Abstract
Cytoplasmic male sterility (CMS) is maternally inherited trait, which hinders the ability to produce viable pollen in plants. It serves as a useful tool for hybrid seed production via exploiting heterosis in crops. The molecular mechanism of CMS and fertility restoration has been investigated in different crops. However, limited number of reports is available on comparison of Ogura- and Polima-CMS with their shared maintainer in Chinese cabbage. We performed transcript profiling of sterile Ogura CMS (Tyms), Polima CMS (22m2) and their shared maintainer line (231–330) with an aim to identify genes associated with male sterility. In this work, we identified 912, 7199 and 6381 DEGs (Differentially Expressed Genes) in 22m2 Vs Tyms, 231–330 VS 22m2 and 231–330 Vs Tyms, respectively. The GO (Gene Ontology) annotation and KEGG pathway analysis suggested that most of the DEGs were involved in pollen development, carbon metabolism, lipase activity, lipid binding, penta-tricopeptide repeat (PPR), citrate cycle and oxidative phosphorylation, which were down-regulated in both CMS lines. This result will provide an important resource for further understanding of functional pollen development, the CMS mechanism and to improve molecular breeding in Chinese cabbage.
Electronic supplementary material
The online version of this article (10.1007/s12298-020-00775-5) contains supplementary material, which is available to authorized users.
Keywords: Transcriptome, Ogura CMS, Polima CMS, Maintainer line
Introduction
Male sterility denotes inability to produce viable pollen in plants. It could be governed by either nuclear gene (s) (genic male sterility/GMS) or interaction between nuclear and cytoplasmic gene (s) (cytoplasmic male sterility/CMS). Both of male sterility systems are used in agriculture for developing hybrid crops with enhanced yield via exploiting heterosis (Tester and Langridge 2010). CMS inherited maternally in higher plants, which is involved in producing either aborted or infertile pollen grains during anther development (Dong et al. 2013). Although several researches have been conducted for more than two centuries but CMS mechanism is not completely understood. CMS is a consequence of interaction of nuclear and mitochondrial genes (Aviv and Galun 1980; Štorchová 2017), the CMS genes are located in mitochondria but their functions are controlled by nuclear gene(s), called as fertility restorer genes.
CMS has been found in more than 150 plant species, which has been evolved through spontaneous mutations or developed by induced mutations, wide-hybridization and protoplast fusion (Hanson and Bentolila 2004; Singh et al. 2015; Yamagishi and Bhat 2014). CMS mostly occurred due to alteration of mitochondria genes but not of nuclear genes (Carlsson and Glimelius 2011). CMS is an interesting topic in the reproductive biology, which is utilized broadly in hybrid breeding and genetic improvement of economic traits (Luo et al. 2013; Pelletier and Budar 2007; Woodson and Chory 2008). CMS phenotypes show a range of reproductive irregularities, like abnormal anthers, non-functional pollen, carpelloid and petaloid stamens (Chase 2007). There are 28 types of CMS in 13 crop species resulted from rearrangement of at least 10 mitochondrial genes (Chen and Liu 2014).
A variety of CMS is available in Brassica species, which are Ogura- CMS (Ogura 1968), Polima- CMS (Fu et al. 1995), nap CMS (Thompson 1972), nig CMS (Pearson 1981), hau CMS (Wan et al. 2008) and Nsa (Liu et al. 2015b). Among them, Ogura- CMS is broadly used in hybrid seed production of Brassica vegetables. Ogura- CMS was first identified in wild radish (Raphanus sativus) (Ogura 1968), which produces few and infertile pollen grains on indehiscent anthers. CMS Brassica napus line was developed through protoplast fusion between the Ogura- CMS containing radish and B. napus lines, thereafter this Ogura- CMS transferred to Chinese cabbage in 1980 through repeated backcrossing (Pelletier et al. 1983). The first F1 hybrid seeds of Chinese cabbage was produced by using Ogura- CMS line (Ke et al. 1992). Ogura CMS is regulated by mitochondrial orf138 locus in Chinese cabbage, but which nuclear genes respond toward mutation of orf138 is still not clear (Wei et al. 2015).
Chinese cabbage is one of the most important and popular leafy vegetable in China, Korea, Japan and East Asian people. The F1 hybrid of this vegetable is produced using either self-incompatible (SI) or Ogura- CMS system. The Ogura- CMS was introgressed from wild radish to Chinese cabbage via B. napus since 1980 and is still widely used in Chinese cabbage production industry (Yamagishi and Bhat 2014). Many genes are associated as network in Ogura- CMS plants compared to the maintainer line (Dong et al. 2013), which are auxin response, ATP synthesis, pollen development and the stress responsive genes. Molecular marker-assisted breeding improved the Ogura- CMS fertility restorer line (Rfo) and mapping of the Rfo in B. juncea (Tian et al. 2014). They are currently using the improved system for developing high yielding hybrid varieties in condiment and canola B. juncea. Research results indicated that the novel allele-specific marker could be used for the Marker Assisted Selection (MAS) of the Rfo gene in B. oleracea, and six Ogura- CMS fertility-restored interspecific hybrids were created for the first time (Yu et al. 2016).
In addition, another CMS system controlled by mitochondrial orf224 was discovered in rapeseed at Huazhong Agricultural University since 1972 (Fu 1981; Wang et al. 2002). Thereafter, it was widely spread over China in hybrid breeding of rapeseed and Chinese cabbage Polima cytoplasm consisting mainly of pol mitotype (Chen et al. 2011) and the sterility occurs due to failure of sporogenous cell differentiation (An et al. 2014). In the pol CMS system, a chimeric ORF is present in upstream of atp6 encoding subunit 6 of mt-ATPase (Singh and Brown 1991). The transcripts of orf224 are altered via affecting the orf224/atp6 in presence of restorer gene Rfp, located in the nucleus. RNA-seq data of polima- CMS and fertile flower bud lines corroborated that a series of associated genes check the energy deficiency in fertile flower bud, which is caused by orf224/atp6 and help to develop functional pollen through mitochondrial-nuclear gene interactions (An et al. 2014). Liu et al. (2016) did not find any orf224/atp6 transcripts in the maintainer line (1141B) but found decreased level of such transcript in Rfp transgenic T1 lines and in the fertility-restored line (B409). Allelism analysis of the Rfp locus in different restorer lines could be helpful for detecting new restorer lines for hybrid Chinese cabbage breeding.
Plant hormone signaling pathway and different hormone synthesis altered significantly in the CMS plants. ABA synthesis becomes higher with lower IAA observed in CMS lines (Polima, Ogura, Nsa) compared to maintainer lines, which indicates ABA and IAA play different roles in fertile pollen development (Ding et al. 2018). In addition, it was reported that transcription factors have also been involved in pollen infertility (Xing et al. 2018). Anther and microspore development-associated genes were identified in Ogura CMS line male fertile line of turnip, which responded to mitochondrial retrograde signaling (Lin et al. 2019). Meanwhile, Ogura- CMS line and its maintainer cabbage lines were analysied by iTRAQ-based proteome technology and found some genes responsible in processing of pollen exine, tetrad wall formation and programmed cell death, which contributed to pollen sterility (Han et al. 2018). To date, no specific comparative transcriptome has been reported for Ogura- and Polima- CMS for underpinning regulatory network of CMS in Chinese cabbage.
