Abstract
Previous studies have demonstrated inhibitory effect of garlic component diallyl trisulfide (DATS) on growth of breast cancer cells in vitro and in vivo. This study investigated the effect of DATS on oncogenic signaling regulated by leptin, which plays an important role in breast carcinogenesis. Leptin-induced phosphorylation and nuclear translocation of STAT3 was inhibited significantly in the presence of DATS in MCF-7 (a luminal-type human breast cancer cell line) and MDA-MB-231 (a basal-like human breast cancer cell line). Leptin-stimulated cell proliferation, clonogenic cell survival, and migration and/or invasion ability in MCF-7 and/or MDA-MB-231 cells were also suppressed by DATS treatment. DATS exposure resulted in inhibition of leptin-stimulated expression of protein and/or mRNA levels of Bcl-2, Bcl-xL, Cyclin D1, vascular endothelial growth factor, and matrix metalloproteinase-2. Western blotting revealed a decrease in protein levels of phosphorylated STAT3 in breast cancer xenografts from DATS-treated mice when compared to controls in vivo. However, the incidence of N-methyl-N-nitrosourea-induced luminal-type breast cancer development in rats was not affected by oral administration of 5 mg/kg or 25 mg/kg DATS. The present study reveals that oncogenic signaling induced by leptin is inhibited in the presence of DATS but higher doses of this phytochemical may be required to achieve chemopreventive activity.
Keywords: Breast cancer, Leptin, STAT3, Diallyl trisulfide, Chemoprevention
INTRODUCTION
Epidemiological studies have implied an inverse relationship between dietary intake of Allium vegetables (e.g., garlic and onions) and the risk of breast cancer, which continues to be a major cause of cancer-associated mortality in women worldwide [1-3]. For example, breast cancer risk was inversely associated with increasing intake of garlic and onions in a French case-control study of 345 pathologically confirmed cases and the same number of controls matched for age and social and economic status [3]. The bioactive phytochemicals with anticancer activity in Allium vegetables include water-soluble (e.g., S-allyl cysteine) and fat-soluble sulfur compounds such as diallyl trisulfide (DATS) but the later class exhibits greater anticancer potency compared with the water-soluble phytochemicals at least in cellular models of cancer [4,5]. The γ-glutamyl-S-alk(en)yl-L-cysteine that is hydrolyzed and oxidized to produce alliin is the odorless precursor of bioactive sulfur compounds in Allium vegetables [4]. Cutting or chewing of Allium vegetables releases a vacuolar enzyme (allinase) that acts upon alliin to give rise to allicin and other alkyl alkane-thiosulfinate compounds [4]. Eventually, various sulfur compounds including DATS are generated due to decomposition of allicin [4].
Growth inhibitory effects and associated mechanisms of DATS have been studied using human breast cancer cell lines and athymic xenograft models [6-9]. DATS treatment resulted in suppression of MCF-7 (a human breast cancer cell line with molecular characteristics of luminal-subtype) cell viability that was accompanied by a decrease in percent of cells in G2 phase and induction of apoptotic cell death [6]. These effects of DATS in MCF-7 cell line were associated with increased expression of FAS, Bax, and p53 and downregulation of Akt and Bcl-2 proteins [6]. Another study implicated pro-apoptotic protein Bim in cytotoxicity of DATS in the MDA-MB-231 cell line [7]. Specifically, DATS treatment caused oxidative stress leading to dissociation of glutaredoxin from apoptosis signal-regulating kinase 1 that resulted in activation of downstream signal transduction of c-Jun N-terminal kinase-Bim pathway [7]. A role for reactive oxygen species-mediated activation of c-Jun N-terminal kinase and activator protein 1 was demonstrated in DATS-induced apoptosis in MCF-7 cells [8]. The in vivo growth of MCF-7 xenografts was inhibited significantly by oral administration of DATS [8]. We showed that DATS-induced apoptosis in MDA-MB-231 and MCF-7 cells was significantly abrogated by ectopic expression of superoxide dismutase due to attenuation of Bak activation [9]. Furthermore, the DATS-mediated inhibition of breast cancer cell migration was also significantly attenuated by superoxide dismutase overexpression [9]. Cellular effects in a non-tumorigenic MCF-10A cell line induced by an environmental carcinogen (benzo[a]pyrene) were reversible following DATS exposure [10]. These studies indicate that DATS is a promising agent potentially useful for treatment and/or prevention of breast cancer. Therefore, elucidation of the mechanism(s) underlying anticancer effect of DATS is/are important for its clinical development.
In this study, we investigated the effect of DATS treatment on leptin-induced signaling in human breast cancer cells in vitro and in vivo. We also tested the possibility of cancer prevention by DATS using a chemically-induced rat model of breast cancer. Impetus for the present study was based on following observations: (a) signal transducer and activator of transcription 3 (STAT3) is a known downstream target of leptin, and DATS treatment inhibits STAT3 activation in cancer cells [11,12], and (b) leptin is known to regulate breast cancer stem-like cells and DATS suppresses self-renewal of this population in breast cancer cells [13,14]. Leptin is a 16 kDa cytokine that exercises its action through binding to its specific receptors on the cell membrane of a variety of tissues [11]. Leptin has been implicated in initiation and progression of breast cancer [11]. Moreover, biological effects of leptin are regulated in cooperation with multiple oncogenes (e.g., STAT3, mitogen-activated protein kinases, phosphoinositide 3-kinase/Akt etc.), cytokines (interleukin-1 and interleukin-8), and growth factors like insulin-like growth factor-1/epidermal growth factor receptor [11]. Leptin is also known to stimulate production of pro-inflammatory cytokines as well as promote T-helper 1 responses [11].
MATERIALS AND METHODS
Ethics statement
The use of rats for determining the effect of DATS administration on incidence of N-methyl-N-nitrosourea (MNU)-induced luminal type breast cancer was approved by the University of Pittsburgh Animal Care and Use Committee (protocol # 18114222).
Reagents
DATS was purchased from LKT Laboratories (St. Paul, MN, USA). Cell culture media were purchased from Cellgro (CORNING, Corning, NY, USA). Leptin was purchased from R&D Systems (Minneapolis, MN, USA). Matrigel was from BD Biosciences (San Jose, CA, USA) and Transwell permeable support was from Costar (CORNING). Antibodies against phospho-STAT3 (Tyr705), phospho-STAT3 (Ser727), and total STAT3 were from Cell Signaling (Danvers, MA, USA). Antibodies against Bcl-xL, Cyclin D1 and phospho-STAT3 (Tyr705) for immunofluorescence microscopy were from Santa Cruz Biotechnology (Dallas, TX, USA). Anti-b-actin antibody was from Sigma (Sigma-Aldrich, St. Louis, MO, USA) and anti-Bcl-2 antibody was from DakoCytomation (Glostrup, Denmark). SYTOX Green reagent and Alexa Fluor 568 goat anti-rabbit antibody were from Invitrogen (Waltham, MA, USA).
