Abstract
Manganese (Mn) is an important micronutrient for plant growth and development and sustains metabolic roles within different plant cell compartments. The metal is an essential cofactor for the oxygen-evolving complex (OEC) of the photosynthetic machinery, catalyzing the water-splitting reaction in photosystem II (PSII). Despite the importance of Mn for photosynthesis and other processes, the physiological relevance of Mn uptake and compartmentation in plants has been underrated. The subcellular Mn homeostasis to maintain compartmented Mn-dependent metabolic processes like glycosylation, ROS scavenging, and photosynthesis is mediated by a multitude of transport proteins from diverse gene families. However, Mn homeostasis may be disturbed under suboptimal or excessive Mn availability. Mn deficiency is a serious, widespread plant nutritional disorder in dry, well-aerated and calcareous soils, as well as in soils containing high amounts of organic matter, where bio-availability of Mn can decrease far below the level that is required for normal plant growth. By contrast, Mn toxicity occurs on poorly drained and acidic soils in which high amounts of Mn are rendered available. Consequently, plants have evolved mechanisms to tightly regulate Mn uptake, trafficking, and storage. This review provides a comprehensive overview, with a focus on recent advances, on the multiple functions of transporters involved in Mn homeostasis, as well as their regulatory mechanisms in the plant’s response to different conditions of Mn availability.
Keywords: manganese transport, manganese uptake, manganese deficiency, manganese toxicity, intracellular distribution, Arabidopsis, rice, barley
Introduction
Manganese (Mn) is an essential element in virtually all living organisms where it can fulfill two different functions: acting as an enzyme cofactor or as a metal with catalytic activity in biological clusters (Andresen et al., 2018). In humans, Mn functions as a cofactor for a variety of enzymes, including arginase, glutamine synthetase, pyruvate carboxylase, and Mn superoxide dismutase (MnSOD). But in comparison to other essential micronutrients, such as iron (Fe) and zinc (Zn), whose deficiencies in humans are responsible for major health problems, Mn deficiency in humans is rare. However, Mn poisoning may be encountered more frequently upon overexposure to this metal causing hepatic cirrhosis, polycythemia, dystonia, and Parkinson-like symptoms (Li and Yang, 2018). In plants, Mn is one of the 17 essential elements for growth and reproduction. It is needed in only small quantities by plants, but is ultimately as critical to growth as are the other nutrients. In photosynthetic organisms, Mn is an essential element of the metalloenzyme cluster of the oxygen-evolving complex (OEC) in photosystem II (PSII). In spite of its importance for photosynthetic activity, Mn homeostasis in plants has been poorly investigated. Nevertheless, Mn deficiency can be a serious plant nutritional disorder in soils with high pH and high partial pressure of O2 (pO2), where the bio-availability of Mn can decrease far below the level that is required for normal plant growth (Broadley et al., 2012). Fertilization with Mn salts at soil level is often not effective, since soluble Mn (Mn2+) is rapidly converted to plant-unavailable Mn oxides, particularly in sandy alkaline soils. (In this review, we use the general term “Mn,” unless we refer to a specific oxidation state). However, it has been argued that the application of Mn fertilizers to the soil can be an effective way to alleviate Mn deficiencies, but only if soil pH is also corrected (White and Greenwood, 2013). Foliar Mn application can supply sufficient Mn to overcome Mn deficiency, but this strategy is expensive and often impractical for farmers on marginal lands. Moreover, foliar Mn sprays are only effective for a limited time period since Mn is very little mobile in the plant and does not remobilize from older leaves to Mn-deficient young leaves (Li et al., 2017). On the other extreme, Mn toxicity can occur in poorly drained and in strongly acidic soils, where it is usually associated with other acidity-related soil fertility problems, such as aluminum toxicity and deficiencies of calcium (Ca), magnesium (Mg), and molybdenum (Mo) (Goulding, 2016).
Depending on Mn availability, plants either need to efficiently acquire and utililize Mn under limiting conditions, or to detoxify the metal under superfluous supply. Transport processes are at the core of those adaptations. In the past, Mn transporters have been identified at the molecular level in many eukaryotic organisms (Pittman, 2005). Furthermore, recent progress in Mn homeostasis has mainly focused on Arabidopsis and rice, which represent dicot and graminaceous monocot plants, respectively (Socha and Guerinot, 2014; Shao et al., 2017). Only recently, transporters involved in uptake and subcellular distribution of Mn have been characterized in a wider range of plant species. This article reviews the current knowledge of Mn uptake, translocation and subcellular distribution, as well as the functions of Mn in different plant species. Table 1 lists the Mn transporters discussed in the text.
TABLE 1.
Manganese transport proteins reviewed in this article.
| Family/name | Organ(s)/tissue(s) expression | Subcellular localization | Gene expression response | Other substrates | References |
| CaCA | |||||
| AtCAX2 | Root (stele and root tip), stem, leaf, flower, pollen, fruit | Tonoplast | Unaffected by +Cu, +Mn, +Zn | Ca, Cd | Hirschi et al., 2000; Shigaki et al., 2003; Pittman et al., 2004; Edmond et al., 2009 |
| AtCAX4 | Root, stem, leaf, flower, fruit | Tonoplast | Up-regulated by +Mn, +Ni, -Ca | Cd, Ca, Zn | Cheng et al., 2002; Koren’kov et al., 2007; Mei et al., 2009 |
| AtCAX5 | Root, stem, leaf, flower, fruit | Tonoplast | Up-regulated by +Mn down-regulated by +Zn | Ca | Edmond et al., 2009 |
| HvCAX2 | Root, leaf, seed | – | Up-regulated by +Ca, +Na unaffected by +Mn | Ca | Edmond et al., 2009 |
| LeCAX2 | Root, leaf, fruit | – | – | Ca | Edmond et al., 2009 |
| OsCAX1a | Root, shoot, flower, seed | Tonoplast | – | Ca | Kamiya et al., 2005 |
| OsCAX3 | Root, shoot, flower, seed | – | – | Ca | Kamiya et al., 2005 |
| OsCAX4 | Root | – | Up-regulated by +Ca | Ca,Cu | Yamada et al., 2014 |
| VvCAX3 | Root, stem, leaf, fruit | Tonoplast | Up-regulated by +Ca, +Na unaffected by +Mn | Ca, Cu, Li, Na | Martins et al., 2017 |
| AtCCX3 | Root, stem, leaf, flower | Tonoplast, endomembranes | Up-regulated by +K, +Na unaffected by +Mn | K, Na | Morris et al., 2008 |
| BICAT | |||||
| AtBICAT1/PAM71/CCHA1 | Leaf | Chloroplast (thylakoid membrane) | Unaffected by +Mn | Ca | Schneider et al., 2016; Wang et al., 2016; Eisenhut et al., 2018; Frank et al., 2019 |
| AtBICAT2/CMT1 | Root, stem, leaf, flower, fruit | Chloroplast (inner envelope) | Down-regulated by +Mn | Ca, Mg | Eisenhut et al., 2018; Frank et al., 2019; Zhang et al., 2018 |
| CDF/MTP | |||||
| AtMTP8 | Root (epidermis, cortex), seed | Tonoplast | Up-regulated by +Mn -Fe | Fe | Eroglu et al., 2016, 2017 |
| AtMTP9 | – | – | Unaffected by +Mn | – | Delhaize et al., 2007; Chu et al., 2017 |
| AtMTP10 | – | – | Unaffected by +Mn | – | Delhaize et al., 2007; Chu et al., 2017 |
| AtMTP11 | Root (root tip), leaf (guard cells) | Golgi/PVC | Unaffected by +Mn | – | Delhaize et al., 2007; Peiter et al., 2007 |
| ShMTP8 | – | Tonoplast | – | – | Delhaize et al., 2003, 2007 |
| BmMTP10 | Root, leaf | Endomembranes* | Up-regulated by +Mn | – | Erbasol et al., 2013 |
| BmMTP11 | Root, leaf | Endomembranes* | Unaffected by +Mn | Ni | Erbasol et al., 2013 |
| OsMTP8.1 | Root, shoot | Tonoplast | – | – | Chen et al., 2013; Takemoto et al., 2017 |
| OsMTP8.2 | Root, shoot | Tonoplast | – | – | Takemoto et al., 2017 |
| OsMTP9 | Root (endodermis, exodermis) | Plasma membrane | Unaffected by +Mn -Mn | Ueno et al., 2015 | |
| OsMTP11 | Root, shoot | Golgi/TGN | Up-regulated by +Mn +Zn +Cd +Ni | Co, Ni | Zhang and Liu, 2017; Ma et al., 2018; Tsunemitsu et al., 2018 |
| HvMTP8.1 | Root, leaf | Golgi | Down-regulated by -Mn (root) down-regulated by +Mn (shoot) | – | Pedas et al., 2014 |
| HvMTP8.2 | Root, leaf | Golgi | Down-regulated by +Mn | – | Pedas et al., 2014 |
| PtMTP11.1 | – | TGN | – | – | Peiter et al., 2007 |
| PtMTP11.2 | – | TGN | – | – | Peiter et al., 2007 |
| PbMTP8.1 | – | – | – | Fe | Hou et al., 2019 |
| PbMTP8.2 | – | – | – | Fe | Hou et al., 2019 |
| PbMTP9 | – | – | – | Fe | Hou et al., 2019 |
| PbMTP10 | – | – | – | Fe | Hou et al., 2019 |
| PbMTP11.1 | – | – | – | – | Hou et al., 2019 |
| PbMTP11.2 | – | – | – | – | Hou et al., 2019 |
| NtMTP8.1 | Root, stem, leaf, flower | – | Up-regulated by +Co +Zn unaffected by +Mn | – | Liu et al., 2019 |
| NtMTP8.4 | Stem, leaf, flower | – | Up-regulated by +Cd +Co +Mn (root) up-regulated by +Cd +Co +Mg +Zn (shoot) |
– | Liu et al., 2019 |
| NtMTP11.1 | Root, stem, leaf, flower | – | Up-regulated by +Co +Cd +Mn +Zn | – | Liu et al., 2019 |
| CsMTP9 | Root (endodermis) | Plasma membrane | Up-regulated by +Cd +Mn +Ni down-regulated by –Mn -Zn |
Cd | Migocka et al., 2015 |
| NRAMP | |||||
| AtNRAMP1 | Root (Cortex, endodermis) > > shoot | Plasma membrane | Up-regulated by -Fe -Mn | Cd, Fe | Curie et al., 2000; Thomine et al., 2000; Cailliatte et al., 2010; Castaings et al., 2016 |
| AtNRAMP2 | Root (pericycle, root tip), leaf vasculature, flower, trichome | TGN | Up-regulated by -Mn down-regulated by -Fe | – | Curie et al., 2000; Alejandro et al., 2017; Gao et al., 2018 |
| AtNRAMP3 | Root (stele), leaf vasculature, developing seed | Tonoplast | Up-regulated by -Fe unaffected by -Mn | Cd, Fe | Thomine et al., 2000, 2003; Lanquar et al., 2005, 2010 |
| AtNRAMP4 | Root (stele), leaf vasculature, developing seed | Tonoplast | Up-regulated by -Fe unaffected by -Mn | Cd, Fe | Thomine et al., 2000; Lanquar et al., 2005, 2010 |
| OsNRAMP3 | Node, leaf vasculature | Plasma membrane | Unaffected by +Mn -Mn | – | Yamaji et al., 2013; Yang et al., 2013 |
| OsNRAMP5 | Root (exodermis, endodermis, stele), panicle | Plasma membrane | Up-regulated by -Fe (shoot) up-regulated by -Fe, -Zn (root) unaffected by -Mn | Cd, Fe | Ishimaru et al., 2012; Sasaki et al., 2012; Yang et al., 2014 |
| OsNRAMP6 | Leaves | Plasma membrane | – | Fe | Peris-Peris et al., 2017 |
| HvNRAMP5 | Root (epidermis, stele) | Plasma membrane | Up-regulated by -Fe unaffected by +Mn | Cd | Wu et al., 2016 |
| BnNRAMP1b | Root, shoot | – | Up-regulated by +Cd | Cd, Zn | Meng et al., 2017 |
| TcNRAMP3 | – | Tonoplast | – | Cd, Fe | Oomen et al., 2009 |
| TcNRAMP4 | – | Tonoplast | – | Cd, Fe, Zn | Oomen et al., 2009 |
| TcNRAMP3 (T. cacao) |
Root, shoot | – | Up-regulated by -Fe unaffected by -Mn | Fe | Ullah et al., 2018 |
| TcNRAMP5 (T. cacao) |
Root | – | Up-regulated by -Fe unaffected by -Mn |
Cd, Fe, Zn | Ullah et al., 2018 |
| TcNRAMP6 (T. cacao) |
Root, shoot | – | – | – | Ullah et al., 2018 |
| LeNRAMP1 | Root | Endomembranes* | up-regulated by -Fe | – | Bereczky et al., 2003 |
| LeNRAMP3 | Root, shoot | Endomembranes* | Up-regulated by -Fe | – | Bereczky et al., 2003 |
| AhNRAMP1 | Root, stem | – | Up-regulated by -Mn, -Zn | Zn | Wang et al., 2019 |
| MbNRAMP1 | Root | Endomembranes* | Up-regulated by -Fe | Fe | Xiao et al., 2008 |
| P-TYPE ATPASE | |||||
| AtECA1 | Root, stem, leaf, guard cells, trichome | ER | – | Ca, Zn | Liang et al., 1997; Wu et al., 2002; Li et al., 2008 |
| AtECA3 | Root (stele), stem, leaf vasculature, guard cells, flower, fruit | Golgi | Unaffected by -Mn | Ca, Zn | Mills et al., 2007; Li et al., 2008 |
| LeLCA1 | – | ER? | – | Ca | Johnson et al., 2009 |
| VIT | |||||
| AtVIT1 | Root, cotyledon, developing seed | Tonoplast | – | Fe | Kim et al., 2006 |
| OsVIT1 | Leaf > > root, stem, panicle, embryo | Tonoplast | Down-regulated by -Fe | Fe, Zn | Zhang et al., 2012; Wang et al., 2017 |
| OsVIT2 | Leaf, stem, panicle, embryo | Tonoplast | Up-regulated by +Fe down-regulated by -Fe | Fe, Zn | Zhang et al., 2012 |
| TaVIT2 | Root, shoot, seed | Tonoplast | – | Fe | Connorton et al., 2017 |
| AtMEB1 | – | ER bodies | – | Fe | Yamada et al., 2013 |
| AtMEB2 | – | ER bodies | – | Fe | Yamada et al., 2013 |
| YSL | |||||
| OsYSL2 | Root (phloem), leaf, vascular bundle, developing seed | Plasma membrane | Up-regulated by -Fe down-regulated by -Mn | Fe | Koike et al., 2004; Yang et al., 2014 |
| OsYSL6 | Root, shoot | Plasma membrane | Unaffected by +Mn -Mn | – | Sasaki et al., 2011 |
| HvYSL2 | Root (endodermis), shoot | – | Up-regulated by -Fe | Fe, Zn, Co, Ni, Cu | Araki et al., 2011 |
| ZIP | |||||
| AtIRT1 | Root (epidermis, cortex) > > shoot | Plasma membrane | Up-regulated by -Fe | Fe, Zn, Co, Ni | Korshunova et al., 1999; Curie et al., 2000; Thomine et al., 2000; Vert et al., 2002 |
| AtZIP1 | Root (stele), leaf vasculature | Tonoplast | Up-regulated by -Fe -Zn (root) up-regulated by -Mn (shoot) down-regulated by -Zn (shoot) | Zn | Milner et al., 2013 |
| AtZIP2 | Root (stele) | Plasma membrane | Down-regulated by -Fe -Mn | Zn | Milner et al., 2013 |
| AtZIP5 | Root, shoot | – | – | – | Milner et al., 2013 |
| AtZIP6 | Root, shoot | – | – | – | Milner et al., 2013 |
| AtZIP7 | Shoot > > root | – | – | Fe, Zn | Milner et al., 2013; Fu et al., 2017 |
| AtZIP9 | Root, shoot | – | – | – | Milner et al., 2013 |
| HvIRT1 | Root (epidermis, cortex**, endodermis**, pericycle), seed | Plasma membrane | Up-regulated by -Fe, -Mn | Fe, Zn | Pedas et al., 2008; Long et al., 2018 |
| LeIRT1 | Root, flower | – | – | Cd, Fe, Zn | Eckhardt et al., 2001 |
| LeIRT2 | Root | – | – | Cd, Fe, Zn | Eckhardt et al., 2001 |
| MtZIP4 | Root***, leaf | – | Down-regulated by -Fe -Mn up-regulated by +Zn | – | López-Millán et al., 2004 |
| MtZIP7 | Leaf | – | Unaffected by -Mn | – | López-Millán et al., 2004 |
| PtIRT1 | Root, leaf | – | Down-regulated by -Mn | Fe, Zn | Fu et al., 2017 |
+ excess, − deficiency, * in yeast cells, ** only under +Fe, *** only under +Zn.
Functions of Manganese in Plants
Mn plays a role in diverse processes of a plant’s life cycle such as photosynthesis, respiration, scavenging of reactive oxygen species (ROS), pathogen defense, and hormone signaling. In Arabidopsis, 398 enzymes are predicted to contain Mn in the metal-binding site (The UniProt Knowledgebase1). Among them, 20% showed an experimental evidence to require Mn as a cofactor. In many enzymes, Mn is interchangeable with other divalent cations such as Ca, cobalt (Co), copper (Cu), Mg, or Zn. In plants, only the OEC in PSII, MnSOD, and oxalate oxidase have been shown to require exclusively Mn.
The most-well-studied function in plant metabolism that depends on Mn is the water-splitting reaction in PSII, which is the first step of photosynthesis. This process requires the tetra-Mn cluster Mn4O5Ca to split two water molecules into four electrons, four protons, and molecular O2 (Bricker et al., 2012). However, the delivery of Mn2+ and Ca2+ to the OEC reaction center in land plants is still under investigation. In the unicellular photosynthetic organism Synechocystis, PratA functions as an assembly factor and chaperone protein for efficient delivery of Mn to the PSII reaction core (Stengel et al., 2012). In green plants, it is speculated that the extrinsic protein PsbP, the closest homolog to PratA, acts as a Mn carrier protein to introduce Mn into the OEC reaction center, where it subsequently stabilizes the Mn cluster in association with PsbO and PsbQ (Bondarava et al., 2007).
Another process in plants dependent on Mn is the detoxification of ROS. In plant cells, ROS are formed mainly in chloroplasts, mitochondria, peroxisomes, and in the cytosol. Mn is a cofactor of MnSODs located in mitochondria as also in peroxisomes (Bowler et al., 1994; Corpas et al., 2017). In addition, oxalate oxidase is a Mn-dependent enzyme which catalyzes the oxidation of oxalate to CO2 coupled with a reduction of O2 to H2O2 (Requena and Bornemann, 1999). Oxalate oxidase is located in the apoplast, where it is involved in pathogen defense likely by the generation of microcidal concentrations of H2O2 and by the formation of effective barriers against pathogen penetration by H2O2-mediated lignification (Lane, 2002). Oxalate oxidase activity has been identified mainly in monocot plant species including wheat, barley, and rice (Svedruzic et al., 2005). Interestingly, germin-like proteins (GLPs) in Arabidopsis, which are homologous to oxalate oxidase, showed neither oxalate oxidase activity nor a Mn-dependent activation (Membré et al., 2000; Li et al., 2016). Intriguingly, the Mn2+ cation itself may act as an antioxidant molecule. In fact, it has been shown in yeast that elevated intracellular Mn was associated with a reduction of oxidative damage in yeast cells (Reddi et al., 2009). It is likely that Mn-phosphate (Mn-P) complexes act as antioxidants whereby the Mn speciation is altered by changes in phosphate concentrations (McNaughton et al., 2010).
