Abstract
DUF538 (domain of unknown function 538) proteins are known as a group of putative hypothetical proteins in a wide range of plant species. They have been identified from some plants challenged with various environmental stresses. However, a little is known about their functional properties. They have been newly predicted to have binding capacity and esterase-type hydrolytic activity towards bacterial lipopolysaccharides and chlorophyll molecules as carboxylic compounds in plants. In the present study, the binding ability and the methylesterase activity of DUF538 proteins towards pectin molecules were also predicted. Their similarities to pectin methylesterases and their binding ability to pectin molecule were predicted using bioinformatic tools as well as the experimental method. A probable cooperation was speculated between DUF538 and pectin methylesterase protein families in cell wall associated defense responses in plants.
Keywords: DUF538, Hydrolysis, Methylesterase, Pectin, Stress protein
Introduction
DUF538 proteins are mostly known as putative hypothetical proteins in wide ranges of mono-cotyledonous and di-cotyledonous plants. These proteins have been characterized from stress challenged plants including those grown under nutrient deficiency, crown gall, mixed elicitors and mild drought stresses (Gholizadeh and Baghbankohnehrouz 2010; Takahashi et al. 2013). Their molecular weights are about 19–21 kDa encoding around 170 amino acids and their structures are mostly dominated by beta-strands (PDB ID: 1ydu).
Despite this information, a little is known about their functional properties. However, some functions have been attributed to DUF538 protein superfamily in plants. DUF538 proteins were predicted to play regulatory roles in different stress-challenged plants based on their phosphorylation potential (Nakagami et al. 2010). They were known as potent activators of the redox system of plant cells (Gholizadeh 2011). Later on, they were suggested to be the functional homologues of bactericidal permeability increasing (BPI) proteins in mammalians innate system (Gholizadeh and Baghbankohnehrouz 2013). They were predicted to bind to lipopolysaccharides of bacteria on their outer leaflet and hydrolyze them until to kill bacteria. By using the bioinformatic tools, the tertiary structures of BPI and DUF538 were suggested to be homologue to the esterase-type lipolytic enzymes including acyl-peptide hydrolase, carboxyesterease type B, entrochelin esterease, and peroxisomal long chain acyl-coA hydrolase (Gholizadeh 2014). Thereby, DUF538 proteins were suggested to utilize lipids or their aromatic compounds to hydrolyze ester bonds in nature. Interestingly, following finding the similarity of DUF538 to type 1 water soluble chlorophyll binding protein (WSCP 1), the binding ability of DUF538 to chlorophyll molecules was also predicted in plant system. DUF538 proteins were shown to contain esterase-type hydrolytic domain that is able to hydrolyze chlorophyll molecules in vitro (Gholizadeh 2017, 2018).
Considering all these investigations to gather, to sketch more about DUF538 protein superfamily, their esterase domain was further investigated. In a survey by bioinformatic tools, it was identified that plant pectin methylesterases contain DUF538 protein domain. Based on this, our attempt was made to examine the binding ability of DUF538 to pectin molecules as well as the methylesterase hydrolytic activity of DUF538 towards pectin. Pectin is a methyl-esterified polysaccharide. Pectin methylesterases (PME) are methyl ester containing hydrolytic enzymes that hydrolysis pectin molecule to pectate and methanol (Micheli 2001). They have been isolated from plants, pathogenic fungi and bacteria (Collmer and keen 1986; Gainvors et al. 1994). They are located in the cell wall as insoluble forms or in the cytosol as soluble isoforms (Kohli et al. 2015). It has been known that the hydrolysis of pectin by PMEs modify the food products qualities. PMEs also are identified as one of the plant defense system against pathogenic fungi or bacteria (Kohli et al. 2015). They are involved in a number of physiological and developmental processes in plants (Wakeley et al. 1998; Futamura et al. 2000; Kohli et al. 2015).
Herein, using computational and experimental recombinant method, it was suggested that DUF538 proteins are able to bind pectin molecules and enable to hydrolyze them in vitro. Considering DUF538 proteins as stress related cytoplasmic proteins, they were speculated to help the catabolism of pectin in the cytosol to recycle carbon in stress-challenged plants.
Materials and methods
Materials
E. coli strain TB1 expressing MBP-fused DUF538 was taken from laboratory stock (Gholizadeh, 2011). For isolation and purification of fusion product, all of the materials and columns were provided in protein fusion and purification system kit (Cat. No. E8000S; NEW ENGLAND, Bio Lab). Pichia pastoris alcohol oxidase, apple pectin with 75% methyl esterification and purpald (4-Amino-3-hydrazino-5-mercapto-1,2,4-triazole, 4-Amino-5-hydrazino-1,2,4-triazole-3-thiol) were purchased from Sigma company. All of the other chemicals were of molecular biology grade.
