Abstract
Platelet-rich-plasma (PRP) is an autologous human platelet concentrate extracted from plasma. PRP has been investigated in order to be used in many fields, with emphasis on the musculoskeletal field applied to sports injuries, as well as on other medical fields such as cardiac surgery, gynecology, pediatric surgery, urology, ophthalmology and plastic surgery. Cancer treatment is another important field where PRP should be investigated; thus, it is important validating PRP preparation protocols to be used in clinical research. Many protocols should be revised since, overall, most studies do not provide necessary information to allow them to be multiplied or replicated. The current review focuses on several topics about cancer, mainly on innovative studies about PRP use as a feasible therapeutic alternative to treat bladder cancer - a field where it could play a key role. Relevant aspects such as platelets' contribution to immune regulation and the supportive role they play in innate and adaptive immune functions are also addressed. Another important topic reviewed in the current study refers to inflammatory process regulation associated with cancer and thrombosis sites, which indicated that tumor-induced platelet activation could be used as an important therapeutic target in the future. New aspects concerning nitric oxide's ability to restrain platelet adhesion and aggregation in order to slow metastasis progress in cancer patients provide an important advantage in cancer treatment. Finally, the current review has pointed out perspectives and the main concerns about, and possibilities of, PRP use in cancer treatment.
Keywords: Biochemistry, Cancer research, Cell biology, Health sciences, Immunology, Platelet-rich-plasma, Cancer
Biochemistry; Cancer Research; Cell biology; Health Sciences; Immunology; Platelet-rich-plasma, cancer.
1. Introduction
Platelet-rich-plasma (PRP) is a blood-derived product obtained through centrifugation, which, in its turn, enables high platelet concentration - three to five times higher than the baseline - in a little portion of plasma. This procedure enables platelet's α-granules degranulation and the release of several important growth factors such as platelet-derived growth factor isomers ([PDGF-αα] [PDGF-αβ] [PDGF-ββ]), vascular endothelial growth factor (VEGF), two transforming growth factor-β isomers ([TGF-β1] [TGF-β2]) and epithelial growth factor (EGF) [1]. PRP is also known by other names such as platelet-rich growth factors (GFs), platelet concentrate and platelet-rich fibrin (PRF) matrix, among others [2].
The term PRP was created by hematologists in the 1970s in order to describe plasma presenting platelet level higher than that related to peripheral blood, which was firstly used in transfusion procedures focused on treating thrombocytopenia patients - a decade later, PRP started being used in maxillofacial surgery. Besides the hemostatic properties capable of inducing fibrin generation, new PRP properties have been discovered in recent years; among them one finds its anti-inflammatory and immunomodulation properties, as well as its cell proliferation capacity [3].
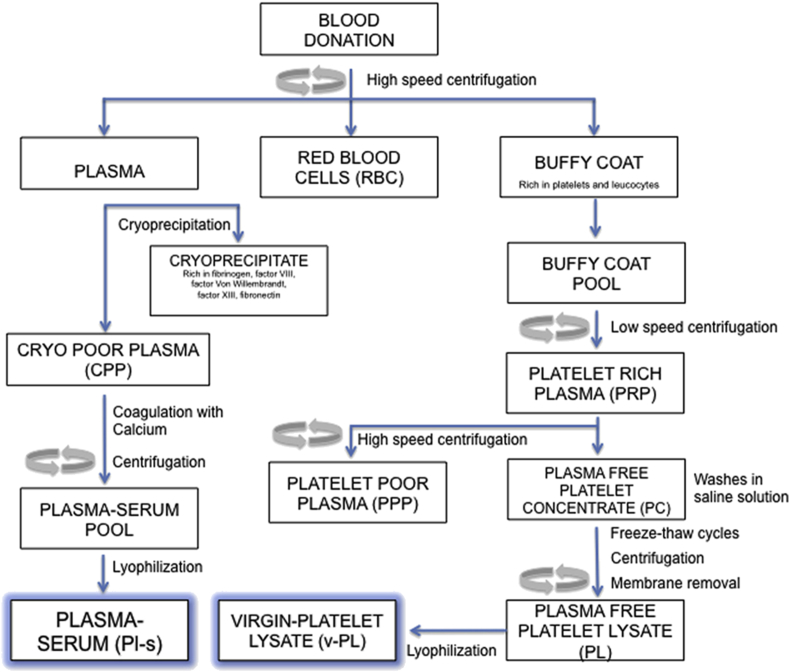
Figure 1 presents a scheme of PRP and PRP-derivatives’ preparation to help better understanding the role played by PRP [4].
Figure 1.
Schematic representation of the manufacturing process of the preparation of PRP. Reproduced from reference 4 under the terms of the Creative Commons Attribution License (CC BY).
PRP has been mainly used in the musculoskeletal field focused on sports injuries [5, 6]. Cardiac surgery, pediatric surgery, plastic surgery, gynecology, ophthalmology and urology are other important medical fields currently using PRP [1, 7, 8]. Overall, protocols about PRP preparation and use in clinical research are significantly inconsistent, since researchers do not provide sufficient information to enable replicating them. Therefore, it is necessary conducting an itemized, accurate, objective and stepwise PRP preparation protocol description to allow comparing results observed by different research groups, as well as to enable their reproducibility [9].
2. The role played by PRP in cancer treatment
It is known that carcinogenesis is a multistep process involving several interaction types between cancer cells and their bio-microenvironment; in many cases, these cancer cells use the host cells for survival purposes. For many decades, platelets have been assumed to have decisive function in cancer metastasis [10, 11, 12]. Many studies have evaluated the effects of platelets on cancer cell properties; however, contradictory results have hindered the definition of the exact effect of platelets on tumor cells [13, 14]. A study focused on evaluating the effects of platelets on HeLa cell culture proliferation, viability and adhesion at different PRP concentrations, as well as on investigating their interactions with antiplatelet drugs (e.g. aspirin), was performed. All data have slightly increased at low PRP concentrations (4.4×105 platelets/μL), whereas contrasting effect was observed at the highest PRP concentration (2×106 platelets/μL). Cell viability, adhesion and proliferation have decreased in patients only treated with 20 mM aspirin, a fact that indicated aspirin cytotoxicity in HeLa cells. On the other hand, all the aforementioned parameters have increased in the presence of PRP. Aspirin administration has decreased cancer cell viability; however, this effect was partially reversed by PRP [15]. More recently, some data have indicated possible mechanisms associated with the role played by platelets in cancer cell treatment [16].
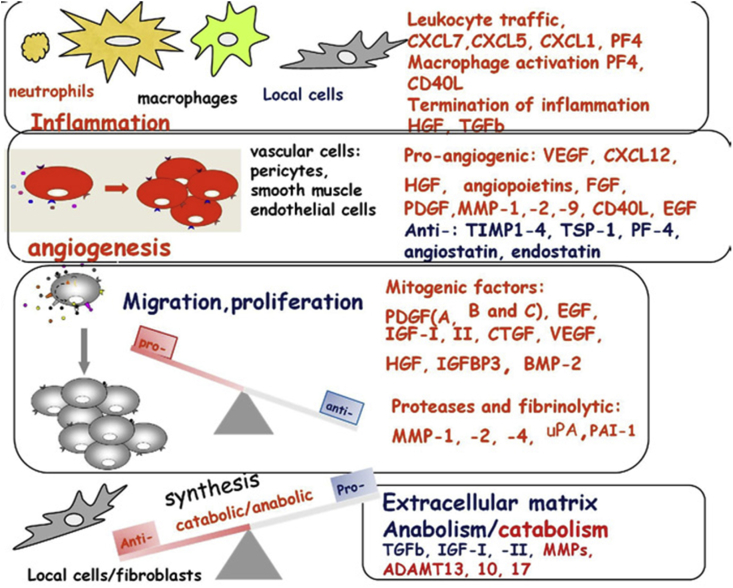
PRP may have opposite effect on tumor growth and metastasis in cancer patients; growth factors released by PRP can contribute to tumor progression. Since PRP has angiogenesis inhibitors and stimulators [17], it is necessary conducting further studies to help better understanding the mechanisms triggered by platelets in order to regulate angiogenesis [18]. It is well known that PRP administration is a promising approach to enable tissue repair, since it has cytokines and growth factors capable of enabling stem cell proliferation and differentiation [19, 20]. PRP controls several biological processes such as angiogenesis, inflammation, cell proliferation, cell migration, as well as extracellular matrix synthesis and remodeling processes, as shown in Figure 2 [18].