Furthermore, Mn is an important cofactor of enzymes involved in isoprenoid biosynthesis (Wilkinson and Ohki, 1988; Köllner et al., 2008). Mn and Mg are two major cofactors of terpene synthases (Rohdich et al., 2006; Köllner et al., 2008). Therefore, it has been proposed that different patterns of plant terpene profiles are often closely correlated with Mg/Mn ratios rather than with concentrations of each cofactor element alone in the growth media (Farzadfar et al., 2017). Beside this, Mn is involved in lignin biosynthesis at two different levels: (i) Mn, besides Mg, can serve as cofactor of the phenylalanine ammonia-lyase (PAL) (Engelsma, 1972), a key enzyme in the phenylpropanoid pathway to produce monolignols; (ii) Lignin can be synthesized from monolignols that are oxidized by Mn3+ into monolignol radicals, which can then be added to existing phenolic groups to form lignin polymers (Önnerud et al., 2002).
Intriguingly, it has been shown that Mn can replace Mg in the active site of some enzymes of the photosynthetic pathway, including Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), changing the functional role of these enzymes (Bloom and Lancaster, 2018; Bloom, 2019). Furthermore, in vitro experiments demonstrated that Mn is an important cofactor in abscisic acid (ABA) and auxin signaling by activating PP2C phosphatases and IAA-amino acid conjugate hydrolases, respectively (LeClere et al., 2002; Meinhard et al., 2002; Schweighofer et al., 2004). Since diverse Golgi-localized glycosyl transferases require Mn for their activity, Mn is also essential for protein glycosylation and the biosynthesis of pectin and hemicellulose polymers (Egelund et al., 2006; Strasser et al., 2007; Basu et al., 2015). Other enzymes that require Mn as a cofactor are purple acid phosphatases (PAPs) (Schenk et al., 1999; Venkidasamy et al., 2019). Also, different decarboxylases (e.g., NAD malic enzyme) and dehydrogenases (e.g., phosphoenolpyruvate carboxylase) of the tricarboxylic acid cycle can be activated by Mn, but in many cases Mn can be replaced by Mg (Burnell, 1988; Gregory et al., 2009).
Another Mn-dependent process lies in the deposition of cuticular waxes in leaves. In barley, it has been demonstrated that Mn deficiency may decrease the cuticular wax layer, which leads to a higher transpiration rate (Hebbern et al., 2009). Moreover, Mn deficiency caused a decrease of cuticular waxes in Arabidopsis leaves (Alejandro et al., 2017).
In addition, Mn plays a role in such diverse processes as chloroplast development (Rohdich et al., 2000; Hsieh et al., 2008), purine and urea catabolism (Werner et al., 2008; Cao et al., 2010), phospholipid biosynthesis (Collin et al., 1999; Nowicki et al., 2005), Ca2+ signaling (Kim et al., 2003; Hashimoto et al., 2012), DNA repair (Takahashi et al., 2007; Szurmak et al., 2008), or histidine biosynthesis (Glynn et al., 2005).
Manganese Deficiency and Toxicity
In plants, Mn deficiency often occurs as a latent disorder, without clear visual symptoms. Thus, the magnitude to which Mn deficiency affects crop yield is difficult to quantify. The critical concentration for Mn deficiency is generally below 10–20 mg.kg–1 dry weight (Broadley et al., 2012). One of the consequences of Mn deficiency in plants is an impaired growth, leading to a decrease in biomass (Longnecker et al., 1991; Hebbern et al., 2005; Pedas et al., 2005). This can be caused by lower numbers of chloroplasts, lower net photosynthetic efficiency, and a decrease in chlorophyll content (Henriques, 2004; Hebbern et al., 2009; Alejandro et al., 2017), as well as higher susceptibility to pathogen infections (Heckman et al., 2003; Heine et al., 2011), imbalance in plant water relations (Ohki, 1985; Hebbern et al., 2009), and decreased tolerance to low temperatures (Ihnatowicz et al., 2014; Stoltz and Wallenhammar, 2014). Mn deficiency leads to a reduced number of Mn-complexes in the PSII core, which causes the destabilization and disintegration of PSII complexes that lowers the net photosynthesis rate (Schmidt et al., 2016). Besides, the disintegration of PSII complexes directly affects the thylakoid structure and promotes chlorophyll degradation leading to the development of characteristic interveinal leaf chlorosis (Papadakis et al., 2007). Due to the low phloem mobility of Mn (Ferrandon and Chamel, 1988; Li et al., 2017), typical symptoms of Mn deficiency first develop in younger leaves. Pale mottled leaves and interveinal chlorosis are the most visible symptoms of the disorder (Schmidt et al., 2016). Under severe Mn deficiency, leaves may also develop gray speck symptoms, which are characterized by brownish or necrotic spots (Broadley et al., 2012). It has been proposed that necrotic spots are a consequence of an increase in free oxygen radicals in damaged chloroplasts and a decrease in MnSOD activity (Broadley et al., 2012; Hajiboland, 2012). Similar responses have been described in Chlamydomonas, where a decrease in photosynthesis and mitochondrial MnSOD function was observed under Mn-deficient conditions (Allen et al., 2007). However, Mn-starved Arabidopsis seedlings showed a decrease in net photosynthesis while there was no loss of MnSOD activity (Lanquar et al., 2010). This suggests that the use of the cellular Mn pool for Mn-requiring metabolic reactions under low Mn conditions is variable among photosynthetic organisms.
In roots, an increase in the frequency of root hairs can be observed under Mn deficiency (Yang et al., 2008). If the deficiency becomes more severe, root tips may develop serious necrosis (Yamaji et al., 2013).
Toxic Mn concentrations are highly dependent on plant species and genotypes (Husted et al., 2009; Broadley et al., 2012; Fernando and Lynch, 2015). Excess Mn may be stored in vacuoles (Dučić and Polle, 2007; Dou et al., 2009), cell walls (Führs et al., 2010), and distributed to different leaf tissues (Fernando et al., 2006a, b). Also, Mn can be chelated in Mn-P complexes in trichomes (McNear and Küpper, 2014; Blamey et al., 2015) and complexed by organic acids in leaves (Lambers et al., 2015). Therefore, it is likely that differences between plant species lie in the cellular distribution and speciation of Mn, dominated by complexes with malate or citrate (Fernando et al., 2010). At the molecular level, excessive Mn can prevent the uptake and translocation of other essential elements such as Ca, Mg, Fe, and P (Alam et al., 2005; St. Clair and Lynch, 2005; Blamey et al., 2015; Lešková et al., 2017), inhibit chlorophyll biosynthesis (Clairmont et al., 1986; Subrahmanyam and Rathore, 2001), cause a decline in the photosynthetic rate (Nable et al., 1988; Amao and Ohashi, 2008), reduce the meristematic cell division in roots by inhibiting auxin biosynthesis (Morgan et al., 1966; Zhao et al., 2017), and lead to an increase in the accumulation of oxidized Mn and oxidized phenolic compounds in the apoplast (Fecht-Christoffers et al., 2006).
The symptoms of Mn toxicity vary widely among plant species, with chlorotic leaves and necrotic spots as the most common symptoms (Millaleo et al., 2010). Accordingly, Mn stress in plants has been explained by two main hypotheses based on either symplastic or apoplastic processes (Fernando and Lynch, 2015). The symplastic hypothesis proposes that Mn toxicity acts via photo-oxidative stress in the chloroplast that causes chlorosis. Conversely, in the apoplastic hypothesis, Mn stress damage is mainly due to the accumulation of Mn oxides, oxidized phenolic compounds, and ROS, in the cell wall, leading to necrosis. Necrotic spots have thus been associated with the accumulation and oxidation of Mn and of oxidized phenolic compounds, while chlorosis has been often attributed to Fe deficiency induced by high Mn. Surprisingly, necrotic spots are high in Mn and appear first on older leaves, whereas in chlorotic areas no Mn accumulation can be observed. It is hence conceivable that the mechanism resulting in necrotic spots differs from that which causes chlorosis.
Several lines of evidence suggest that the early target of Mn toxicity is the photosynthetic process (Millaleo et al., 2010). In fact, plants exposed to Mn excess showed a decline in net photosynthesis rate and chlorophyll content (Li et al., 2010). Although in chloroplasts the occurrence of thylakoid swelling has been associated with the administration of excess Mn (Lidon et al., 2004; Doncheva et al., 2009), the target of Mn in these photosynthetic membranes is still unclear. It has been proposed that the oxidation of Mn in chloroplasts by light-activated chlorophyll generates ROS, and thereby damages chlorophyll (Panda et al., 1987; Baldisserotto et al., 2007). At the same time, Mn may substitute Mg in chlorophyll molecules or bind to ferredoxin in the thylakoid matrix, eventually destroying the ultrastructure of chloroplasts (Panda et al., 1987; Hauck et al., 2003). Moreover, the lack of physiologically active Fe would be a secondary effect of Mn toxicity (Nian, 1989; Huang et al., 2016), which might block chlorophyll synthesis and the correct assembly of photosystem I (PSI) (Chereskin and Castelfranco, 1982; Cornah et al., 2002; Millaleo et al., 2013). Consequently, photoinhibition of PSII would likely be a late side effect of Mn exposure.
Manganese Dynamics in Soil
Mn is one of the most abundant and widely distributed metals in nature and comprises about 0.1% of the Earth’s crust (Emsley, 2003). The element is found in minerals, combined with other elements such as oxygen, sulfur, carbon, silicon, and chlorine (Turekian and Wedepohl, 1961). Mn can exist in 11 oxidation states, ranging from –3 to +7, but in soils, Mn is mainly present as +2 (e.g., Mn2+), +3 (e.g., Mn2O3) and +4 (e.g., MnO2). Availability of Mn to plants depends on its oxidation state: Mn2+ is the only plant-available form and can be readily transported into root cells and translocated to the shoot, whereas the oxidized species Mn(III) and Mn(IV) form insoluble oxides that rapidly sediment (Stumm and Morgan, 1996). Thus, Mn dynamics in soils are mostly represented by the concept of a balance between soluble Mn2+ and insoluble Mn oxides (MnOx).
Mn deficiency in plants is particularly prevalent in alkaline soils, in which the oxidization of Mn2+ to unavailable MnOx is favored. Such soils are common in the northern part of Europe, the UK, USA, China, and in southern Australia (Husted et al., 2009; George et al., 2014). In addition to pH, the oxygen level (pO2) in soil and soil microorganisms are also relevant factors of Mn dynamics in soils. Beside this, root exudates can modify the Mn availability in the rhizosphere. The Mn redox status in soils involves primarily the competition of soluble highly mobile oxidants, such as molecular O2, and soluble reducing organic molecules, derived from soil organic matter and biological sources. In aerobic soils, due to the high mobility and redox potential of O2, Mn oxidation is favored rather than reduction of MnOx by microorganisms (Lovely, 1995). By contrast, waterlogging causes a reduction of MnOx most likely by decreasing the O2 concentration, leading to an increase of plant-available Mn2+ in soil solution up to toxic levels (Khabaz-Saberi et al., 2006).
However, it has been proposed that the oxidation of Mn2+ in environments with abundant O2 is sluggish, particularly in the absence of biological catalysts, and that the oxidation of Mn2+ in soil is carried out predominantly by microorganisms (Sparrow and Uren, 2014). Indeed, microorganisms can catalyze Mn2+ oxidation in order of 103 times faster than abiotic oxidation (Morgan, 2005). Some microorganisms produce enzymes which directly oxidize Mn2+, or produce extracellular superoxide leading to the production of insoluble MnOx (Hansel et al., 2012; Zhang et al., 2014). Conversely, other microorganisms can reduce MnOx and thereby increase Mn availability for plant uptake (Rengel, 2015). Also, environmental conditions (temperature, humidity) affect soil Mn availability by modulating the activity of Mn-oxidizing microorganisms (Sparrow and Uren, 2014). Therefore, the population of Mn-oxidizing and Mn-reducing microorganisms is a key factor in the availability of soil Mn for plant uptake.
Another important factor of Mn dynamics in soils is the exudation of protons (H+), carboxylates, and enzymes by plant roots. Proton exudation increases the Mn availability in the rhizosphere by exchanging Mn that is immobilized by negatively charged organic matter and clay minerals, and also by lowering the pH of alkaline soils (Rengel, 2015). Mn uptake by some Mn-hyperaccumlators, such as Phytolacca species, is based on a strong rhizosphere acidification (Lambers et al., 2015). Noteworthy, Mn availability is also increased by root exudation of carboxylates which chelate Mn and reduce Mn(IV) to Mn2+ in either acidic or alkaline soils (Jauregui and Reisenauer, 1982; Gherardi and Rengel, 2004).
Phytate (inositol hexaphosphate) is generally the dominant form of organic P in soils and has the potential to complex Mn2+ and other divalent cations. Exudation of phytases, which catalyze the degradation of phytate thus increases the Mn availability by releasing Mn2+ (George et al., 2014).
The release of carboxylates into the rhizosphere is a mechanism for the acquisition of not only Mn, but in particular of P. Carboxylates mobilize P absorbed to Fe/Al hydroxides by ligand exchange, especially under low P availability (Nuruzzaman et al., 2006). By contrast, elevated levels of soil-P lead to a reduction of carboxylate and phytase exudation, in turn decreasing Mn2+ acquisition (Lambers et al., 2015; Giles et al., 2017). However, hydroponic experiments in barley, where most likely no Mn–P complexes were formed, have also shown a decrease in Mn uptake under elevated P supply (Pedas et al., 2011). Hence, it has been proposed that there is a competition between Mn2+ and P during Mn uptake.
Manganese Transport Proteins in Plants
As mentioned above, reduced Mn (Mn2+) is the only available form for plants. To maintain an optimal supply, acquisition from the rhizosphere and distribution of Mn have to be regulated. One of the most important mechanisms to regulate the acquisition from the soil is the uptake by specific transporters into root cells (Figure 1). Once Mn reaches the symplast, the main pathways for the translocation and distribution of Mn in the whole plant involve transport toward and into the xylem, transfer to the phloem, and translocation to and between the different tissues. However, most of the Mn transporters and mechanisms required for Mn loading into the xylem of the root stele, for loading Mn into the phloem, and for Mn transport into the plant cell in shoots have not been identified yet. Interestingly, the mobility of Mn in the phloem is supposed to be low (Li et al., 2017), but there is evidence that a small amount of Mn may be recycled via the phloem. In fact, it has been reported that after application of 52Mn to a cut barley leaf, radioactivity was detectable in the discrimination center of the shoot, in other leaves, as also in root tips (Tsukamoto et al., 2006). These results suggested that 52Mn was transported by unknown transporters with low affinity.
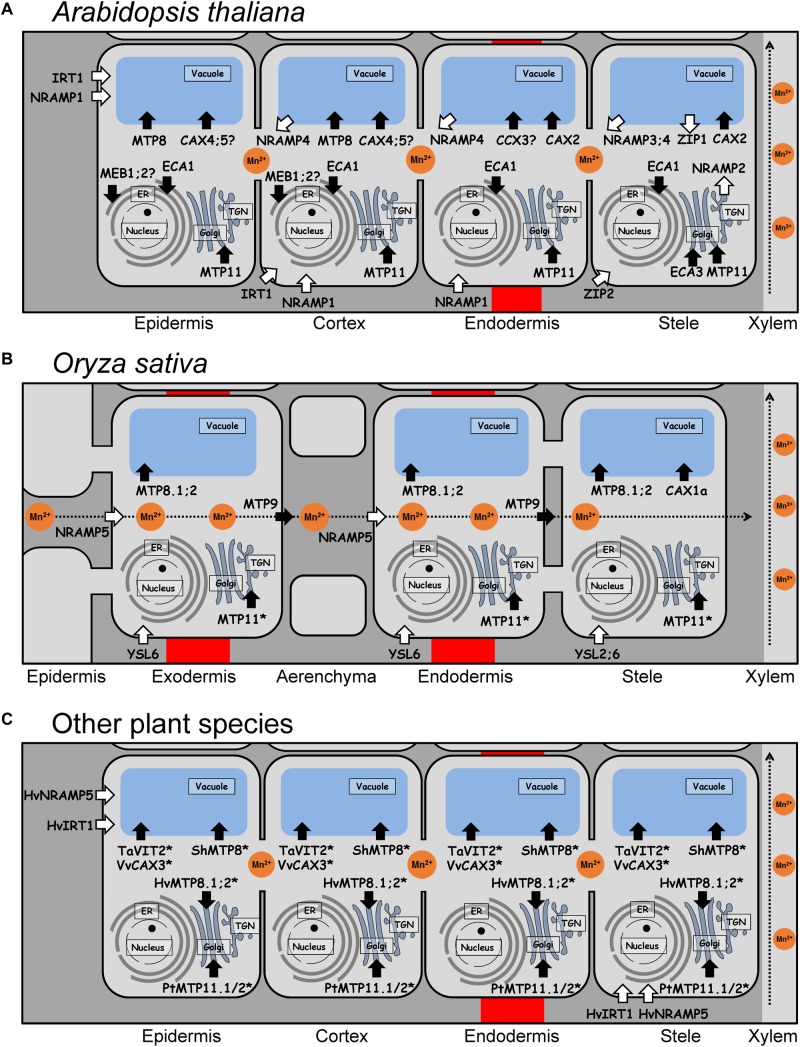
FIGURE 1.
Tissue specificity and subcellular localization of Mn transport proteins in roots of different plant species. (A) Mn transport proteins in epidermis, endodermis, cortex, and stele (including pericycle) of Arabidopsis roots. (B) Mn transport proteins in exodermis, endodermis, and stele of rice roots. Radial transport of Mn2+ is carried out by OsNRAMP5 and OsMTP9, which are polarly localized transporters at both the exodermis and the endodermis, providing a unidirectional flux of Mn from the soil to the stele (indicated as dashed arrow). (C) Mn transport proteins in epidermis, endodermis, cortex, and stele of roots of other plant species. (A–C) White arrows indicate import into the cytosol, black arrows indicate export out of the cytosol. Transport proteins with yet unknown root tissue specificity are marked by asterisks. Proteins which subcellular localization only shown in yeast but not in plants are indicated by a question mark. Hv, Hordeum vulgare; Pt, Populus trichocarpa; Sh, Stylosanthes hamata; Ta, Triticum aestivum; Vv, Vitis vinifera.
An intriguing property of Mn transport across membranes is that most of the proteins that transport Mn are not specific for the metal. Plant Mn2+ transporters can transport, to varying extent, other divalent cations, such as Fe2+, Zn2+, Cu2+, Cd2+, Ca2+, Co2+, and Ni2+. The physiological relevance of this low specificity needs further investigations, including the modification of specificity. For instance, in the Mn/Fe transporter AtMTP8, Fe transport activity was inhibited by introducing point mutations in different Fe-binding domains without affecting its Mn2+ transport capability (Chu et al., 2017).
