Abstract
The study aims to determine the timing of application for high efficacy of Metarhizium anisopliae as a biocontrol agent. A field experiment was undertaken with M. anisopliae applied to the soil at five intervals during the peanut crop lifecycle, at seed germination (day 0) through to pod filling period [75 days after sowing (DAS)], and assessed the change of M. anisopliae density by sampling rhizospheric soil, subsequently at regular intervals and testing counts (CFU/g dry soil) through to harvest. The crop was sown into soil with an established white grub population, with larval density determined at harvest when the trial was concluded. Applications at 0, 15 and 30 days in the crop growth cycle, saw M. anisopliae mean propagule counts drop significantly after 15 days before increasing over the following 15–45 days. We observed an elevated mean increase in counts 30–45 days after application at the early flowering stage (30 DAS). Irrespective of application timing, in general, M. anisopliae densities declined to less than the initial 10% in the late stages of peanut development. At harvest, larval densities in all M. anisopliae treatments were significantly less compared to the control, with the highest mortality (72%) in M. anisopliae treatment applied at early flowering (30 DAS). Relationship analysis showed that white grub density was significantly related to peanut yield. A regression of yield on number of damaged pods also supported that treatment at the early flowering caused the highest impact in terms of reducing damage to pods and improving yield. These results suggest that applying M. anisopliae at the early flowering stage optimizes survival of M. anisopliae in the soil profile, meaning greater probability of larvae contacting the pathogen, leading to greater mortality.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02178-5) contains supplementary material, which is available to authorized users.
Keywords: Entomopathogens, Insect-pathogen interactions, Peanut phenophases, Integrated pest management (IPM), Rhizosphere, Holotrichia sp.
Introduction
White grubs (Coleoptera: Scarabaeidae), comprise a complex of scarab species including Holotrichia parallela Motschulsky, H. oblita Faldermann, Anomala corpulenta Motschulsky, and A. exoleta Faldermann with the soil dwelling larval stage presenting a significant pest problem to peanut production in China (Hu 2004; Luo et al. 2009; Han et al. 2011; Jiang et al. 2013). Larvae usually damage young seedlings, roots, and pods of peanut sometimes resulting in significant yield losses of 48% and greater (Xu 1991; Jiang et al. 2013). The predominant white grub pest species in peanut crops are H. parallela and H. oblita. These scarabs have one generation per year, with the third instar larvae overwintering at 50–60 cm in the soil profile before moving closer to the surface to resume feeding and development in spring (March–May). After pupation, emergence of adults usually occurs in early to middle of June (early summer). Adults can fly and feed (mainly on tree species), mate and return to the field to lay eggs. Oviposition peaks approximately 2 weeks after adult emergence. Out of the three larval stages, the second and early third instars severely affect peanut crop. Therefore, the late instar grubs occurring in spring attack peanut seeds and seedlings, but later in spring, the growing plants and fruit of peanuts are damaged by the new spring larval generation.
Normally organophosphate insecticides including phoxim, chlorpyrifos or thiamethoxam are applied by the growers to quickly control the larvae, however these compounds are highly toxic and randomly affect the non-target species (Hallmann et al. 2014; Douglas et al. 2015; Wood and Goulson 2017), whereas below ground, these chemicals widely impact on both farmland ecology and surrounding landscape (Imfeld and Vuilleumier 2012; Shao and Zhang 2017). To ensure food safety and security, efforts have been made by the European Union (Sattler et al. 2007; Schäfer et al. 2019) and more recently China (Jin and Zhou 2018) to minimize the impact of insecticides on the wider environment.
Generally, entomopathogenic fungi are considered important alternatives to insecticides, providing effective control of a target pest minimizing yield losses while maintaining agro-ecosystems. Metarhizium anisopliae (Metchnikoff) Sorokin (Hypocreales: Clavicipitaceae) is an entomopathogenic fungus with many strains shown to significantly affect mortality in across range of target insect species both under laboratory and field conditions (Pu and Li 1996; Skinner et al. 2014; Dar et al. 2017). Field and laboratory trials have also demonstrated the effectiveness of M. anisopliae to kill larvae and reduce damage to populations on limited scale to conserve the sustainable agro-ecological environment (Liu et al. 2011; Nong et al. 2011; Chelvi et al. 2011; Skinner et al. 2014).
The effectiveness of pest control and the virulence of M. anisopliae in the soil are determined partly by a minimum population baseline of pest defined as the quantity necessary to establish successful infection and maintain long-term pest control in the field (Paulitz 2000). In addition, to successfully control the pests, an effective contact is required between M. anisopliae and the target organism. In this regard, the ability of the fungus to persist in the soil is an important variable in effective control. The fungal population displays a variable maintenance under field conditions, depending on soil moisture, temperature, acidity, organic matter and availability of arthropod organisms (Hu and St. Leger 2002; Pilz et al. 2011; Tiago et al. 2012). It has been shown that M. anisopliae can survive at low levels in soil for one year with an extended life of about 3.5 years (Milner et al. 2003; Bruck 2005). When applied to peanut roots, Liu et al. (2016) observed a decline in M. anisopliae counts (colony forming units (CFUs)/g soil) to 50% after 36.8 days, with a further significant decline after 43 days, such that counts 60 and 90 days after application had declined to 10% of the original.
The ecological relationship between entomopathogens and their target hosts, are critical to developing a strategy for efficient and long-term biological control (St. Leger 2008; Bruck 2009). Thus, timing of M. anisopliae application could be particularly important for survival of the entomopathogen and maintenance of optimum density (Liu et al. 2016), which may provide effective suppression of the grub population to minimize peanut yield losses.
The entomopathogenic fungus, M. anisopliae has considerable potential to control soil-dwelling pests, providing a viable alternative to chemical pesticides. White grubs can seriously affect peanut production, attacking seedlings, roots and pods. In this field experiment, we investigated the population dynamics of M. anisopliae applied at different stages of peanut development, starting at the time of sowing through to pod filling. At harvest, we also measured peanut yield parameters, as well as white grub larval population from each treatment, to determine the effect of timing of M. anisopliae application on larval densities and yield responses.
Materials and methods
Field site
The experiment was conducted in a homogeneous area of 35 m × 120 m (total area 4200 m2) on the research farm of the Cangzhou Academy of Agriculture and Forestry Science, Hebei Province, China. The field site was un-irrigated, previously sown in maize, and was ploughed in spring (early April). A base fertilizer was applied during cultivation, and left fallow until significant rain occurred later in April. The site was sown 28 April 2016 following the rain event. Preliminary sampling indicated that white grub larvae were present in the field prior to sowing.