Figure 2.
Schematic representation of the multifunctional roles of PRP upon the release of molecules and growth factor involved in different biological processes, including: inflammation, angiogenesis, cell proliferation and formation of the extracellular matrix. Growth factors are involved in the healing response. Reproduced with modification from reference 18 with permission from Elsevier.
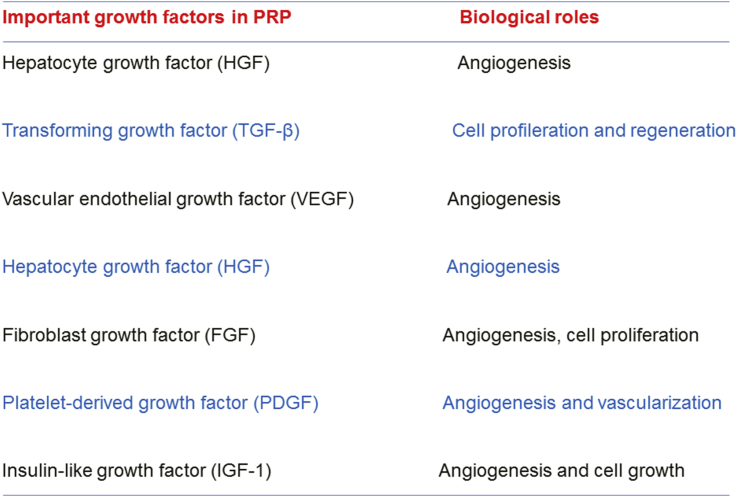
Thus, preliminary data deriving from clinical settings have shown that fat grafting-PRP combination in breast reconstruction after tumor removal has led to ambiguous results [19]. PRP has been reported to induce angiogenesis and stem cell differentiation. In fact, endothelial cells close to the application site are stimulated by PRP and favor the formation of new blood vessels [21]. Thus, PRP may induce tumor growth. the main growth factors released by PRP are represented in Figure 3. If one takes into consideration that PRP has different growth factors, its administration in sites previously affected by malignant tumors may trigger the neoplastic proliferation from residual cells [19] - the platelet-derived growth factor signaling pathway increases luminal breast cancer cell proliferation [19]. A study has investigated the direct contact between platelets-fibrin bundles primes metastasis deriving from PRP and tumor stroma [16]. PRP administration has stimulated the reversal of fibroblasts in benign (breast) phyllod tumors from mesenchymal to epithelial phenotype, a fact that has represented a critical step in cell survival. The angiogenesis process in the co-cultured platelet-fibrin-breast tumor cells/human umbilical vein endothelial cells interface was investigated. Breast tumor cells deriving from Luminal B and HER2 cells have shown significant number of endothelial cell capillary-like tubes. The authors suggested that the investigated interface contributes to the endothelial cell attachment process and provides a source of growth factors. In addition, the substitution of breast tumor cells from supplementation of fetal bovine serum by PRP is an important approach adopted to enable mimicking real multifactorial tumor microenvironments. However, current cell culture models need to mimic environments in vivo in order to validate whether results ex vivo reflect systems taking place in vivo [16]. This validation is necessary because breast cancer has different histopathological aspects and presents several prognostic results based on positive or negative ER tumor subtypes. Consequently, no single model would be enough to express this complex disease.
Figure 3.
Important growth factors found in PRP and their main biological roles.
On the other hand, PRP effectiveness in promoting and accelerating tissue regeneration due to effects of platelet-derived growth factors has been reported. Barbieri et al. [22] have observed the beneficial effects of PRP-gel on tumor relapse rates in mouse models with human fibrosarcoma. Mice treated with PRP presented significant decrease in tumor growth and lesion recurrence in comparison to the control group, although the immune-histochemical analysis did not show differences in cellular morphology between PRP-treated and control animals. Overall, the aforementioned authors have shown that PRP use for tissue regeneration purposes may also impair tumor growth [22].
It is known that neutropenia and thrombocytopenia are severe events associated with anticancer chemotherapy, since they can reduce the efficiency of therapy outcomes. However, according to Schiffer et al. [23], patients treated with platelets from external donors presented bleeding and thrombocytopenia due to exhaustive chemotherapy. More recently, platelet concentrate transfusion (PCT) in patients with bleeding issues was investigated. Corrected count increment (CCI), rather than bleeding time (BT), was the best parameter used to assess the efficiency of platelet concentrate transfusion (PCT) therapy in this study. However, the authors suggested that results should be carefully interpreted and that clinical and laboratory results of all patients should be monitored. In addition, they suggested that it is necessary improving the current therapy guidelines in order to enable platelet therapy application in the near future [24].
3. The role played by platelets in immunity
Platelets contribute to immune regulation [25] by participating in adaptive and innate immune functions [26]. This is clear from sterilization responses on wounds, as well as with vesicle-mediated transfer of proteins on the surface of immune cells in association with stimulation [27]. Platelets also present significant content of transforming growth factor-β (TGFβ), which acts as modulatory T cell immunosuppressive factor and regulates cell homeostasis [28]. TGF-β1 receptor blockade is a beneficial effect that prevents ovarian cancer progression due to platelet-derived TGF-β1 [29]. Functional disability in normal survey and elimination pathways enables the development of pro-tumorigenic microenvironments. Tumor immune surveillance and tumor cell-platelet crosstalk may hamper immune cell recognition or the connection of immune system-effector cells to tumors [25].
The main clinically-relevant function of CD8+ T cells lies on eliminating cancer cells; and this is the focus of immunotherapies applied to cancer patients [30]. Lack of activated CD8+ T cells in the tumor stroma of colorectal cancer patients has indicated disease relapse within 5 years, whereas long-term disease-free survival was observed in patients presenting these T cells [31, 32]. According to Rachidi et al. [33], platelets can directly suppress immune cells or kill cancer cells by inhibiting and suppressing the CD8 function - similar to CD4 T cells mediated via lactate and TGFβ. Platelets are also capable of protecting tumor cells from cytotoxic NK cell activity by armoring them or by transferring the major histocompatibility complex (MHCI) to the surface of tumor cells [34].
It is known that programmed death-ligand 1 (PD-L1) expression in tumor cells plays an important role in cancer immunotherapy due to interaction with cytotoxic CD8+ T cells that attract the programmed cell death 1 (PD-1) receptor to the surface [35]. According to Kleinovink et al. [36], PD-L1 (indicative marker of immune suppression) expressed in non-tumor cells has also shown inhibitory effect on the cytotoxic response of CD8+ T cells in the tumor. Immune checkpoint inhibitors acting on PD-L1-immune cells interaction have been extensively used in clinical studies focused on mitigating immune cell tumor suppression [37, 38, 39, 40, 41, 42]. Therapies based on the application of blocking antibodies (PD-L1) have shown different responses in many patients, which led to the development of different strategies aimed at improving immunotherapy effectiveness by reaching more pathways accounting for tumor growth suppression and/or tumor cell death [43, 44, 45, 46].