Diverse families of transport proteins are known to be components of the Mn homeostatic network in plants and can be classified into importers and exporters. Importers translocate Mn from the extracellular space or from internal compartments into the cytosol, whereas exporters are responsible for the exclusion of Mn out of the cytosol into intracellular compartments or into the apoplast. The Natural Resistance Associated Macrophage Protein (NRAMP) family, the Zinc-Regulated Transporter/Iron-Regulated Transporter (ZRT/IRT)-related Protein (ZIP) family, and the Yellow Stripe-Like (YSL) family have members involved in the transport of Mn2+ into the cytosol. In contrast, the Cation Diffusion Facilitator/Metal Transport Protein (CDF/MTP) family, the Vacuolar Iron Transporter (VIT) family, the Ca2+/Cation Antiporter (CaCA) superfamily, the Bivalent Cation Transporter (BICAT) family, and the P2A-type ATPase family have members involved in the transport of Mn2+ out of the cytosol. Figure 1 shows the tissue specificity and subcellular localization of Mn transport proteins in roots of Arabidopsis, rice, and other plant species. Genes encoding encoding Mn transport proteins in aerial parts of the plant species are depicted in Figure 2, and the subcellular localization of these proteins is displayed in Figure 3. The NRAMP family has been characterized in a number of species including bacteria, fungi, plants, and animals. These proteins act as metal/H+ symporters and are capable of transporting divalent metal cations (Fe2+, Mn2+, Zn2+, Cd2+, Co2+, Ni2+, Cu2+) into the cytosol (Nevo and Nelson, 2006), with the exceptions of OsNRAMP4 (syn. OsNrat1), that transports the trivalent cation Al3+ (Xia et al., 2010; Li et al., 2014), and OsNRAMP1, that appears to mediate also As(III) transport (Tiwari et al., 2014).
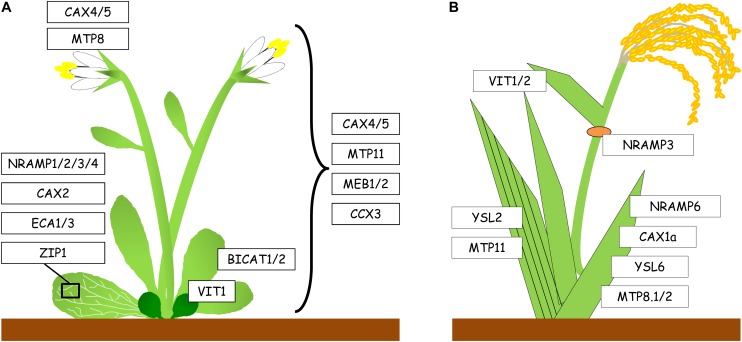
FIGURE 2.
Expression of genes encoding Mn transport proteins in aerial parts of Arabidopsis and rice. (A) Mn transport proteins in shoots of Arabidopsis. Transporters listed on the left are expressed in the vasculature of leaves, transporters listed on the right are expressed throughout the above-ground tissues of the plant. In vegetative tissues, AtVIT1 is only expressed in cotyledons. (B) Mn transport proteins in shoots of rice. OsMTP11 and OsYSL2 are expressed in the vasculature. OsNRAMP3 is expressed in the first node (orange). OsYSL6 and OsMTP8.1/2 are expressed in older leaves, whereas expression of OsVIT1/2 is more pronounced in younger leaves.
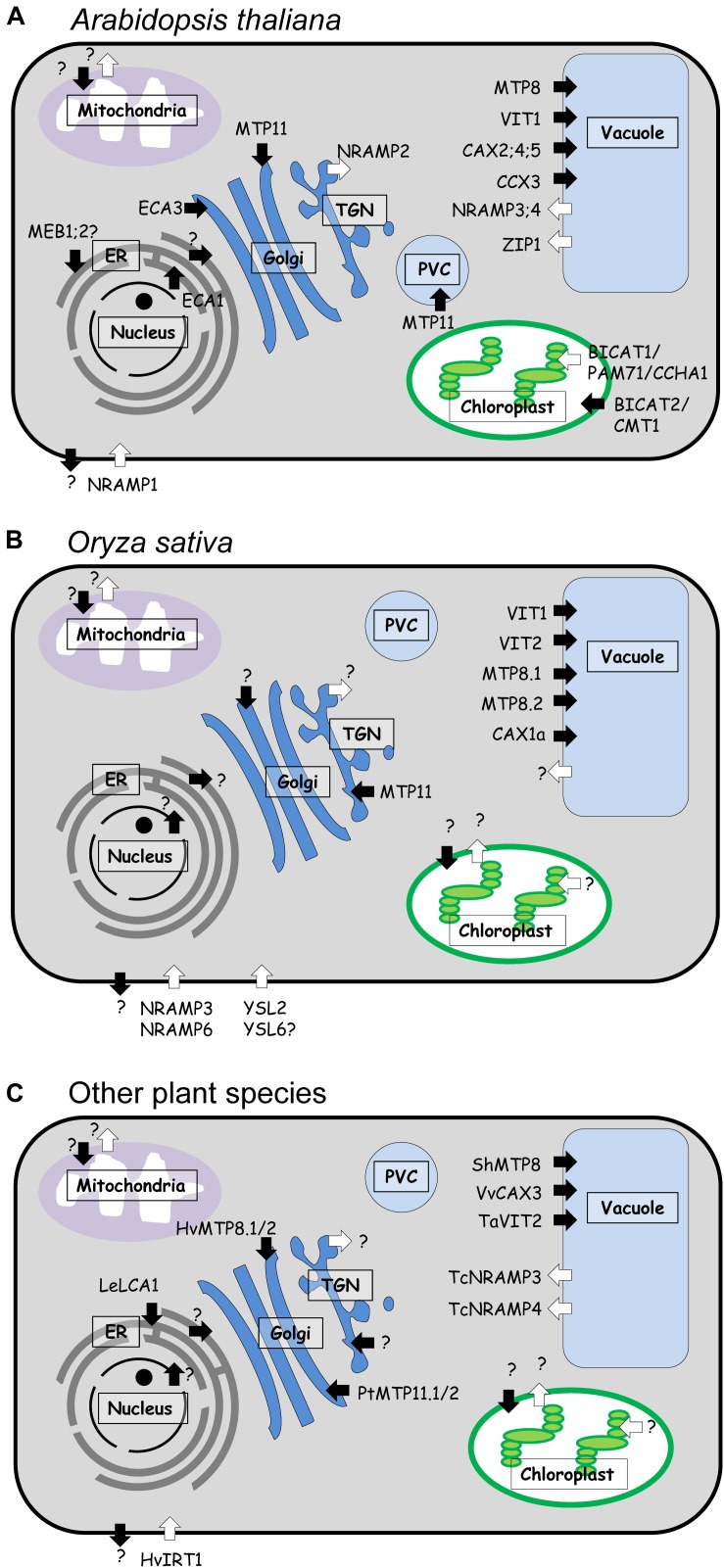
FIGURE 3.
Subcellular localization of Mn transport proteins in aerial parts of different plant species. (A) Arabidopsis, (B) rice, and (C) other species. White arrows represent import into the cytosol, black arrows represent export from the cytosol. Transport pathways with uncharacterized Mn transporters are indicated by a question mark. Hv, Hordeum vulgare; Le, Lycopersicum esculentum (syn. Solanum lycopersicum); Pt, Populus trichocarpa; Sh, Stylosanthes hamata; Ta, Triticum aestivum; Vv, Vitis vinifera.
The ZIP transporters have been found widely in bacteria, fungi, plants, and animals and are predicted to be involved in Fe2+, Zn2+, Cd2+, Co2+, Cu2+, and Mn2+ transport. They have 8 transmembrane domains (TMD) with extracellular N- and C-termini and a cytosolic histidine-rich loop (Guerinot, 2000). YSL transporters are related to the OligoPeptide Transporter (OPT) family and are exclusively found in plants, bacteria, fungi, and archaea. Members of the YSL family are predicted to transport metals (Mn2+, Zn2+, Cu2+, Ni2+, Cd2+, Fe2+) complexed to non-proteinogenic amino acids, such as nicotianamine (NA) or phytosiderophores (PS) (Schaaf et al., 2004).
The CDF family has members in all organisms. Most CDFs are Metal2+/H+(K+) antiporters and mediate the efflux of Zn2+, Co2+, Fe2+, Cd2+, Ni2+, and/or Mn2+. Most of them have six TMDs with histidine-rich regions at their cytosolic N- and/or C-terminus and additionally between the 4th and 5th TMD. Based on phylogenetic relationships, the CDF family can be classified, corresponding to the main transported metal, into three major subgroups: Zn-CDFs, Fe/Zn-CDFs, and Mn-CDFs (Montanini et al., 2007).
BICAT proteins, also denominated Photosynthesis-Affected Mutant71 (PAM71) or Chloroplast Manganese Transporter (CMT) proteins, belong to the Uncharacterized Protein Family 0016 (UPF0016), which represents a new family of cation transporters present in all eukaryotes and prokaryotes, except in Lactobacillales and Bacillales (Demaegd et al., 2013, 2014; Hoecker et al., 2017). The protein structure of the plant homologs is characterized by two clusters of three transmembrane domains separated by a central loop. BICAT proteins act as Mn2+ and Ca2+ transporters (Hoecker et al., 2017; Frank et al., 2019).
CaCA transporters are present in all kingdoms of life and have a conserved core structure of ten transmembrane domains and two conserved α-repeat regions containing acidic amino acids (Pittman and Hirschi, 2016). In particular, the Cation/Ca2+ exchanger (CCX) and the H+/Cation exchanger (CAX) families, which are both members of the CaCA superfamily, are present within all plants.
VIT transporters are found in plants, fungi, and bacteria, but are absent in animals. Members of the VIT family in plants share a high degree of sequence similarity and most of them are capable to transport Fe2+ in addition to Mn2+, but their biological functions have been poorly investigated (Cao, 2019).
Plant P-type Ca2+-ATPases are divided into two groups, 2A and 2B, whereby the first one does not contain an N-terminal autoinhibitory domain (Johnson et al., 2009). Those P2A-type Ca2+-ATPases have also a role in Mn2+ transport.
Manganese Uptake From the Soil and Translocation to the Shoot
Despite the importance of Mn in plant physiology, our knowledge of systems mediating Mn uptake followed by translocation from the roots to the shoot is limited. This is mainly due to the lack of information about the expression and subcellular localization of Mn transporters in most plants. In fact, only few Mn transporters involved in uptake and root radial transport have been identified so far. Uptake of Mn2+ has been assumed to be mediated by plasma membrane Ca2+ channels, which are generally permeable to Mn2+ (Wymer et al., 1997; White et al., 2002). However, this is likely to be a minor Mn2+ uptake pathway due to its competition with Ca2+, only relevant when Mn2+ is present in high concentrations. However, in soil, Mn2+ is usually far less abundant than Ca2+ (Broadley et al., 2012). Since this assumption, few Mn transporters have been identified mainly in A. thaliana and rice. Their tissue-specific localizations in roots are shown in Figure 1A for Arabidopsis, in Figure 1B for rice, and in Figure 1C for other plant species including barley, wheat, tomato, poplar, and grapevine.
In Arabidopsis, there is plenty of evidence that Mn2+ uptake is mainly mediated by AtNRAMP1 (Figure 1A), which is localized in the plasma membrane of epidermis and cortex cells in roots, and less in vascular tissues of young leaves (Cailliatte et al., 2010; Castaings et al., 2016). Under Mn-deficient conditions, the expression of AtNRAMP1 is moderately up-regulated, and nramp1 knockout mutants accumulate less Mn in shoots under Mn deficiency, which points to a function of this protein as a high-affinity Mn2+ uptake transporter (Cailliatte et al., 2010). NRAMP1 orthologs in cacao, rapeseed, and peanut are also expressed in roots and complement a Mn uptake-deficient yeast strain, smf1, lacking a Mn transporter located in the plasma membrane (Meng et al., 2017; Ullah et al., 2018; Wang et al., 2019). In addition, active Mn2+ uptake may be accomplished by the ZIP transporter AtIRT1 (Castaings et al., 2016), that is considered as the major Fe uptake transporter of dicots. However, AtIRT1 is not strongly selective for Fe2+, but also transports Zn2+, Cu2+, Co2+, Ni2+, and Mn2+ (Korshunova et al., 1999). IRT1 orthologs expressed in roots have also been identified in tomato and trifoliate orange, but their function in Mn2+ uptake remains to be confirmed (Eckhardt et al., 2001; Fu et al., 2017). Furthermore, two ZIP transporters expressed in the root stele are described to be involved in root-to-shoot translocation of Mn in Arabidopsis (Milner et al., 2013). AtZIP1 is a transporter localized to the tonoplast and probably involved in remobilizing Mn2+ from vacuoles to the cytosol in root stele cells, whereas AtZIP2 is localized to the plasma membrane and may mediate Mn2+ uptake into cells of the root stele. Other Mn2+ transporters of the AtZIP family are also expressed in roots, but their function in Mn2+ uptake and remobilization is still unknown (Milner et al., 2013).
In comparison with other plant species, in rice, transporters involved in uptake, xylem loading, and root-to-shoot translocation of Mn have been functionally characterized more extensively (Shao et al., 2017). The first Mn2+ transporter identified in rice roots was OsNRAMP5, which contributes to Mn2+ uptake and translocation (Sasaki et al., 2012). Further investigations showed that Mn2+ uptake and translocation works in conjunction with another transporter next to OsNRAMP5, namely OsMTP9 (Ueno et al., 2015). OsNRAMP5, localized to the plasma membrane of the distal side of the exo- and endodermis was shown to be responsible for the import of Mn2+ from the soil solution (Figure 1B). Subsequently, OsMTP9, localized to the plasma membrane of the proximal side of these cell layers, mediates the export of Mn2+ into the stele (Sasaki et al., 2012; Ueno et al., 2015). Knockout of either OsMTP9 or OsNRAMP5 significantly decreased Mn2+ uptake and root-to-shoot translocation, indicating that both transporters are responsible for transporting Mn2+ from the soil to the xylem.
Interestingly, in barley, the plasma membrane transporter HvIRT1 is implicated in the uptake and translocation of Mn2+, but not Fe2+ (Long et al., 2018). HvIRT1 is constitutively expressed in cells of the epidermis and in pericycle funder cells (Figure 1C), but under Mn deficiency, its expression is extended to the entire pericycle and the cortex. In hvirt1 RNAi lines, a reduced shoot Mn concentration was observed without changes in Fe or Zn concentrations. Barley is characterized as a strategy II plant species that requires HvYSL transporters for the uptake of Fe3+ complexed with phytosiderophores (PS) (Araki et al., 2011). Therefore, the Fe transport function of HvIRT1 has become redundant because Fe is acquired via strategy II processes. Although HvIRT1 may transport Fe in yeast (Pedas et al., 2008), HvIRT1 plays actually a key role in uptake and root-to-shoot translocation of Mn rather than Fe. HvNRAMP5 is another Mn transporter localized to the plasma membranes of cells in root tips of barley (Wu et al., 2016). In contrast to HvIRT1, its expression is slightly upregulated by Fe deficiency, but not by Mn deficiency. Since HvIRT1 is up-regulated by Mn deficiency and HvNRAMP5 is constitutively expressed in roots (Wu et al., 2016), both transporters may play different roles. Therefore, it has been suggested that HvNRAMP5 may confer constitutive Mn2+ uptake, while HvIRT1 plays a role in Mn2+ uptake only under Mn-deficient conditions (Wu et al., 2016). The barley transporter HvYSL2 appears to mediate the transport of PS complexes with metals, including Mn, in the endodermis, and it may thus be involved in the transport of minerals from the cortex to the pericycle (Araki et al., 2011). However, further analyses of HvYSL2 are required to fully understand its biological role in barley.
Once Mn has been absorbed by the root, it needs to be translocated to the shoot. To date, the molecular basis of xylem loading of Mn is still unclear, and there is no clear evidence of which Mn complex is required to translocate Mn to the shoot via the xylem. Nevertheless, transporters of the YSL family may be involved in Mn translocation. In fact, during senescence, Mn concentration decreased in Arabidopsis wild type leaves, whereas no change was observed in the ysl1ysl3 double mutant (Waters et al., 2006). Hence, it has been proposed that both AtYSL1 and AtYSL3 are putative Mn-nicotianamine (Mn2+-NA) transporters, but so far, there is no direct evidence supporting this hypothesis. In rice, Mn2+ is transferred by OsNRAMP3 from the xylem to the phloem at the basal node, followed by its distribution to young leaves, panicles and root tips (Yamaji et al., 2013). However, at high Mn availability, Mn is distributed to mature tissues. Therefore, in rice nodes OsNRAMP3 functions as a switch for Mn distribution, whereby the protein is activated or deactivated in response to fluctuating Mn concentrations (Figure 2B). Moreover, a rice YSL transporter, OsYSL2, was proposed to be implicated in long-distance transport and distribution of Mn, since it may transport Mn2+-NA, as well as Fe2+-NA complexes (Koike et al., 2004). Because OsYSL2 is localized in phloem companion cells, it is probably involved in phloem loading of Mn2+-NA (Ishimaru et al., 2010), although its exact role in rice needs to be further investigated.
Manganese Uptake by Leaf Cells
As described above, a number of plasma membrane transporters involved in Mn2+ import into the cytosol of root cells has been characterized. In contrast, the identity of Mn2+ transporters in the plasma membrane of leaf cells remains largely elusive. Recently, it has been reported that OsNRAMP6, which is expressed in shoots, is localized to the plasma membrane (Figure 3B) and functions as Fe and Mn2+ transporter when expressed in yeast (Peris-Peris et al., 2017). Interestingly, OsNRAMP6 rescued, to some extent, the growth of yeast strains mutated in SMF1 (plasma membrane Mn2+ transporter) or SMF2 (Mn2+ transporter in intracellular vesicles) under Mn-limited conditions. In plants, OsNRAMP6 accumulated in vesicles in the vicinity of the plasma membrane. Whether these vesicles represent an anterograde or retrograde trafficking stage of OsNRAMP6, depending on the Mn2+ status, remains to be elucidated. Moreover, an OsNRAMP6 knock-out plant showed enhanced resistance to infection by the rice fungus M. oryzae and a reduced biomass compared to wild type plants (Peris-Peris et al., 2017). Therefore, it is likely that OsNRAMP6 plays a role in regulating the Mn and/or Fe contents in infected tissues which would boost the expression of defense genes. Based on the decreased root and shoot biomass of the nramp6 mutant under non-stress conditions, it was hypothesized that OsNRAMP6 functions as a Mn uptake transporter. Future studies are needed to establish the importance of OsNRAMP6 in cellular Mn2+ uptake and plant growth.
A second mechanism for Mn uptake into leaf cells of rice may be conferred by the OsYSL6 transporter, which was described to transport Mn2+-NA complexes from the leaf apoplast to the symplast (Sasaki et al., 2011). OsYSL6 is expressed in roots and shoots, particularly in older leaves. Due to the ability of OsYSL6 to increase Mn2+ influx when expressed in the smf1 yeast mutant, it is likely localized in the plasma membrane (Sasaki et al., 2011). ysl6 mutant plants accumulate high Mn concentrations in the apoplast of shoots and exhibit symptoms of Mn toxicity. Therefore, it is likely that OsYSL6 translocates Mn2+ from the apoplast to the symplast where it is sequestered under Mn excess. However, since its expression level remains unchanged under either Mn deficiency or excess, OsYSL6 may also act as a constitutive Mn importer in leaf cells.