Cell lines and treatment
The MCF-7 and MDA-MB-231 cells were obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained as suggested by the supplier. Both these cell lines were last authenticated by us in March of 2017. Stock solution of DATS was prepared in dimethyl sulfoxide (DMSO), and an equal volume of DMSO (0.1%) was added to controls.
For treatment, cells were seeded and then serum starved for 16 hours. The cells were treated with or without DATS (40 μmol/L) for 2 hours followed by exposure to leptin (100 ng/mL) for specified time for each experiment.
Western blotting
Whole lysates from cells treated with DMSO (control) or DATS and/or leptin were prepared as described by us previously [15]. Lysate proteins were resolved by polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membrane. After blocking with 5% non-fat dry milk, the membrane was incubated with the desired primary antibody for overnight at 4°C. The membrane was then treated with secondary antibody and the bands were visualized by enhanced chemiluminescence method.
Immunocytochemistry for nuclear localization of P-STAT3
Cells (1 × 105) were plated on coverslips and allowed to attach by overnight incubation. The following day, cells were serum-starved for 16 hours and pretreated with DATS for 2 hours prior to exposure to leptin for 3 hours. The cells were fixed with 2% paraformaldehyde for 1 hour at room temperature and permeabilized with 0.05% Triton X-100 for 10 minutes. The cells were then incubated with PBS containing 0.5% bovine serum albumin and 0.15% glycine for 1 hour followed by overnight incubation with anti-phospho (P)-STAT3 antibody at 4°C. The cells were then treated with 2 μg/mL of Alexa Fluor 568–conjugated secondary antibody for 1 hour at room temperature. After washing with PBS, the cells were counterstained with 0.5 μmol/L SYTOX Green for 5 minutes at room temperature. Subsequently, the cells were mounted and observed under a Leica DC300F fluorescence microscope at 100× objective magnification.
Cell viability and clonogenic assays
Cells were seeded in 12-well plates at a density of 105 cells/well. After 16 hours of serum starvation, the cells were treated with or without DATS (40 μmol/L) for 2 hours and then treated with leptin (100 ng/mL) for 22 hours in the absence or presence of DATS. The effects of leptin and/or DATS treatments on cell viability was determined by trypan blue dye exclusion assay as described by us previously [5]. For clonogenic assay, the cells were plated in 6-well plates at a density of 500 cells/well and treated with leptin and/or DATS as described for the cell viability assay. Medium containing DATS and/or leptin was replaced every 3 days. After 12 days, the medium was removed and colonies were stained with 0.5% crystal violet. The colonies were counted under a microscope.
Reverse transcription-PCR
Total RNA from MCF-7 and MDA-MB-231 cells treated with DMSO, leptin and/or DATS was extracted with the use of the RNeasy kit (Qiagen, Hilden, Germany). The cDNA was synthesized with the use of 1 μg of total RNA using SuperScript III reverse transcriptase with oligo(dT)20 primer. Specific primers and amplification conditions were as follow: Bcl-2 (forward) 5’-TGCACCTGACGCCCTTCAC-3’ and (reverse) 5’-TAGCTGATTCGACGTTTTGCCTGA-3’; Bcl-xL (forward) 5’-CCCAGAAAGGATACAGCTGG-3’ and (reverse) 5’-GCGATCCGACTCACCAATAC-3’; Cyclin D1 (forward) 5’-TGTTTGCAAGCAGGACTTTG-3’ and (reverse) 5’-ACGTCAGCCTCCACACTCTT-3’; matrix metalloproteinase (MMP)-2 (forward) 5’- AGATCTTCTTCTTCAAGGACCGGTT-3’ and (reverse) 5’-GGCTGGTCAGTGGCTTGGGGTA-3’; MMP-9 (forward) 5’-GCGGAGATTGGGAACCAGCTGTA-3’ and (reverse) 5’-GACGCGCCTGTGTACACCCACA-3’; VEGF (forward) 5’-CCATGAACTTTCTGCTGTCTTGG-3’ and (reverse) 5’-CTCACCGCCTCGGCTTGTCAC-3’; glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (forward) 5’- GGACCTGACCTGCCGTCTAGAA-3’ and (reverse) 5’- GGTGTCGCTGTTGAAGTCAGAG-3’. Amplification conditions were: Bcl-2: 94°C/15 seconds, 56°C/30 seconds, 72°C/30 seconds, 28 cycles; Bcl-xL: 94°C/15 seconds, 68°C/1 minute, 72°C/1 minute, 28 cycles; Cyclin D1: 94°C/40 seconds, 59°C/40 seconds, 72°C/1 minute, 32 cycles; MMP-2: 94°C/45 seconds, 60°C/1 minute, 72°C/1 minute, 30 cycles; MMP-9: 94°C/30 seconds, 68°C/1 minute, 72°C/30 seconds, 34 cycles; VEGF: 94°C/45 seconds, 60°C/1 minute, 72°C/1 minute, 34 cycles; GAPDH: 94ºC/30 seconds, 60ºC/30 seconds, 72ºC/30 seconds, 30 cycles.
Determination of cell migration and invasion
For cell migration assay, we used 24-well plate inserts with 8.0 μm pore size polycarbonate filters (Costar; CORNING). For cell invasion assay, filters were pre-coated with 25 μL diluted Matrigel in serum-free medium (1 : 2) for 5 hours at 37°C. Serum starved MDA-MB-231 cells were seeded in upper chamber in 150 μL of serum-free media, while media supplemented with 10% FBS (600 μL) was placed in the lower chamber. The following procedures were the same for both cell migration and cell invasion assays. Twenty-four hour following treatment with DMSO, DATS and/or leptin, the cells remaining in the upper chamber were gently removed with a cotton swap. Cells that migrated or invaded through the Matrigel to the lower chamber were fixed in methanol, stained with hematoxylin and eosin and photographed. The migrated or invaded cells from each filter were counted under a microscope at 100× magnification. The experiments were performed in triplicate and repeated to ensure reproducibility of the results.
RNA interference
The MDA-MB-231 (5 × 104) cells were seeded in 6-well plates and transfected at ~50% confluency with 100 nmol/L of control siRNA or STAT3-targeted siRNA using Oligofectamine. Twenty-four hours after transfection, the cells were serum starved for 16 hours and then treated with DMSO or DATS/and or leptin for 24 hours. After treatment, the cells were processed for immunoblotting and invasion assay.
Analysis of the RNA-Seq data from The Cancer Genome Atlas
Association of STAT3 expression with that of Bcl-2, Bcl-xL (BCL2L1), Cyclin D1 (CCND1), VEGF, MMP-2, and MMP-9 was determined from the breast cancer RNA-Seq data from The Cancer Genome Atlas (TCGA) with the help of University of California Santa Cruz Xena Browser (http://xena.ucsc.edu/public-hubs/).