There may be several reasons why failures in patients' response to anti-[PD1/PD-L1] monotherapy take place; among them one finds differential PD-L1 expression in tumors, presence of limiting marks in immune cells and insignificant amount of tumor-infiltrating lymphocytes, among other factors. In addition, the expression level of PD-L1 or positive PD-L1 circulating tumor cells (CTCs) after anti-PD-L1 therapy in lung cancer patients presenting ongoing sickness indicates evasion of the mechanism to check point therapy [47]. There may be association between the number of circulating platelets and unresponsive CTCs, and it can be a possible mechanism used by CTCs to escape from immune vigilance. It is possible to achieve this by becoming trapped in platelet aggregates, or by hiding themselves by expressing platelet proteins. Data have indicated that patients presenting high platelet counts show poor response to PD-L1 therapy. Higher platelet/lymphocyte ratios before treatment have led to shorter overall survival rates and metastatic progression in lung cancer patients - this outcome was observed for patients subjected to nivolumab therapy [48, 49]. Based on these data, limiting the number of platelets to promote or maintain immunotherapy responses is a significant alternative [25]. In fact, PRP's ability to inhibit IL-6 release from human dendritic cells may prevent local excessive tissue infarction, inflammatory hemorrhage and necrosis in human lung [50].
4. PRP applications in cancer patients
A method focused on treating cancer patients with PRP was reported. Concentrated platelets were broken by means of ultrasound in order to produce a platelet lysate, which was formulated into an injectable preparation to be directly administered to the cancer cells, i.e., injected into the tumor [51]. Another method based on PRP composition was used in patients needing treatment for brain (glioblastoma), lung, breast and colon cancer [52].
Gentilli et al. performed a study focused on evaluating the effect of using fat grafted with PRP on fat volume enhancement in patients subjected to breast restoration after breast soft-tissue defects and on patients affected by breast cancer reconstruction outcomes [53]. According to the aforementioned authors, based on sequential treatments aimed at obtaining natural results with no noticeable scars, it was possible repairing fat volume in breast hypoplasia patient; however, their study did not make any comments about breast cancer reconstruction in the presence of PRP [53]. Spartalis et al. [54] have questioned the innovative results observed by Gentilli et al. [53] based on the following arguments: it is known that growth factor release can stimulate angiogenesis due to PRP. VEGF and PDGF induce tumor lymphangiogenesis and lead to high nodal metastasis rate. They stated that breast tumors testing positive for PDGF presented significantly lower response to chemotherapy and significantly shorter survival length. Besides, Spartalis et al. [54] have pointed out that the TGF-β ligand regulates different cell behaviors such as migration, cell differentiation and apoptosis due to multifunctional growth factors. Based on these facts, they advocated that PRP application should be contraindicated for patients undergoing cancer resection given the presence of growth factors capable of inducing tumor proliferation; they strongly believe that Gentilli et al. [53] should have taken into consideration this important point. Alternatively, the inclusion of patients affected by breast cancer reconstruction outcomes should have been avoided [54].
However, new facts totally contradict the hypothesis suggested by Spartalis et al. [54]. As previously mentioned, the likely association of increased risk of cancer with increased serum insulin-like growth factor-1 (IGF-1) and C-reactive protein (CRP) levels in cancer patients has been reported. A study focused on evaluating the effect of a single intramuscular PRP injection in PRGF on serum IGF-1 and CRP levels in dogs was performed. According to the aforementioned study, 16 dogs were divided into two different groups; they were injected with different PRGF doses in the lumbar muscle. The CRP and IGF-1 levels recorded for dogs subjected to all treatments were monitored based on blood analyses conducted at baseline and after injection. IGF-1 and CRP did not change, regardless of the application protocol. Clinical PRGF dose application did not lead to significant changes in systemic IGF-1 concentrations or to detectable inflammation in any dog. Systemic IGF-1 and CRP concentrations did not show significant changes after i.m. PRGF application in dogs; thus, it was not possible inducing cancer or inflammation in them [55].
Accordingly, many studies about cancer induction, recurrence and post-operative survival rate are focused on tumor stroma, since it is a crucial parameter for tumor development [56, 57]. Molecules and growth factors such as VEGF, PDGF, EGFR (HER) and TGF-b are mediators in interactions leading to tumor cell immortality, such as angiogenesis, lymphangiogenesis, proliferation, defective differentiation and apoptosis cancel [54, 58]. Data available in the literature have indicated that patients with breast tumors testing positive for PDGF present significantly low response to chemotherapy and shorter survival length. On the other hand, PDGF plasma levels also correlate to short survival ratio [59]. Experimental data have also shown that breast tumor stroma increases luminal breast cancer cell proliferation/angiogenesis over PDGF signaling pathway [60]. Therefore, PRP administration to sites previously affected by malignant tumors could trigger neoplastic proliferation in residual cells. Although the PRP-related malignant transformation hypothesis was not confirmed, it is of great importance to eliminate any chance of recurrence in patients who have theoretically undergone complete tumor burden excision. Therefore, further experiments in vitro and animal studies should be encouraged to thoroughly investigate PRP interactions in cancer excision sites. Since there is no established indication of PRP use in breast reconstruction processes, PRP use should not yet be recommended for patients undergoing resection due to cancer [19].
Breast cancer cell proliferation, cancer cell-induced endothelial tube formation in vitro, and tumor growth in vivo were investigated in the presence of protease-activated receptor 1-stimulated platelet releasate (PAR1-PR; rich in pro-angiogenic factors) or of PAR4-PR (rich in anti-angiogenic factors). Both factors have similarly enhanced the proliferation of MCF-7 and MDA-MB-231 breast cancer cells. Cancer cells have induced capillary-like tube formation of endothelial cells that were further enhanced by pro-angiogenic factor-rich PAR1-PR. VEGF receptor blockade inhibited PAR1-PR/PAR4-PR-enhanced cancer cell proliferation. Integrin occluded by RGDS had effects similar to that of VEGF inhibition. Proto-oncogene tyrosine-protein kinase (Src) and regulated kinase (ERK) inhibition diminished, whereas PI3K and PKC blockade eliminated platelet releasate-enhanced cancer cell proliferation. A model for subcutaneous implantation of MDA-MB-231 cells in nude mice was used in this case; PAR1-PR has significantly enhanced tumor growth in comparison to PAR4-PR, and seemed to achieve the overdone by fomenting more significant tumor angiogenesis. Platelet releasate from PRP has increased breast cancer cell proliferation through VEGF–integrin associative signaling. Pro-angiogenic factor-rich platelet releasate has efficiently improved cancer cell-induced angiogenesis and, consequently, overdo tumor growth in vivo [61].
It is known that cancer stem cells (CSCs) are associated with cancer regression [62]. It happens due to the signal transduction pathway among CSCs, which induces platelet activation and platelet-secreted growth factors capable of enhancing cancer cell growth [63, 64].
A study has analyzed the effect of using platelet lysate (PL) from PRP deriving from human venous blood donors, as well as its platelet-derived growth factor-AB (PDGF-AB) content as tumor microenvironment on (CD24-/CD44+) BCSC proliferation [62]. Breast cancer (BC) patients presented higher platelet counts and PDGF-AB levels in PL related to healthy donors. PLs from BC patients were more efficient in stimulating BCSCs proliferation than those of healthy donors; besides, they recorded lower population doubling time (PDT) value. Results indicated cell proliferation and PDT correlation to PDGF-AB level. This outcome has indicated that PDGF-AB played a key role in BCSCs proliferation; however, PL did not have effect on BCSCs viability (Note: see ref [65] for differences in platelet lysate and platelet releasate).