Fungal strain and conidial culture
The M202-1 of Metarhizium anisopliae (CGMCC4275) was sourced from the China General Microbiological Culture Collection. Initially isolated from an infected white grub, the strain was found to be highly virulent, and characterized on nucleotide sequence of EF-1α gene region and related morphological features of conidiophores (Bischoff et al. 2009). The strain has the ability of potential biological control agent against white grubs assessed under laboratory and limited field conditions (Liu et al. 2011; Nong et al. 2011). Using the process of liquid–solid two phase fermentation, the strain was cultured to obtain conidia (Nong et al. 2007), the conidial powder then quantified to 1.76 × 1010 conidia/g and stored at 4 °C until used in the following experiment.
Fungal application treatments at different stages of peanut development
The peanut (Arachis hypogaea L.) Chinese cultivar ‘Jihua 6’ was used in this field experiment. Jihua 6 is the commonly grown peanut cultivar, which is acceptable to farmers for their fruit shape and market demand. The experiment was conducted in a homogeneous area of 35 m × 120 m (total area 4200 m2) on the research farm of the Cangzhou Academy of Agriculture and Forestry Science, Hebei Province, China. There were 342 sowing rows with about 35 cm inter-row spacing; whereas plants were sown 23 cm apart within each row with a total of 150 sowing points (= plants). The different treatments of M. anisopliae were arranged in the central 130 rows, which were nominally divided into five blocks as five repeats. The six timing treatments were conducted in each block. One row including all seeding points (plants) represented one timing treatment with two buffer rows between treatment rows. Five rows along both sides of each of the five blocks were allocated to each sampling interval (Fig. 1).
Fig. 1.
Schematic view of the configuration of M. anisopliae treatments in the field. Treatment rows are marked with pinhead bold line whereas, simple line refers to interval rows. 130 plant rows of peanut were nominally divided into five blocks as five repeats. The six timing (day 0, 15, 30, 45, 60, 75 during the peanut growth cycle) treatments were conducted in each block. One row including all seeding points (plants) represented one timing treatment with two buffer rows between treatment rows. Five rows along both sides of each of the five blocks were allocated to each sampling interval
For each treatment, M. anisopliae conidia were prepared in a suspension of 0.1% Tween-80 aqueous solution that had 1.0 × 107 colony forming units (CFU)/mL. A suspension of 100 mL of this solution was then applied to the soil in a 5 cm radius around each seeding point or plant by a small garden water can. For the designated control rows located along both sides of the treatment rows, 100 mL of 0.1% Tween-80 solution was applied to the soil around each seeding point or plant using the method described above. Treatments were applied at key phenological growth stages of peanut, commencing with applications at the germination [0 days after sowing (DAS)], seedling 8-leaf (15 DAS), early flowering (30 DAS), late flowering (45 DAS), pod-bearing (60 DAS) and pod-filling (75 DAS) stage. The subsequent management of the crop was maintained by conventional methods as required.
Collection of soil samples
Samples were collected at the time of application and subsequently at 15 days intervals, following treatment until harvest at 135 DAS. The amount of soil collected from around the plants depended on growth stage. At the time of sowing, and following application of M. anisopliae, approximately 20–30 g of soil was collected from around the seed within each treatment. Thereafter, rhizosphere soil was collected. The general method involved randomly selecting ten seedling points or plants to sample in each treatment row of each block, with soil collected at a depth of 3–8 cm using a hand trowel. Post germination, a hand trowel was used to remove the plant and surrounding soil. Plants were gently shaken to remove loose soil leaving only the rhizosphere soil tightly adhering to the roots, which was collected using a fine paint brush. The amount of soil collected from the rhizosphere ranged from 1–8 g with the smallest volume (1 g), collected from seedling 8-leaf plants due to the low volume of root biomass. The soil collected from around seeds on the day of M. anisopliae application was considered to be equivalent to the rhizosphere soil in subsequent samples. Equal amounts of soil from two random plants were mixed as one sample, so five repeat per every treatment row. A total of 195 soil samples were obtained from 390 plants of 5 plots. Each sample was sealed in a plastic bag and stored at 4 °C in a chiller (0:24 L:D) until processed.
Root development
At intervals during the first 20 days and subsequently coinciding with soil sampling, five plants were taken from each treatment and the extent of root development on plants was recorded. The removal of plants from the ground was performed as described above for collection of soil rhizosphere.
Detection of M. anisopliae in soil samples
Each soil sample was well mixed using a glass rod, and a 0.5 ± 0.001 g sub-sample weighed, placed in a special glass weighing bottle with sealable cover and baked for 2 h at 140 °C, and then re-weighed to determine soil dry weight. The remaining soil was tested to determine the presence of M. anisopliae. Each sample weighed to 0.5 g equivalent soil dry weight, after which 0.1% Tween-80 aqueous solution was added to obtain a tenfold dilution. This dilution was agitated in a vortex for three min and then serially diluted from 1:9 and 1:1 to achieve a 200-fold dilution. One hundred µL of the 200-fold dilution was taken and inoculated onto 5 identical (replicates) plates Petri dishes (90 mm × 10 mm) with selective media (CD-SPA) containing 20 g sucrose, 10 g peptone, 0.167 g chloromycetin, 100 mg dodine, and 18 g agar per 1000 mL following the procedure outlined by Liu et al. (2014). Plates were incubated at 28 °C for 4 days, after which colonies were observed and counted to determine density per CFUs per gram of dried soil (CFU/g). The population dynamics of the applied M. anisopliae were evaluated and compared in different treatments.
M. anisopliae impact on white grub larval populations
To determine the effect of M. anisopliae treatments on white grub larval densities, 15 randomly selected peanut plants were chosen from within each treatment row on 135th day of peanut development and coinciding with peanut yield assessments. A 23 cm × 23 cm square quadrat (0.053 m−2), was centered on a plant and soil removed to a depth of 25 cm using a hand trowel, after which the number of white grub larvae in the square was counted. For peanut, total pods and damaged pods were counted and full pods and nuts were weighed after counting. For analysis, three plants were combined into one sample to provide five replicates per treatment. Total pods are all pods counted, full pods are pods with fully developed seeds and nuts the total dried weights (g) of the shelled seed, pooled from three plants.