Computational analysis
Celosia cristata DUF538 protein domain analysis was carried out by using CDART (conserved domain architectural tool) at https://www.ncbi.nlm.blast.com/. Celosia DUF538 protein, the C-terminus end of Brassica napus pectin methylesterase and Arabidopsis thaliana pectin esterase sequences were subjected to homology modeling by using Modeller software (Webb and Sali 2016). The best pdb template for modeling was found by BLAST server (Altschul et al. 1997). For modeling the structure of pectin ligand, its monomer was taken from Pubchem: https://pubchem.ncbi.nlm.nih.gov/compound/Pectin, and then its polymer structure was modeled and optimized using Hyperchem: www.hyper.com. Molecular docking analysis between test proteins and ligand was carried out by Autodock VINA software (Trott and Olson 2010). For each structure, 10 models with three replicates were considered. Molecular dynamics (MD) was performed using GROMACS 5.0.7 package under an amber.ff99SB force field (Abraham et al. 2015). The Antechamber software was utilized for pectin GAFF topologies generation using calculated partial charges with restrained electrostatic potential method. By using the acpype tool, the GAFF topologies were converted into GROMACS format. The Particle-Mesh Ewald procedure was used with a cut-off of 10 Å. Treatment of Van der Waals forces was a cut-off of 10 Å. Also, modeled structures of three proteins were used for theoretical studies. To update the neighbor list, the frequency was 5. In order to calculate the total charges of test molecules, the protonation state of GROMACS package was utilized. In the first step of MD simulation, by using the steepest descent and conjugate gradients algorithms, the entire system was minimized. Secondly, using a force constant of 1000 kJ/mol nm, the heavy atoms were restrained and the solvent and ions were evolved. This was through the NVT and NPT ensembles for 100 ps. In order to equilibrate the temperature inside the system, the Berendsen temperature coupling method was used in the NVT ensemble and the system was maintained under 1 atm pressure with allowed compressibility ranging from 4.5 × 10−5 under NPT. The equilibrium geometry was obtained at 298 K and 1 atm by increasing the temperature and resigning the velocities of the system based on Maxwell–Boltzmann distribution and equilibration for 100 ps. The temperature and pressure couplings were set to 0.1 ps and 2 ps, respectively. During the equilibration step, the Berendsen algorithm was used for thermostat and barostat. The bonds were constrained through the LINCS algorithm (Hess et al. 1997). In production phase, MD simulation was carried out in a 50 ns under NPT. The temperature and pressure stabilities were retained by Nosé–Hoover thermostat and Parrinello–Rahman barostat. In order to constrain the hydrogen-containing bonds lengths, the LINCS algorithm was utilized. To neutralize the charge of the system, Na+ and Cl− were used.
Cloning and expression of DUF538
Cloning and expression of Celosia cristata DUF538 cDNA had been carried out from leaf materials in the previous research work (Gholizadeh 2011). The procedure had been performed in pMALc2X cloning and expression vector system of E. coli. TB1 strain of E. coli had been used as transformed competent cells.
Fused DUF538 extraction and purification
In order to extract the fused product of DUF538, the stock recombinant bacterial cells were allowed to grow in 500 mL of glucose and ampicillin containing rich broth medium. The expression of fused product was induced by the addition of 0.3 mM IPTG for a period of 8 h. For harvesting and precipitation of the cells, the cell containing medium was centrifuged at 4,000 × g for 10 min. This was followed by dissolving the obtained pellet in 25 mL of protein extraction buffer consisting of 20 mM of Tris-Cl, 200 mM of NaCl, 1 mM of EDTA, 1 mM of sodium azide, and 10 mM of BME. Thereafter, the sample was allowed to freeze at − 20 °C in the same extraction buffer and sonicated in short pulses of 15 s. The obtained sample was centrifuged at 10,000×g at 4 °C for 20 min, and the supernatant was utilized as crude protein extract. The fused product was purified from the crude protein extract by single-step affinity chromatography. For this, the purification columns were packed with amylase resins having specificity for maltose-binding protein. The amylase-bound fusion protein was eluted out from the column by the buffer consisting of crude extraction buffer plus 10 mM of maltose. The obtained fused protein was analyzed for its homogeneity by separating on 10% polyacrylamide gel (SDS-PAGE) (Laemmli 1970).
Pectin methylesterase test of fused DUF538
In order to test the pectin methylesterase activity, the lyophilized fused DUF538 protein was dissolved in 200 mM phosphate buffer (pH 7.2). To 10 μL of purified DUF538 containing solution (containing 15 μg of fused protein), 1% pectin was added and kept at 30 °C for 30 min. After incubation period, the possible desterified methanol was tested using alcohol oxidase and purpald. For this assay, a mixture containing 90 μL of 200 mM phosphate buffer (pH 7.2), 10 μL of 0.01 unit/μL alcohol oxidase in 200 mM phosphate buffer (pH 7.2), 50 μL methanol contain solution and H2O to a final volume of 200 μL was prepared and incubated at 30 °C for 10 min. After incubation, 200 μL of 5 mg/mL purpald solution in 0.5 N NaOH was added, vortexed vigorously and incubated at 30 °C for 30 min. The sample was removed from the water bath and H2O was added to a 1 mL final volume. The absorbance of the sample was then determined at 550 nm. The experiment was repeated with five varying concentrations of fused protein including 5, 10, 15, 25, 30 μg/10 μL. The MBP containing buffer and protein-free mixture were considered as control samples. Each experiment was repeated three times and data were presented as the mean values ± SD on the graph.
