Abstract
This study was performed to investigate the constituents, in vitro antifungal activity and phytotoxicity potential of the essential oil from Juniperus polycarpos var. turcomanica leaves. The essential oil was analyzed by GC–FID, and GC/MS, which predominantly contains α-pinene (51.21%), germacrene–B (4.80%), and ∆-cadinene (2.56%). The antifungal activity of the essential oil against some phytopathogenic fungi, including Alternaria alternata, Colletotrichum trichellum, Curvularia fallax, Cytospora sacchari, Fusarium oxysporum, and Macrophomina phaseolina was performed through disk diffusion and agar dilution assays. The essential oil of J. polycarpos var. turcomanica had high antifungal activity against tested phytopathogenic fungi. The most susceptible fungi to the essential oil were C. trichellum in agar dilution and M. phaseolina and C. fallax in disk diffusion methods, whereas, the most resistant fungus to the essential oil was obtained from A. alternata in both assays. Screening methods had an influence on antifungal activity of the essential oil as most of the tested fungi in this study were shown to be more resistant in disc diffusion methods. According to the phytotoxic assay results, the essential oil from J. polycarpos var. turcomanica had high phytotoxicity against three species of weeds, including P. oleracea L., A. retroflexus L., and D. stramonium L. The results of this research suggest that the herbicidal and antifungal activities of the essential oil from J. polycarpos var. turcomanica can be attributed to its major group of constituents, monoterpenes hydrocarbons.
Keywords: Essential oil, Α-Pinene, Phytopathogenic fungi, Monoterpenes hydrocarbons
Introduction
Due to the diversity and abundant medicinal and physiological effects of Juniperus, these plants have been utilized in many different fields such as in the pharmaceutical, food, cosmetic, organic, and herbal fungicide and pesticide industries (Bakkali et al. 2008). The genus Juniperus from Cupressaceae family includes more than 60–70 species throughout the world and they are mostly dispersed in the northern hemisphere (Adams 2008). Five Juniperus species namely J. communis L., J. foetidissima Willd., J. excelsa M.-Bieb., J. oxycedrus L., J. sabina L. and J. oblonga M.-Bieb. (= J. communis) were listed in the Flora of Iran. J. polycarpos C. Koch, which is from central Asia, is a common, multi-seeded juniper (Adams 2001). One of the native juniper species in Iran is J. polycarpos. This species is naturally dispersed through Arassbaran, the northern parts of Khorasan, and the southern slopes of the Alborz mountain chain. J. polycarpos var. turcomanica (B. Fedtsch.) R.P. Adams is found in the northeastern region of Iran neighboring Turkmenistan (Adams 2008). This evergreen shrub is one of the rare conifers native to Iran and that grows from northern to southern Iran (Adams and Hojjati 2012).
The Juniperus genus is widely used in cosmetics, folk medicine, cooking, and food production (Lesjak et al. 2011). The constituents of the essential oil from the leaves of different Juniperus species have been reported previously, and analysis of the leaf essential oil of J. polycarpus var. turcomanica from southern Iran showed that it consisted of α-pinene (45%), germacrene D-4-ol (8.9%) and germacrene B (2.8%) (Adams 2008; Adams and Hojjati 2013). Moreover, its essential oil from the northeast of Iran during growing season included α-pinene (41.8–75.4%), germacrene B (1–5.8%), camphor (0.7–4.5%), and α-cadinol (0.4–4.8%) (Moghaddam et al. 2018).
Competition and/or allelopathy interfere with the growth of plants. The phytotoxic characteristics of terpenoids in medicinal and aromatic plants have been examined in previous studies (Kordali et al. 2008; Mabrouk et al. 2013). According to a previous study (Langenheim 1994), volatile allelochemicals of plants can be released into the soil by aqueous filtering. Therefore, the allelochemicals in the soil and thus the level of phytotoxicity will increase. The allelochemicals reduce the seed germination and subsequent growth in many crops and weeds (Langenheim 1994).
Different testing methods are used to evaluate the in vitro antimicrobial activity of essential oils or extracts as potential antimicrobial agents. Each method has individual effects on the specific fungi. The disk-diffusion assay has many advantages in comparison to other methods as it has the ability to test very large numbers of microorganisms and antimicrobial agents, has a low cost, is simple, and its results can be explained easily (Balouiri et al. 2016). The agar dilution method, in contrast, shows greater tolerance to some fungi (Moghaddam et al. 2015).
Based on the authors’ knowledge, there has been no previous research about antifungal activity of leaf essential oil of J. polycarpos var. turcomanica on the studied fungi by various methods, nor any report on its phytotoxicity effect against weeds. The objectives of this experiment were: (i) to assess the chemical constituents of essential oil from the J. polycarpos var. turcomanica leaves; (ii) to investigate antifungal activity of the J. polycarpos var. turcomanica essential oil against some phytopathogenic fungi; and (iii) to evaluate the phytotoxicity effects of the J. polycarpos essential oil on three main weeds: Portulaca oleracea L., Amaranthus retroflexus L. and Datura stramonium L.
Materials and methods
Plant materials
The J. polycarpos var. turcomanica fresh leaves were collected from plants grown in the Hezarmasjed Mountains in the north of the Khorasan province, in the northeast of Iran. The voucher specimen (Code. 24273) was deposited in the herbarium of the Research Center for Plants Sciences, Ferdowsi University of Mashhad, Mashhad, Iran.
Extraction of essential oil
Essential oil was extracted from the fresh leaves of the plants by hydrodistillation (1: 15) for 4 h using a Clevenger type apparatus. Then, the oil was collected over the water, separated and dried over anhydrous sodium sulfate. The essential oil was stored at 4 ºC in dark sealed glass bottles for chemical analysis and biological testing.
Identification of the constituents of essential oil
The essential oil from J. polycarpos var. turcomanica was analyzed by gas chromatography (GC). The GC analysis was carried out on an Agilent Technologies 7890 equipped with a flame ionization detector (GC-FID). Volatile components were separated on a HP-5MS capillary column (30 m, 0.25 mm i.d.; 0.25 mm film thickness). Helium with purity of 99.999% was used as the carrier gas at a flow of 0.8 mL min−1. The oven temperature was increased from 60° to 280 °C at a rate of 4 °C/minute and held isothermally at 280 °C for 10 min. The injector and detector temperatures were set at 280 °C and 300 °C, respectively. The split ratio was 1:100 such that 1 µL essential oil sample was injected manually in the split mode. GC-MS analysis was done on the Agilent Technologies 5975 Mass system. The mass spectra were recorded at 70 eV, and the mass range was from m/z 50–550.
The identification of the constituents was based on the comparison of their mass spectra with those of a computer library search (NIST 08 and Willey registry of mass spectral data 9th edition) and confirmed by comparing their retention indices (determined with reference to homologous series of C5-C24n-alkanes), either with those of authentic compounds or with data published in the literature (Adams 2007; McLafferty 2009). The peak area percentages were computed from HP–5MS column without the use of FID response factors.
Antifungal activity assays
The phytopathogenic fungi used in this study were Alternaria alternata, Curvularia fallax, Cytospora sacchari, Colletotrichum trichellum, Fusarium oxysporum, and Macrophomina phaseolina, which were obtained from the collection of the Department of Plant Diseases, Tehran University, Iran. Antifungal activity of essential oil was conducted against pathogenic fungi by two different screening methods in four replications.