In recent years, high-throughput sequencing methods such as Illumina SOLEXA, ABI’s SOLiD (Sequencing by Oligonucleotide Ligation and Detection) and Roche 454 obviously increased the efficiency and reduced the cost of sequencing, making the study of transcriptomes and even genome easier and more feasible (Schuster 2008). Understanding of the molecular mechanisms of CMS and identify the differentially expressed genes controlling pollen development and abortion in sterile and fertile lines, respectively is required. We used transcriptome sequencing to compare transcript profiles of flower bud of sterile Ogura- CMS (Tyms), Polima- CMS (22m2) and their shared maintainer (231–330) lines with aim to determine critical genes and pathways associated with male sterility and fertility restoration. Some of them were further verified by real-time quantitative PCR (qRT-PCR). Our results help to elucidate the CMS mechanism uses in the breeding of Chinese cabbage.
Materials and methods
Plant material and sample collection
The flower buds of an Ogura- CMS line (Tyms), Polima- CMS line (22m2) and maintainer line (231–330) of Chinese cabbage were used in this study. All of the three lines are isogenic with different cytoplasmic background. The plants were cultivated in the experimental plots, Henan Academy of Agricultural Sciences (Yuanyang, Henan Province, China). Buds with a length of 4–5 mm were stripped from ten different plants for RNA extraction and sequencing. Fifty buds for each line consisted of five buds from each plant and were gathered together, kept at − 80 °C for further use after snap-freezing in liquid nitrogen.
RNA extraction, library construction and sequencing
Total RNA was extracted with TRIzol reagent (Invitrogen, USA) following the manufacturer’s instructions, and pretreated with RNase-free DNase I (Takara, Japan) to remove contaminated genomic DNA. The treated RNA was then subjected to poly-A selection, fragmentation, random priming and first- as well as second- strand cDNA synthesis using Illumina Gene Expression Sample Prep kit (CA, USA). Double-stranded cDNA was fragmented by nebulization and used for mRNA library construction according to the Illumina paired-end sample preparation protocol, using custom multiplex-indexed Solexa adaptors and sequenced using the Illumina Genome Analyzer paired-end pipeline.
Processing of data
The raw reads of Illumina platform were checked for quality control (QC), filtered into clean reads and aligned to the reference sequences of Brassica rapa Genome (http://brassicadb.org/brad/index.php). The alignment data was utilized to calculate distribution of reads on reference genes and performed analysis for coverage. Gene expression level within a given sample was calculated using values for a gene’s uniquely aligned reads per kilobase transcriptome per million mapped reads (RPKM) (Mortazavi et al. 2008). Statistical comparisons among Tyms, 22m2 and 231–330 were performed using IDEG6 software (Romualdi et al. 2003). The general Chi square method and false discovery rate (FDR) were applied to determine the Q-value threshold. Uni-genes were considered as differentially expressed when the RPKM between Tyms, 22m2 and 231–330 displayed more than two-fold change with less than 10−2 FDR. Sequences of the DEGs were sequence aligned against non-redundant (Nr) protein database in NCBI using the BLASTx algorithm with the threshold 1e−10.
PCR and quantitative real-time PCR
To analyze the mRNAs, 1.0 μg of total RNA was polyadenylated using a cDNA synthesis kit (Takara Inc., Dalian, China). The poly-A tail-amended total RNA was reverse-transcribed by PrimeScript™ RT reagent kit with gDNA eraser using a RT primer mix. The qPCR was performed on a LightCycler® 96 System (Roche, Basel, Switzerland) using SYBR® Premix Ex Taq™ (TaKaRa, Dalian, China). The primers of the selected gene subjected to the target analysis are listed in Supplementary Table S1 and β-actin was used as an internal control for calculating relative expression following 2−ΔΔCt method (Livak and Schmittgen 2001). In qPCR, the reaction was performed using 20 μL reaction-mixes, including 10 μL of SYBR® Premix Ex Taq™ (Tli RNaseH Plus), 0.8 μL of 10 mM forward primer, 0.8 μL of 10 mM reverse primer, 2.0 μL of the cDNA (30 ng/μL) sample and 6.4 μL of dH2O. Three independent technical replicates were used in qPCR. qPCR was performed with an initial denaturation at 95 °C for 5 min, followed by 45 cycles of denaturation at 95 °C for 10 s, annealing at 58 °C for 10 s, and extension at 72 °C for 15 s. For amplifying the orf specific primers, 20 μL PCR mix that contained 8 μL premix (TaKaRa, Dalian, China), 1 μL forward (10 mM) and 1 μL reverse (10 mM) primers, 9 μL DDH2O and 1 μL (50 ng/μL) template DNA was used. The thermal cycle profile was fixed as; initial denaturation 95 °C for 5 min followed by 35 cycles of denaturation 95 °C for 1 min, annealing 55 °C for 1 min and elongation 72 °C for 1 min and final elongation 72 °C for 5 min. The PCR Amplicon was visualized under UV after 30 min electrophoresis (100 V) on 1.2% agarose after staining with GelRed (Biotium, Inc., CA, USA).
Paraffin sectioning and microscopic observation
The paraffin sectioning and microscopic observations were carried out according to the method described by Xiaochun (Wei et al. 2015). Sterile and fertile flower buds were fixed and embedded in paraffin using beeswax. Thin (0.8 μm) sections were prepared with an Ultracut Eultra microtome (Leica, Germany), stained with 0.5% hematoxylin and photographed under a LEICA DMI3000B microscope (Leica, Germany).
Result
Phenotypic and marker based characterization of sterile and fertile floral buds
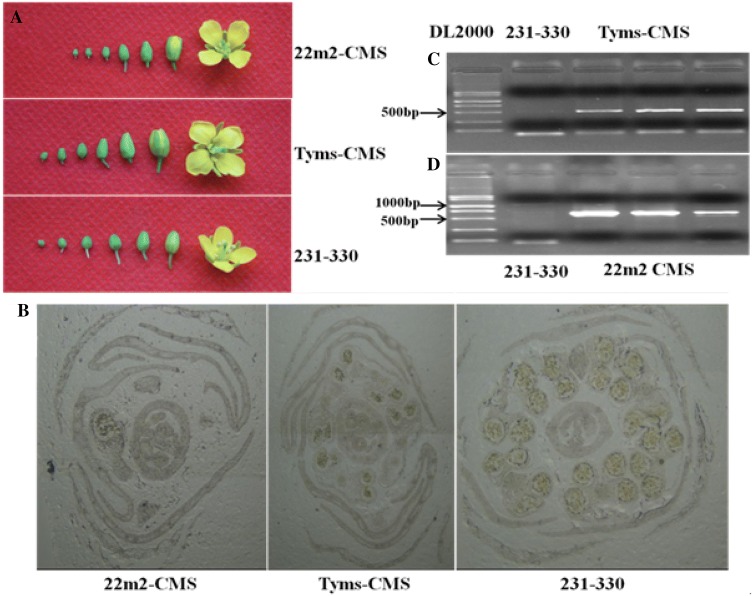
The morphology, microscopy and orf PCR amplicon of Ogura- CMS line (Tyms), Polima- CMS line (22m2) and maintainer line (231–330) are presented in Fig. 1a–d. The flower organ in Chinese cabbage comprised the sepal, petal, stamen, and pistil. The male reproductive organ normally developed in rapeseed flower with four long and two short stamens. Each stamen had the filament and anther (Fig. 1a). At late uni-nucleate stage, no mature pollen was formed in the Polima CMS line (22m2) and a little mature pollen was formed in the Ogura- CMS line (Tyms), while normal pollen development was observed in the maintainer line (231–330) (Fig. 1b). Ogura- CMS specific primers on orf138 amplified in CMS line Tyms (Fig. 1c), indicating the presence of orf138 causing sterility of this genotype. Meanwhile, Polima CMS specific primers on orf224 amplified in CMS line (22m2) indicating orf224 for sterility (Fig. 1D).
