis. Pharmacoeconomics. 2017;35(10):1047–1062. doi: 10.1007/s40273-017-0529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NHS England and NHS Improvement. What is a biosimilar medicine? 2015. https://www.england.nhs.uk/wp-content/uploads/2015/09/biosimilar-guide.pdf. Accessed 14 Mar 2019.
- 7.Moorkens E, Vulto AG, Huys I. Biosimilars regulatory frameworks for marketing authorisation of biosimilars: where do we go from here? Eur Pharm Law Rev. 2018;2(3):149–154. doi: 10.21552/eplr/2018/3/6. [DOI] [Google Scholar]
- 8.Isaacs J, Gonçalves J, Strohal R, Castañeda-Hernández G, Azevedo V, Dörner T, et al. The biosimilar approval process: how different is it? Consid Med. 2017;1(1):3–6. doi: 10.1136/conmed-2017-100003. [DOI] [Google Scholar]
- 9.de Ridder L, Assa A, Bronsky J, Romano C, Russell RK, Afzal NA, et al. Use of biosimilars in pediatric inflammatory bowel disease: an updated position statement of the Pediatric IBD Porto Group of ESPGHAN. J Pediatr Gastroenterol Nutr. 2019;68(1):144–153. doi: 10.1097/MPG.0000000000002141. [DOI] [PubMed] [Google Scholar]
- 10.Rugo HS, Rifkin RM, Declerck P, Bair AH, Morgan G. Demystifying biosimilars: development, regulation and clinical use. Future Oncol. 2018 doi: 10.2217/fon-2018-0680. [DOI] [PubMed] [Google Scholar]
- 11.Derbyshire M. Patent expiry dates for biologicals: 2018 update. GaBi J. 2019;8(1):24–31. doi: 10.5639/gabij.2019.0801.003. [DOI] [Google Scholar]
- 12.Derbyshire M. Patent expiry dates for biologicals: 2017 update. GaBi J. 2018;7(1):29–34. doi: 10.5639/gabij.2018.0701.007. [DOI] [Google Scholar]
- 13.Harston A. How the U.S. compares to Europe on biosimilar approvals and products in the pipeline. 2019. https://www.biosimilarsip.com/2019/05/07/how-the-u-s-compares-to-europe-on-biosimilar-approvals-and-products-in-the-pipeline-4/. Accessed 26 Aug 2019.
- 14.Heredia E, Ribeiro A. Discount offered by first and subsequent biosimilars in the US, EU and LATAM: impact trends of originator starting price, market dynamics and regulations. Value Health. 2018;21(Suppl. 1):S103–S104. doi: 10.1016/j.jval.2018.04.700. [DOI] [Google Scholar]
- 15.Moorkens E, Vulto AG, Huys I, Dylst P, Godman B, Keuerleber S, et al. Policies for biosimilar uptake in Europe: an overview. PLoS One. 2017;12(12):e0190147. doi: 10.1371/journal.pone.0190147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reuters. Humira biosimilars available at up to 80 percent discount in Europe: AbbVie. Business News. 2018. https://www.reuters.com/article/us-abbvie-results-humira/humira-biosimilars-available-at-up-to-80-percent-discount-in-europe-abbvie-idUSKCN1N71NZ?il=0. Accessed 14 Mar 2019.
- 17.IMS Report. Delivering on the potential of biosimilar medicines. 2016. https://www.medicinesforeurope.com/wp-content/uploads/2016/03/IMS-Institute-Biosimilar-Report-March-2016-FINAL.pdf. Accessed 14 Mar 2019.
- 18.IQVIA Report. The impact of biosimilar competition in Europe. Presentation by Per Troein, European Commission. London: IQVIA; 2018.
- 19.de Mora F. Biosimilars: a Value Proposition. BioDrugs. 2019 doi: 10.1007/s40259-019-00360-7. [DOI] [PubMed] [Google Scholar]
- 20.IQVIA Report. Advancing Biosimilar Sustainability in Europe: a multi-stakeholder assessment. 2018. https://www.iqvia.com/institute/reports/advancing-biosimilar-sustainability-in-europe. Accessed 14 Mar 2019.
