Abstract
We describe a patient who developed severe tracheal stenosis while on treatment for pulmonary tuberculosis. Bronchoscopic-guided balloon dilatation succeeded in managing this disorder. Diagnosis of tracheobronchial tuberculosis requires a high index of suspicion because symptoms are usually attributed to co-existing pulmonary disease and airway lesions are not detectable on chest x-ray. Interventional bronchoscopy is employed to restore airway patency once significant stenosis develops. Should bronchoscopic measures fail, surgical options can be considered.
Keywords: Bronchoscopy, Tracheal stenosis, Tuberculosis
1. Introduction
Central airway obstruction (CAO) is generally defined as occlusion of >50% of the trachea, mainstem bronchi, bronchus intermedius or a lobar bronchi [1,2]. It can result from a variety of malignant and non-malignant disorders [1,2], malignant CAO occurring more frequently than benign obstruction [3]. Benign forms of CAO mainly include post-intubation and post-tracheostomy tracheal stenosis, tuberculosis and transplant-related stenoses, and idiopathic cases [2]. Herein, we report the case of a patient with tuberculous subglottic stenosis who experienced a favorable outcome following bronchoscopic dilatation.
2. Case report
A 52-year-old smoker woman presented to our hospital with a 6-week history of anorexia, weight loss, fatigue, productive cough, exertional dyspnea, and hoarseness. She was diagnosed with pulmonary and probable laryngeal tuberculosis and was commenced on a 6-month, antituberculous drug regimen. Clinical course was uneventful and she was discharged 7 days after admission without breathlessness and with minimal dysphonia.
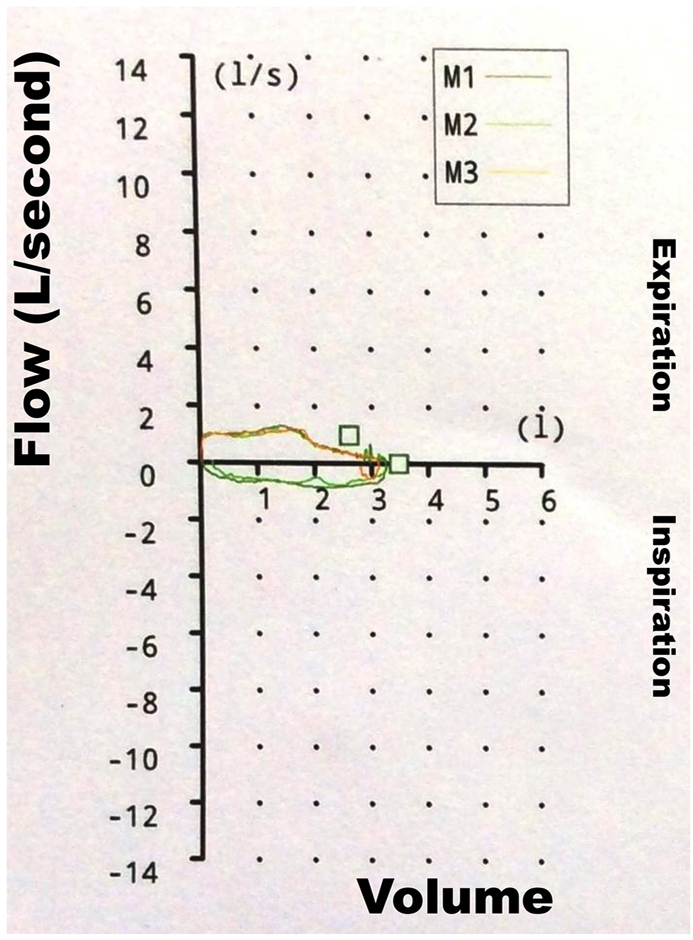
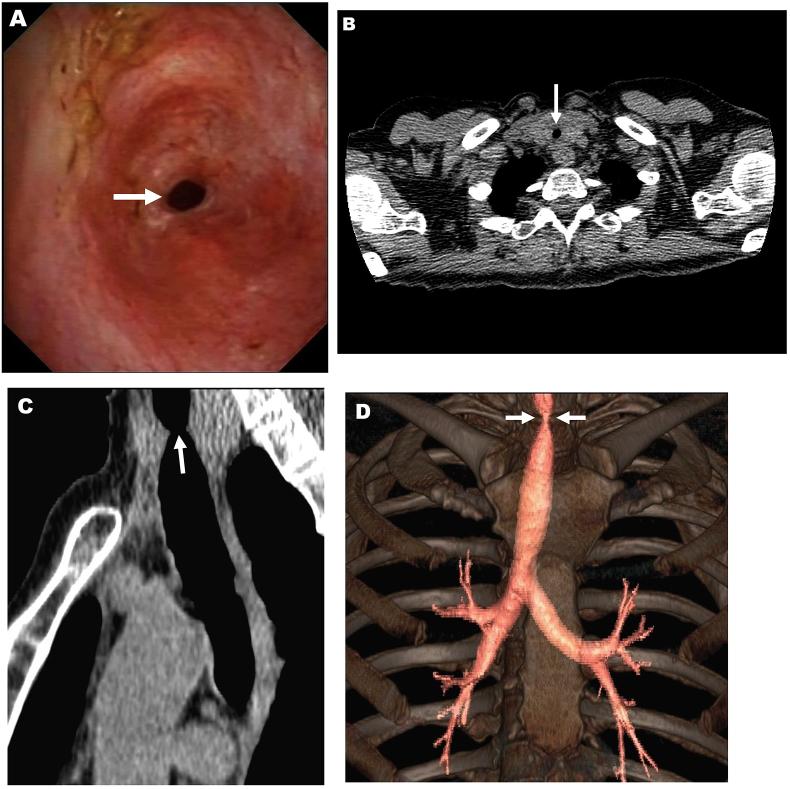