Results and discussion
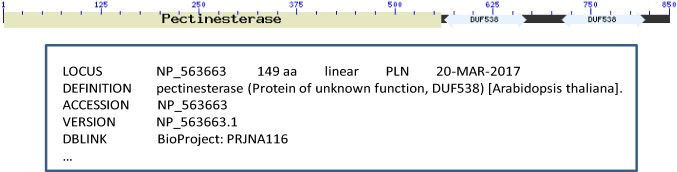
In the present study, by a survey in protein databases using CDART protein domain prediction online server, it was clarified that plant pectin methylesterases contain DUF538 domain in their carboxy termini. For example, the presence of DUF538 domain in pectin methyesterase from Brassica napus is shown in Fig. 1. This pectin methylesterase was composed of two sections including N-terminus and C-terminus parts. The N-terminal section belongs to pectin methylesterase inhibitor (PMEI) family. The C-terminal part belongs to pectin methylesterase (PME) family. Due to the similarity of DUF538 protein to C-terminus part of pectin methylesterases and because of the location of catalytic domain of pectin methylesterases at their carboxy termini, it was proposed that DUF538 proteins may act as pectin methylesterases. On the other hand, the N-terminus part of pectin methylesterases is cleaved during translocation of PMEs towards plant cell wall and the mature part (without the N-terminus section) of the PMEs is only found and located in the cell wall. Thereby, DUF538 protein family was predicted to be similar to the mature pectin methylesterases. Besides the C-terminus part of Brassica pectin methylesterase, our BLAST search data revealed that Arabidopsis pectinesterase protein is also homologue to DUF538 proteins with Expect value of 1e−46 (Fig. 1). These data together suggest that DUF538 might act as pectin methylesterase.
Fig. 1.
Protein domain analysis of Brassica napus pectin methylesterase and BLAST result. (Upper) Protein domain analysis by CDART server at NCBI identifying the presence of DUF538 domain in the C-terminal end of Brassica napus pectin methylesterase. (Lower) BLAST search data showing the identity between Arabidopsis thaliana pectinesterase and DUF538 protein family
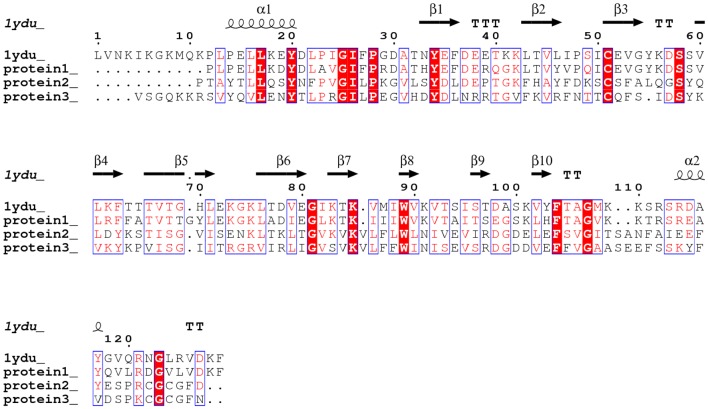
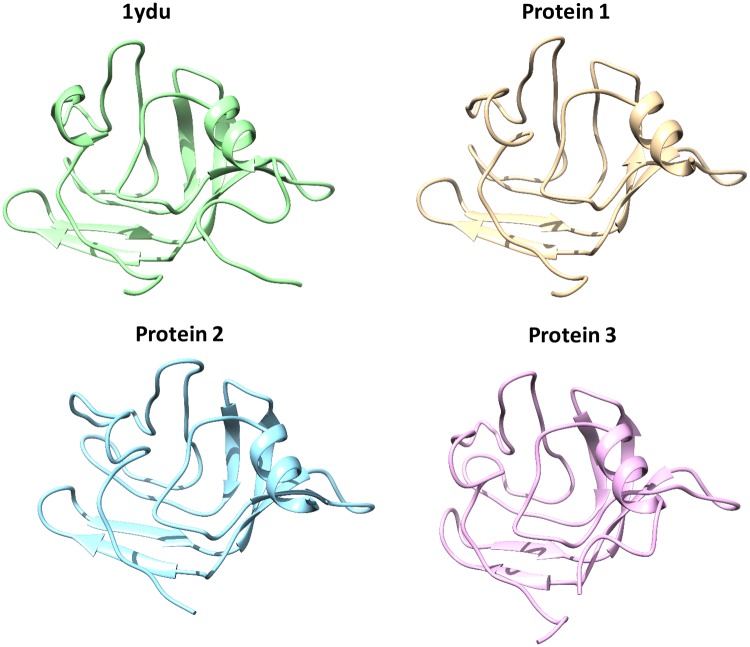
Comparison of the amino acid sequences between Celosia cristata DUF538 protein (protein 1), C-terminal end of Brassica PME (protein 2) and Arabidopsis pectinesterase (protein 3) by BLAST server revealed that they are homologous structures and 1ydu (Arabidopsis DUF538 protein) is the best pdb template for all three (Fig. 2). Prediction of their three-dimensional structures by homology modeling identified that they have homologous 3D structures, too. Similar to 1ydu, all three structures were found to be β-dominated, showing the same 3D patterns and orientations (Fig. 3).