Chemoprevention study in rats
To determine the in vivo efficacy of DATS for chemoprevention of luminal type breast cancer, a Sprague–Dawley rat model of breast cancer induced by MNU was used. MNU was freshly prepared in 0.9% sodium chloride solution (Sigma) in the dark prior to injection. Female rats (21-day old) were injected intraperitoneally with MNU (50 mg/kg body weight). One week after MNU injection, the rats were randomly divided into 3 groups. The control group of rats (n = 21) were orally administered with 100 μL of corn oil (vehicle for DATS) 5 times/wk. The second and third groups of rats were orally treated with 100 mL of corn oil containing 5 mg DATS/kg body weight (n = 20) or 25 mg DATS/kg body weight (n = 20) 5 times/wk. The body weights of rats of each group were recorded at weekly intervals. Each rat of every group was palpated daily for the presence of mammary tumor. Two rats from the control group and one rat from the 25 mg DATS/kg body weight group were sacrificed early (at 7-9 weeks post-MNU injection) due to tumor burden. Rest of the rats were sacrificed at 11 weeks after MNU injection.
Statistical analysis
GraphPad Prism (ver. 7.02; https://www.graphpad.com/scientific-software/prism/) was used for statistical analyses. ANOVA followed by Bonferroni’s test was used for multiple group comparisons. Student’s t-test was used for comparison of two groups. Fisher’s exact test was used for statistical comparison of tumor incidence in the rat study.
RESULTS
DATS inhibited leptin-stimulated activation of STAT3 in breast cancer cells
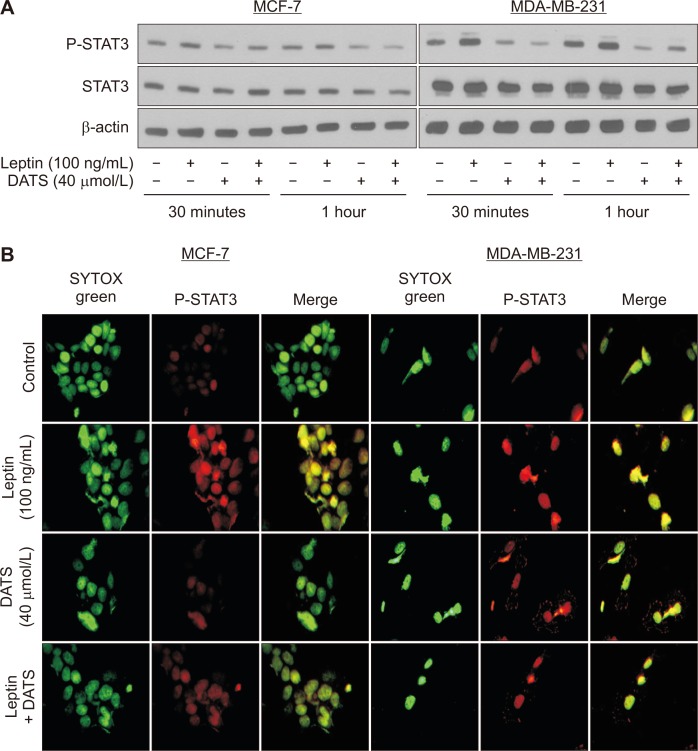
We used 40 μmol/L DATS concentration for in vitro studies based on its pharmacokinetics in rats after administration of a single 10 mg dose [16]. The maximum blood concentration of DATS was found to be ~31 μmol/L [16]. Leptin treatment alone resulted in an increase in phosphorylation of STAT3, a measure of its activation, in MCF-7 and MDA-MB-231 cells that was not due to induction of total STAT3 protein (Fig. 1A). Constitutive phosphorylation of STAT3 was markedly suppressed following DATS treatment in both cell lines (Fig. 1A). Furthermore, leptin-stimulated activation of STAT3 was also decreased in the presence of DATS (Fig. 1A).
Figure 1. Diallyl trisulfide (DATS) treatment inhibits constitutive as well as leptin-induced STAT3 phosphorylation at Tyr-705 in human breast cancer cells.
(A) Immunoblotting for phospho (P)-STAT3, STAT3, and β-actin using lysates from 16-hour serum starved MCF-7 (left panel) and MDA-MB-231 (right panel) cells. Cells were first serum starved for 16 hours and then exposed to dimethyl sulfoxide (DMSO; control) or 40 µmol/L DATS for 2 hours and then further treated for 30 minutes or 1 hour after adding 100 ng/mL leptin. Experiment was repeated twice. (B) Immunocytochemistry for P-STAT3 (red fluorescence) in MCF-7 (left panel) and MDA-MB-231 (right panel) cells. Cells were serum starved for 16 hours and then treated with DMSO (control) or 40 µmol/L DATS for 2 hours and further incubated for 3 hours after adding 100 ng/mL leptin. SYTOX Green was used to stain nucleus. Images were captured at 100 × objective magnification. Experiment was repeated twice with comparable results.
Extracellular signal-regulated kinase (ERK) is another downstream target of leptin in cancer cells [17]. We therefore examined the effects of leptin and/or DATS treatment on protein levels of phosphorylated-ERK and total ERK in MCF-7 and MDA-MB-231 cells by western blotting. However, the results were not consistent (data not shown). As the effects of leptin and/or DATS were consistent for STAT3 in both cell lines, we focused on this pathway for further investigation.
DATS inhibited leptin-stimulated nuclear translocation of STAT3 in breast cancer cells
STAT3 is a transcription factor and hence its nuclear translocation is essential for its activity. Figure 1B depicts results of immunocytochemistry for P-STAT3 in DMSO-treated control MCF-7 and MDA-MB-231 cells and those treated with leptin and/or DATS. Consistent with Western blotting results, leptin treatment alone caused an increase in nuclear staining for phosphorylated STAT3 in MCF-7 and MDA-MB-231 cells reflected by merging of red fluorescence (P-STAT3) and SYTOX Green fluorescence (a nuclear stain) (Fig. 1B). The leptin-stimulated nuclear localization of P-STAT3 was decreased by DATS treatment (Fig. 1B). DATS alone was also effective in reducing the nuclear level of P-STAT3 (Fig. 1B). Collectively, these results indicated inhibition of leptin-stimulated activation of STAT3 following DATS treatment in a luminal-type (MCF-7) and a basal-like (MDA-MB-231) human breast cancer cell line.
