Abstract
Transition metals play an important role in a wide variety of biological processes, but their functions are dependent on the quantity and the type of species present. Specific forms of arsenic (As) and chromium (Cr) are associated with oxidative stress, cellular damage and inflammation. The aim of this research was to test in a food system whether, in the presence of hydrolyzed oat proteins, arsenic or chromium will exist predominantly in a specific oxidative state, and to evaluate the potential implication of promoting or decreasing oxidative stress. Eight hydrolyzed proteins with different degrees of radical scavenging activities were produced by combining two extraction methods and four proteases. The addition of hydrolysates to ground chicken meat decreased lipid hydroperoxides by up to 50% when stored at 4 °C but had no effect at -20 °C. The ratio of pentavalent arsenic (As(V)) to arsenobetaine (AsB) in meat was about 2:1 but in the presence of the hydrolysates, meanwhile, the amount of AsB detected was 3-fold higher depending on the storage condition. This was due to better extraction of AsB in the presence of hydrolysates rather than to the conversion of other species. Data on chromium showed that Cr(VI) contents decreased from 14.3 ± 0.1 to 6.3 ± 0.5 μg/g while concentrations of Cr(III) increased from 2.8 ± 0. 2 to 8.6 ± 0.7 μg/g. In summary, the addition of hydrolyzed oat proteins to chicken meat enhanced the extraction of AsB, and had little effect on arsenic speciation during storage meanwhile, there was a reduction of Cr(VI) to Cr(III) which was in part due to the relative content of thiol groups. Additionally, there was a reduction of lipid oxidation in meats that contained the oat protein hydrolysates.
Keywords: Food science, Food chemistry, Speciation, Hydroperoxides, Lipid oxidation, Antioxidant free thiols, Aromatic amino acids, UV absorption
Food science, Food chemistry, Speciation, Hydroperoxides, Lipid oxidation, Antioxidant free thiols, Aromatic amino acids, UV absorption
1. Introduction
Foods have now been recognized to provide the human body with more than just energy and essential nutrients. Specifically, naturally occurring food components have been demonstrated to possess health-promoting properties in chronic conditions such as cardiovascular diseases, hypertension and inflammation (Ortiz-Martinez et al., 2014; Walters, Udenigwe, et al., 2018). These beneficial effects have been attributed not only to various phenolic molecules, but also to fibers and, more recently to peptides released from the hydrolysis of proteins (Sadiq Butt et al., 2008). Cereals are staple foods and constitute an important source of energy and nutrients for populations worldwide. Oat is the sixth highest consumed type of cereal, and its use in human nutrition has been increasing due to the discovery of functional and biological activities of some of its constituents like glucans, polyphenols, peptides (Espinosa et al., 2019; Ryan et al., 2011). Most of the active molecules of oats and other cereals are concentrated in the bran, which is a by-product generated during their processing (Sadiq Butt et al., 2008). The amount of proteins in oats is high relative to other cereals with the main group being globulins (50–80%) followed by albumins (1–12%), prolamins (4–15%) and glutenins (<10%) (Espinosa et al., 2019). Proteins in general have nutritive and functional properties like foaming or emulsification, however, many researchers have digested the food proteins with proteases from various sources to release peptides with biological functions (Carrasco-Castilla et al., 2012; Kamdem and Tsopmo, 2019; Walters, Udenigwe, et al., 2018). Activities of hydrolyzed proteins often reported in the literature include antioxidant, anti-inflammatory and antihypertensive properties (Kamdem and Tsopmo, 2019; Park et al., 2014). There are also reports that hydrolyzed proteins can chelate transition metal ions, thereby, enhancing their absorption, or reducing their toxicity and pro-oxidant effects. Radical reactions initiated by metals can contribute to the oxidation of lipids in foods or biological systems and to the oxidation of nucleotides.
Many metals are involved in metabolic and cellular reactions. For example, they play a role in processes such as energy production, primary and secondary metabolisms, gene regulation or signal transduction (Hänsch and Mendel, 2009; Walters, Esfandi, et al., 2018). The function of chromium includes potentiating insulin response and normal glucose metabolism, while copper is a cofactor of redox enzymes (e.g. cytochrome c oxidase, superoxide dismutase), and cobalt is part of the structure of vitamin B12 (Jomova and Valko, 2011). The function of minerals meanwhile is dependent on their oxidative state, their concentration and the presence of chelators. Chromium (Cr) oxidative states range from -2 to +6 with the trivalent form (Cr(III)) being involved in energy metabolism while the hexavalent (Cr(VI)) species is mainly characterized by its toxicity and carcinogenicity. Arsenic also has several oxidation states with arsenate (As(V)) and arsenite (As(III)) being the main ones. The relative concentrations of the metal oxidative state vary depending on the redox environment and hence the need to quantify all species instead of just the total amount. The toxicity of chromium and arsenic are related to their ability to induce oxidative stress through the generation of reactive oxygen species such as superoxide anion radical (O2─•), singlet oxygen (1O2) and hydroxyl radical (HO•). Dimethylarsinic peroxyl [(CH3)2AsOO•] and dimethylarsinic[(CH3)2As•] radicals have been reported as oxidative arsenic products (Kitchin and Conolly, 2010). The radical scavenging properties of hydrolyzed food proteins have been reported (Park et al., 2014; Zhou et al., 2013) and there are literature data on their ability to convert toxic Cr(VI) to the biologically relevant Cr(III) (Leung et al., 2018; Tsopmo et al., 2014). However, there is no data on how they may affect either arsenic or the relative concentration of different oxidative states (chromium, arsenic) in a food system. The objective of this work was then to determine the effect of the addition of hydrolyzed oat proteins on arsenic and chromium speciation in ground meat, and also the effect on the oxidative stability of the meat.