Previous results observed for PRP application in cancer cases were reinforced by Dias et al. [66], who presented data about an alternative therapy for non-muscle invasive bladder cancer (NMIBC) in animal models based on the combination of Bacillus Calmette-Guerin (BCG) intravesical immunotherapy and PRP. Results in vitro have shown that PRP administration in association, or not, with BCG leads to expressive cytotoxicity in HTB-9 cells (bladder carcinoma cells). Results observed for rats subjected to PRP application in vivo in association with BCG have shown that 70% of animals has presented better histopathological recovery from neoplastic lesions than the ones subjected to monotherapy. This outcome resulted in the therapeutic association of different activation levels of immune system-mediated proteins such as toll-like 2 and 4, which led to enhanced TRIF, IRF3, MyD88, and IFN-γ immunoreactivities. Thus, these results have suggested that interferon signaling-pathway activation through PRP treatment, in association with BCG immunotherapy, may be a new therapeutic option for NMIBC.
5. Platelet activation use as cancer therapy modality
Platelets' contribution to regulate inflammation processes associated with thrombosis and cancer has suggested that tumor-induced platelet activation is an important therapeutic target [67]. However, this approach must be carefully planned to avoid the risk of bleeding; therefore, it is necessary taking into consideration the significance of the role played by platelets as hemostasis controller. Overall, low-molecular weight substances, such as heparin, were used in clinical trials focused on investigating cancer-associated thrombosis; however, results have been questioned [68, 69]. Acetylsalicylic acid, also known as aspirin, is a well-known pharmaceutical compound that has been used for centuries to treat patients with coagulopathies (e.g., severe coronary syndrome). It inhibits COX-enzymes that produce thromboxane A2, which, in its turn, is an effective platelet activation and aggregation inducer. In addition, the protective effect of aspirin on the blocking setting, mainly on colorectal cancer patients, was previously addressed in the twentieth century. Studies on population and clinical situations have also shown that aspirin reduced the risk of colon cancer progression and suppressed cancer growth and invasiveness [70]. It is also known that aspirin inhibits platelets’ ability to stimulate cancer metastasis in mice [71]. According to Cao et al. [13], regular aspirin use by approximately 150,000 individuals - who were monitored for more than thirty years - was correlated to significantly diminished risk of cancer development, mainly in the gastrointestinal tract. Then, the long-term application of low aspirin doses can inhibit cancer metastasis and significantly reduce cancer incidence. This outcome suggested that platelet activation was a potential target and prognosis marker for cancer treatment [72].
Several clinical studies are currently in progress; they aim at assessing the therapeutic effect of aspirin-based treatment on cancer patients [73]. Clopidogrel (inhibitor of the P2Y12 receptor) application to target ADP-mediated platelet activation, either alone or in association with aspirin is another platelet activation inhibition strategy that will likely be effective in treating cancer [74]. This statement was substantiated by results recorded for HBV transgenic mice treated with aspirin/clopidogrel, which delayed or prevented hepatocellular carcinoma development and improved models’ overall survival [75].
A review of recent studies about the role played by platelets in tumor microenvironments, such as angiogenesis and metastasis, as well as in targeting platelets for cancer treatment, mainly in drug-resistant patients, was performed. Therefore, targeting tumor–platelet interactions and, more specifically, targeting platelet-originated growth factors may be a promising approach to help enhancing tumor sensitivity to chemotherapy [76].
6. Cancer, nitric oxide and PRP interactions
It is known that metastasis is the main cause of cancer-related deaths [77]. Metastasis is a dynamic process comprising many important stages, as follows: tumor cells separation from the initial site, invasion of hosts' blood vessels, migration into hosts’ blood stream, travel throughout the circulatory system, binding or adhesion to capillaries in remote organs, spread out and, finally, proliferation inside foreign tissues. Tumor survival in the bloodstream is rarely seen due to immune system action or shear forces; very low percentages (<0.01%) of these cells lead to metastasis. Tumor cell-induced platelet aggregation favors hematogenous metastasis, since it enhances the capture of tumor cell emboli in the bloodstream. Interestingly, platelet aggregation protects tumor cells from immunological attacks.
Data analyzed by Suzuki-Inoue et al. [78] and Tsuruo and Fujita [77] have suggested that the platelet activation receptor C-type lectin-like receptor 2 (non-classical C-type lectin), which acts as receptor in the platelet membrane (CLEC-2), is a physiological target protein of podoplanin. This receptor is also involved in podoplanin-induced platelet aggregation, tumor metastasis and in other cellular responses; therefore, podoplanin (Aggrus) may work as an important target for drugs used to block metastasis [77]. In addition, it is known that tumors cause venous thromboembolism due to the pro-coagulation activity and induce metastasis in cancer patients because of the interaction between endothelial (ECs) and cancer cells, a fact that triggers the extravasation process. EC-based activation and release of the pro-coagulation protein von Willebrand factor (VWF) is fundamental for malignancy. The use of human tumor samples in melanoma cells activated by ECs in murine models (mice) results in the release of VWF fibers and in platelet aggregation in tumor microvessels. Human blood and tumor tissue samples have shown that VWF release is associated with proteolytic activity inhibition. The expression of a disintegrin-like and metalloproteinase with thrombospondin type I repeats motif 13 (ADAMTS13) protein and forms a pro-coagulant milieu. The use of heparin (tinzaparin) - which blocks EC activation - impaired VWF network formation and suppressed tumor progression in a mouse model. Thus, based on the authors, targeting EC activation enables new therapeutic strategies capable of mitigating angiogenesis and coagulation in tumors [10].
Recently, a study has shown that the growth of podoplanin-positive lung squamous carcinoma (LSCC) cells was suppressed by podoplanin knockout in vivo, but not in vitro. Similarly, podoplanin promoted cell growth in vivo due to ectopic expression and favored intratumoral platelet activation. In addition, LSCC cells led to platelet aggregation mediated by podoplanin (PMPA). Releasates from platelets during PMPA led to LSCC cell growth in vitro. The Phospho-RTK (phospho-receptor-tyrosine-kinase) array analysis has shown that the epidermal growth factor receptor (EGFR) phosphorylation of LSCC cells was responsible for growth promotion induced by platelet releasates. The use of podoplanin-neutralizing antibody has reduced the growth of LSCC tumor xenografts via EGFR phosphorylation suppression. Data have indicated that podoplanin in LSCC increased cell growth by inducing PMPA in vivo and enabled malignant advancement [79].
It is clear that platelet aggregation plays an important role in cancer through metastasis. Nitric oxide (NO) suppresses platelet aggregation and adhesion; thus, it can be used to mitigate metastasis progress in cancer patients. NO is a free radical playing a dual role in cancer biology, since it can act as tumor repressor or suppressor [80]. Cellular microenvironment, NO concentration, location and timing determine the dichotomous effects of NO [81]. Overall, low NO concentrations promote tumor growth, angiogenesis, cell invasion and metastasis [82]. According to reports, low NO concentrations (pico-nanomolar range) have anti-apoptotic effects [83]. On the other hand, NO, which is mainly produced by inducible nitric oxide synthase (iNOs), promotes apoptosis and leads to antitumor effects when it is used at high concentrations (micro molar range) [84]. Thus, NO plays a dual role in cancer biology, depending on its concentration. NO's ability to overcome tumor resistance has been recently reported, and such ability proved to be a new way to evaluate NO/NO donors based on a new oncopreventive approach [85].
Patients presenting acute respiratory distress syndrome (ARDS), who did not have preexisting coagulation disorders, were subjected to NO inhalation treatment, which had beneficial effects on the oxygenation of arterial and pulmonary circulation. These effects were associated with significant platelet aggregation suppression [86]. The use of glyceryl trinitrate (GTN), diethylamine diazeniumdiolate (DEA/NO), sodium nitroprusside (SNP), S-nitrosoglutathione (GSNO) and S-nitrosothiol (RIG200) in ADP and human platelet-rich plasma activated by collagen was investigated. GTN was a very weak platelet aggregation inhibitor, whereas other NO donors suppressed platelet aggregation, regardless of the agonist. NO scavengers, such as hemoglobin, have suppressed the effect, except at high DEA/NO concentrations. Only SNP-mediated inhibition by soluble guanylate cyclase inhibitor (1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one, ODQ) was cGMP-dependent. Specific cGMP-independent effects of S-nitrosothiols have suggested that different NO-related mediators were responsible for their actions [87].
