Abstract
The aim of this study was to investigate soil lead pollution on biochemical properties and gene expression pattern of antioxidant enzymes in three wheat cultivars (Morvarid, Gonbad and Tirgan) at flag leaf sheath swollen stage. Lead (Pb(NO3)2) was used at four different concentrations (0, 15, 30 and 45 mg/kg of soil). The leaf and roots samples were taken at late-booting stage (Zadoks code, GS: 45). The results showed that lead heavy metal toxicity increased the expression of some genes and the activity of key enzymes of the antioxidant defense system in wheat. Moreover, the cell oxidation levels (MDA, LOX) enhanced under lead stress conditions. The relative gene expression and activity of antioxidant enzymes (CAT, SOD, GPX and APX) increased significantly in the both leaves and root tissues under lead stress conditions. The level of gene expression and enzymatic activity were higher in the root than the leaf tissue. There was no significant difference among cultivars in each of lead concentrations but Morvarid and Tirgan cultivars had more tolerance to toxic concentrations of lead when compared to Gonbad cultivar.
Keywords: Triticum aestivum L., Lead, Gene expression, Antioxidant enzymes
Introduction
Wheat (Triticum aestivum L.) is regarded as a main resource for food in most of the countries in the world, and also the main source of calories and proteins (Chaves et al. 2013). Environmental pollution with heavy metals has an ecological significance, as it contaminates agriculture, animals and humans. Heavy metals can damage photosynthetic pigments and reduce photosynthetic efficiency of growing plants (Shu et al. 2012). Lead (Pb) is not only an unessential element for plants, but also a soil contaminant; it can enter the plants and then the human body (Jaishnkar et al. 2014). Lead metal ions interact with cellular components including DNA and nuclear proteins, leading to DNA damage and organizational changes that may induce carcinogenesis (Beyersmann and Hartwig 2008). The level of reactive oxygen species (ROS) may be increased in lead-polluted plants (Sytar et al. 2013). ROS can promote the oxidation of DNA, proteins and fatty acids. It can inhibit the photosynthetic activity and ATP production. ROS has signaling roles in adapting plants to stress conditions (Sharma et al. 2012). Several enzymes are involved in plant antioxidant system, among which are ascorbate peroxidase (APX), catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR), guaiacol peroxidase (GPX), and peroxiredoxins (PRX). SODs are the first step of cellular defense to clean ROSs; they convert superoxide (O2−) and water (H2O) to hydrogen peroxide (H2O2) and molecular oxygen (O2). H2O2 is catalyzed by CAT into H2O and ½ O2 (Lamhamdi et al. 2011). APX is a chloroplast enzyme that quickly convert H2O2 into water and oxygen, a reaction mediated by ascorbate (AsA) as a specific electron donor. APX needs to overlap with GPX for detoxification of H2O2 (Sofo et al. 2015).
ROS can damage plant cells and disrupt cell structure (Tao et al. 2015). The level of ROS can be determined by evaluating thiobarbituric acid-reactive materials (TBARM) content (Kumar et al. 2013). Moreover, it was observed that stressful conditions significantly enhanced the activity of lipoxygenases (LOXs), which is an important enzyme related to the plant cell membrane lipid destruction (Lim et al. 2015). Concerns about increasing levels of heavy metals, especially lead, in Iranian soils has recently led to the Environmental Protection Administration (EPA) of the Islamic Republic of Iran to begin a collaborative research programme to establish the presence of heavy metals in Iranian soils, such as Golestan province. There are well-defined area of high soil lead levels with a series of regional hotspots clusters distributed along the central-southern part of Golestan and in the northeast, which are associated to the region of Golestan where mineral materials extractions have taken place for years (Mirzaei et al. 2014).
There are several reports about the effect of lead stress conditions on wheat seedling stages (Lamhamdi et al. 2011; Kaur et al. 2012). However, the gene expression pattern of the enzymes involved in antioxidant defense at booting stages still remains unknown. The aim of this study was to investigate soil lead stress conditions on biochemical properties and gene expression pattern of antioxidant enzymes in three wheat cultivars, Morvarid Gonbad and Tirgan, which are widely cultivated in Golestan province, Iran.
Materials and methods
Plant material and growth conditions
Three spring wheat cultivars (Triticum aestivum L.), Gonbad, Morvarid and Tirgan, were grown in a greenhouse in a completely randomized design with four replications. These cultivars are widely cultivated in Golestan province, Iran. Seeds were surface sterilized by submerging them in 0.01% HgCl2 solution for 3 min, rinsed with distilled water, and then placed on moist Whatman germination papers in petri dishes to germinate. The seeds were grown in a controlled environment under 16 h in light (28 °C) and 8 h in dark (18 °C). The relative humidity was 60–70% and the light intensity was approximately 350 μmol/ms. Germinated seeds were planted in pots containing five kg of loamy-clay soils. Lead nitrate treatment Pb(NO3)2 included concentrations of 0, 15, 30 and 45 mg/kg of soil, and the samples were taken at late-booting stage (Zadoks code, GS: 45). All plants had grown 72–75 days. Tissue samples (leaves and roots) were kept at − 80° C for the measurement of biochemical traits and RNA extraction.