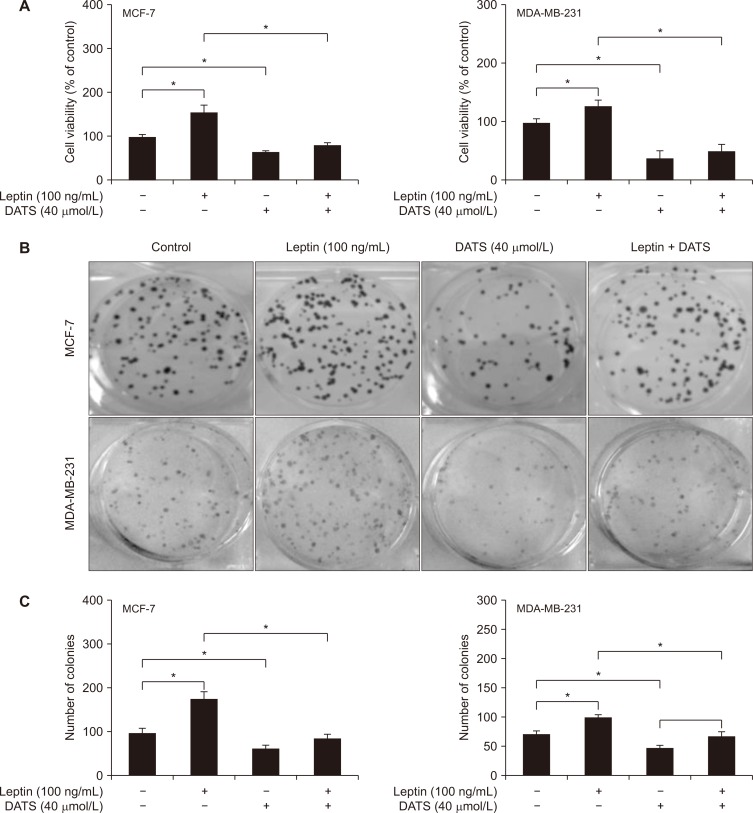
DATS inhibited leptin-stimulated survival of breast cancer cells
The viability of MCF-7 and MDA-MB-231 cells was increased significantly following leptin stimulation (Fig. 2A). DATS treatment alone resulted in inhibition of cell viability (Fig. 2A). The leptin-stimulated survival of MCF-7 and MDA-MB-231 cells was also decreased in the presence of DATS (Fig. 2A). Figure 2B shows images of colonies of MCF-7 and MDA-MB-231 cells following 12 days of treatment with leptin and/or DATS or solvent control. Consistent with cell viability data (Fig. 2A), the number of colonies was increased in the presence of leptin (Fig. 2C). The leptin-stimulated clonogenic survival of MCF-7 and MDA-MB-231 cells was decreased significantly by DATS treatment (Fig. 2C).
Figure 2. Diallyl trisulfide (DATS) treatment inhibits basal as well as leptin-induced cell growth in human breast cancer cells.
(A) Quantitation of cell viability of 16-hour serum-starved MCF-7 (left panel) and MDA-MB-231 (right panel) cells treated with dimethyl sulfoxide (DMSO; control) or 40 µmol/L DATS for 2 hours and then further incubated for 22 hours after adding 100 ng/mL leptin. Results shown are mean ± SD (n = 3) and statistical analysis was performed by one-way ANOVA followed by Bonferroni’s multiple comparisons test (*P < 0.05). Experiment was repeated twice with consistent results. (B) Representative images of colonies from 16-hour serum-starved MCF-7 (upper panel) and MDA-MB-231 (lower panel) cells treated with DMSO (control) or 40 µmol/L DATS for 2 hours and then further incubated for 12 days after adding 100 ng/mL leptin. Media containing agents was changed every third day. (C) Quantitation of colony formation from data shown in panel B. Results shown are mean ± SD (n = 3) and statistical analysis was performed by one-way ANOVA followed by Bonferroni’s multiple comparisons test (*P < 0.05). Experiment was repeated twice with consistent results.
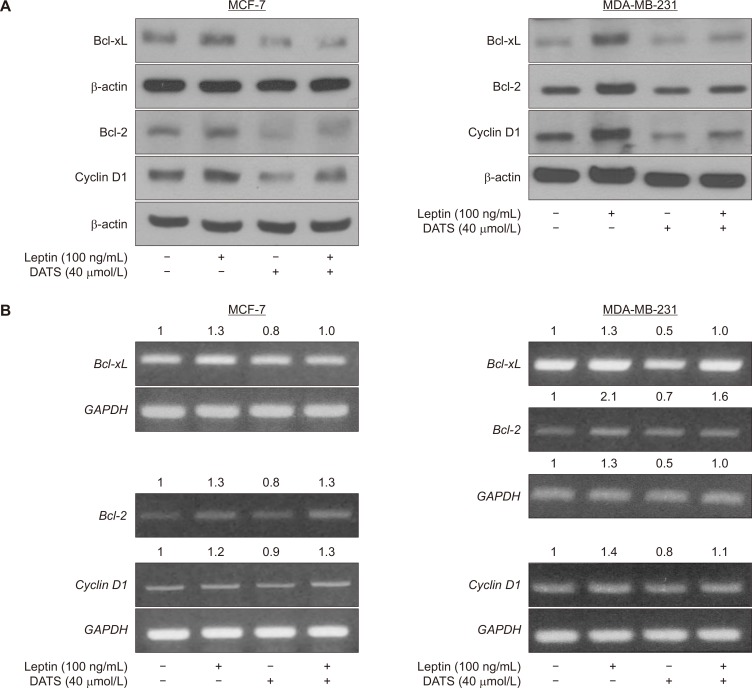
DATS inhibited leptin-stimulated expression of pro-survival proteins in breast cancer cells
The expression of pro-survival proteins, including Bcl-2, Bcl-xL, and Cyclin D1 was increased after leptin treatment in MCF-7 and MDA-MB-231 cells (Fig. 3A). Leptin-stimulated induction of these proteins was decreased by DATS (Fig. 3A). DATS treatment alone also caused a decrease in the levels of these proteins in both cells (Fig. 3A). The results of reverse transcription-PCR for Bcl-2, Bcl-xL, and Cyclin D1 mRNA expression in leptin and/or DATS treated cells (Fig. 3B) were generally consistent with the results of Western blotting data (Fig. 3A). These results may explain DATS-mediated inhibition of leptin-stimulated survival of MCF-7 and MDA-MB-231 cells.
Figure 3. Diallyl trisulfide (DATS) treatment inhibits basal as well as leptin-stimulated expression of pro-survival proteins regulated by STAT3.
(A) Immunoblotting for Bcl-2, Bcl-xL, Cyclin D1, and β-actin using lysates from 16-hour serum starved MCF-7 (left panel) and MDA-MB-231 (right panel) cells treated with dimethyl sulfoxide (DMSO; control) or 40 µmol/L DATS for 2 hours and then further incubated for 22 hours after adding 100 ng/mL leptin. Experiment was repeated at least twice. (B) Reverse transcription-PCR analysis for Bcl-2, Bcl-xL, Cyclin D1, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) using 16-hour serum starved MCF-7 (left panel) and MDA-MB-231 (right panel) cells treated with DMSO (control) or 40 µmol/L DATS for 2 hours and then further incubated for 22 hours after adding 100 ng/mL leptin. Numbers above bands indicate change in expression relative to solvent control treated cells. Experiment was repeated at least twice.