Fig. 2.
Sequence alignment between test proteins and 1ydu template. Analysis of test proteins structures by BLAST showing the high level of structural homology between 1ydu (Arabidopsis thaliana DUF538) and test proteins (Celosia cristata DUF538 as protein 1, C-terminus of Brassica napus pectin metylesterase as protein 2 and Arabidopsis thaliana pectinesterase as protein 3)
Fig. 3.
Comparison of three-dimensional structures of 1ydu and test proteins. The modeled 3D-structures of 1ydu and test proteins showing the three-dimensional structural similarity between 1ydu (Arabidopsis thaliana DUF538) and test proteins (Celosia cristata DUF538 as protein 1, C-terminus of Brassica napus pectin metylesterase as protein 2 and Arabidopsis thaliana pectinesterase as protein 3)
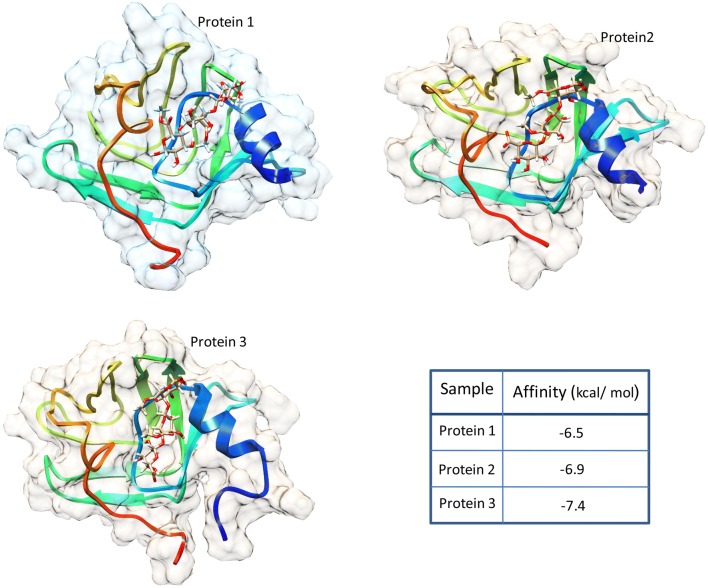
Molecular docking analysis by using Autodock VINA between all three proteins and pectin molecule as common ligand revealed that they all have the similar pattern of docking. The results showed that they have the similar binding site for pectin molecule (Fig. 4). The affinities between test proteins and pectin were predicted to be − 7.4, − 6.9 and − 6.5 kcal/mol for Arabidopsis pectinesterase, the C-terminus of Brassica PME and Celosia DUF538, respectively.
Fig. 4.
Molecular docking analysis between test proteins and pectin ligand. Molecule docking result by Autodock VINA showing the similar patterns of interactions between pectin molecule and three test proteins including Celosia cristata DUF538 as protein 1, C-terminus of Brassica napus pectin metylesterase as protein 2 and Arabidopsis thaliana pectinesterase as protein 3
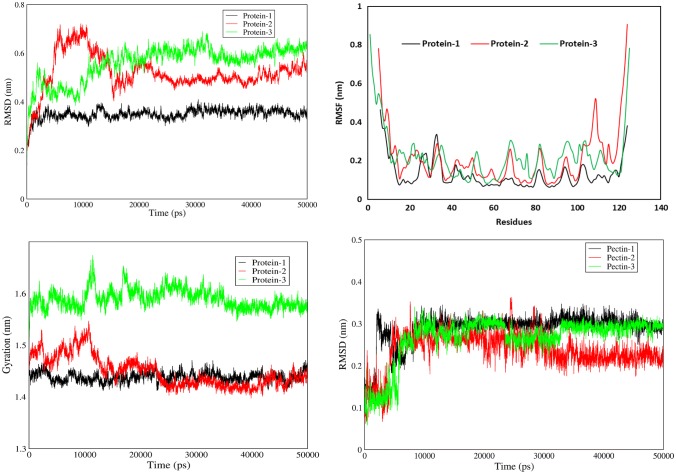
The molecular interactions of all three test proteins with pectin ligand were analyzed by molecular dynamics simulation using GROMACS. Analysis of the RMSD (Root-mean-square deviation) of α carbon atom positions between all three proteins during 50 ns simulation time revealed that DUF538 protein has more constant values than the C-terminus of Brassica PME and Arabidopsis pectinesterase. The highest RMSD values were calculated to be 0.35, 0.7 and 0.67 nm for Celosia DUF538, the C-terminus of Brassica PME and Arabidopsis pectinesterase, respectively (Fig. 5). Besides RMSD, analysis of Root-mean-square fluctuation (RMSF) values of different residues also revealed that DUF538 residues have more constant values than those of the C-terminus of Brassica PME and Arabidopsis pectinesterase. But, the RMSF values of all three proteins were predicted to be fluctuated at the terminal residues in comparison to middle parts (Fig. 5). This means that the N and C terminus parts of all three test proteins are positioned towards the environment. It also indicates that the middle parts of all proteins are more folded or interacted. Data analysis showed that the terminal residues of Brassica PME have more fluctuations in comparision to Arabidopsis pectinesterase residues. Therefore, it seems that incomplete PME is more fluctuated. Analysis of gyration radius of the test proteins revealed that the Arabidopsis pectinesterase and Brassica PME have higher gyration radius values (1.58 and 1.47 nm vs 1.44 nm) in comparision to Celosia DUF538 protein (Fig. 5). Data analysis indicated that the gyration radiuses of Brassica PME and Arabidopsis pectinesterase are more fluctuated during molecular dynamics as compared to Celosia DUF538. As a result of this analysis, DUF538 protein is more stable than Brassica PME and Arabidopsis pectinesterase. RMSD of ligand analysis revealed that Celosia DUF538 has more ligand RMSD value (0.3 nm vs 0.2 and 0.1 nm) than those of Brassica PME and Arabidopsis pectinesterase. The result also showed that ligand RMSD values of Brassica PME and Arabidopsis pectinesterase are more fluctuated than that of Celosia DUF538, showing the structural stability of Celosia DUF538.