Agar dilution method
Antifungal activity of the essential oil was tested against phytopathogenic fungi using a range of different concentrations (100, 200, 400, and 800 µLL−1) on potato dextrose agar (PDA). The pure essential oil was dissolved in absolute ethyl alcohol and 5% Tween 20 (v/v) in a 1:1 ratio, was added to the culture medium at 40–45 °C, and was poured into Petri dishes. When the medium had solidified, the fungi were inoculated with mycelium, which was taken from the edge of seven-day-old fungal stock cultures. Disks (5 mm) of mycelial were inverted and placed in the center of each Petri dish, with the mycelium facing the medium. We prepared two controls: fresh medium with ethyl alcohol + 5% Tween 20, as well as an aqueous solution with PDA medium only. The cultures were incubated in the dark at 25 ± 2 °C. Then the colony radii were evaluated after 1, 3, and 6 days. The mycelial growth inhibition percentage was calculated from the mean values of colony diameter of treated and control groups (ethyl alcohol + 5% Tween 20).
Disc diffusion method
The inoculums for the disc diffusion assay were prepared using a suitable stock culture. 5 mm plugs of fungus were removed from each fungal colony and were placed onto a petri dish containing PDA. A single sterile (6 mm) filter paper disc, onto which 100, 200, 400 or 800 µLL−1 of essential oil was pipetted and placed on the inner surface of the Petri dishes. Sterile distilled water-soaked filter paper was considered as the control group. The dishes were immediately sealed with Parafilm and incubated at 25 ± 2 °C. The fungi growth (in mm) was measured at 1, 3, and 6 days. The mycelial growth inhibition percentage was calculated from the mean values of colony diameter of the treated and the control groups.
Phytotoxicity assay
The phytotoxicity potential of the J. polycarpos var. turcomanica essential oil was obtained from the leaves on the seed germination, the shoot and radicle growth of three important weeds including P. oleracea L., A. retroflexus L. and D. stramonium L. were investigated.
All concentrations (50, 100, 200, and 400 µLL−1) of the essential oil were obtained by dispersing as an emulsion in deionized water using Tween 80 (Inouye et al. 2001). We also had two control treatments: 1) Distlled water, 2) Distlled water + Tween. All seeds of the weeds were scarified with sulfuric acid and surface sterilized with sodium hypochloride (2%). 25 seeds for each treatment, were placed in 9 cm diameter sterile petri dishes lined with double-sterile filter paper (Whatman No. 2) in four replications. 5 mL of solution was added to each Petri dish. Dishes prepared without solvent were used as a negative control. Dishes were sealed with parafilm to prevent water loss, and they were stored in a germination cabinet in the dark at 25 °C for 7 days. A seed on which the radical appeared was considered germinated. In each treatment, the germination percentage was settled as well as the measuring radicle and the shoot length after a week. Afterwards, the seedlings of P. oleracea, A. retroflexus and D. stramonium were collected. The shoot and radical lengths, and the fresh and dry weights were measured per petri dish to evaluate the phytotoxic activity of the J. polycarpos var. turcomanica essential oil. The inhibitory or stimulatory effects were computed using the following equation, with minor modifications from Chung et al. (2001):
where ESO (essential oil effect) is the parameter measured in the presence of J. polycarpos var. turcomanica essential oil and Ce (control effect), the parameter measured in the presence of distilled water.
Statistical analysis
The data for inhibitory effects of J. polycarpos var. turcomanica essential oil on the mycelia of fungi, and the statistics on seedlings growth of weeds were subjected to analysis of variance (ANOVA) using Minitab 17 software. The means comparisons were made using Bonferroni multiple range tests at p ≤ 0.05. All data are reported as mean ± standard deviation.
Results
Yield and chemical composition of the essential oil
J. polycarpos var. turcomanica provided a yield level of 2 (mL per 100 g fresh matter) essential oil. The results obtained by GC–FID, and GC/MS analysis of J. polycarpos var. turcomanica oil are presented in Table 1. The major essential oil compounds were α-pinene (51.21%) and germacrene–B (4.80%). Our findings have shown that the major groups of essential oil compounds in J. polycarpos var. turcomanica were monoterpene hydrocarbons (60.67%) and sesquiterpene hydrocarbons (18.56%), which were followed by oxygenated sesquiterpenes.
Table 1.
Chemical compositions of the essential oil from the Juniperus polycarpos var. turcomanica leaf
| No | Component | RTa | RIb | RIc | Percentage | Methods of identificationd |
|---|---|---|---|---|---|---|
| Monoterpene hydrocarbons | 60.67 | |||||
| 1 | α-Thujene | 3.96 | 931 | 929.20 | 0.42 | MS, LRI |
| 2 | α-Pinene | 4.20 | 939 | 941.20 | 51.21 | MS, LRI |
| 3 | Camphene | 4.37 | 953 | 949.92 | 0.65 | MS, LRI |
| 4 | Verbenene | 4.47 | 967 | 955.67 | 0.23 | MS, LRI |
| 5 | Sabinene | 4.84 | 976 | 974.17 | 0.10 | MS, LRI |
| 6 | β-Pinene | 4.92 | 980 | 978.25 | 1.10 | MS, LRI |
| 7 | β-Myrcene | 5.12 | 991 | 988.36 | 1.82 | MS, LRI, S |