The NO donor MAHMA NONOate (Z-1-{N-methyl-N-[6-(N-methylammoniohexyl) amino]}diazen-1-ium-1,2-diolate) has inhibited rat platelet aggregation in a similar manner to that observed for GSNO. Sodium nitroprusside and glyceryl trinitrate were poor platelet aggregation suppressors. Therefore, rat platelet aggregation suppression by MAHMA NONOate (a NO donor) was widely ODQ-resistant, regardless of the guanylate cyclase. It was suggested that SERCA (sarco-endoplasmic reticulum calcium-ATPase) activation might act as inhibition mechanism [88].
Thrombogenicity was monitored in an animal model (porcine) and evaluated ex vivo for platelet thrombus formation in Badimon perfusion chamber. A LA816 (S-nitrosothiol) infusion was administered to pigs for 2 h LA816/clopidogrel and acetylsalicylic acid (ASA)-clopidogrel led to thrombus mass reduction by ~ 45% in comparison to placebo control perfusions. The treatment based on oral ASA-clopidogrel and intravenous LA816 association led to important additional decrease by 25% in platelet deposition. Intravenous LA816 application did not change animals’ blood pressure or heart rate. In addition, acute NO donation through LA816 did not change hemodynamic parameters and enabled restraining effect similar to that recorded for chronic treatment with ASA-clopidogrel. Besides, LA816 enabled platelet inhibitory effects similar to the ones observed for the associated blockade of cyclooxygenase and P2y(12) receptor (platelet-ADP receptor and ADP-induced platelet aggregation inhibitor) [89].
Crane et al. [90] reported dose-response curves for DEA/NO (diethylamine diazeniumdiolate), SNVP (S-nitroso-N-valerylpenicillamine) and SIN-1 (3-morpholinosydnonomine) in PRP and washed platelets (WP) in the front of ODQ. All NO donors presented GMP-independent platelet aggregation inhibition due to PRP; however, DEA/NO has shown cGMP-independent aggregation inhibition due to WP. Washed platelets (WP) reconstitution based on plasma factors has shown that copper content in plasma protein ‘caeruloplasmin’ (CP) catalyzed NO release from SNVP, whereas superoxide dismutase (Cu/Zn SOD) exposed NO generated from SIN-1. Increased extracellular NO production is correlated to switch into an independent cGMP effect with both NO donors. A study on Fura-2-loaded WP (fluorescence chromophore for calcium signaling) has shown that DEA/NO suppressed Ca2+ signaling in platelets via cGMP-independent mechanism, only in NO donors. However, SNVP and SIN-1 pre-incubations with CP and SOD, respectively, led to GMP-independent inhibition of intra-platelet Ca2+ trafficking by NO donors. Results suggested that extracellular NO is necessary to enable cGMP-independent inhibition of platelet activation. Plasma components may play significant pharmacological role in stimulating cGMP-independent signaling by S-nitrosothiols or peroxynitrite generators.
Knockout mice for endothelial NOS [(eNOS−/−)] or inducible NOS [iNOS−/−)] by diethylenetriamine/NO (DETA/NO) and congeners of wild-type (WT) were treated with endothelin-1 (ET-1) and activated an enhancement in plasma levels of 6-keto prostaglandin F1α (6-keto PGF1α) in eNOS and WT mice but not in animals with iNOS. The agonist ‘bradykinin’ triggered F1α release and suppressed platelet aggregation in all investigated mice strains. In addition, platelet aggregation in vitro induced by ADP was likely reduced by iloprost in WT and iNOS mice. ET-1 expressively increased 8-isoprostane plasma levels in WT in another experiment; however, it was not the case in iNOS mice. WT mice subjected to a three-week treatment with antioxidants presented suppressed ET-1 capacity to increase plasma F1α. It was the first time iNOS accounted for controlling ET-1 release induced by prostacyclin and for suppressing platelet aggregation in mice [91].
NO generation in individual platelets went through collagen substrate adhesion under flow conditions. This process was followed by the use of fluorescent probe, 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM). Moreover, NO generation increased in the presence of L-arginine (substrate of iNOS) and diminished in the presence of L-NG-monomethyl arginine (iNOS inhibitor); however, it was not the case in the presence of inactive enantiomer D-NG-monomethyl arginine. Platelet surface coverage (deposition) determined by mepacrine-labeled platelets was inversely proportional to NO generation. There was correlation between Ca2+ increase and NO generation, which indicated that platelet NO generation was caused by increased intracytoplasmic Ca2+. These data provided one of the first neat demonstrations of platelet NO generation activated by interaction with activating surface under flow conditions. In addition, they indicated that intra-platelet Ca2+ increase leads to NO generation, which successively modulates thrombus size [92]. These data have shown the important role played by NO against PRP aggregation.
Flierl et al. [93] observed that platelet activation increase in a murine model of congestive heart failure (CHF) can be efficiently modulated by pentaerythritol tetranitrate (PETN). Therefore, chronic NO application based on PETN has diminished the activation of circulating platelets, although platelet reactivity caused ADP stimulation in cardiovascular phenotype of CHF rats. The aforementioned authors suggested that PETN may provide a useful approach to help keeping platelet integrity and avoiding thromboembolic complications in CHF patients. As previously described, tumor cell-induced platelet aggregation favors metastasis, since it enhances the capture of tumor cell emboli in microcirculation; NO donors have inhibited these processes and appeared to be an interesting new strategy to treat cancer in association with PRP.
7. Conclusions and perspectives
Important developments in recent years have been observed in medical fields such as cardiac surgery, pediatric surgery, plastic surgery, gynecology, ophthalmology, urology and oncology, based on PRP use. As previously stated in the current review, protocols focused on PRP use in cancer patients are yet to be properly validated in clinical studies, although recent studies have provided enough information to validate and reproduce them.
Innovative studies focused on investigating alternative therapies to treat bladder cancer based on PRP use appeared to provide an important step in compressing PRP use in cancer patients. Advances in PRP studies have contributed to improve immune regulation. Thus, two topics have caught the scientific community's attention: the first topic refers to innate and adaptive immune functions, whereas the second one refers to the role played by platelets in regulating inflammation processes associated with cancer and thrombosis. Studies about the second topic have indicated that tumor-induced platelet activation reemerges as an important therapeutic target. It is important emphasizing that it is necessary restricting platelet activation in cancer patients at the time to outline new therapeutic approaches to treat them. Important new reports on the action of, and role played by, nitric oxide in platelet adhesion and aggregation, as well as in mitigating metastasis progress in cancer patients, have been exhaustively reviewed.
Although PRP use in cancer patients is a promising approach that needs to be further explored, caution must be taken since PRP may have opposite effects on tumor growth and metastasis. PRP has several angiogenesis stimulators (Figure 3), which can contribute to tumor progression. Pro-angiogenic factor-rich platelet releasate can efficiently improve cancer cell-induced angiogenesis; thus, it can accelerate tumor growth in vivo - the application of PRP derivatives should be done with caution. It is worth emphasizing that PRP use in cancer patients is only starting; therefore, it is necessary conducting further studies focused on addressing the following key points: (i) how safe and efficient is PRP use in cancer patients? (ii) Does PRP use apply to different cancer types? (iii) What are the potential side effects of PRP treatment? Finally, we hope that the present study can contribute to the study of PRP use in cancer patients.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by Grants 2018/08194-2 from SãoPaulo Research Foundation (FAPESP). NanoBioss/SisNano (CNPq-Brazil, Process number 402280/2013–0, 313117/2019–5). INOMAT (CNPq, Proc. 573644/2008–0), CNPq 404815/2018–9.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to thank Good Deal for revising the text.
