Abstract
In plants, previous studies show that telomerase activity contributes to the maintenance of telomeric length for the proper development of organs and tissues. In this work, we investigated telomerase activity in A. tequilana during several years of cultivation. We found that during growth of the leaf there are two crucial phases: (1) the onset of cell elongation in 3 years and (2) differentiation of vascular bundles in 6 years. This coincides with the ages where the highest telomerase activity is seen. Therefore indicates that telomerase is associated with cellular activities such as; elongation, division, and cell differentiation. Likewise, we detected high activity during the period of vegetative growth, indicating that telomerase also contributes to telomeric maintenance on the leaf in A. tequilana.
Electronic supplementary material
The online version of this article (10.1007/s12298-020-00781-7) contains supplementary material, which is available to authorized users.
Keywords: Agave tequilana, Telomerase activity, Development, Growth, Leaf, Cell proliferation
Introduction
Telomeres are specialized structures located at the physical ends of eukaryotic chromosomes. They are formed by repeated sequences and specific proteins that provide stability in chromosomes (Greider 1991). The telomeric sequence varies between eukaryotes, but most of the higher plants share the Arabidopsis (TTTAGGG)n (Richards and Ausubel 1988) type, except for a group of monocotyledonous plants of the order Asparagales, including the Agave genus, that shares the human (TTAGGG)n type (Sýkorová et al. 2006). Telomere maintenance is achieved by a specialized enzyme, telomerase, which is a ribonucleoprotein complex that involves two subunits: the TERT protein (telomerase reverse transcriptase) subunit responsible for the synthesis of telomeric DNA and the RNA-TER (telomerase RNA) subunit used as a template for synthesis. This enzyme compensates for the inability of DNA polymerase (DNA pol) to complete telomeric DNA synthesis (Greider and Blackburn 1985; Greider 2010).
In plants, as in humans, telomerase activity is dependent on cell proliferation, as observed in diverse species, such as Nicotiana tabacum, Glycine max, Daucus carota, Brassica oleracea, Zea may and Hordeum vulgare ((Fajkus et al. 1996; Fitzgerald et al. 1996; Killan et al. 1998); during the cell cycle, the activity of telomerase showed a slight increase with each regeneration. However, the telomere length remained stable during both processes, indicating that there is a telomerase regulatory mechanism that can compensate for the replicative loss of telomeric DNA (Fajkus et al. 1998a). This dependence on the cell cycle suggests that telomerase is regulated by the development of organs and tissues, as demonstrated in BY-2 tobacco cells (Tamura et al. 1999a; Yang et al. 2002).
Plasticity in plants requires a more flexible expression of telomerase in comparison with that in animals, mainly because the dynamics of growth and development depend on each species and there are species that have prolonged life cycles compared to others. Therefore, the development of their tissues can take several years in some cases (Nicholls et al. 2011; Procházková Schrumpfová et al. 2019). The activity of telomerase has been widely studied in several plant species but is still unclear for those species with a long life cycle and slow development of tissues, especially trees, due to the difficulty in obtaining plant material. Studies conducted on Gingko biloba leaves (Song et al. 2010) and Pinus longaeva needles (Flanary and Kletetschka 2005) show that telomerase activity depends on cell proliferation and differentiation (Flanary and Kletetschka 2005; Song et al. 2011). In this sense, the process of cell proliferation involves active division and cell elongation and requires a perfect coordination between cell proliferation and differentiation at each stage of development, which affects morphogenesis (Cho et al. 2007; Gonzalez et al. 2010). In animals, telomere shortening has been correlated with age in some tissues; likewise, telomere length is associated with lifespan, while telomerase expression changes along with an increase in the sizes of organs and tissues (Gomes et al. 2011). In plants, it has been shown mainly in trees with a long life cycle that active telomerase and maintenance of telomere length during development and growth are necessary to maintain the life of the plant (Riha et al. 1998; Song et al. 2011; Mu et al. 2015).
Recently, in mammals, the absence of telomerase was found to suppress the proliferative capacity of epidermal stem cells in mice. The telomere shortening affected hair growth, and conversely, overexpression of TERT promoted cell proliferation; with telomere length changes, there was an improvement in hair growth. Therefore, telomeres and telomerase are important in tissue development (Flores et al. 2005).
In our group, we recently performed different analyses focused on the dynamics of telomere length in A. tequilana plants aged 1–6 years under field conditions and found that the telomere length is maintained in a range of 25 to 31 kb (Rescalvo-Morales et al. 2019). Additionally, different tissues (leaves, stems, and roots) were compared in 1- to 3-year-old A. tequilana and A. fourcroydes grown under greenhouse conditions; a similar behaviour was observed with those plants under field conditions, regardless of the species and tissue analysed (Rescalvo-Morales et al. 2016), which reflects that telomeric tissue-length maintenance in A. tequilana and A. fourcroydes is necessary for correct development.