Statistical analysis
Counts of M. anisopliae from each soil and plant sample were used to determine means and standard deviations and were corrected against controls using Abbott’s formula (Abbott 1925). One-way analysis of variance (ANOVA), followed by Tukey’s honestly significant difference test for mean comparisons and single-factor regression using Statistical Analysis System (SAS) software version 8.0 (SAS Institute Inc., Cary, NC, USA) was used. Data was subjected to normality and homogeneity test as per Gomez and Gomez (1984). Values were then used to compare and evaluate the effect of M. anisopliae application at different stages on the fungal survival and pathogenicity against white grub larval densities. The exponential decay of Metarhizium count for the last four assessments (excluding seed or cotyledon stage) was fitted by regressing log count with a common slope (P < 0.001) for the day factor, but there were separate intercepts for each plant stage.
Relationship among the numbers of damaged pods and peanut weight were explored and fitted by regression within each block. For changes in Metarhizium counts over time, the log of Metarhizium counts was analyzed as an ANOVA at each sampling time using block as a blocking factor. This was done to avoid repeated measures and analyzing the treatments that were present at each sample time. Means and standard error of the mean (SEM) at each time were determined for the survivorship curves. ANOVA was carried out on grub counts with the mean and SEM calculated, and then interaction of grub density and peanut yield was checked through statistical analysis.
Results
Persistence of M. anisopliae applied at different peanut phenophases
M. anisopliae applications were carried out at 0, 15, 30, 45, 60, and 75 DAS, which corresponded to the germination, seedling 8-leaf, early flowering, late flowering, pod bearing, and pod filling phenophases, respectively. Soil around the plants was sampled at the time of application, then approximately every 15 days through to harvest i.e. 135th days. Due to wet weather and consequent muddy conditions, no sampling could be carried out at 120 days.
Results showed that the CFUs ranged from 5.23 × 104–13.47 × 104 g−1 of soil depending on sample timing and crop development with significant differences in M. anisopliae population dynamics. Overall, as shown in Fig. 2, at the first three applications coinciding with germination (0 DAS), seedling 8-leaf (15 DAS) and early flowering (30 DAS), a sharp decline in M. anisopliae counts was subsequently observed in the next sample (after 15 days), before exhibiting a rapid increase in propagules/g over the next 1–3 sampling periods (Fig. 2). Irrespective of application time, M. anisopliae counts decreased in the later stages of peanut development. In the final harvest, only the early flowering application maintained 21.7% of the initial densities of M. anisopliae, whereas other stage treatments showed less than 10% of the initial densities (Fig. 2).
Fig. 2.
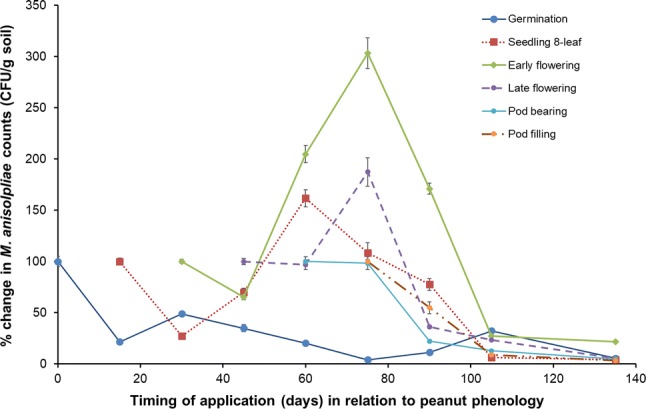
Population dynamics of M. anisopliae (log CFU/g soil) applied at six times during the peanut crop life cycle. Timing of application shown in relation to phenological growth stages, in which the germination, seedling 8-leaf, early flowering, late flowering, pod bearing and pod filling phenophases were at 0, 15, 30, 45, 60, and 75 DAS, respectively. Means ± SE are shown
When applied at germination, M. anisopliae counts (CFU/g dry soil) rapidly decreased by an average of 21.6% over the first 15 DAS, coinciding with seed sprouting and seedling emergence (Fig. 2). At 30 DAS, with approximately 30% of plants flowering, mean M. anisopliae counts modestly recovered to 49.0% of the initial counts, followed by a slow decline through to the pod filling stage (75 DAS), after which there was a rise then fall in counts until harvest (Fig. 2). When M. anisopliae was applied at the seedling 8-leaf stage (15 DAS), the fungal population showed a rapid decline (27.4%) in the initial 15 days, followed by an increase of 161.5% over the initial count of application during the flowering and pod-bearing stages (30–60 DAS). This was followed by a marked decline in counts with a slope of − 0.9615 during pod filling and maturity stages (60–105 DAS), following the linear regression y = − 25.975x + 111.14 (R2 = 0.978). Similarly, M. anisopliae applied at the early flowering stage (30 DAS), showed a moderate decline in counts in the first 15 days, to 65.1% of the initial amount, followed by a rebound during the pod bearing and pod filling development stages (45–75 DAS). The highest peak reached 303.3% of the initial count and was recorded 45 days after application, with a subsequent decline through to harvest (Fig. 2). By comparison, when applied at the late flowering stage (45 DAS), M. anisopliae counts remained stable in the initial 15 days (96.8% of the initial amount), followed by a 187.2% increase of the initial count amount from the pod-bearing to pod-filling periods (60–75 DAS). When applied at the pod-bearing (60 DAS) and pod-filling (75 DAS) stages, M. anisopliae counts were found to subsequently decline across the remaining sampling intervals (Fig. 2).
Relationship between M. anisopliae population dynamics and timing of application
Based on exponentially unstable decay, the survivorship curves for the different Metarhizium treatments are given in Fig. 3. In the initial four treatment applications, there was a consistent trend of a decline in counts phenological phase treatments, the population were seen in a zigzag expression having upwards process corresponds to the flowering season or continued to the pod stage. Over the last four dates on average, for all stages except ‘seed or cotyledon’, the half-life of M. anisopliae CFU/g in soil was 9.47 days (95% CI 8.90–10.12). Three of the most significant phases for treatments times with enhanced M. anisopliae counts were following applications at seedling 8-leaf stage, early flowering, and late flowering periods. Although there was a decline in mean counts 15 days after application, peak population counts occurred 30–45 days following application, corresponding to peanut flowering or pod-bearing development, although a decline was apparent after pod filling. An application at the pod filling stage (75 DAS), showed a continuous decline in M. anisopliae counts through to harvest. This response was also observed in treatments applied earlier in crop development (Fig. 3).