Determination of lead accumulation
Determination of lead content in root and leaf samples was done described in Verma and Dubey (2003). Briefly, fresh samples were surface sterilized with 1 M HCl then with 1 mM Na2EDTA to resolve excess surface bound lead, then dried in 70 °C. Dried samples were finely powdered and then digested by H2SO4. Digested samples were dissolved in distilled water and lead content was determined after the realization of a standard curve using atomic absorption spectrometer. All measurements were performed in four replicates and it was expressed in mg/g dry weight.
Chlorophyll content measurements
Chlorophyll content were measured using the Porra et al. (1989) method. Leaf samples (50 mg) were homogenized using acetone 80% (10 ml) and centrifuged at 10,000 g for 15 min. The absorbance of the solution was recorded by spectrophotometer (Uvikon, Kontron, Zurich, Switzerland) at wavelengths of 646.6 and 663.6 nm.
Determination of TBARM
The measurements of oxidative stress, malondialdehyde (MDA), final stable product and large oxidation molecules have been measured. TBARM (Thiobarbituric acid-reactive material) content was determined using the method described by Hagege et al. (1990). Tissue samples (50 mg) was homogenized using 1 ml trichloroacetic acid (10% w/v). The homogenate was washed with 10 ml acetone, vortexed, and then centrifuged at 5000 g for 15 min. The pellet fraction was suspended in 5 ml acetone, vortexed, and then centrifuged at 5000 g for 10 min. Pellet washing was repeated four times. Then, 3 ml of hypo-phosphoric acid (1%) and 1 ml of thiobarburic acid (w/v 0.6%) were added to the pellet to make a suspension. The suspension which was placed at 100 °C for 30 min. The reaction was then stopped by rapid cooling of the pipes on ice. The absorbance of the resulting solution was measured at 532 and 590 wavelengths using spectrophotometer (Uvikon, Kontron, Zurich, Switzerland). Finally, TBARM content was expressed as μM per 1 g of plant fresh weight (FW).
Determination of LOX
The method described by Zhuang et al. (1994) with some modifications was used to quantify LOX. Briefly, 2.5 g sample was dissolved in pure cold water and then centrifuged for 10 min at about 12,000 g. The top solution was removed and the rest was purified using PD 10 gel column. An equal amount of potassium phosphate buffer (6.50 Mm) was added. The solution was homogenized using sodium salt buffer and linoleic acid (80 nmol) and the absorbance measured at 234 nm using spectrophotometer (Uvikon, Kontron, Zurich, Switzerland). LOX activity was expressed as nM/mg of protein/min.
Antioxidant enzyme activity
Superoxide dismutase (SOD) activity was determined using the method described by Giannopolitis and Ries (1977). Reaction mixture (3 ml) contained 50 mM potassium phosphate buffer (pH 7.8), 2 μM riboflavin, 0.1 mM EDTA, 13 mM methionine, 75 μM nitroblue tetrazolium chloride (NBT), 0.05 M sodium carbonate (pH 10.2) and enzyme extract (100 μl). The reaction mixture was illuminated for 15 min at light intensity of 5000 lx. The photo reduction of NBT (formation of purple formazone) was measured at 560 nm. One unit of SOD activity is defined as the amount of enzyme required to 50% inhibition in NBT reduction. SOD activity was expressed as unit/mg protein.
Catalase (CAT) activity was determined using H2O2 at 240 nm absorbance (Loggini et al. 1999). The reaction mixture (3 ml, pH 7.2) contained 50 mM phosphate buffer 15 mM H2O2 and 100 μl of crude enzyme extract. The activity was calculated using the extinction coefficient 39,400 M per cm. The CAT activity was expressed as μM/mg of protein/min.
Ascorbate peroxidase activity (APX) was determined upon decrease of ascorbate level. Reaction buffer (3 ml) was composed of 50 mM potassium phosphate buffer (pH 7.2), 0.5 mM ascorbic acid, H2O2 and 100 μl of crude enzyme extract by Nakano and Asada (1981). The activity was calculated using the extinction coefficient 2800 M−1 cm−1. Finally, APX activity was expressed as nM/mg of protein/min.
The reaction mixture for guaiacol peroxidase (GPX) activity was composed of 50 mM potassium phosphate, 9 mM guaïacol buffer (pH 7. 2), 50 μL H2O2 and 100 mL of crude enzyme extract. The enzyme activity was measured by monitoring the increase in absorbance at 470 nm extinction coefficient of 2470 M per cm during polymerization of guaïacol (Hiner et al. 2000). Finally, GPX activity was expressed as μM/mg of protein/min.