| 8 | α-Phellandrene | 5.51 | 1005 | 1006.09 | 0.12 | MS, LRI |
| 9 | ∆-3-Carene | 5.65 | 1011 | 1011.39 | 0.02 | MS, LRI |
| 10 | α-Terpinene | 5.98 | 1018 | 1016.31 | 0.1 | MS, LRI |
| 11 | p-Cymene | 6.10 | 1026 | 1028.46 | 0.92 | MS, LRI |
| 12 | Limonene | 6.20 | 1031 | 1032.34 | 1.93 | MS, LRI, S |
| 13 | (E)-β-Ocimene | 6.30 | 1040 | 1039.21 | 0.12 | MS, LRI |
| 14 | γ-Terpinene | 6.84 | 1062 | 1056.43 | 0.74 | MS, LRI |
| 15 | α-Terpinolene | 7.51 | 1088 | 1081.68 | 1.19 | MS, LRI |
| Oxygenated monoterpenes | 5.00 | MS, LRI | ||||
| 16 | 1,8-Cineole | 6.15 | 1033 | 1030.43 | 0.05 | MS, LRI |
| 17 | Camphor | 9.27 | 1143 | 1141.54 | 2.32 | MS, LRI, S |
| 18 | Borneol | 9.90 | 1165 | 1161.74 | 0.20 | MS, LRI |
| 19 | Terpinen-4-ol | 10.23 | 1177 | 1172.70 | 0.23 | MS, LRI |
| 20 | α-Terpineol | 10.65 | 1189 | 1186.25 | 0.24 | MS, LRI |
| 21 | Myrtenol | 10.72 | 1194 | 1194.12 | 0.32 | MS, LRI |
| 22 | Verbenone | 10.93 | 1204 | 1209.23 | 1.24 | MS, LRI |
| 23 | Bornyl acetate | 13.75 | 1285 | 1286.43 | 0.30 | MS, LRI |
| 24 | Thymol | 13.89 | 1290 | 1287.81 | 0.05 | MS, LRI, S |
| 25 | Carvacrol | 14.19 | 1298 | 1297.04 | 0.05 | MS, LRI |
| Sesquiterpene hydrocarbons | 18.56 | |||||
| 26 | ∆-Elemene | 15.24 | 1339 | 1330.94 | 0.78 | MS, LRI |
| 27 | α-Cubebene | 16.41 | 1351 | 1368.83 | 0.11 | MS, LRI |
| 28 | β-Elemene | 16.94 | 1375 | 1385.82 | 0.69 | MS, LRI |
| 29 | α-Gurjunene | 17.30 | 1409 | 1429.35 | 0.41 | MS, LRI |
| 30 | β-Caryophyllene | 17.76 | 1418 | 1412.24 | 1.70 | MS, LRI, S |
| 31 | γ-Elemene 18.20 | 1433 | 1426.68 | 1.24 | MS, LRI | |
| 32 | α-Humulene 18.77 | 1447 | 1444.96 | 0.24 | MS, LRI | |
| 33 | Germacrene D 19.63 | 1480 | 1474.23 | 1.66 | MS, LRI | |
| 34 | β-Selinene | 19.77 | 1485 | 1478.79 | 0.26 | MS, LRI |
| 35 | α-Selinene | 20.04 | 1494 | 1487.66 | 0.47 | MS, LRI |
| 36 | ∆-Selinene | 20.13 | 1495 | 1484.06 | 0.26 | MS, LRI |
| 37 | α-Muurolene | 20.24 | 1499 | 1501.63 | 0.40 | MS, LRI |
| 38 | Germacrene A | 20.32 | 1503 | 1505.43 | 0.34 | MS, LRI |
| 39 | γ-Cadinene | 20.61 | 1513 | 1506.53 | 1.73 | MS, LRI |
| 40 | ∆-Cadinene | 20.92 | 1524 | 1516.73 | 2.56 | MS, LRI |
| 41 | Elemicin | 21.31 | 1554 | 1553.54 | 0.91 | MS, LRI |
| 42 | Germacrene B | 21.81 | 1560 | 1561.05 | 4.80 | MS, LRI |
| Oxygenated sesquiterpenes | 6 | |||||
| 43 | Elemol | 21.69 | 1547 | 1542.40 | 1.32 | MS, LRI |
| 44 | Caryophyllene oxide | 22.60 | 1581 | 1574.86 | 0.14 | MS, LRI |
| 45 | t-Muurolol | 23.97 | 1642 | 1642.45 | 1.72 | MS, LRI |
| 46 | α-Cadinol | 24.27 | 1653 | 1650.00 | 1.23 | MS, LRI |
| 47 | t-Cadinol | 24.63 | 1661 | 1666.00 | 1.59 | MS, LRI |
| Total | 90.23 | |||||
| Essential oil yield | 2%(v/w) | |||||
aRetention time (min)
bLinear retention indices measured on a HP-5 MS capillary column
cLinear retention indices reported in the NIST Chemistry WebBook Database and citations therein
dMS, identification performed by GC–MS spectrometry library, AMDIS software; LRI, a book by Dr. Robert P. Adams and NIST Chemistry Webbook; S, authentic standards
Antifungal activity of essential oil
The antifungal activities of the essential oil from the J. polycarpos var. turcomanica leaves against phytopathogenic fungi was tested at different concentrations by two different antifungal screening assays, which are depicted in Table 2. All of the tested essential oil concentrations inhibited the growth of fungi and showed moderate to high antifungal activity in comparison with the control. The resistance variability of each fungus against the tested fungi was observed by the percentage of mycelia growth inhibition that related to the essential oil concentrations and times.
Table 2.
Antifungal activity of Juniperus polycarpos var. turcomanica essential oil by two different methods
| Fungi | Concentration (µLL−1) | Percent inhibition of mycelia growtha | |||||
|---|---|---|---|---|---|---|---|
| Method | |||||||
| Agar dilution | Disc diffusion | ||||||
| Day | Day | ||||||
| 1 | 3 | 6 | 1 | 3 | 6 | ||
| Alternaria alternata | 100 | 74.4 ± 2.72 bb | 49.6 ± 8.76 d | 27.7 ± 6.66 f | 58.8 ± 2.50 b-e | 43.5 ± 9.56 fg | 27.5 ± 7.11 h |
| 200 | 100.0 ± 0.00 a | 50.0 ± 4.22 d | 39.4 ± 3.79 e | 67.1 ± 6.14a-c | 46.9 ± 3.56 ef | 28.6 ± 6.18 h | |
| 400 | 100.0 ± 0.00 a | 75.9 ± 4.07 b | 51.4 ± 2.41 d | 71.8 ± 3.60 ab | 57.7 ± 8.31c-e | 31.1 ± 7.03gh | |
| 800 | 100.0 ± 0.00 a | 77.6 ± 3.54 b | 65.6 ± 0.91 c | 77.0 ± 6.09 a | 61.1 ± 6.51b-d | 49.4 ± 5.25 d-f | |
| Control (tween + alcohol) | 3.7 ± 1.1 g | 2.3 ± 1.2 g | 0.0 ± 0.0 g | – | – | – | |
| Control | 0.0 ± 0.0 g | 0.0 ± 0.0 g | 0.0 ± 0.0 g | 0.0 ± 0.0 i | 0.0 ± 0.0 i | 0.0 ± 0.0 i | |
| Macrophomina phaseolina | 100 | 71.5 ± 4.03 d | 56.9 ± 2.07 e | 44.7 ± 4.29 g | 100.0 ± 0.00 a | 76.9 ± 7.88 de | 65.0 ± 4.67 f |
| 200 | 87.0 ± 9.45 b | 69.4 ± 5.77 cd | 53.3 ± 2.41 f | 100.0 ± 0.00 a | 89.9 ± 1.04b | 73.1 ± 3.67e | |
| 400 | 100.0 ± 0.0 a | 77.3 ± 4.73 c | 62.8 ± 2.65 d | 100.0 ± 0.00 a | 100.0 ± 0.00 a | 81.4 ± 2.46 cd | |
| 800 | 100.0 ± 0.0 a | 85.8 ± 4.47 b | 69.2 ± 1.38 cd | 100.0 ± 0.00 a | 100.0 ± 0.00 a | 85.0 ± 3.79 bc | |
| Control (tween + alcohol) | 3.8 ± 1.2 h | 2.2 ± 1.4 h | 0.8 ± 0.5 h | – | – | – | |
| Control | 0.0 ± 0.0 h | 0.0 ± 0.0 h | 0.0 k ± 0.0 h | 0.0 ± 0.0 g | 0.0 ± 0.0 g | 0.0 ± 0.0 g | |