- 21.Sun D, Andayani TM, Altyar A, MacDonald K, Abraham I. Potential cost savings from chemotherapy-induced febrile neutropenia with biosimilar filgrastim and expanded access to targeted antineoplastic treatment across the European Union G5 countries: a simulation study. Clin Ther. 2015;37(4):842–857. doi: 10.1016/j.clinthera.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Gulacsi L, Brodszky V, Baji P, Rencz F, Pentek M. The rituximab biosimilar CT-P10 in rheumatology and cancer: a budget impact analysis in 28 European countries. Adv Ther. 2017;34(5):1128–1144. doi: 10.1007/s12325-017-0522-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aapro M, Cornes P, Abraham I. Comparative cost-efficiency across the European G5 countries of various regimens of filgrastim, biosimilar filgrastim, and pegfilgrastim to reduce the incidence of chemotherapy-induced febrile neutropenia. J Oncol Pharm Pract. 2012;18(2):171–179. doi: 10.1177/1078155211407367. [DOI] [PubMed] [Google Scholar]
- 24.Jha A, Upton A, Dunlop WC, Akehurst R. The budget impact of biosimilar infliximab (Remsima®) for the treatment of autoimmune diseases in five European countries. Adv Ther. 2015;32(8):742–756. doi: 10.1007/s12325-015-0233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abraham I, Han L, Sun D, MacDonald K, Aapro M. Cost savings from anemia management with biosimilar epoetin alfa and increased access to targeted antineoplastic treatment: a simulation for the EU G5 countries. Future Oncol. 2014;10(9):1599–1609. doi: 10.2217/fon.14.43. [DOI] [PubMed] [Google Scholar]
- 26.ESN. Switch management between similar biological medicines. A communication guide for nurses. 2018. http://www.esno.org/assets/biosimilar-nurses-guideline-final.pdf. Accessed 14 Mar 2019.
- 27.Gonzalez-Fernandez M, Villamanan E, Jimenez-Nacher I, Moreno F, Plasencia C, Gaya F, et al. Cost evolution of biological agents for the treatment of spondyloarthritis in a tertiary hospital: influential factors in price. Int J Clin Pharm. 2018;40(6):1528–1538. doi: 10.1007/s11096-018-0703-z. [DOI] [PubMed] [Google Scholar]
- 28.Kanters TA, Stevanovic J, Huys I, Vulto AG, Simoens S. Adoption of biosimilar infliximab for rheumatoid arthritis, ankylosing spondylitis, and inflammatory bowel diseases in the EU5: a budget impact analysis using a Delphi panel. Front Pharmacol. 2017;8:322. doi: 10.3389/fphar.2017.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simoens S. Biosimilar medicines and cost-effectiveness. Clinicoecon Outcomes Res. 2011;3:29–36. doi: 10.2147/CEOR.S12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baji P, Gulacsi L, Brodszky V, Vegh Z, Danese S, Irving PM, et al. Cost-effectiveness of biological treatment sequences for fistulising Crohn’s disease across Europe. United Eur Gastroenterol J. 2018;6(2):310–321. doi: 10.1177/2050640617708952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garattini L, van de Vooren K, Curto A. Cost-effectiveness of trastuzumab in metastatic breast cancer: mainly a matter of price in the EU? Health Policy. 2015;119(2):212–216. doi: 10.1016/j.healthpol.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 32.NICE. TNF-alpha inhibitors for ankylosing spondylitis and non-radiographic axial spondyloarthritis. 2016. https://www.nice.org.uk/guidance/ta383/resources/tnfalpha-inhibitors-for-ankylosing-spondylitis-and-nonradiographic-axial-spondyloarthritis-pdf-82602848027077. Accessed 14 Mar 2019.
- 33.NICE. Erythropoiesis-stimulating agents (epoetin and darbepoetin) for treating anaemia in people with cancer having chemotherapy. https://www.nice.org.uk/guidance/ta323/resources/erythropoiesisstimulating-agents-epoetin-and-darbepoetin-for-treating-anaemia-in-people-with-cancer-having-chemotherapy-pdf-82602485230021. Accessed 14 Mar 2019.