Intracellular Transport of Manganese
Once Mn has entered a plant cell, it must be moved to the appropriate location for the adequate supply of Mn-dependent targets or for storage. Mn is present in all cellular compartments, including ER, Golgi apparatus, mitochondria, plastids, and peroxisomes, where it performs specific cellular functions (see section Functions of Manganese in Plants), whereas vacuoles can serve as a reservoir to regulate cellular Mn homeostasis. Consequently, cells contain a plethora of transporters that are responsible for the distribution of Mn to the different compartments. Figure 3 shows an overview of Mn transporters previously described in different plant species and their subcellular localization. To structure the description of these Mn transporters in the following sections, they are organized based on their demonstrated or putative subcellular localization.
Vacuoles as Manganese Stores
Vacuoles generally function as a primary compartment for metal internalization to avoid metal toxicity. In the case of Mn, vacuoles also serve as a temporal Mn storage pool for a proper distribution to other organelles, e.g., chloroplasts (Lanquar et al., 2010). Many transporters have been described to contribute to vacuolar Mn sequestration and unloading processes.
In the tropical legume Stylosanthes hamata, a plant tolerant to acidic soils in which high concentrations of plant-available Mn2+ can occur, the tonoplast-localized Mn transporter ShMTP8 was identified (Delhaize et al., 2003). This protein was the first characterized member of the Mn-CDF subgroup and is involved in Mn detoxification by sequestering Mn into vacuoles. Sequence analysis of ShMTP8 showed that this protein lacks the complete N-terminal sequence and the histidine-rich loop common for members of the CDF family. When expressed in Arabidopsis, ShMTP8 conferred tolerance to Mn toxicity (Delhaize et al., 2003). In pear and tobacco, members of the Mn-CDF subclade have also been described as Mn transporters, but their subcellular localization and relevance in Mn homeostasis is unknown (Hou et al., 2019; Liu et al., 2019). In Arabidopsis, the Mn-CDF subclade of the CDF/MTP family has four members, AtMTP8 through AtMTP11 (Montanini et al., 2007). All of them were shown to transport Mn2+ in yeast (Chu et al., 2017), but so far, only AtMTP8 and AtMTP11 have been described in more detail (Figure 3A). AtMTP8 was characterized as Mn2+ and Fe2+ transporter localized in the tonoplast (Eroglu et al., 2016; Chu et al., 2017; Eroglu et al., 2017). AtMTP8 expression was specific to cells of the epidermis and the cortex in roots and strongly induced by Fe deficiency (Eroglu et al., 2016). Moreover, mtp8 mutants showed chlorosis and critically low Fe levels in shoots on media with limited Fe availability, if Mn was present in the medium. This further demonstrated a Mn-specific role of AtMTP8 during Fe limitation, which lies in the detoxification of Mn taken up by the non-specific Fe transporter AtIRT1. In accord with a function of AtMTP8 in Mn detoxification, growth of mtp8 mutants was impaired by high Mn, and AtMTP8 expression was increased under excess Mn2+ supply (Eroglu et al., 2016). Besides its role in the Fe deficiency response, AtMTP8 plays a second role during seed development. An analysis of metal localization in the embryo by μXRF tomography showed that AtMTP8 is responsible for the specific accumulation of Mn in subepidermal cells on the abaxial side of the cotyledons and in cortical cells of the hypocotyl (Chu et al., 2017; Eroglu et al., 2017). In mutant embryos lacking the vacuolar Fe/Mn transporter AtVIT1, AtMTP8 built up Fe hotspots in those AtMTP8-expressing cell types, suggesting that AtMTP8 transports Fe in addition to Mn. This was supported by complementation of the Fe-sensitive yeast strain ccc1. An mtp8vit1 double mutant showed a homogeneous distribution of both metals in all cell types of the embryo, demonstrating that both are the primary transporters determining Mn and Fe allocation (Chu et al., 2017; Eroglu et al., 2017). Mn2+ transport and vacuolar localization were also demonstrated for MTP8 orthologs in rice (Figure 3B). OsMTP8.1 was highly expressed in older leaves, and it was induced and repressed by high and low Mn2+ levels, respectively (Chen et al., 2013). OsMTP8.2 was expressed in roots and shoots, and it showed lower expression levels than OsMTP8.1 (Takemoto et al., 2017). The mtp8.1mtp8.2 double mutant suffered from necrotic leaf blades. Since Mn concentrations were comparable to those in healthy wild type plants, it has been suggested that this phenotype was the result of an insufficient Mn sequestration in the double mutant. Unlike AtMTP8 in Arabidopsis, there is no evidence that OsMTP8.1 and OsMTP8.2 are able to transport Fe or that their transcript levels are increased upon Fe deficiency (Chen et al., 2013; Takemoto et al., 2017).
VIT proteins in plants are mainly Fe transporters (Cao, 2019). AtVIT1, the most-studied VIT transporter in Arabidopsis, was identified as a vacuolar Fe transporter that is responsible for the localization of Fe primarily to the provascular strands of the embryo in seeds (Kim et al., 2006). The ability of AtVIT1 to transport Mn2+ was shown by metal analysis of vacuoles from the Fe-sensitive yeast strain ccc1 expressing AtVIT1 (Kim et al., 2006). Interestingly, seeds of a vit1 mutant showed a localization of Fe that coincided with the localization of Mn in the subepidermal cells on the abaxial side of the cotyledons and that was dependent on AtMTP8, as discussed above (Chu et al., 2017; Eroglu et al., 2017). VIT transporters of rice, OsVIT1 and OsVIT2, not only partially rescued the Fe2+-sensitive phenotype, but also the Zn2+-sensitive phenotypes of yeast mutant strains. Similar to AtVIT1, an analysis of vacuolar metal composition of these cells showed an increased accumulation of Mn (Zhang et al., 2012). Moreover, OsVIT1 and OsVIT2 were shown to be highly expressed in flag leaf blades and leaf sheath. Consistent with these results, decreased accumulation of Fe and Zn was observed in flag leaves of osvit1 and osvit2 mutants. However, both mutants showed no significant change of Mn in these tissues or in grains (Zhang et al., 2012). These results suggest that OsVIT1 and OsVIT2 may function primarily in flag leaves to mediate vacuolar sequestration of Fe and Zn. Moreover, the Fe and Zn accumulation in seeds and the decrease of those metals in flag leaves (the source organ) of osvit1 and osvit2 mutants indicates an indirect involvement of both gene products in translocation of both metals to sink organs, since they are only weakly expressed in embryos, which is in contrast to AtVIT1 (Zhang et al., 2012). Finally, there are two VIT homologs in wheat, which are located to the tonoplast (Figure 3C), but only one of them, namely TaVIT2, was shown to complement a Mn-sensitive yeast strain, pmr1. TaVIT2 was also able to transport Fe2+ and has been employed for biofortification of wheat grains with Fe (Connorton et al., 2017).
Members of the CAX family are metal transporters that mediate efflux of cations into the vacuole (Martinoia et al., 2012). All plant CAX transporters characterized to date appear to be able to transport Ca2+. Nevertheless, a broad metal substrate range, including Mn2+, is a common characteristic of these proteins. Based on amino acid sequences, the plant CAX family is divided into two clusters, type 1-A and 1-B. Their capability to transport Mn2+ is not related to their phylogenetic association, with AtCAX4, OsCAX1a, and VvCAX3 being members of the type 1-A subfamily and AtCAX2, AtCAX5, OsCAX3, and OsCAX4 being members of the type 1-B subfamily (Martins et al., 2017). AtCAX2 transports a range of cations into the vacuole, including Mn2+, Zn2+, and Cd2+ (Koren’kov et al., 2007). Knockout analysis suggested that AtCAX2 does not play a major physiological role in Ca homeostasis, but is more important for vacuolar Mn accumulation (Pittman et al., 2004). However, vacuolar Mn2+ transport was not completely abolished in cax2. AtCAX5 is a likely candidate for this residual activity. AtCAX5 showed a lower Ca2+ and Mn2+ transport activity than AtCAX2 as also a reduced Ca2+ transport capacity (Edmond et al., 2009). Despite the significant sequence similarity and substrate specificity of AtCAX2 and AtCAX5, a clear distinction appeared in their expression pattern and transcriptional regulation. AtCAX5 was expressed in all tissues of Arabidopsis seedlings, particularly in the stem and the root, and at a lower level in the leaf. In addition, there was an increase in AtCAX5 transcripts under conditions of excess Mn, and a reduction in response to excess Zn. In contrast, AtCAX2 was detected at fairly low levels in all tissues, but was not greatly induced by any metal (Edmond et al., 2009). The relevance of these CAX transporters in intracellular Mn homeostasis is unclear. Since only the Arabidopsis cax2 mutant showed a growth defect under Mn deficiency (Connorton et al., 2012), future genetic analysis by using cax2cax5 double mutant plants may uncover the physiological function of these transporters in plants.
CAX2-like transporters of other species, as tomato LeCAX2 and barley HvCAX2, transport Ca2+ and Mn2+ into yeast vacuoles upon heterologous expression, but with different transport kinetics (Edmond et al., 2009). HvCAX2 is expressed ubiquitously in roots, shoot, immature spikes, and in seeds, preferentially in the embryo rather than the endosperm. Transcripts of HvCAX2 increased under excess Ca2+ and Na+. Likewise, LeCAX2 was also expressed in roots and to a higher extend in leaves and fruits (Edmond et al., 2009).
AtCAX4 was expressed at low level in all tissues, and expression increased after Mn2+, Na+, and Ni2+ treatment (Cheng et al., 2002). Specifically, AtCAX4 transcript levels increased in the root apex and lateral root primordia upon Mn2+ and Ni2+ treatment and decreased if Ca2+ was depleted. Mutation of AtCAX4 led to an arrested growth in seedlings under excess Cd2+, Mn2+, and auxin (Mei et al., 2009). Previously, a link between auxin-regulated plant development, cytosolic Ca2+, and kinases was described (Robert and Offringa, 2008). Therefore, these findings suggest that the cax4 mutant may have increased cytosolic Ca2+ levels (because of reduced Ca2+ transport into the vacuole), that cause an impaired auxin gradient and altered root development (Mei et al., 2009). Alternatively, the auxin sensitivity phenotype may be a result of altered Mn homeostasis in cax4 mutants, since Mn has an influence on auxin responses and auxin metabolism (Mei et al., 2009). VvCAX3 is a ubiquitously expressed vacuolar transporter for both, monovalent and divalent cations in grapevine. Expression of VvCAX3 in yeast restored the growth on media with high Na+, Li+, Cu2+, and Mn2+ concentrations (Martins et al., 2017). Interestingly, expression of VvCAX3 decreased during the development in parallel with Ca2+ accumulation in the fruit. It is likely that VvCAX3 is not only involved in Ca2+ sequestration, but also in the detoxification of trace elements and the mitigation of salt stress, since its transcript level increased under NaCl treatment (Martins et al., 2017).
Other members of the type 1-B subfamily of CAX transporters are OsCAX3 and OsCAX4 in rice. OsCAX3 is expressed in all plant tissues, and the protein has a capacity to transport Mn2+. Interestingly, when expressed in yeast, next to Mn tolerance OsCAX3 also conferred Ca2+ tolerance, but to a lower extent compared to other OsCAX transporters. Therefore, OsCAX3, as AtCAX2, might preferentially transport Mn2+, rather than Ca2+, into the vacuole (Kamiya et al., 2005). Nevertheless, future work is needed to confirm its vacuolar localization. By contrast, expression of OsCAX4 provides Mn and Cu tolerance to yeast, and OsCAX4 transcripts were increased upon prolonged salt stress in planta (Yamada et al., 2014). A type 1-A transporter of rice, OsCAX1a, transports Ca2+ and Mn2+ into yeast vacuoles, but is involved mainly in Ca2+ homeostasis in plant cells under high concentrations of Ca2+ (Kamiya et al., 2006). Further analysis of the function of OsCAX1a in planta showed that the transcript level in roots was increased by Ca2+ and decreased by Mn2+, Ni2+, Mg2+, and Cu2+, while shoots showed only an increase of OsCAX1a transcript levels after treatment with Ca2+. The decreased expression of OsCAX1a in response to other divalent cations might be a mechanism to keep high concentrations of cytosolic Ca2+ in order to protect the cell from toxic levels of ions like Mn2+ (Kamiya et al., 2006). Therefore, OsCAX1a may transport Ca2+ into the vacuole under Ca2+ toxicity or regulate Ca2+ homeostasis in the cytosol. In rice, further studies using combined mutants of OsCAX1a, OsCAX3, and OsCAX4 are needed to confirm their relevance in plant Mn2+ and Ca2+ homeostasis.
Members of the CCX family were previously described as transporters of the CAX family. However, because they are phylogenetically closer to the mammalian plasma membrane K+-dependent Na+/Ca2+ exchangers (NCXs), this gene family, which has five members in Arabidopsis (CCX1-5), was re-classified (Pittman and Hirschi, 2016). Expression of AtCCX3 was induced in roots and flowers upon treatment with Mn2+, and in yeast the expression of AtCCX3 complemented strains defective in Mn2+ export (Morris et al., 2008). Tobacco cells expressing AtCCX3 showed enhanced Mn levels, further suggesting an ability of AtCCX3 to transport Mn2+. The transcript level of AtCCX3 increased in plants upon a treatment with Na+, K+, and Mn2+, albeit a mutation in AtCCX3 provoked no discernible growth abnormalities under those conditions (Morris et al., 2008).
The genome of Arabidopsis contains six genes encoding NRAMP transporters, whereby AtNRAMP3 and AtNRAMP4 have been localized to the tonoplast (Thomine et al., 2003; Lanquar et al., 2005). The NRAMP homologs TcNRAMP3 and TcNRAMP4 from Thlaspi caerulescens were also localized to the tonoplast (Oomen et al., 2009). These NRAMP transporters from both species are involved in the transport of Cd2+, Fe2+, and Mn2+ (Thomine et al., 2000; Oomen et al., 2009; Pottier et al., 2015). In addition, AtNRAMP4 and TcNRAMP4 also transport Zn2+ when expressed in yeast (Lanquar et al., 2004; Oomen et al., 2009; Pottier et al., 2015). AtNRAMP3 and AtNRAMP4 protein levels in leaves were unaffected by Mn deficiency, but expression of AtNRAMP4 was induced under Fe-limited conditions (Lanquar et al., 2010). Both proteins were shown to be responsible for the retrieval of Fe from vacuoles during seed germination and to mediate the export of vacuolar Mn in photosynthetic tissues of adult plants (Lanquar et al., 2005, 2010). The nramp3nramp4 double mutant had comparable Mn concentrations in leaf mesophyll cells as wild type plants, but it showed a strong accumulation of Mn in the vacuoles (Lanquar et al., 2010). Under Mn deficiency, the double mutant showed a decreased growth which was correlated with reduced photosynthetic activity as a consequence of a shortage of Mn for the formation of OEC complexes in PSII. In contrast, nramp3nramp4 did not show altered mitochondrial MnSOD activity under Mn deficiency (Lanquar et al., 2010). These results suggest an important role for AtNRAMP3/AtNRAMP4-dependent Mn transit through the vacuole prior to the import into chloroplasts of mesophyll cells.
Manganese Accumulation in Chloroplasts
Mn plays an extremely important role in chloroplast function, as it is essential for photosynthetic activity. There is a high demand for Mn in the photosynthetic apparatus, mainly for the formation of the Mn4Ca -cluster in the OEC of PSII, which is essential for water splitting. In spite of the importance of Mn for chloroplast function, only recent studies have reported the identification and characterization of Mn transporters in chloroplast membranes. Two members of the BICAT family, which are related to the GDT1 protein in yeast (Demaegd et al., 2013) and the Mnx protein in the cyanobacterium Synechocystis (Brandenburg et al., 2017), are involved in Mn loading of the chloroplast and the distribution within this organelle (Figure 3A).
The Arabidopsis AtBICAT1 (syn. PAM71, CCHA1) protein is localized in the thylakoid membrane and transports Ca2+ and Mn2+ into the thylakoid lumen (Schneider et al., 2016; Wang et al., 2016; Frank et al., 2019). AtBICAT1 complemented the Mn-sensitive phenotype of the pmr1 yeast mutant and mediated Ca2+ influx upon expression in E. coli. bicat1 knockout mutants showed slightly pale green leaves along with compromised growth. Interestingly, growth retardation and photosynthetic activity of bicat1 could be partially restored by exogenous treatment with Mn2+ (Schneider et al., 2016). In addition, transient elevations of the stromal free Ca2+ concentration, induced by a light-to-dark shift, were increased in bicat1 mutants (Frank et al., 2019). Based on these findings, AtBICAT1 presumably functions in Mn2+ and Ca2+ flux into thylakoids for assembly of the Mn4Ca-cluster, and also in the homeostasis of stromal Ca2+, which regulates numerous processes in chloroplasts (Sello et al., 2018).
AtBICAT2 (syn. PAM71HL, CMT1), the second member of this family, was also described to fulfill functions in chloroplast Mn and Ca2+ homeostasis (Eisenhut et al., 2018; Frank et al., 2019). In contrast to AtBICAT1, it resides in the chloroplast envelope. Like AtBICAT1, the expression of AtBICAT2 can also alleviate the Mn2+ and Ca2+ sensitivity phenotypes of the pmr1 and pmr1gdt1 yeast mutants, respectively. Disruption of AtBICAT2 resulted in strong chlorosis, severely impaired plant growth, defective thylakoid stacking, and severe reduction of PS II complexes, resulting in diminished photosynthetic activity (Eisenhut et al., 2018; Frank et al., 2019). Consistent with a reduced oxygen evolution capacity, bicat2 mutant chloroplasts contained less Mn than those of the wild type (Zhang et al., 2018). Also, mutants were defective in Ca2+ uptake across the chloroplast envelope and showed a strongly dampened dark-induced Ca2+ signal in the stroma (Frank et al., 2019). As consequence, the disruption of AtBICAT2 should also lead to a decreased Ca2+ import into the thylakoid lumen. Taken together, these results indicate that AtBICAT2 functions as an inner envelope transporter responsible for chloroplast Mn2+ and Ca2+ uptake. Ca2+ dynamics and Mn concentrations in both, stroma and lumen, were likely diminished in bicat2 mutants, which may explain the stronger phenotype compared to that of bicat1 mutants. The phenotype of bicat1bicat2 double mutants suggest that AtBICAT2 is the limiting step in Mn2+ and Ca2+ delivery to the chloroplast. Further work is needed to unravel the bi-functionality and regulation of BICATs in Ca2+ and Mn2+ homeostasis in chloroplasts.
