Abstract
Market is increasingly demanding vegetables with high quality and nutraceutical characteristics. It was demonstrated that leafy vegetables can get benefit from biostimulants, for the reduction of nitrate concentration and the increment of antioxidants, with potential benefit for human health. The research purpose was to investigate on the role of a novel plant-based biostimulant in affecting nitrogen and carbon metabolism in wild rocket (Diplotaxis tenuifolia L.). Foliar spray treatments were performed with extracts obtained from borage (Borago officinalis L.) leaves and flowers. To evaluate the treatments effect, in vivo determinations (chlorophyll a fluorescence and chlorophyll content) were performed. At harvest, nitrate concentration, sucrose, total sugars, chlorophyll, and carotenoids levels were measured in leaves. In order to characterize the mechanism of action also at molecular level, a set of genes encoding for some of the key enzymes implicated in nitrate and carbon metabolism was selected and their expression was measured by qRT-PCR. Interesting results concerned the increment of sucrose, coherent with a high value of Fv/Fm, in addition to a significant reduction of nitrate and ABA than control, and an enhanced NR in vivo activity. Also, genes expression was influenced by extracts, with a more pronounced effect on N related genes.
Electronic supplementary material
The online version of this article (10.1007/s12298-020-00783-5) contains supplementary material, which is available to authorized users.
Keywords: Borago officinalis L., Diplotaxis tenuifolia L., Biostimulant, Nitrate, Gene expression
Introduction
Wild rocket (Diplotaxis tenuifolia L.) is a fast-growing crop belonging to the Brassicaceae family and widely cultivated as baby leaf. It is a low calories vegetable and is considered a potential health promoting produce with diuretic, stimulant, depurative, and stomachic properties. To be specific, it is a good source of ascorbic acid, flavonoids, carotenoids, and glucosinolates, with demonstrated antioxidant, pharmaceutical, and anti-cancer properties (D’Antuono et al. 2009; Jakse et al. 2013; Cavaiuolo and Ferrante 2014; Tripodi et al. 2017). Rocket hyper-accumulates nitrate (NO3−) (Di Gioia et al. 2013), which is an important intermediate molecule with key functions in nitrogen metabolism, amino acids formation and in plant physiology. At the same time, after consumption, in the acid environment of the stomach, nitrate-derived nitrite (NO2−) can represent a threat for the consumers as it can combine with free amines forming nitrosamines, which are recognized to be carcinogenic and thus potentially toxic to human health (Santamaria et al. 2001; D’Anna et al. 2003; Ferrante et al. 2002). On the other hand, some researches reported that nitrate conversion to nitrite could play an important antimicrobial role in the stomach (McKnight et al. 1999). Nitrate concentration depends on several factors, like season of cultivation, light intensity and quality, temperature, fertilization, storage conditions of the crop after harvest (Premuzic et al. 2001; Frezza et al. 2005; Magnani et al. 2007; Kim and Ishii 2007; Colla et al. 2018 and references therein), as well as genotype (Anjana and Iqbal 2007). In Europe, for the commercialization of leafy vegetables, the nitrate concentration in foods is limited by a specific regulation (Reg. No. 1258/2011). The limits for rocket range from 7000 NO3− [mg kg−1 FW] (harvest from October to March) to 6000 NO3− [mg kg−1 FW] (harvest from April to September). For these reasons, to understand the nitrate metabolism in rocket is highly important to develop agronomical strategies to be applied to control its accumulation and, at the same time, maintain high quality and yields.
Nitrogen (N) is a fundamental macronutrient for plants, which is required for the synthesis of amino and nucleic acids, and it is an essential nutrient for cellular metabolism (Parker and Newstead 2014). Its absorption at the root level affects plant growth and consequently the productivity of crops (Krapp et al. 2014; O’Brien et al. 2016). Nitrate is actively transported through the plasma membrane of the epidermal and cortical cells of the roots across the proton symporters (NO3−:2H+) or Cl− canal (2NO3−:H+); this active action exploits the driving force of transmembrane different potential, created due to the ATP hydrolysis by H+ ATPase of the plasma membrane. The transport is controlled by a large family of nitrate transporters (NTR) (EC 7.3.2.4), including NTR1, which transports nitrate, histidine, and nitrite, and belongs to the subgroup of nitrate/nitrite transporters (Pao 1998); NTR2, which belongs to the subgroup of proton dependent oligopeptide transporters and is responsible for the transportation of peptides, amino acids, nitrate, chlorate, and nitrite. After its uptake, NO3− can be loaded and stored inside the cell vacuoles to accomplish osmotic functions, can go back to the soil via apoplast, can be translocated via xylem and transported to other tissues, or it can be reduced by different redox reactions so as to be assimilated (Jakse et al. 2013). These redox reactions are catalyzed by specific enzymes, are energy dependent and generally uses NAD(P)H+ as electron donor. Nitrate metabolism starts in the cytosol with the reduction of nitrate to nitrite by the action of the enzyme nitrate reductase, NR (EC 1.6.6.1). After that, nitrite is transported into the chloroplast of the leaf, or in the plastid of the root, to be reduced to ammonium by the second enzyme of the pathway, the nitrite reductase NiR (EC 1.6.6.4). Nitrite and ammonium ions are cytotoxic because they lead to pH changes and induce a rise in reactive nitrogen species and oxidative damages, so they cannot be accumulated inside the cell (Chow and Hong 2002). For the above reasons, their incorporation into organic compounds must be relatively fast (Chow and Hong 2002). Ammonium then triggers the “Glutamine Synthetase/Glutamine Oxoglutarate Aminotransferase” cycle (GS/GOGAT). The enzyme glutamine oxoglutarate aminotransferase is also known as glutamate synthase (EC 1.4.1.14), GOGAT. Ammonium is converted to glutamine (Stitt 1999) in the cytosol or in the chloroplasts/plastids, by the enzyme glutamine synthetase (EC 6.3.1.2) (GS), which presents two active isoforms, one cytosolic and another chloroplastic/plastidial, called GS1 and GS2, respectively (Lancien et al. 2000). The condensation of ammonium with glutamate to obtain glutamine requires ATP (Temple et al. 1998) as source of energy. At this step, if α-ketoglutarate (or oxo-glutarate) and energy are available from photosynthesis process, two amide groups of glutamine can be transferred, by GOGAT, to α-ketoglutarate (or oxo-glutarate) (Temple et al. 1998). One of the two molecules of glutamate can accept NH4+ during another GS/GOGAT cycle, while the other can be converted to amino acids by transaminases and then transformed in proteins in order to be effectively used by the plant. Also other three enzymes probably participate to the process of ammonium assimilation: cytosolic asparagine synthetase (AS) (EC 6.3.5.4), plastidial carbamoylphosphate synthase (CPSase) (EC 6.3.4.16) and mitochondrial NADH-glutamate dehydrogenase (GDH) (EC 1.4.1.2) (Masclaux-Daubresse et al. 2010). AS, using ammonia as substrate, catalyzes the transfer of the amide group of glutamine and a molecule of aspartate to create glutamate and asparagine (Masclaux-Daubresse et al. 2010). Carbamoylphosphate synthase (CPSase) uses bicarbonate, ATP, ammonium or the amide group of glutamine to catalyze the formation of carbamoylphosphate, a precursor of citrulline and arginine (Masclaux-Daubresse et al. 2010). GDH can catalyze the de-amination of glutamate or, alternatively, incorporate ammonium into glutamate in the presence of high ammonium levels due to stress conditions (Masclaux-Daubresse et al. 2010). In the mesophyll of the cells, there is high activity of GS2, while GS is low in leaves, being generally limited to the phloem; these two isoenzymes have an organ-specific expression pattern (Edwards and Coruzzi 