Gene expression analysis
To evaluate the expression of genes, total RNA was extracted from 100 mg of samples prepared using the Biozol kit (Cinagen, Iran). After DNaseI treatment, extracted RNA was quantified using spectrophotometer (Uvikon, Kontron, Zurich, Switzerland) and its quality was verified using 1.5% agarose gel electrophoresis. The cDNA strand was synthesized from 2 μg total RNA as the template with an RT reagent kit (Fermentas company, Vilnius, Lithuania), in accordance with the manufacturer’s protocol.
The Specific primers for Quantitative real-time (qRT-PCR) PCR were designed using Primer3 software. The sequence of the primers used to assess the gene expression pattern of antioxidant enzymes (SOD, CAT, APX and GPX) are shown in Table 1. The housekeeping gene GAPDH was used as endogenous control (Gonçalves et al. 2005). The qRT-PCR was implemented in an iCycler iQ5 thermocycler (Bio Rad Company). The quantity of mRNAs was measured by SYBR Green method using SYBR Biopars Kit (GUASNR, Iran). Each sample was evaluated in 3 repetitions and two biological replications. Relative gene expression was calculated by Pfaffl et al. (2001) formula, 2−ΔΔCT in relation to control samples (0, non-use of lead).
Table 1.
Features of primers applied in qRT-PCR experiment
| Primer | Primers pair sequence (5′–3′) | Tm value (°C) |
|---|---|---|
| SOD for | 5′CACACACCAAACCACACTATCCA3′ | 58.4 |
| SOD rev | 5′TGTCTACTCGGACAAATCATGC3′ | 58.4 |
| CAT for | 5′CCATGAGATCAAGGCCATCT3′ | 61 |
| CAT rev | 5′ATCTTACATGCTCGGCTTGG3′ | 61 |
| APX for | 5′GCCATGGCTAAGAACTACCCC3′ | 59 |
| APX rev | 5′CGCAGTTCTTCTCGGCGAT3′ | 59 |
| GPX for | 5′ACCGTGAGCGAGGACTACCT3′ | 56 |
| GPX rev | 5′CGTCAAGTGAGCCTTAGC3′ | 56 |
| GAPDH for | 5′TCACTGACAAGGACAAGGCTG3′ | 64 |
| GAPDH rev | 5′CTGGCTTCGCAAGTCTAACAG3′ | 64 |
Statistical analysis
Statistical analysis was carried out using the SAS 9.1 software (SAS Institute Inc., Cary, NC, USA), in which the comparison of means was carried out by least significant difference (LSD) test at 0.05 probability level. Analysis of gene expression data was performed using REST software (Pfaffl et al. 2002).
Results
Lead accumulation
In both leaves and roots tissues, the lead content increased upon increase of lead concentrations in all the cultivars. There was no significant difference among cultivars in each of lead concentrations. The lead content in all concentrations of lead in root tissue was significantly higher than leaf (Fig. 1).
Fig. 1.

The amount of lead content in wheat leaves and roots. The vertical lines represent ± SE of the mean of four replicates. Significant difference at P < 0.05 level
Effects of lead on chlorophyll content
In wheat leaves, the chlorophyll content decline significantly at 15, 30 and 45 mg/kg in three cultivars, compared to the control conditions. While, the severity of the reduction in chlorophyll content was higher in the Gonbad than Morvarid and Tirgan (Fig. 2).
Fig. 2.

Effects of different concentrations of lead on chlorophyll content in wheat leaves. The vertical lines represent ± SE of the mean of four replicates. Significant difference at P < 0.05 level
Effects of lead on lipid peroxidation
With increasing lead concentration, MDA content increased significantly in root tissue at concentrations of 30 and 45 mg/kg. Also, the highest MDA content was found in root tissue in Gonbad cultivar at 15, 30 and 45 mg/kg Pb. MDA content in wheat roots was significantly higher than the leaves at all concentrations of lead. With increasing lead concentrations, the MDA content increased slightly in the three cultivars in root (Fig. 3). It was revealed that the LOX activity in the cultivars treated with 15, 30 and 45 mg/kg lead was significantly increased in both leaves and root tissues when compared to control conditions (Fig. 4).
Fig. 3.

Effects of different concentrations of lead on the TBARM activity in leaf and root tissues of wheat. The vertical lines represent ± SE of the mean of four replicates. Significant difference at P < 0.05 level
Fig. 4.

