Abstract
We report a case of a collision tumor, a meningioma complicated with metastasis from a primary renal cell carcinoma. A 75-year-old man, with known history of renal cell carcinoma, and 10-year history of stable meningioma developed neurological symptoms. Computed tomography and magnetic resonance imaging revealed left frontal intracranial extra-axial mass with imaging criteria suspicious for an atypical meningioma or hemangiopericytoma. Given the history of a known primary, the possibility of brain metastasis was included. Pathology confirmed the presence of metastatic renal cell carcinoma nidus with a surrounding meningioma. Tumor-to-tumor metastasis or collision tumor is a very rare phenomenon. Atypical radiologic findings with positive history of a primary extracranial tumor should raise the suspicion of potential metastases.
Keywords: Meningioma, Renal cell carcinoma, Tumor to tumor metastasis, Collision tumor
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; CT, Computed tomography; RCC, Renal cell carcinoma; WHO, World Health Organization
Introduction
Meningiomas are the most common primary extra-axial intracranial tumors, accounting for approximately 37.1% of all primary brain tumors as reported from Central Brain Tumor Registry of the United States. Approximately 99% (36.7%/37.1%) are benign and the remaining 1% (0.4%/37.1%) is malignant [1]. Brain metastases are the most common brain tumor in adults, yet there is no national wide registry for metastatic disease. Estimates of frequency vary significantly [2]. As meningiomas and brain metastases are the most frequent type of brain tumors, the coexistence of both tumors can happen theoretically. This phenomenon could occur in 2 ways; the first type is called a collision tumor where two neoplasms coexist with each other. The second type is called tumor-to-tumor metastasis, which has to meet certain specific criteria. For tumor-to-tumor metastasis to occur, there has to be more than 1 primary tumor and the recipient
tumor must be a true neoplasm, and a donor tumor must be the source of true metastasis [1].
This report presents a case of a collision tumor in which a renal cell carcinoma metastasized into a long-standing meningioma. We have found only ten cases in the English literature that described similar findings.
Case report
A 75-year-old white Caucasian male presented with 2-week history of vague memory loss and urinary frequency. He had a 10-year medical history of a stable intracranial mass. This patient underwent right radical nephrectomy in July 2015 for T3, Fuhrman grade 3, multifocal clear, and papillary renal cell carcinoma. He also had the history of a Gleason 6 prostate cancer, and was on active surveillance.
Computed tomography (CT) head without contrast was performed and revealed a large (5.5 × 4.0 cm in axial plane) left frontal intracranial mass. Associated surrounding vasogenic edema, 7 mm right midline shift, and calvarial invasion were present (Fig. 1a). Based on these findings, the diagnostic impression included the possibility of brain metastasis, given the history of known primary tumor, and less likely primary brain neoplasm. He was transferred to the neurosurgery service at Missouri University Hospital, where the patient presented with speech difficulties and diffuse motor weakness, worse on the right.
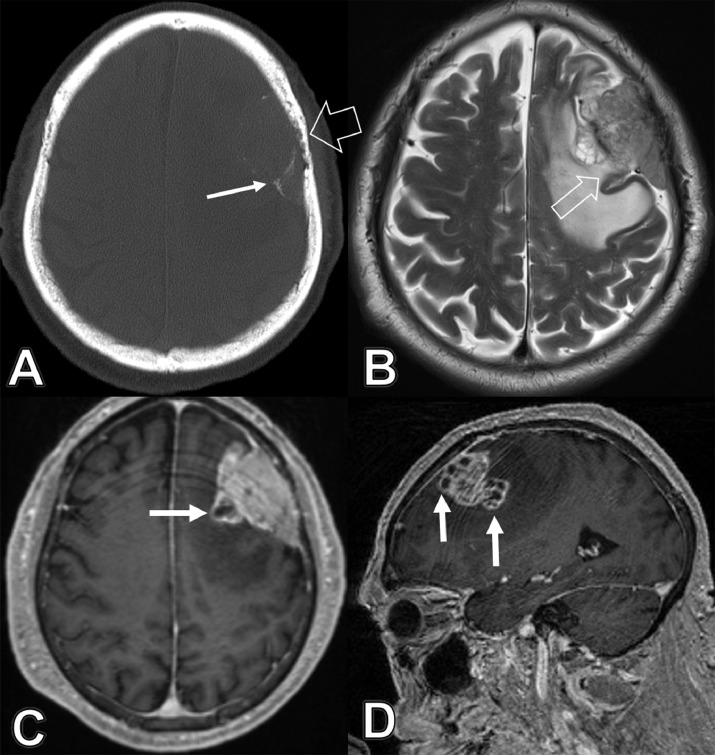
Fig. 1.