1990). Therefore, GS1 is the major form of GS located in plant roots, it is very important for the primary nitrogen assimilation and its expression is metabolically regulated by both nitrogen and carbon availability (Sun et al. 2010). GS2 plays a crucial role in re-assimilation of NH4+ released via photorespiration in plants. Glutamate synthase is present in two isoforms in plants: Fd-GOGAT, that uses ferredoxin as electron donor, and NADH-GOGAT, with NADH as co-factor. Generally, the first one is in the chloroplasts, while NADH-GOGAT is localized in the plastids of non-photosynthetic tissues (Masclaux-Daubresse et al. 2010). As a general rule, the reduction of nitrate occurs more effectively in leaves than in roots, due to the close dependence on photosynthesis for reductants, energy, and carbon skeleton (Chen et al. 2004). It is known that there is a close connection between nitrogen and carbon metabolism (Goel et al. 2016); in particular, the flows of nitrate and ammonium are integrated within the organic acids metabolism (Vance and Gantt 1992) and the incorporation of ammonium, which occurs mainly in the GS/GOGAT cycle, requires the availability of carbon skeletons, specifically of α-ketoglutarate. This organic acid is originated in the Krebs cycle by the enzyme isocitrate dehydrogenase, IDH (EC 1.1.1.42), and in the ammonium cycle, by aspartate aminotransferase, AspAT (EC 2.6.1.1). Thus, the α-ketoglutarate appears to be an important player in the regulation of nitrogen and carbon metabolism (Lancien et al. 1999). Moreover, the formation of α-ketoglutaric acid, which is probably a limiting factor for GOGAT activity, is regulated by a series of factors, including the availability of nitrate and the accumulation of ammonium (Lam et al. 1996). Another key enzyme, phosphoenolpyruvate carboxylase (PEPC) (EC 4.1.1.31) catalyzes important reaction in plants primary metabolism and has been shown to play a crucial role in regulating carbon and nitrogen metabolism in Arabidopsis (Shi et al. 2015). In C4 and CAM plants, PEPC is responsible for the carbon fixation during the photosynthesis, while in non-photosynthetic tissues and in C3 plants, its role is to provide intermediate molecules for the Krebs cycle, catalyzing the β-carboxylation of phosphoenolpyruvate to oxaloacetate, which in turn is converted to malate by malate dehydrogenase (Sánchez et al. 2006). Four genes encoding for four different PEPC isoforms were found in Arabidopsis, PPC1, PPC2, PPC3 and PPC4. Among those genes, PPC1 and PPC2 have been suggested to be the most representative in leaves (Shi et al. 2015), while PPC3 was more expressed in roots (Sánchez et al. 2006). A novel role in the adaptation to salt and drought stress has been proposed for the PPC4 gene (Sánchez et al. 2006).
Considering all these factors, the central role of photosynthesis in regulating plant growth, yield and the coordinated regulation of the carbon and nitrogen metabolism is clear. In fact, the increment of photosynthetic capacity has been shown to facilitate nitrate metabolism and regulate C/N balance in Arabidopsis (Otori et al. 2017). A good photosynthesis rate is strictly related to the activity of the enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) (EC 4.1.1.39), which catalyzes the first step in net photosynthetic CO2 assimilation and photorespiratory carbon oxidation in C3 plants. To maintain and promote its activity is very important to obtain a good plants yield (Spreitzer and Salvucci 2002; Raines 2011) and to enhance carbon and nitrogen metabolism.
Abscisic acid (ABA) is a versatile phytohormone that regulates several cellular and molecular processes during plants development and in response to stress conditions (Zhu 2002; Kiba et al. 2012). The involvement of this hormone in nitrogen acquisition is becoming more evident considering that changes in ABA content have been reported to be linked to nitrogen signaling in many plant species (Radin et al. 1982; Palmer 1985; Peuke et al. 1994; Brewitz et al. 1995; Wilkinson and Davies 2002). However, to date there is no clear relationship between tissue nitrate levels and the ABA response.
Taking into account the commercial importance of rocket, it is important to look for strategies aiming to decrease nitrate concentration in leaves and to enhance the nutrient use efficiency of crop, the yield as well as the produce quality. Biostimulants may influence plant metabolism, acting on the regulation of key enzymes involved in N assimilation (Schiavon et al. 2008; Ertani et al. 2009) and C metabolism (Rouphael and Colla 2018 and reference therein). They can also trigger the activity of the enzymes of Krebs cycle, contributing to the interplay of C and N metabolisms (Schiavon et al. 2008; Santi et al. 2017), which is strictly linked to plant productivity.
The purpose of this work was to investigate on the role of a novel plant-based biostimulant in affecting nitrogen and carbon metabolism in rocket.
Foliar spray treatments were performed with a biostimulant formulation obtained from borage leaves and flowers. To evaluate the effect of borage extracts on rocket, non-destructive in vivo determinations (chlorophyll a fluorescence and chlorophyll content) were measured during cultivation. At harvest, nitrate concentration, sucrose, total sugars, chlorophyll, and carotenoids levels were measured in leaves. In addition to these analyses, a set of genes encoding for some of the key enzymes involved in nitrate and carbon metabolism was selected and their expression was measured by quantitative qRT-PCR, in order to characterize the mechanism of action of the biostimulant at molecular level.
Materials and methods
Plant material, treatments, and sampling
Rocket (Diplotaxis tenuifolia L. ‘Frastagliata’) was grown in a floating hydroponic system in an experimental glasshouse at the University of Milan, under monitored conditions (20 ± 1 °C, 55 ± 5% RH, 400 Wm−2 and 16 h photoperiod). By mid-December, seeds were sown in polystyrene trays (51.5 × 32.5 cm, 228 holes, expected plant density was 1150 plants m−2) on perlite substrate and placed in tanks filled with a modified Hoagland’s nutrient solution. The concentrations of nutrients in the solution, expressed as mM, were: 12 N-NO3, 3.8 N-NH4, 2.8 P, 8.4 K, 3.5 Ca, 1.4 Mg and Hoagland’s concentration for micronutrients. Oxygen was supplied by bubbling air in the nutrient solution so that the oxygen concentration was around 5–6 mg/L.
Borage extracts were obtained using borage (Borago officinalis L.) plants harvested in open field in Lodi province, during the flowering stage. Borage flowers or leaves were separately minced, macerated in deionized water (500 g in 1 L) for 25 days, in the dark, at room temperature (RT) (Bulgari et al. 2017). The aqueous extracts were filtered and properly diluted in water (10 mL L−1) to be used for treatments. No surfactant was used, since in any of the preliminary trials, no problems in dispersion of foliar spray were observed.
Extracts were sprayed between 09:00 and 10:00 a.m. onto rocket leaves until run-off, 35 days after sowing and 1 day before harvest (45 days after sowing). Harvesting was performed when the baby leaf commercial stage was reached. Treatment conditions were: water (control); 10 mL L−1 of borage leaf extract (LE); 10 mL L−1 of borage flower extract (FE). After harvest, leaves were gently rinsed with distilled water, blotted with paper towels, immediately frozen in liquid nitrogen and stored at − 80 °C. For the gene expression analysis, sampling was performed 2–4–6–9 and 24 hours (h) after the second treatment, to evaluate the possible fluctuations of genes expression over time and to identify the timing of gene activation following the application of the extracts. The in vivo nitrate reductase activity assay was performed the day after (24 h) the second treatment, at T0 (condition of dark), T1 (2 h of light exposure), and T2 (4 h of light exposure). Samples for biochemical determinations (chlorophyll and carotenoids, nitrate, sucrose, total sugars and abscisic acid) were collected 24 h after the last treatment and stored at − 20 °C until used for laboratory analyses.