Fig. 5.
RMSD, RMSF and Gyration analysis. (Upper left) RMSD values of test proteins showing the structural stability of Celosia cristata DUF538 protein as protein 1 in comparision to C-terminus of Brassica napus pectin metylesterase as protein 2 and Arabidopsis thaliana pectinesterase as protein 3 during simulation time. (Upper right) RMSF values of test proteins showing the structural stability of Celosia cristata DUF538 protein as protein 1. (Lower left) Gyration values of test proteins showing the structural stability of Celosia cristata DUF538 protein as protein 1. (Lower right) RMSD values of ligand showing the stability of DUF538-pectin interactive structure as compared to others
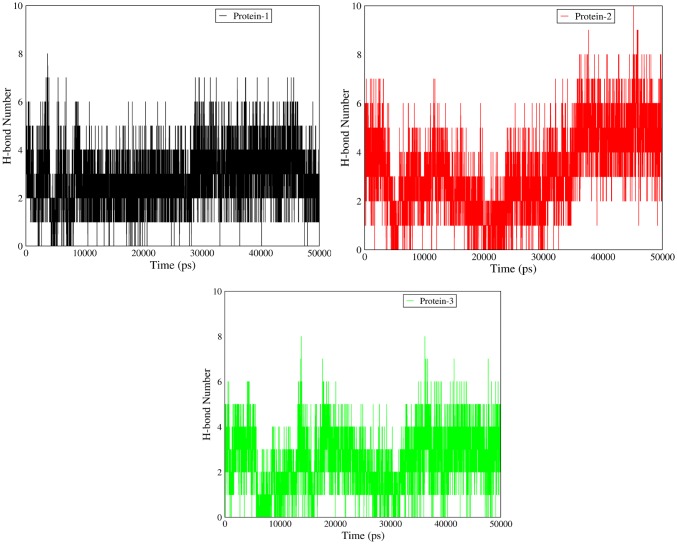
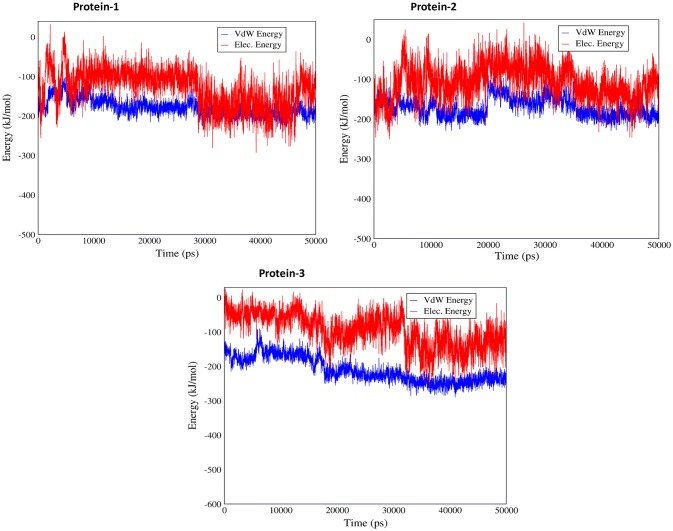
The interaction between test proteins and ligand was further analyzed by hydrogen bond establishment. Data analysis showed that Brassica PME has generally more hydrogen connection (6 vs 4) as compared to Celosia DUF538 and Arabidopsis pectinesterase (Fig. 6). Thereby, the C-terminus end of Brassica PME seems to establish their protein–ligand interaction through hydrogen bonds. Analysis of the protein–ligand interaction by Van der Waals and electrostatics connections revealed that all three proteins establish their interactions with pectin more through electrostatic connections rather than Van der Waals (− 100 vs − 200 kcal/mol) (Fig. 7). The result also showed that test proteins have different patterns of Van der Waals and electrostatics energies fluctuations during simulation time.