| Curvularia fallax | 100 | 73.7 ± 2.48 bc | 49.3 ± 11.19 d | 27.7 ± 6.66 f | 100.0 ± 0.00 a | 76.9 ± 7.88 de | 65.0 ± 4.67 f |
| 200 | 100.0 ± 0.00 a | 50.0 ± 4.22 d | 39.4 ± 3.79 e | 100.0 ± 0.00 a | 89.9 ± 1.04 b | 73.1 ± 3.67 e | |
| 400 | 100.0 ± 0.00 a | 73.4 ± 6.04 bc | 53.9 ± 5.94 d | 100.0 ± 0.00 a | 100.0 ± 0.00 a | 81.4 ± 2.46 cd | |
| 800 | 100.0 ± 0.00 a | 77.6 ± 3.54 b | 66.6 ± 0.91c | 100.0 ± 0.00 a | 100.0 ± 0.00 a | 85.0 ± 3.79 bc | |
| Control (tween + alcohol) | 3.5 ± 1.4 g | 2.2 ± 1.1 g | 0.0 ± 0.0 g | – | – | – | |
| Control | 0.0 ± 0.0 g | 0.0 ± 0.0 g | 0.0 ± 0.0 g | 0.0 ± 0.0 g | 0.0 ± 0.0 g | 0.0 ± 0.0 g | |
| Cytospora sacchari | 100 | 100.0 ± 0.00 a | 75.0 ± 4.07 c | 65.6 ± 5.51d | 72.8 ± 4.20 cd | 63.5 ± 3.79 ef | 48.7 ± 8.07 g |
| 200 | 100.0 ± 0.00 a | 100.0 ± 0.00 a | 75.6 ± 2.59 c | 100.0 ± 0.00 a | 71.5 ± 2.96 cd | 59.2 ± 2.46 f | |
| 400 | 100.0 ± 0.00 a | 100.0 ± 0.00 a | 83.6 ± 1.05 b | 100.0 ± 0.00 a | 75.3 ± 2.75 c | 67.0 ± 1.06 de | |
| 800 | 100.0 ± 0.00 a | 100.0 ± 0.00 a | 87.8 ± 2.38 b | 100.0 ± 0.00 a | 90.6 ± 1.92 b | 75.8 ± 1.90 c | |
| Control (tween + alcohol) | 4.1 ± 1.6 e | 3.7 ± 1.3 ef | 2.5 ± 1.2 ef | – | – | – | |
| Control | 0.0 ± 0.0 f | 0.0 ± 0.0 f | 0.0 ± 0.0f | 0.0 ± 0.0 h | 0.0 ± 0.0 h | 0.0 ± 0.0 h | |
| Fusarium oxysporum | 100 | 100.0 ± 0.00 a | 70.0 ± 2.37 b | 61.1 ± 0.89 b | 100.0 ± 0.00 a | 68.0 ± 3.33d | 51.7 ± 2.67 f |
| 200 | 100.0 ± 0.00 a | 77.9 ± 4.30 ab | 70.0 ± 3.24 b | 100.0 ± 0.00 a | 76.6 ± 8.61c | 59.2 ± 2.24 e | |
| 400 | 100.0 ± 0.00 a | 100.0 ± 0.00 a | 80.6 ± 1.42 ab | 100.0 ± 0.00 a | 86.4 ± 2.91b | 71.1 ± 2.84 cd | |
| 800 | 100.0 ± 0.00 a | 100.0 ± 0.00 a | 65.6 ± 2.42 b | 100.0 ± 0.00 a | 90.4 ± 1.47 b | 76.4 ± 1.43 d | |
| Control (tween + alcohol) | 3.9 ± 1.3 c | 3.6 ± 1.4 c | 2.2 ± 1.1c | – | – | – | |
| Control | 0.0 ± 0.0c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 g | 0.0 ± 0.0 g | 0.0 ± 0.0 g | |
| Colletotricbum trichellum | 100 | 100.0 ± 0.00 a | 100.0 ± 0.00 a | 100.0 ± 0.00 a | 66.1 ± 6.93 bc | 50.6 ± 4.16 ef | 41.7 ± 7.98 f |
| 200 | 100.0 ± 0.00 a | 100.0 ± 0.00 a | 100.0 ± 0.00 a | 71.3 ± 6.29 bc | 62.9 ± 4.56 cd | 54.8 ± 3.93de | |
| 400 | 100.0 ± 0.00 a | 100.0 ± 0.00 a | 100.0 ± 0.00 a | 74.6 ± 4.17 b | 63.3 ± 6.11 cd | 61.4 ± 1.39 cd | |
| 800 | 100.0 ± 0.00 a | 100.0 ± 0.00 a | 100.0 ± 0.00 a | 100.0 ± 0.00 a | 66.2 ± 7.41 bc | 67.2 ± 1.43bc | |
| Control (tween + alcohol) | 4.5 ± 1.3 b | 3.8 ± 1.2 c | 2.7 ± 1.2 d | – | – | – | |
| Control | 0.0 ± 0.0 e | 0.0 ± 0.0 e | 0.0 ± 0.0 e | 0.0 ± 0.0 g | 0.0 ± 0.0 g | 0.0 ± 0.0 g | |
aData are mean ± SD (standard deviation). (n = 4)
bMeans comparison were done between different concentrations of Juniperus oil in various days for each fungus. Means with different letters about each fungus in each method are statistically significant at 5% level probability
Based on the variance analysis, a significant effect (p ≤ 0.05) was observed which was connected to the concentration, time, fungi screening techniques, and corresponding interactions. The variation of the tolerances versus the essential oil is a result of the proportions inhibition of mycelia growth of different fungi.
Agar dilution method
The results of inhibition percent of mycelia growth showed that different concentrations had a different impact on each fungus (Table 2). In the agar dilution method, the antifungal activity of the essential oil at 100, 200, 400, and 800 µLL−1 concentrations against all of the examined fungi was observed as a result of the concentrations and times from 27.7% to 100% mean values. The most susceptible fungus to the essential oil was C. trichellum at all the tested concentrations and days (at 1, 3, and 6 day). Furthermore, A. alternata and C. fallax with 27.7 ± 6.66 were the most resistant fungi at the lowest concentration (100 µLL−1) of the essential oil after 6 days (Table 2).
Disk diffusion method
The percent inhibition of mycelia growth in this method was shown in Table 2. A broad differentiation was observed among different fungi. Four concentrations of the essential oil had control on all of the tested fungi. The antifungal activity of the essential oils at 100, 200, 400, and 800 (µLL−1) concentrations was observed against the tested fungi with inhibition of mycelia growth percentage from 27.5% to 100% at different concentrations and times. Our findings showed that, after six-days, C. fallax and M. phaseolina were the most susceptible fungi (with 86.0 and 85.0 ± 3.79 inhibition percent, respectively) at the highest concentration (800 µLL−1). Also, at the highest concentration (800 µLL−1) after six-days, A. alternata was the most resistant fungus with a 49.4 ± 5.25 percent inhibition of mycelia growth (Table 2).
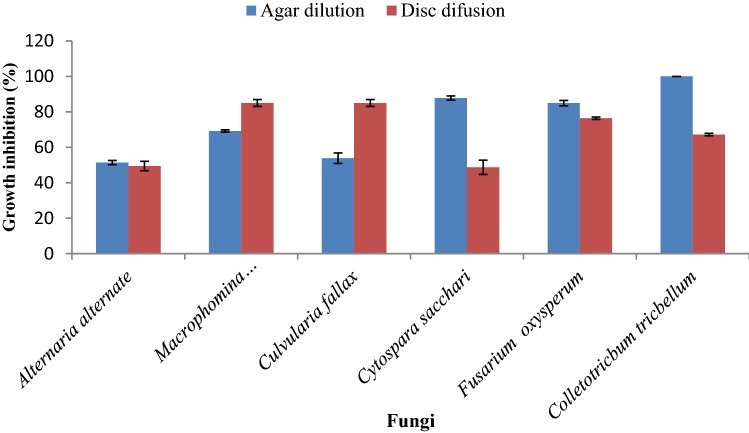
On the basis of the applied methods, the susceptibility and resistance of the tested fungi were different. At the highest concentration (800 µLL−1) of the essential oil after six-days, a comparison between the two methods showed that the agar dilution was the better method for inhibiting the growth of C. sacchar, F. oxysporum and C. trichellum fungi whereas disc diffusion was the better method for M. phaseolina and C. fallax fungi. On the other hand, in the case of A. alternata, there was no significant difference between two methods (Fig. 1).
Fig. 1.