Fig. 1.
Phenotypic and marker based characterization of fertile and sterile floral buds of CMS and maintainer lines; (a) different developmental stages of flower buds of CMS and maintainer lines, (b) cross section of fertile and sterile floral buds observed under microscope, (c) PCR amplicons of Ogura- CMS line and (d) Polima- CMS line in comparison to maintainer line, three repeated PCR products were used in gel electrophoresis for both CMS lines
RNA-seq data analysis
To obtain the transcriptome involved in Ogura- CMS, Polima CMS and their maintainer of Chinese cabbage, high-throughput RNA sequencing was performed using Illumina platform with poly-A enriched RNAs (mRNA-Seq) from the flower buds of an Ogura- CMS line (Tyms), Polima- CMS line (22m2) and maintainer line (231–330) of Chinese cabbage. A total of 74417542 reads were generated by 100 bp paired end sequencing from three cDNA libraries. Of these, 25106780, 25015186 and 24295576 reads were generated from the 22m2, 231–330 and Tyms lines, respectively. The average percentage of total reads from all libraries was remapped to reference transcripts and found ~ 74% coverage, while 26% of the reads did not match with the reference genome (Supplementary Table S2). Forty-seven percent (47%) of the total genes was matched with reference genome and 90 ~ 100% of them had Q value > 20% (i.e. those reads with an average quality score > 20) with > 40% GC content, indicates that the sequencing accuracy and quality are sufficient for further analysis (Supplementary Figure S1). In addition, 16.84%, 15.55% and 16.95% of total reads for 22m2, 231–330 and Tyms, respectively were found as the coverage of intergenic sequences. While, 2.06%, 2.1% and 2.13% of 22m2, 231–330 and Tyms, respectively were recognized as intronic sequences (Supplementary Table S2).
Functional annotation of DEGs
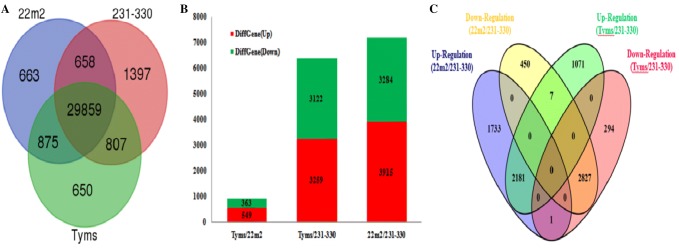
After mapping the RNA-Seq reads to the reference genome with TopHat, transcripts were assembled. RPKM was used to quantify the expression level of unigenes. The expression level detected by RNA-seq is highly sensitive. A total of 96,967 genes were identified in the three cDNA libraries, of which 32,055, 32,721 and 32,191 unigenes were generated from 22m2, 231–330 and Tyms lines, respectively. Among them, 29,859 genes were co-expressed in the three genotypes (Fig. 2a). In the process of DEG screening, we used “FDR < 0.001 and FC (fold change) > 2” as the threshold to determine the significant differences in gene expression. FC was the ratio of RPKM between sterile [Ogura- CMS (Tyms) and Polima- CMS (22m2)] and fertile [maintainer (231–330)] lines. In 22m2 Vs Tyms, we identified 912 DEGs, including 549 (60.2%) up-regulated genes and 363 (39.8%) down-regulated genes, while 875 genes were found as co-expressed genes in the 22m2 Vs Tyms. 231–330 Vs Tyms and 231–330 Vs 22m2 had 6381 and 7199 DEGs, respectively. Among them 3259 (51.1%) and 3915 (54.4%) were induced; 3122 (48.9%) and 3284 (45.6%) were suppressed, respectively (Fig. 2b). In addition with Venn diagram, Volcano plot also confirmed the abundance of the DEGs between CMS and fertile lines (Supplementary Figure S2 A-C). Interestingly, we found 2181 and 1733 DEGs up-regulated in 231–330 Vs Tyms and 231–330Vs 22m2, respectively; while 2827 and 294 DEGs were down-regulated in 231–330 Vs 22m2 and 231–330 Vs Tyms, respectively (Fig. 2C).
Fig. 2.
Differentially expressed genes (DEGs) in the sterile Ogura CMS (Tyms), Polima CMS (22m2) and their shared maintainer (231–330) lines; (a) number of unigenes in the sterile Ogura CMS (Tyms), Polima CMS (22m2) and their shared maintainer line (231–330), (b) number of DEGs of in 22m2 Vs Tyms, 231–330 Vs Tyms and 231–330 Vs 22m2 and (c) number of up-regulated and down-regulated DEGs of 231–330 Vs Tyms and 231–330 Vs 22m2
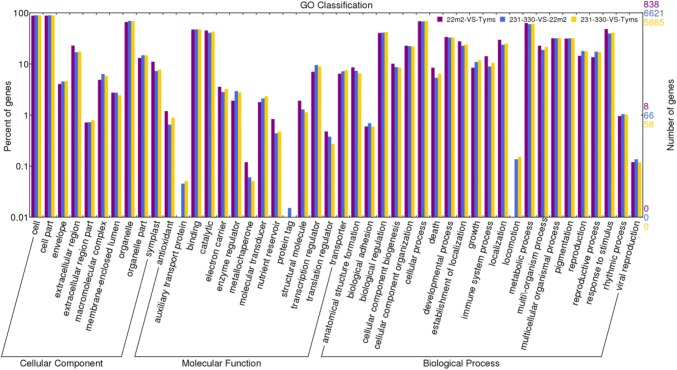
GO (Gene Ontology) is an international standardized gene function classification system, which describes properties of genes and their products in any organism. To create a gene expression profile of CMS and maintainer lines, we used Web Gene Ontology Annotation Plot (WEGO) for gene annotation analysis. To screen the candidate genes from DEGs for functional annotation, we analyzed 912, 7199 and 6381 DEGs in 22m2Vs Tyms, 231–330 Vs 22m2 and 231–330 Vs Tyms, respectively. 22m2 Vs Tyms, 231–330 Vs 22m2, and 231–330 Vs Tyms had 838, 6621 and 5885 DEGs, respectively that were assigned to all three categories; such as cellular component, molecular function and biological process. In each of the three main categories of the GO classification, there were 10, 14 and 22 functional groups, respectively (Fig. 3). The DEGs in cell, cell part, organelle, binding, catalytic, metabolic process, cellular process and response to stimulus played important roles in pollen development. The GO term with p ≤ 0.05 was defined as significantly enriched DEGs via GO enrichment significance analysis. For molecular function, significantly enriched GO terms of DEGs included oxidoreductase, electron carrier, lipase, lipid binding and GDP-dissociation inhibitor activities. For enriched biological processes, 10 GO terms of DEGs were involved in pollen development, pollen tube development, pollen wall assembly, cell morphogenesis, cellular component morphogenesis, cellular process involved in reproduction, ion transport, micro-gametogenesis, male gamete generation and catalytic activity. These results provided a comprehensive view for screening candidate genes related to metabolic processes of pollen development, which could be potential for further in depth analysis of causal factor of CMS in Chinese cabbage.