Agave tequilana var. Azul is a socially and economically important species for the Mexican tequila industry. A. tequilana is a perennial monocarpic species with a cycle of more than 12 years. The vegetative phase lasts between 6 and 7 years and is followed by the sexual phase. Leaves develop in a rosette arrangement and are maintained for most of the life cycle (Little and Gentry 2006; Valenzuela 2011). One of the main characteristics of the genus Agave and other CAM species is the succulence of its leaves, which are composed of undifferentiated mesophyll cells with enlarged vacuoles (Dodd et al. 2002; Winter et al. 2015). Enlarged vacuoles are essential for water storage and required to initiate cell elongation, increasing the size of the mesophilic cells and the thickness of the leaf (Elizabeth et al. 2005).
The formation of vascular bundles and mesophilic cells involves the processes of elongation, differentiation, and active division, which stimulate an increase in plant size. Therefore, we used the A. tequilana leaf as a model to evaluate its morphology, tissue cell components and telomerase activity using quantitative TRAP (qTRAP, quantitative telomerase repeat amplification protocol) during the vegetative development period (1–6 years) under field conditions to determine the participation of telomerase during plant growth and whether the structural changes throughout plant development are related to the patterns of telomerase activity.
Materials and methods
Plant material
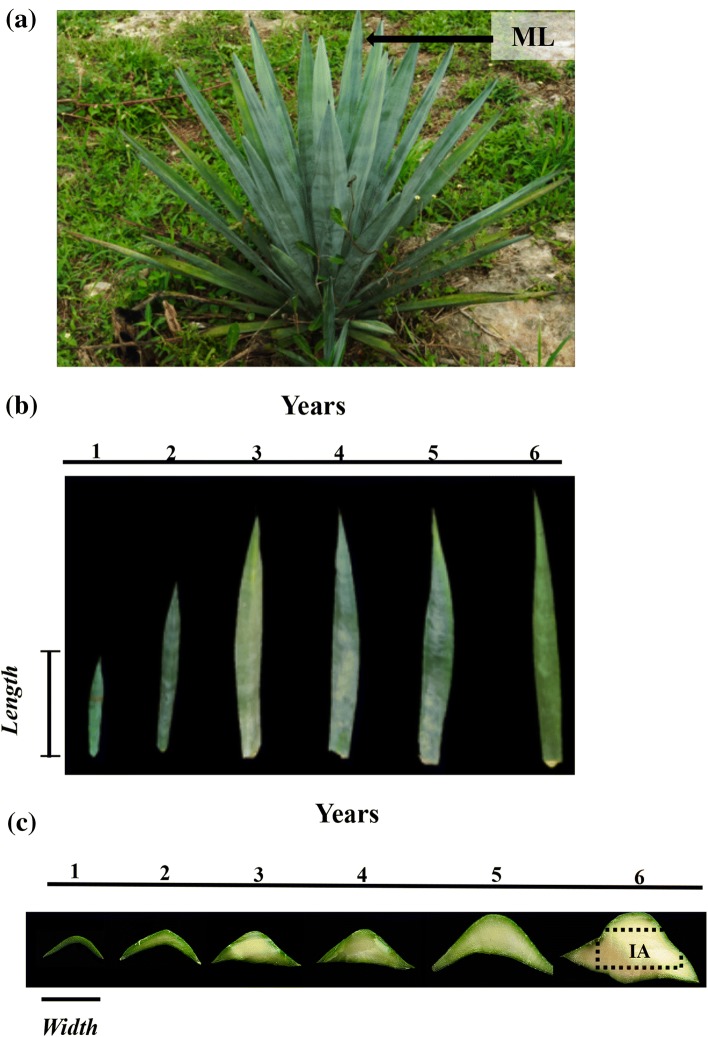
Plants (A. tequilana) were collected in the “Mayapan” hacienda, located in Valladolid, Yucatan. We collected the Middle leaf (ML) of plants (Fig. 1a) with different age 1–6 years old from three different plants (three biological replicates). The material used for the TRAP test was characterized by morphology (Fig. 1b, c), length (Fig. 1b) and width (size; Fig. 1c) of the leaf by age. The leaf area (leaf length × width) was determined, using Pearson’s correlation, the increase in leaf area for age was determined. For all measurements, an ANOVA and Tukey analysis were applied, using Rstudio for the analyzes and GraphPad Prism version 6.0 for the graphs.
Fig. 1.
Plant of A. tequilana, with average leaf length and width and estimation of the foliar area; a 3-year-old plant of A. tequilana and the position of the leaf taken for this study at different ages, b, c representation of the mean leaf of one to 6 years old plants b length and c width of leaf
The leaf material divided into three different sections, Internal apical (IA), Internal basal (IB), and External apical (EA) where cytological differences reported by Palomino et al. (2012). The IA region (Fig. 1c) used for this work due to it contains the highest number of cells in G2, in plants from 1 to 6 years old. The plant material was kept at low temperatures during transport to avoid changes in any activity, stored in − 80 °C.