Contributor Information
Amedea B. Seabra, Email: amedea.seabra@ufabc.edu.br.
Nelson Durán, Email: nelsonduran1942@gmail.com, nduran@unicamp.br.
References
- 1.Choi J., Minn K.W., Chang H. The efficacy and safety of platelet-rich plasma and adipose-derived stem cells: an update. Arch Plast Surg. 2012;39:585–599. doi: 10.5999/aps.2012.39.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alves R., Grimalt R. A review of platelet-rich plasma: history, biology, mechanism of action, and classification. Skin Appendage Disord. 2018;4:18–24. doi: 10.1159/000477353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conde-Montero E., Fernandez-Santos M.E., Suarez-Fernandez R. Platelet-rich plasma: applications in dermatology. Actas Dermosifiliogr. 2015;106:104–111. doi: 10.1016/j.ad.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Muraglia A., Nguyen V.T., Nardini M. Culture medium supplements derived from human platelet and plasma: cell commitment and proliferation support. Front. Bioeng. Biotechnol. 2017;5:66. doi: 10.3389/fbioe.2017.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch M.D., Bashir S. Applications of platelet rich plasma in dermatology: a critical appraisal of the literature. J. Dermatol. Treat. 2016;27:285–289. doi: 10.3109/09546634.2015.1094178. [DOI] [PubMed] [Google Scholar]
- 6.Hussain N., Johal H., Bhandari M. An evidence-based evaluation on the use of platelet rich plasma in orthopedics – a review of the literature. J Soc Intern Chirur Orthoped Traumatol (SICOT J) 2017;3:57. doi: 10.1051/sicotj/2017036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andia E., Rubio-Azpeitia J., Martin I. Current concepts and translational uses of platelet rich plasma biotechnology. In: Ekinci D., editor. Biotechnology. InTech; 2015. https://www.intechopen.com/books/biotechnology/current-concepts-and-translational-uses-of-lateletrich-plasma-biotechnology [Google Scholar]
- 8.Andia I. Platelet-rich plasma biology. In: Alves R., Grimalt R., editors. Clinical Indications and Treatment Protocols with Platelet-Rich Plasma in Dermatology. Barcelona, Ediciones Mayo; 2016. pp. 3–15. [Google Scholar]
- 9.Chahla J., Cinque M.E., Piuzzi N.S. A call for standardization in platelet-rich plasma preparation protocols and composition reporting a systematic review of the clinical orthopaedic literature. J Bone Joint Surg Am. 2017;99:1769–1779. doi: 10.2106/JBJS.16.01374. [DOI] [PubMed] [Google Scholar]
- 10.Bauer A.T., Suckau J., Frank K. On Willebrand factor fibers promote cancer-associated platelet aggregation in malignant melanoma of mice and humans. Blood. 2015;125:3153–3163. doi: 10.1182/blood-2014-08-595686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goubran H.A., Burnouf T., Radosevic M. The platelet-cancer loop. Eur. J. Intern. Med. 2013;24:393–400. doi: 10.1016/j.ejim.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Yang A.-J., Wang M., Wang Y. Cancer cell-derived von Willebrand factor enhanced metastasis of gastric adenocarcinoma. Oncogenesis. 2018;7:12. doi: 10.1038/s41389-017-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao Y., Nishihara R., Wu K. Population-wide impact of long-term use of aspirin and the risk for cancer. JAMA Onco. 2016;2:762–769. doi: 10.1001/jamaoncol.2015.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y., Zhang H. Platelet-induced inhibition of tumor cell growth. Thromb. Res. 2008;123:324–330. doi: 10.1016/j.thromres.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Mehta N., Muthusamy S., Bhatia A. Do platelets inhibit the effect of aspirin on cancer cells? Cancer Microenviron. 2015;8:119–122. doi: 10.1007/s12307-015-0169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrade S.S., Sumikawa J.T., Castro E.D. Interface between breast cancer cells and the tumor microenvironment using platelet-rich plasma to promote tumor angiogenesis - influence of platelets and fibrin bundles on the behavior of breast tumor cells. Oncotarget. 2017;8:16851–16874. doi: 10.18632/oncotarget.15170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson J.E., Zurakowski D., Italiano J.E. Normal ranges of angiogenesis regulatory proteins in human platelets. Am. J. Hematol. 2010;85:487–493. doi: 10.1002/ajh.21732. [DOI] [PubMed] [Google Scholar]
- 18.Andia I., Sánchez M., Maffulli N. Basic science: molecular and biological aspects of platelet-rich plasma therapies. Operat. Tech. Orthop. 2012;22:3–9. [Google Scholar]
- 19.Spartalis E., Tsilimigras D.I., Charalampoudis P. The “Yin and Yang” of platelet-rich plasma in breast reconstruction after mastectomy or lumpectomy for breast cancer. Anticancer Res. 2017;37:6557–6562. doi: 10.21873/anticanres.12112. [DOI] [PubMed] [Google Scholar]
- 20.Syllaios A., Tsimpoukelis A., Vagios I. Breast reconstruction with autologous fat combined with platelet rich plasma: fighting between medical novelty and cancer biology. JBUON. 2019;24:1516–1520. [PubMed] [Google Scholar]
- 21.Eppley B.L., Pietrzak W.S., Blanton M. Platelet-rich plasma: a review of biology and applications in plastic surgery. Plast. Reconstr. Surg. 2006;118:147e–159e. doi: 10.1097/01.prs.0000239606.92676.cf. [DOI] [PubMed] [Google Scholar]
- 22.Barbieri A., Bimonte S., Loquercio G. The effects of the use of platelet-rich plasma gel on local recurrence in an animal model of human fibrosarcoma. Infect. Agents Canc. 2019;14:21. doi: 10.1186/s13027-019-0237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiffer C.A., Anderson K.C., Bennett C.L. Platelet transfusion for patients with cancer: clinical practice guidelines of the American society of clinical oncology. J. Clin. Oncol. 2001;19:1519–1538. doi: 10.1200/JCO.2001.19.5.1519. [DOI] [PubMed] [Google Scholar]
- 24.Nikolić L.I., Nedeljković N.D., Jelić S.B. Chemotherapy induced thrombocytopenia treated by four types of platelets concentrates. Hosp. Pharm. 2015;2:297–307. [Google Scholar]
- 25.Kanikarla-Marie P., Lam M., Sorokin A.V. Platelet Metabolism and other targeted drugs; potential impact on immunotherapy. Front Oncol. 2018;8:107. doi: 10.3389/fonc.2018.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali R.A., Wuescher L.M., Worth R.G. Platelets: essential components of the immune system. Curr. Trends Immunol. 2015;16:65–78. [PMC free article] [PubMed] [Google Scholar]
- 27.Laffont B., Corduan A., Rousseau M. Platelet microparticles reprogram macrophage gene expression and function. Thromb. Haemostasis. 2016;115:311–323. doi: 10.1160/TH15-05-0389. [DOI] [PubMed] [Google Scholar]
- 28.Li M.O., Wan Y.Y., Sanjabi S. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 29.Hu Q., Hisamatsu T., Haemmerle M. Role of platelet-derived Tgfβ1 in the progression of ovarian cancer. Clin. Canc. Res. 2017;23:5611–5621. doi: 10.1158/1078-0432.CCR-16-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson S.R., Yuan J., Teague R.M. Targeting CD8+ T-cell tolerance for cancer immunotherapy. Immunotherapy. 2014;6:833–852. doi: 10.2217/imt.14.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mlecnik B., Tosolini M., Kirilovsky A. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J. Clin. Oncol. 2011;29:610–618. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 32.Curtis N.J., Primrose J.N., Thomas G.J. The adaptive immune response to colorectal cancer: from the laboratory to clinical practice. Eur. J. Surg. Oncol. 2012;38:889–896. doi: 10.1016/j.ejso.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Rachidi S., Metelli A., Riesenberg B. Platelets subvert T cell immunity against cancer via GARP-TGFbeta axis. Sci Immunol. 2017;2:1–12. doi: 10.1126/sciimmunol.aai7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Placke T., Örgel M., Schaller M. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Canc. Res. 2012;72:440–448. doi: 10.1158/0008-5472.CAN-11-1872. [DOI] [PubMed] [Google Scholar]