Fig. 3.
Gene ontology assignment of functional enrichment with up- and down-regulated DEGs in 22m2 Vs Tyms, 231–330 Vs 22m2 and 231-330 Vs Tyms using flower buds. The later axis and vertical axis show the functional classification of genes and the percentage of total number of genes in a particular annotation, respectively
Kyoto encyclopedia of genes and genomes (KEGG) pathway analysis
KEGG pathway-based analysis allowed knowing the biological functions of DEGs. KEGG analysis provides information and further understanding on how male sterile plants regulate their biological functions and synthesize secondary metabolites (Yang et al. 2015). The KEGG pathways with Q ≤ 0.05 indicated significantly enriched KEGG pathway and their involved DEGs. A total of 544, 4113 and 3699 DEGs were mapped into 105, 124, 122 KEGG pathways for 22m2 Vs Tyms, 231–330 Vs 22m2 and 231–330 Vs Tyms, respectively. Among them 13, 30, 31 KEGG pathways were enriched significantly in 22m2 Vs Tyms, 231–330 Vs 22m2 and 231–330 Vs Tyms, respectively (Supplementary Table S3). Most of the DEGs were involved in pentose and glucuronate inter-conversions, glycosaminoglycan degradation, amino sugar and nucleotide sugar metabolism, starch and sucrose metabolism, ether lipid metabolism, linoleic acid metabolism, glycerophospholipid metabolism, glycosphingolipid biosynthesis—ganglio series.
22m2 Vs Tyms, 231–330 Vs 22m2 and 231–330 Vs Tyms had 557, 4401 and 4142 DEGs, respectively in COG classification belonging to 25 COG categories. Among 25 COG categories, the largest group was ′general function prediction only′ followed by ′transcription′, ′signal transduction mechanisms′, ′replication, recombination and repair′ and ′carbohydrate transport and metabolism′. However, we did not find any DEGs in the N (cell motility), W (extracellular structure) and Y (nuclear structure) categories (Fig. 4).
Fig. 4.
Frequency of the DEGs of 22m2 Vs Tyms, 231–330 vs 22m2 and 231–330 Vs Tyms in COG functional categories
Genes related to pollen development
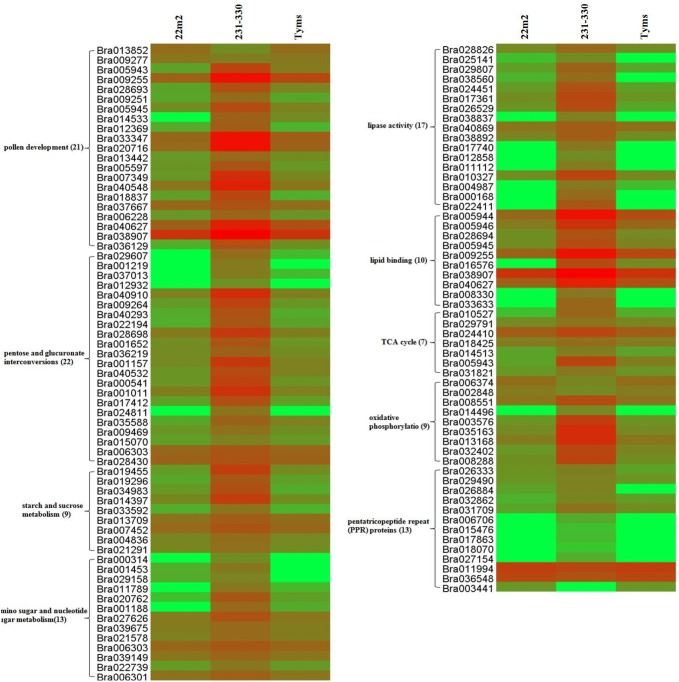
Pollen development is a fundamental and complex process in flowering plants, which is essential for sexual propagation and evolution and has been characterized in the model species Arabidopsis thaliana. Therefore, all of the unigenes identified in this study were annotated to the TAIR database and 21 genes were found to be involved in regulating pollen development (Fig. 5, Supplementary Table S4). In the heat-map, we found two genes significantly up-regulated in both the CMS lines compared to their fertile maintainer line. Of which, one gene encoded tapetum determinant 1 (TPD1; Bra013852) and other gene encoded a putative leucine-rich repeat receptor protein kinase (EMS1; Bra009277). In the heat-map, 19 unigenes, such as GRP17 (Bra005943, Bra009255 and Bra028693), GRP19 (Bra009251 and Bra005945), EXL4 (Bra014533 and Bra012369), PGA4 (Bra033347), MF6t (Bra020716), QRT3 (Bra013442), MYB101 (Bra005597), BAN102 (Bra007349), AGP11 (Bra040548), FU (Bra018837), ARL (Bra037667), EXO70C2 (Bra006228 and Bra040627), LTP12 (Bra038907), DAN1 (Bra036129) were down-regulated in both of the CMS lines, but upregulated in the maintainer line. These results indicate that these 19 uni-genes mainly participated in the formation and differentiation of tapetum, pollen development and reproductive development in fertile line and vice versa in the CMS lines.
Fig. 5.
Clustering heat map of DEGs in Ogura CMS line (Tyms), Polima CMS line (22m2) and maintainer line (231–330) of Chinese cabbage. Left side of the figure shows gene ID and one row for one gene, three columns represent the genotype name from which 4–5 mm size flower buds were collected for RNA sequencing, the genes that are enclosed by the brackets are the gene number identified in different functional group assigned based on non-redundant (Nr) protein database (NCBI) database. Green and red colors indicate up- and down-regulation of the genes, respectively
DEGs involved in carbon metabolism
Pollen fertility and the microspore development are regulated via abundance of starch accumulation during micro-sporogenesis. In KEGG analysis, a number of genes showed differential expression between CMS and their maintainer lines. These genes are involved in starch and sucrose metabolism, pentose and glucuronate inter-conversions, amino sugar and nucleotide sugar metabolism. Among them, 22 genes down-regulated in CMS lines for pentose and glucuronate inter-conversions (Fig. 5, Supplementary Table S4). Of which, 14 genes (Bra029607, Bra001219, Bra037013, Bra012932, Bra040910, Bra009264, Bra040293, Bra022194, Bra028698, Bra001652, Bra036219, Bra001157, Bra040532 and Bra000541) encoded pectinesterase family protein, 6 genes (Bra001011, Bra017412, Bra024811, Bra035588, Bra009469 and Bra015070) encoded pectate lyase family protein, 2 genes (Bra006303 and Bra028430) encoded UDP-glucose 6-dehydrogenase. Moreover, 9 genes related to starch and sucrose metabolism were significantly down-regulated in both CMS lines (Fig. 5, Supplementary Table S4). Among them, six genes (Bra019455, Bra019296, Bra034983, Bra014397, Bra033592 and Bra013709) encoded glycosyl hydrolase family 9 protein, one gene (Bra007452) encoded pfkB-type carbohydrate kinase family protein and two genes (Bra004836 and Bra021291) were involved in beta-glucosidase. Thirteen genes related to amino sugar and nucleotide sugar metabolism showed down-regulation in both of the CMS lines (Fig. 5, Supplementary Table S4). Among them, three genes (Bra039149, Bra022739 and Bra006301) encoded alpha-1,4-galacturonosyltransferase, two genes (Bra020762 and Bra001188) encoded galacturonosyltransferase-like 4, two genes (Bra039675 and Bra021578) encoded UDP-glucose 4,6-dehydratase, the gene Bra027626 encoded UDP-glucose 4-epimerase, one gene (Bra006303) was involved in UDP-glucose 6-dehydrogenase, two genes (Bra000314 and Bra001453) encoded chitinase and each of the gene Bra029158 and Bra011789 encoded fructokinase and hexokinase, respectively. We found 4 genes (Bra020716, Bra007665, Bra033347 and Bra030510) which were linked to pollen development and participating in starch and sucrose metabolism as well as pentose to glucuronate inter-conversion.