Effect of different methods of antifungal activity assay on percentage of growth inhibition in the studied fungi after 6 days at the highest concentration (800 µL.L−1). (n = 4)
The results indicated that J. polycarpos var. turcomanica essential oil exhibited different antifungal activities against various plant pathogenic fungi. In addition, the different concentrations showed numerous results on each fungus. Therefore, increasing the oil concentration causes an enhancement of the zones of growth inhibition in all cases. Moreover, the resistances of all of the tested fungi were increased as the time passed. In order to develop new antifungal agents to control important fungal diseases of plants, it is recommended to investigate the effect of essential oil of J. polycarpos var. turcomanica against other serious fungi. Our investigation showed that different antifungal activity methods had various effects on the percent inhibition of mycelia growth. Therefore, the most resistant and susceptible fungi were different depending on the method used.
Phytotoxicity potential
The phytotoxic effects of the essential oil of J. polycarpos var. turcomanica at four concentrations are summarized in Table 3.
Table 3.
Allelopathic effect of the leaf essential oil of Juniperus polycarpos var. turcomanica on germination characteristics of Portulaca oleracea, Amaranthus retroflexus and Datura stramonium
| Concentration (µLL−1) | Seed germination (%) | Shoot length (mm) | Radicle length (mm) | Root length/stem length | Fresh weight (g) | Dry weight (g) |
|---|---|---|---|---|---|---|
| Portulaca oleracea | ||||||
| Control (Distilled water) | 85.33 ± 6.1 ab | 3.5 ± 0.3 a | 2.8 ± 0.3 a | 1.52 ± 0.09 a | 0.076 ± 0.005 a | 0.004±0.0 a |
| Control (Distilled water + Tween) | 74.7a ± 2.3 b | 2.8 ± 0.4 a | 2.3 ± 0.3 a | 1.4 ± 0.45 a | 0.060 ± 0.002 b | 0.0034 ± 0 ab |
| 50 | 44.0 ± 4.0 c | 1.9 b | 1.2 ± 0.3 b | 0.4 ± 0.04 b | 0.043 ± 0.003 c | 0.0026 ± 0 bc |
| 100 | 33.3 ± 2.3 d | 1.7 ± 0.5 b | 0.8 ± 0.3 b | 0.3 ± 0.06 b | 0.400 ± 0.001 c | 0.0020 ± 0 c |
| 200 | 0 e | 0 c | 0 c | 0 c | 0 d | 0 d |
| 400 | 0 e | 0 c | 0 c | 0 c | 0 d | 0 d |
| Amaranthus retroflexus | ||||||
| Control (Distilled water) | 85.33 ± 6.11 a | 2.9 ± 0.6 a | 2.8 ± 0.3 a | 1.52 ± 0.06 a | 0.076 ± 0.005 a | 0.004 ± 0 a |
| Control (Distilled water + Tween) | 74.7 ± 2.3 ab | 2.4 ± 0.1 ab | 2.3 ± 0.3 ab | 1.4 ± 0.45 a | 0.060 ± 0.002 b | 0.0080 ± 0.1 a |
| 50 | 61.3 ± 6.1 bc | 2.0 ± 0.2 b | 1.7 ± 0.2 bc | 0.7 ± 0.11 b | 0.056 ± 0.0046 bc | 0.0036 ± 0 a |
| 100 | 50.7 ± 4.6 cd | 1.97 ± 0.2 b | 1.6 ± 0.3 bc | 0.7 ± 0.08 b | 0.050 ± 0.002 bd | 0.0034 ± 0 a |
| 200 | 40.0 ± 4.0 d | 1.87 ± 0.2 b | 1.5 ± 0.3 c | 0.6 ± 0.08 b | 0.047 ± 0.001 cd | 0.0027 ± 0 a |
| 400 | 22.7 ± 6.1 e | 1.7 ± 0.5 b | 1.2 ± 0.2 c | 0.6 ± 0.06 b | 0.040 ± 0.003 d | 0.0010 ± 0 a |
| Datura stramonium | ||||||
| Control (Distilled water) | 86.67 ± 4.6 a | 3.3 ± 0.2 a | 2.8 ± 0.3 a | 1.52 ± 0.09 a | 0.077 ± 0.005 a | 0.004 ± 0 a |
| Control (Distilled water + Tween) | 73.3 ± 2.3 ab | 3.0 ± 0.2 ab | 2.6 ± 0.8 ab | 1.4 ± 0.45 ab | 0.063 ± 0.002 b | 0.0034 ± 0 ab |
| 50 | 70.7 ± 6.1 ab | 2.7 ± 0.1 ab | 2.3 ± 0.2 ab | 1.1 ± 0.12 ab | 0.060 ± 0.001 bc | 0.0031 ± 0 bc |
| 100 | 66.7 ± 4.6 bc | 2.3 ± 0.1 bc | 2.3 ± 0.3 ab | 0.9 ± 0.02 bc | 0.060 ± 0.00 bc | 0.0025 ± 0 cd |
| 200 | 52.0 ± 6.9 b | 1.9 ± 0.2 c | 2.3 ± 0.32 ab | 0.7 ± 0.09 c | 0.050 ± 0.001 cd | 0.0021 ± 0 de |
| 400 | 33.3 ± 6.1 d | 1.7 ± 0.5 c | 2.1 ± 0.1 bb | 0.6 ± 0.03 c | 0.046 ± 0.003 d | 0.0015 ± 0 e |
aData are mean ± SD (standard deviation). (n = 4)
bMeans with different letters in a column are statistically significant at 5% level probability
In accordance with the phytotoxic assay results, the essential oil of J. polycarpos var. turcomanica indicated high phytotoxic activity against P. oleracea with 33.3% seed germination at 100 µLL−1, whereas A. retroflexus and D. stramonium with 22.7% and 33.33% seed germination at the highest concentration (400 µLL−1), respectively. By increasing the essential oil concentration, the radicle and shoot length, as well as the fresh and dry weight of the seedlings were significantly stunted. Concentrations higher than 100 µLL−1 completely inhibited seed germination, and radicle and shoot growth of P. oleracea. However, the essential oil from the leaves of J. polycarpos var. turcomanica displayed different phytotoxic effects on P. oleracea, A. retroflexus and D. stramonium seeds. Its toxicity in P. oleracea appeared at a concentration higher than 100 µLL−1. Therefore, the phytotoxic influence on P. oleracea, A. retroflexus and D. stramonium germination, and the seedling growth had a significant difference based on the essential oil concentration and plant species.
Our results revealed that the constituents separated from J. polycarpos var. turcomanica essential oil have significantly delayed the seed germination of the four weeds, and P. oleracea, A. retroflexus and D. stramonium seedlings growth. These inhibitory activities appeared, mainly, because of the toxic compounds present in them.