Manganese Transport Proteins in Endomembranes
The Arabidopsis CDF protein AtMTP11 was shown to exhibit an important role in Mn detoxification. The promoter of AtMTP11 had a high activity in root tips, shoot margins, and hydathodes, but not in epidermal cells and trichomes. Expression of AtMTP11 in yeast complemented the Mn2+ hypersensitivity of the pmr1 mutant (Peiter et al., 2007). In Arabidopsis, mtp11 mutants were hypersensitive to elevated Mn2+ levels, whereas AtMTP11-overexpressing lines were hypertolerant (Delhaize et al., 2007; Peiter et al., 2007). As AtMTP11 appeared to be localized to the prevacuolar compartment (PVC) or to the Golgi apparatus (Figure 3A), it was suggested that it functions in the accumulation of excess Mn either into vacuoles via the PVC or in the secretion to the apoplastic space via vesicle-mediated exocytosis (Delhaize et al., 2007; Peiter et al., 2007). The latter pathway was supported by increased Mn concentrations in mtp11 mutants (Peiter et al., 2007). Two poplar homologs of this protein, PtMTP11.1 and PtMTP11.2, were also able to complement the Mn-sensitive yeast mutant pmr1 and targeted to the same Golgi compartments as AtMTP11 (Figure 3C; Peiter et al., 2007). Those genes are therefore likely to function in a similar way. Another MTP11 ortholog in rice, OsMTP11, was also described to be involved in Mn detoxification. OsMTP11 is induced by high Mn and expressed specifically in conducting tissues (Zhang and Liu, 2017). Interestingly, epigenetic mechanisms (e.g., DNA methylation) were a major factor regulating the expression level of OsMTP11 (Zhang and Liu, 2017). Knockdown of OsMTP11 resulted in growth inhibition in the presence of high concentrations of Mn2+ and also led to increased accumulation of Mn in shoots and roots. By contrast, the overexpression of OsMTP11 enhanced Mn tolerance of rice and decreased the accumulation of Mn in shoots and roots (Ma et al., 2018). Stable expression of OsMTP11-GFP in Arabidopsis and transient expression of this construct in rice and tobacco protoplasts showed that OsMTP11 was also located to the Golgi (Figure 3B; Farthing et al., 2017). However, recent studies suggested that OsMTP11 localized to the trans-Golgi network (TGN) when it was expressed in rice protoplasts and tobacco epidermal cells (Ma et al., 2018). Surprisingly, in tobacco epidermal cells, OsMTP11 relocalized to the plasma membrane upon treatment with high extracellular Mn2+ concentrations. These findings suggest that OsMTP11 is required for Mn homeostasis and contributes to Mn2+ tolerance in rice (Ma et al., 2018).
In the sugar beet relative B. vulgaris spp. maritima, the MTP11 homologs BmMTP10 and BmMTP11 were also characterized as Mn2+ transporters by complementation of the pmr1 yeast strain (Erbasol et al., 2013). In barley, transient expression in onion epidermal cells showed that the HvMTP8.1 and HvMTP8.2 proteins, which are most closely related to the vacuolar AtMTP8 (see section Vacuoles as Manganese Stores), were localized to the Golgi apparatus and also complemented the pmr1 strain (Pedas et al., 2014). However, the function of those CDF proteins in planta is still unclear.
The Arabidopsis genome encodes 15 P-type Ca2+-ATPases, of which the P2A -type or ECA (ER-type Calcium ATPase) subfamily has two members involved in endomembrane Mn2+ transport (Kamrul Huda et al., 2013). AtECA1 and AtECA3 both function as a pump for Ca2+ and Mn2+, and localize to the ER and Golgi apparatus, respectively (Figure 3A). Furthermore, AtECA3 was shown to be localized also in subpopulations of endosomes or PVC (Li et al., 2008). AtECA1 and AtECA3 expression in the K616 yeast mutant, defective in the Golgi Ca2+ and Mn2+ pump PMR1 and in the vacuolar Ca2+ pump PMC1, increased its tolerance to toxic levels of Mn2+ and to Ca2+ deficiency (Liang et al., 1997; Wu et al., 2002; Mills et al., 2007). Another ER-localized ECA transporter in tomato, LCA1, also complemented the growth of K616 yeast under conditions of high Mn2+ and low Ca2+ (Johnson et al., 2009). eca1 mutants of Arabidopsis showed impaired growth under Ca2+ deprivation and Mn2+ excess. On high-Mn2+ media, root hair elongation was inhibited in the mutants, suggesting an impairment in tip growth (Wu et al., 2002). Taken together, AtECA1 and LeLCA1 are likely involved in the transport of Ca2+ and Mn2+ from the cytosol into the ER. On the other hand, eca3 was shown to be sensitive to Mn deficiency and also to Mn toxicity, but not to Ca2+ deficiency (Mills et al., 2007; Li et al., 2008). Thus, the phenotype of eca3, observed under Mn-deficient conditions, may be due to a reduction of the Mn content in the Golgi. Based on these studies, CDF and ECA proteins may be responsible for Mn2+ loading of Golgi-related vesicular compartments, for delivering Mn to Mn-dependent enzymes and/or for detoxification of Mn via a secretory pathway.
In Arabidopsis, AtNRAMP2 is located in the TGN (Figure 3A) and was shown to be involved in the intracellular allocation of Mn (Alejandro et al., 2017; Gao et al., 2018). AtNRAMP2 is mainly expressed in the vasculature of roots and shoots and was barely induced upon Mn deficiency. Nevertheless, nramp2 mutants showed a hypersensitivity to Mn deficiency and a reduction in Mn contents in vacuoles and chloroplasts with an accompanying reduction in PSII activity under those conditions (Alejandro et al., 2017). Surprisingly, the nramp2nramp3nramp4 triple mutant did not exhibit higher sensitivity to Mn deficiency than the single nramp2 and double nramp3nramp4 mutants (Alejandro et al., 2017). This suggests that the three transporters act in the same pathway to deliver Mn to the chloroplasts.
NRAMP transporters from tomato (LeNRAMP1 and LeNRAMP3) and apple (MbNRAMP1) were also able to transport Mn2+ and located to intracellular vesicles when expressed in yeast cells, but their function in Mn homeostasis is still unclear (Bereczky et al., 2003; Xiao et al., 2008).
ER bodies are fusiform compartments connected to the ER that were found specifically in Brassicales (Matsushima et al., 2003). It has been proposed that ER bodies might play a role in the defense against pathogens and herbivores (Yamada et al., 2011). In Arabidopsis, two VIT transporters, AtMEB1 (Membrane protein of Endoplasmic reticulum Body) and AtMEB2, that localize to the ER body membrane but not to the ER network, have been identified (Yamada et al., 2013). Heterologous expression of AtMEB1 and AtMEB2 in yeast suppressed Fe and Mn toxicity, suggesting that they possibly act as metal transporters. ER bodies are present in hypocotyls of seedlings where they disappear during plant development. In contrast, in roots, ER bodies are constitutively present. Therefore, it has been suggested that MEB transporters may be involved in the sequestration of metals into root ER bodies under metal stress conditions (Yamada et al., 2013).
Manganese Homeostasis in Other Organelles
In mitochondria, Mn is crucial for the activity of MnSOD which scavenges ROS generated within the citric acid cycle and the electron transport chain. However, the transport mechanisms for Mn2+ in plant mitochondria still remain to be elucidated. To date, no transporter involved in Mn2+ transport to and from plant mitochondria has been characterized. Nevertheless, in humans, a mitochondrial Ca2+ uniporter (MCU) that is responsible for Ca2+ and Mn2+ loading into the mitochondria has been described (Kirichok et al., 2004). Arabidopsis has six MCU isoforms which are predicted to be localized in mitochondria, except one, cMCU, that is targeted to the chloroplast (Stael et al., 2012). The cMCU protein mediates Ca2+ fluxes across the chloroplast envelope (Teardo et al., 2019). In humans, MCU is part of a complex named MCUC (MCU complex) which includes other subunits, including the EF hand-containing proteins MICU1 and MICU2 (Ca2+-sensing inhibitory subunit). MICU1 plays a decisive role in ion specificity of MCU, allowing it to distinguish between Ca2+ vs. Mn2+ (Kamer et al., 2018). In Arabidopsis, a MICU homolog, AtMICU, that binds Ca2+ and localizes to the mitochondria, was described (Wagner et al., 2015). These findings provoke the idea that a conserved uniporter system, similar to MCUC in humans, may mediate Ca2+ and Mn2+ uptake by plant mitochondria.
In peroxisomes, Mn is important as a cofactor of the peroxisomal MnSOD. This enzyme is present in some plant species, including pea, cucumber, and pepper (Corpas et al., 2017). However, so far, transporters to load Mn into the peroxisomes are still missing.
Conclusion and Perspectives
The relevance of manganese as a micronutrient of plants is still largely underestimated. For a long time, the accepted dogma among animal and plant biologists has been that the physiological requirement for Mn by living cells is low and that Mn uptake exceeds the requirement. However, in natural and in agricultural settings, Mn availability can be a seriously limiting factor for plant growth, which necessitates the operation of high-affinity Mn transporters in roots and efficient mechanisms of Mn distribution in the plant to cope with Mn shortage. Crops with improved Mn uptake capacity and Mn use efficiency will achieve higher growth and yield under suboptimal Mn availability, primarily by providing sufficient Mn to PSII and thus increasing their photosynthetic efficiency.
There are many unresolved issues in our understanding of Mn transport and homeostasis, such as the transport of Mn into the xylem – Is it mediated by a vesicle-based secretory mechanism or by plasma membrane-bound exporters? How Mn is imported by different cell types in the shoot is another open question. Most Mn2+ transporters are not specific, but also able to move other divalent cations, like Ca2+, Fe2+, Zn2+, or Cu2+, but so far it is still unclear if and how they discriminate between the cations. In humans, the substrate selectivity of a mitochondrial Mn transporter is altered by interacting EF hand proteins (Kamer et al., 2018). In this respect, the ability of diverse Ca2+ transport proteins to also permeate Mn2+ (or vice versa) is particular interesting. The possibility that Ca2+ and Mn2+ share transport pathways mediated by ECAs, BICATs, CAXs, and Ca2+ channels, implies an interference of Mn2+ in Ca2+ signaling. In fact, Mn2+ has been demonstrated to act as a signaling agent per se in humans (Wang et al., 2018).
The sensing and signaling of the plant’s Mn status is another area yet to be explored: On which level is Mn transport activity regulated by the nutritional status of the plant? This review pointed out some transcriptional changes of Mn transporter-encoding genes under Mn-toxic and Mn-deficient conditions, but the signaling networks that underlie these changes are still missing. Furthermore, a recent systems-wide analysis indicated that Mn deficiency responses are primarily regulated at the posttranscriptional level (Rodríguez-Celma et al., 2016). Moreover, our understanding of how Mn transport proteins are regulated is very rudimentary. In this respect, a couple of proteins that regulate the subcellular localization of AtNRAMP1 have been identified, revealing links to inositol phosphate and choline homeostasis (Agorio et al., 2017; Gao et al., 2017). As another fascinating case, it has been demonstrated that phosphorylation, ubiquitination, and metal binding modify the stability and localization of AtIRT1, an Fe2+ transporter that contributes to the uptake of Mn2+ (Dubeaux et al., 2018). However, for the vast majority of Mn transporters, posttranslational modifications and protein-protein interactions are unknown.
Future research has to be focused on all these fundamental aspects. The function, regulation, and co-operation of Mn2+ transport proteins in different plant tissues upon Mn deficiency and excess ought to be understood at transcriptional and at protein level. Such studies will eventually elucidate the mechanisms controlling Mn acquisition, subcellular compartmentation, and homeostasis in plants, a knowledge that can be harnessed to develop Mn-efficient germplasm of staple crops.
Author Contributions
SA, SH, and BM drafted the manuscript. SA, SH, BM, and EP finalized the manuscript, approved the final version of the manuscript, and agreed to be accountable for all aspects of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding. This work was supported by the European Regional Development Fund (ERDF) within the State Research Focus Program “Molecular Biosciences as a Motor for a Knowledge-Based Economy” (Grant No. ZS/2016/06/79740 to EP). We acknowledge the financial support within the funding program Open Access Publishing by the German Research Foundation (DFG).
http://www.uniprot.org, accessed 20 October 2019
References
- Agorio A., Giraudat J., Bianchi M. W., Marion J., Espagne C., Castaings L., et al. (2017). Phosphatidylinositol 3-phosphate–binding protein AtPH1 controls the localization of the metal transporter NRAMP1 in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 114 E3354–E3363. 10.1073/pnas.1702975114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam S., Akiha F., Kamei S., Huq S. M. I., Kawai S. (2005). Mechanism of potassium alleviation of manganese phytotoxicity in barley. J. Plant Nutr. 28 889–901. 10.1081/pln-200055572 [DOI] [Google Scholar]
- Alejandro S., Cailliatte R., Alcon C., Dirick L., Domergue F., Correia D., et al. (2017). Intracellular distribution of manganese by the trans-Golgi network transporter NRAMP2 is critical for photosynthesis and cellular redox homeostasis. Plant Cell 29 3068–3084. 10.1105/tpc.17.00578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M. D., Kropat J., Tottey S., Del Campo J. A., Merchant S. S. (2007). Manganese deficiency in Chlamydomonas results in loss of photosystem II and MnSOD function, sensitivity to peroxides, and secondary phosphorus and iron deficiency. Plant Physiol. 143 263–277. 10.1104/pp.106.088609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amao Y., Ohashi A. (2008). Effect of Mn ion on the visible light induced water oxidation activity of photosynthetic organ grana from spinach. Catal. Commun. 10 217–220. 10.1016/j.catcom.2008.08.022 [DOI] [Google Scholar]
- Andresen E., Peiter E., Küpper H. (2018). Trace metal metabolism in plants. J. Exp. Bot. 69 909–954. 10.1093/jxb/erx465 [DOI] [PubMed] [Google Scholar]
- Araki R., Murata J., Murata Y. (2011). A novel barley yellow stripe 1-like transporter (HvYSL2) localized to the root endodermis transports metal-phytosiderophore complexes. Plant Cell Physiol. 52 1931–1940. 10.1093/pcp/pcr126 [DOI] [PubMed] [Google Scholar]
- Baldisserotto C., Ferroni L., Anfuso E., Pagnoni A., Fasulo M. P., Pancaldi S. (2007). Responses of Trapa natans L. floating laminae to high concentrations of manganese. Protoplasma 231 65–82. 10.1007/s00709-007-0242-2 [DOI] [PubMed] [Google Scholar]
- Basu D., Tian L., Wang W., Bobbs S., Herock H., Travers A., et al. (2015). A small multigene hydroxyproline-O-galactosyltransferase family functions in arabinogalactan-protein glycosylation, growth and development in Arabidopsis. BMC Plant Biol. 15:295. 10.1186/s12870-015-0670-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereczky Z., Wang H. Y., Schubert V., Ganal M., Bauer P. (2003). Differential regulation of nramp and irt metal transporter genes in wild type and iron uptake mutants of tomato. J. Biol. Chem. 278 24697–24704. 10.1074/jbc.m301365200 [DOI] [PubMed] [Google Scholar]
- Blamey F. P. C., Hernandez-Soriano M., Cheng M., Tang C., Paterson D., Lombi E., et al. (2015). Synchrotron-based techniques shed light on mechanisms of plant sensitivity and tolerance to high manganese in the root environment. Plant Physiol. 169 2006–2020. 10.1104/pp.15.00726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom A. J. (2019). Metal regulation of metabolism. Curr. Opin. Chem. Biol. 49 33–38. 10.1016/j.cbpa.2018.09.017 [DOI] [PubMed] [Google Scholar]
- Bloom A. J., Lancaster K. M. (2018). Manganese binding to Rubisco could drive a photorespiratory pathway that increases the energy efficiency of photosynthesis. Nat. Plants 4 414–422. 10.1038/s41477-018-0191-0 [DOI] [PubMed] [Google Scholar]
- Bondarava N., Un S., Krieger-Liszkay A. (2007). Manganese binding to the 23 kDa extrinsic protein of photosystem II. Biochim. Biophys. Acta Bioenerg. 1767 583–588. 10.1016/j.bbabio.2007.01.001 [DOI] [PubMed] [Google Scholar]
- Bowler C., Van Camp W., Van Montagu M., Inzé D., Asada K. (1994). Superoxide dismutase in plants. Crit. Rev. Plant Sci. 13 199–218. [Google Scholar]
- Brandenburg F., Schoffman H., Kurz S., Krämer U., Keren N., Weber A. P. M., et al. (2017). The Synechocystis manganese exporter Mnx is essential for manganese homeostasis in cyanobacteria. Plant Physiol. 173 1798–1810. 10.1104/pp.16.01895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker T. M., Roose J. L., Fagerlund R. D., Frankel L. K., Eaton-Rye J. J. (2012). The extrinsic proteins of Photosystem II. Biochim. Biophys. Acta Bioenerg. 1817 121–142. [DOI] [PubMed] [Google Scholar]
- Broadley M., Brown P., Cakmak I., Rengel Z., Zhao F. (2012). “Function of nutrients: micronutrients,” in Marschner’s Mineral Nutrition of Higher Plants, 3rd Edn, ed. Marschner P. (Oxford: Elsevier; ), 191–249. [Google Scholar]
- Burnell J. N. (1988). “The biochemistry of manganese in plants,” in Manganese in Soils and Plants, eds Graham R. D., Hannam R. J., Uren N. C. (Dordrecht: Springer; ), 125–137. [Google Scholar]