DEGs involved in lipid metabolism
In lipid metabolism, fatty acids are converted to fatty alcohols and plays an important role in developing anthers of fertile plant (Shi et al. 2011). Based on GO annotation and KEGG analysis, we found 27 DEGs involved in lipid metabolism (Fig. 5, Supplementary Table S4). Among them, 17 and 10 DEGs significantly down- and up-regulated in two CMS lines (Tyms and 22m2) and their maintainer line (231–330), respectively. Among 17 down-regulated DEGs, 8 genes (Bra028826, Bra025141, Bra029807, Bra038560, Bra024451, Bra017361, Bra026529 and Bra038837) encoded hydrolase family protein, 4 genes (Bra040869, Bra038892, Bra017740 and Bra012858) encoded putative ROP family GTPase, 2 genes (Bra011112 and Bra010327) were involved in phospholipase A2 family protein, 2 genes (Bra004987 and Bra000168) encoded phosphoinositide-specific phospholipase C family protein and 1 gene (Bra022411) encoded phospholipase C. Moreover, 10 DEGs were found in lipid binding function, among them, 2 genes (Bra005944 and Bra005946) were related to oleosin-like protein 4 genes (Bra028694, Bra005945, Bra009255 and Bra016576) encoded pollen coat oleosin-glycine rich protein, 2 genes (Bra038907 and Bra040627) were involved in lipid transfer protein, 1 gene (Bra008330) was related to pleckstrin homology domain-containing protein and 1 gene (Bra033633) was involved in glycolipid transfer protein (GLTP) family. A total 8 DEGs (Bra038837, Bra017740, Bra012858, Bra011112, Bra000168, Bra022411, Bra008330 and Bra033633) did not express in two types of CMS lines (Ogura- CMS; Tyms and Polima- CMS; 22m2), which might be the putative candidate genes involved in pollen development and maturation.
DEGs involved in tricarboxylic acid (TCA) cycle and oxidative phosphorylation
Adequate energy supply is required for pollen development, which is hampered in shortage of energy (Wang et al. 2016). The organelles mitochondria produces cellular energy through different metabolic pathways, like glycolysis, oxidative phosphorylation and TCA cycle (Verdin et al. 2010). We identified 7 DEGs involved in TCA cycle, most of them were down-regulated in two CMS lines (Fig. 5, Supplementary Table S4). Among them, IDH-III (Bra010527) and IDH-VI (Bra029791) encoded isocitrate dehydrogenase, PCK2 (Bra024410) related to putative phosphoenolpyruvate carboxykinase (ATP-dependent), CSY5 (Bra014513) encoded citrate synthase 5, ACLA-1 (Bra018425) regulated subunit A of the trimeric protein ATP citrate lyase, GRP17 (Bra005943) related to glycine-rich protein with an oleosin domain, unigene (Bra031821) encoded spindle assembly abnormal protein. Citrate synthase is the key enzyme of TCA cycle, which functions in the formation of citrate using acetyl-CoA and oxaloacetic acid as substrate. We also identified 9 DEGs related to the electron transport chain and oxidative phosphorylation, which were down-regulated in two types of CMS lines compared to fertile maintainer line (Fig. 5, Supplementary Table S4). For instance, 6 DEGs [ATP6 (Bra013168), ATP7 (Bra014496), ATP8 (Bra008288), ATP9 (Bra003576 and Bra035163), VHA-E2 (Bra032402)] encoded subunits of ATPase, NDB4 (Bra006374) involved in NADH dehydrogenase, COX15 (Bra002848) encoded cytochrome-C oxidase subunit XV assembly protein and PPA1 (Bra008551) encoded subunits of pyrophosphorylase.
Differentially expressed pentatricopeptide repeat (PPR) proteins
Proteins encoding the PPR motif are predicted as site-specific and RNA-binding adaptor proteins, which mediated the interactions between RNA substrates and relative enzymes (Du et al. 2016). All the cloned fertility restorer (Rf) genes encode a pentatricopeptide repeat-containing (PPR) protein, except Rf2 of maize, bvORF20 of sugar beet, and Rf17 and Rf2 of rice (Zhang et al. 2017). Most PPR genes are localized in mitochondria and chloroplasts become functioning in regulation of plant growth and development as well as fertility restoration for CMS (Han et al. 2016). We identified 10 genes (Bra026333, Bra029490, Bra026884, Bra032862, Bra031709, Bra006706, Bra015476, Bra017863, Bra018070 and Bra027154) encoding PPR proteins, which were down-regulated; while 3 genes (Bra011994, Bra036548 and Bra003441) were up-regulated in two types of CMS lines (Fig. 5, Supplementary Table S4). Among the 13 genes, 5 genes (Bra006706, Bra015476, Bra017863, Bra018070 and Bra027154) did not induce at all in two types of CMS lines but one gene (Bra003441) in the fertile maintainer line. These PPR proteins encoded genes could be potential candidates for further confirming the link between nuclear and mitochondrial gene expression.
Validation of expression patterns of genes by qRT-PCR
The qRT-PCR was performed to validate the RNA-seq results using 22 DEGs, involved in pollen development, carbon metabolism, lipid metabolism, ATPase and transcription factors. Among these 22 genes, most of them were down-regulated in CMS lines compared to their maintainer, except one gene named CCS1 (Bra014184), which showed up-regulation in both of the CMS lines. In addition, most of the genes with induced expression in qRT-PCR also showed high RPKM values in RNA-seq result in both of the CMS and the fertile maintainer lines. The expression patterns of the selected genes shared similar trends for qRT-PCR and Solexa sequencing, suggesting that Solexa sequencing could detect genes from Chinese cabbage flowers accurately and successfully (Fig. 6).
Fig. 6.
Twenty-two DEGs involved in pollen development, carbon metabolism, lipid metabolism, ATPase and transcription factors were selected for validation and showed the same tendency with RNA-seq; (a) relative expression of DEGs by qRT-PCR using two CMS (Tyms and 22m2) and one maintainer (231–330) lines, (b) RPKM values of the 22 DEGS selected from RNA-seq data of two CMS (Tyms and 22m2) and one maintainer (231–330) lines
Discussion
Utilization of CMS in Chinese cabbage is a better way to produce hybrid seeds compared to traditional SI (Self-Incompatibility) system. Several studies have been conducted to explore the physiological, genetic and molecular basis of CMS and fertility restoration, however the molecular mechanisms regarding occurrence of CMS are still not clear in Chinese cabbage (Du et al. 2016; Wang et al. 2016; Xie et al. 2016). Currently, RNA-Seq technology has been used at transcriptome level or whole genome analysis in many plants to predict the putative candidate genes, which regulated particular trait of interest (Lu et al. 2017). Therefore, a comparative transcriptional analysis of Chinese cabbage flower buds from an Ogura- CMS line (Tyms), Polima- CMS line (22m2) and the maintainer line (231–330) was performed to identify and characterize the DEGs associated with occurrence of CMS using next-generation sequencing technology. A total of 96,967 genes were identified in three cDNA libraries; there were 912, 7199 and 6381 DEGs found in 22m2 Vs Tyms, 231–330 Vs 22m2 and 231–330 Vs Tyms, respectively. Among them, up- and down-regulated genes might make difference between Ogura- and Polima- CMS lines, while the genes from the co-expressed fraction might regulate the CMS system for both of the CMS lines (22m2 and Tyms).