2. Materials and methods
2.1. Chemicals and reagents
The standards for metal species were arsenic oxide (As(III), As2O3), sodium arsenate dibasic heptahydrate (As(V), Na2HAsO4.7H2O); Cr(III) and Cr(VI) in the form of Cr(NO3)3 and K2CrO4, obtained from Sigma Aldrich (Oakville, ON, Canada) while dimethylarsinic acid (DMA) or cacodylic acid and arsenobetaine (AsB) were Sigma Aldrich (Milwaukee, WI, USA). Disodium methyl arsenate or monomethylarsonate (MMA) was purchase from Agilent Technologies Canada Inc. (Mississauga, ON, Canada).
Medium oat bran flour (Product number 112-001) containing 16% total dietary fiber, 9.2% moisture, 14% Protein, and 5.5% beta-glucan was donated by Richardson Milling (Portage la Prairie, MB, Canada). Viscozyme L® 100 Fungal Beta Glucanase (FBG)/g, cellulase 700 Endo-Glucanase Units (EGU)/g, flavourzyme 500 units/g, papain 1500 units/g, alcalase 2.4 units/g, protamex 1.5 units/g, pyrogallol, Tris–HCL, 1,10-phenanthroline, and Trolox were obtained from Sigma Aldrich (Oakville, ON, Canada). 2,2′-Azobis (2- amidinopropane) dihydrochloride (AAPH) was obtained from Wako Chemical (Richmond, VA, USA). SDS-PAGE reagents were obtained from Bio-Rad Laboratories Inc. (Mississauga, ON, Canada). Fluorescein, hydrogen peroxide and ferrous sulfate heptahydrate were purchased from Fisher Scientific Co. (Nepean, ON, Canada). Spectrophotometric measurements were performed on the BioTek Epoch™ UV–Vis microplate reader while incubations were done on a MaxQ™ 8000 model incubator from Fisher Scientific (Nepean, ON, Canada).
2.2. Extraction and hydrolysis of proteins
Defatted medium oat bran samples were mixed with deionized water 1:10 (w/v) ratios and blended to obtain homogeneous slurries. To break down polysaccharides, viscozyme or cellulase was added at 3 FGG/g and 20 EGU/g, respectively, according to a previous study (Esfandi et al., 2019) and incubated at 45 °C for 90 min. Proteins were then solubilized at pH 9.5 over 30 min incubation (45 °C). Non-soluble residues were removed by centrifugation at 2500g for 20 min at 4 °C. The pH of each supernatant was adjusted to 4.0 and centrifuged at 10000g for 40 min at 4 °C. The protein isolates (pellet) were collected, washed with water (pH 4.0) and centrifuged for 10 min at 1100g, followed by suspension in water (pH 7.0) and freeze-drying to yield two oat bran protein isolates (OBPIs), one extracted with viscozyme and the other with cellulase. Protein contents of OBPI and their hydrolysates were determined based on the Lowry procedure (Lowry et al., 1951). The preparation of hydrolyzed proteins was done by treating each OBPI with four proteases individually. The amount of each protease added was 3% (w/w) based on previous work. The hydrolysis was performed on 0.5 g of OBPI suspended in water (1:20 ratio) for 4 h at the optimum temperature and pH for the proteases; alcalase (pH 8.0, 60 °C), papain and protamex (pH 7.0, 60 °C), and flavourzyme (pH 7.0, 50 °C). At the end of the hydrolysis, each sample was heated at 90 °C for 10 min to inactivate the protease, followed by cooling to 4 °C and centrifugation to remove enzymes and non-digested proteins. The resulting oat bran protein hydrolysate (OBPH) samples were freeze-dried and stored at -20 °C.
2.3. Gel electrophoresis, intrinsic absorbance, and thiols
The relative molecular weight of polypeptides in OBPI and OBPH samples was determined using the well-established sodium dodecyl sulfate-polyacrylamide gel electrophoresis SDS-PAGE method (Laemmli, 1970). The composition of proteins in isolates and hydrolysates were analyzed on SDS-PAGE slab gels, which consisted of 12% separating gel (pH 8.6) and 5% stacking gel (pH 6.8). Samples (1 mg/mL) were prepared under reducing conditions in buffer made of 125 mM Tris-HCl (pH 6.8), 1% (w/v) SDS, 5% (v/v) glycerol, and 2% (v/v) 2-mercaptoethanol (2-ME). Ten microliters were loaded in each lane of the gel. After electrophoresis, the gels were stained in Coomassie Brilliant Blue R-250 and destained in a solution containing 10% acetic acid and 20% methanol. Gel slabs containing each protein bands were compared to a standard mixture to determine relative molecular weights.