- Cailliatte R., Schikora A., Briat J.-F., Mari S., Curie C. (2010). High-affinity manganese uptake by the metal transporter NRAMP1 is essential for Arabidopsis growth in low manganese conditions. Plant Cell 22 904–917. 10.1105/tpc.109.073023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F. Q., Werner A. K., Dahncke K., Romeis T., Liu L. H., Witte C. P. (2010). Identification and characterization of proteins involved in rice urea and arginine catabolism. Plant Physiol. 154 98–108. 10.1104/pp.110.160929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J. (2019). Molecular evolution of the vacuolar iron transporter (VIT) family genes in 14 plant species. Genes 10:144. 10.3390/genes10020144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaings L., Caquot A., Loubet S., Curie C. (2016). The high-affinity metal transporters NRAMP1 and IRT1 team up to take up iron under sufficient metal provision. Sci. Rep. 6 37222–37222. 10.1038/srep37222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Fujii Y., Yamaji N., Masuda S., Takemoto Y., Kamiya T., et al. (2013). Mn tolerance in rice is mediated by MTP8.1, a member of the cation diffusion facilitator family. J. Exp. Bot. 64 4375–4387. 10.1093/jxb/ert243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N.-H., Pittman J. K., Shigaki T., Hirschi K. D. (2002). Characterization of CAX4, an Arabidopsis H+ /cation antiporter. Plant Physiol. 128 1245–1254. 10.1104/pp.010857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chereskin B. M., Castelfranco P. A. (1982). Effects of iron and oxygen on chlorophyll biosynthesis. Plant Physiol. 69 112–116. 10.1104/pp.69.1.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H.-H., Car S., Socha A. L., Hindt M. N., Punshon T., Guerinot M. L. (2017). The Arabidopsis MTP8 transporter determines the localization of manganese and iron in seeds. Sci. Rep. 7 11024–11024. 10.1038/s41598-017-11250-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clairmont K. B., Hagar W. G., Davis E. A. (1986). Manganese toxicity to chlorophyll synthesis in tobacco callus. Plant Physiol. 80 291–293. 10.1104/pp.80.1.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin S., Justin A. M., Cantrel C., Arondel V., Kader J. C. (1999). Identification of AtPIS, a phosphatidylinositol synthase from Arabidopsis. Eur. J. Biochem. 262 652–658. 10.1046/j.1432-1327.1999.00378.x [DOI] [PubMed] [Google Scholar]
- Connorton J. M., Jones E. R., Rodríguez-Ramiro I., Fairweather-Tait S., Uauy C., Balk J. (2017). Wheat vacuolar iron transporter TaVIT2 transports Fe and Mn and is effective for biofortification. Plant Physiol. 174 2434–2444. 10.1104/pp.17.00672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connorton J. M., Webster R. E., Cheng N., Pittman J. K. (2012). Knockout of multiple Arabidopsis cation/H+ exchangers suggests isoform-specific roles in metal stress response, germination and seed mineral nutrition. PLoS One 7:e47455. 10.1371/journal.pone.0047455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornah J. E., Roper J. M., Singh D. P., Smith A. G. (2002). Measurement of ferrochelatase activity using a novel assay suggests that plastids are the major site of haem biosynthesis in both photosynthetic and non-photosynthetic cells of pea (Pisum sativum L.). Biochem. J. 362 423–432. 10.1042/bj3620423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas F. J., Barroso J. B., Palma J. M., Rodriguez-Ruiz M. (2017). Plant peroxisomes: a nitro-oxidative cocktail. Redox Biol. 11 535–542. 10.1016/j.redox.2016.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curie C., Alonso J. M., Le Jean M., Ecker J. R., Briat J.-F. (2000). Involvement of NRAMP1 from Arabidopsis thaliana in iron transport. Biochem. J. 347:749. 10.1042/0264-6021:3470749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E., Gruber B. D., Pittman J. K., White R. G., Leung H., Miao Y., et al. (2007). A role for the AtMTP11 gene of Arabidopsis in manganese transport and tolerance. Plant J. 51 198–210. 10.1111/j.1365-313x.2007.03138.x [DOI] [PubMed] [Google Scholar]
- Delhaize E., Kataoka T., Hebb D. M., White R. G., Ryan P. R. (2003). Genes encoding proteins of the cation diffusion facilitator family that confer manganese tolerance. Plant Cell 15 1131–1142. 10.1105/tpc.009134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaegd D., Colinet A. S., Deschamps A., Morsomme P. (2014). Molecular evolution of a novel family of putative calcium transporters. PLoS One 9:e100851. 10.1371/journal.pone.0100851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaegd D., Foulquier F., Colinet A. S., Gremillon L., Legrand D., Mariot P., et al. (2013). Newly characterized Golgi-localized family of proteins is involved in calcium and pH homeostasis in yeast and human cells. Proc. Natl. Acad. Sci. U.S.A. 110 6859–6864. 10.1073/pnas.1219871110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doncheva S., Poschenrieder C., Stoyanova Z., Georgieva K., Velichkova M., Barceló J. (2009). Silicon amelioration of manganese toxicity in Mn-sensitive and Mn-tolerant maize varieties. Environ. Exp. Bot. 65 189–197. 10.1016/j.envexpbot.2008.11.006 [DOI] [Google Scholar]
- Dou C. M., Fu X. P., Chen X. C., Shi J. Y., Chen Y. X. (2009). Accumulation and detoxification of manganese in hyperaccumulator Phytolacca americana. Plant Biol. 11 664–670. 10.1111/j.1438-8677.2008.00163.x [DOI] [PubMed] [Google Scholar]
- Dubeaux G., Neveu J., Zelazny E., Vert G. (2018). Metal sensing by the IRT1 transporter-receptor orchestrates its own degradation and plant metal nutrition. Mol. Cell 69 953–964. 10.1016/j.molcel.2018.02.009 [DOI] [PubMed] [Google Scholar]
- Dučić T., Polle A. (2007). Manganese toxicity in two varieties of Douglas fir (Pseudotsuga menziesii var. viridis and glauca) seedlings as affected by phosphorus supply. Funct. Plant Biol. 34 31–40. [DOI] [PubMed] [Google Scholar]
- Eckhardt U., Mas Marques A., Buckhout T. J. (2001). Two iron-regulated cation transporters from tomato complement metal uptake-deficient yeast mutants. Plant Mol. Biol. 45 437–448. [DOI] [PubMed] [Google Scholar]
- Edmond C., Shigaki T., Ewert S., Nelson M. D., Connorton J. M., Chalova V., et al. (2009). Comparative analysis of CAX2-like cation transporters indicates functional and regulatory diversity. Biochem. J. 418 145–154. 10.1042/BJ20081814 [DOI] [PubMed] [Google Scholar]
- Egelund J., Petersen B. L., Motawia M. S., Damager I., Faik A., Olsen C. E., et al. (2006). Arabidopsis thaliana RGXT1 and RGXT2 encode Golgi-localized (1,3)-alpha-D-xylosyltransferases involved in the synthesis of pectic rhamnogalacturonan-II. Plant Cell 18 2593–2607. 10.1105/tpc.105.036566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhut M., Hoecker N., Schmidt S. B., Basgaran R. M., Flachbart S., Jahns P., et al. (2018). The plastid envelope CHLOROPLAST MANGANESE TRANSPORTER1 is essential for manganese homeostasis in Arabidopsis. Mol. Plant 11 955–969. 10.1016/j.molp.2018.04.008 [DOI] [PubMed] [Google Scholar]
- Emsley J. (2003). Nature’s Building Blocks: An A-Z Guide to the Elements. Oxford: Oxford University Press. [Google Scholar]
- Engelsma G. (1972). A possible role of divalent manganese ions in the photoinduction of phenylalanine ammonia-lyase. Plant Physiol. 50 599–602. 10.1104/pp.50.5.599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbasol I., Bozdag G. O., Koc A., Pedas P., Karakaya H. C. (2013). Characterization of two genes encoding metal tolerance proteins from Beta vulgaris subspecies maritima that confers manganese tolerance in yeast. Biometals 26 795–804. 10.1007/s10534-013-9658-7 [DOI] [PubMed] [Google Scholar]
- Eroglu S., Giehl R. F. H., Meier B., Takahashi M., Terada Y., Ignatyev K., et al. (2017). Metal tolerance protein 8 mediates manganese homeostasis and iron reallocation during seed development and germination. Plant Physiol. 174 1633–1647. 10.1104/pp.16.01646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu S., Meier B., von Wirén N., Peiter E. (2016). The vacuolar manganese transporter MTP8 determines tolerance to iron deficiency-induced chlorosis in Arabidopsis. Plant Physiol. 170 1030–1045. 10.1104/pp.15.01194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farthing E. C., Menguer P. K., Fett J. P., Williams L. E. (2017). OsMTP11 is localised at the Golgi and contributes to Mn tolerance. Sci. Rep. 7:15258. 10.1038/s41598-017-15324-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzadfar S., Zarinkamar F., Hojati M. (2017). Magnesium and manganese affect photosynthesis, essential oil composition and phenolic compounds of Tanacetum parthenium. Plant Physiol. Biochem. 112 207–217. 10.1016/j.plaphy.2017.01.002 [DOI] [PubMed] [Google Scholar]
- Fecht-Christoffers M. M., Führs H., Braun H. P., Horst W. J. (2006). The role of hydrogen peroxide-producing and hydrogen peroxide-consuming peroxidases in the leaf apoplast of cowpea in manganese tolerance. Plant Physiol. 140 1451–1463. 10.1104/pp.105.070474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando D. R., Bakkaus E. J., Perrier N., Baker A. J., Woodrow I. E., Batianoff G. N., et al. (2006a). Manganese accumulation in the leaf mesophyll of four tree species: a PIXE/EDAX localization study. New Phytol. 171 751–758. 10.1111/j.1469-8137.2006.01783.x [DOI] [PubMed] [Google Scholar]
- Fernando D. R., Batianoff G. N., Baker A. J., Woodrow I. E. (2006b). In vivo localization of manganese in the hyperaccumulator Gossia bidwillii (Benth.) N. Snow & Guymer (Myrtaceae) by cryo-SEM/EDAX. Plant Cell Environ. 29 1012–1020. 10.1111/j.1365-3040.2006.01498.x [DOI] [PubMed] [Google Scholar]
- Fernando D. R., Lynch J. P. (2015). Manganese phytotoxicity: new light on an old problem. Annals Bot. 116 313–319. 10.1093/aob/mcv111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando D. R., Mizuno T., Woodrow I. E. (2010). Characterization of foliar manganese (Mn) in Mn (hyper) accumulators using X−ray absorption spectroscopy. New Phytol. 188 1014–1027. 10.1111/j.1469-8137.2010.03431.x [DOI] [PubMed] [Google Scholar]
- Ferrandon M., Chamel A. R. (1988). Cuticular retention, foliar absorption and translocation of Fe, Mn and Zn supplied in organic and inorganic form. J. Plant Nutr. 11 247–263. 10.1080/01904168809363800 [DOI] [Google Scholar]
- Frank J., Happeck R., Meier B., Hoang M. T. T., Stribny J., Hause G., et al. (2019). Chloroplast-localized BICAT proteins shape stromal calcium signals and are required for efficient photosynthesis. New Phytol. 221 866–880. 10.1111/nph.15407 [DOI] [PubMed] [Google Scholar]
- Fu X.-Z., Zhou X., Xing F., Ling L.-L., Chun C.-P., Cao L., et al. (2017). Genome-wide identification, cloning and functional analysis of the Zinc/Iron-Regulated Transporter-Like Protein (ZIP) gene family in trifoliate orange (Poncirus trifoliata L. Raf.). Front. Plant Sci. 8:588. 10.3389/fpls.2017.00588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Führs H., Behrens C., Gallien S., Heintz D., Van Dorsselaer A., Braun H. P., et al. (2010). Physiological and proteomic characterization of manganese sensitivity and tolerance in rice (Oryza sativa) in comparison with barley (Hordeum vulgare). Ann. Bot. 105 1129–1140. 10.1093/aob/mcq046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Xie W., Yang C., Xu J., Li J., Wang H., et al. (2018). NRAMP2, a trans-Golgi network-localized manganese transporter, is required for Arabidopsis root growth under manganese deficiency. New Phytol. 217 179–193. 10.1111/nph.14783 [DOI] [PubMed] [Google Scholar]
- Gao Y. Q., Chen J. G., Chen Z. R., An D., Lv Q. Y., Han M. L., et al. (2017). A new vesicle trafficking regulator CTL1 plays a crucial role in ion homeostasis. PLoS Biol. 15:e2002978. 10.1371/journal.pbio.2002978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George T. S., French A. S., Brown L. K., Karley A. J., White P. J., Ramsay L., et al. (2014). Genotypic variation in the ability of landraces and commercial cereal varieties to avoid manganese deficiency in soils with limited manganese availability: is there a role for root-exuded phytases? Physiol. Plant. 151 243–256. 10.1111/ppl.12151 [DOI] [PubMed] [Google Scholar]
- Gherardi M. J., Rengel Z. (2004). The effect of manganese supply on exudation of carboxylates by roots of lucerne (Medicago sativa). Plant Soil 260 271–282. 10.1023/b:plso.0000030182.11473.3b [DOI] [Google Scholar]
- Giles C. D., Brown L. K., Adu M. O., Mezeli M. M., Sandral G. A., Simpson R. J., et al. (2017). Response-based selection of barley cultivars and legume species for complementarity: root morphology and exudation in relation to nutrient source. Plant Sci. 255 12–28. 10.1016/j.plantsci.2016.11.002 [DOI] [PubMed] [Google Scholar]
- Glynn S. E., Baker P. J., Sedelnikova S. E., Davies C. L., Eadsforth T. C., Levy C. W., et al. (2005). Structure and mechanism of imidazoleglycerol-phosphate dehydratase. Structure 13 1809–1817. 10.1016/j.str.2005.08.012 [DOI] [PubMed] [Google Scholar]
- Goulding K. W. T. (2016). Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 32 390–399. 10.1111/sum.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory A. L., Hurley B. A., Tran H. T., Valentine A. J., She Y. M., Knowles V. L., et al. (2009). In vivo regulatory phosphorylation of the phosphoenolpyruvate carboxylase AtPPC1 in phosphate-starved Arabidopsis thaliana. Biochem. J. 420 57–65. 10.1042/BJ20082397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot M. L. (2000). The ZIP family of metal transporters. Biochim. Biophys. Acta Biomembr. 1465 190–198. 10.1016/s0005-2736(00)00138-3 [DOI] [PubMed] [Google Scholar]
- Hajiboland R. (2012). “Effect of micronutrient deficiencies on plants stress responses,” in Abiotic Stress Responses in Plants, eds Ahmad P., Prasad M. (New York, NY: Springer; ), 283–329. 10.1007/978-1-4614-0634-1_16 [DOI] [Google Scholar]
- Hansel C. M., Zeiner C. A., Santelli C. M., Webb S. M. (2012). Mn(II) oxidation by an ascomycete fungus is linked to superoxide production during asexual reproduction. Proc. Natl. Acad. Sci. U.S.A. 109 12621–12625. 10.1073/pnas.1203885109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K., Eckert C., Anschütz U., Scholz M., Held K., Waadt R., et al. (2012). Phosphorylation of calcineurin B-like (CBL) calcium sensor proteins by their CBL-interacting protein kinases (CIPKs) is required for full activity of CBL-CIPK complexes toward their target proteins. J. Biol. Chem. 287 7956–7968. 10.1074/jbc.M111.279331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck M., Paul A., Gross S., Raubuch M. (2003). Manganese toxicity in epiphytic lichens: chlorophyll degradation and interaction with iron and phosphorus. Environ. Exp. Bot. 49 181–191. 10.1016/s0098-8472(02)00069-2 [DOI] [Google Scholar]
- Hebbern C. A., Laursen K. H., Ladegaard A. H., Schmidt S. B., Pedas P., Bruhn D., et al. (2009). Latent manganese deficiency increases transpiration in barley (Hordeum vulgare). Physiol. Plant. 135 307–316. 10.1111/j.1399-3054.2008.01188.x [DOI] [PubMed] [Google Scholar]
- Hebbern C. A., Pedas P., Schjoerring J. K., Knudsen L., Husted S. (2005). Genotypic differences in manganese efficiency: field experiments with winter barley (Hordeum vulgare L.). Plant Soil 272 233–244. 10.1007/s11104-004-5048-9 [DOI] [Google Scholar]
- Heckman J. R., Clarke B. B., Murphy J. A. (2003). Optimizing manganese fertilization for the suppression of take-all patch disease on creeping bentgrass. Crop Sci. 43 1395–1398. 10.2135/cropsci2003.1395 [DOI] [Google Scholar]
- Heine G., Max J. F. J., Führs H., Moran-Puente D. W., Heintz D., Horst W. J. (2011). Effect of manganese on the resistance of tomato to Pseudocercospora fuligena. J. Plant Nutr. Soil Sci. 174 827–836. 10.1002/jpln.201000440 [DOI] [Google Scholar]
- Henriques F. S. (2004). Reduction in chloroplast number accounts for the decrease in the photosynthetic capacity of Mn-deficient pecan leaves. Plant Sci. 166 1051–1055. 10.1016/j.plantsci.2003.12.022 [DOI] [Google Scholar]
- Hirschi K. D., Korenkov V. D., Wilganowski N. L., Wagner G. J. (2000). Expression of Arabidopsis CAX2 in tobacco. Altered metal accumulation and increased manganese tolerance. Plant Physiol. 124 125–134. 10.1104/pp.124.1.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoecker N., Leister D., Schneider A. (2017). Plants contain small families of UPF0016 proteins including the PHOTOSYNTHESIS AFFECTED MUTANT71 transporter. Plant Signal. Behav 12:e1278101. 10.1080/15592324.2016.1278101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L., Gu D., Li Y., Li J., Li J., Chen X., et al. (2019). Phylogenetic and expression analysis of Mn-CDF transporters in pear (Pyrus bretschneideri Rehd.). Plant Mol. Biol. Report. 37 98–110. 10.1007/s11105-019-01142-9 [DOI] [Google Scholar]
- Hsieh M. H., Chang C. Y., Hsu S. J., Chen J. J. (2008). Chloroplast localization of methylerythritol 4-phosphate pathway enzymes and regulation of mitochondrial genes in ispD and ispE albino mutants in Arabidopsis. Plant Mol. Biol. 66 663–673. 10.1007/s11103-008-9297-5 [DOI] [PubMed] [Google Scholar]
- Huang Y. L., Yang S., Long G. X., Zhao Z. K., Li X. F., Gu M. H. (2016). Manganese toxicity in sugarcane plantlets grown on acidic soils of southern China. PLoS One 11:e0148956. 10.1371/journal.pone.0148956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husted S., Laursen K. H., Hebbern C. A., Schmidt S. B., Pedas P., Haldrup A., et al. (2009). Manganese deficiency leads to genotype-specific changes in fluorescence induction kinetics and state transitions. Plant Physiol. 150 825–833. 10.1104/pp.108.134601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihnatowicz A., Siwinska J., Meharg A. A., Carey M., Koornneef M., Reymond M. (2014). Conserved histidine of metal transporter AtNRAMP1 is crucial for optimal plant growth under manganese deficiency at chilling temperatures. New Phytol. 202 1173–1183. 10.1111/nph.12737 [DOI] [PubMed] [Google Scholar]
- Ishimaru Y., Masuda H., Bashir K., Inoue H., Tsukamoto T., Takahashi M., et al. (2010). Rice metal-nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. Plant J. 62 379–390. 10.1111/j.1365-313X.2010.04158.x [DOI] [PubMed] [Google Scholar]
- Ishimaru Y., Takahashi R., Bashir K., Shimo H., Senoura T., Sugimoto K., et al. (2012). Characterizing the role of rice NRAMP5 in manganese, iron and cadmium transport. Sci. Rep. 2:286. 10.1038/srep00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauregui M. A., Reisenauer H. M. (1982). Dissolution of oxides of manganese and iron by root exudate components. Soil Sci. Soc. Am. J. 46 314–317. 10.2136/sssaj1982.03615995004600020020x [DOI] [Google Scholar]