Non-destructive measurements
Chlorophyll a fluorescence
At harvest, before cutting rocket leaves, chlorophyll a fluorescence was measured using a hand-portable fluorometer (Handy PEA, Hansatech, Kings Lynn, UK). Leaves were dark-adapted for 30 min. Using a leaf clip (4 mm diameter), a rapid pulse of high-intensity light of 3000 μmol m−2 s−1 (600 W m−2) was administered to the leaf inducing fluorescence (Murchie and Lawson 2013). The fluorescence parameters were calculated automatically by the used device: in particular, we considered data related to the maximum quantum efficiency of PSII (Fv/Fm), performance index (PI), and the number of reactive centers per cross-section (RC/CSm).
Chlorophyll measurements in vivo
Chlorophyll content was estimated in vivo with a chlorophyll meter (CL-01, Hansatech, UK), at harvest. This device provides an indication of green color of leaves and it determines relative chlorophyll content using dual wavelength optical absorbance (620 nm and 940 nm wavelength) (Wood et al. 1993).
Destructive analyses
Chlorophyll (a + b) and total carotenoids determination
Leaf tissue (30–50 mg) was extracted in 100% (v/v) methanol, for 24 h at 4 °C in a dark room; afterwards quantitative determination was carried out. Absorbance readings were measured at 665.2 nm and 652.4 nm for chlorophyll (a + b) and 470 nm for total carotenoids. Pigment levels were calculated by Lichtenthaler's formula (1987) and expressed on the basis of tissue fresh weight.
Sucrose and total sugars levels
About 1 g of leaf tissue was homogenized in 4 mL of distilled water and centrifuged at 4000×g for 15 min at RT. Sucrose and total sugars were assayed according to the resorcinol method and anthrone assay, respectively (Yemm and Willis 1954; Cocetta et al. 2015). Absorbance was read at 500 nm for sucrose and at 620 nm for total sugars and the levels were calculated referring to sucrose or glucose calibration curves, respectively.
Nitrate reductase in vivo activity and nitrate levels
Nitrate reductase in vivo activity was performed as described by Aslam et al. (1984) modified. Fresh leaves were immediately put in ice at dark. Leaves were quickly cut in little disks of 5 mm of diameter and put in 15 mL tubes to reach 0.8 mg of fresh weight. After that, tubes were closed and placed in ice in order to maintain inactive the enzyme. The control samples were immediately boiled in water for 5 min in order to denature the enzyme. The incubation medium was composed of potassium phosphate 100 mM (pH 7.5); 5% v/v isopropanol and 30 mM potassium nitrate. 1 mL of the reaction buffer was added to the tubes placed in ice and then the tubes were transferred in a water bath at 30 °C for 30 min. After incubation, the reaction was stopped with 1 mL of 1% sulfanilamide in HCl 3.0 N, and 0.02% N naftin etilen diamide as indicator of nitrites content. Tubes were left in the dark for 30 min to wait for the color development and then spectrophotometric readings were made at 540 nm. The calibration was carried out with a standard solution of sodium nitrite.
Nitrate concentration was measured by the salicyl-sulphuric acid method (Cataldo et al. 1975). 1 g of fresh leaf tissue was homogenized (mortar and pestle) in 4 mL of distilled water. The extract was centrifuged at 4000×g for 15 min at RT (ALC centrifuge-model PK130R) and the recovered supernatant was used for the colorimetric determination. 12 μL of sample were added to 80 μL of 5% (w/v) salicylic acid dissolved in H2SO4 plus 3 mL of 1.5 N NaOH. The samples were cooled at room temperature and absorbance at 410 nm was measured. Nitrate concentration was calculated referring to a KNO3 standard calibration curve [0, 1, 2.5, 5, 7.5, 10 mM KNO3].
Abscisic acid concentration
ABA was determined by an indirect enzyme linked immuno-sorbent assay (ELISA) based on the use of DBPA1 monoclonal antibody, raised against S( +)-ABA (Vernieri et al. 1989). Rocket (1 g) was homogenized (mortar and pestle) in 4 mL of distilled water. The extract was centrifuged at 3000×g for 15 min at RT (ALC centrifuge-model PK130R) and the recovered supernatant was used for the analysis. The ELISA was performed according to the method described by Borghesi et al. (2016).
RNA extraction and qRT-PCR
About 100 mg of ground tissues were used for the extraction of total RNA using the Spectrum Plant Total RNA Kit with on-column DNase-treatment (Sigma) according to manufacture instructions. RNA concentration and integrity were assessed by NanoDrop N-1000 spectrophotometer (NanoDrop technologies). 3 µg of RNA were reversely transcribed to cDNA using the SuperScript® III cDNA Synthesis Kit according to the manufacturer’s instruction (Invitrogen). qRT-PCR analysis was performed using the SYBR® Green PCR Master Mix (Applied Biosystems) in 20 µL reaction mix consisting of 2 µL of cDNA (1:20 dilution), 10 µL of 1 Master Mix, 0.4 µM of forward and reverse primers, and sterile water up to 20 µL. Analysis was performed using a ABI7300 (Applied Biosystem) thermocycler. Temperature profiles consisted of an initial step at 50 °C for 2 min, followed by denaturation at 95 °C for 2 min, and by 40 cycles of denaturation (95 °C for 15 s) and annealing/extension (60 °C for 1 min).
Gene expression analyses were performed using gene-specific primers for: nitrate reductase (DtNR), nitrite reductase (DtNiR), glutamyne synthetase (DtGS1), glutamate synthase (DtGLU), nitrate transporter (DtNTR), isocitrate dehydrogenase (DtIDH), phosphoenolpyruvate carboxylase 2 and 4 (DtPEPC2-like, DtPEPC4-like), and Rubisco (DtRuBisCO) (Supplemental material—Supplemental Table S1).
Primers were designed based on the RNAseq library recently built by using the Illumina RNA-Seq technology and providing sequence information and expression levels (RPKM) of Diplotaxis tenuifolia L. transcriptome (Cavaiuolo et al. 2017). Expression levels were calculated using the delta–delta Ct (ΔΔCt) method. The reported values are means ± SE (n = 6). Actin and elongation factor (EF-1α) were tested to be used as housekeeping gene (Supplemental Table S1). Due to the highest stability in its expression levels, EF1-α was used for the calculations.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 6. All data were subjected to one or two-way ANOVA and differences among means were determined by Bonferroni’s post-test. Additional information is reported in the figure legends.
Results
Chlorophyll a fluorescence
As regards the chlorophyll a fluorescence parameters measured at harvest, the maximum quantum efficiency of PSII (Fv/Fm) of rocket leaves showed a significant increment in response to FE application in comparison to control (Fig. 1a). The performance index (PI) did not show any significant difference, even if values were slightly higher in treated leaves (Fig. 1b) and the same pattern was noticeable in the number of reaction centers per cross section (RC/CSm) (Fig. 1c).
Fig. 1.

Chlorophyll a fluorescence parameters, Fv/Fm (a), PI (b), and RC/CSm (c), measured in vivo in rocket leaves treated with water (control), 10 mL L−1 borage LE or FE. Values are means ± SE (n = 3). Data were subjected to one-way ANOVA with Bonferroni’s post-test (P < 0.05). Different letters, when present, indicate statistical differences among treatments.
Chlorophylls and carotenoids
Leaf pigments, such as chlorophylls and carotenoids, have important role in light harvesting and energy transmission, but they also contribute to the product visual appearance and hence to the quality of vegetables.