- 35.Chen D.S., Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;3:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Kleinovink J.W., Marijt K.A., Schoonderwoerd M.J.A. PD-L1 expression on malignant cells is no prerequisite for checkpoint therapy. OncoImmunology. 2017;6 doi: 10.1080/2162402X.2017.1294299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doi T., Piha-Paul S.A., Jalal S.I. Safety and antitumor activity of the anti-programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J. Clin. Oncol. 2018;36:61–66. doi: 10.1200/JCO.2017.74.9846. [DOI] [PubMed] [Google Scholar]
- 38.Kaufman H.L., Hunger M., Hennessy M. Nonprogression with avelumab treatment associated with gains in quality of life in metastatic Merkel cell carcinoma. Future Oncol. 2018;14:255–266. doi: 10.2217/fon-2017-0470. [DOI] [PubMed] [Google Scholar]
- 39.Li C., Zhang N., Zhou J. Peptide blocking of PD-1/PD-L1 interaction for cancer immunotherapy. Cancer Immunol Res. 2018;6:178–188. doi: 10.1158/2326-6066.CIR-17-0035. [DOI] [PubMed] [Google Scholar]
- 40.Ling D.C., Bakkenist C.J., Ferris R.L. Role of immunotherapy in head and neck cancer. Semin. Radiat. Oncol. 2018;28:12–16. doi: 10.1016/j.semradonc.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Reck M. Pembrolizumab as first-line therapy for metastatic non-small- cell lung cancer. Immunotherapy. 2018;10:93–105. doi: 10.2217/imt-2017-0121. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y., Wu L., Tian C. PD-1-PD-L1 immune-checkpoint blockade in malignant lymphomas. Ann. Hematol. 2018;97:229–237. doi: 10.1007/s00277-017-3176-6. [DOI] [PubMed] [Google Scholar]
- 43.Hu Z., Ott P.A., Wu C.J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 2018;18:168–182. doi: 10.1038/nri.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hou Y., Nitta H., Wei L. Evaluation of immune reaction and PD-L1 expression using multiplex immunohistochemistry in HER2-positive breast cancer: the association with response to anti-HER2 neoadjuvant therapy. Clin. Breast Canc. 2018;18:e237–e244. doi: 10.1016/j.clbc.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ray A., Das D.S., Song Y. Combination of a novel HDAC 6 inhibitor ACY-241 and anti-PD-L1 antibody enhances anti-tumor immunity and cytotoxicity in multiple myeloma. Leukemia. 2018;32:843–846. doi: 10.1038/leu.2017.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu X., Xu J., Cai H. Carboplatin and programmed death-ligand 1 blockade synergistically produce a similar antitumor effect to carboplatin alone in murine ID8 ovarian cancer model. J. Obstet. Gynaecol. Res. 2018;44:303–311. doi: 10.1111/jog.13521. [DOI] [PubMed] [Google Scholar]
- 47.Nicolazzo C., Raimondi C., Mancini M. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor nivolumab. Sci. Rep. 2016;6:31726. doi: 10.1038/srep31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diem S., Schmid S., Krapf M. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Canc. 2017;111:176–181. doi: 10.1016/j.lungcan.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 49.Russo A., Franchina T., Ricciardi G.R.R. Baseline neutrophilia, derived neutrophil-to-lymphocyte ratio (dNLR), platelet-to-lymphocyte ratio (PLR), and outcome in non small cell lung cancer (NSCLC) treated with nivolumab or docetaxel. J. Cell. Physiol. 2018;233:6337–6343. doi: 10.1002/jcp.26609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Czakai K., Dittrich M., Kaldorf M. Influence of platelet-rich plasma on the immune response of human monocyte-derived dendritic cells and macrophages stimulated with Aspergillus fumigates. Inter J Med Microbiol. 2017;307:95–107. doi: 10.1016/j.ijmm.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 51.Mishra, A. Method of treating cancer using platelets releasate. US Patent 2010; 20100135969.
- 52.Mishra A.: Method of treating cancer using platelet compositions. US Patent 2013; 2013/012 1979 A1.
- 53.Gentile P., Di Pasquali C., Bocchini I. Breast reconstruction with autologous fat graft mixed with platelet-rich plasma. Surg. Innovat. 2013;20:370–376. doi: 10.1177/1553350612458544. [DOI] [PubMed] [Google Scholar]
- 54.Spartalis E.D., Tomos P., Dimitroulis D. Platelet-rich plasma in surgical oncology. Surg. Innovat. 2014;21:441. doi: 10.1177/1553350613520516. [DOI] [PubMed] [Google Scholar]
- 55.Vilar J.M., Damiá E., Rubio M. Therapeutic doses of plasma rich in growth factors cannot provoke cancer by means of the IGF-1 pathway or inflammation in dogs. J. Appl. Anim. Res. 2017;45:490–493. [Google Scholar]
- 56.Spartalis E.D., Tomos P., Konofaos P. Breast reconstruction with autologous fat graft; does plateletrich plasma affect patient’s survival? Int. J. Clin. Exp. Med. 2014;7:329–330. [PMC free article] [PubMed] [Google Scholar]
- 57.Guo X., Wu Y., Hathaway H.J. Microenvironmental control of the breast cancer cell cycle. Anat. Rec. 2012;295:553–562. doi: 10.1002/ar.22417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levva S., Kotoula V., Kostopoulos I. Prognostic evaluation of epidermal growth factor receptor (EGFR) genotype and phenotype parameters in triple-negative breast cancers. Cancer Genom Proteom. 2017;14:181–195. doi: 10.21873/cgp.20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seymour L., Dajee D., Bezwoda W.R. Tissue platelet derivedgrowth factor (PDGF) predicts for shortened survival and treatment failure in advanced breast cancer. Breast Canc. Res. Treat. 1993;26:247–252. doi: 10.1007/BF00665802. [DOI] [PubMed] [Google Scholar]
- 60.Pinto M.P., Dye W.W., Jacobsen B.M. Malignant stroma increases luminal breast cancer cell proliferation and angiogenesis through platelet-derived growth factor signaling. BMC Canc. 2014;14:735. doi: 10.1186/1471-2407-14-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang L., Luan Y., Miao X. Platelet releasate promotes breast cancer growth and angiogenesis via VEGF–integrin cooperative signaling. Br. J. Canc. 2017;117:695–703. doi: 10.1038/bjc.2017.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kartolo W.A., Pawitan J.A., Harahap A.R. PDGF-AB rich-trombocyte lysate supplementation from breast cancer patients increased the proliferation of breast cancer stem cells. Med J Indones. 2018;27:19–25. [Google Scholar]