Fig. 6.
Changes in the number of hydrogen bonds. The number of hydrogen bonds formation was analyzed in test proteins–ligand interactions, showing the high level of fluctuation in C-terminus of Brassica napus pectin metylesterase as protein 2 during simulation time. Protein 1 and protein 3 show the similar pattern of fluctuation
Fig. 7.
Analysis of electrostatic and Van der Waals energies fluctuations. Electrostatic and Van der Waals connection analysis showing the high level of electrostatic connection in all test proteins–ligand interactions during simulation time
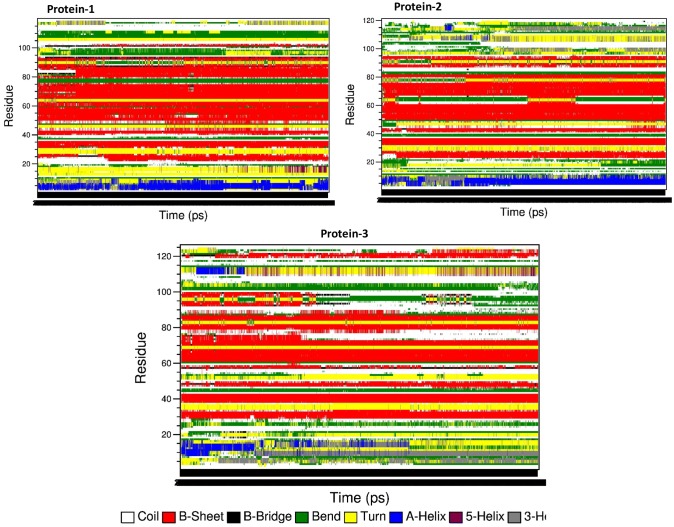
Analysis of secondary structural changes during molecular dynamics time revealed that Celosia DUF538 has more stable secondary structure than Brassica PME and Arabidopsis pectinesterase (Fig. 8). Arabidopsis pectinesterase showed the high level of secondary structure fluctuation during simulation time.
Fig. 8.
Analysis of secondary structure fluctuation. Secondary structure analysis showing the high structural fluctuation in Arabidopsis pectinesterase as protein 3 in comparision to C-terminal of Brassica PME as protein 2 and Celosia DUF538 as protein 1 during simulation time
Following the computational-based predictions showing the structural similarity between DUF538 protein and pectin methylesterase-type enzymes, we used experimental tools for the better understanding of the computated structures. For the most experimental studies, the efficiently produced and purified heterologous products with correctly folded structures are needed. With regard to DUF538, we had previously attained the successful heterologous expression of MBP-fused Celosia DUF538 protein in E. coli expressing system (Gholizadeh 2011). Thereby, in the present study, the previously prepared DUF538 expressing bacteria were used for the next experimental steps As compared to other fusion tags, MBP tag was selected for this study because of its high level expression and enhancement of folding. Because of the difficulty of expressing plant PME and formation of inclusion bodies in Escherichia coli cells (Ding et al. 2000; Peng et al. 2005), selection of MBP solubility tag for expressing PME homologue seemed to be most suitable. For this, the recombinant TB1 cells were grown in 0.3 mM final concentration of IPTG induced condition. After crude protein extraction, the purification process was performed by affinity chromatography. The crude and purified products of fusion proteins were subjected to SDS-PAGE analysis (Fig. 9). The purified MBP-fused DUF538 protein (as test sample), MBP (as control sample) and protein-free buffer solution (as control sample) were tested for pectin methylesterase activity. The absorbance of the samples at 550 nm was considered as criteria for the activity assessment. The obtained result showed that the OD550 value of fused protein containing sample is 0.54 in compare to controls. Control samples did not exhibit absorbance at 550 nm. For further analysis, the concentration dependent experiment was arranged. The experiment was carried out with five varying concentrations of fused protein including 5, 10, 15, 20, 25 and 30 μg/μL. The obtained data revealed that the absorbance of the test samples increases with the increasing concentrations of fused protein in the mixture (Fig. 9). The similar experiment was repeated for MBP-containing mixture, showing no absorbance increase at 550 nm. These experiments data indicated that DUF538 protein might act as pectin methylesterase and might be able to hydrolyze pectin molecule.
Fig. 9.