The chlorophyll content determined in vivo (Fig. 2a) and the chlorophyll a + b concentration determined with destructive method (Fig. 2b) showed the same trend; borage treatments (in particular FE) slightly diminished pigments in rocket leaves compared to control, but differences were not significant. Total carotenoids concentration (Fig. 2c) showed an opposite behavior; in fact, borage treatments enhanced carotenoids content, in particular LE, even if not significantly. The carotenoids concentration ranged from 0.15 to 0.25 mg g−1 FW.
Fig. 2.
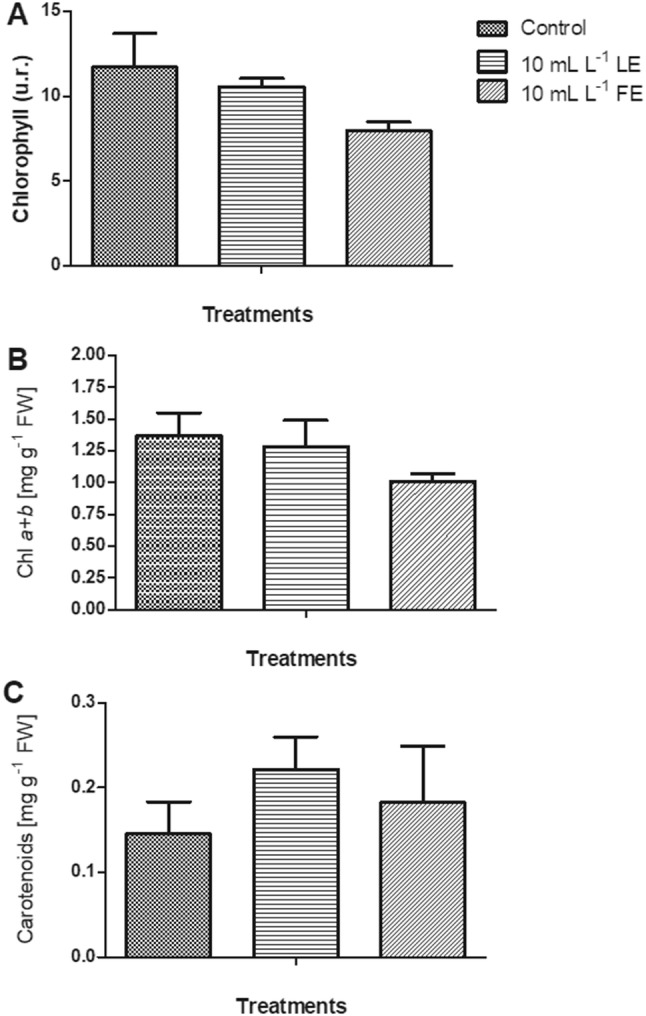
Chlorophyll content determined in vivo (a), chlorophyll a + b (b), and carotenoids concentration (c) in rocket leaves treated with water (control), 10 mL L−1 borage LE or FE. Values are means ± SE (n = 3). Data were subjected to one-way ANOVA with Bonferroni’s post-test (P < 0.05)
Abscisic acid levels
ABA is an important plant hormone that regulates the crop water balance, and its levels increase under stress conditions. The application of FE borage treatment allowed reducing the concentration of free abscisic acid compared to control and LE-treated plants (Fig. 3) with values more than halved, suggesting that this biostimulant allowed keeping lower ABA levels. Leaves of rocket plants treated with LE borage treatment showed ABA levels similar to control.
Fig. 3.
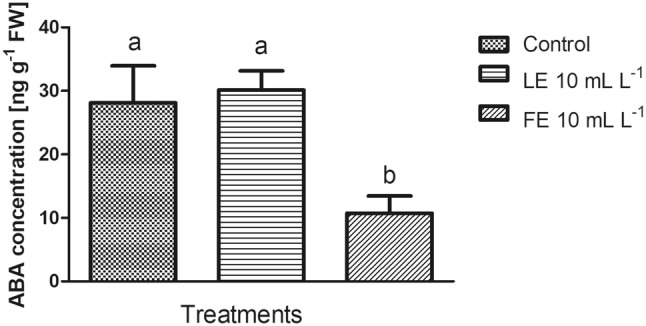
Abscisic acid (ABA) concentrations in rocket leaves treated with water (control), 10 mL L−1 borage LE or FE. Values are means ± SE (n = 3). Data were subjected to one-way ANOVA with Bonferroni’s post-test (P < 0.05). Different letters, when present, indicate statistical differences among treatments
Sucrose and total sugar concentration
Sugars are important metabolites indispensable for plant metabolism, but they have also a crucial role as signaling molecules. Moreover, in leafy vegetables, they represent the source of energy during storage and they have fundamental nutritional value in human diet, as well.
Figure 4a showed that FE exerted a positive effect on sucrose concentration in rocket leaves, confirmed by statistical analyses. The leaf sucrose concentration in FE treatment was higher than 300 mg kg−1 FW. Borage extracts slightly enhanced also total sugars concentrations than control but, in this case, differences were not significant (Fig. 4b). Total sugars in average were 2 g kg−1
Fig. 4.

Sucrose (a) and total sugars (b) concentration of rocket leaves treated with water (control), 10 mL L−1 borage LE or FE. Values are means ± SE (n = 3). Data were subjected to one-way ANOVA with Bonferroni’s post-test (P < 0.05). Different letters, where present, represent significant differences among treatments
Nitrate concentration and nitrate reductase in vivo activity
Nitrate is an important source of nitrogen for plant growth, but the accumulation in leaves can be a problem for leafy vegetables commercialization. Borage extracts were used for evaluating their ability in improving nitrate assimilation rate. The nitrate concentration ranged from 2800 to 5500 mg kg−1 FW (Fig. 5). It is interesting to observe that biostimulant treated plants showed nitrate levels halved than untreated control, and this difference was confirmed by statistical analysis. Borage extracts had a positive effect in the enhancement of nitrate assimilation.
Fig. 5.

Nitrate concentration of rocket leaves treated with water (control), 10 mL L−1 borage LE or FE. Values are means ± SE (n = 3). Data were subjected to one-way ANOVA with Bonferroni’s post-test (P < 0.05). Different letters represent significant differences among treatments
Nitrate concentration is a balance between the amount of nitrate absorbed and that assimilated. The key enzyme of nitrate assimilation is nitrate reductase. Figure 6 showed the activity of the enzyme nitrate reductase in vivo, monitored at three different time points (0, 2, and 4 h of light exposure), the day after the second treatment. FE determined a significant increment of the activity, at 2 h and 4 h compared to control. LE presented an intermediate activity, with a peak at 4 h. Control showed the lowest values of activity. These results were coherent with the halved nitrate concentration reported above.
Fig. 6.
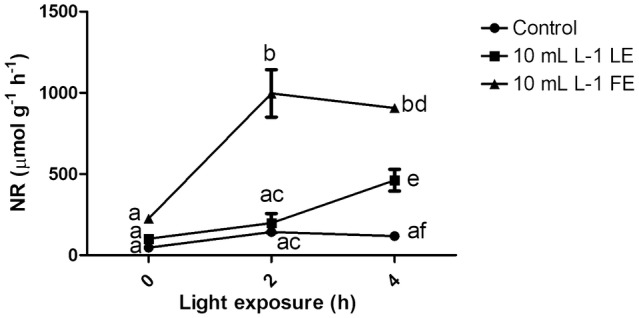
Nitrate reductase in vivo activity measured in rocket leaves treated with water (control), 10 mL L−1 borage LE or FE at three different time points (0, 2, and 4 h of light exposure). Values are means ± SE (n = 3). Data were subjected to two-way ANOVA (P < 0.0001) with Bonferroni’s post-test. Different letters represent significant differences among treatments and time
Genes expression analysis
Nitrate assimilation depends from the expression of genes that belong to the nitrate uptake, reduction and incorporation in sugars for the biosynthesis of amino acids. These sugars derive from the primary metabolism that is regulated by different enzymes and related genes.