A 75-year-old male with previous history of renal cell carcinoma and long-standing intracranial meningioma, presented with 2-week history of vague memory loss and urinary frequency. (A) Axial CT head bone window image demonstrates the mass with peripheral and internal calcific foci “white arrow” as well as erosive/permeative changes of overlying calvarial bone, reflecting bone invasion “wide arrow.” (B) Axial T2 weighted magnetic resonance image shows heterogeneous hyperintense signal of the mass with anteromedial area of cystic changes and focal interruption of the capsule “arrow,” concerning of infiltration of the underlying left frontal lobe. Note the extensive parenchymal edema within left frontal lobe. (C) Axial and (D) sagittal MPRAGE after gadolinium administration demonstrate the cystic changes along the anteromedial margin of the mass, atypical for a meninigioma, and concerning for other alternative diagnoses such as atypical meninigioma, hemangiopericytoma, or metastasis.
MRI of the brain without and with IV gadolinium administration was ordered and confirmed the large left frontal extra-axial space-occupying tumor. This mass had heterogeneous signal on T2/fluid attenuated inversion recovery images with areas of cystic changes along its medial margin (Fig. 1b, c and d). There was a focal interruption of its margin with possible infiltration of the adjacent left superior frontal gyrus (Fig. 1b). Evidence of invasion of overlying frontal calvarial bone was also noted. Associated subcortical vasogenic edema and right midline shift were confirmed (Fig. 1b).
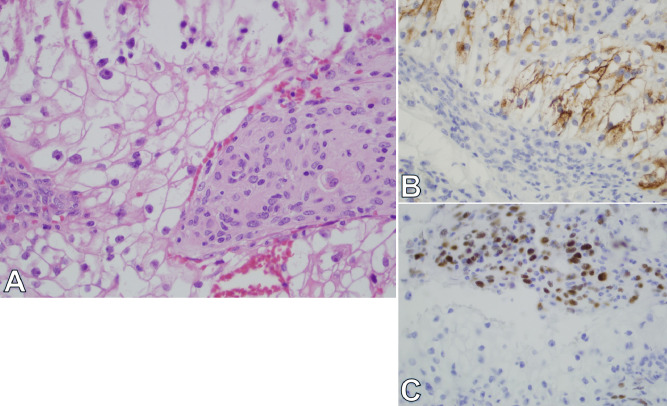
Diagnostic possibilities included aggressive atypical meningioma versus hemangiopericytoma or a metastatic lesion given the history of a known primary tumor. Evaluation with whole body positron emission tomography-CT was ordered to rule out metastatic disease, which was negative. The patient was scheduled for left frontal craniotomy and tumor resection on December 2018. Two left frontal tumor pathology specimens were obtained; the first was diagnosed as a meningioma “WHO grade 1.” The second specimen was a collision tumor, proven to be a metastatic clear cell renal carcinoma infiltrated into the meningioma (Fig. 2a). Immunostains demonstrated that the clear cell tumor was immunoreactive for cytokeratin 7, CD10, and renal cell carcinoma (RCC) markers—these are all typical for renal cell carcinoma—whereas the meningioma in the same sections was immunonegative for these markers (Fig. 2b and c). The meningioma had, at least focally, cells with nuclear immunoreactivity for progesterone receptors. Given the confirmation of metastasis, the patient was referred to the radiation oncology service for three doses stereotactic radiosurgery to the tumor bed. The patient had significant expressive aphasia post operatively and right sided weakness, which improved dramatically during the recovery period.
Fig. 2.
A 75-year-old male with previous history of renal cell carcinoma and long-standing intracranial meningioma, presented with 2-week history of vague memory loss and urinary frequency. (A) Review of H&E stained sections demonstrates a collision of a meningioma with a metastatic carcinoma composed of clear cells. (B and C) Immunohistochemistry with progesterone receptor antibodies highlights only meningioma cells with nuclear positivity. RCC antibodies highlight the clear cells of the carcinoma. These cells were also immunoreactive for cytokeratin 7 and CD10, consistent with renal cell carcinoma.
Discussion
Meningiomas are classified into 3 grades according to the World Health Organization Classification [1]. Benign meningioma (WHO grade1) represents nearly 90% of all meningiomas. The imaging features of typical meningioma are: a broad dural-based round or oval mass with a characteristic appearance of a dural tail on postcontrast images. The most frequent location is supratentorial, but they can be found in the cerebellopontine angle or intraventricularly. The classic CT criteria includes iso to hyperdense appearance on noncontrast CT, and homogenous enhancement after intravenous contrast administration. Associated calcifications, and possible hyperostosis of adjacent calvarial bone, are better demonstrated on CT.