Fig. 3.

Mean (± SE) survivorship curves for M. anisopliae (CFU/g soil) treatments applied at 0 (germination), 15 (seedling 8-leaf), 30 (early flowering), 45 (late flowering), 60 (pod bearing) and 75 (pod filling) DAS. Whereas, DAS refers to days after sowing
M. anisopliae impact on white grub larval populations
Mean larval density in all M. anisopliae treatments was significantly decreased compared to the control (Table 1). However, application at the early flowering stage (30 DAS), resulted the substantial reduction in larval populations (71.6%), followed by an application at the late flowering stage which resulted in a mean 56.8% reduction density (Table 1). Across all M. anisopliae treatments, the highest larval densities were found in the pod filling (18.2 larvae/m2) and pod bearing (18.9 larvae/m2) growth stages, although not significantly higher than the remaining M. anisopliae treatment, with the exception of the early flowering treatment (9.8 larvae/m2).
Table 1.
Effect of timing of M. anisopliae application in relation to peanut growth stages on white grub larval densities (mean ± SD) measured following peanut harvest (135 days)
| Phenological stage | Days after sowing | Density (larvae/m−2) | % reduction in population | Significance (LSD 5%) |
|---|---|---|---|---|
| Control | 34.5 ± 6.0 | – | a | |
| Germination | 0 | 17.9 ± 3.1 | 48.1 | b |
| Seedling 8-leaf | 15 | 17.6 ± 1.8 | 49.0 | b |
| Early flowering | 30 | 9.8 ± 1.2 | 71.6 | c |
| Late flowering | 45 | 14.9 ± 3.9 | 56.8 | b |
| Pod bearing | 60 | 18.2 ± 2.7 | 47.2 | b |
| Pod filling | 75 | 18.9 ± 6.7 | 45.2 | b |
The overall impact of treatments on peanut production parameters is shown in Table 2. Compared to the control, the Metarhizium treatments significantly reduced the percentage of damaged pods (14.3–50.0%). Treatments made at 0, 15 during initial phase of germination (0 DAS), seedling 8-leaf (15 DAS) and early flowering (30 DAS), ultimately boosted the number of pods (22.9–66.1%), improved the weight of pods (83.9–161%), and increased number of kernels (93.4–189.2%). However, maximum peanut yield and least damage were recorded from the early flowering (30 days) treatment (Table 2). White grub larval density was significantly related to peanut yield. This association has been established because of grub density and plant responses, where grub density significantly affected the plant production parameters such as peanut weight, mean number of damaged pods, number of damaged pods and yield (Fig. 4). The treatments enhanced range of nut weight and minimized the number of damaged pods. A regression of yield on number of damaged pods with a common slope and separate intercepts for each block gives nut weight reduced by 0.64 ± 0.06 g for an increase of one damaged pod. The results showed that treatment at the early flowering stage (30 DAS) caused the highest impact in terms of reducing damage to pods and improving yield. Treatment at seeding (0 DAS) and seedling (15 DAS) stages produced the next best plant responses with reductions in damage of 36.7% and 34.8%, respectively.
Table 2.
Effect of M. anisopliae applied at different stages of the peanut phenology and consequent plant damage and yield response in relation to feeding by white grub larvae
| Treatment time | Total pods | Damaged pods | Full pods | Nuts | ||||
|---|---|---|---|---|---|---|---|---|
| Numbers | % increase1 | % damaged | % reduction1 | Weight (g) | % increase1 | Weight (g) | % increase1 | |
| Germination (day 0) | 34.4 ± 6.8bc | 22.9 | 37.4 ± 3.3d | 36.7 | 60.6 ± 12.8b | 98.2 | 21.6 ± 4.7b | 93.4 |
| Seedling 8-leaf (day 15) | 35.8 ± 3.8b | 27.9 | 38.6 ± 3.2d | 34.8 | 56.3 ± 7.2b | 83.9 | 22.1 ± 2.7b | 97.6 |
| Early flowering (day 30) | 46.5 ± 4.9a | 66.1 | 29.5 ± 3.2e | 50.0 | 80.2 ± 8.4a | 161.9 | 32.3 ± 3.2a | 189.2 |
| Late flowering (day 45) | 30.1 ± 3.3 cd | 7.4 | 43.9 ± 4.1c | 25.8 | 38.0 ± 4.8c | 24.2 | 17.3 ± 2.2bc | 55.2 |
| Pod bearing (day 60) | 30.0 ± 6.7 cd | 7.1 | 47.3 ± 4.8bc | 20.1 | 34.7 ± 9.4c | 13.4 | 16.6 ± 4.3bc | 48.7 |
| Pod filling (day 75) | 30.4 ± 3.8bcd | 8.7 | 50.7 ± 4.5b | 14.3 | 32.5 ± 4.4c | 6.0 | 14.2 ± 1.8bc | 27.1 |
| Control | 28.0 ± 1.5d | 0.0 | 59.1 ± 6.5a | 0.0 | 30.6 ± 5.2c | 0.0 | 11.2 ± 1.6c | 0.0 |
Values are based on mean scores of three plants with five replicates per treatment. Total pods are all pods counted from three plants; full pods the number containing seeds, and nuts the dried weights of the shelled seed
1% decrease in damage indexed against control treatment. Data within columns followed by different letters are significantly different at α = 0.05
Fig. 4.