Histology
The histological analysis used to analyze the cellular components of the internal apical region (IA) in a leaf of A. tequilana from 1 to 6-years-old plants (Fig. 3). The samples were fixed in FAA (10% formaldehyde/5% acetic acid/50% ethanol/35% distilled water) for 5 days, then dehydrated through graduated series of ethanol (30%, 50%, 70%, 85% and 96%) and embedded in plastic resin (JB-4 Glycol Methacrylate, PolyScience®, Warrenton, PA).
Fig. 3.
Size increase (elongation, and vascularization) in A. tequilana leaf. a, b 1–2 years tissue, mesophilic cells size 20–25 μm (red arrowhead), abundance of vascular bundles (black arrowhead) and identification of epidermal cells (yellow arrowhead). c 3-year-old tissue, vascular bundles (gray arrowhead), spongy mesophilic cells size 35–40 μm (red arrowhead) and elongation of vascular bodies (black arrowhead). d, e 4 and 5 years, mesophilic cells size 40 - 45 μm (red arrowhead) and vascular bundles (black arrowhead). f 6 years, mesophilic cells 50–65 µm (red arrowhead) and vascular bundles (black arrowhead); cross-sectional comparison at 20 μm. g 1-year vascular beam, (black arrowhead) h vascular beam size 3 years, with intermediate development (black arrowhead) i mature vascular bundle 6 years, completely differentiated; longitudinal section at 100 μm, j 1 year, it is observed how the cells that make up the vascular beam are not yet compartmentalized, k 3 years, cells of the vascular bundle, l 6 years, differentiation of the vascular bundle (black arrowhead)
Tissues are sectioned at a thickness of 3 μm and stained with methylene blue (M9140) as described by Pérez-Núñez et al. (2006), analyzed in Zeiss Axioplan 2 upright light/fluorescence microscope, captured images using the AxioVision 4.8 software, and explained in objective 20 and 100 μm.
TRAP assay
For telomerase activity assay (TRAP), the protein extracts prepared as described previously in Fitzgerald et al. (1996), Sýkorová et al. (2006). Briefly, 200 mg of leaf tissue were ground in mortar with liquid nitrogen, resuspend in 200 µl buffer W [50 mM Tris-Ac, pH 7.5/5 mM MgCl2/100 mM potassium glutamate/20 mM EGTA/1.5% PVP-40/10% glycerol/0.1 mM PSMF/1 mM DTT/0.6 vanadyl ribonucleotide complex (SIGMA®)]. It was centrifuged at 17,500×g for 15 min followed by protein precipitation with PEG-8000 added to a final concentration of 10%. Mix for 30 min at 4 °C, and spin at 17,500×g for 5 min. The pellet was resuspended in 20 µl buffer W, mixed for 30 min at 4 °C. After centrifugation, total protein concentration determined the soluble fraction of telomerase enriched protein extract (TEX) with the Bradford method (Ismail 1976).
Conventional TRAP assay was performed using telomerase extracts containing 100 ng of total protein mixed with 1 µl of 10 µM substrate primer (TS21 primer: 5′-GAC AAT CCG TCG AGC AGA GTT-3′, Fitzgerald et al. 1996) in 46 µl of TRAP buffer and incubated at 26 °C for 45 min (extension step). After the extension step, the telomerase inactivation at 95 °C for 10 min, and samples cooled to 80 °C (hot start). The PCR amplification step started by adding a mix containing 1 µl of 10 µM reverse primer (HUTPR: 5′-CCG AAT TCA ACC CTA AAA CCC TAA ACC CTA AAC CCC-3′; Sýkorová et al. 2006) and 2 units of Taq polymerase (Invitrogen®) and the reaction mixture was subjected to 35 cycles of 95 °C/30 s, 65 °C/30 s, 72 °C/30 s followed by final extension at 72 °C/5 min. TRAP products were run on 12.5% PAGE in 0.5 TBE and stained with ethidium bromide; the gels scanned by a ChemiDoc® XRS + Imager (#170-8265), Bio-Rad. Conventional TRAP assay used for evaluation of telomerase activity in all samples before evaluating in qTRAP.
q-TRAP assay
Quantitative measurement of telomerase activity was performed using the SYBR Green Master mix® (Applied Biosystems), TS21, and TELPR (concentration of each primer in the reaction mix was 0.25 µM). After extension step (45 min at 26 °C) and enzymatic inactivation (15 min at 95 °C), extension products were amplified in 30 cycles of 30 s at 95 °C, 60 secs at 60 °C (at the end of this step, fluorescence was measured). For standard curve determination, dilutions containing 500 ng, 200 ng, 100 ng, 50 ng and 25 ng of total protein in 1 µl of TEX of apical meristem tissue (active cell division), for each TEX dilution was carried out in triplicate.