In male sterility system, the anthers of flower become dysfunctional through either failure to produce functional pollen or no pollen under natural conditions (Liu et al. 2015a; Pei et al. 2017). As anthers carry the pollen grain, therefore we compared structural differences inside the anther between both CMS and maintainer lines (Fig. 1b), and there was difference between them, maintainer line had sign of developing pollen but CMS was without such sign of development. Pollen formation in flowering plants depends on the differentiation and interaction of the reproductive cells named microsporocytes and the somatic cells named tapetum. In the developmental process, the sterile anthers produced little or no pollen with normal pistil (An et al. 2014). The mutant of FUSED (FU) gene belonging to the Ser/Thr protein kinase family, dominantly inhibits male meiotic cytokinesis in Arabidopsis (Oh et al. 2014). In this work, FU (Bra018837) was not expressed in both of the CMS lines and might inhibit male meiotic cytokinesis in plants, leading to male sterility. Meanwhile, we found low expression of EXO70C2 (Bra006228) gene in sterile lines; i.e., approximately 1/20th of the maintainer line (231–330). EXO70C2 is a member of the EXO70 gene family associated with reproductive development and has been co-expressed in anthers (Li et al. 2010).
Tapetum function and pollen coat formation are the major processes in pollen development immediately after completion of meiosis, (Dong et al. 2013). TPD1 is a small secreted cysteine-rich protein ligand that interacts with the leucine-rich repeat (LRR) domain of the EMS1 receptor kinase at two sites. Tapetal cell differentiation requires reproductive cell secreted TPD1, which illuminates a novel mechanism whereby signals from reproductive cells determine the somatic cell fate in plant sexual reproduction (Huang et al. 2016). EMS1 controls somatic and reproductive cell fates in the Arabidopsis anther (Zhao et al. 2002). In this study, TPD1 (Bra013852) and EMS1 (Bra009277) were up-regulated in maintainer line compared to both CMS lines and TPD1 showed more up-regulation than EMS1. The pollen coat covering the surface of pollen grains has many important roles in pollination, which depends on pollen coat enriched with lipids and proteins (Pacini and Hesse 2002). In the Arabidopsis genome, oleosin glycine-rich protein genes, like Atgrp6 (GRP16), Atgrp7 (GRP17) and Atgrp8 (GRP18) are present in clusters and expressed specifically in the tapetum cells (Franco et al. 2002).
Rearrangements of mitochondrial genome could alter the expression of genes involved in respiration and ATP synthesis, which effect ATP formation and physiological processes in mitochondria. ATP production or carbohydrate accumulation becomes weak in the flowers of CMS cabbage and broccoli (Pei et al. 2017; Wang et al. 2016). In this study, 6 DEGs associated in TCA cycle were significantly down-regulated in both CMS lines. DEGs involved in citrate synthase, isocitrate dehydrogenase and phosphoenolpyruvate carboxykinase. DEGs were involved in the oxidative phosphorylation pathway, which affects NADH dehydrogenase, pyrophosphorylase and ATP synthase; and found down-regulation of 9 DEGs in both CMS lines. A series of enzymes catalyze the synthesis of glucose, starch and other saccharides involved in carbohydrate metabolism during pollen development for supplying energy (Miernyk et al. 2011). We found a number of genes involved in pentose and glucuronate inter-conversions, starch and sucrose metabolism, and amino sugar and nucleotide sugar metabolism was significantly down-regulated in CMS lines compared to maintainer. Further investigations on them will help to illustrate the primary targets and downstream components of CMS-associated mitochondrial signaling pathways in CMS lines.
Nuclear genes, especially PPR encoded genes control the biosynthesis and function of mitochondrial ORF (Andersson et al. 2003). In Arabidopsis, 60% of 450 PPR proteins are associated in regulating functions of mitochondrial ORF (Du et al. 2016). Previous studies confirmed that PPR genes can restore pollen fertility by inhibiting the expression of mitochondrial CMS genes in plants (O’Toole et al. 2008; Wang et al. 2017). In this study, 13 PPR proteins showed differential expression between two CMS lines and their maintainer line. These PPR genes are predicted as candidate for further analyzing the link between nuclear and mitochondrial gene expression.
Conclusion
In this work, we used transcriptome sequencing techniques to analyze the differential gene expression in buds of Ogura- CMS (Tyms), Polima- CMS (22m2) and maintainer line (231–330). We identified 912, 7199 and 6381 DEGs in 22m2 Vs Tyms, 231–330 Vs 22m2 and 231–330 Vs Tyms, respectively. The DEGs are characterized to be involved in diverse molecular functions through GO and metabolic pathway analyses. Transcriptional profiling corroborated that many DEGs involved in pollen development, carbon metabolism, lipid metabolism, TCA and oxidative phosphorylation. Our findings would be potential source for elucidating molecular mechanisms and functional characterization of the candidate genes leading to CMS in Chinese cabbage and other Brassica crops.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The data of this project has been deposited at NCBI under the accessions; SRR2132359, SRR2132463 and SRR4299394.
Funding
This work was supported by the National Key Research and Development Program of China (2017YFD0101802, 2016YFD0100204-18), Henan Provincial Science and Technology Open Cooperation Project (182106000052), Special Funds for Scientific Research and Development of Henan Academy of Agricultural Sciences (2019CY016), the Science Foundation of Henan Province (162300410162).
Compliance with ethical standards
Conflict of interest
There is no conflict of interests among authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaochun Wei, Yanyan Lv, Yanyan Zhao have equally contributed to this work.
Contributor Information
Fang Wei, Email: fangwei@zzu.edu.cn.
Xiaowei Zhang, Email: xiaowei5737@163.com.