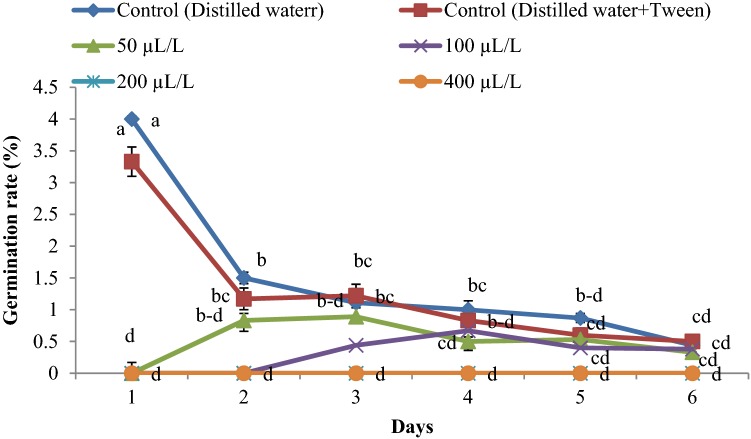
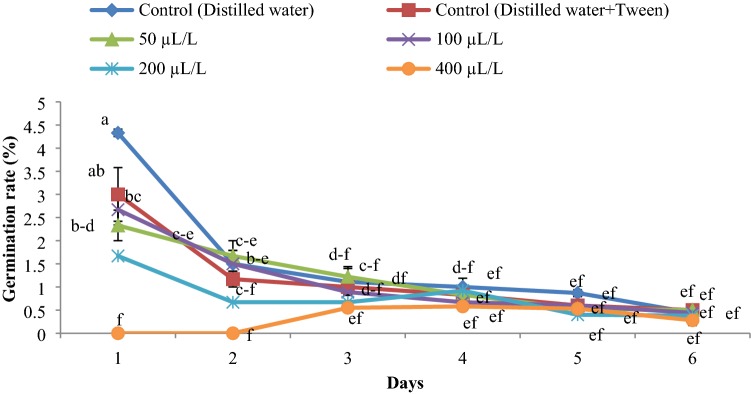
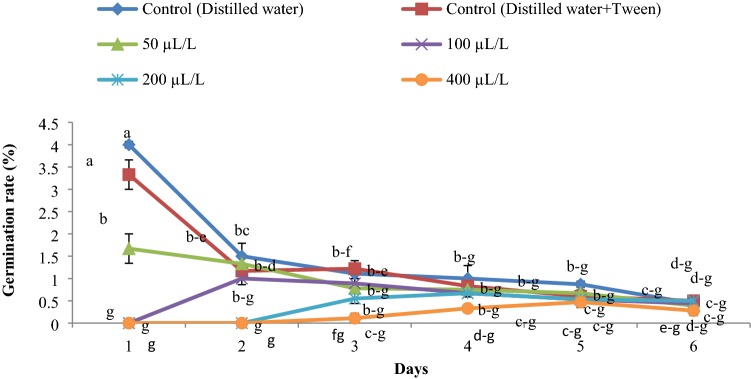
The germination rate was reduced with an increase in essential oil concentration (Figs. 2, 3, 4). The highest rate of germination was obtained in the control group. The lowest germination rate was observed at an essential oil concentration of 400 µLL−1 for all weed seeds.
Fig. 2.
Germination rate of Portulaca oleracea influenced by the essential oil concentrations (0, 50, 100, 200 and 400 µLL−1) of Juniperus polycarpos var. turcomanica leaves. (n = 4)
Fig. 3.
Germination rate of Datura stramonium influenced by the essential oil concentrations (0, 50, 100, 200 and 400 µLL−1) of Juniperus polycarpos var. turcomanica leaves. (n = 4)
Fig. 4.
Germination rate of Amaranthus retroflexus influenced by the essential oil concentrations (0, 50, 100, 200 and 400 µLL−1) of Juniperus polycarpos var. turcomanica leaves. (n = 4)
Discussion
Chemical composition of the essential oil
Due to the major and minor components of essential oil of Juniperus species from different regions of Iran, there are significant variations between the chemical constituents of the essential oils (Mazloomifar et al. 2004). In conformity to our results, the leaf essential oil of J. navicularis mainly consisted of monoterpene hydrocarbons (67.1–88.0%) (Cavaleiro et al. 2003). In agreement with our findings, the main compound of the leaf essential oils from J. excelsa subsp. polycarpos (Moein et al. 2010), J. phoenicea L. (Derwich et al. 2010; Ait-Ouazzou et al. 2012) and J. excelsa (Ehsani et al. 2012) was α-pinene, which was present in different percentages. By comparing the essential oil components of Juniperus species from different studies qualitatively (Derwich et al. 2010; Salehi Shanjani et al. 2010; Ait-Ouazzou et al. 2012), significant variation in different compositions were observed. While some constituents have been determined as the main compounds in previous studies, they were not present in our specimen. These variations could be caused by the collection of plants from different regions, the harvesting period, and the fresh or dried herbage (Salehi Shanjani et al. 2010). Results of previous investigations showed that many factors can explain the essential oil constituent variability. The essential oil constituents are influenced by the species and genotype, the environmental and ecological conditions, the growth regulators, the age of the plants, the growth stage, the method of drying, and the extraction methods (Moghaddam et al. 2007; Ghasemi Pirbalouti et al. 2013; Memarzadeh et al. 2015; Bajalan et al. 2018).
Antifungal activity of essential oil
To better control plant diseases, synthetic fungicides are widely used. However, these chemicals can cause environmental pollution due to their slow biodegradation and pose a risk to the development of resistant microorganisms (Kordali et al. 2008). The antimicrobial and antifungal activities of the plant essential oils from J. excelas (Lesjak et al. 2011; Tumen et al. 2012; Khan et al. 2012) and J. macrocarpa Sibth. (Lesjak et al. 2014) were evaluated before.
In a previous study on anti-phytopathogenic activity of peppermint (Mentha piperita L.) essential oil, it was shown that M. phaseolina had a greater tolerance among the chosen concentration range and at the highest concentration. Moreover, Fusarium oxysporum f.sp. ciceris had a complete inhibitory effect (Moghaddam et al. 2013). The antimicrobial activity of the essential oils is related to their constituents and their concentrations. Two or three main components at high concentration (up to 80%) usually described the essential oil properties (Bakkali et al. 2008). Moreover, some types of synergism between the minor compounds and the other active compounds might exist (Deba et al. 2008). Results of previous studies (Kordali et al. 2008; Diánez et al. 2018; Wang et al. 2018) indicated that the inhibitory effects of the essential oils, which were isolated from different plant species, contain mainly aromatic monoterpenes on the mycelia growth of different phytopathogenic fungal species. These variations are dependent of different compounds, such as their antifungal constituents, their structural formation, and their functional groups. Likewise, there might be synergistic interactions between the different constituents (Bajpai et al. 2013a).
According to the distinctive concentrations, different results were obtained because of the configuration of hydrogen bonds between the hydroxyl groups of essential oil phenolics (Kim et al. 2008). Furthermore, monoterpenes in the essential oils increased the concentration of lipidic peroxides, like hydroxyl, alkoxyl and alkoperoxyl radicals, and they bring about cell death (Lucini et al. 2006). Recently, an investigation has reported that the major biological agents of essential oils with therapeutic activity are monoterpene and sesquiterpene hydrocarbons, and their oxygenated derivatives, which exhibit characteristics like strong microbial pathogens growth and toxicity against plant pathogenic fungi.