MRI is the modality of choice for radiologic diagnosis and surveillance of meningiomas. It is superior to CT in characterization of intracranial masses, detection of small subtle meningiomas as well as discrimination between small parafalcine meningiomas from focal calcifications of the falx cerebri. Meningiomas typically appear as well circumscribed masses and have homogenous iso-hypointense signal on T1 weighted images, and iso-hyperintense in T2 weighted images, relative to the cerebral gray matter. These low-grade tumors have homogenous enhancement after intravenous gadolinium administration. The appearance of a dural tail after contrast administration is suggestive, but not a specific finding, for meningioma [1].
Imaging criteria which suggests aggressive or atypical behavior include: presence of ill-defined margins, distinct heterogenous intratumoral components within meningioma, significant mass effect on the adjacent brain parenchyma, disproportionate subcortical vasogenic edema, relatively heterogeneous enhancement, increased relative cerebral blood volume on perfusion MRI, evidence of neovascularization, dural penetration, and infiltration of adjacent brain parenchyma. CT can more precisely evaluate the degree of osseous destructions and invasion of overlying calvarial bone. The constellation of findings should raise suspicion for more aggressive and high-graded tumors such as atypical or malignant meningiomas, hemangiopericytomas, meningeal metastasis or a metastatic lesion within a meningioma “collision tumor.” The possibility of metastasis should be evoked especially for a patient with a known primary tumor. It should be noted that some cases were reported in absence of positive history of a primary tumor [3].
The mechanism behind this phenomenon is not understood because of its rarity, but pathological specificity suggests an exact mechanism should occur. There are several theories that could explain this phenomena. Meningiomas have indolent growth pattern and low metabolic rate compared to the surrounding tissue; this may represent a good environment for metastatic seeding and growth. Meningiomas also have a rich vascular supply, which could increase the odds of providing a nidus for a circulating tumor metastasis. The histologic characteristics of meningioma with high collagen and lipid content, also represent a favorable fertile soil for metastatic seeding [4].
It is interesting to note that sometimes brain metastasis of RCC may look like a meningioma especially if it metastasized to the dura. It can have a homogenous contrast enhancement, and a dural tail sign that are usual findings in meningioma. Patricio Tagle et al [5] illustrated 4 cases of brain metastasis where the primary preoperative diagnosis was meningiomas. One of them was a fronto-orbital RCC metastasis where vascular supply was from middle meningeal artery and from internal maxillary artery, features that are suggestive of a meningioma. Brain lesions can be the first manifestations where the radiologic diagnosis suggests a meningioma, but a dural-based metastasis cannot be excluded. Therefore, it is important to maintain a broad differential diagnosis and general cancer screening in patients with initial presentation of an extra-axial dural-based tumor [1].
Conclusion
Atypical radiologic findings with positive history of a primary tumor should raise the suspicion of a collision tumor or dural metastasis. Metastatic lesion in a meningioma is a very rare phenomenon without clear established mechanism.
Footnotes
Declaration of competing interest: All the authors have declared that they have no conflict of interest.
Ethical approval: All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study. All authors have substantial role in idea formulation, data collection, manuscript writing, editing, and revision. All authors have read the final manuscript, approved it, and accepted for publication.
Funding sources: None.
Acknowledgements: None.
Contributor Information
Ayman Nada, Email: anada@health.missouri.edu.
Ahmed Abdelrahman, Email: ahactc@missouri.edu.
Christopher Cunningham, Email: cunninghamchr@health.missouri.edu.
Joseph Cousins, Email: cousinsj@health.missouri.edu.
References
- 1.Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20(suppl_4):iv1–i86. doi: 10.1093/neuonc/noy131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox BD, Cheung VJ, Patel AJ, Suki D, Rao G. Epidemiology of metastatic brain tumors. Neurosurg Clin N Am. 2011;22(1):1–6. doi: 10.1016/j.nec.2010.08.007. v. [DOI] [PubMed] [Google Scholar]
- 3.Nowosielski M, Galldiks N, Iglseder S, Kickingereder P, von Deimling A, Bendszus M. Diagnostic challenges in meningioma. Neuro Oncol. 2017;19(12):1588–1598. doi: 10.1093/neuonc/nox101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zakhari N, Torres C, Castillo M, Nguyen TB. Uncommon cranial meningioma: key imaging features on conventional and advanced imaging. Clin Neuroradiol. 2017;27(2):135–144. doi: 10.1007/s00062-017-0583-y. [DOI] [PubMed] [Google Scholar]
- 5.Tagle P, Villanueva P, Torrealba G, Huete I. Intracranial metastasis or meningioma? An uncommon clinical diagnostic dilemma. Surg Neurol. 2002;58(3–4):241–245. doi: 10.1016/s0090-3019(02)00831-5. [DOI] [PubMed] [Google Scholar]