The relative amount of aromatic amino acids OBPI and OBPH were determined by measuring their intrinsic absorbance (Bhattacharya et al., 2011). Samples were dissolved in 0.1 M sodium phosphate buffer (pH 7.0) to give a final concentration of 0.5 mg/mL. The absorbance was measured at 280 nm in triplicate on the BioTek Epoch microplate reader. The amount of sulfhydryl groups in the samples were also measured using the Ellman's method (Ellman, 1959). In this case, OBPI and OBPH (1 mg/mL) were dissolved in 0.1 M potassium phosphate buffer, pH 8.0 containing 1 mM EDTA. The measurement was performed at 280 nm and, sulfhydryl groups in the isolates and hydrolysates were calculated using data generated from a cysteine standard (0–1.5 mM).
2.4. Processing of meat samples for analysis of arsenic and chromium species
Chicken breast meat samples were purchased at a local grocery store in Ottawa (Ontario, Canada) and use the same day. First, 5 g meat sample and 5 mg each OBPI or OBPH were homogenized in triplicate to give a smooth paste-like sample. Then, homogenates were divided into three aliquots (1 g each), two of which were stored separately at 4 and -20 °C for 24 h before analysis. The third was analyzed without prior storage. The extraction was based on previous work that pain to extract arsenic in chicken meat (Liu et al., 2015). In summary, 0.5 g stored and non-stored samples were mixed and vortexed with 5 mL of papain (1 mg/mL in 50 mM NH4HCO3). They were then incubated in a water bath for 1 h at 63 °C, followed by 10 min sonication at an amplitude of 50. The samples were centrifuged at 4000 rpm for 20 min and then filtered through 0.45 μm syringe for quantification of As and Cr species.
2.5. Analysis of arsenic and chromium species by HPLC-ICP-MS
The determination of chromium and arsenic species using HPLC-ICP-MS was based on previously validated methods (Séby et al., 2003; Van Hulle et al., 2002). The mass spectrometry module was an Agilent 7500cx octopole reaction system inductively coupled plasma mass spectrometer (ICP-MS). It was connected to an Agilent 1200 series high-performance liquid chromatography (HPLC), equipped with an auto-sampler and temperature column control compartment. The HPLC separation was achieved through an ion pair reserve-phase process using a Phenomenex ODS-3 column (150 × 4.6 mm, 3 μm). The mobile phase contained 5 mmol/L tetrabutylammonium (TBA), 5% methanol, and 3 mmol/L malonic acid, with a flow rate of 1.2 mL/min. Arsenic and chromium species were separated over 14 and detected at m/z 75 and 52. Calibration standards (0.5–10 μg/L) of As(III), As(V), MMA, DMA, AsB Cr(III), and Cr(VI) in 0.1% nitric acid containing 1 mM EDTA were made daily and used to calculate the concentration of each species in (ng/g of ground meat).
2.6. Lipid peroxidation assay
Hydroperoxides in chicken breast samples were analyzed to test the antioxidant activity of the hydrolyzed oat proteins under conditions similar to those used to assess the effect on As and Cr. The assay was modified from a literature procedure that used ferrous oxide xylenol (FOX) (Nourooz-Zadeh, 1998). Briefly, FOX reagent was prepared by mixing one part of a solution (2.5mM ferrous ammonium sulphate and 1.0 mM xylenol orange) in 250 mM H2SO4 with nine parts of β-Hydroxytoluene (5 mM in methanol). The calibration standard curve was made from a stock solution of 30% H2O2 with eight concentrations (5–200 μM). Lipids were extracted from the chicken breast (1 g) that was treated with or without OBPI and OBPH (1 mg/g) and stored as above (4 °C and -20 °C) using 5 mL of chloroform–methanol (2:1). FOX reagent (1.140 mL) was mixed with lipid extract or standard (0.06 mL), followed by the incubation for 30 min at room temperature and absorbance reading at 560 nm. Lipid peroxide concentrations were calculated using the calibration curve and expressed as micro-moles of H2O2 per gram of wet weight. Meat not treated with OBPI and OBPH were also prepared and used as controls.
2.7. Measurement of in vitro radical scavenging activities of OBPIs and OBPHs
A potassium phosphate buffer solution (75 mM at pH 7.4) was used to dissolve OBPI and OBPH samples and reagents for oxygen radical absorbance capacity (ORAC) and hydroxyl radical (HO•) assays. The determination ROO• radical scavenging activity using the ORAC assay was carried out using fluorescein probe (0.08 μM), standard Trolox (6.25–100 μM), and isolate/hydrolysate (0.1 mg/mL) (Jodayree et al., 2012). In the HO• assay, 50 μL of OBPI/OBPH (1 mg/mL) or buffer (blank and control) were added to a 96-well clear microplate. This was followed by the addition of 50 μL of 1,10-phenanthroline (3 mM) and 50 μL of 3 mM aqueous Ferrous (II) and finally by 50 μL of 0.03% aqueous hydrogen peroxide (in sample (SPL) and control (CTL)) or water (in blank, blk). The plate was sealed and incubated for 1 h at 37 °C. The absorbance (A) was measured at 536 nm to calculate the HO• scavenging activity as a percentage inhibition using the following formula (Vanvi and Tsopmo, 2016).
2.8. Statistical analysis
Data (n = 3) are shown as mean ± standard deviation. Statistical differences were evaluated by a one-way analysis of variance (ANOVA) using SPSS 11.0 (SPSS Inc., Chicago). Tukey's Honest Significance Differences (HSD) was used to determine the significant difference between groups (p < 0.05). The linear regression was also performed using the SPSS software.