References
- An H, Yang Z, Yi B, Wen J, Shen J, Tu J, Ma C, Fu T. Comparative transcript profiling of the fertile and sterile flower buds of pol CMS in B. napus. BMC Genom. 2014;15:258. doi: 10.1186/1471-2164-15-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson SG, Karlberg O, Canback B, Kurland CG (2003) On the origin of mitochondria: a genomics perspective. Philosophical transactions of the Royal Society of London Series B, Biological Sciences 358: 165–177; discussion 177–169 [DOI] [PMC free article] [PubMed]
- Aviv D, Galun E. Restoration of fertility in cytoplasmic male sterile (CMS) Nicotiana sylvestris by fusion with X-irradiated N. tabacum protoplasts. Theoretical Appl Genetics. 1980;58:121–127. doi: 10.1007/BF00263102. [DOI] [PubMed] [Google Scholar]
- Carlsson J, Glimelius K. Cytoplasmic male-sterility and nuclear encoded fertility restoration. In: Kempken F, editor. Plant Mitochondria. New York: Springer; 2011. pp. 469–491. [Google Scholar]
- Chase CD. Cytoplasmic male sterility: a window to the world of plant mitochondrial–nuclear interactions. Trends Genetics. 2007;23:81–90. doi: 10.1016/j.tig.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu Y-G. Male sterility and fertility restoration in crops. Annual Rev Plant Biol. 2014;65:579. doi: 10.1146/annurev-arplant-050213-040119. [DOI] [PubMed] [Google Scholar]
- Chen J, Guan R, Chang S, Du T, Zhang H, Xing H. Substoichiometrically different mitotypes coexist in mitochondrial genomes of Brassica napus L. PLoS ONE. 2011;6:e17662. doi: 10.1371/journal.pone.0017662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B, Hao M, Mei D, Zaman QU, Sang S, Wang H, Wang W, Fu L, Cheng H, Hu Q. Transcriptome and hormone comparison of three cytoplasmic male sterile systems in Brassica napus. Int J Mol Sci. 2018;19:4022. doi: 10.3390/ijms19124022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Kim WK, Lim YP, Kim YK, Hur Y. Ogura-CMS in Chinese cabbage (Brassica rapa ssp. pekinensis) causes delayed expression of many nuclear genes. Plant Sci Int J Exp Plant Biol. 2013;199–200:7–17. doi: 10.1016/j.plantsci.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Du K, Liu Q, Wu X, Jiang J, Wu J, Fang Y, Li A, Wang Y. Morphological structure and transcriptome comparison of the cytoplasmic male sterility line in Brassica napus (SaNa-1A) derived from somatic hybridization and its maintainer line SaNa-1B. Front Plant Sci. 2016;7:1313. doi: 10.3389/fpls.2016.01313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco LO, de Manes OCL, Hamdi S, Sachetto-Martins G, Oliveira DE DE. Distal regulatory regions restrict the expression of cis-linked genes to the tapetal cells. FEBS Lett. 2002;517:13–18. doi: 10.1016/s0014-5793(02)02557-7. [DOI] [PubMed] [Google Scholar]
- Fu T. Production and research of rapeseed in the People’s Republic of China. Eucarpia Cruciferae Newsl. 1981;6:6–7. [Google Scholar]
- Fu T, Yang G, Yang X, Ma C. The discovery, research and utilization of pol cytoplasmic male sterile in Brassica napus. Prog Nat Sci Commun State Key Lab. 1995;5:287–293. [Google Scholar]
- Han Z, Qin Y, Kong F, Deng Y, Wang Z, Shen G, Wang J, Duan B, Li R. Cloning and expression analysis of eight upland cotton pentatricopeptide repeat family genes. Appl Biochem Biotechnol. 2016;180:1243–1255. doi: 10.1007/s12010-016-2164-y. [DOI] [PubMed] [Google Scholar]
- Han F, Zhang X, Yang L, Zhuang M, Zhang Y, Li Z, Fang Z, Lv H. iTRAQ-based proteomic analysis of Ogura-CMS cabbage and its maintainer line. Int J Mol Sci. 2018;19:3180. doi: 10.3390/ijms19103180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MR, Bentolila S. Interactions of mitochondrial and nuclear genes that affect male gametophyte development. Plant Cell. 2004;16:S154–S169. doi: 10.1105/tpc.015966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zhang T, Linstroth L, Tillman Z, Otegui MS, Owen HA, Zhao D. Control of anther cell differentiation by the small protein ligand TPD1 and its receptor EMS1 in Arabidopsis. PLoS Genet. 2016;12:e1006147. doi: 10.1371/journal.pgen.1006147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke G, Zhao Z, Song Y, Zhang L, An DL. Breeding of alloplasmic male sterile line CMS3411-7 in Chinese cabbage (Brassica campestris L. ssp. pekinensis (Lour) Olsson) and its application. Acta Hortic Sin. 1992;16:333–340. [Google Scholar]
- Li S, van Os GM, Ren S, Yu D, Ketelaar T, Emons AM, Liu CM. Expression and functional analyses of EXO70 genes in Arabidopsis implicate their roles in regulating cell type-specific exocytosis. Plant Physiol. 2010;154:1819–1830. doi: 10.1104/pp.110.164178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Miao Y, Su S, Xu J, Jin L, Sun D, Peng R, Huang L, Cao J. Comprehensive analysis of Ogura cytoplasmic male sterility-related genes in turnip (Brassica rapa ssp. rapifera) using RNA sequencing analysis and bioinformatics. PLoS ONE. 2019;14:e0218029. doi: 10.1371/journal.pone.0218029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Pang C, Wei H, Song M, Meng Y, Ma J, Fan S, Yu S. iTRAQ-facilitated proteomic profiling of anthers from a photosensitive male sterile mutant and wild-type cotton (Gossypium hirsutum L.) Journal of proteomics. 2015;126:68–81. doi: 10.1016/j.jprot.2015.05.031. [DOI] [PubMed] [Google Scholar]
- Liu J, Xiang R, Wang W, Mei D, Li Y, Mason AS, Fu L, Hu Q. Cytological and molecular analysis of Nsa CMS in Brassica napus L. Euphytica. 2015;206:279–286. [Google Scholar]
- Liu Z, Yang Z, Wang X, Li K, An H, Liu J, Yang G, Fu T, Yi B, Hong D. A mitochondria-targeted PPR protein restores pol cytoplasmic male sterility by reducing orf224 transcript levels in oilseed rape. Mol Plant. 2016;9:1082–1084. doi: 10.1016/j.molp.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu X, Zhou X, Cao Y, Zhou M, McNeil D, Liang S, Yang C. RNA seq analysis of cold and drought responsive transcriptomes of Zea mays ssp. mexicana L. Front Plant Sci. 2017;8:136. doi: 10.3389/fpls.2017.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Xu H, Liu Z, Guo J, Li H, Chen L, Fang C, Zhang Q, Bai M, Yao N, Wu H, Wu H, Ji C, Zheng H, Chen Y, Ye S, Li X, Zhao X, Li R, Liu YG. A detrimental mitochondrial-nuclear interaction causes cytoplasmic male sterility in rice. Nat Genet. 2013;45:573–577. doi: 10.1038/ng.2570. [DOI] [PubMed] [Google Scholar]
- Miernyk JA, Pretova A, Olmedilla A, Klubicova K, Obert B, Hajduch M. Using proteomics to study sexual reproduction in angiosperms. Sex Plant Reprod. 2011;24:9–22. doi: 10.1007/s00497-010-0149-5. [DOI] [PubMed] [Google Scholar]
- Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- Ogura H. Studies on the new male-sterility in Japanese Radish, with special reference to the utilization of this sterility towards the practical raising of hybrid seeds. Mem Fac Agric Kagoshima Univ. 1968;6:39–78. [Google Scholar]
- Oh SA, Bourdon V, Dickinson HG, Twell D, Park SK. Arabidopsis fused kinase TWO-IN-ONE dominantly inhibits male meiotic cytokinesis. Plant Reprod. 2014;27:7–17. doi: 10.1007/s00497-013-0235-6. [DOI] [PubMed] [Google Scholar]