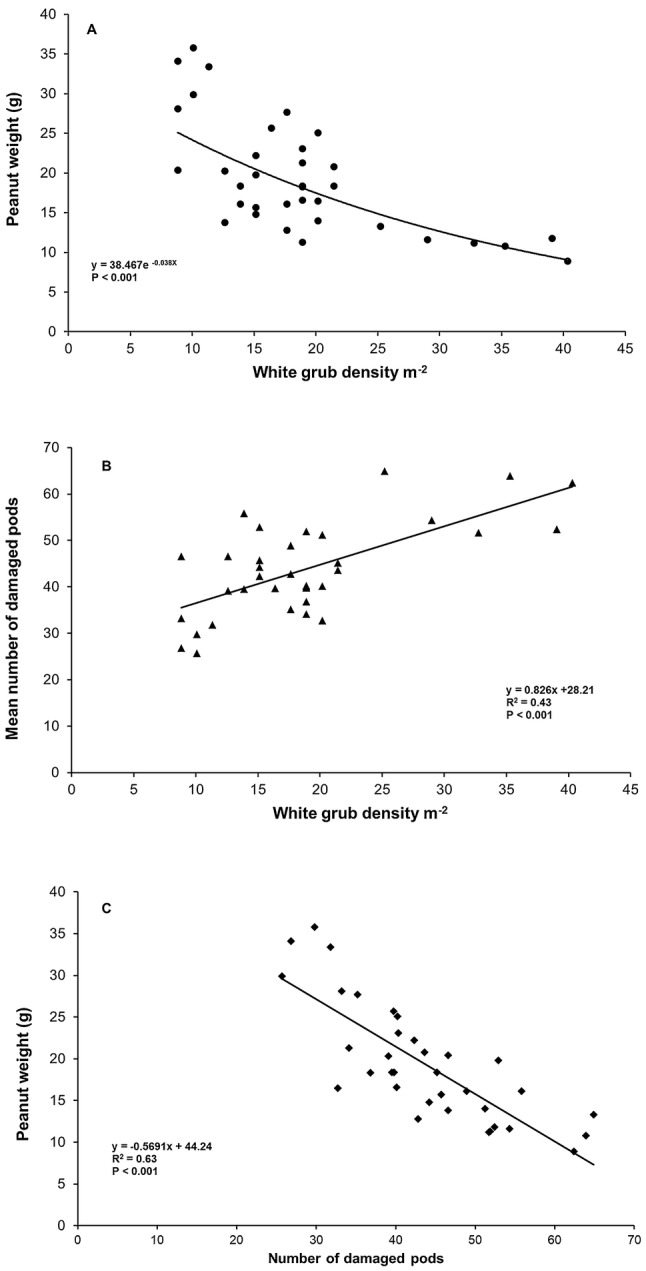
Relationship of white grub density with peanut yield parameters. a White grub larval density with nut weight; b association of white grub density with number of damaged pods; c association of number of damaged pods with peanut yield
Plotting grub numbers against peanut yield showed that applying M. anisopliae 30 DAS provided the highest yield response, compared to the other treatment times (Figure S1). The impact of treatment on soil just after 30th days has also been observed in relation with the responses of plant production parameters (Table 2).
Root development process
The entire process of seed germination took approximately 16 days, with the radicle emerged 2 DAS with 2–3 cm elongation observed approximately 4 DAS (Figure S2b). Fully developed laterals approximately 1 mm in length were seen within 5–6 DAS followed by elongation from 3 to 8 mm in 9–10 DAS, with mean counts of 17.4 ± 1.50 laterals per plant (Figure S2c–e). Lateral density increased significantly and secondary fine laterals visible after 12 DAS (Figure S2 f, g). The root system developed completely with a robust taproot and dense laterals 15–16 DAS (Figure S2 h); afterwards the laterals became denser at 30 DAS. At this stage, approximately 30% of plants were flowering on the primary branches. At 45 DAS, the root system continues to expand slightly but the cortex becomes harder. At this time, the plants are in full bloom with the carpophores (pegs) visible and penetrating the soil, and pods beginning to develop. Between 60–75 DAS, the pods were fully developed, while the surface of the root system has begun to darken in color. In the last period until harvest (75–135 DAS), coinciding with ongoing development and ripening of pods through to maturity, there was shrinkage of the senescing root system and cortical.
Discussion
Species of the entomopathogenic fungi Metarhizium are ubiquitous in soils, (Quesada-Moraga et al. 2007; Vega et al. 2009), but consistent infection of scarabs is generally limited M. anisopliae (Jackson and Klein 2006). The fungus has evolved a close association between insect hosts and plants (St. Leger 2008; Johnson and Rasmann 2015), and in some cases providing dual use not only entomopathogens, but as endophytes to benefit plant growth (Jaber and Ownley 2018). In addition, M. anisopliae has also evolved an ability to survive in soil for long periods even in the absence of a suitable insect host, utilizing plant-released soil carbon (Bidochka et al. 2001; St. Leger 2008), with some strains found to be rhizosphere competent (Hu and St. Leger 2002; St. Leger 2008). Maintaining a critical level of inoculum in the soil is crucial for M. anisopliae to successfully infect white grub larvae and effect high mortality (Paulitz 2000). To understand Metarhizium survival dynamics under diversified environmental factors, a required level of entomopathogen inoculum needs to be present to effectively control the target pest and maintain viability for prolonged periods when hosts are absent (Maute et al. 2017; Martínez-García et al. 2018). Virulence of entomopathogens to target species can be highly variable, because of intrinsic resistance to generalist pathogens (Jackson and Klein 2006). In addition, there is a range of abiotic and biotic factors that impact on survival of conidia and virulence including humidity, temperature, soil types and depth, pesticides, competition with other microorganisms and distribution in the soil profile in relation to suitable hosts (Kessler et al. 2004; Quesada-Moraga et al. 2007; Jaronski 2010; Boetel et al. 2012; Wang et al. 2019; Yang et al. 2019). The results of this experiment showed that the timing of M. anisopliae applications during the course of peanut growth, significantly affected proliferation of propagules. Apart from treatments at 45 and 60 DAS (late flowering and pod bearing), respectively, across all treatments, there was a significant decline in propagules in the first 15 days of application but both marked increases then decreases in propagules over time. Liu et al. (2016) found that time, distance from root and soil depth had significant effects on M. anisopliae survival. Similar to this study, in some cases there was a rapid decline in CFU/g soon after application, followed by a more shallow decline in counts, after which propagule CFU/g increased.
Research to understand the interaction between entomopathogenic fungi and plants has been undertaken by several researchers in relation to M. anisopliae. M. anisopliae can survive and grow by utilizing free carbon in the rhizosphere and interacting with the roots (St. Leger 2008). Higher survival of M. anisopliae was found around the rhizosphere of cabbages compared to non-rhizosphere soils (St. Leger 2008). By comparison, Liu et al. (2016) found that the decline in M. anisopliae counts at the rhizosphere zone was more rapid than observed distal from the plant. It was concluded that the rhizosphere maintains survival and promoted M. anisopliae propagules in the soil profile (Hu and St. Leger 2002). Gene expression analysis has also indicated that M. anisopliae can grow on soybean root exudates, with expression different from that found on the insect epidermis or in the haemolymph, which indirectly supports dependency of entomopathogenic M. anisopliae on available nutrients in rhizosphere (Wang and St. Leger 2007). The association between the fungus and plants has also been demonstrated, with germination of M. anisopliae in the presence of root exudates of beans (Pava-Ripoll et al. 2011). Our findings suggested that M. anisopliae colonization and proliferation were promoted during the peanut flowering and pod-bearing stages. However, following pod-filling the number of M. anisopliae propagules declined rapidly. Although not tested for in this study, we postulate that the composition of root exudates at different peanut growth stages may be important factors for the population dynamics of M. anisopliae. Comparing the fungal population dynamics to the root development progress from the germinating seed, we found that the upward phase of the fungal propagules corresponded closely to the flowering and pod bearing stages (30–60 days of peanut development). Potentialy, the root exudates produced in this stage of peanut development may specifically promote colonization and proliferation of M. anisopliae.