3. Results
3.1. Characterisation of hydrolyzed proteins
Polysaccharide degrading enzymes cellulase and viscozyme were used to prepare two protein isolates CPI and VPI with soluble protein contents of 77.9 ± 1.3 and 82.1 ± 1.9%, respectively. The isolated proteins were individually treated with proteases of different specificities to release peptides that might affect metal species in meat in addition to a potential reduction of the oxidation of fatty acids. Alcalase, papain, flavourzyme, and protamex protein hydrolysates from CPI were indexed CAL, CPA, CFL and CPRO, respectively, while those from VPI treated brans were named VAL, VPA, VFL and VPRO. Proteins and their hydrolysates were characterized based on their relative molecular weights, soluble protein, aromatic amino acid and free thiol contents. Protein contents of the hydrolysates (Table 1) ranged from 19.6 ± 1. 2% to 77.9 ± 1.2% and these represented a significant drop from the content of proteins before treatment with proteases. The decrease of proteins after hydrolysis is likely due to the removal of undigested proteins by centrifugation and to the presence of salts used to adjust pH at various steps. Protein contents of protamex and flavourzyme hydrolysates were not affected by the extraction procedure; meanwhile, for alcalase and papain, the protein contents were higher when viscozyme was used for the extraction. In fact, polypeptide composition data from SDS-PAGE showed that both CPI and VPI had globulin subunits around 19, 20, 27 and 35 kDa, but in addition, there was another band at 60 kDa in the VPI protein sample. Amongst the proteases used, flavourzyme is the only one with endo- and exo-peptidase and its action likely released more free amino acids and hence the lower content of soluble proteins in its hydrolysates.
Table 1.
Free sulfhydryl groups, protein convents and UV absorbance of oat bran protein isolates and their hydrolysates. Data are means (n = 3) ± STDEV.
| Samples | Free Thiol (-SH) (μM cysteine) | Protein Content (%) | Absorbance 280nm |
|---|---|---|---|
| CAL | 17.6 ± 2.6 | 35.8 ± 0.5 | 0.85 ± 0.01 |
| CPA | 10.7 ± 1.0 | 39.5 ± 5.0 | 1.10 ± 0.05 |
| CFL | 29.3 ± 2.7 | 21.1 ± 1.9 | 1.05 ± 0.02 |
| CPRO | 13.1 ± 1.6 | 28.9 ± 1.9 | 0.94 ± 0.02 |
| CPI | 55.1 ± 3.1 | 77.9 ± 1.3 | 1.09 ± 0.14 |
| VAL | 27.2 ± 1.8 | 50.0 ± 0.8 | 0.44 ± 0.01 |
| VPA | 11.7 ± 1.0 | 55.9 ± 0.8 | 0.51 ± 0.01 |
| VFL | 30.3 ± 2.7 | 19.6 ± 1.2 | 0.76 ± 0.01 |
| VPRO | 24.5 ± 3.2 | 22.3 ± 0.5 | 0.58 ± 0.01 |
| VPI | 11.4 ± 1.6 | 45.1 ± 0.9 | 1.04 ± 0.08 |
The relative content of aromatic amino acids (AAA) (Table 1) showed a difference between the isolates (CPI, VPI). The treatment with proteases significantly decreased AAA contents of VPI hydrolysates but only minimally affected CPI hydrolysates. Phenylalanine, tyrosine and tryptophan are the three amino acids with an absorbance at 280 nm; however, tryptophan and tyrosine possess high absorbance (Eftink, 2002). In proteins that consist of both tryptophan and tyrosine, tryptophan always dominates and gives the strongest absorption at wavelength 280 nm (Walters, Esfandi, et al., 2018). Differences between CPI and VPI hydrolysates can be attributed to the relative number of AAA residues because research has shown that absorbance is low in polypeptides that contain tyrosine and no tryptophan (Walters, Esfandi, et al., 2018). The conformation of the polypeptides is also important as AAA residues on the surface will contribute more to the absorbance. Contrary to the AAA data, three (VAL, VFL, VPRO) of the four hydrolysates with higher concentrations of free thiol or sulfhydryl (–SH) groups (Table 1) were derived from VPI proteins. Aromatic amino acids of protein hydrolysates are important for their antioxidant activities through proton and electron donation, while the thiol functional group is important both antioxidant and metal chelation or reduction (Pérez-González et al., 2015). It was therefore expected that the hydrolysates prepared here will have different effects in meat.