For the normalization of the Relative telomerase activity, we used apical meristem at a concentration of 100 ng TEX extract in a plant of 1–3 years old. From leaf tissue (IA), we used 100 ng of TEX of 1–6 years old plants. For normalizer tissue and leaf tissues in all ages evaluated, we use biological, and statistics replicates. The qTRAP assay was performed using cycler Rotor-Gene 3000 (Qiagen) and software version 2.1.0.9. Ct values and relative telomerase activity were calculated by the delta-delta Ct method, the normalization follows approach Pffaafl 2004 comparing with the expression of telomerase activity in apical meristem and the statistical evaluation ANOVA and Tukey were calculated with GraphPad Prism version 6.0.
Results
To describe in detail the growth of the leaf during the vegetative period, material from A. tequilana plants aged 1- to 6-years-old was collected and screened based on morphology and changes in specific tissue components in the internal apical region (IA) of the leaf. During the period of 1–6 years, there was a total increase of 4.5 times the leaf area, with a strong correlation between age and leaf area, with an R2 value of 0.90 (Fig. 2a). This indicates that there was a continuous increase in the leaf area during this period.
Fig. 2.
Linear correlation and statistical analysis (ANOVA and Tukey comparative) of leaf area in leaf 1–6 years of A. tequilana; a linear correlation of leaf area, with a value of R2 = 0.90, showing an increase in leaf area with respect to age, b ANOVA and Tukey comparative by age of foliar area
The length (Fig. S1A) and width (Fig. S1B) of leaves of different ages showed an increase of 2.5 times in length and 1.8 times in width in leaves from 1 to 6 years old (Table 1). Additionally, the analysis of the data shows that the most substantial increase in leaf size occurs during the first 4 years of growth under field conditions, in comparison with years five and six, during which the growth was slower, increasing only 7% for length (Fig. S1A) and 1% for width (Fig. S1B). However, leaves began to show an increase in the thickness of the internal apical region (IA) after 3 years, and after 6 years, the most significant increase in the area (IA) was shown (Fig. 1b, c). Therefore, an increase in the length and width of the leaf occurred in addition to an increase in leaf thickness (Fig. 1c).
Table 1.
Length, width and leaf area in A. tequilana; average and standard deviation of the leaf size by the age of length, width and leaf area in with the biological replicas
| Age (years) | Length (cm) | Width (cm) | Leaf area (cm) |
|---|---|---|---|
| 1 | 44.0 ± 10.6 | 4.5 ± 0.81 | 232.8 ± 35.1 |
| 2 | 76.9 ± 3.6 | 6.3 ± 0.71 | 480.1 ± 34.9 |
| 3 | 91.3 ± 8.5 | 6.6 ± 0.67 | 609.5 ± 115.4 |
| 4 | 104.8 ± 2.3 | 8.2 ± 0.47 | 887.6 ± 72.8 |
| 5 | 107.0 ± 7.1 | 8.0 ± 0.78 | 858.6 ± 23.3 |
| 6 | 114.7 ± 9.1 | 8.1 ± 0.21 | 932.8 ± 81.2 |
The analysis of specific tissue/cellular components showed that the spongy mesophyll cells (red arrowhead) and vascular bundles (black arrowhead) increased in size over the period of 1 to 6 years (Fig. 3a–f). The 1- and 2-year-old plants showed mesophyll cells of approximately 20-25 μm in size, with some degree of leaf epidermis development (yellow arrowhead, Fig. 3a–b). In 3-year-old plants, the size of the mesophyll cells increased compared to that of the 1–2-year-old plants, reaching 30–45 μm (Fig. 3c). In 4- and 5-year-old plants, the size of the mesophyll cells was still between 40 and 45 μm (Fig. 3d, e). Finally, 6-year-old tissue presented a remarkable increase in the intracellular space of the mesophyll to an approximate size of 40–60 μm (Fig. 3f).
In 1- and 2-year-old plants, a smaller size was observed than in 3-year-olds. In 3-year-old plants, immature vascular bundles (id) (gray arrowhead) were observed (Fig. 3c), meaning that in these plants, not only the size of the vascular bundles but also the formation of new vascular bundles was promoted (Fig. 3h). In 4- to 5-year-old plants, an increase in the size of the vascular bundles was still observed (Fig. 3d, e). In the leaves of the 6-year-old plants, most of the vascular bundles were mature; therefore, all the ducts of the vasculature shown in the longitudinal section were totally defined at 20 μm (Fig. 3f), and they were entirely elongated, mainly in the primary (p.f.c) and secondary fibre layer (s.f.c) (Fig. 3j). The comparison of the vascular bundles at one, three and 6 years of age revealed that at 6 years of age, all the vascular bundles that form the A. tequilana fibre were totally differentiated (Fig. 3j–l).