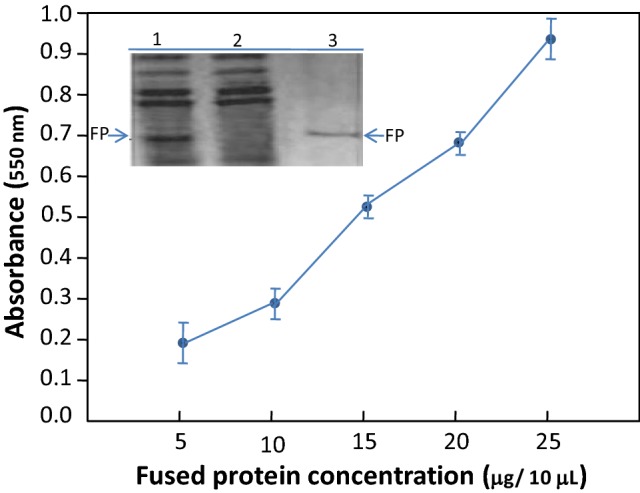
SDS-PAGE analysis and pectin methylesterase activity assay. The expressed and purified fused protein was analyzed by 10% polyacrylamide gel. (Lane 1) Recombinant clone after induction with IPTG, (lane 2) transformed cells containing vector alone after induction with IPTG. (Lane 3) Purified fusion protein (FP). Pectin methylesterase activity assay was carried out by measuring the absorbance of the samples containing varying concentrations of the fused DUF538 protein at 550 nm
According to our previous results, carboxylesterase enzymes were predicted to be the best functional match for DUF538 superfamily (Gholizadeh 2014). These enzymes catalyze carboxylic esters to carboxylates and alcohols. DUF538 protein family had been already known to act on bacterial lipopolysaccharides and chlorophyll molecules in plant system (Gholizadeh and Baghbankohnehrouz 2013). Both lipopolysaccharides and chlorophyll molecules belong to carboxylic compounds and contain carboxylic ester bonds that could be targeted by DUF538 protein family as possible carboxylesterase. From this point of view, considering pectin as a carboxylic compound, the presently predicted pectin methylesterase function for DUF538 could be acceptable.
Based on present primary and tertiary structural similarity search data, it was revealed that plant DUF538 proteins are structurally similar to plant methylesterases. In plants, two groups of PME have identified. Group 1 PME have no N-terminus PRO region or no PME inhibitor domain, while group 2 PME contain 1–3 PRO region or PME inhibitor domain at their N-terminal part (Camardella et al. 2000). The PRO region shows a low level of amino acid identity between its different isoforms (Markovic and Janecek 2004; Bosch and Hepler 2005). Present similarity search data showed that DUF538 proteins are similar to the group 1 PME or C-terminal active part of group 2 PME. Based on our molecular docking data, DUF538 protein similar to the pectin methylesterase and pectinesterase is able to bind pectin molecule, showing the similar pattern of molecular interaction. Besides this, the molecular dynamics data analysis revealed that DUF538 protein structure is more stable and makes the stable interaction with pectin in comparision to the C-terminus end of PME and pectinesterases. These data together with our experimental data analysis strongly suggest that Plant DUF538 protein family might act as pectin methylesterase.
DUF538 proteins have been previously known to be expressed as stress-induced proteins in plants (Gholizadeh and Baghbankohnehrouz 2010; Takahashi et al. 2013). Also considering the stress-specific expression patterns for plant pectin methylesterases/pectinesterases (Pelloux et al. 2007), it seems that both DUF538 and pectin methylesterases/pectinesterases are involved in cell wall degradation under stress condition in plant system. Because cell wall acts as a barrier between the inside of the plant cells and its environment, its modifications are often associated with plant defense responses (Vorwerk et al. 2004). Despite plant pectin methylesterases/pectinesterases, DUF538 are known to be cytosolic proteins (Gholizadeh 2011). Thereby, DUF538 is suggested to act as cytosolic defense proteins and exert their defense responses to the cell wall during stress conditions in plants. It was speculated that DUF538 may help the catabolism of pectin in the cytosol to recycle carbon in stress-challenged plants. However, its mechanism of defense response needs to be more identified in details.
Conclusions
By using bioinformatics and experimental tools, DUF538 protein family were predicted to act as pectin methylesterases. Since both DUF538 and pectin methylesterases/pectinesterases are already known to belong to stress related proteins, thereby their possible cooperation in cell wall associated defense responses were predicted in plants. However the details of their cooperation are recommended to be investigated and identified in the future.
Acknowledgements
The author of this paper is thankful to the Research Institute for Fundamental Sciences (RIFS) (Grant No. 6906), University of Tabriz for the financial support.