3.2. Effect of protein isolates and hydrolysates on arsenic in meat
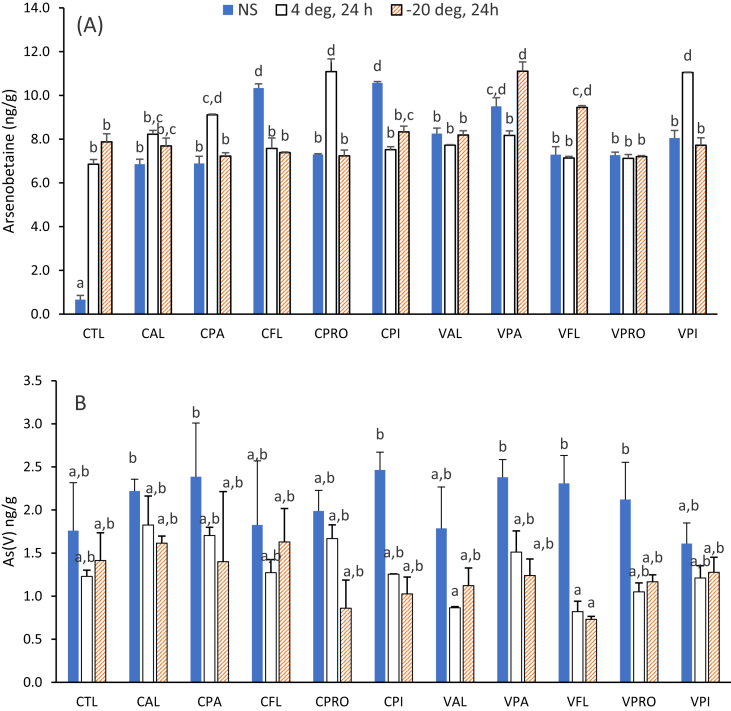
Arsenic is a toxic and carcinogenic metal, especially in the trivalent (As(III)) and pentavalent (As(V)) forms. It is, therefore, of interest not only to quantify total contents, but also the level of individual species. This work then focussed on the effect of hydrolyzed oat proteins on speciation rather than on the total of arsenic or chromium. The aim being to assess whether, in the presence of hydrolyzed oat proteins, arsenic or chromium will exist predominantly in a specific oxidative state. The speciation of arsenic was then conducted in chicken breast meat and of the five species (As(III), AsB, DMA, MMA, As(V)) analyzed, arsenobetaine (AsB) and As(V) were present (Figure 1). Data for AsB and As(V). Their quantification was carried out in chicken beast meats supplemented with oat proteins/hydrolysates immediately after addition and after storage for 24 h at 4 °C or -20 °C are displayed in Figure 2. The addition of oat protein isolates or hydrolysates significantly increased the amount of AsB in all non-stored samples (Figure 2A). The AsB content was 2.7-fold less than that of As(V) in the control meat sample; meanwhile, upon treatment with oat proteins, the concentration of AsB was 2.9–5.5-fold higher relative to As(V). Overall, concentrations of AsB ranged from 6.9 ± 0.3 to 11.1 ± 0.5 ng/g except for one control (0.7 ± 0.2 ng/g). The control with the low AsB was analyzed immediately after homogenization, while the others that were after 24 h storage at 4 or -20 °C had concentrations of 6.9 and 7.9 ng/g, respectively. It then appeared that more time was needed to lyse tissues and enhance the release of AsB. The addition of oat protein hydrolysates had minimal effect on the extraction AsB.
Figure 1.
HPLC-ICP-MS chromatograms of arsenics at m/z 75. Top: Standard solution containing 5 μg/L of As(III), As(V), DMA (dimethylarsinic acid) and MMA (monomethylarsonate), AsB (arsenoberatine). Bottom: Extract from control chicken breast meat sample.
Figure 2.
Arsenic species in chicken breast meats with or without the addition of oat proteins or hydrolysates. A) Arsenobtaine (AsB); B) pentavalent arsenic (As(V)). Oat bran proteins extracted with cellulase (CPI) and viscozyme (VPI). CAL, CPA, CFL, CPRO are alcalase, papain, flavourzyme, and protamex hydrolysates from CPI; corresponding hydrolysates from VPI are VAL, VPA, VFL and VPRO; CTL: control meat (no supplementation). Meat samples were analyzed immediately after processing (NS: non-stored) or stored for 24 h at 4 °C and -20 °C before analysis. Data are means (N = 3) ± STDEV. Values with different letters are significant different as determined with Tukey's Honest Significance Differences (HSD) in one-way ANOVA (p < 0.05).
The analysis of As(V) immediately after homogenization of the chicken meat samples was not influenced by the addition of hydrolyzed oat proteins. The amount of As(V) decreased in samples that were stored at +4 or -20 °C for 24 h and was significant for proteins extracted with viscozyme followed hydrolysis with either alcalase or flavourzyme (Figure 2B). The decrease of As(V) could be due to reduction by thiol-containing peptides, but this did not result in quantifiable amounts of As(III). Additionally, no correlation was found between the concentration of AsB/As(V) and the amount of thiols present in the oat proteins and hydrolysates. There was a difference in speciation of arsenic for the 4 °C control meat relative to the initial control. The values (AsB, As(V)) were 6.9 ± 0.2 and1.23 ± 0.32 ng/g at 4 °C (Figure 2B) versus 0.66 ± 0.19 and 1.76 ± 0.56 ng/mL before storage.
The total amount of arsenic (<13 ng/g), obtained from this study (90% of which are AsB) are low compared to tolerance levels (50–200 ng/g) for food products from animals treated with veterinary drugs containing arsenic (U.S. ATSDR, 2010). Arsenobetaine (AsB) is the most abundant form of arsenic in meats or seafoods, and it is considered non-toxic because it is not metabolized in humans. Studies have reported total arsenic content of freeze-dried chicken breast tissues to be 168 and 270 ng/g but also 48 ± 5 ng/g for AsB (Pizarro et al., 2003; Sánchez-Rodas et al., 2006). These reports are based on dry weight and the wet weight as in the present work. The variation of AsB or total arsenic in chicken is affected by feeding and the use of arsenic-containing veterinary drugs. Antibiotic-free and organic chicken meat generally contain lower arsenic contents (Liu et al., 2016; Nachman et al., 2013). Three of the species analyzed As(III), MMA and DMA were not found in the tested chicken meat samples. There are literature of their present most often, when chickens received feeds containing arsenic veterinary drugs such as Roxarsone (Liu et al., 2015). The nature of the veteran drugs, their concentration and the duration affect the amount of As species in the chicken tissue.