Based on these morphological and histological observations, we showed that A. tequilana leaf growth is continuous, but this species requires a period of approximately 6 years to elongate and differentiate its cellular structures that result in the formation of internal leaf tissue. During this growth phase, a series of molecular mechanisms are triggered that require active division to carry out the production of fibre and the cellular expansion of tissues. Characterization of the plant material allowed us to determine that during the growth of the A. tequilana leaf, there are two phases. The first phase is elongation and tissue formation, which lasts up until age three (Fig. 3c), and the second phase is elongation and tissue differentiation, which lasts up to 6 years (Fig. 3l).
We established an experimental method for the quantitative measurement of telomerase activity in A. tequilana. Calculation of the amplification efficiency of the qTRAP assay was performed using a standard curve with meristem tissue (TEX) with between 25 to 500 ng of total protein. The linearity of the standard curve was 0.97 (Fig. S2), which confirmed that the telomerase activity detection was proportional to the amount of protein added to the samples (Table S1), and potential impurities (e.g., secondary metabolites in protein extracts) did not influence the quantitative analysis. Therefore, our data suggest that qTRAP is an efficient method to define telomerase activity in A. tequilana tissues. Subsequently, we examined the telomerase activity in meristem tissues from plants of different ages (1–3 years, Fig. S2). The Ct value for the different ages was constant. Thus, the level of telomerase activity in the meristematic tissue can be used as a reference for measurement of the relative telomerase activity in the leaves of A. tequilana plants.
Agave tequilana is a long-living plant that grows slowly but continuously. Due to its rosette growth habit, the new leaves form the central part of the stem and are arranged in a spiral from the centre outwards. Our objective was to investigate whether the activity of leaf telomerase correlates with the age of A. tequilana plants. As a preliminary test, we investigated the activity of telomerase by conventional and quantitative TRAP in the central, medium and basal leaves of 1- and 4-year-old plants (the period during which we observed the increase in leaf size). We detected telomerase activity in the leaves; however, no differences in the telomerase activity were found with regard to the position of the leaves (data not shown). Therefore, we decided to use the IA within the middle leaves of A. tequilana, which represents a tissue that actively divides and shows a variation in the content of its G0/G1 cells (Palomino et al. 2012).
The analysis of telomerase by conventional TRAP showed the expected pattern of telomere products in all samples of tissues from 1- to 6-year-old plants (Fig. S3). We observed that telomerase was present and active at all ages analysed, including in older plants (6 years old; Fig. S3). The quantification of telomerase activity in the leaves of 1- to 6-year-old plants showed that the highest relative activity occurred in both 3- and 6-year-old plants (Fig. 4). We found that during the vegetative phase of development (1 to 3 years old), the relative activity of telomerase in leaves was proportional to the increase in the age of the plant. The values of ddCt = 0.049, 0.025, and 0.014 for age and telomerase activity for 4- and 5-year-olds, respectively, were similar to that observed for 1-year-old tissue, which was ddCt = 0.052 (Table S2).
Fig. 4.

Relative leaf telomerase activity of 1–6 years in A. tequilana; detectable telomerase activity observed in leaf at all analyzed ages normalized with apical meristem activity, statistical analysis ANOVA and comparative Tukey show a value of p < 0.0001 *** in 3 and 6 years, ages with higher telomerase activity in comparison with the other periods analyzed
Discussion
In this study, leaf size increased with respect to the age of the plant, and structural changes in the apical internal region during the period of vegetative growth (1–6 years) of A. tequilana plants were observed. In the analysis of telomerase activity, for all ages analysed, there was no difference in activity relative to telomerase in meristematic tissue. Sýkorová previously demonstrated that telomerase is active in species of the genus Agave, but no information was reported in relation to age and tissue development (Sýkorová et al. 2006). Rescalvo-Morales reported that during the vegetative period of A. tequilana plants under field conditions (1–6 years), the telomere length was maintained with age (Rescalvo-Morales et al. 2019). Telomeric maintenance during tissue development, which contributes to the preservation of tissues during growth, has been observed in Melandrium album (Riha et al. 2007). Additionally, maintenance of telomere length during the growth or life cycle of trees with telomerase activity is necessary for the maintenance of their long life cycles as seen, for example, in Pinus aristata, Pinus monticola, Pinus resinous, Pinus taeda, Pinus palustris (Flanary and Kletetschka 2005), Pinus tabulaeformis (Mu et al. 2015), and G. biloba (Song et al. 2010). When observing the maintenance of telomere regions in the leaves of A. tequilana, the activity of telomerase in the medium leaves of 1- to 6-year-old plants could be used as a control for the telomerase activity in A. tequilana under field conditions.