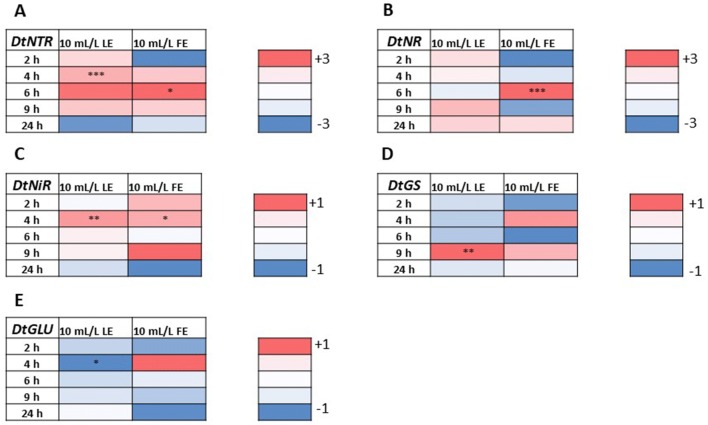
In order to verify the effect of borage extracts at molecular level, the expression of some key genes involved in nitrate and carbon metabolism was studied in response to the different treatments. Changes in the expression of genes involved in nitrogen and carbon metabolism have been presented as heat maps in Figs. 7 and 8. The complete results of the gene expression analyses and the details of statistical analysis are reported in supplemental material (Supplemental Figure S1). Borage extracts had a significant effect on the expression of genes involved in nitrate assimilation (Fig. 7). In case of DtNTR, LE strongly induced the expression of the gene 4 h after the treatment application, while FE had the same effect after 6 h (Fig. 7a). The same treatment induced the expression of DtNR at the same time-point (Fig. 7b), while DtNiR expression was induced by both treatments 4 h after the application (Fig. 7c). DtGS was up-regulated in response to LE after 9 h, while DtGLU was repressed by the same treatment already after 4 h (Fig. 7d, e).
Fig. 7.
Heat map illustrating variations in the expression (Log fold change, compared to control) of genes involved in nitrogen metabolism, in rocket leaves treated with 10 mL L−1 borage LE or FE. For each gene, high expression is depicted as intense red color, and low expression as intense blue color. Data were compared by using two-way ANOVA, with Bonferroni’s post-test. Asterisks represent significant differences compared to untreated control
Fig. 8.
Heat map illustrating variations in the expression (Log fold change, compared to control) of genes involved in carbon metabolism, in rocket leaves treated with 10 mL L−1 borage LE or FE. For each gene, high expression is depicted as intense red color, and low expression as intense blue color. Data were compared by using two-way ANOVA, with Bonferroni’s post-test. Asterisks represent significant differences compared to untreated control
Regarding the expression of genes involved in C metabolism, LE determined the significant down-regulation of DtRuBisco 6 h after treatment (Fig. 8a), DtIDH was only affected by FE with a slight activation 2 h after the application (Fig. 8b). No significant changes were observed in the expression of DtPEPC2-like gene, while DtPEPC4-like expression was stimulated 2 h after LE treatment and after 4 h from the FE application, with a stronger up-regulation compared to control (Fig. 8d).
Discussion and conclusions
Today, one of the main goals in vegetables production is to obtain a produce with high quality and nutraceutical characteristics (Tarantino et al. 2015), without affecting the yield, for an increasingly demanding market (Ragaert et al. 2004; Ramos et al. 2013). It was demonstrated that leafy vegetables can get benefit from biostimulants application, with particular reference to the reduction of nitrate concentration (Vernieri et al. 2005; Liu et al. 2007; Liu and Lee 2012; Dudaš et al. 2016; Fiorentino et al. 2018), the increase of yield, and the increment of many antioxidant compounds with potential health benefit for human (Bulgari et al. 2015; Colla et al. 2015).
In this sense, the results obtained in this experiment suggest that borage extracts could exert a positive effect on rocket leaves. From the biochemical point of view, it was possible to note that the mean of carotenoids concentration was slightly enhanced by both borage extracts, even if not in a significant way. Sucrose level was enhanced by FE application, instead the total sugars concentration was not significantly affected, even if an upward trend was observable. In addition, the increment of sucrose concentration was coherent with the high value of Fv/Fm registered than control. Recently, maximum PSII efficiency measurement was proposed as a reliable metric to monitor parameters closely related to the functioning of photosynthetic apparatus (such as plant growth, gas exchange, activity of certain enzymes, biomass yield and the amounts of elements), thus representing a good indicator of vigor, growth, and overall plant health status (Romanowska-Duda et al. 2019). It is possible to speculate that treated plants had a greater photosynthetic activity which led to a greater sucrose accumulation in leaves, as observed by Otori et al. (2017) in Arabidopsis. This hypothesis was supported also by a slight rise of PI level in rocket leaves; this index is an overall evaluation of leaf functionality and health status of plants. At molecular level, a decrement in the transcript levels of genes implicated in the Calvin cycle was attributed to an intracellular unbalanced C/N ratio such as high C and low N (Rideout et al. 1992; Otori et al. 2017). As reported by Rook et al. (2006), high sugar levels could result in a negative feedback on the expression of photosynthetic genes. In a study on maize, Sheen (1990) showed that the transcriptional activity of seven photosynthetic genes was repressed by sucrose, glucose, and acetate. In our material, a lower DtRuBisCO expression was observable in treated plants, as time passed by. In fact, 6 h after LE treatments, this down-regulation was even statistically relevant. At the same time, an up-regulation in most of the studied genes involved in N metabolism was observed, as well as a substantial decrement in nitrate concentration. Considering the crop selected for the experiment, rocket, the most interesting result was precisely the decrement of nitrate level observed after the extracts application, confirmed also by the increment of the in vivo activity of the NR enzyme. Regarding this point, between the two borage extracts, the FE seemed to have higher effectiveness. A similar influence was highlighted by Ertani et al. (2009) on maize plantlets, in consequence of treatment with proteins hydrolyzed; an increase in the activity of nitrate reductase and glutamine synthetase, and a reduction of nitrate accumulation in roots and leaves occurred. Schiavon et al. (2008) observed that the activity of enzymes involved in C metabolism and N reduction (among which nitrate and nitrite reductase) was affected in a positive way by alfaalfa protein hydrolysed. A lower nitrate concentration was observed in fennel (Tarantino et al. 2015) after the application of biostimulant. The decrease of nitrate levels in rocket leaves could be due to the activation of nitrate assimilation pathway for the amino acids biosynthesis, as reported in several scientific papers (Ertani et al. 2009, 2013; Baglieri et al. 2014; Calvo et al. 2014) and a regulation of C/N balance, as mentioned earlier. Similar results were obtained on spinach, in which an amino acids based biostimulant increased NR activity and lowered the nitrate leaves content (Kunicki et al. 2010). As mentioned before, FE induced a significant increment of sucrose concentration; this increase may explain the enhanced nitrogen assimilation, remarking the effect on N metabolism and C metabolism, as well. The availability of more C skeletons (sugars) promotes nitrate assimilation (Schiavon et al. 2008; Colla et al. 2015) and more energy for amino acids and protein biosynthesis is available.