- Johnson N. A., Liu F., Weeks P. D., Hentzen A. E., Kruse H. P., Parker J. J., et al. (2009). A tomato ER-type Ca2 +-ATPase, LCA1, has a low thapsigargin-sensitivity and can transport manganese. Arch. Biochem. Biophys. 481 157–168. 10.1016/j.abb.2008.11.010 [DOI] [PubMed] [Google Scholar]
- Kamer K. J., Sancak Y., Fomina Y., Meisel J. D., Chaudhuri D., Grabarek Z., et al. (2018). MICU1 imparts the mitochondrial uniporter with the ability to discriminate between Ca2 + and Mn2 +. Proc. Natl. Acad. Sci. U.S.A. 115 E7960–E7969. 10.1073/pnas.1807811115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya T., Akahori T., Ashikari M., Maeshima M. (2006). Expression of the vacuolar Ca2 +/H+ exchanger, OsCAX1a, in rice: cell and age specificity of expression, and enhancement by Ca2 +. Plant Cell Physiol. 47 96–106. 10.1093/pcp/pci227 [DOI] [PubMed] [Google Scholar]
- Kamiya T., Akahori T., Maeshima M. (2005). Expression profile of the genes for rice cation/H+ exchanger family and functional analysis in yeast. Plant Cell Physiol. 46 1735–1740. 10.1093/pcp/pci173 [DOI] [PubMed] [Google Scholar]
- Kamrul Huda K. M., Yadav S., Akhter Banu M. S., Trivedi D. K., Tuteja N. (2013). Genome-wide analysis of plant-type II Ca2 + ATPases gene family from rice and Arabidopsis: potential role in abiotic stresses. Plant Physiol. Biochem. 65 32–47. 10.1016/j.plaphy.2013.01.002 [DOI] [PubMed] [Google Scholar]
- Khabaz-Saberi H., Setter T. L., Waters I. (2006). Waterlogging induces high to toxic concentrations of iron, aluminum, and manganese in wheat varieties on acidic soil. J. Plant Nutr. 29 899–911. 10.1080/01904160600649161 [DOI] [Google Scholar]
- Kim K. N., Lee J. S., Han H., Choi S. A., Go S. J., Yoon I. S. (2003). Isolation and characterization of a novel rice Ca2 +-regulated protein kinase gene involved in responses to diverse signals including cold, light, cytokinins, sugars and salts. Plant Mol. Biol. 52 1191–1202. 10.1023/b:plan.0000004330.62660.a2 [DOI] [PubMed] [Google Scholar]
- Kim S. A., Punshon T., Lanzirotti A., Li L., Alonso J. M., Ecker J. R., et al. (2006). Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science 314 1295–1298. 10.1126/science.1132563 [DOI] [PubMed] [Google Scholar]
- Kirichok Y., Krapivinsky G., Clapham D. E. (2004). The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427 360–364. 10.1038/nature02246 [DOI] [PubMed] [Google Scholar]
- Koike S., Inoue H., Mizuno D., Takahashi M., Nakanishi H., Mori S., et al. (2004). OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant J. 39 415–424. 10.1111/j.1365-313x.2004.02146.x [DOI] [PubMed] [Google Scholar]
- Köllner T. G., Held M., Lenk C., Hiltpold I., Turlings T. C. J., Gershenzon J., et al. (2008). A maize (E)-β-caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. Plant Cell 20 482–494. 10.1105/tpc.107.051672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren’kov V., Park S., Cheng N. H., Sreevidya C., Lachmansingh J., Morris J., et al. (2007). Enhanced Cd2 +-selective root-tonoplast-transport in tobaccos expressing Arabidopsis cation exchangers. Planta 225 403–411. 10.1007/s00425-006-0352-7 [DOI] [PubMed] [Google Scholar]
- Korshunova Y. O., Eide D., Clark W. G., Guerinot M. L., Pakrasi H. B. (1999). The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol. Biol. 40 37–44. [DOI] [PubMed] [Google Scholar]
- Lambers H., Hayes P. E., Laliberté E., Oliveira R. S., Turner B. L. (2015). Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends Plant Sci. 20 83–90. 10.1016/j.tplants.2014.10.007 [DOI] [PubMed] [Google Scholar]
- Lane B. G. (2002). Oxalate, germins, and higher-plant pathogens. IUBMB Life 53 67–75. 10.1080/15216540211474 [DOI] [PubMed] [Google Scholar]
- Lanquar V., Lelièvre F., Barbier-Brygoo H., Thomine S. (2004). Regulation and function of AtNRAMP4 metal transporter protein. Soil Sci. Plant Nutr. 50 1141–1150. 10.1080/00380768.2004.10408587 [DOI] [Google Scholar]
- Lanquar V., Lelièvre F., Bolte S., Hamès C., Alcon C., Neumann D., et al. (2005). Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J. 24 4041–4051. 10.1038/sj.emboj.7600864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanquar V., Schnell Ramos M., Lelièvre F., Barbier-Brygoo H., Krieger-Liszkay A., Krämer U., et al. (2010). Export of vacuolar manganese by AtNRAMP3 and AtNRAMP4 is required for optimal photosynthesis and growth under manganese deficiency. Plant Physiol. 152 1986–1999. 10.1104/pp.109.150946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClere S., Tellez R., Rampey R. A., Matsuda S. P. T., Bartel B. (2002). Characterization of a family of IAA-amino acid conjugate hydrolases from Arabidopsis. J. Biol. Chem. 277 20446–20452. 10.1074/jbc.m111955200 [DOI] [PubMed] [Google Scholar]
- Lešková A., Giehl R. F. H., Hartmann A., Fargašová A., von Wirén N. (2017). Heavy metals induce iron deficiency responses at different hierarchic and regulatory levels. Plant Physiol. 174 1648–1668. 10.1104/pp.16.01916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Wang P., Menzies N. W., Lombi E., Kopittke P. M. (2017). Effects of changes in leaf properties mediated by methyl jasmonate (MeJA) on foliar absorption of Zn, Mn and Fe. Ann. Bot. 120 405–415. 10.1093/aob/mcx063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. Y., Liu J., Dong D., Jia X., Mccouch S. R., Kochian L. V. (2014). Natural variation underlies alterations in Nramp aluminum transporter (NRAT1) expression and function that play a key role in rice aluminum tolerance. Proc. Natl. Acad. Sci. U.S.A. 111 6503–6508. 10.1073/pnas.1318975111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Xu X., Chen C., Shen Z. (2016). Genome-wide characterization and expression analysis of the Germin-like protein family in rice and Arabidopsis. Int. J. Mol. Sci. 17:1622. 10.3390/ijms17101622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Yang X. (2018). The essential element manganese, oxidative stress, and metabolic diseases: links and interactions. Oxid. Med. Cell. Longev. 2018:7580707. 10.1155/2018/7580707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Chen L. S., Jiang H. X., Tang N., Yang L. T., Lin Z. H., et al. (2010). Effects of manganese-excess on CO2 assimilation, ribulose-1,5-bisphosphate carboxylase/oxygenase, carbohydrates and photosynthetic electron transport of leaves, and antioxidant systems of leaves and roots in Citrus grandis seedlings. BMC Plant Biol. 10:42. 10.1186/1471-2229-10-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Chanroj S., Wu Z., Romanowsky S. M., Harper J. F., Sze H. (2008). A distinct endosomal Ca2 +/Mn2 + pump affects root growth through the secretory process. Plant Physiol. 147 1675–1689. 10.1104/pp.108.119909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F., Cunningham K. W., Harper J. F., Sze H. (1997). ECA1 complements yeast mutants defective in Ca2 + pumps and encodes an endoplasmic reticulum-type Ca2 +-ATPase in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 94 8579–8584. 10.1073/pnas.94.16.8579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidon F. C., Barreiro M. G., Ramalho J. C. (2004). Manganese accumulation in rice: implications for photosynthetic functioning. J. Plant Physiol. 161 1235–1244. 10.1016/j.jplph.2004.02.003 [DOI] [PubMed] [Google Scholar]
- Liu J., Gao Y., Tang Y., Wang D., Chen X., Yao Y., et al. (2019). Genome-wide identification, comprehensive gene feature, evolution, and expression analysis of plant metal tolerance proteins in tobacco under heavy metal toxicity. Front. Genet. 10:345. 10.3389/fgene.2019.00345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long L., Persson D. P., Duan F., Jørgensen K., Yuan L., Schjoerring J. K., et al. (2018). The iron-regulated transporter 1 plays an essential role in uptake, translocation and grain-loading of manganese, but not iron, in barley. New Phytol. 217 1640–1653. 10.1111/nph.14930 [DOI] [PubMed] [Google Scholar]
- Longnecker N. E., Graham R. D., Card G. (1991). Effects of manganese deficiency on the pattern of tillering and development of barley (Hordeum vulgare c.v. galleon). Field Crops Res. 28 85–102. 10.1016/0378-4290(91)90076-8 [DOI] [Google Scholar]
- López-Millán A. F., Ellis D. R., Grusak M. A. (2004). Identification and characterization of several new members of the ZIP family of metal ion transporters in Medicago truncatula. Plant Mol. Biol. 54 583–596. 10.1023/b:plan.0000038271.96019.aa [DOI] [PubMed] [Google Scholar]
- Lovely D. R. (1995). Microbial reduction of iron, manganese, and other metals. Adv. Agron. 54 175–231. 10.1016/s0065-2113(08)60900-1 [DOI] [Google Scholar]
- Ma G., Li J., Li J., Li Y., Gu D., Chen C., et al. (2018). OsMTP11, a trans-Golgi network localized transporter, is involved in manganese tolerance in rice. Plant Sci. 274 59–69. 10.1016/j.plantsci.2018.05.011 [DOI] [PubMed] [Google Scholar]
- Martinoia E., Meyer S., De Angeli A., Nagy R. (2012). Vacuolar transporters in their physiological context. Annu. Rev. Plant Biol. 63 183–213. 10.1146/annurev-arplant-042811-105608 [DOI] [PubMed] [Google Scholar]
- Martins V., Carneiro F., Conde C., Sottomayor M., Gerós H. (2017). The grapevine VvCAX3 is a cation/H+ exchanger involved in vacuolar Ca2 + homeostasis. Planta 246 1083–1096. 10.1007/s00425-017-2754-0 [DOI] [PubMed] [Google Scholar]
- Matsushima R., Hayashi Y., Yamada K., Shimada T., Nishimura M., Hara-Nishimura I. (2003). The ER body, a novel endoplasmic reticulum-derived structure in Arabidopsis. Plant Cell Physiol. 44 661–666. 10.1093/pcp/pcg089 [DOI] [PubMed] [Google Scholar]
- McNaughton R. L., Reddi A. R., Clement M. H. S., Sharma A., Barnese K., Rosenfeld L., et al. (2010). Probing in vivo Mn2 + speciation and oxidative stress resistance in yeast cells with electron-nuclear double resonance spectroscopy. Proc. Natl. Acad. Sci U.S.A. 107 15335–15339. 10.1073/pnas.1009648107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNear D. H., Küpper J. V. (2014). Mechanisms of trichome-specific Mn accumulation and toxicity in the Ni hyperaccumulator Alyssum murale. Plant Soil 377 407–422. 10.1007/s11104-013-2003-7 [DOI] [Google Scholar]
- Mei H., Cheng N. H., Zhao J., Park S., Escareno R. A., Pittman J. K., et al. (2009). Root development under metal stress in Arabidopsis thaliana requires the H+/cation antiporter CAX4. New Phytol. 183 95–105. 10.1111/j.1469-8137.2009.02831.x [DOI] [PubMed] [Google Scholar]
- Meinhard M., Rodriguez P. L., Grill E. (2002). The sensitivity of ABI2 to hydrogen peroxide links the abscisic acid-response regulator to redox signalling. Planta 214 775–782. 10.1007/s00425-001-0675-3 [DOI] [PubMed] [Google Scholar]
- Membré N., Bernier F., Staiger D., Berna A. (2000). Arabidopsis thaliana germin-like proteins: common and specific features point to a variety of functions. Planta 211 345–354. 10.1007/s004250000277 [DOI] [PubMed] [Google Scholar]
- Meng J. G., Zhang X. D., Tan S. K., Zhao K. X., Yang Z. M. (2017). Genome-wide identification of Cd-responsive NRAMP transporter genes and analyzing expression of NRAMP1 mediated by miR167 in Brassica napus. Biometals 30 917–931. 10.1007/s10534-017-0057-3 [DOI] [PubMed] [Google Scholar]
- Migocka M., Papierniak A., Kosieradzka A., Posyniak E., Maciaszczyk-Dziubinska E., Biskup R., et al. (2015). Cucumber metal tolerance protein CsMTP9 is a plasma membrane H+-coupled antiporter involved in the Mn2 + and Cd2 + efflux from root cells. Plant J. 84 1045–1058. 10.1111/tpj.13056 [DOI] [PubMed] [Google Scholar]
- Millaleo R., Reyes-Díaz M., Alberdi M., Ivanov A. G., Krol M., Hüner N. P. A. (2013). Excess manganese differentially inhibits photosystem I versus II in Arabidopsis thaliana. J. Exp. Bot. 64 343–354. 10.1093/jxb/ers339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millaleo R., Reyes-Diaz M., Ivanov A. G., Mora M. L., Alberdi M. (2010). Manganese as essential and toxic element for plants: transport, accumulation and resistance mechanisms. J. Soil Sci. Plant Nutr. 10 470–481. 10.4067/s0718-95162010000200008 [DOI] [Google Scholar]
- Mills R. F., Doherty M. L., Lopez-Marques R. L., Weimar T., Dupree P., Palmgren M. G., et al. (2007). ECA3, a Golgi-localized P2A-type ATPase, plays a crucial role in manganese nutrition in Arabidopsis. Plant Physiol. 146 116–128. 10.1104/pp.107.110817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner M. J., Seamon J., Craft E., Kochian L. V. (2013). Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J. Exp. Bot. 64 369–381. 10.1093/jxb/ers315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanini B., Blaudez D., Jeandroz S., Sanders D., Chalot M. (2007). Phylogenetic and functional analysis of the Cation Diffusion Facilitator (CDF) family: improved signature and prediction of substrate specificity. BMC Genomics 8:107. 10.1186/1471-2164-8-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. J. (2005). Kinetics of reaction between O2 and Mn(II) species in aqueous solutions. Geochim. Cosmochim. Acta 69 35–48. 10.1016/j.gca.2004.06.013 [DOI] [Google Scholar]
- Morgan P. W., Joham H. E., Amin J. V. (1966). Effect of manganese toxicity on the indoleacetic acid oxidase system of cotton. Plant Physiol. 41 718–724. 10.1104/pp.41.4.718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J., Tian H., Park S., Sreevidya C. S., Ward J. M., Hirschi K. D. (2008). AtCCX3 is an Arabidopsis endomembrane H+-dependent K+ transporter. Plant Physiol. 148 1474–1486. 10.1104/pp.108.118810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nable R. O., Houtz R. L., Cheniae G. M. (1988). Early inhibition of photosynthesis during development of Mn toxicity in tobacco. Plant Physiol. 86 1136–1142. 10.1104/pp.86.4.1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo Y., Nelson N. (2006). The NRAMP family of metal-ion transporters. Biochim. Biophys. Acta Mol. Cell Res. 1763 609–620. 10.1016/j.bbamcr.2006.05.007 [DOI] [PubMed] [Google Scholar]
- Nian L. S. (1989). Manganese induced iron chlorosis in pineapple and its control by foliar application of iron-citrate. J. Plant Nutr. Soil Sci. 152 125–126. 10.1002/jpln.19891520122 [DOI] [Google Scholar]
- Nowicki M., Müller F., Frentzen M. (2005). Cardiolipin synthase of Arabidopsis thaliana. FEBS Lett. 579 2161–2165. 10.1016/j.febslet.2005.03.007 [DOI] [PubMed] [Google Scholar]
- Nuruzzaman M., Lambers H., Bolland M. D. A., Veneklaas E. J. (2006). Distribution of carboxylates and acid phosphatase and depletion of different phosphorus fractions in the rhizosphere of a cereal and three grain legumes. Plant Soil 281 109–120. 10.1007/s11104-005-3936-2 [DOI] [Google Scholar]
- Ohki K. (1985). Manganese deficiency and toxicity effects on photosynthesis, chlorophyll, and transpiration in Wheat. Crop Sci. 25 187–191. 10.2135/cropsci1985.0011183x002500010045x [DOI] [Google Scholar]
- Önnerud H., Zhang L., Gellerstedt G., Henriksson G. (2002). Polymerization of monolignols by redox shuttle–mediated enzymatic oxidation. Plant Cell 14 1953–1962. 10.1105/tpc.001487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen R. J. F. J., Wu J., Lelièvre F., Blanchet S., Richaud P., Barbier-Brygoo H., et al. (2009). Functional characterization of NRAMP3 and NRAMP4 from the metal hyperaccumulator Thlaspi caerulescens. New Phytol. 181 637–650. 10.1111/j.1469-8137.2008.02694.x [DOI] [PubMed] [Google Scholar]
- Panda S., Mishra A. K., Biswal U. C. (1987). Manganese induced peroxidation of thylakoid lipids and changes in chlorophyll-α fluorescence during aging of cell free chloroplasts in light. Phytochemistry 26 3217–3219. 10.1016/s0031-9422(00)82472-3 [DOI] [Google Scholar]
- Papadakis I. E., Giannakoula A., Therios I. N., Bosabalidis A. M., Moustakas M., Nastou A. (2007). Mn-induced changes in leaf structure and chloroplast ultrastructure of Citrus volkameriana (L.) plants. J. Plant Physiol. 164 100–103. 10.1016/j.jplph.2006.04.011 [DOI] [PubMed] [Google Scholar]
- Pedas P., Hebbern C. A., Schjoerring J. K., Holm P. E., Husted S. (2005). Differential capacity for high-affinity manganese uptake contributes to differences between barley genotypes in tolerance to low manganese availability. Plant Physiol. 139 1411–1420. 10.1104/pp.105.067561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedas P., Husted S., Skytte K., Schjoerring J. K. (2011). Elevated phosphorus impedes manganese acquisition by barley plants. Front. Plant Sci. 2:37. 10.3389/fpls.2011.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedas P., Stokholm M. S., Hegelund J. N., Ladegård A. H., Schjoerring J. K., Husted S. (2014). Golgi localized barley MTP8 proteins facilitate Mn transport. PLoS One 9:e113759. 10.1371/journal.pone.0113759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedas P., Ytting C. K., Fuglsang A. T., Jahn T. P., Schjoerring J. K., Husted S. (2008). Manganese efficiency in barley: identification and characterization of the metal ion transporter HvIRT1. Plant Physiol. 148 455–466. 10.1104/pp.108.118851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiter E., Montanini B., Gobert A., Pedas P., Husted S., Maathuis F. J. M., et al. (2007). A secretory pathway-localized cation diffusion facilitator confers plant manganese tolerance. Proc. Natl. Acad. Sci. U.S.A. 104 8532–8537. 10.1073/pnas.0609507104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris-Peris C., Serra-Cardona A., Sánchez-Sanuy F., Campo S., Ariño J., San Segundo B. (2017). Two NRAMP6 isoforms function as iron and manganese transporters and contribute to disease resistance in rice. Mol. Plant Microbe Interact. 30 385–398. 10.1094/MPMI-01-17-0005-R [DOI] [PubMed] [Google Scholar]
- Pittman J. K. (2005). Managing the manganese: molecular mechanisms of manganese transport and homeostasis. New Phytol. 167 733–742. 10.1111/j.1469-8137.2005.01453.x [DOI] [PubMed] [Google Scholar]