While M. anisopliae dynamics may be associated with peanut root development and exudates around the rhizosphere, there appeared to be critical period in the timing of M. anisopliae applications and reduction in white grub larval populations. According to the historical occurrence of the local populations, there were two dominant species, Holotrichia parallela and H. oblita, in the peanut field. At the time of sowing, a portion of the white grub population comprised a mix of larvae and pupae, with larvae feeding on seeds and seedlings, prior to pupation. The impact of white grub larvae on peanut yields is strongly linked to the phenology of both insect and plant. Emergence of first generation larvae occurs during the early flowering stage (days 30–44), while first and early second instar larvae occur during the pod bearing stage (days 60–74) and attack the root system. Laboratory bioassays have indicated that 1st and 2nd instars are more susceptible to infection by M. anisopliae, than later instars (Nong et al. 2011), with higher infection rates improving M. anisopliae persistence and proliferation in the soil profile. During pod filling (days 75–89), larvae are 2nd and 3rd instars and feed primarily on the developing pods, which is when the most significant economic crop damage occurs (Jiang et al. 2013; Feng et al. 2012). While all applications from day 0 to 75 DAS, resulted in significant declines in larval populations compared to the control, there was a significant reduction in larvae at 30 DAS or at the early flowering stage, which was reflected in significant yield responses in weight of full pods and nuts. The next best yield response was seen for plants that had been treated 15 DAS (seedling 8-leaf stage), followed by an application at the time of sowing. Applying M. anisopliae during the middle and late stages of peanut development did not improve yields, as even significant control of grubs by M. anisopliae following an application was negated by cumulative damage that occurred prior to entomopathogen treatment. While previous research has also indicated that applying M. anisopliae at sowing resulted between a 24–42% increase the number of peanut seeds and a 33–40% increase in DM yield (Liu et al. 2011), these results indicate that controlling larval populations from 30 DAS was critical to substantive yield increases. However, it is important to point out that M. anisopliae counts made at the rhizosphere are a measure of population propagule density, but cannot be directly linked to subsequent larval mortality. This is because larvae are mobile in the soil profile and while feeding on the root system, without the ability to visualise their position in the profile are direct link to counts in the rhizosphere and subsequent mortality would be tenous. In addition, repellency to high concentrations of M. anisopliae in soil, for up to 20 days, has been demonstrated by larvae of Japanese beetle (Popillia japonica Newman) (Villani et al. 1994). Potentially, reduced root feeding in the presence of M. anisopliae that may contribute to the improved yields observed in the study.
Conclusions
This research has shown that the timing of M. anisopliae application to peanut crops has an important influence on survival and proliferation in soil. There was also compelling evidence that the M. anisopliae strain M202-1 was highly pathogenic to white grub larvae, with significant reductions in populations across treatment times. In particular, an application to the plant root zone 30 days after sowing (early flowering), produced a mean larval mortality of 72% and a significant yield response compared to applications earlier or later in the peanut growth cycle. For growers, this result offers a promising alternative to insecticides, but repeatability of results under different abiotic conditions, including soils type and moisture regimes is required to ensure that effective economic control can be achieved. Understanding the effect of intercropping, cultivation and seasonality on conidia survival and viability in fields and the development of a formulated M. anisopliae bio-pesticide that can be applied at sowing to provide entomopathogen activity beyond 30 days after sowing more areas for future research that contribute to a peanut IPM program.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Chunqin Liu and her team members of Cangzhou Agricultural and Forest Science Academy, Hebei, China for participating in the soil treatment and planting management. Thanks to Chikako van Koten (AgResearch Ltd.) for assistance in analysis of white grub and peanut data. We would also like to thank LetPub for providing linguistic assistance during the preparation of this manuscript.
Author contributions
Conceptualization, XL, XN and ZZ; Formal analysis, QW; Investigation, XL, XL and XN; Methodology, ZZ; Resources, GW, HU, QW and HVH; Supervision, XN; Validation, HVH; Visualization, HVH; Writing—original draft, XL, XN and ZZ; Writing—review and editing, XN, MRM and HU.
Funding
This study was financially supported by National Key R&D Program of China under project Nos. 2018YFD0201000 and 2017YFD0201205, and the Earmarked Fund for China Agriculture Research System (CARS-34-07B).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Xingjia Li and Xiangqun Nong equally contributed to the study.
Contributor Information
Xingjia Li, Email: lixingjiabj@163.com.
Xun Liu, Email: lxun1208@163.com.
Xiangqun Nong, Email: xqnong@sina.com.
Guangjun Wang, Email: wangguangjun@caas.cn.
Mark Richard McNeill, Email: mark.mcneill@agresearch.co.nz.
Hidayat Ullah, Email: shabkadar@yahoo.com.
Qinglei Wang, Email: wqlei02@163.com.
Harold V. Henderson, Email: harold.henderson@agresearch.co.nz
Zehua Zhang, Email: zhangzehua@caas.cn.