Furthermore, analogous to observation with several other species like lettuce (Bulgari et al. 2017), rocket (Vernieri et al. 2005), tomato (Zodape et al. 2011), bean plants (Abbas 2013), biostimulants increase the chlorophyll content and the photosynthetic activity. Hence, the nitrate organication is probably enhanced since the NR enzyme uses the electrons from the photosynthetic machinery.
Borage treatments influenced the nitrate assimilation pathway also at molecular level. In fact, we observed that the gene expression of DtNR, DtNiR, partially of DtGLU, and DtNTR was affected by extracts application. Results confirm that borage extracts have a role in the physiological processes in which the considered genes are involved. Moreover, considering the well-established role of ABA as a regulator of multiple aspects of plant growth (Lu et al. 2015), a link between ABA levels and nitrogen status in rocket leaves emerges from this study. It is widely accepted that in stressful conditions causing a depression of photosynthesis, such as low soil water potential, low air humidity and strong sunlight, the maximum quantum efficiency of photosystem II and stomatal conductance tend to decrease and concomitantly an increase of ABA levels was observed (Zhu 2002; Xu and Shen 2005). Moreover, in some circumstances, high nitrate availability alters osmotic potential of plant cells and stimulates endogenous ABA accumulation (Ondzighi-Assoume et al. 2016). For example, during lateral root development at high nitrate concentrations, the inhibitory effect of high nitrate on lateral root growth has been shown to require ABA synthesis, suggesting the involvement of ABA and nitrate in the same osmotic stress response pathway (Signora et al. 2001; Guan 2017).
In our study, plants did not experience stressful conditions and the use of borage extracts, in particular the flowers one (FE), significantly reduced endogenous ABA content. It is interesting to speculate that ABA homeostasis might play a role to optimize plant responses to different environmental inputs, linking for example changes in its content to the nitrogen status and to improve overall plant health condition, as well. These facts depict in rocket, treated with FE borage extract, an interesting area for future applied research in sustainable crop production, even in stressful conditions.
In conclusion, borage extracts seem to coordinate and optimize responses at a whole plant level on rocket, and to improve the quality and nutraceutical properties of this vegetable commodity. However, it is important to consider that extracts showed a different effectiveness on some of the biochemical parameters examined and on the genes up and down regulation, confirming that FE and LE cause a variable response, probably due to the bioactive compounds contained therein. A deep investigation on extracts composition will be necessary in order to detect the molecules of interest of the two aqueous extracts. Results encourage further investigations on borage extracts, considering that they may specifically improve N use efficiency in rocket plants, suggesting a reasonable exploitation of these treatments in the production of this species.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author's contribution
RB: performed the experiment and analytical determinations, the elaboration and the interpretation of data, and contributed to manuscript writing; GC: contributed to molecular analysis, data interpretation and manuscript writing; AT: contributed to ABA extraction and determination, and manuscript writing; AF: responsible for the research activities, experimental plan, and revision of the final manuscript. All authors read and approved the final version of the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbas SM. The influence of biostimulants on the growth and on the biochemical composition of Vicia faba cv. Giza 3 beans. Rom Biotechnol Lett. 2013;18(2):8061–8068. [Google Scholar]
- Anjana SU, Iqbal M. Nitrate accumulation in plants, factors affecting the process, and human health implications: a review. Agron Sustain Dev. 2007;27(1):45–57. [Google Scholar]
- Aslam M, Huffaker RC, Rains DW. Early effects of salinity on nitrate assimilation in barley seedlings. Plant Physiol. 1984;76(2):321–325. doi: 10.1104/pp.76.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglieri A, Cadili V, Monterumici CM, Gennari M, Tabasso S, Montoneri E, Nardi S, Negre M. Fertilization of bean plants with tomato plants hydrolysates: Effect on biomass production, chlorophyll content and N assimilation. Sci Hortic. 2014;176:194–199. [Google Scholar]
- Borghesi E, Ferrante A, Gordillo B, Rodríguez-Pulido FJ, Cocetta G, Trivellini A, Mensuali-Sodi A, Malorgio F, Heredia FJ. Comparative physiology during ripening in tomato rich-anthocyanins fruits. Plant Growth Regul. 2016;80(2):207–214. [Google Scholar]
- Brewitz E, Larsson C-M, Larsson M. Influence of nitrogen supply on concentrations and translocation of abscisic acid in barley (Hordeum vulgare) Physiol Plant. 1995;95:499–506. [Google Scholar]
- Bulgari R, Cocetta G, Trivellini A, Vernieri P, Ferrante A. Biostimulants and crop responses: a review. Biol Agric Hortic. 2015;31:1–17. [Google Scholar]
- Bulgari R, Morgutti S, Cocetta G, Negrini N, Farris S, Calcante A, Spinardi A, Ferrante A. Evaluation of borage extracts as potential biostimulant using a phenomic, agronomic, physiological, and biochemical approach. Front Plant Sci. 2017;8:935. doi: 10.3389/fpls.2017.00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo P, Nelson L, Kloepper JW. Agricultural uses of plant biostimulants. Plant Soil. 2014;383(1–2):3–41. [Google Scholar]
- Cataldo DA, Haroon M, Sehrader LE, Youngs VL. Rapid colorimetric determination of nitrate in plant tissue by titration of salicylic acid. Commun Soil Sci Plant Anal. 1975;6:71–80. [Google Scholar]
- Cavaiuolo M, Ferrante A. Nitrates and glucosinolates as strong determinants of the nutritional quality in rocket leafy salads. Nutrients. 2014;6:1519–1538. doi: 10.3390/nu6041519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaiuolo M, Cocetta G, Spadafora ND, Müller CT, Rogers HJ, Ferrante A. Gene expression analysis of rocket salad under pre-harvest and postharvest stresses: a transcriptomic resource for Diplotaxis tenuifolia. PloS One. 2017;12(5):e0178119. doi: 10.1371/journal.pone.0178119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BM, Wang ZH, Li SX, Wang GX, Song HX, Wang XN. Effects of nitrate supply on plant growth, nitrate accumulation, metabolic nitrate concentration and nitrate reductase activity in three leafy vegetables. Plant Sci. 2004;167(3):635–643. [Google Scholar]
- Chow CK, Hong CB. Dietary vitamin E and selenium and toxicity of nitrite and nitrate. Toxicology. 2002;180(2):195–207. doi: 10.1016/s0300-483x(02)00391-8. [DOI] [PubMed] [Google Scholar]
- Cocetta G, Rossoni M, Gardana C, Mignani I, Ferrante A, Spinardi A. Methyl jasmonate affects phenolic metabolism and gene expression in blueberry (Vaccinium corymbosum) Physiol Plant. 2015;153:269–283. doi: 10.1111/ppl.12243. [DOI] [PubMed] [Google Scholar]
- Colla G, Nardi S, Cardarelli M, Ertani A, Lucini L, Canaguier R, Rouphael Y. Protein hydrolysates as biostimulants in horticulture. Sci Hortic. 2015;196:28–38. [Google Scholar]