During plant development, plasticity and specific growth, considerable variability in the activation of telomerase throughout the growth of the organs and tissues is observed, specifically in species with a long life cycle (Flanary and Kletetschka 2005). Species with short life cycles show low or undetectable levels of telomerase (Fitzgerald et al. 1996; Heller et al. 1996). In contrast, G. biloba exhibits high telomerase activity only in the early stages of growth, and telomerase is undetectable at the end of its life cycle (Song et al. 2011), reflecting that telomerase activity can be dependent on the state of leaf growth. Likewise, it has been shown that loss of TERT function (AtTERT−/−) in A. thaliana causes, in addition to telomere shortening, a decrease in root length in successive generations, indicating that maintenance of the telomere length and the activity of telomerase are necessary to maintain correct root growth in the plant. It has been demonstrated in cells with extremely short telomeres that telomerase activity is necessary to maintain constant division (González-García et al. 2015) to avoid cellular senescence (Heacock et al. 2007). These studies suggest that cells need active telomerase and a minimal but stable length of telomeres for each cell division. We morphologically characterized the growth of the A. tequilana leaf as having two phases. The first phase is the elongation of mesophyll cells with the formation of vascular bundles in the IA tissue and lasts up to 3 years of age (Fig. 3c). The second phase is the elongation phase of mesophyll cells and the differentiation of vascular bundles in the IA tissue and lasts up to 6 years of age (Fig. 3l). During these transition phases of growth, telomerase activity also increased between the third and sixth years of age. Thus, telomerase activity occurs in the leaf tissue during the leaf growth period in A. tequilana. We suggest that telomerase activity is associated with cell proliferation processes during the leafy vegetative period.
Our histological observations and characterization of the size of the plant material revealed a constant increase related to the age of the plant, similar to several reports in succulent species, wherein the increase in leaf size was associated with generation and elongation of the spongy tissue (mesophyll) (Elizabeth et al. 2005). With the increase in the size of CAM sheets, multiple processes are involved, such as active division, elongation and cell differentiation (Gonzalez 2010). In A. thaliana, any disturbance in the molecular mechanism that controls cell proliferation affects the final sizes of organs and tissues (Horiguchi et al. 2005; Horiguchi et al. 2006; Horiguchi and Tsukaya 2011). The overexpression of transcription factors that direct growth (GRF5) and the genes responsible for the transport of auxins (AVP1) increase the leaf area and the ratio between the length and width of the plant (Gonzalez et al. 2010). Moreover, there is evidence that auxins are involved in cell division and elongation processes in the tobacco cell line VBI-0 (Prisca Campanoni and Peter Nick 2005). The activity of telomerase in tobacco TBY-2 cell lines is dependent on the concentration of auxins (Tamura et al. 1999b) during the differentiation and dedifferentiation processes of these same lines. The changes in telomerase activity are related to dramatic changes in cell division rates, while telomere length remains stable in both processes, suggesting the existence of a telomerase regulatory mechanism in plant cells that compensates for the replicative loss of telomeric DNA and thus provides genomic stability in the cell (Fajkus et al. 1998b). The molecular mechanisms that regulate plant growth in species with a long life cycle have not yet been fully explored. Additionally, the mechanism for the maintenance of genetic stability and the interaction between proteins that are responsible for the correct development of tissues and organs are topics that have not yet been addressed. Undoubtedly, determining how telomerase contributes to the growth of long-lived species under natural conditions throughout their development can help us understand the molecular signalling that leads to senescence in prolonged development. In trees, this theory is called “limitation hydraulics” and suggests that cessation of growth and mortality is due to the limitation of water and nutrient transport and extrinsic factors to which the trees are exposed and not their age per se. (Mencuccini 2003; Mencuccini et al. 2005).
Finally, we attempted to understand how telomeric regions are increased and regulated. Alternative mechanisms help to maintain genomic stability during development in the Agave genus, including in a diverse number of polyploid species. In plants, as reported in Arabidopsis thaliana, there are alternative mechanisms that help restore telomere length due to genomic instability resulting from the telomerase negative gene (AtTERT) (Kuchař and Fajkus 2004). Polypoid species of Agave could have alternative mechanisms for the maintenance of telomeres.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Dr. Antonio Rescalvo Morales and Dr. Laura Espinosa Barrera, for the technical guidance, Eva Sykorova PhD, Miloslava Fojtova PhD, Peter Fijkus PhD for academic feedback during the internship in qTRAP. This project was funded by CONACyT project CB-2012-180757-Z and by the grant awarded. We like to thank Goty Bautelpacher, owner of the hacienda “MAYAPAN” farm for the plant material.