References
- Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18:265–267. doi: 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- Bidochka MJ, Kamp AM, Lavender TM, Dekoning J, De Croos JNA. Habitat association in two genetic groups of the insect-pathogenic fungus Metarhizium anisopliae: uncovering cryptic species? Appl Environ Microbiol. 2001;67:1335–1342. doi: 10.1128/AEM.67.3.1335-1342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff JF, Rehner SA, Humber RA. A multilocus phylogeny of the Metarhizium anisopliae lineage. Mycologia. 2009;101:512–530. doi: 10.3852/07-202. [DOI] [PubMed] [Google Scholar]
- Boetel MA, Majumdar A, Jaronski ST, Horsley RD. Cover crop and conidia delivery system impacts on soil persistence of Metarhizium anisopliae (Hypocreales: Clavicipitaceae) in sugarbeet. Biocontrol Sci Tech. 2012;22:1284–1304. doi: 10.1080/09583157.2012.725127. [DOI] [Google Scholar]
- Bruck DJ. Ecology of Metarhizium anisopliae in soilless potting media and the rhizosphere: implications for pest management. Biol Cont. 2005;32:155–163. doi: 10.1016/j.biocontrol.2004.09.003. [DOI] [Google Scholar]
- Bruck DJ. Fungal entomopathogens in the rhizosphere. In: Roy HE, Vega FE, Chandler D, Goettel MS, Pell J, Wajnberg E, editors. The Ecology of Fungal Entomopathogens. Dordrecht: Springer; 2009. pp. 103–112. [Google Scholar]
- Chelvi CT, Thilagaraj WR, Nalini R. Field efficacy of formulations of microbial insecticide Metarhizium anisopliae (Hyphocreales: Clavicipitaceae) for the control of sugarcane white grub Holotrichia serrata F (Coleoptera: Scarabidae) J Biopestic. 2011;4(2):186–189. [Google Scholar]
- Dar SA, Rather BA, Ajaz AK. Insect pest management by entomopathogenic fungi. J Entomol Zool Stud. 2017;5(3):1185–1190. [Google Scholar]
- Douglas MR, Rohr JR, Tooker JF. Neonicotinoid insecticide travels through a soil food chain, disrupting biological control of non-target pests and decreasing soya bean yield. J Appl Ecol. 2015;52:250–260. doi: 10.1111/1365-2664.12372. [DOI] [Google Scholar]
- Feng XJ, Liu CQ, Pu NN, Liu YT, Xu GC, Wang QL. Application of Beauveria bassiana in controlling Holotrichia parallela. J Hebei Agri Sci. 2012;16(4):64–66. [Google Scholar]
- Gomez KA, Gomez AA. Statistical procedures for agricultural research. New York: Wiley; 1984. [Google Scholar]
- Hallmann CA, Foppen RPB, van Turnhout CAM, de Kroon JE. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature. 2014;511:341–343. doi: 10.1038/nature13531. [DOI] [PubMed] [Google Scholar]
- Han XQ, Wu ZH, Zhang SQ, Zhang YX. Investigation of dominant species of white grubs in peanut field and field control techniques in the eastern of Hebei province. J Hebei Agric Sci. 2011;15:27–29. [Google Scholar]
- Hu G, St. Leger RJ. Field studies using a recombinant mycoinsecticide (Metarhizium anisopliae) reveal that it is rhizosphere competent. Appl Environ Microbiol. 2002;68(12):6383–6387. doi: 10.1128/AEM.68.12.6383-6387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu QB. Research advance of occurrence and control of underground pest grub in China. Hubei Agric Sci. 2004;6:87–92. [Google Scholar]
- Imfeld G, Vuilleumier S. Measuring the effects of pesticides on bacterial communities in soil: a critical review. Eur J Soil Biol. 2012;49:22–30. doi: 10.1016/j.ejsobi.2011.11.010. [DOI] [Google Scholar]
- Jaber LR, Ownley BH. Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? Biol Cont. 2018;116:36–45. doi: 10.1016/j.biocontrol.2017.01.018. [DOI] [Google Scholar]
- Jackson TA, Klein MG. Scarabs as pests: a continuing problem. Coleopt Bull. 2006;60(mo 5):102–119. doi: 10.1649/0010-065X(2006)60[102:SAPACP]2.0.CO;2. [DOI] [Google Scholar]
- Jaronski ST. Ecological factors in the inundative use of fungal entomopathogens. Biocontrol. 2010;55:159–185. doi: 10.1007/s10526-009-9248-3. [DOI] [Google Scholar]
- Jiang YP, Ju Q, Jiang XJ, Zhao ZQ, Li X, Lu JJ, Jiang YS, Ni WL, Chen ZD, Wang GT. Survey of peanut yield loss caused by white grub and its effect factors analysis. J Peanut Sci. 2013;42:42–46. [Google Scholar]
- Jin S, Zhou F. Zero growth of chemical fertilizer and pesticide use: China's objectives, progress and challenges. J Resour Ecol. 2018;9:50–59. doi: 10.5814/j.issn.1674-764x.2018.01.006. [DOI] [Google Scholar]
- Johnson SN, Rasmann S. Root-feeding insects and their interactions with organisms in the rhizosphere. Annu Rev Entomol. 2015;60:517–535. doi: 10.1146/annurev-ento-010814-020608. [DOI] [PubMed] [Google Scholar]
- Kessler P, Enkerli J, Schweizer C, Keller S. Survival of Beauveria brongniartii in the soil after application as a biocontrol agent against the European cockchafer Melolontha melolontha. Biocontrol. 2004;49:563–581. doi: 10.1023/B:BICO.0000036441.40227.ed. [DOI] [Google Scholar]
- Liu X, Nong X, Liu C, Xi G, Zhang X, Zhang Z. Biocontrol of peanut white grubs, Holotrichia parallela, using entomopathogenic fungus Metarhizium anisopliae at sowing period of peanut. Chin J Biol Cont. 2011;27:485–489. [Google Scholar]
- Liu X, Nong X, Su Y, Li X, Zhang Z. An efficiently selective medium with dodine for quantitative isolation of Metarhizium anisolpliae in soil. Chin J Biol Control. 2014;30:552–557. [Google Scholar]
- Liu X, Nong X, Wang Q, Li X, Wang G, Cao G, Zhang Z. Persistence and proliferation of a Chinese Metarhizium anisopliae ss isolate in the peanut plant root zone. Biocontrol Sci Tech. 2016;26(6):746–758. doi: 10.1080/09583157.2016.1155106. [DOI] [Google Scholar]
- Luo Z, Li K, Cao Y, Yin J, Zhang J, Zhang J, Shang G. Investigations on soil-inhabiting pests in peanut fields in Henan. Plant Prot. 2009;35:104–108. [Google Scholar]
- Martínez-García LB, Korthals G, Brussaard L, Jørgensen HB, De Deyn GB. Organic management and cover crop species steer soil microbial community structure and functionality along with soil organic matter properties. Agr Ecosyst Environ. 2018;263:7–17. doi: 10.1016/j.agee.2018.04.018. [DOI] [Google Scholar]