3.3. Chromium speciation
Chromium toxicity is similar to that of arsenic with its most oxidized form, hexavalent chromium (Cr (VI)) being responsible for the carcinogenic effect (Kitchin and Conolly, 2010). In solution, hydrolyzed food proteins can affect the concentration of chromium species because they can reduce Cr(VI) to Cr(III) (Leung et al., 2018). Their effect in a food system is, however, not known. The addition of oat proteins/hydrolysates to chicken breast meat had a significant effect on chromium speciation. Overall, there was a reduction in the concentration of Cr(VI) that was accompanied by an increase in levels of Cr(III) (Figure 3). In control meat samples, Cr(III) concentrations were 2.76 ± 0.15 to 3.38 ± .011 (Figure 3A) while Cr(VI) values were 11.5 ± 0.3 to 14.3 ± 0.1 ng/g (Figure 3B). The addition of proteins shifted the values to 3.25 ± 0.05–8.6 ± 0.72 ng/g for Cr(III) and 5.0 ± 0.6–10.4 ± 0.9 ng/g for Cr(III). Those data showed that there was a reduction of Cr(VI) to Cr(III) immediately after the addition of hydrolysates and after storage at 4 °C and -20 °C. They also showed that the reduction was greater after storage for most hydrolysates. Studies have shown that reductants of Cr(VI) include small thiol molecules such as glutathione but also ascorbate (Shi, 1999; Wiegand et al., 1984). There are also data on the reduction of Cr(VI) by hydrolyzed food proteins and cysteine tripeptides derived from rye secalin proteins (Leung et al., 2018), but this is the first report of their effect in a food system.
Figure 3.
Chromium III (A) and VI (B) in chicken breast meats with or without oat proteins or hydrolysates. Oat bran proteins extracted with cellulase (CPI) and viscozyme (VPI). CAL, CPA, CFL, CPRO are alcalase, papain, flavourzyme, and protamex hydrolysates from CPI; corresponding hydrolysates from VPI are VAL, VPA, VFL and VPRO; CTL: control meat (no supplementation). Meat samples were analyzed immediately after processing (NS: non-stored) or stored for 24 h at 4 °C and -20 °C before analysis. Data are means (n = 3) ± STDEV. Values with different letters are significant different as determined with Tukey's Honest Significance Differences (HSD) in one-way ANOVA (p < 0.05).
The total level chromium in chicken tissues (meat, liver, kidney, heart, skin) were 28–75 ng/g of wet weight (Uluozlu et al., 2009). Other works reported 14–66 ng/g and 10–16 ng/g fresh weight in pig and cattle meats, respectively (Jorhem et al., 1989) while the concentration Cr(III) in plants such as Zingiber was 2.6 ± 0.5 ng/g (Soylak and Unsal, 2010). The values of chromium in the present work are on the lower end of those literature data. The toxicity of the two mineral (Cr, As) in biological systems is due to their binding to protein, leading to a loss of function but also to the generation of reactive oxygen species (Kitchin and Wallace, 2008). The excess oxidant (superoxide anion and hydroxyl radicals) overwhelms the redox environment, and the consequence is damage to lipids and nucleotides. It was, therefore, of interest to also evaluate the antioxidant properties of the hydrolyzed oat proteins.
3.4. Inhibition of lipid peroxidation in meat
Fatty acids in meat and other food products undergo degradation reactions that lead to rancidity and decrease shelf life. Although these reactions are greater at higher temperatures, studies on biochemical changes in meats during frozen storage have shown that lipid oxidation reaction occurs (Soyer et al., 2010). Data on the ability of hydrolysates to inhib lipid peroxidation in chicken breast meats are shown in Figure 4. The amount of hydroperoxides in the meat was 0.37 ± 0.04 μM H2O2 eq./g. This value decreased (p < 0.05) immediately after the addition of four of the protein hydrolysates to 0.17–0.22 μM H2O2 eq./g while there was no change due to the presence of other hydrolysates (Figure 4). The non-hydrolyzed proteins caused an increase upon their addition to meat. During storage at 4 °C, the amount of hydroperoxides increased by 2.5-fold in the control meat. Proteins hydrolyzed with alcalase (CAL, VAL) or papain (CPA, VPA) limited the increased to about 1.5-fold while other hydrolysates decreased hydroperoxide values below the control value. In the meat samples stored at -20 °C, some hydrolyzed proteins inhibited the increase in the concentration of hydroperoxides and, when there was an increase, it was significantly less compared to the samples stored at 4 °C. Independently of the storage conditions, the lower amount of hydroperoxides was in the meat sample containing alcalase (CAL, VAL) and papain (CPA, VPA) hydrolysates.
Figure 4.