Effects of different concentrations of lead on the content LOX in leaf and root tissues of wheat. The vertical lines represent ± SE of the mean of four replicates. Significant difference at P < 0.05 level
Genes expression analysis and activity assay of the antioxidant enzymes
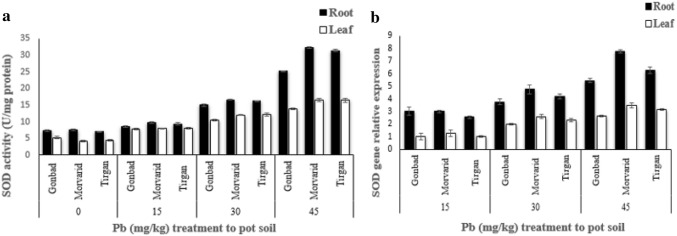
The activity of the SOD enzyme increased in both leaf and root tissues, depending on the concentrations of lead. SOD activity was higher in root than the leaf tissue. The highest enzymatic activity was observed in root of Morvarid and Tirgan cultivars at 45 mg/kg soil (Fig. 5a). In general, SOD gene expression increased upon increase of lead concentrations in both leaf and root tissues. Compared to leaf samples, root samples exhibited higher level of SOD gene expression. At the concentration of 45 mg/kg soil in both leaf and root tissues, the Morvarid and Tirgan revealed that the highest gene expression, compared to Gonbad (Fig. 5b).
Fig. 5.
Effects of different concentrations of lead on SOD enzyme activity (a) and SOD gene expression (b) leaf and root tissues of wheat. The vertical lines represent ± SE of the mean of four replicates. Significant difference at P < 0.05 level
In all the cultivars, the CAT enzyme activity increased upon increase of lead concentrations in both leaves and roots. The CAT enzyme activity was generally higher in root samples than leaf samples. The minimum CAT activity (in both leaf and root tissues) in 45 mg/kg soil belonged to Gonbad cultivar (Fig. 6a). In both leaf and root tissues, CAT gene expression increased up to 30 mg/kg soil and then decreased. Regardless of lead concentrations, CAT gene expression was higher in root tissue than leaf tissue (Fig. 6b).
Fig. 6.
Effects of different concentrations of lead on CAT enzyme activity (a) and CAT gene expression (b) leaf and root tissues of wheat. The vertical lines represent ± SE of the mean of four replicates. Significant difference at P < 0.05 level
In both leaves and roots tissues, GPX activity and GPX gene expression increased upon increase of lead concentrations in all the cultivars. Regardless of lead concentrations, GPX activity and GPX gene expression was significantly more active in root tissues than leaf. The maximum GPX activity and GPX gene expression was observed in root of Morvarid cultivar at 45 mg/kg soil (Fig. 7a, b).
Fig. 7.
Effects of different concentrations of lead on GPX enzyme activity (a) and GPX gene expression (b) leaf and root tissues of wheat. The vertical lines represent ± SE of the mean of four replicates. Significant difference at P < 0.05 level
APX enzyme activity was found to be affected by lead concentrations. In both leaf and root samples APX activity increased up to 30 mg/kg soil and then decreased in concentration of 45 mg/kg soil, compared to control conditions. The level of activity of APX enzyme in all concentrations of lead in root tissue was significantly higher than leaf. The APX enzyme in Morvarid cultivar was the most active in root tissue at a concentration of 30 mg/kg soil (Fig. 8a). The expression of APX gene in the root tissue was significantly higher than the leaf. The expression of APX gene in root tissue increased with increasing lead concentrations, then decreased at a concentration of 45 mg/kg soil. In the leaf tissue, the amount of expression of the gene increased slightly, while at a concentration of 45 mg/kg soil significantly decreased, so that the expression of the APX gene in the Gonbad cultivar had the greatest decrease compared to control conditions (Fig. 8b).
Fig. 8.
Effects of different concentrations of lead on APX enzyme activity (a) and APX gene expression (b) leaf and root tissues of wheat. The vertical lines represent ± SE of the mean of four replicates. Significant difference at P < 0.05 level
Discussion
Despite organic contaminants that are degraded into much smaller safe molecules, heavy metals remain intact and contaminate the soil (Meagher and Heaton 2005). High concentrations of lead triggers the production of ROS, increasing the activity of antioxidant enzymes in plants. Antioxidant enzymes eliminate ROS, thus reducing damage to membranes and oxidative stress, resulting in greater resistance to oxidative stress (Mittler 2002).