- 34.IQVIA. Delivering on the potential of biosimilar medicines: the role of functioning competitive markets. 2016. https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/delivering-on-the-potential-of-biosimilar-medicines.pdf. Accessed 14 Mar 2019.
- 35.Simon Kucher & Partners. Payers’ price & market access policies supporting a sustainable biosimilar medicines market. 2016. https://www.medicinesforeurope.com/wp-content/uploads/2016/09/Simon-Kucher-2016-Policy-requirements-for-a-sustainable-biosimilar-market-FINAL-report_for-publication2.pdf. Accessed 14 Mar 2019.
- 36.Simoens S, Le Pen C, Boone N, Breedveld F, Celano A, Llombart-Cussac A, et al. How to realize the potential of off-patent biologicals and biosimilars in Europe? Guidance to policymakers. GaBi J. 2018;7(2):70–74. doi: 10.5639/gabij.2018.0702.014. [DOI] [Google Scholar]
- 37.Razanskaite V, Bettey M, Downey L, Wright J, Callaghan J, Rush M, et al. Biosimilar infliximab in inflammatory bowel disease: outcomes of a managed switching programme. J Crohns Colitis. 2017;11(6):690–696. doi: 10.1093/ecco-jcc/jjw216. [DOI] [PubMed] [Google Scholar]
- 38.Franken MG, Kanters TA, Coenen JL, de Jong P, Koene HR, Lugtenburg PJ, et al. Potential cost savings owing to the route of administration of oncology drugs: a microcosting study of intravenous and subcutaneous administration of trastuzumab and rituximab in the Netherlands. Anticancer Drugs. 2018;29(8):791–801. doi: 10.1097/CAD.0000000000000648. [DOI] [PubMed] [Google Scholar]
- 39.Jackisch C, Muller V, Dall P, Neumeister R, Park-Simon TW, Ruf-Dordelmann A, et al. Subcutaneous trastuzumab for HER2-positive breast cancer - evidence and practical experience in 7 German centers. Geburtshilfe Frauenheilkd. 2015;75(6):566–573. doi: 10.1055/s-0035-1546172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lietzen E. A solution in search of a problem at the biologics frontier. U Ill L Rev Online. 2018;19–35. University of Missouri School of Law Legal Studies Research Paper No 2018-05. https://illinoislawreview.org/online/a-solution-in-search-of-a-problem-at-the-biologics-frontier/. Accessed 11 Nov 2019.
- 41.Bocquet F, Loubiere A, Fusier I, Cordonnier AL, Paubel P. Competition between biosimilars and patented biologics: learning from European and Japanese experience. Pharmacoeconomics. 2016;34(11):1173–1186. doi: 10.1007/s40273-016-0428-6. [DOI] [PubMed] [Google Scholar]
- 42.Gascon P, Tesch H, Verpoort K, Rosati MS, Salesi N, Agrawal S, et al. Clinical experience with Zarzio® in Europe: what have we learned? Support Care Cancer. 2013;21(10):2925–2932. doi: 10.1007/s00520-013-1911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawalec P, Stawowczyk E, Tesar T, Skoupa J, Turcu-Stiolica A, Dimitrova M, et al. Pricing and reimbursement of biosimilars in Central and Eastern European Countries. Front Pharmacol. 2017;8:288. doi: 10.3389/fphar.2017.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vogler S, Dedet G, Pedersen HB. Financial burden of prescribed medicines included in outpatient benefits package schemes: comparative analysis of co-payments for reimbursable medicines in European countries. Appl Health Econ Health Policy. 2019 doi: 10.1007/s40258-019-00509-z. [DOI] [PubMed] [Google Scholar]
- 45.Dylst P, Vulto A, Simoens S. Barriers to the uptake of biosimilars and possible solutions: a Belgian case study. Pharmacoeconomics. 2014;32(7):681–691. doi: 10.1007/s40273-014-0163-9. [DOI] [PubMed] [Google Scholar]
- 46.Kristensen LE, Alten R, Puig L, Philipp S, Kvien TK, Mangues MA, et al. Non-pharmacological effects in switching medication: the nocebo effect in switching from originator to biosimilar agent. BioDrugs. 2018;32(5):397–404. doi: 10.1007/s40259-018-0306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]