Abbreviations
- TRAP assay
Telomerase repeated amplification protocol
- qTRAP assay
Quantitative telomerase repeated amplification protocol
- A. tequilana
Agave tequilana var. Azul
- IA
Internal apical region
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Campanoni P, Nick P. Auxin-dependent cell division and cell elongation. 1-naphthaleneacetic acid and 2,4-dichlorophenoxyacetic acid activate different pathways. Plant Physiol. 2005;137:939–948. doi: 10.1104/pp.104.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KH, Jun SE, Jeong SJ, et al. Developmental processes of leaf morphogenesis in Arabidopsis. J Plant Biol. 2007;50:282–290. [Google Scholar]
- Dodd AN, Borland AM, Haslam RP, et al. Crassulacean acid metabolism: plastic, fantastic. J Exp Bot. 2002;53:569–580. doi: 10.1093/jexbot/53.369.569. [DOI] [PubMed] [Google Scholar]
- Fajkus J, Kovarík A, Královics R. Telomerase activity in plant cells. FEBS Lett. 1996;391:307–309. doi: 10.1016/0014-5793(96)00757-0. [DOI] [PubMed] [Google Scholar]
- Fajkus J, Fulnečková J, Hulánová M, et al. Plant cells express telomerase activity upon transfer to callus culture, without extensively changing telomere lengths. Mol Gen Genet. 1998;260:470–474. doi: 10.1007/s004380050918. [DOI] [PubMed] [Google Scholar]
- Fitzgerald MS, McKnight TD, Shippen DE. Characterization and developmental patterns of telomerase expression in plants. Proc Natl Acad Sci USA. 1996;93:14422–14427. doi: 10.1073/pnas.93.25.14422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanary BE, Kletetschka G. Analysis of telomere length and telomerase activity in tree species of various life-spans, and with age in the bristlecone pine Pinus longaeva. Biogerontology. 2005;6:101–111. doi: 10.1007/s10522-005-3484-4. [DOI] [PubMed] [Google Scholar]
- Flores I, Cayuela ML, Blasco MA. Molecular biology: effects of telomerase and telomere length on epidermal stem cell behavior. Science. 2005;309(80):1253–1256. doi: 10.1126/science.1115025. [DOI] [PubMed] [Google Scholar]
- Gomes NMV, Ryder OA, Houck ML, et al. Comparative biology of mammalian telomeres: hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell. 2011;10:761–768. doi: 10.1111/j.1474-9726.2011.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez N, De Bodt S, Sulpice R, et al. Increased leaf size: different means to an end. Plant Physiol. 2010;153:1261–1279. doi: 10.1104/pp.110.156018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-García MP, Pavelescu I, Canela A, et al. Single-cell telomere-length quantification couples telomere length to meristem activity and stem cell development in Arabidopsis. Cell Rep. 2015 doi: 10.1016/j.celrep.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greider CW. Telomeres. Curr Opin Cell Biol. 1991;3:444–451. doi: 10.1016/0955-0674(91)90072-7. [DOI] [PubMed] [Google Scholar]
- Greider CW. Telomerase discovery: the excitement of putting together pieces of the puzzle (Nobel lecture) Angew Chem Int Ed. 2010;49:7422–7439. doi: 10.1002/anie.201002408. [DOI] [PubMed] [Google Scholar]
- Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- Heacock ML, Idol RA, Friesner JD, et al. Telomere dynamics and fusion of critically shortened telomeres in plants lacking DNA ligase IV. Nucleic Acids Res. 2007;35:6490–6500. doi: 10.1093/nar/gkm472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K, Kilian A, Piatyszek MA, Kleinhofs A. Telomerase activity in plant extracts. Mol Gen Genet. 1996;252:342–345. doi: 10.1007/BF02173780. [DOI] [PubMed] [Google Scholar]
- Horiguchi G, Tsukaya H. Organ size regulation in plants: insights from compensation. Front Plant Sci. 2011;2:1–6. doi: 10.3389/fpls.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi G, Kim GT, Tsukaya H. The transcription factor AtGRF5 and the transcription coactivator AN3 regulate cell proliferation in leaf primordia of Arabidopsis thaliana. Plant J. 2005;43:68–78. doi: 10.1111/j.1365-313X.2005.02429.x. [DOI] [PubMed] [Google Scholar]
- Horiguchi G, Ferjani A, Fujikura U, Tsukaya H. Coordination of cell proliferation and cell expansion in the control of leaf size in Arabidopsis thaliana. J Plant Res. 2006;119:37–42. doi: 10.1007/s10265-005-0232-4. [DOI] [PubMed] [Google Scholar]
- Ismail AM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Killan A, Heller K, Kleinhofs A. Development patterns of telomerase activity in barley and maize. Plant Mol Biol. 1998;37:621–628. doi: 10.1023/a:1005994629814. [DOI] [PubMed] [Google Scholar]