The photosynthetic apparatus is adversely affected by lead toxicity. Plants exposed to lead stress show a decline in photosynthetic rate which results from distorted chloroplast, restrained synthesis of chlorophyll, plastoquinone and carotenoids, obstructed electron transport, inhibited activities of Calvin cycle enzymes, as well as deficiency of CO2 as a result of stomatal closure (Sharma and Dubey 2005). Lead stress inhibits chlorophyll synthesis by causing impaired uptake of essential elements such as magnesium and iron (Naz et al. 2015). It damages the photosynthetic apparatus due to its affinity for protein N- and S- ligands (Sharma and Dubey 2005). The result showed that the leaf chlorophyll content decreased significantly in three cultivars especially Gonbad cultivar, under lead stress conditions (Fig. 2). Lead stress has been associated with reduction in chlorophyll content, which is due decrease of the number of grana in chloroplast, also the key nutrients (Mg, Fe and Cu) have been replaced by lead and lead toxicity declined Mg, Fe and Cu content (Akinci et al. 2010). The effect of heavy metals is associated with increased ROS aggregation in plant cells and induction of oxidative stress that leads to photosynthetic dysfunction (Balakhnina and Nadezhkina 2017). Heavy metals increase the resistance of stomata and decrease transpiration. Therefore, it prevents the flow of water and nutrients from soil to the plant and represses growth and biomass accumulation (Islam et al. 2008). Lead (Pb) physically block the uptake of water from root to shoot, that is associated to the rate of photosynthesis, specially associated to decreased water content, CO2 absorption and ability to maintain cell turgor and cell plasticity (Azmat et al. 2009). Leaf samples exposed to lead stress showed a number of morphological changes in the leaf epidermis structure, including a reduction in the cell size, most abundant wax coating, and an increase in the density of trichomes and stomata (number per unit area) with synchronized reduction in the size of the guard cells tissues (Weryszko-Chmielewska and Chwil 2005). It is assumed that these events are the plant adaptation mechanisms that reduce the content of the toxic metal in internal leaf tissues (Weryszko-Chmielewska and Chwil 2005).
Lipid peroxidation is regarded as indicator for oxidative stress, involves oxidative degradation of polyunsaturated fatty acyl residues of membranes (Girroti 1990). Lead stress induces lipid peroxidation, decreases the level of saturated fatty acids and increases the content of unsaturated fatty acids of membrane in plant (Halliwell and Gutteridge 2015). Heavy metals can induce the LOX activity producing hydroperoxide derivates, which destroys membranes and induce death of cells (Rucinska and Gwozdz 2005). Moreover, hydroperoxides production LOX activity strongly correlated also with superoxide anion generation in senescing plant tissues (Gallego et al. 1996). Our result showed, under different concentrations of lead, LOX was higher than control (Fig. 3). It clearly resulted due to the increase in level of ROS, because it is well known that lead stress has the ability to bind thiols groups and disrupts redox status of the cell thereby enhancing the production of ROS (Serida et al. 2008). It suggests that lead stress caused severe oxidative stress as it generated more MDA with increasing lead concentration. The effect of heavy metals on LOX activity have been reported in various studies (Serida et al. 2008; Tamas et al. 2008; Sfaxi-Bousbih et al. 2010).
We observed over-expression of genes and increased activity of antioxidant enzymes (SOD, CAT, GPX and APX) following lead stress conditions in both leaf and root tissues. Increase in SOD activity is due to counteracting oxidative stress and protecting the plant’s defense system from oxidative damage (Malecka et al. 2012). SOD in many cellular organs is considered as the first defense against ROS. Generally, superoxide is the first free radicals that are produced during stress. SOD quickly converts superoxide radicals into hydrogen peroxide and oxygen. By eliminating superoxide, SOD plays a critical role in antioxidant systems (Gill and Tuteja 2010).
The CAT gene expression and CAT enzymatic activity increases after exposing to lead (Fig. 5). Our findings were consistent with previous reports (Malar et al. 2014). Catalase is the main enzyme in neutralizing ROS toxicity, and reducing H2O2 content in plant cells. CAT converts H2O2 into water and oxygen and play important roles in plant adaptation to environmental stresses. At high lead concentrations, H2O2 and other free radicals are increased in the cell, inhibiting metabolism and decreasing the activity of CAT (Du et al. 2008). In both leaf and root tissues, CAT activity and CAT gene expression increased up to 30 mg/kg soil and then decreased (Fig. 5). Decrease of CAT activity in high lead concentrations is probably due to the reduction of enzyme synthesis or the change in the enzyme subunit composition, or the activation of peroxisome proteases. CAT contains Fe and Lead reduces Fe, resulting in decrease of CAT enzymatic activity (Rebecca and Geoffrey 2017).
We found that lead increases GPX enzymatic activity and GPX gene expression in both leaf and root tissues (Fig. 6) that was consistent with the findings of previous studies (Malar et al. 2014). GPX is one of the major enzymes involved in ROS under stress. H2O2 decomposition by GPX is dependent on oxidation of the substrate. GPX can act as effective receptor for ROS and peroxidation radicals by increasing heavy metals in plant cells (Sofo et al. 2015). APX, a key enzyme in the ascorbate cycle, is found in chloroplasts and cytosol. APX converts H2O2 into water by mediation of ascorbate (AsA) as a particular electron donor (Sofo et al. 2015). The enzyme activity increases with excessive concentrations of ROS. Despite CAT, which can destroy H2O2, this enzyme needs to act with GPX to detoxify H2O2. Increased activity of APX in plants after exposure to lead treatment has also been reported in another study (Malar et al. 2014). Increase of antioxidant enzyme activity after increase of lead concentrations in wheat activates defense mechanisms and protects the plant against oxidative stresses.