Next, we analyzed the association of STAT3 expression with that of Bcl-2, Bcl-xL (BCL2L1), Cyclin D1 (CCND1), VEGF, MMP-2, and MMP-9 from the breast cancer RNA-Seq data from TCGA. Statistically significant positive association was discernible between STAT3 and Bcl-2 or MMP-2. On the other hand, STAT3 expression was negatively associated with that of Bcl-xL, VEGFB, and MMP-9 (Figure S1).
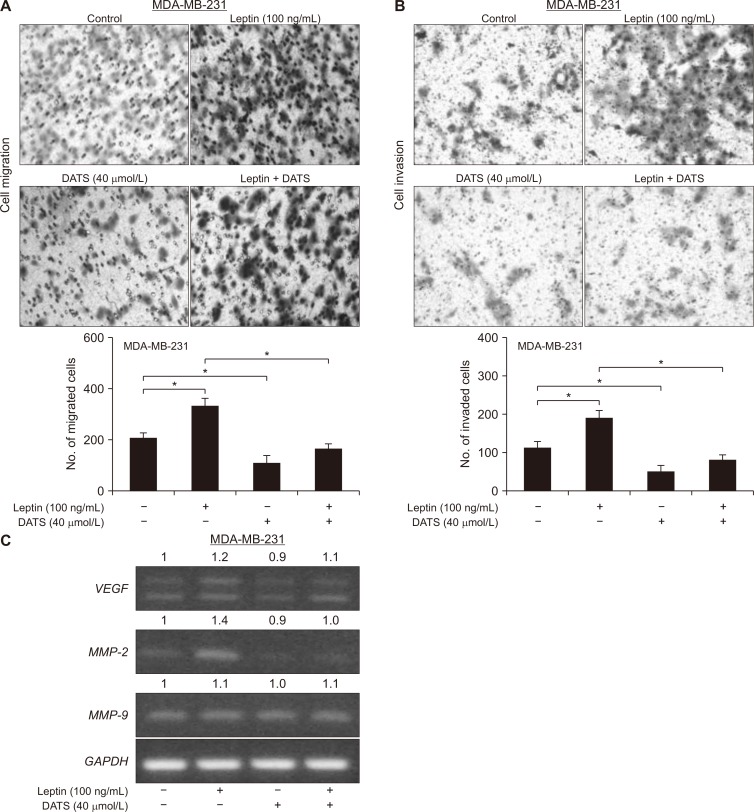
DATS inhibited leptin-stimulated migration and invasion ability of breast cancer cells
As can be seen in Figure 4A, migration of MDA-MB-231 cells was increased significantly by leptin exposure that was sensitive to inhibition by DATS treatment. Similarly, leptin-mediated increase in invasion of MDA-MB-231 cells was inhibited significantly in the presence of DATS (Fig. 4B). DATS treatment alone also inhibited migration and invasion of MDA-MB-231 cells. Induction of pro-metastasis genes, including VEGF and MMP-2 resulting from leptin treatment was suppressed in the presence of DATS (Fig. 4C). These results provided mechanistic insights into the DATS-mediated inhibition of leptin-stimulated migration and invasion of MDA-MB-231 cells. However, MMP-9 may not be involved in DATS-mediated inhibition of leptin-stimulated cell invasion. Finally, these experiments were not done with MCF-7 cells as they migrate and invade poorly as compared to MDA-MB-231 cells.
Figure 4. Diallyl trisulfide (DATS) treatment inhibits leptin-stimulated migration and invasion of MDA-MB-231 cells.
(A) Representative H & E-stained images (× 100 magnification) showing cell migration and its quantification. Cells were serum starved for 16 hours and then treated with dimethyl sulfoxide (DMSO; control) or 40 µmol/L DATS for 24 hours in the absence or presence of 100 ng/mL leptin. Results are shown as mean ± SD (n = 3) and statistical analysis was performed by one-way ANOVA followed by Bonferroni’s multiple comparisons test (*P < 0.05). Experiment was repeated thrice with consistent results. (B) Representative H & E-stained images (× 100 magnification) showing cell invasion and its quantification. Cells were serum starved for 16 hours and then treated with DMSO (control) or 40 µmol/L DATS for 24 hours in the absence or presence of 100 ng/mL leptin. Results are shown as mean ± SD (n = 2-3) and statistical analysis was performed by one-way ANOVA followed by Bonferroni’s multiple comparisons test (*P < 0.05). Experiment was repeated thrice with consistent results. (C) Reverse transcription-PCR analysis for VEGF, matrix metalloproteinase MMP-2, MMP-9, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) using lysates from 16-hour serum starved MDA-MB-231 cells treated with DMSO (control) or 40 µmol/L DATS for 2 hours and then further incubated for 22 hours after adding 100 ng/mL leptin. Numbers above bands indicate change in expression relative to solvent control treated cells. Experiment was repeated thrice with consistent results.
Effect of RNA interference of STAT3 on invasion of MDA-MB-231 cells
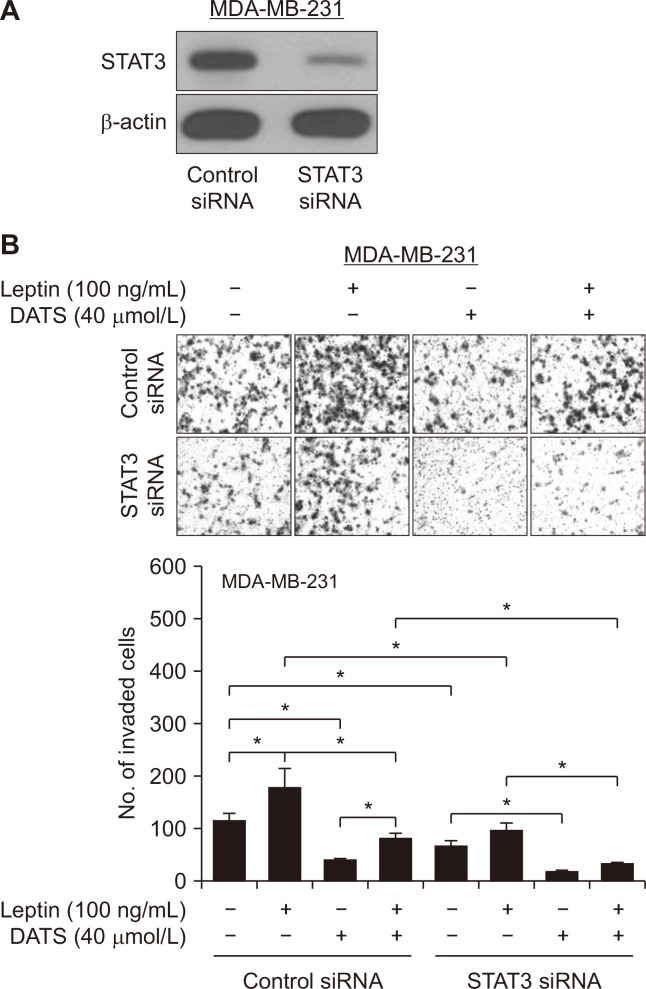
Figure 5A shows knockdown of STAT3 protein after transfection of MDA-MB-231 cells with a STAT3-targeted siRNA when compared to cells transfected with a control non-specific siRNA. RNA interference of STAT3 decreased the ability of leptin to increase cell invasion (Fig. 5B). On the other hand, DATS-mediated inhibition of leptin-stimulated invasion of MDA-MB-231 cells was augmented by knockdown of STAT3 protein (Fig. 5B). As DATS still inhibits the invasiveness of the cells even in the absence of STAT3, these findings suggest that DATS exerts antiproliferative and anti-migrative effects, at least in part, independently of targeting STAT3.