- Pittman J. K., Hirschi K. D. (2016). Phylogenetic analysis and protein structure modelling identifies distinct Ca2 +/cation antiporters and conservation of gene family structure within Arabidopsis and rice species. Rice 9:3 10.1186/s12284-016-0075-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman J. K., Shigaki T., Marshall J. L., Morris J. L., Cheng N., Hirschi K. D. (2004). Functional and regulatory analysis of the Arabidopsis thaliana CAX2 cation transporter. Plant Mol. Biol. 56 959–971. 10.1007/s11103-004-6446-3 [DOI] [PubMed] [Google Scholar]
- Pottier M., Oomen R., Picco C., Giraudat J., Scholz-Starke J., Richaud P., et al. (2015). Identification of mutations allowing natural resistance associated macrophage proteins (NRAMP) to discriminate against cadmium. Plant J. 83 625–637. 10.1111/tpj.12914 [DOI] [PubMed] [Google Scholar]
- Reddi A. R., Jensen L. T., Naranuntarat A., Rosenfeld L., Leung E., Shah R., et al. (2009). The overlapping roles of manganese and Cu/Zn SOD in oxidative stress protection. Free Radic. Biol. Med. 46 154–162. 10.1016/j.freeradbiomed.2008.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengel Z. (2015). Availability of Mn, Zn and Fe in the rhizosphere. J. Soil Sci. Plant Nutr. 15 397–409. [Google Scholar]
- Requena L., Bornemann S. (1999). Barley (Hordeum vulgare) oxalate oxidase is a manganese-containing enzyme. Biochem. J. 343 185–190. 10.1042/bj3430185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert H. S., Offringa R. (2008). Regulation of auxin transport polarity by AGC kinases. Curr. Opin. Plant Biol. 11 495–502. 10.1016/j.pbi.2008.06.004 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Celma J., Tsai Y.-H., Wen T.-N., Wu Y.-C., Curie C., Schmidt W. (2016). Systems-wide analysis of manganese deficiency-induced changes in gene activity of Arabidopsis roots. Sci. Rep. 6 35846–35846. 10.1038/srep35846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohdich F., Lauw S., Kaiser J., Feicht R., Köhler P., Bacher A., et al. (2006). Isoprenoid biosynthesis in plants - 2C-methyl-D-erythritol-4-phosphate synthase (IspC protein) of Arabidopsis thaliana. FEBS J. 273 4446–4458. 10.1111/j.1742-4658.2006.05446.x [DOI] [PubMed] [Google Scholar]
- Rohdich F., Wungsintaweekul J., Eisenreich W., Richter G., Schuhr C. A., Hecht S., et al. (2000). Biosynthesis of terpenoids: 4-diphosphocytidyl-2C-methyl-D-erythritol synthase of Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 97 6451–6456. 10.1073/pnas.97.12.6451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A., Yamaji N., Xia J., Ma J. F. (2011). OsYSL6 is involved in the detoxification of excess manganese in rice. Plant Physiol. 157 1832–1840. 10.1104/pp.111.186031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A., Yamaji N., Yokosho K., Ma J. F. (2012). Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 24 2155–2167. 10.1105/tpc.112.096925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf G., Ludewig U., Erenoglu B. E., Mori S., Kitahara T., von Wirén N. (2004). ZmYS1 functions as a proton-coupled symporter for phytosiderophore- and nicotianamine-chelated metals. J. Biol. Chem. 279 9091–9096. 10.1074/jbc.m311799200 [DOI] [PubMed] [Google Scholar]
- Schenk G., Ge Y., Carrington L. E., Wynne C. J., Searle I. R., Carroll B. J., et al. (1999). Binuclear metal centers in plant purple acid phosphatases: Fe-Zn in sweet potato and Fe-Zn in soybean. Arch. Biochem. Biophys. 370 183–189. 10.1006/abbi.1999.1407 [DOI] [PubMed] [Google Scholar]
- Schmidt S. B., Jensen P. E., Husted S. (2016). Manganese deficiency in plants: The impact on photosystem II. Trends Plant Sci. 21 622–632. 10.1016/j.tplants.2016.03.001 [DOI] [PubMed] [Google Scholar]
- Schneider A., Steinberger I., Herdean A., Gandini C., Eisenhut M., Kurz S., et al. (2016). The evolutionarily conserved protein PHOTOSYNTHESIS AFFECTED MUTANT71 is required for efficient manganese uptake at the thylakoid membrane in Arabidopsis. Plant Cell 28 892–910. 10.1105/tpc.15.00812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighofer A., Hirt H., Meskiene I. (2004). Plant PP2C phosphatases: Emerging functions in stress signaling. Trends Plant Sci. 9 236–243. 10.1016/j.tplants.2004.03.007 [DOI] [PubMed] [Google Scholar]
- Sello S., Moscatiello R., Mehlmer N., Leonardelli M., Carraretto L., Cortese E., et al. (2018). Chloroplast Ca2 + fluxes into and across thylakoids revealed by thylakoid-targeted aequorin probes. Plant Physiol. 177 38–51. 10.1104/pp.18.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J. F., Yamaji N., Shen R. F., Ma J. F. (2017). The key to Mn homeostasis in plants: regulation of Mn transporters. Trends Plant Sci. 22 215–224. 10.1016/j.tplants.2016.12.005 [DOI] [PubMed] [Google Scholar]
- Shigaki T., Pittman J. K., Hirschi K. D. (2003). Manganese specificity determinants in the Arabidopsis metal/H+ antiporter CAX2. J. Biol. Chem. 278 6610–6617. 10.1074/jbc.M209952200 [DOI] [PubMed] [Google Scholar]
- Socha A. L., Guerinot M. L. (2014). Mn-euvering manganese: the role of transporter gene family members in manganese uptake and mobilization in plants. Front. Plant Sci. 5:106. 10.3389/fpls.2014.00106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow L. A., Uren N. C. (2014). Manganese oxidation and reduction in soils: effects of temperature, water potential, pH and their interactions. Soil Res. 52 483–494. 10.1071/SR13159 [DOI] [Google Scholar]
- St.Clair S. B., Lynch J. P. (2005). Element accumulation patterns of deciduous and evergreen tree seedlings on acid soils: implications for sensitivity to manganese toxicity. Tree Physiol. 25 85–92. 10.1093/treephys/25.1.85 [DOI] [PubMed] [Google Scholar]
- Stael S., Wurzinger B., Mair A., Mehlmer N., Vothknecht U. C., Teige M. (2012). Plant organellar calcium signalling: an emerging field. J. Exp. Bot. 63 1525–1542. 10.1093/jxb/err394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A., Gügel I. L., Hilger D., Rengstl B., Jung H., Nickelsen J. (2012). Initial steps of photosystem II de novo assembly and preloading with manganese take place in biogenesis centers in Synechocystis. Plant Cell 24 660–675. 10.1105/tpc.111.093914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltz E., Wallenhammar A. C. (2014). Manganese application increases winter hardiness in barley. Field Crops Res. 164 148–153. 10.1016/j.fcr.2014.05.008 [DOI] [Google Scholar]
- Strasser R., Bondili J. S., Vavra U., Schoberer J., Svoboda B., Glossl J., et al. (2007). A unique β1,3-galactosyltransferase is indispensable for the biosynthesis of N-glycans containing Lewis a structures in Arabidopsis thaliana. Plant Cell 19 2278–2292. 10.1105/tpc.107.052985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm W., Morgan J. J. (1996). Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters. New York, NY: Wiley. [Google Scholar]
- Subrahmanyam D., Rathore V. S. (2001). Influence of manganese toxicity on photosynthesis in ricebean (Vigna umbellata) seedlings. Photosynthetica 38 449–453. [Google Scholar]
- Svedruzic D., Jonsson S., Toyota C. G., Reinhardt L. A., Ricagno S., Lindqvist Y., et al. (2005). The enzymes of oxalate metabolism: unexpected structures and mechanisms. Arch. Biochem. Biophys. 433 176–192. 10.1016/j.abb.2004.08.032 [DOI] [PubMed] [Google Scholar]
- Szurmak B., Wysłouch-Cieszyńska A., Wszelaka-Rylik M., Bal W., Dobrzańska M. (2008). A diadenosine 5′,5″-P1P4 tetraphosphate (Ap4A) hydrolase from Arabidopsis thaliana that is activated preferentially by Mn2 + ions. Acta Biochim. Polon. 55 151–160. 10.18388/abp.2008_3173 [DOI] [PubMed] [Google Scholar]
- Takahashi S., Sakamoto A. N., Tanaka A., Shimizu K. (2007). AtREV1, a Y-family DNA polymerase in Arabidopsis, has deoxynucleotidyl transferase activity in vitro. Plant Physiol. 145 1052–1060. 10.1104/pp.107.101980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto Y., Tsunemitsu Y., Fujii-Kashino M., Mitani-Ueno N., Yamaji N., Ma J. F., et al. (2017). The tonoplast-localized transporter MTP8.2 contributes to manganese detoxification in the shoots and roots of Oryza sativa L. Plant Cell Physiol. 58 1573–1582. 10.1093/pcp/pcx082 [DOI] [PubMed] [Google Scholar]
- Teardo E., Carraretto L., Moscatiello R., Cortese E., Vicario M., Festa M., et al. (2019). A chloroplast-localized mitochondrial calcium uniporter transduces osmotic stress in Arabidopsis. Nat. Plants 5 581–588. 10.1038/s41477-019-0434-8 [DOI] [PubMed] [Google Scholar]
- Thomine S., Lelièvre F., Debarbieux E., Schroeder J. I., Barbier-Brygoo H. (2003). AtNRAMP3, a multispecific vacuolar metal transporter involved in plant responses to iron deficiency. Plant J. 34 685–695. 10.1046/j.1365-313X.2003.01760.x [DOI] [PubMed] [Google Scholar]
- Thomine S., Wang R., Ward J. M., Crawford N. M., Schroeder J. I. (2000). Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc. Natl. Acad. Sci. U.S.A. 97 4991–4996. 10.1073/pnas.97.9.4991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari M., Sharma D., Dwivedi S., Singh M., Tripathi R. D., Trivedi P. K. (2014). Expression in Arabidopsis and cellular localization reveal involvement of rice NRAMP, OsNRAMP1, in arsenic transport and tolerance. Plant Cell Environ. 37 140–152. 10.1111/pce.12138 [DOI] [PubMed] [Google Scholar]
- Tsukamoto T., Nakanishi H., Kiyomiya S., Watanabe S., Matsuhashi S., Nishizawa N. K., et al. (2006). 52Mn translocation in barley monitored using a positron-emitting tracer imaging system. Soil Sci. Plant Nutr. 52 717–725. 10.1111/j.1747-0765.2006.00096.x [DOI] [Google Scholar]
- Tsunemitsu Y., Genga M., Okada T., Yamaji N., Ma J. F., Miyazaki A., et al. (2018). A member of cation diffusion facilitator family, MTP11, is required for manganese tolerance and high fertility in rice. Planta 248 231–241. 10.1007/s00425-018-2890-1 [DOI] [PubMed] [Google Scholar]
- Turekian K. K., Wedepohl K. H. (1961). Distribution of the elements in some major units of the earth’s crust. GSA Bull. 72 175–192. [Google Scholar]
- Ueno D., Sasaki A., Yamaji N., Miyaji T., Fujii Y., Takemoto Y., et al. (2015). A polarly localized transporter for efficient manganese uptake in rice. Nat. Plants 1:15170. 10.1038/nplants.2015.170 [DOI] [PubMed] [Google Scholar]
- Ullah I., Wang Y., Eide D. J., Dunwell J. M. (2018). Evolution, and functional analysis of natural resistance-associated macrophage proteins (NRAMPs) from Theobroma cacao and their role in cadmium accumulation. Sci. Rep. 8:14412. 10.1038/s41598-018-32819-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkidasamy B., Selvaraj D., Ramalingam S. (2019). Genome-wide analysis of purple acid phosphatase (PAP) family proteins in Jatropha curcas L. Int. J. Biol. Macromol. 123 648–656. 10.1016/j.ijbiomac.2018.11.027 [DOI] [PubMed] [Google Scholar]
- Vert G., Grotz N., Dédaldéchamp F., Gaymard F., Guerinot M., Lou B. J., et al. (2002). IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14 1223–1233. 10.1105/tpc.001388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S., Behera S., De Bortoli S., Logan D. C., Fuchs P., Carraretto L., et al. (2015). The EF-hand Ca2 + binding protein MICU choreographs mitochondrial Ca2 + dynamics in Arabidopsis. Plant Cell 27 3190–3212. 10.1105/tpc.15.00509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Guan Y., Lv M., Zhang R., Guo Z., Wei X., et al. (2018). Manganese increases the sensitivity of the cGAS-STING pathway for double-stranded DNA and is required for the host defense against DNA viruses. Immunity 48 675.e7–687.e7. 10.1016/j.immuni.2018.03.017 [DOI] [PubMed] [Google Scholar]
- Wang C., Xu W., Jin H., Zhang T., Lai J., Zhou X., et al. (2016). A putative chloroplast-localized Ca2 +/H+ antiporter CCHA1 is involved in calcium and pH homeostasis and required for PSII function in Arabidopsis. Mol. Plant 9 1183–1196. 10.1016/j.molp.2016.05.015 [DOI] [PubMed] [Google Scholar]
- Wang N., Qiu W., Dai J., Guo X., Lu Q., Wang T., et al. (2019). AhNRAMP1 enhances manganese and zinc uptake in plants. Front. Plant Sci. 10:415. 10.3389/fpls.2019.00415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhong F., Woo C. H., Miao Y., Grusak M. A., Zhang X., et al. (2017). A rapid and efficient method to study the function of crop plant transporters in Arabidopsis. Protoplasma 254 737–747. 10.1007/s00709-016-0987-6 [DOI] [PubMed] [Google Scholar]
- Waters B. M., Chu H. H., Didonato R. J., Roberts L. A., Eisley R. B., Lahner B., et al. (2006). Mutations in Arabidopsis Yellow Stripe-Like1 and Yellow Stripe-Like3 reveal their roles in metal ion homeostasis and loading of metal ions in seeds. Plant Physiol. 141 1446–1458. 10.1104/pp.106.082586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner A. K., Sparkes I. A., Romeis T., Witte C. P. (2008). Identification, biochemical characterization, and subcellular localization of allantoate amidohydrolases from Arabidopsis and soybean. Plant Physiol. 146 418–430. 10.1104/pp.107.110809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. J., Bowen H. C., Demidchik V., Nichols C., Davies J. M. (2002). Genes for calcium-permeable channels in the plasma membrane of plant root cells. Biochim. Biophys. Acta Biomembr. 1564 299–309. 10.1016/S0005-2736(02)00509-6 [DOI] [PubMed] [Google Scholar]
- White P. J., Greenwood D. J. (2013). “Properties and management of cationic elements for crop growth,” in Soil Conditions and Plant Growth, eds Gregory P. J., Nortcliff S. (Oxford: Blackwell Publishing; ), 160–194 10.1002/9781118337295.ch6 [DOI] [Google Scholar]
- Wilkinson R. E., Ohki K. (1988). Influence of manganese deficiency and toxicity on isoprenoid syntheses. Plant Physiol. 87 841–846. 10.1104/pp.87.4.841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Yamaji N., Yamane M., Kashino-Fujii M., Sato K., Ma J. F. (2016). The HvNramp5 transporter mediates uptake of cadmium and manganese, but not iron. Plant Physiol. 172 1899–1910. 10.1104/pp.16.01189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Liang F., Hong B., Young J. C., Sussman M. R., Harper J. F., et al. (2002). An endoplasmic reticulum-bound Ca2 +/Mn2 + pump, ECA1, supports plant growth and confers tolerance to Mn2 + stress. Plant Physiol. 130 128–137. 10.1104/pp.004440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymer C. L., Bibikova T. N., Gilroy S. (1997). Cytoplasmic free calcium distributions during the development of root hairs of Arabidopsis thaliana. Plant J. 12 427–439. 10.1046/j.1365-313X.1997.12020427.x [DOI] [PubMed] [Google Scholar]
- Xia J., Yamaji N., Kasai T., Ma J. F. (2010). Plasma membrane-localized transporter for aluminum in rice. Proc. Natl. Acad. Sci. U.S.A. 107 18381–18385. 10.1073/pnas.1004949107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H., Yin L., Xu X., Li T., Han Z. (2008). The iron-regulated transporter, MbNRAMP1, isolated from Malus baccata is involved in Fe, Mn and Cd trafficking. Ann. Bot. 102 881–889. 10.1093/aob/mcn178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Hara-Nishimura I., Nishimura M. (2011). Unique defense strategy by the endoplasmic reticulum body in plants. Plant Cell Physiol. 52 2039–2049. 10.1093/pcp/pcr156 [DOI] [PubMed] [Google Scholar]
- Yamada K., Nagano A. J., Nishina M., Hara-Nishimura I., Nishimura M. (2013). Identification of two novel endoplasmic reticulum body-specific integral membrane proteins. Plant Physiol. 161 108–120. 10.1104/pp.112.207654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N., Theerawitaya C., Cha-Um S., Kirdmanee C., Takabe T. (2014). Expression and functional analysis of putative vacuolar Ca2 +-transporters (CAXs and ACAs) in roots of salt tolerant and sensitive rice cultivars. Protoplasma 251 1067–1075. 10.1007/s00709-014-0615-2 [DOI] [PubMed] [Google Scholar]
- Yamaji N., Sasaki A., Xia J. X., Yokosho K., Ma J. F. (2013). A node-based switch for preferential distribution of manganese in rice. Nat. Commun. 4:2442. 10.1038/ncomms3442 [DOI] [PubMed] [Google Scholar]
- Yang M., Zhang W., Dong H., Zhang Y., Lv K., Wang D., et al. (2013). OsNRAMP3 is a vascular bundles-specific manganese transporter that is responsible for manganese distribution in rice. PLoS One 8:e83990. 10.1371/journal.pone.0083990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Zhang Y., Zhang L., Hu J., Zhang X., Lu K., et al. (2014). OsNRAMP5 contributes to manganese translocation and distribution in rice shoots. J. Exp. Bot. 65 4849–4861. 10.1093/jxb/eru259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T. J. W., Perry P. J., Ciani S., Pandian S., Schmidt W. (2008). Manganese deficiency alters the patterning and development of root hairs in Arabidopsis. J. Exp. Bot. 59 3453–3464. 10.1093/jxb/ern195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Zhang C., Liu C., Jing Y., Wang Y., Jin L., et al. (2018). Inner envelope CHLOROPLAST MANGANESE TRANSPORTER 1 supports manganese homeostasis and phototrophic growth in Arabidopsis. Mol. Plant 11 943–954. 10.1016/j.molp.2018.04.007 [DOI] [PubMed] [Google Scholar]
- Zhang M., Liu B. (2017). Identification of a rice metal tolerance protein OsMTP11 as a manganese transporter. PLoS One 12:e0174987. 10.1371/journal.pone.0174987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Xu Y. H., Yi H. Y., Gong J. M. (2012). Vacuolar membrane transporters OsVIT1 and OsVIT2 modulate iron translocation between flag leaves and seeds in rice. Plant J. 72 400–410. 10.1111/j.1365-313X.2012.05088.x [DOI] [PubMed] [Google Scholar]
- Zhang Z., Yin H., Tan W., Koopal L. K., Zheng L., Feng X., et al. (2014). Zn sorption to biogenic bixbyite-like Mn2O3 produced by Bacillus CUA isolated from soil: XAFS study with constraints on sorption mechanism. Chem. Geol. 389 82–90. 10.1016/j.chemgeo.2014.09.017 25987886 [DOI] [Google Scholar]
- Zhao J., Wang W., Zhou H., Wang R., Zhang P., Wang H., et al. (2017). Manganese toxicity inhibited root growth by disrupting auxin biosynthesis and transport in Arabidopsis. Front. Plant Sci. 8:272. 10.3389/fpls.2017.00272 [DOI] [PMC free article] [PubMed] [Google Scholar]