- Kuchař M, Fajkus J. Interactions of putative telomere-binding proteins in Arabidopsis thaliana: Identification of functional TRF2 homolog in plants. FEBS Lett. 2004;578:311–315. doi: 10.1016/j.febslet.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Little EL, Gentry HS. Agaves of continental North America. Bull Torrey Bot Club. 2006 [Google Scholar]
- Mencuccini M. The ecological significance of long-distance water transport: short-term regulation, long-term acclimation and the hydraulic costs of stature across plant life forms. Plant Cell Environ. 2003;26:163–182. [Google Scholar]
- Mencuccini M, Martínez-Vilalta J, Vanderklein D, et al. Size-mediated ageing reduces vigour in trees. Ecol Lett. 2005;8:1183–1190. doi: 10.1111/j.1461-0248.2005.00819.x. [DOI] [PubMed] [Google Scholar]
- Mu Y, Ren L, Hu X et al (2015) Season-specific changes in telomere length and telomerase activity in Chinese Pine (Pinus tabulaeformis Carr.). Физиoлoгия pacтeний. 10.7868/s0015330315040144
- Nelson EA, Sage TL, Sage RF. Functional leaf anatomy of plants with crassulacean acid metabolism. Funct Plant Biol. 2005;2:409–419. doi: 10.1071/FP04195. [DOI] [PubMed] [Google Scholar]
- Nicholls C, Li H, Wang JQ, Liu JP. Molecular regulation of telomerase activity in aging. Protein Cell. 2011;2:726–738. doi: 10.1007/s13238-011-1093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomino G, Dolezel J, Mèndez I, Rubluo A. Nuclear genome size analysis of Agave tequilana Weber. Caryologia. 2012 [Google Scholar]
- Pérez-Núñez MT, Chan JL, Sáenz L, et al. Improved somatic embryogenesis from Cocos nucifera (L.) plumule explants. Vitr Cell Dev Biol Plant. 2006;42:37–43. [Google Scholar]
- Procházková Schrumpfová P, Fojtová M, Fajkus J. Telomeres in plants and humans: not so different, not so similar. Cells. 2019;8:58. doi: 10.3390/cells8010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescalvo-Morales A, Monja-Mio KM, Herrera-Herrera G, et al. Analysis of telomere length during the organogenesis induction of Agave fourcroydes Lem and Agave tequilana Weber. Plant Cell Tissue Organ Cult. 2016;127:135–143. [Google Scholar]
- Rescalvo-Morales A, Monja-Mio KM, Robert ML, Sánchez-Teyer LF. Telomere length in Agave tequilana Weber plants during the in vitro to ex vitro transition. Plant Cell Tissue Organ Cult. 2019 [Google Scholar]
- Richards EJ, Ausubel FM. Isolation of a higher eukaryotic telomere from Arabidopsis thaliana. Cell. 1988 doi: 10.1016/0092-8674(88)90494-1. [DOI] [PubMed] [Google Scholar]
- Riha K, Fajkus J, Siroky J, Vyskot B. Developmental control of telomere lengths and telomerase activity in plants. Plant Cell. 1998;10:1691–1698. doi: 10.1105/tpc.10.10.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riha K, Fajkus J, Siroky J, Vyskot B. Developmental control of telomere lengths and telomerase activity in plants. Plant Cell. 2007 doi: 10.1105/tpc.10.10.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Liu D, Chen X, et al. Change of season-specific telomere lengths in Ginkgo biloba L. Mol Biol Rep. 2010;37:819–824. doi: 10.1007/s11033-009-9627-y. [DOI] [PubMed] [Google Scholar]
- Song H, Liu D, Li F, Lu H. Season- and age-associated telomerase activity in Ginkgo biloba L. Mol Biol Rep. 2011;38:1799–1805. doi: 10.1007/s11033-010-0295-8. [DOI] [PubMed] [Google Scholar]
- Sýkorová E, Leitch AR, Fajkus J. Asparagales telomerases which synthesize the human type of telomeres. Plant Mol Biol. 2006;60:633–646. doi: 10.1007/s11103-005-5091-9. [DOI] [PubMed] [Google Scholar]
- Tamura K, Liu H, Takahashi H. Auxin induction of cell cycle regulated activity of tobacco telomerase. J Biol Chem. 1999;274:20997–21002. doi: 10.1074/jbc.274.30.20997. [DOI] [PubMed] [Google Scholar]
- Valenzuela A. A new agenda for blue agave landraces: food, energy and tequila. GCB Bioenergy. 2011;3:15–24. [Google Scholar]
- Winter K, Holtum JAM, Smith JAC. Tansley insight Crassulacean acid metabolism: a continuous or discrete trait? Evolution (N Y) 2015 doi: 10.1111/nph.13446. [DOI] [PubMed] [Google Scholar]
- Yang SW, Jin E, Chung IK, Kim WT. Cell cycle-dependent regulation of telomerase activity by auxin, abscisic acid and protein phosphorylation in tobacco BY-2 suspension culture cells. Plant J. 2002;29:617–626. doi: 10.1046/j.0960-7412.2001.01244.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.