Figure 5. STAT3 knockdown augments diallyl trisulfide (DATS)-mediated inhibition of leptin-stimulated cell invasion in MDA-MB-231 cells.
(A) Immunoblotting for STAT3 and β-actin in MDA-MB-231 cells transfected with control or STAT3-targeted siRNA. (B) Representative H & E-stained images (× 100 magnification) and quantification of cell invasion in 16-hour serum-starved transiently transfected MDA-MB-231 cells with control or STAT3 siRNA and treated for 24 hours with dimethyl sulfoxide (control) or 40 µmol/L DATS in the absence or presence of 100 ng/mL leptin. Results are shown as mean ± SD (n = 3) and statistical analysis was performed by one-way ANOVA followed by Bonferroni’s multiple comparisons test (*P < 0.05). Experiment was repeated twice with consistent results.
DATS treatment differentially inhibited phosphorylation of STAT3 in vivo
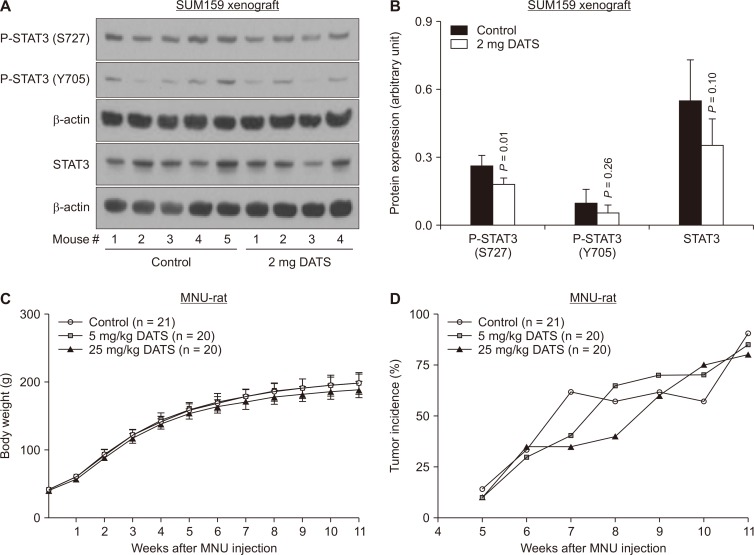
We have shown previously that oral treatment with 2 mg DATS (in 100 μL of PBS beginning on the day of tumor cell implantation) 3 times/wk decreases tumor take (incidence) of a basal-like SUM159 xenografts by 50% when compared to control mice [14]. The average wet weight of the SUM159 xenografts was also lower in DATS-treated mice compared with control mice [14]. Supernatants from SUM159 xenografts from this study were used to check the levels of phosphorylated and total STAT3. The level of P-STAT (S727), bit not P-STAT (Y705), was significantly lower (Fig. 6A and 6B).
Figure 6. Diallyl trisulfide (DATS) administration inhibits phosphorylation of STAT3 at Ser-727 in SUM159 xenografted mice and shows no signs of toxicity in N-methyl-N-nitrosourea (MNU)-induced Sprague–Dawley rats.
(A) Immunoblots showing protein levels of P-STA3 (S727), P-STAT3 (Y705), and total STAT3 using tumor lysates from SUM159 xenografts. (B) The bar graph shows quantitation of immunoreactive bands for P-STAT3 (S727), P-STAT3 (Y705), and total STAT3. Results shown are mean ± SD (n=5 for control group and n=4 for DATS treatment group). Statistical analysis was performed by unpaired Student’s t-test. (C) Body weight over time in MNU-injected rats fed vehicle (control) or DATS (5 or 25 mg/kg body weight). Results shown are mean ± SD (n = 21 for control, n = 20 for 5 mg/kg DATS, and n = 20 for 25 mg/kg DATS). Statistical analysis was performed by one-way ANOVA followed by Dunnett’s test. (D) Palpable mammary tumor incidence over time in MNU-injected rats fed vehicle or DATS (5 or 25 mg/kg body weight). Statistical analysis was performed by Fisher’s exact test.
N-methyl-N-nitrosourea-induced breast cancer formation in rats was not prevented by oral administration of DATS
We next proceeded to determine whether DATS administration could prevent cancer development using a well-established rat model of chemically-induced breast cancer. DATS administration did not cause weight loss (Fig. 6C) or any other obvious side effects like water and food withdrawal or gastrointestinal issues such as diarrhea or bleeding. Despite an early promising effect (e.g., 7 weeks post-MNU injection), the overall incidence of breast cancer was comparable in control and DATS-treated rats (Fig. 6D). The tumor volume also did not differ significantly between control and DATS-treated rats (data not shown). These results indicated that DATS was ineffective in preventing MNU-induced luminal-type tumors in a rat model at least at the tested doses. Because the mammary cancer incidence was not affected, expression of STAT3 or its target proteins was not determined from rat tumor tissues.
DISCUSSION
Leptin is an adipokine that signals by binding to its receptor Ob-R and regulates energy balance in normal subjects [18]. Leptin has an oncogenic role in breast cancer and is one of the mechanistic links explaining obesity-breast cancer connection [19]. A study using 76 invasive ductal carcinomas and 32 samples of corresponding normal mammary gland showed weak expression of Ob-R in normal mammary cells but its overexpression in 83% of ductal carcinoma cells [20]. Moreover, overexpression of leptin was also reported in 92% of ductal carcinoma specimens [20]. Leptin not only promotes growth and invasion of breast cancer cells but also stimulates epithelial-mesenchymal transition [21-23]. The present study demonstrates, for the first time that oncogenic effects of leptin are counteracted by a safe phytochemical from garlic that is a key ingredient of human diet for many cultures worldwide.
STAT3 is one of the oncogenes that is activated by leptin in breast and other cancers [11,17,24]. STAT3 is overexpressed in breast cancer and it is a highly investigated therapeutic target for this malignancy [25]. In this study, we show that DATS treatment inhibits activation of STAT3 in breast cancer cells in vitro as well as in SUM159 xenografts in vivo. The leptin-mediated activation of STAT3 is also suppressed in the presence of DATS. Inhibition of STAT3 signaling following DATS treatment has been reported in other cell types including mouse colitis and prostate cancer [12,26]. Activation of STAT3 is mediated by Janus activated kinase (JAK) [27]. It is possible that DATS treatment inhibits JAK to suppress phosphorylation of STAT3 in breast cancer cells. Even though further work is necessary to explore this mechanistic possibility, we have shown previously that DATS treatment decreases interleukin 6-stimulated phosphorylation of JAK2 in prostate cancer cells [12].