Terpenes with aromatic rings and phenolic hydroxyl groups are capable of forming hydrogen bonds with the active sites of the target enzymes. They may describe the antimicrobial activity as well as the monoterpene hydrocarbons (Khalighi-Sigaroodi et al. 2005). The mechanisms of antifungal activity of monoterpenes are not fully understood. However, several reports concluded that essential oils, such as lipophilic agents, perform their action at the level of the membrane and the membrane-embedded enzymes. The lipophilic characteristics of the hydrocarbon skeleton of essential oils and the hydrophilic characteristics of their functional groups are very important (Böhme et al. 2014). Essential oils mainly act against the cell cytoplasmic membrane of microorganisms and monoterpenes to destroy the cellular integrity by inhibiting the respiration process in the microbial cell and the inactivation and/or the synthesis inhibition of intracellular and extracellular enzymes. The hydrophobicity of essential oils cause cell membrane permeability to increase by accumulating in cell membranes and thereby disturbing the cell membrane structures (Bajpai et al. 2013b). Therefore, the oxygenate monoterpenes, monoterpene hydrocarbons, and sesquiterpenes have an important role in antifungal properties of J. polycarpus var. turcomanica. The decrease or structural changes in the major constituents and possible functional synergistic interactions between the compounds have an important role in antifungal activity (Kim et al. 2008). In addition, monoterpenes disrupt the microbial cytoplasmic membranes; therefore, high impermeability of the membrane against protons and larger ions are lost (Lopes-Lutz et al. 2008). Furthermore, the method which is used to examine the antifungal activity of the essential oil, can influence the results obtained. In previous research, two assays were performed to examine the antifungal activity of Echinophora platyloba DC. essential oil. The experiments demonstrated that the consequences were different, and that each method had a special effect on the particular fungi (Moghaddam et al. 2015). The variations between the most susceptible and resistant fungi between each method was caused by the differences between the constituents of the essential oil and their interactions with each fungus. In addition, the morphological and physiological characteristics of the fungal hyphae cause an exhibition of different sensitivities to various pathogens. Generally, the dose, the fungus species and the day of observation can affect the percentages of inhibition. The agar dilution and disk diffusion methods seemed to give an indication of the susceptibility of particular fungi. Therefore, both methods can be useful in the initial screening of the antifungal activity of essential oils (Moghaddam et al. 2015).
Agar dilution assay has been used to report antifungal activity of essential oils. It was studied that by changing the concentration of Tween 80, it was possible to influence the susceptibility of the fungi to the different essential oils. For instance, it has been suggested that Tween 80 may increase fungi growth or modify cell membrane permeability. Also, it may act antagonistically against the active essential oil constituents or it may allow better spreading of components through the agar. The major factors in testing procedures are the variation in mycelia growth, the time of exposure of fungi to the essential oil, and the application and quantity of an emulsifier. All these factors considerably influence the outcome produced (Grayer and Kokubun 2001).
The differentiation between the most resistant fungi in each method is caused by the production of more enzymes by the fungi which catalyze the oxidation, and therefore the inactivation of the added essential oil. Moreover, growth inhibition of the fungal pathogens may be caused by the emulsion that destroys the cell wall and the cell membrane to different degrees due to various capacities to penetrate essential oil into the chitin based cell walls of fungal hyphae (Grayer and Kokubun 2001). Thus, as reported in this research, each method influenced the individual fungi differently.
Phytotoxicity potential
Chemical herbicides intensively contaminate the soil and the groundwater, as well as the development of weed resistance (Duke et al. 2000), and the risk of toxic residues in crops, which are increased at high concentrations. Applications of synthetic herbicides have a negative influence on the environment, so the need for natural herbicides has increased (Duke et al. 2000). In fact, the reduction in seed germination and shoot length may be connected with the reduced rate of cell division and cell elongation due to the presence of the allelochemicals (Uniyal and Chhetri 2010). In conformity with our results, there were a few reports related to toxic compounds of essential oils (e.g. caryophyllene oxide, limonene, spathulenol, etc.), as well as phytotoxic properties (Chung et al. 2001; Mabrouk et al. 2013). Some chemical compounds could be responsible for high phytotoxic potential of essential oils. Hence, low abundance control of J. polycarpos var. turcomanica in its habitat and poor diversity of other species surrounding it, is caused by its allochemicals.
Conclusion
The sensitization of the importance of fungal infections and some troubles in their treatment, cause an increase in interest of finding new fungicides to replace the chemical ones. Some chemical composition could be responsible for high phytotoxic potential of essential oils. Thus, from to the findings of this experiment, it can be concluded that the essential oil of J. polycarpos var. turcomanica leaves is a suitable alternative component to synthetic fungicides for application in agro industries. Moreover, the control of many phytopathogens has caused serious destruction in crops; therefore, novel types of selective and natural fungicides are screened and developed. Finally, the phytotoxic activity of J. polycarpos var. turcomanica essential oil on different plants can help to produce new herbicide for weed control.
Acknowledgements
The authors appreciate Alice Bouchard from Department of Experimental Surgery, McGill University, Canada due to editing the manuscript.
Abbreviations
- EO
Essential oil
- GC
Gas chromatography
- GC–MS
Gas chromatography–mass spectrometry
- FID
Flame ionization detector
- PDA
Potato dextrose agar
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adams RP. Geographic variation in leaf essential oils and RAPDs of Juniperus polycarpos K. Koch in central Asia. Biochem Syst Ecol. 2001;29:609–619. doi: 10.1016/s0305-1978(00)00098-3. [DOI] [PubMed] [Google Scholar]
- Adams RP. Identification of essential oil components by gas chromatography/mass spectroscopy. Carol Stream: Allured Publishing Corporation; 2007. [Google Scholar]
- Adams RP. The Junipers of the world: the genus Juniperus. 2. Victoria: Trafford Publications; 2008. [Google Scholar]
- Adams RP, Hojjati F (2012) Taxonomy of Juniperus in Iran: Insight from DNA
- Adams RP, Hojjati F. Taxonomy of Juniperus in Iran: Insight from DNA sequencing. Phytologia. 2013;94:219–227. [Google Scholar]
- Ait-Ouazzou A, Loran S, Arakrak A, Laglaoui A, Herrera A, Pagan R, Conchello P, Rota C. Evaluation of the chemical composition and antimicrobial activity of Mentha pulegium, Juniperus phoenicea, and Cyperus longus essential oils from Morocco. Food Res Int. 2012;45:313–319. [Google Scholar]
- Bajalan I, Rouzbahani R, Ghasemi Pirbalouti A, Maggi F. Quali-quantitative variation of essential oil from Iranian rosemary (Rosmarinus officinalis L.) accessions according to environmental factors. J Essent Oil Res. 2018;30:16–24. [Google Scholar]