- O’Toole N, Hattori M, Andres C, Iida K, Lurin C, Schmitz-Linneweber C, Sugita M, Small I. On the expansion of the pentatricopeptide repeat gene family in plants. Mol Biol Evol. 2008;25:1120–1128. doi: 10.1093/molbev/msn057. [DOI] [PubMed] [Google Scholar]
- Pacini E, Hesse M. Types of pollen dispersal units in orchids, and their consequences for germination and fertilization. Ann Botany. 2002;89:653–664. doi: 10.1093/aob/mcf138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson O. Nature and mechanisms of cytoplasmic male sterility in plants: a review. HortScience (USA) 1981;16:482–487. [Google Scholar]
- Pei X, Jing Z, Tang Z, Zhu Y. Comparative transcriptome analysis provides insight into differentially expressed genes related to cytoplasmic male sterility in broccoli (Brassica oleracea var. italica) Sci Horticult. 2017;217:234–242. [Google Scholar]
- Pelletier G, Budar F. The molecular biology of cytoplasmically inherited male sterility and prospects for its engineering. Curr Opin Biotechnol. 2007;18:121–125. doi: 10.1016/j.copbio.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Pelletier G, Primard C, Vedel F, Chetrit P, Remy R, Rousselle Renard M. Intergeneric cytoplasmic hybridization in cruciferae by protoplast fusion. Mol General Genet MGG. 1983;191:244–250. [Google Scholar]
- Romualdi C, Bortoluzzi S, D’Alessi F, Danieli GA. IDEG6: a web tool for detection of differentially expressed genes in multiple tag sampling experiments. Physiol Genomics. 2003;12:159–162. doi: 10.1152/physiolgenomics.00096.2002. [DOI] [PubMed] [Google Scholar]
- Schuster SC. Next-generation sequencing transforms today’s biology. Nat Methods. 2008;5:16–18. doi: 10.1038/nmeth1156. [DOI] [PubMed] [Google Scholar]
- Shi J, Tan H, Yu XH, Liu Y, Liang W, Ranathunge K, Franke RB, Schreiber L, Wang Y, Kai G, Shanklin J, Ma H, Zhang D. Defective pollen wall is required for anther and microspore development in rice and encodes a fatty acyl carrier protein reductase. Plant Cell. 2011;23:2225–2246. doi: 10.1105/tpc.111.087528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Brown GG. Suppression of cytoplasmic male sterility by nuclear genes alters expression of a novel mitochondrial gene region. Plant Cell. 1991;3:1349–1362. doi: 10.1105/tpc.3.12.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, Singh SP, Pandey T, Singh RR, Sawant SV. A novel male sterility-fertility restoration system in plants for hybrid seed production. Scientific Rep. 2015;5:11274. doi: 10.1038/srep11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Štorchová H. The role of non-coding RNAs in cytoplasmic male sterility in flowering plants. Int J Mol Sci. 2017;18(11):2429–2441. doi: 10.3390/ijms18112429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester M, Langridge P. Breeding technologies to increase crop production in a changing world. Science. 2010;327:818–822. doi: 10.1126/science.1183700. [DOI] [PubMed] [Google Scholar]
- Thompson K. Cytoplasmic male-sterility in oil-seed rape. Heredity. 1972;29:253–257. [Google Scholar]
- Tian E, Roslinsky V, Cheng B. Molecular marker-assisted breeding for improved Ogura CMS restorer line (RfoRfo) and mapping of the restorer gene (Rfo) in Brassica juncea. Mol Breed. 2014;34:1361–1371. [Google Scholar]
- Verdin E, Hirschey MD, Finley LW, Haigis MC. Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci. 2010;35:669–675. doi: 10.1016/j.tibs.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Z, Jing B, Tu J, Ma C, Shen J, Yi B, Wen J, Huang T, Wang X, Fu T. Genetic characterization of a new cytoplasmic male sterility system (hau) in Brassica juncea and its transfer to B. napus. Theor Appl Genet. 2008;116:355–362. doi: 10.1007/s00122-007-0673-3. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ma S, Wang M, Zheng X, Gu M, Hu S. Sequence analysis of the gene correlated with cytoplasmic male sterility (CMS) in rape-seed (Brassica napus) Polima and Shaan 2A. Chinese Sci Bullet. 2002;47:124–127. [Google Scholar]
- Wang S, Wang C, Zhang X-X, Chen X, Liu J-J, Jia X-F, Jia S-Q. Transcriptome de novo assembly and analysis of differentially expressed genes related to cytoplasmic male sterility in cabbage. Plant Physiol Biochem. 2016;105:224–232. doi: 10.1016/j.plaphy.2016.04.027. [DOI] [PubMed] [Google Scholar]
- Wang ZW, De Wang C, Cai QZ, Mei SY, Gao L, Zhou Y, Wang T. Identification of promoter exchange at a male fertility restorer locus for cytoplasmic male sterility in radish (Raphanus sativus L.). Mol Breed. 2017;37:82. [Google Scholar]
- Wei X, Zhang X, Yao Q, Yuan Y, Li X, Wei F, Zhao Y, Zhang Q, Wang Z, Jiang W, Zhang X. The miRNAs and their regulatory networks responsible for pollen abortion in Ogura-CMS Chinese cabbage revealed by high-throughput sequencing of miRNAs, degradomes, and transcriptomes. Front Plant Sci. 2015;6:894. doi: 10.3389/fpls.2015.00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson JD, Chory J. Coordination of gene expression between organellar and nuclear genomes. Nat Rev Genet. 2008;9:383–395. doi: 10.1038/nrg2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Zhang W, Wang Y, Xu L, Zhu X, Muleke EM, Liu L. Comprehensive transcriptome-based characterization of differentially expressed genes involved in microsporogenesis of radish CMS line and its maintainer. Funct Integr Genomics. 2016;16:529–543. doi: 10.1007/s10142-016-0504-1. [DOI] [PubMed] [Google Scholar]
- Xing M, Sun C, Li H, Hu S, Lei L, Kang J. Integrated analysis of transcriptome and proteome changes related to the Ogura cytoplasmic male sterility in cabbage. PLoS ONE. 2018;13:e0193462. doi: 10.1371/journal.pone.0193462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi H, Bhat SR. Cytoplasmic male sterility in Brassicaceae crops. Breed Sci. 2014;64:38–47. doi: 10.1270/jsbbs.64.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yao G, Yue W, Zhang S, Wu J. Transcriptome profiling reveals differential gene expression in proanthocyanidin biosynthesis associated with red green skin color mutant of pear (Pyrus communis L.). Front Plant Sci. 2015;6:795. doi: 10.3389/fpls.2015.00795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HL, Fang ZY, Liu YM, Yang LM, Zhuang M, Lv HH, Li ZS, Han FQ, Liu XP, Zhang YY. Development of a novel allele-specific Rfo marker and creation of Ogura CMS fertility-restored interspecific hybrids in Brassica oleracea. Theor Appl Genet. 2016;129:1625–1637. doi: 10.1007/s00122-016-2728-9. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wu J, Dai Z, Qin M, Hao L, Ren Y, Li Q, Zhang L. Allelism analysis of BrRfp locus in different restorer lines and map-based cloning of a fertility restorer gene, BrRfp1, for pol CMS in Chinese cabbage (Brassica rapa L.) Theor Appl Genet. 2017;130:539–547. doi: 10.1007/s00122-016-2833-9. [DOI] [PubMed] [Google Scholar]
- Zhao DZ, Wang GF, Speal B, Ma H. The excess microsporocytes1 gene encodes a putative leucine-rich repeat receptor protein kinase that controls somatic and reproductive cell fates in the Arabidopsis anther. Genes Dev. 2002;16:2021–2031. doi: 10.1101/gad.997902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.