STAT3 is known to regulate expression of many cancer relevant genes [28-30]. STAT3 occupancy at the promoter of Bcl-2 was shown in HeLa cells by chromatin immunoprecipitation assay [28]. Breast cancer TCGA data also shows excellent positive association between expression of STAT3 and Bcl-2. In agreement with these results, we also found that leptin-mediated activation of STAT3 was accompanied by induction of Bcl-2. Likewise, the positive association for expression of STAT3 with that of MMP-2 in breast cancer TCGA is consistent with the published literature [29]. A role for STAT3 in regulation of MMP-2 expression was established from an electrophoretic mobility shift assay [30]. Surprisingly, analysis of the breast cancer TCGA did not reveal statistically significant positive association for STAT3 expression with that of Bcl-xL, Cyclin D1, VEGF, and MMP-9 even though literature suggests regulatory function of STAT3 for these genes [11,30-33]. The reasons for these discrepancies are not clear but may be explained by the fact that leptin can activate other signaling pathways and transcription factors [18,19,21-24]. Nevertheless, the present study reveals that DATS inhibits constitutive as well as leptin-inducible expression of many pro-survival and pro-metastatic proteins leading to inhibition of cell proliferation, migration, and invasion.
It was surprising to us that DATS administration did not prevent development of MNU-induced breast cancer in rats because this agent is highly effective for prevention or therapy of breast and other cancers [8,34,35]. Moreover, DATS is known to inhibit estrogen receptor α expression and activity in MCF-7 and T47D cells [36] and MNU-induced mammary tumors in rats are estrogen receptor α positive with similarities to luminal-type disease [37-39]. For example, 17β-estradiol-stimulated growth of MCF-7 cells implanted on the left or right thoracic area in female Balb/c nude mice was inhibited by >80% by oral administration of 5 μmol DATS/kg body weight only twice per week for 1 month [8]. Noticeably, 50% of the DATS-treated mice were alive after 12 weeks but none of the control mice survived at this time point [8]. Likewise, lung and liver metastasis induced by orthotopic injection of MDA-MB-231 cells into the mammary fat pad of Balb/c nude mice was inhibited by daily oral administration of DATS at 25 mg/kg and 50 mg/kg body weight although the growth of primary tumor was not affected [34]. Interestingly, the DATS-mediated inhibition of pulmonary metastasis in the MDA-MB-231 model was accompanied by a statistically significant inhibition of MMP-2 and MMP-9 protein level at the 50 mg/kg dose [34]. DATS treatment (5-20 μM for 24 hours) inhibited dissemination and metastasis of MDA-MB-231 cells in a zebrafish model that was associated with suppression of MMP-2 and MMP-9 activity [35]. In another study, the incidence as well as burden of poorly differentiated prostate adenocarcinoma and pulmonary metastasis multiplicity were suppressed significantly following oral administration of 1 mg (about 40 mg DATS/kg body weight for a 25 g mouse) and 2 mg DATS (about 80 mg DATS/kg body weight for a 25 g mouse) [40]. It is possible that DATS concentrations higher than 25 mg/kg body weight might be required for prevention of MNU-induced mammary cancer in rats.
In conclusion, the present study reveals that DATS is a potent inhibitor of leptin-stimulated activation of STAT3 in vitro in estrogen-responsive and estrogen-independent human breast cancer cells. Further work is necessary to determine the in vivo efficacy of DATS for prevention of MNU-induced breast cancer in rats at doses above 25 mg/kg. In future studies, it would also be worthwhile investigating the promotion of experimentally induced mammary carcinogenesis by leptin and its possible modulation by DATS.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.15430/JCP.2020.25.1.1
ACKNOWLEDGMENTS
This study was supported by the USPHS grant RO1 CA219180 awarded by the National Cancer Institute. This research used the Flow Cytometry Facility and the Tissue and Research Pathology Facility supported in part by Cancer Center Support Grant from the National Cancer Institute (P30 CA047904).
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Challier B, Perarnau JM, Viel JF. Garlic, onion and cereal fibre as protective factors for breast cancer: a French case-control study. Eur J Epidemiol. 1998;14:737–47. doi: 10.1023/A:1007512825851. [DOI] [PubMed] [Google Scholar]
- 2.Levi F, La Vecchia C, Gulie C, Negri E. Dietary factors and breast cancer risk in Vaud, Switzerland. Nutr Cancer. 1993;19:327–35. doi: 10.1080/01635589309514263. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 4.Antony ML, Singh SV. Molecular mechanisms and targets of cancer chemoprevention by garlic-derived bioactive compound diallyl trisulfide. Indian J Exp Biol. 2011;49:805–16. [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao D, Choi S, Johnson DE, Vogel VG, Johnson CS, Trump DL, et al. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23:5594–606. doi: 10.1038/sj.onc.1207747. [DOI] [PubMed] [Google Scholar]
- 6.Malki A, El-Saadani M, Sultan AS. Garlic constituent diallyl trisulfide induced apoptosis in MCF7 human breast cancer cells. Cancer Biol Ther. 2009;8:2175–85. doi: 10.4161/cbt.8.22.9882. [DOI] [PubMed] [Google Scholar]
- 7.Lee BC, Park BH, Kim SY, Lee YJ. Role of Bim in diallyl trisulfide-induced cytotoxicity in human cancer cells. J Cell Biochem. 2011;112:118–27. doi: 10.1002/jcb.22896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Na HK, Kim EH, Choi MA, Park JM, Kim DH, Surh YJ. Diallyl trisulfide induces apoptosis in human breast cancer cells through ROS-mediated activation of JNK and AP-1. Biochem Pharmacol. 2012;84:1241–50. doi: 10.1016/j.bcp.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Chandra-Kuntal K, Lee J, Singh SV. Critical role for reactive oxygen species in apoptosis induction and cell migration inhibition by diallyl trisulfide, a cancer chemopreventive component of garlic. Breast Cancer Res Treat. 2013;138:69–79. doi: 10.1007/s10549-013-2440-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nkrumah-Elie YM, Reuben JS, Hudson A, Taka E, Badisa R, Ardley T, et al. Diallyl trisulfide as an inhibitor of benzo(a)pyrene-induced precancerous carcinogenesis in MCF-10A cells. Food Chem Toxicol. 2012;50:2524–30. doi: 10.1016/j.fct.