Lipid hydroperoxide concentrations in chicken breast meats with or without oat proteins or hydrolysates. Oat bran proteins extracted with cellulase (CPI) and viscozyme (VPI). CAL, CPA, CFL, CPRO are alcalase, papain, flavourzyme, and protamex hydrolysates from CPI; corresponding hydrolysates from VPI are VAL, VPA, VFL and VPRO; CTL: control meat (no supplementation). Meat samples were analyzed immediately after processing (NS, non-stored), or stored for 24 h at 4 °C and -20 °C before analysis. Data were obtained on triplicate samples and expressed as means ± STDEV. Values with different letters are significant different as determined with Tukey's Honest Significance Differences (HSD) in one-way ANOVA (p < 0.05).
The presence of aromatic or hydrophobic amino acids within the sequence of peptides in hydrolyzed proteins can enhance their interaction with lipids and potentially improve their properties to protect lipid oxidation. Amongst the most actives hydrolysates, CPA has the higher aromatic amino acid (AAA) contents, but its activity was similar to those of CAL, VAL and VPA with lower AAA content. This could be explained by the fact that in addition to the hydrophobic interaction of peptides with lipids, the location of the proton donating AAA is important. The hydrolysis improved the activity of both cellulase (CPI), and viscozyme (VPI) extracted proteins. Protein isolates and hydrolysates from other food sources have been shown to inhibit lipid peroxidation in food systems. For example, wheat hydrolysates decreased lipid oxidation in pork meat patties (Park et al., 2012) while the incorporation of rice hydrolyzed proteins into ground beef also decreased the oxidation of lipid (Zhou et al., 2013). Like in this study, the decrease was observed after 24 h storage at 4 °C; meanwhile, the literature work quantified the oxidation based on the amount of malondialdehyde (an end-product of lipid oxidation) rather than hydroperoxides which are primary oxidation products. A previous work obtained hydroperoxide values of 0.74–1.85 μM H2O2 eq/g in chicken meat depending on the processing method (Fratianni et al., 2010) while in this work values were 0.17–1.84 μM H2O2 eq/g.
3.5. Radical scavenging properties of protein hydrolysates
Peroxyl (ROO•) and hydroxyl (HO•) radicals and two species that are commonly associated with oxidative degradation of foods, including the generation of lipid hydroperoxides. It was, therefore, of interest to assess the radical scavenging activity of the oat proteins and their hydrolysates. The action of protease on the isolated proteins VPI or CPI increased their ROO• scavenging activities from 107 ± 12 and 305 ± 28μM Trolox equivalents (TE)/g, respectively to 406–641 μM TE/g (Figure 5A). This is in agreement with literature data on ROO• scavenging properties of hydrolyzed plant proteins (Wattanasiritham et al., 2016; Wongekalak et al., 2011). In fact, the hydrolysis can release small peptides that possess proton donating ability and consequently have a better interaction with the peroxyl radical. Although CPI had a higher ROO• scavenging power compared to VPI, most CPI-hydrolysates had lower activities compared to those derived from VPI. This is likely because of the differences in protein compositions, as shown by gel electrophoresis data, and the differences in specificities of the proteases. The HO• radical is the most reactive free radical formed from the reaction of hydrogen peroxide and free transition metals. It can oxidatively damage polypeptides and lipids in foods and, if consumed, oxidized foods can, in turn, cause damage to biological molecules (Jodayree et al., 2012; Vanvi and Tsopmo, 2016). Contrary to peroxyl radicals, non-hydrolyzed proteins (CPI, VPI) had higher HO• scavenging activities than their hydrolysates (Figure 5B). This is likely because the HO• assay is dependent on both the ability of the antioxidant molecule to donate protons and to chelation ferrous ions. Intact proteins might have had better chelating properties, which can explain their higher inhibition of HO• radicals.
Figure 5.
Peroxyl (A) and hydroxyl (B) radical scavenging activity of oat bran proteins extracted with cellulase (CPI) and viscozyme (VPI). CAL, CPA, CFL, CPRO are alcalase, papain, flavourzyme, and protamex hydrolysates from CPI; corresponding hydrolysates from VPI are VAL, VPA, VFL and VPRO. Each sample was analyzed in triplicate and is expressed as means ± STDEV. Values with different letters are significant different as determined with Tukey's Honest Significance Differences (HSD) in one-way ANOVA (p < 0.05).
The presence of aromatic amino acids (AAA) and thiol residues within the peptide sequences can enhance the radical scavenging properties of protein hydrolysates, mainly through a proton transfer mechanism. This work found a significant correlation (F(1,8) = 6.69, F = 0.03, r = 0.45) between ROO• scavenging data and the content of AAA based on UV absorbance but not with the HO• data (F(1,8) = 0.01, F = 0.9). The HO• scavenging power of the hydrolysates then likely relied on their ability to chelate ferrous ions. There was no correlation of antioxidant data with the amount of free thiol groups, although the relation with HO• data was better compared to the one with ROO• data.
4. Conclusion
This study detected two species of arsenic and two species of chromium in chicken meat samples. Their concentrations are within the range of the literature data. The addition of hydrolyzed oat bran proteins to the meat had minimal effects on the concentration or ratio of arsenic of arsenobetaine to arsenic(V), but there was some conversion of chromium-VI to chromium-III, which is beneficial as the former is the toxic form of chromium. The addition of hydrolyzed oat proteins also better preserved the meat samples by reducing the level of lipid oxidation.
Declarations
Author contribution statement
Adenike Shittu: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Ramak Esfandi: Analyzed and interpreted the data; Wrote the paper.