- Colla G, Kim HJ, Kyriacou MC, Rouphael Y. Nitrate in fruits and vegetables. Sci Hortic. 2018;237:221–238. [Google Scholar]
- D'Anna F, Miceli A, Vetrano F (2003) First results of floating system cultivation of Eruca sativa L. In: Pardossi E et al (ed) Proceeding on greenhouse salinity, Acta Hortic, 609:361–364
- D'Antuono LF, Elementi S, Neri R. Exploring new potential health-promoting vegetables: glucosinolates and sensory attributes of rocket salads and related Diplotaxis and Eruca species. J Sci Food Agric. 2009;89:713–722. [Google Scholar]
- Di Gioia F, Gonnella M, Santamaria P. Contribution of leafy vegetables to dietary nitrate intake and regulations. In: Umar S, Anjum NA, Khan NA, editors. Nitrate in leafy vegetables: toxicity and safety measures. New Delhi: I.K. International Publishing; 2013. pp. 2–16. [Google Scholar]
- Dudaš S, Šola I, Sladonja B, Erhatić R, Ban D, Poljuha D. The effect of biostimulant and fertilizer on “low input” lettuce production. Acta Bot Croat. 2016;75:253–259. [Google Scholar]
- Edwards JW, Coruzzi GM. Cell-specific gene expression in plants. Annu Rev Genet. 1990;24(1):275–303. doi: 10.1146/annurev.ge.24.120190.001423. [DOI] [PubMed] [Google Scholar]
- Ertani A, Cavani L, Pizzeghello D, Brandellero E, Altissimo A, Ciavatta C, Nardi S. Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maize seedlings. J Plant Nutr Soil Sci. 2009;172(2):237–244. [Google Scholar]
- Ertani A, Schiavon M, Muscolo A, Nardi S. Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant Soil. 2013;364(1–2):145–158. [Google Scholar]
- Ferrante A, Incrocci L, Maggini R, Tognoni F, Serra G (2002) Preharvest and postharvest strategies for reducing nitrate content in rocket (Eruca sativa). In: XXVI international horticultural congress: issues and advances in postharvest horticulture, vol 628, pp 153–159
- Fiorentino N, Ventorino V, Woo SL, Pepe O, De Rosa A, Gioia L, Romano I, Lombardi N, Napolitano M, Colla G, Rouphael Y. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve N uptake efficiency, yield, and nutritional quality of leafy vegetables. Front Plant Sci. 2018;9:743. doi: 10.3389/fpls.2018.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frezza D, León A, Logegaray V, Chiesa A, Desimone M, Diaz L. Soilless culture technology for high quality lettuce. Acta Hortic. 2005;697:43. [Google Scholar]
- Goel P, Bhuria M, Kaushal M, Singh AK. Carbon: nitrogen interaction regulates expression of genes involved in N-uptake and assimilation in Brassica juncea L. PloS one. 2016;11(9):e0163061. doi: 10.1371/journal.pone.0163061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan P. Dancing with hormones: a current perspective of nitrate signaling and regulation in arabidopsis. Front Plant Sci. 2017;28(8):169. doi: 10.3389/fpls.2017.01697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakse M, Hacin J, Kacjan NM. Production of rocket (Eruca sativa Mill) on plug trays and on a floating system in relation to reduced nitrate content. Acta Agric Slov. 2013;101(1):59. [Google Scholar]
- Kiba T, Feria-Bourrellier AB, Lafouge F, Lezhneva L, Boutet-Mercey S, Orsel M, et al. The Arabidopsis nitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen-starved plants. Plant Cell. 2012;24:245–258. doi: 10.1105/tpc.111.092221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-J, Ishii G. Effect of storage temperature and duration on glucosinolate, total vitamin C and nitrate contents in rocket salad (Eruca sativa Mill) J Sci Food Agric. 2007;87(6):966–973. [Google Scholar]
- Krapp A, David LC, Chardin C, Girin T, Marmagne A, Leprince AS, Chaillou S, Ferrario-Méry S, Daniel-Vedele F. Nitrate transport and signalling in Arabidopsis. J Exp Bot. 2014;65(3):789–798. doi: 10.1093/jxb/eru001. [DOI] [PubMed] [Google Scholar]
- Kunicki E, Grabowska A, Sekara A, Wojciechowska R. The effect of cultivar type, time of cultivation, and biostimulant treatment on the yield of spinach (Spinacia oleracea L.) Folia Hortic. 2010;22:9–13. [Google Scholar]
- Lam HM, Coschigano KT, Oliveira IC, Melo-Oliveira R, Coruzzi GM. The molecular-genetics of nitrogen assimilation into amino acids in higher plants. Annu Rev Plant Biol. 1996;47(1):569–593. doi: 10.1146/annurev.arplant.47.1.569. [DOI] [PubMed] [Google Scholar]
- Lancien M, Ferrario-Méry S, Roux Y, Bismuth E, Masclaux C, Hirel B, Gadal P, Hodges M. Simultaneous expression of NAD-dependent isocitrate dehydrogenase and other Krebs cycle genes after nitrate resupply to short-term nitrogen-starved tobacco. Plant Physiol. 1999;120(3):717–726. doi: 10.1104/pp.120.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancien M, Gadal P, Hodges M. Enzyme redundancy and the importance of 2-oxoglutarate in higher plant ammonium assimilation. Plant Physiol. 2000;123(3):817–824. doi: 10.1104/pp.123.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic membranes. Methods Enzymol. 1987;148:350–382. [Google Scholar]
- Liu XQ, Lee KS. Effect of mixed amino acids on crop growth. In: Aflakpui G, editor. Agricultural science. Rijeka: InTech Europe Publisher; 2012. pp. 119–158. [Google Scholar]
- Liu XQ, Ko KY, Kim SH, Lee KS. Effect of amino acid fertilization on nitrate assimilation of leafy radish and soil chemical properties in high nitrate soil. Commun Soil Sci Plant Anal. 2007;39(1–2):269–281. [Google Scholar]
- Lu Y, Yamaguchi J, Sato T. Integration of C/N-nutrient and multiple environmental signals into the ABA signaling cascade. Plant Signal Behav. 2015;10(12):e1048940. doi: 10.1080/15592324.2015.1048940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani G, Filippi F, Borghesi E, Vitale M (2007) Impact of sunlight spectrum modification on yield and quality of ready-to-use lettuce and rocket salad grown on floating system. In: International symposium on high technology for greenhouse system management: greensys, vol 801, pp 163–170
- Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A. Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann Bot. 2010;105(7):1141–1157. doi: 10.1093/aob/mcq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight GM, Duncan CW, Leifert C, Golden MH. Dietary nitrate in man: friend or foe? Br J Nutr. 1999;81:349–358. doi: 10.1017/s000711459900063x. [DOI] [PubMed] [Google Scholar]
- Murchie EH, Lawson T. Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Exp Bot. 2013;64(13):3983–3998. doi: 10.1093/jxb/ert208. [DOI] [PubMed] [Google Scholar]
- O'Brien JA, Vega A, Bouguyon E, Krouk G, Gojon A, Coruzzi G, Gutiérrez RA. Nitrate transport, sensing, and responses in plants. Mol Plant. 2016;9(6):837–856. doi: 10.1016/j.molp.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Ondzighi-Assoume CA, Chakraborty S, Harris JM. Environmental nitrate stimulates root tip abscisic acid accumulation via release from inactive stores. Plant Cell. 2016;28:729–745. doi: 10.1105/tpc.15.00946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otori K, Tanabe N, Maruyama T, Sato S, Yanagisawa S, Tamoi M, Shigeoka S. Enhanced photosynthetic capacity increases nitrogen metabolism through the coordinated regulation of carbon and nitrogen assimilation in Arabidopsis thaliana. J Plant Res. 2017;130(5):909–927. doi: 10.1007/s10265-017-0950-4. [DOI] [PubMed] [Google Scholar]
- Palmer CE. The relationship of abscisic acid to nitrate reductase activity in the potato plant. Plant Cell Physiol. 1985;26:1167–1174. [Google Scholar]