The results showed an increased uptake of lead with increase in lead concentration. Also, the lead was less translocated from root to leaf, therefore the absorbed lead was accumulated at greater level in root than in leaf (Fig. 1). It has demonstrated that lead is absorbed mainly by root hairs, where different root tissues act as barriers to apoplastic and symplastic lead transport and hence lead transport to shoot gets limited (Trvedi and Erdei 1992; Kabata-Pendias 2010). A significantly higher concentration of lead in root than in shoot was observed by the other researchers (Mishra and Singhal 1992; Verma and Dubey 2003; Sekara et al. 2005; Bharwana 2013).
Lead stress increases the formation of ROS in plants leading to oxidative stress, such as hydrogen peroxide (Malecka et al. 2012). This hydrogen peroxide can lead to membrane degradation and lipid peroxidation (increased levels of MDA and LOX). When the levels of ROSs are high, the activity of antioxidant enzymes increases. It seems that these events will happen in succession. Biochemical tolerance to lead stress conditions is related to the capacity of the plants to activation of the antioxidant defense system (Sharma and Dubey 2005). In general, our results showed that expression and activity of antioxidant enzymes (SOD, CAT, GPX and APX) increased following plant treatment with lead.
There was no significant difference among cultivars in each of lead concentrations. However, Morvarid and Tirgan cultivars were found to be more tolerant to toxic concentrations of lead when compared to Gonbad cultivar. Due to the fact that there is a well-defined area of high soil lead levels with a series of regional hotspots clusters distributed along the central-southern part of Golestan and in the northeast (Mirzaei et al. 2014). Therefore, it is recommended to avoid cultivating the Gonbad cultivar in these hotspots regions.
Conclusion
The presence of lead in soil can trigger the expression of the key enzymes involved in plant defense mechanism, including antioxidant enzymes. The levels of antioxidant enzymes involved in detoxification of H2O2 (SOD, CAT, GPX and APX) increased in wheat leaf and root tissues after exposing to toxic amounts of lead. No significant difference was observed among cultivars in each of lead concentrations. Root samples showed a higher level of enzymatic activity and gene expression in antioxidant enzyme when compared to leaf tissues. Morvarid and Tirgan cultivars had more tolerance to toxic concentrations of lead when compared to Gonbad cultivar.
Acknowledgements
We thank Seed and Plant Improvement Institute (SPII) for providing the genetic materials. This work was supported by a grant from Gorgan University of Agricultural Sciences and Natural Resources.
Abbreviations
- Pb(NO3)2
Lead (II) nitrate
- GS
Growth stage
- MDA
Malondialdehyde
- LOX
Lipoxygenase
- CAT
Catalase
- SOD
Superoxide dismutase
- GPX
Guaiacol peroxidase
- APX
Ascorbate peroxidase
- ROS
Reactive oxygen species
- DNA
Deoxy ribonucleic acid
- ATP
Adenosine tri phosphate
- TBARM
Thiobarbituric acid-reactive materials
- O2−
Superoxide
- H2O
Water
- O2
Oxygen
- H2O2
Hydrogen peroxide
- EPA
Environmental protection administration
- HgCl2
Mercury (II) chloride
Author contributions
Study conception and design: SN, SB and AY. Acquisition of data: HK and SB. Analysis and interpretation of data: HK and SN. Drafting of manuscript: HK, SB. All authors read and approved the final manuscript.
Funding
This study has been supported by Gorgan University of Agricultural Sciences and Natural Resources (Grant Number: 95-354-71).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Saeid Navabpour, Email: s.navabpour@gau.ac.ir.
Ahad Yamchi, Email: yamchi@gau.ac.ir.
Saeed Bagherikia, Email: s.bagherikia@areeo.ac.ir.
Haniyeh Kafi, Email: kafi.haniyeh@gmail.com.