- 63.Klevebring F., Rosin G., Ma R. Sequencing of breast cancer stem cell populations indicates a dynamic conversion between differentiation states in vivo. Breast Cancer Res. 2014;15(R72) doi: 10.1186/bcr3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tesfamariam B. Involvement of platelets in tumor cell metastasis. Pharmacol. Ther. 2016;157:112–119. doi: 10.1016/j.pharmthera.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 65.Martinez S.F., editor. Vol. 27. Elsevier, Inc.; 2016. p. 32. (Physical Medicine and Rehabilitation Clinucs of North America. Regenerative Medicine). number 4. [Google Scholar]
- 66.Dias L.P., Luzo A.C.M., Volpe B.B. Effects of intravesical therapy with platelet-rich plasma (PRP) and Bacillus Calmette-Guérin (BCG) in non-muscle invasive bladder cancer. Tissue Cell. 2018;52:17–27. doi: 10.1016/j.tice.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 67.Olsson A.K., Cedervall J. The pro-inflammatory role of platelets in cancer. Platelets. 2018;29:569–573. doi: 10.1080/09537104.2018.1453059. [DOI] [PubMed] [Google Scholar]
- 68.Zhang N., Lou W., Ji F. Low molecular weight heparin and cancer survival: clinical trials and experimental mechanisms. J. Canc. Res. Clin. Oncol. 2016;142:1807–1816. doi: 10.1007/s00432-016-2131-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elalamy I., Mahe I., Ageno W. Long-term treatment of cancer-associated thrombosis: the choice of the optimal anticoagulant. J. Thromb. Haemostasis. 2017;15:848–857. doi: 10.1111/jth.13659. [DOI] [PubMed] [Google Scholar]
- 70.Lichtenberger L.M., Fang D., Bick R.J. Unlocking Aspirin’s chemopreventive activity: role of irreversibly inhibiting platelet cyclooxygenase-1. Canc. Prev. Res. 2017;10:142–152. doi: 10.1158/1940-6207.CAPR-16-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guillem-Llobat P., Dovizio M., Bruno A. Aspirin prevents colorectal cancer metastasis in mice by splitting the crosstalk between platelets and tumor cells. Oncotarget. 2016;7:32462–32477. doi: 10.18632/oncotarget.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang S., Li Z., Xu R. Human cancer and platelet interaction, a potential therapeutic target. Int. J. Mol. Sci. 2018;19:1246. doi: 10.3390/ijms19041246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Santilli F., Boccatonda A., Davi G. Aspirin, platelets, and cancer: the point of view of the internist. Eur. J. Intern. Med. 2016;34:11–20. doi: 10.1016/j.ejim.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 74.Elaskalani O., Berndt M.C., Falasca M. Targeting platelets for the treatment of cancer. Cancers. 2017;9:94. doi: 10.3390/cancers9070094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sitia G., Aiolfi R., Di Lucia P. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sc USA. 2012;109:E2165–E2172. doi: 10.1073/pnas.1209182109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huong P.T., Nguyen L.T., Nguyen X.-B. The role of platelets in the tumor-microenvironment and the drug resistance of cancer cells. Cancers. 2019;11:240. doi: 10.3390/cancers11020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsuruo T., Fujita N. Platelet aggregation in the formation of tumor metastasis. Proc Jpn Acad Ser. B. 2008;84:189–198. doi: 10.2183/pjab/84.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suzuki-Inoue K., Kato Y., Inoue O. Involvement of the snake toxin receptor clec-2, in podoplanin-mediated platelet activation, by cancer cells. J. Biol. Chem. 2007;282:25993–26001. doi: 10.1074/jbc.M702327200. [DOI] [PubMed] [Google Scholar]
- 79.Miyata K., Takemoto A., Okumura S. Podoplanin enhances lung cancer cell growth in vivo by inducing platelet aggregation. Sci. Rep. 2017;7:4059. doi: 10.1038/s41598-017-04324-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Somasundaram V., Basudhar G., Bharadwaj J.H. Molecular mechanisms of nitric oxide in cancer progression. Antioxidants Redox Signal. 2019;30:1124–1143. doi: 10.1089/ars.2018.7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choudhari S.K., Chaudhary M., Bagde S. Nitric oxide and cancer: a review. World J. Surg. Oncol. 2013;118:11. doi: 10.1186/1477-7819-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seabra A.B., Durán N. Nanoparticulated nitric oxide donors and their biomedical applications. Mini Rev. Med. Chem. 2017;17:216–223. doi: 10.2174/1389557516666160808124624. [DOI] [PubMed] [Google Scholar]
- 83.Seabra A.B., de Lima R., Calderón M. Nitric oxide releasing nanomaterials for cancer treatment: current status and perspectives. Curr. Top. Med. Chem. 2015;15:298–308. doi: 10.2174/1568026615666150108122918. [DOI] [PubMed] [Google Scholar]
- 84.González R., Molina-Ruiz F.J., Bárcena J.A. Regulation of cell survival, apoptosis, and epithelial-to-mesenchymal transition by nitric oxide dependent post-translational modifications. Antioxidants Redox Signal. 2018;29:1312–1332. doi: 10.1089/ars.2017.7072. [DOI] [PubMed] [Google Scholar]
- 85.Pieretti J.C., Pelegrino M.T., Nascimento M.H.M. Small molecules for great solutions: can nitric oxide-releasing nanomaterials overcome drug resistance in chemotherapy? Biochem. Pharmacol. 2020 doi: 10.1016/j.bcp.2019.113740. in press. [DOI] [PubMed] [Google Scholar]
- 86.Samama C.M., Diaby M., Fellahi J.-V. Inhibition of platelet aggregation by inhaled nitric oxide in patients with acute respiratory distress syndrome. Anesthesiology. 1995;83:56–65. doi: 10.1097/00000542-199507000-00007. [DOI] [PubMed] [Google Scholar]
- 87.Sogo N., Magid K.S., Shaw C.A. Inhibition of human platelet aggregation by nitric oxide donor drugs: relative contribution of cGMP-independent mechanisms. Biochem. Biophys. Res. Commun. 2000;279:412–419. doi: 10.1006/bbrc.2000.3976. [DOI] [PubMed] [Google Scholar]
- 88.Homer K.L., Wanstall J.C. Inhibition of rat platelet aggregation by the diazeniumdiolate nitric oxide donor MAHMA NONOate. Br. J. Pharmacol. 2002;137:1071–1081. doi: 10.1038/sj.bjp.0704971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vilahur G., Segalés E., Salas E. Effects of a novel platelet nitric oxide donor (LA816), Aspirin, clopidogrel, and combined therapy in inhibiting flow- and lesion-dependent thrombosis in the porcine ex vivo model. Circulation. 2004;110:1686–1693. doi: 10.1161/01.CIR.0000142296.19558.99. [DOI] [PubMed] [Google Scholar]
- 90.Crane M.S., Rossi A.G., Megson A.L. A potential role for extracellular nitric oxide generation in cGMP-independent inhibition of human platelet aggregation: biochemical and pharmacological considerations. Br. J. Pharmacol. 2005;144:849–859. doi: 10.1038/sj.bjp.0706110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carrier E., Brochu I., de Brum-Fernandes A.J. The inducible nitric-oxide synthase modulates endothelin-1-dependent release of prostacyclin and inhibition of platelet aggregation ex vivo in the mouse. J. Pharmacol. Exp. Therapeut. 2007;323:972–978. doi: 10.1124/jpet.107.125690. [DOI] [PubMed] [Google Scholar]
- 92.Cozzi M.R., Guglielmini G., Battiston M. Visualization of nitric oxide production by individual platelets during adhesion in flowing blood. Blood. 2015;125:697–705. doi: 10.1182/blood-2014-06-579474. [DOI] [PubMed] [Google Scholar]
- 93.Flierl U., Fraccarollo D., Widder J.D. The nitric oxide donor pentaerythritol tetranitrate reduces platelet activation in congestive heart failure. PloS One. 2015;10 doi: 10.1371/journal.pone.0123621. [DOI] [PMC free article] [PubMed] [Google Scholar]