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atoum MF, Alzoughool F, Al-Hourani H. Linkage between obesity leptin and breast cancer. Breast Cancer (Auckl) 2020;14:1178223419898458. doi: 10.1177/1178223419898458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandra-Kuntal K, Singh SV. Diallyl trisulfide inhibits activation of signal transducer and activator of transcription 3 in prostate cancer cells in culture and in vivo. Cancer Prev Res (Phila) 2010;3:1473–83. doi: 10.1158/1940-6207.CAPR-10-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crean-Tate KK, Reizes O. Leptin regulation of cancer stem cells in breast and gynecologic cancer. Endocrinology. 2018;159:3069–80. doi: 10.1210/en.2018-00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SH, Kaschula CH, Priedigkeit N, Lee AV, Singh SV. Forkhead Box Q1 is a novel target of breast cancer stem cell inhibition by Diallyl Trisulfide. J Biol Chem. 2016;291:13495–508. doi: 10.1074/jbc.M116.715219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–7. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 16.Sun X, Guo T, He J, Zhao M, Yan M, Cui F, et al. Determination of the concentration of diallyl trisulfide in rat whole blood using gas chromatography with electron-capture detection and identification of its major metabolite with gas chromatography mass spectrometry. Yakugaku Zasshi. 2006;126:521–7. doi: 10.1248/yakushi.126.521. [DOI] [PubMed] [Google Scholar]
- 17.Saxena NK, Sharma D, Ding X, Lin S, Marra F, Merlin D, et al. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67:2497–507. doi: 10.1158/0008-5472.CAN-06-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maroni P. Leptin, adiponectin, and Sam68 in bone metastasis from breast cancer. Int J Mol Sci. 2020;21:E1051. doi: 10.3390/ijms21031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barone I, Giordano C, Bonofiglio D, Andò S, Catalano S. Leptin, obesity and breast cancer: progress to understanding the molecular connections. Curr Opin Pharmacol. 2016;31:83–9. doi: 10.1016/j.coph.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin Cancer Res. 2004;10:4325–31. doi: 10.1158/1078-0432.CCR-03-0749. [DOI] [PubMed] [Google Scholar]
- 21.Chen C, Chang YC, Liu CL, Chang KJ, Guo IC. Leptin-induced growth of human ZR-75-1 breast cancer cells is associated with up-regulation of cyclin D1 and c-Myc and down-regulation of tumor suppressor p53 and p21WAF1/CIP1. Breast Cancer Res Treat. 2006;98:121–32. doi: 10.1007/s10549-005-9139-y. [DOI] [PubMed] [Google Scholar]
- 22.Huang Y, Jin Q, Su M, Ji F, Wang N, Zhong C, et al. Leptin promotes the migration and invasion of breast cancer cells by upregulating ACAT2. Cell Oncol (Dordr) 2017;40:537–47. doi: 10.1007/s13402-017-0342-8. [DOI] [PubMed] [Google Scholar]
- 23.Wei L, Li K, Pang X, Guo B, Su M, Huang Y, et al. Leptin promotes epithelial-mesenchymal transition of breast cancer via the upregulation of pyruvate kinase M2. J Exp Clin Cancer Res. 2016;35:166. doi: 10.1186/s13046-016-0446-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haque I, Ghosh A, Acup S, Banerjee S, Dhar K, Ray A, et al. Leptin-induced ER-α-positive breast cancer cell viability and migration is mediated by suppressing CCN5-signaling via activating JAK/AKT/STAT-pathway. BMC Cancer. 2018;18:99. doi: 10.1186/s12885-018-3993-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin JJ, Yan L, Zhang J, Zhang WD. STAT3 as a potential therapeutic target in triple negative breast cancer: a systematic review. J Exp Clin Cancer Res. 2019;38:195. doi: 10.1186/s13046-019-1206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HJ, Lee HG, Choi KS, Surh YJ, Na HK. Diallyl trisulfide suppresses dextran sodium sulfate-induced mouse colitis: NF-κB and STAT3 as potential targets. Biochem Biophys Res Commun. 2013;437:267–73. doi: 10.1016/j.bbrc.2013.06.064. [DOI] [PubMed] [Google Scholar]
- 27.Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334(Pt 2):297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi HJ, Han JS. Overexpression of phospholipase D enhances Bcl-2 expression by activating STAT3 through independent activation of ERK and p38MAPK in HeLa cells. Biochim Biophys Acta. 2012;1823:1082–91. doi: 10.1016/j.bbamcr.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F, Sawaya R, et al. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene. 2004;23:3550–60. doi: 10.1038/sj.onc.1207383. [DOI] [PubMed] [Google Scholar]
- 30.Carpenter RL, Lo HW. STAT3 target genes relevant to human cancers. Cancers (Basel) 2014;6:897–925. doi: 10.3390/cancers6020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–15. doi: 10.1016/S1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 32.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–8. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 33.Song Y, Qian L, Song S, Chen L, Zhang Y, Yuan G, et al. Fra-1 and Stat3 synergistically regulate activation of human MMP-9 gene. Mol Immunol. 2008;45:137–43. doi: 10.1016/j.molimm.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Zhao Y, Wei Z, Tao L, Sheng X, Wang S, et al. Targeting thioredoxin system with an organosulfur compound, Diallyl Trisulfide (DATS), attenuates progression and metastasis of triple-negative breast cancer (TNBC) Cell Physiol Biochem. 2018;50:1945–63. doi: 10.1159/000494874. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Zhu P, Wang Y, Wei Z, Tao L, Zhu Z, et al. Antimetastatic therapies of the polysulfide diallyl trisulfide against triple-negative breast cancer (TNBC) via suppressing MMP2/9 by blocking NF-κB and ERK/MAPK signaling pathways. PLoS One. 2015;10:e0123781. doi: 10.1371/journal.pone.0123781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hahm ER, Singh SV. Diallyl trisulfide inhibits estrogen receptor-α activity in human breast cancer cells. Breast Cancer Res Treat. 2014;144:47–57. doi: 10.1007/s10549-014-2841-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson HJ, Adlakha H. Dose-responsive induction of mammary gland carcinomas by the intraperitoneal injection of 1-methyl-1-nitrosourea. Cancer Res. 1991;51:3411–5. [PubMed] [Google Scholar]
- 38.Russo J, Gusterson BA, Rogers AE, Russo IH, Wellings SR, van Zwieten MJ. Comparative study of human and rat mammary tumorigenesis. Lab Invest. 1990;62:244–78. doi: 10.1007/978-1-4612-0485-5_15. [DOI] [PubMed] [Google Scholar]
- 39.Chan MM, Lu X, Merchant FM, Iglehart JD, Miron PL. Gene expression profiling of NMU-induced rat mammary tumors: cross species comparison with human breast cancer. Carcinogenesis. 2005;26:1343–53. doi: 10.1093/carcin/bgi100. [DOI] [PubMed] [Google Scholar]
- 40.Singh SV, Powolny AA, Stan SD, Xiao D, Arlotti JA, Warin R, et al. Garlic constituent diallyl trisulfide prevents development of poorly differentiated prostate cancer and pulmonary metastasis multiplicity in TRAMP mice. Cancer Res. 2008;68:9503–11. doi: 10.1158/0008-5472.CAN-08-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.