Abbreviations
- BME
Beta mercaptoethanol
- DUF
Domain of unknown function
- EDTA
Ethylene diamine tetraacetic acid
- MBP
Maltose binding protein
- OD
Optical density
- PAGE
Polyacryl amide gel electrophoresis
- SD
Standard deviation
- SDS
Sodium dodecyl sulfate
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abraham, MJ, Spoel D Van der, Lindahl E, Hess B (2015) GROMACS user manual, Version 5.0. 7, Search PubMed, pp 85–86
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Hepler PK. Pectin methylesterases and pectin dynamics in pollen tube. Plant Cell. 2005;17:3219–3226. doi: 10.1105/tpc.105.037473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camardella L, Carratore V, Ciardiello MA, Balestrieri C, Giovane A. Kiwi protein inhibitor of pectin methylesterase-amino acid sequence and structural import two disulfide bridges. Eur J Biochem. 2000;267:4561–4565. doi: 10.1046/j.1432-1327.2000.01510.x. [DOI] [PubMed] [Google Scholar]
- Collmer N, Keen T. The role of pectic enzymes in plant pathogenesis. Annu Rev Phytopathol. 1986;24:383–409. [Google Scholar]
- Ding JL, Lee TT, Wang MM, Tai SS, Tzen JT. Cloning and expression of an acidic pectin methylesterase from jelly fig (Ficus awkeotsang) J Agric Food Chem. 2000;48:3052–3057. doi: 10.1021/jf000273d. [DOI] [PubMed] [Google Scholar]
- Futamura N, Mori H, Kouchi H, Shinohara K. Male flower specific expression of genes for polygalacturonase, pectin methylesterase and beta-3-glucanase in a diocious willow (Salix gilgiana Seemen) Plant Cell Physiol. 2000;41:16–26. doi: 10.1093/pcp/41.1.16. [DOI] [PubMed] [Google Scholar]
- Gainvors A, Frezier V, Lemaresquier H, Lequart C, Aigle M, Belarbi A. Detection of polygalacturonase, pectin lyase and pectin esterase activities in a Saccharomyces cerevisiae strain. Yeast. 1994;10:1311–1319. doi: 10.1002/yea.320101008. [DOI] [PubMed] [Google Scholar]
- Gholizadeh A. Heterologous expression of stress-responsive DUF538 domain containing protein and its morpho-biochemical consequences. Prot J. 2011;30:351–358. doi: 10.1007/s10930-011-9338-9. [DOI] [PubMed] [Google Scholar]
- Gholizadeh A. Prediction of tertiary structure homology between bactericidal/permeability increasing protein of innate immune system and hydrolase enzymes. Int J Biosci. 2014;5:1–6. [Google Scholar]
- Gholizadeh A. Plants water soluble chlorophyll binding proteins act as enzyme-inhibitor pairs. Russ J Plant Physiol. 2017;64:91–99. [Google Scholar]
- Gholizadeh A. Chlorophyll binding ability of non-chloroplastic DUF538 protein superfamily in plants. Proc Natl Acad Sci India Sec B. 2018;88:967–976. [Google Scholar]
- Gholizadeh A, BaghbanKohnehrouz B. Identification of DUF538 cDNA clone from Celosia cristata expressed sequences of none stressed and stressed leaves. Russ J Plant Physiol. 2010;57:247–252. [Google Scholar]
- Gholizadeh A, Baghbankohnehrouz S. DUF538 protein super family is predicted to be the potential homologue of bactericidal/permeability-increasing protein in plant system. Protein J. 2013;32:163–171. doi: 10.1007/s10930-013-9473-6. [DOI] [PubMed] [Google Scholar]
- Hess B, Bekker H, Berendsen HJ, Fraaije JG. LINCS: a linear constraint solver for molecular simulations. J Comput chem. 1997;18:463–1472. [Google Scholar]
- Kohli P, Kalia M, Gupta R. Pectin methylesterases: a review. J Bioprocess Biotech. 2015;5:1–7. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markovic O, Janecek S. Pectin methylesterases: sequence-structural features and phylogenetic relationships. Carbohydr Res. 2004;339:2281–2295. doi: 10.1016/j.carres.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Micheli F. Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 2001;6:414–419. doi: 10.1016/s1360-1385(01)02045-3. [DOI] [PubMed] [Google Scholar]
- Nakagami H, Sugiyama N, Mochida K, Daudi A, Yoshida Y, Toyoda T, Tomita M, Ishihama Y, Shirasu K. Large-scale comparative phosphoproteomics identifies conserved phosphorylation sites in plants. Plant Physiol. 2010;153:1161–1174. doi: 10.1104/pp.110.157347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux J, Rusterucci C, Mellerowicz EJ. New insights into pectin methylesterase structure and function. Trends Plant Sci. 2007;12:268–277. doi: 10.1016/j.tplants.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Peng CC, Hsiao ES, Ding JL, Tzen JT. Functional expression in Pichia pastoris of an acidic pectin methylesterase from jelly fig (Ficus awkeotsang) J Agric Food Chem. 2005;53:5612–5616. doi: 10.1021/jf0504622. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Yoshikawa M, Kamada A, Ohtsuki T, Uchida A, Nakayama K, Satoh H. The photoconvertible water-soluble chlorophyll-binding protein of Chenopodium album is a member of DUF538, a superfamily that distributes in Embryophyta. J Plant Physiol. 2013;170:1549–1552. doi: 10.1016/j.jplph.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorwerk S, Somerville S, Somerville C. The role of plant cell wall polysaccharide composition in disease resistance. Trends Plant Sci. 2004;9:203–209. doi: 10.1016/j.tplants.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Wakeley PR, Rogers HJ, Rozycka M, Greenland AJ, Hussey PJ. A maize pectin methylesterase-like gene, ZmC5, specifically expressed in pollen. Plant Mol Biol. 1998;37:187–192. doi: 10.1023/a:1005954621558. [DOI] [PubMed] [Google Scholar]
- Webb B, Sali A. Comparative protein structure modeling using MODELLER. Curr Protoc Bioinform. 2016;54:5.6.1–5.6.37. doi: 10.1002/cpbi.3. [DOI] [PMC free article] [PubMed] [Google Scholar]