References
- Akinci IE, Akinci S, Yilmaz K. Response of tomato (Solanum lycopersicum L.) to lead toxicity: growth, element uptake, chlorophyll and water content. Afr J Agric Res. 2010;5:416–423. [Google Scholar]
- Azmat R, Haider S, Riaz M. An inverse relation between Pb 2+ and Ca 2+ ions accumulation in Phaseolus mungo and Lens culinaris under Pb stress. Pak J Botany. 2009;41:2289–2295. [Google Scholar]
- Balakhnina TI, Nadezhkina ES. Effect of selenium on growth and antioxidant capacity of Triticum aestivum L. during development of lead-induced oxidative stress. Russ J Plant Physiol. 2017;64:215–223. [Google Scholar]
- Beyersmann D, Hartwig A. Carcinogenic metal compounds: recent insight into molecular and cellular mechanisms. Arch Toxicol. 2008;82:481–493. doi: 10.1007/s00204-008-0313-y. [DOI] [PubMed] [Google Scholar]
- Bharwana S. Alleviation of lead toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes suppressed lead uptake and oxidative stress in cotton. J Bioremed Biodeg. 2013;4:4160–4172. doi: 10.1016/j.ecoenv.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Chaves MS, Martinelli JA, Wesp-Guterres C, Graichen FAS, Brammer SP, Scagliusi SM, Lau EY. The importance for food security of maintaining rust resistance in wheat. Food Secur. 2013;5:157–176. [Google Scholar]
- Du YY, Wang PC, Chen J, Song CP. Comprehensive functional analysis of the catalase gene family in Arabidopsis thaliana. J Integr Plant Biol. 2008;50:1318–1326. doi: 10.1111/j.1744-7909.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- Gallego SM, Benavides MP, Tomaro ML. Effect of heavy metal ion excess on sunflower leaves: evidence for involvement of oxidative stress. Plant Sci. 1996;121:151–159. [Google Scholar]
- Giannopolitis CN, Ries SK. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Girroti AW. Photodynamic lipid peroxidation in biological systems. Photochem Photobiol. 1990;51:497–509. doi: 10.1111/j.1751-1097.1990.tb01744.x. [DOI] [PubMed] [Google Scholar]
- Gonçalves S, Cairney J, Maroco J, Oliveira MM, Miguel C. Evaluation of control transcripts in real-time RT-PCR expression analysis during maritime pine embryogenesis. Planta. 2005;222:556–563. doi: 10.1007/s00425-005-1562-0. [DOI] [PubMed] [Google Scholar]
- Hagege D, Nouvelot A, Boucaud J, Gaspar T. Malondialdehyde titration with thiobarbiturate in plant extracts: avoidance of pigment interference. Phytochem Anal. 1990;1:86–89. [Google Scholar]
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. New York: Oxford University Press; 2015. [Google Scholar]
- Hiner A, Rodríguez-López JN, Arnao MB, Raven EL, García-Cánovas F, Acosta M. Kinetic study of the inactivation of ascorbate peroxidase by hydrogen peroxide. Biochem J. 2000;348:321–332. [PMC free article] [PubMed] [Google Scholar]
- Islam E, Liu D, Li T, Yang X, Jin X, Mahmood Q, Tian S, Li J. Effect of Pb toxicity on leaf growth, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J Hazard Mater. 2008;154:914–926. doi: 10.1016/j.jhazmat.2007.10.121. [DOI] [PubMed] [Google Scholar]
- Jaishnkar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Environ Exp Bot Interdiscipl Toxicol. 2014;7:60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabata-Pendias A. Trace elements in soils and plants. London: CRC Press; 2010. [Google Scholar]
- Kaur G, Singh HP, Batish DR, Kumar RK. Growth, photosynthetic activity and oxidative stress in wheat (Triticum aestivum) after exposure of lead to soil. J Environ Biol. 2012;33:265–269. [PubMed] [Google Scholar]
- Kumar SR, Mahata KR, Pratap Singh D. Differential responses of antioxidant system and photosynthetic characteristics in four rice cultivars differing in sensitivity to sodium chloride stress. Acta Physiol Plant. 2013;10:2915–2926. [Google Scholar]
- Lamhamdi M, Bakrim A, Aarab A, Lafont R, Sayah F. Lead phytotoxicity on wheat (Triticum aestivum L.) seed germination and seedlings growth. CR Biol. 2011;334:118–126. doi: 10.1016/j.crvi.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Lim CW, Han SW, Hwang IS, Kim DS, Hwang BK, Lee SC. The pepper lipoxygenase Ca LOX1 plays a role in osmotic, drought and high salinity stress response. Plant Cell Physiol. 2015;56:930–942. doi: 10.1093/pcp/pcv020. [DOI] [PubMed] [Google Scholar]
- Loggini B, Scartazza A, Brugnoli E, Navari-Izzo F. Antioxidative defense system, pigment composition, and photosynthetic efficiency in two wheat cultivars subjected to drought. Plant Physiol. 1999;119:1091–1100. doi: 10.1104/pp.119.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malar S, Vikram SS, Favas PJ, Perumal V. Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths [Eichhornia crassipes (Mart.)] Bot Stud. 2014;55:1–11. doi: 10.1186/s40529-014-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecka A, Piechalak A, Mensinger A, Hanć A, Baralkiewicz D, Tomaszewska B. Antioxidative defense system in Pisum sativum roots exposed to heavy metals (Pb, Cu, Cd, Zn) Polish J Environ Stud. 2012;21:1721–1730. [Google Scholar]