- Bajpai VK, Sharma A, Baek KH. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control. 2013;32:582–590. [Google Scholar]
- Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils—a review. Food Chem Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhme K, Barros-Velázquez J, Calo-Mata P, Aubourg SP. Antimicrobial compounds. Berlin: Springer; 2014. Antibacterial, antiviral and antifungal activity of essential oils: mechanisms and applications; pp. 51–81. [Google Scholar]
- Cavaleiro C, Salgueiro LR, da Cunha AP, Figueiredo AC, Barroso JG, Bighelli A, Casanova J. Composition and variability of the essential oils of the leaves and berries from Juniperus navicularis. Biochem Syst Ecol. 2003;31:193–201. [Google Scholar]
- Chung IM, Ahn JK, Yun SJ. Assessment of allelopathic potential of barnyard grass (Echinochloa crus-galli) on rice (Oryza sativa L.) cultivars. Crop Protect. 2001;20:921–928. [Google Scholar]
- Deba F, Xuan TD, Yasuda M, Tawata S. Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidens pilosa Linn. var. radiata. Food Control. 2008;19:346–352. [Google Scholar]
- Derwich E, Benziane Z, Boukir A. Chemical composition of leaf essential oil of Juniperus phoenicea and evaluation of its antibacterial activity. Int J Agric Biol. 2010;12:199–204. [Google Scholar]
- Diánez F, Santos M, Parra C, Navarro MJ, Blanco R, Gea FJ. Screening of antifungal activity of 12 essential oils against eight pathogenic fungi of vegetables and mushroom. Lett Appl Microbiol. 2018;67:400–410. doi: 10.1111/lam.13053. [DOI] [PubMed] [Google Scholar]
- Duke SO, Dayan FE, Romagni JG, Rimando AM. Natural products as sources of herbicides: current status and future trends. Weed Res. 2000;40:99–111. [Google Scholar]
- Ehsani E, Akbari K, Teimouri M, Khadem A. Chemical composition and antibacterial activity of two Juniperus species essential oils. Afr J Microbiol Res. 2012;6:6704–6710. [Google Scholar]
- Ghasemi Pirbalouti A, Hashemi M, Taherian Ghahfarokhi F. Essential oil and chemical compositions of wild and cultivated Thymus daenensis Celak and Thymus vulgaris L. Ind Crop Prod. 2013;48:43–48. [Google Scholar]
- Grayer RJ, Kokubun T. Plant–fungal interactions: the search for phytoalexins and other antifungal compounds from higher plants. Phytochemistry. 2001;56:253–263. doi: 10.1016/s0031-9422(00)00450-7. [DOI] [PubMed] [Google Scholar]
- Inouye S, Tsuruoka T, Uchida K, Yamaguchi H. Effect of sealing and Tween 80 on the antifungal susceptibility testing of essential oils. Microbiol Immunol. 2001;45(3):201–208. doi: 10.1111/j.1348-0421.2001.tb02608.x. [DOI] [PubMed] [Google Scholar]
- Khalighi-Sigaroodi F, Hadjiakhoondi A, Shahverdi HR, Mozaffarian VA, Shafiee A. Chemical composition and antimicrobial activity of the essential oil of Ferulago bernardii Tomk. and M. Pimen. DARU J Pharm Sci. 2005;13:100–104. [Google Scholar]
- Khan M, Khan AU, Rehman NU, Gilani AH. Pharmacological explanation for the medicinal use of Juniperus excelsa in hyperactive gastrointestinal and respiratory disorders. J Nat Med. 2012;66:292–301. doi: 10.1007/s11418-011-0605-z. [DOI] [PubMed] [Google Scholar]
- Kim J, Lee Y, Lee S, Shin S, Park I. Fumigant antifungal activity of plant essential oils and components from West Indian bay (Pimenta racemosa) and thyme (Thymus vulgaris) oils against two phytopathogenic fungi. Flavour Frag J. 2008;23:272–277. [Google Scholar]
- Kordali S, Cakir A, Ozer H, Cakmakci R, Kesdek M, Mete E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour Technol. 2008;99:8788–8795. doi: 10.1016/j.biortech.2008.04.048. [DOI] [PubMed] [Google Scholar]
- Langenheim JH. Higher plant terpenoids: a phytocentric overview of their ecological roles. J Chem Ecol. 1994;20:1223–1280. doi: 10.1007/BF02059809. [DOI] [PubMed] [Google Scholar]
- Lesjak M, Beara I, Orcic D, Anackov G, Balog K, Franciskovic M, Mimica-Dukic N. Juniperus sibirica Burgsdorf. as a novel source of antioxidant and antiinflammatory agents. Food Chem. 2011;124:850–856. [Google Scholar]
- Lesjak MM, Beara IN, Orčic DZ, Petar KN, Simin ND, Emilija SD, Mimica-Dukic NM. Phytochemical composition and antioxidant, anti-inflammatory and antimicrobial activities of Juniperus macrocarpa Sibth. et Sm. J Funct Foods. 2014;7:257–268. [Google Scholar]
- Lopes-Lutz D, Alviano DS, Alviano CS, Kolodziejczyk PP. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Photochem. 2008;69:1732–1738. doi: 10.1016/j.phytochem.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Lucini EI, Zunino MP, Lopez ML, Zygadlo JA. Effect of mono-terpenes on lipid composition and sclerotial development of Sclerotium cepivorum Berk. J Phytopathol. 2006;154:441–446. [Google Scholar]
- Mabrouk S, Bel Hadj Salah K, Elaissi A, Jlaiel L, Ben Jannet H, Aouni M, Harzallah-Skhiri F. Chemical composition and antimicrobial and allelopathic activity of Tunisian Conyza sumatrensis (Retz.) E. Walker essential oils. Chem Biodivers. 2013;10:209–223. doi: 10.1002/cbdv.201200117. [DOI] [PubMed] [Google Scholar]
- Mazloomifar H, Saber-Tehrani M, Rustaiyan A, Masoudi S. Constituents of the essential oil of Echinophora platyloba DC. growing wild in Iran. J Essent Oil Res. 2004;16:284–285. [Google Scholar]
- McLafferty FW. Wiley registry of mass spectral data. 9. Hoboken: Wiley; 2009. p. 66. [Google Scholar]
- Memarzadeh SM, Ghasemi Pirbalouti A, AdibNejad M. Chemical composition and yield of essential oils from Bakhtiari savory (Satureja bachtiarica Bunge.) under different extraction methods. Ind Crop Prod. 2015;76:809–816. [Google Scholar]
- Moein MR, Ghasemi Y, Moein S, Nejati M. Analysis of antimicrobial, antifungal and antioxidant activities of Juniperus excelsa M. B subsp. polycarpos (K. Koch) Takhtajan essential oil. Pharmacogn Res. 2010;2:128–131. doi: 10.4103/0974-8490.65505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam M, Omidbaigi R, Sefidkon F. Changes in content and chemical composition of Tagetes minuta oil at various harvest times. J Essent Oil Res. 2007;19:18–20. [Google Scholar]
- Moghaddam M, Pourbaige M, Kourosh Tabar H, Farhadi N, Ahmadi Hosseini SM. Composition and antifungal activity of peppermint (Mentha piperita) essential oil from Iran. J Essent Oil Bear Plant. 2013;16:506–512. [Google Scholar]
- Moghaddam M, Taheri P, Ghasemi Pirbalouti A, Mehdizadeh L. Chemical composition and antifungal activity of essential oil from the seed of Echinophora platyloba DC. against phytopathogens fungi by two different screening methods. LWT- Food Sci Technol. 2015;61:536–542. [Google Scholar]
- Moghaddam M, Ghasemi Pirbalouti A, Farhadi N. Seasonal variation in Juniperus polycarpos var. turcomanica essential oil from northeast of Iran. J EO Oil Res. 2018;30:225–231. [Google Scholar]
- Salehi Shanjani P, Mirza M, Calagari M, Adams RP. Effects drying and harvest season on the essential oil composition from foliage and berries of Juniperus excelsa. Ind Crop Prod. 2010;32:83–87. [Google Scholar]
- Tumen I, Süntar I, Keles H, Akkol EK. A therapeutic approach for wound healing by using essential oils of Cupressus and Juniperus species growing in Turkey. Evid Based Complement Altern Med. 2012;2012:1–7. doi: 10.1155/2012/728281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uniyal AK, Chhetri S. An assessment of phytotoxic potential of promising agro forestry trees on germination and growth pattern of traditional field crops of Sikkim Himalaya, India. Am Euras J Agric Environ Sci. 2010;9:70–78. [Google Scholar]
- Wang K, Jiang S, Pu T, Fan L, Su F, Ye M. Antifungal activity of phenolic monoterpenes and structure-related compounds against plant pathogenic fungi. Nat Prod Res. 2018;33:1423–1430. doi: 10.1080/14786419.2017.1419232. [DOI] [PubMed] [Google Scholar]