- Pao SS, Paulsen IT, Saier MH. Major facilitator superfamily. Microbiol Mol Biol Rev. 1998;62:1–34. doi: 10.1128/mmbr.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JL, Newstead S. Molecular basis of nitrate uptake by the plant nitrate transporter NRT1.1. Nature. 2014;507(7490):68–72. doi: 10.1038/nature13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuke AD, Jeschk WD, Hartung W. The uptake and flow of C, N and ions between roots and shoots in Ricinus communis L. III. Long-distance transport of abscisic acid depending on nitrogen nutrition and salt stress. J Exp Bot. 1994;45:741–747. [Google Scholar]
- Premuzic Z, Gárate A, Bonilla I (2001) Yield and quality of greenhouse lettuce as affected by form of N fertilizer and light supply. In: Plant nutrition, Springer, Netherlands, pp 300–301
- Radin JW, Parker LL, Guinn G. Water relations of cotton plants under nitrogen deficiency: V. Environmental control of abscisic acid accumulation and stomatal sensitivity to abscisic acid. Plant Physiol. 1982;70:1066–1070. doi: 10.1104/pp.70.4.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragaert P, Verbeke W, Devlieghere F, Debevere J. Consumer perception and choice of minimally processed vegetables and packaged fruits. Food Qual Prefer. 2004;15(3):259–270. [Google Scholar]
- Raines CA. Increasing photosynthetic carbon assimilation in C3 plants to improve crop yield: current and future strategies. Plant Physiol. 2011;155(1):36–42. doi: 10.1104/pp.110.168559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos B, Miller FA, Brandão TR, Teixeira P, Silva CL. Fresh fruits and vegetables—an overview on applied methodologies to improve its quality and safety. Innov Food Sci Emerg Technol. 2013;20:1–15. [Google Scholar]
- Rideout JW, Raper CD, Jr, Miner GS. Changes in ratio of soluble sugars and free amino nitrogen in the apical meristem during floral transition of tobacco. Int J Plant Sci. 1992;153:78–88. doi: 10.1086/297008. [DOI] [PubMed] [Google Scholar]
- Romanowska-Duda Z, Grzesik M, Janas R. Maximal efficiency of PSII as a marker of sorghum development fertilized with waste from a biomass biodigestion to methane. Front Plant Sci. 2019;8(9):1920. doi: 10.3389/fpls.2018.01920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook F, Hadingham SA, Li Y, Bevan MW. Sugar and ABA response pathways and the control of gene expression. Plant Cell Environ. 2006;29(3):426–434. doi: 10.1111/j.1365-3040.2005.01477.x. [DOI] [PubMed] [Google Scholar]
- Rouphael Y, Colla G. Synergistic biostimulatory action: designing the next generation of plant biostimulants for sustainable agriculture. Front Plant Sci. 2018;9:1655. doi: 10.3389/fpls.2018.01655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez R, Flores A, Cejudo FJ. Arabidopsis phosphoenolpyruvate carboxylase genes encode immunologically unrelated polypeptides and are differentially expressed in response to drought and salt stress. Planta. 2006;223(5):901–909. doi: 10.1007/s00425-005-0144-5. [DOI] [PubMed] [Google Scholar]
- Santamaria P, Gonnella M, Elia A, Parente A, Serio F. Ways of reducing rocket salad nitrate content. Acta Hortic. 2006;548:529–536. [Google Scholar]
- Santi C, Zamboni A, Varanini Z, Pandolfini T. Growth stimulatory effects and genome-wide transcriptional changes produced by protein hydrolysates in maize seedlings. Front Plant Sci. 2017;8:433. doi: 10.3389/fpls.2017.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. Metabolic repression of transcription in higher plants. Plant Cell. 1990;2:1027–1038. doi: 10.1105/tpc.2.10.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Yi K, Liu Y, Xie L, Zhou Z, Chen Y, Hu Z, Zheng T, Liu R, Chen Y, Chen J. Phosphoenolpyruvate carboxylase in Arabidopsis leaves plays a crucial role in carbon and nitrogen metabolism. Plant Physiol. 2015;167:671–681. doi: 10.1104/pp.114.254474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavon M, Ertani A, Nardi S. Effects of an alfaalfa protein hydrolysate on the gene expression and activity of enzymes of TCA cycle and N metabolism in Zea mays L. J Agric Food Chem. 2008;56:11800–11808. doi: 10.1021/jf802362g. [DOI] [PubMed] [Google Scholar]
- Signora L, De Smet I, Foyer CH, Zhang H. ABA plays a central role in mediating the regulatory effects of nitrate on root branching in Arabidopsis. Plant J. 2001;28:655–662. doi: 10.1046/j.1365-313x.2001.01185.x. [DOI] [PubMed] [Google Scholar]
- Spreitzer RJ, Salvucci ME. Rubisco: structure, regulatory interactions, and possibilities for a better enzyme. Ann Rev Plant Biol. 2002;53(1):449–475. doi: 10.1146/annurev.arplant.53.100301.135233. [DOI] [PubMed] [Google Scholar]
- Stitt M. Nitrate regulation of metabolism and growth. Curr Opin Plant Biol. 1999;2:178–186. doi: 10.1016/S1369-5266(99)80033-8. [DOI] [PubMed] [Google Scholar]
- Sun Y, Fan XY, Cao DM, Tang W, He K, Zhu JY, et al. Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev Cell. 2010;19(5):765–777. doi: 10.1016/j.devcel.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantino E, Disciglio G, Frabboni L, Libutti A, Gatta G, Gagliaridi A, Tarantino A. Effect of biostimulants application on quali-quantitative characteristics of cauliflower, pepper, and fennel crops under organic and conventional fertilization. Environment. 2015;10:13. [Google Scholar]
- Temple SJ, Vance CP, Gantt JS. Glutamate synthase and nitrogen assimilation. Trends Plant Sci. 1998;3(2):51–56. [Google Scholar]
- Tripodi P, Francese G, Mennella G. Rocket salad: crop description, bioactive compounds and breeding perspectives. Adv Hortic Sci. 2017;31(2):107–113. [Google Scholar]
- Vance CP, Gantt JS. Control of nitrogen and carbon metabolism in root nodules. Physiol Plant. 1992;85(2):266–274. [Google Scholar]
- Vernieri P, Borghesi E, Ferrante A, Magnani G. Application of biostimulants in floating system for improving rocket quality. J Food Agric Environ. 2005;3:86–88. [Google Scholar]
- Vernieri P, Perata P, Armellini D, Bugnoli M, Presentini R, Lorenzi R, et al. Solid phase radioimmunoassay for the quantitation of abscisic acid in plant crude extracts using a new monoclonal antibody. J Plant Physiol. 1989;134:441–446. [Google Scholar]
- Wilkinson S, Davies WJ. ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant, Cell Env. 2002;25:195–210. doi: 10.1046/j.0016-8025.2001.00824.x. [DOI] [PubMed] [Google Scholar]
- Wood CW, Reeves DW, Himelrick DG (1993) Relationships between chlorophyll meter readings and leaf chlorophyll concentration, N status, and crop yield: a review. In: Proceedings of the agronomy society of New Zealand, vol 23, pp 1–9
- Xu D, Shen Y. External and internal factors responsible for midday depression of photosynthesis. In: Pessarakli M, editor. Handbook of photosynthesis. 2. Boca Raton: CRC Press; 2005. pp. 287–297. [Google Scholar]
- Yemm EW, Willis AJ. The estimation of carbohydrates in plant extracts by anthrone. Biochem J. 1954;57:508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zodape ST, Gupta A, Bhandari SC, Rawat US, Chaudhary DR, Eswaran K, Chikara J. Foliar application of seaweed sap as biostimulant for enhancement of yield and quality of tomato (Lycopersicon esculentum Mill.) J Sci Ind Res. 2011;70:215–219. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.