- Meagher RB, Heaton AC. Strategies for the engineered phytoremediation of toxic element pollution: mercury and arsenic. J Ind Microbiol Biotechnol. 2005;32:502–513. doi: 10.1007/s10295-005-0255-9. [DOI] [PubMed] [Google Scholar]
- Mirzaei R, Ghorbani H, Moghaddas NH, Martín JAR. Ecological risk of heavy metal hotspots in topsoils in the Province of Golestan, Iran. J Geochem Explor. 2014;147:268–276. [Google Scholar]
- Mishra RK, Singhal GS. Function of photosynthetic apparatus of intact wheat leaves under high light and heat stress and its relationship with peroxidation of thylakoid lipids. Plant Physiol. 1992;98:1–6. doi: 10.1104/pp.98.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Naz A, Khan S, Muhammad S, Khalid S, Alam S, Siddique S, Ahmed T, Scholz M. Toxicity and bioaccumulation of heavy metals in spinach (Spinacia oleracea) grown in a controlled environment. Int J Environ Res Public Health. 2015;12:7400–7416. doi: 10.3390/ijerph120707400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:445–455. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:336–347. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra R, Thompson W, Kriedemann P. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim et Biophys Acta-Bioenerg. 1989;975:384–394. [Google Scholar]
- Rebecca EK, Geoffrey EH. An assessment of techniques to manipulate oxidative stress in animals: current topics in medicinal chemistry. Funct Ecol. 2017;31:9–21. [Google Scholar]
- Rucinska R, Gwozdz EA. Influence of lead on membrane permeability and lipoxygenase activity in lupine roots. Biol Plant. 2005;49:617–619. [Google Scholar]
- Sekara A, Poniedzialeek M, Ciura J, Jedrszczyk E. Cadmium and lead accumulation and distribution in the organs of nine crops: implications for phytoremediation. Polish J Environ Stud. 2005;14:509–516. [Google Scholar]
- Serida K, Ali MB, Hahn EJ, Paek KY. Copper toxicity in Withania somnifera: growth and antioxidant enzymes responses of in vitro grown plants. Environ Exp Bot. 2008;64:279–285. [Google Scholar]
- Sfaxi-Bousbih A, Chaoui A, El Ferjani E. Cadmium impairs mineral and carbohydrate mobilization during the germination of bean seeds. Ecotoxicol Environ Saf. 2010;73:1123–1129. doi: 10.1016/j.ecoenv.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Sharma P, Dubey RS. Lead toxicity in plants. Braz J Plant Physiol. 2005;17:35–52. [Google Scholar]
- Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage and antioxidative defense mechanism in plants under stressful conditions. J Bot. 2012 doi: 10.1155/2012/217037. [DOI] [Google Scholar]
- Shu X, Yin L, Zhang Q, Wang W. Effect of Pb toxicity on leaf growth, antioxidant enzyme activities, and photosynthesis in cuttings and seedlings of Jatropha curcas L. Environ Manag. 2012;19:893–902. doi: 10.1007/s11356-011-0625-y. [DOI] [PubMed] [Google Scholar]
- Sofo A, Scopa A, Nuzzaci M, Vitti A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int J Mol Sci. 2015;16:13561–13578. doi: 10.3390/ijms160613561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sytar O, Kumar A, Latowski D, Kuczynska P, Strzałka K, Prasad M. Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol Plant. 2013;35:985–999. [Google Scholar]
- Tamas L, Dudíkova J, Durcekova K, Huttova J, Mistrik I, Zelinova V. The impact of heavy metals on the activity of some enzymes along the barley root. Environ Exp Bot. 2008;62:86–91. [Google Scholar]
- Tao L, Guo M, Ren J. Effects of cadmium on seed germination, coleoptile growth, and root elongation of six pulses. Pol J Environ Stud. 2015;24:295–299. [Google Scholar]
- Trvedi S, Erdei L. Effects of cadmium and lead on the accumulation of Ca2+ and K+ and on the influx and translocation of K+ in wheat of low and high K+ status. Physiol Plant. 1992;84:94–100. [Google Scholar]
- Verma S, Dubey R. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003;164:645–655. [Google Scholar]
- Weryszko-Chmielewska E, Chwil M. Lead-induced histological and ultrastructural changes in the leaves of soybean (Glycine max (L.) Merr.) Soil Sci Plant Nutr. 2005;51:203–212. [Google Scholar]
- Zhuang H, Barth M, Hildebrand D. Packaging influenced total chlorophyll, soluble protein, fatty acid composition and lipoxygenase activity in broccoli florets. J Food Sci. 1994;59:1171–1182. [Google Scholar]