At a 3 month follow-up visit, the patient complained of worsening exertional dyspnea for the previous month, without other associated symptoms. Chest x-ray displayed no signs suggestive of active pulmonary tuberculosis. Dual bronchodilator therapy was prescribed but, as dyspnea turned out to be refractory, spirometry was done. The flow-volume loop was diagnostic of fixed upper airway obstruction (Fig. 1). Bronchoscopy revealed an approximate 90% tracheal stenosis about 3.5 cm below the glottis (Fig. 2, A) which prevented bronchoscope from passing through the stenosis. A subsequent chest CT scan showed severe proximal tracheal narrowing (Fig. 2, B-D). Tracheal aspirate samples were negative for acid-fast bacilli and their culture did not yield Mycobacterium tuberculosis. Rigid bronchoscopy with balloon dilatation (Storz, California, US) under general anesthesia was scheduled. The first session was initially performed with a bronchoscope of 7.5 mm outer diameter, then substituted for another of 8.5 mm. Following this session, residual stenosis was almost 30% of the normal tracheal lumen and patient's dyspnea alleviated considerably. One year after the first session, however, a second bronchoscopic dilatation was undertaken because her usual exertional dyspnea had recently worsened. This second intervention was done using a bronchoscope of 12 mm external diameter, residual stenosis being around 20% of the normal tracheal lumen. Eventually, she became symptomless. There were no adjunctive treatments given. When last seen, 1 year after the second bronchoscopic session, she remained asymptomatic.
Fig. 1.
Pulmonary function testing.
Flow-volume loop showing flow limitation and marked flattening of the inspiratory and expiratory limbs.
Fig. 2.
Tuberculous tracheal involvement in a 52-year-old woman.
(A) Bronchoscopic appearance of the proximal trachea revealing a severe, ring-like stenosis (arrow). A CT scan of the patient's chest showing a striking tracheal stenosis at thoracic inlet (arrows) (B, axial and C, sagittal views; D, 3D reconstruction).