- Maute K, French K, Story P, Bull CM, Hose GC. Short and long-term impacts of ultra-low-volume pesticide and biopesticide applications for locust control on non-target arid zone arthropods. Agr Ecosyst Environ. 2017;240:233–243. doi: 10.1016/j.agee.2017.02.024. [DOI] [Google Scholar]
- Milner RJ, Samson P, Morton R. Persistence of conidia of Metarhizium anisopliae in sugarcane fields: effect of isolate and formulation on persistence over 3.5 years. Biocontrol Sci Technol. 2003;13(5):507–516. doi: 10.1080/0958315031000140965. [DOI] [Google Scholar]
- Nong X, Liu C, Lu X, Wang Q, Wang G, Zhang Z. Laboratory evaluation of entomopathogenic fungi against the white grubs, Holotrichia oblita and Anomala corpulenta (Coleoptera: Scarabaeidae) from the field of peanut, Arachis hypogaea. Biocontrol Sci Technol. 2011;21(5):593–603. doi: 10.1080/09583157.2011.566324. [DOI] [Google Scholar]
- Nong X, Tu X, Zhang Z, Li C. Factors affecting solid fermentation of Metarhizium anisopliae R8–4. Chin J Biol Control. 2007;23:228–232. [Google Scholar]
- Paulitz TC. Population dynamics of biocontrol agents and pathogens in soils and rhizospheres. Eur J Plant Pathol. 2000;106:401–413. doi: 10.1023/A:1008733927515. [DOI] [Google Scholar]
- Pava-Ripoll M, Angelini C, Weiguo Fang W, Wang S, Posada FJ, St. Leger R. The rhizosphere-competent entomopathogen Metarhizium anisopliae expresses a specific subset of genes in plant root exudate. Microbiology. 2011;157:47–55. doi: 10.1099/mic.0.042200-0. [DOI] [PubMed] [Google Scholar]
- Pilz C, Enkerli J, Wegensteiner R, Keller S. Establishment and persistence of the entomopathogenic fungus Metarhizium anisopliae in maize fields. J Appl Entomol. 2011;135:393–403. doi: 10.1111/j.1439-0418.2010.01566.x. [DOI] [Google Scholar]
- Pu ZL, Li ZZ. Insect mycology. Hefei: Anhui Science and Technology Press; 1996. pp. 362–454. [Google Scholar]
- Quesada-Moraga E, Navas-Cortés JA, Maranhao EAA, Ortiz-Urquiza A, Santiago-Álvarez C. Factors affecting the occurrence and distribution of entomopathogenic fungi in natural and cultivated soils. Mycol Res. 2007;111:947–966. doi: 10.1016/j.mycres.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Sattler C, Kächele H, Verch G. Assessing the intensity of pesticide use in agriculture. Agr Ecosyst Environ. 2007;119:299–304. doi: 10.1016/j.agee.2006.07.017. [DOI] [Google Scholar]
- Schäfer RB, Liess M, Altenburger R, Filser J, Hollert H, Roß-Nickoll M, Schäffer A, Scheringe M. Future pesticide risk assessment: narrowing the gap between intention and reality. Environ Sci Eur. 2019;31:21. doi: 10.1186/s12302-019-0203-3. [DOI] [Google Scholar]
- Shao H, Zhang Y. Non-target effects on soil microbial parameters of the synthetic pesticide carbendazim with the biopesticides cantharidin and norcantharidin. Sci Rep. 2017;7(5521):1–12. doi: 10.1038/s41598-017-05923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M, Parker BL, Kim JS. Role of entomopathogenic fungi in integrated pest management. In: Integrated Pest Management. Cambridge: Academic Press; 2014. pp. 169–191. [Google Scholar]
- St. Leger RJ. Studies on adaptations of Metarhizium anisopliae to life in the soil. J Invertebr Pathol. 2008;98:271–276. doi: 10.1016/j.jip.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Tiago PV, Carneiro-Leao MP, Malosso E, De Oliveira NT, Lima EADLA. Persistence and effect of Metarhizium anisopliae in the fungal community of sugarcane soil. Biocontrol. 2012;57:653–661. doi: 10.1007/s10526-012-9445-3. [DOI] [Google Scholar]
- Vega FE, Goettel MS, Blackwell M, Chandler D, Jackson MA, Keller S, Koike M, Maniania NK, Monzón A, Ownley BH, Pell JK, Rangel DEN, Roy HE. Fungal entomopathogens: new insights on their ecology. Fungal Ecol. 2009;2:149–159. doi: 10.1016/j.funeco.2009.05.001. [DOI] [Google Scholar]
- Villani MG, Krueger SR, Schroeder PC, Consolie F, Consolie NH, Preston-Wilsey LM, Roberts DW. Soil application effects of Metarhizium anisopliae on Japanese beetle (Coleoptera: Scarabaeidae) behavior and survival in turfgrass microcosms. Environ Entomol. 1994;23:502–513. doi: 10.1093/ee/23.2.502. [DOI] [Google Scholar]
- Wang C, St. Leger RJ. The MAD1 adhesin of Metarhizium anisopliae links adhesion with blastospore production and virulence to insects, and the MAD2 adhesin enables attachment to plants. Eukaryot Cell. 2007;6(5):808–816. doi: 10.1128/EC.00409-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Fahad S, Saud S, Kamran M, Khan A, Khan MN, Hammad HM, Nasim W. Morphological acclimation to agronomic manipulation in leaf dispersion and orientation to promote “Ideotype” breeding: evidence from 3D visual modeling of “super” rice (Oryza sativa L.) Plant Physiol Biochem. 2019;135:499–510. doi: 10.1016/j.plaphy.2018.11.010. [DOI] [PubMed] [Google Scholar]
- Wood TJ, Goulson D. The environmental risks of neonicotinoid pesticides: a review of the evidence post 2013. Environ Sci Pollut Res. 2017;24:17285–17325. doi: 10.1007/s11356-017-9240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z (1991) White grub, an important insect pest of peanut and their control in China. In: Proceedings of a workshop on integrated pest management and insecticide resistance management (IPM/IRM) in legume crops in Asia, Chiang Mai, March 1991. International Crops Research Institute for the Semi-Arid Tropics, Patancheru, Andhra Pradesh 502324, India. p 15.
- Yang H, Qin CS, Chen YM, Zhang GY, Dong LH, Wan SQ. Persistence of Metarhizium (Hypocreales: Clavicipitaceae) and Beauveria bassiana (Hypocreales: Clavicipitaceae) in tobacco soils and potential as biocontrol agents of Spodoptera litura (Lepidoptera: Noctuidae) Environ Entomol. 2019;48:147–155. doi: 10.1093/ee/nvy161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