3. Discussion
Tracheobronchial tuberculosis is described in approximately 10–40% of the patients with pulmonary tuberculosis [4]. Diagnosis requires a high index of suspicion because symptoms are usually attributed to co-existing pulmonary disease and airway lesions are not detectable on chest x-ray [5]. Fibrotic tracheobronchial stenosis is one of the most frequent long term complications of tracheobronchial tuberculosis and it can result in significant morbidity [4,5]. Patients with pulmonary tuberculosis and continuous respiratory symptoms during or after treatment should be assessed for tracheobronchial involvement. Interventional bronchoscopy is employed to restore airway patency once significant stenosis develops [6]. Should bronchoscopic measures fail, surgical options can be considered.
Contributorship
Ana C Ruiz: Care for the patient, Substantial contribution to study conception and design, Acquisition of data, Analysis and interpretation of data, Drafting of the manuscript, Critical revision of the manuscript for important intellectual content, Administrative, technical, or material support, Final approval of the version to be published, Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Miguel F Carrascosa: Substantial contribution to study conception and design, Acquisition of data, Analysis and interpretation of data, Drafting of the manuscript, Critical revision of the manuscript for important intellectual content, Administrative, technical, or material support, Study supervision, Final approval of the version to be published, Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Juan L García-Rivero: Substantial contribution to study conception and design, Acquisition of data, Analysis and interpretation of data, Critical revision of the manuscript for important intellectual content, Administrative, technical, or material support, Study supervision, Agreement to be accountable for all aspects of the work in ensuring that questions related to the, accuracy or integrity of any part of the work are appropriately investigated and resolved.
Gerardo B Rodríguez: Substantial contribution to study conception and design, Acquisition of data, Analysis and interpretation of data, Critical revision of the manuscript for important intellectual content, Administrative, technical, or material support, Final approval of the version to be published, Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Marta Cano Hoz: Substantial contribution to study conception and design, Acquisition of data, Analysis and interpretation of data, Administrative, technical, or material support, Final approval of the version to be published, Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Elena Casuso Sáenz: Substantial contribution to study conception and design, Acquisition of data, Analysis and interpretation of data, Administrative, technical, or material support, Study supervision, Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of competing interest
We declare no competing interests. In this regard and more specifically:
There were no funding sources in the writing of the manuscript or the decision to submit it for publication.
The authors of the manuscript have not been paid to write this article by a pharmaceutical company or other agency.
References
- 1.Ernst A., Feller-Kopman D., Becker H.D., Mehta A.C. Central airway obstruction. Am. J. Respir. Crit. Care Med. 2004;169:1278–1297. doi: 10.1164/rccm.200210-1181SO. [DOI] [PubMed] [Google Scholar]
- 2.Murgu S.D., Egressy K., Laxmanan B., Doblare G., Ortiz-Comino R., Hogarth D.K. Central airway obstruction. Benign strictures, tracheobronchomalacia, and malignancy-related obstruction. Chest. 2016;150:426–441. doi: 10.1016/j.chest.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Semaan R., Yarmus L. Rigid bronchoscopy and silicone stents in the management of central airway obstruction. J. Thorac. Dis. 2015;7(Suppl 4):S352–S362. doi: 10.3978/j.issn.2072-1439.2015.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pathak V., Shepherd R.W., Shojaee S. Tracheobronchial tuberculosis. J. Thorac. Dis. 2016;8:3818–3825. doi: 10.21037/jtd.2016.12.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siow W.T., Lee P. Tracheobronchial tuberculosis: a clinical review. J. Thorac. Dis. 2017;9:E71–E77. doi: 10.21037/jtd.2017.01.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang Y., You X., Sha W., Xiao H. Bronchoscopic balloon dilatation for tuberculosis-associated tracheal stenosis: a two case report and a literature review. J. Cardiothorac. Surg. 2016;11:21. doi: 10.1186/s13019-016-0417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]