Apollinaire Tsopmo: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Discovery Grants from the National Science and Engineering Research Council of Canada (NSERC) to A.T (No: 371908).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Bhattacharya M., Jain N., Bhasne K., Kumari V., Mukhopadhyay S. pH-Induced conformational isomerization of bovine serum albumin studied by extrinsic and intrinsic protein fluorescence. J. Fluoresc. 2011;21(3):1083–1090. doi: 10.1007/s10895-010-0781-3. [DOI] [PubMed] [Google Scholar]
- Carrasco-Castilla J., Hernández-Álvarez A.J., Jiménez-Martínez C., Jacinto-Hernández C., Alaiz M., Girón-Calle J., Vioque J., Dávila-Ortiz G. Antioxidant and metal chelating activities of peptide fractions from phaseolin and bean protein hydrolysates. Food Chem. 2012;135(3):1789–1795. doi: 10.1016/j.foodchem.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Eftink M.R. Topics in Fluorescence Spectroscopy. Kluwer Academic Publishers; 2002. Intrinsic fluorescence of proteins; pp. 1–15. [Google Scholar]
- Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82(Issue 1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Esfandi R., Willmore W.G., Tsopmo A. Peptidomic analysis of hydrolyzed oat bran proteins, and their in vitro antioxidant and metal chelating properties. Food Chem. 2019;279:49–57. doi: 10.1016/j.foodchem.2018.11.110. [DOI] [PubMed] [Google Scholar]
- Espinosa G.Y.C., Walters M.E., Tsopmo A. Oats. In: Mir S.A., Manickavasagan A., Shah M.A., editors. Whole Grains: Processing, Product Development, and Nutritional Aspects. CRC Press; 2019. pp. 129–150. [Google Scholar]
- Fratianni F., De Martino L., Melone A., De Feo V., Coppola R., Nazzaro F. Preservation of chicken breast meat treated with thyme and balm essential oils. J. Food Sci. 2010;75(8):M528–M535. doi: 10.1111/j.1750-3841.2010.01791.x. [DOI] [PubMed] [Google Scholar]
- Hänsch R., Mendel R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl) Curr. Opin. Plant Biol. 2009;12(3):259–266. doi: 10.1016/j.pbi.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Jodayree S., Smith J.C., Tsopmo A. Use of carbohydrase to enhance protein extraction efficiency and antioxidative properties of oat bran protein hydrolysates. Food Res. Int. 2012;46(1):69–75. [Google Scholar]
- Jomova K., Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283(2–3):65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Jorhem L., Sundström B., Åstrand C., Haegglund G. The levels of zinc, copper, manganese, selenium, chromium, nickel, cobalt, and aluminium in the meat, liver and kidney of swedish pigs and cattle. Z. Lebensm. Unters. Forsch. 1989;188(1):39–44. doi: 10.1007/BF01027620. [DOI] [PubMed] [Google Scholar]
- Kamdem J.P., Tsopmo A. Reactivity of peptides within the food matrix. J. Food Biochem. 2019;43(1) doi: 10.1111/jfbc.12489. [DOI] [PubMed] [Google Scholar]
- Kitchin K.T., Conolly R. Arsenic-induced carcinogenesissoxidative stress as a possible mode of action and future research needs for more biologically based risk assessment. Chem. Res. Toxicol. 2010;23(2):327–335. doi: 10.1021/tx900343d. [DOI] [PubMed] [Google Scholar]
- Kitchin K.T., Wallace K. The role of protein binding of trivalent arsenicals in arsenic carcinogenesis and toxicity. J. Inorg. Biochem. 2008;102(3):532–539. doi: 10.1016/j.jinorgbio.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leung R., Venus C., Zeng T., Tsopmo A. Structure-function relationships of hydroxyl radical scavenging and chromium-VI reducing cysteine-tripeptides derived from rye secalin. Food Chem. 2018;254:165–169. doi: 10.1016/j.foodchem.2018.01.190. [DOI] [PubMed] [Google Scholar]
- Liu Q., Peng H., Lu X., Le X.C. Enzyme-assisted extraction and liquid chromatography mass spectrometry for the determination of arsenic species in chicken meat. Anal. Chim. Acta. 2015;888:1–9. doi: 10.1016/j.aca.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Liu Q., Peng H., Lu X., Zuidhof M.J., Li X.-F., Le X.C. Arsenic species in chicken breast: temporal variations of metabolites, elimination kinetics, and residual concentrations. Environ. Health Perspect. 2016;124(8):1174–1181. doi: 10.1289/ehp.1510530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry O., Rosebrough N., Farr A., Randall R. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:144–149. [PubMed] [Google Scholar]
- Nachman K.E., Baron P.A., Raber G., Francesconi K.A., Navas-Acien A., Love D.C. Roxarsone, inorganic arsenic, and other arsenic species in chicken: a U.S.-based market basket sample. Environ. Health Perspect. 2013;121(7):818–824. doi: 10.1289/ehp.1206245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourooz-Zadeh J. Ferrous ion oxidation in presence of xylenol orange for detection of lipid hydroperoxides in plasma. Methods Enzymol. 1998;300:58–62. doi: 10.1016/s0076-6879(99)00113-5. [DOI] [PubMed] [Google Scholar]
- Ortiz-Martinez M., Winkler R., García-Lara S. Preventive and therapeutic potential of peptides from cereals against cancer. J. Proteom. 2014;111:165–183. doi: 10.1016/j.jprot.2014.03.044. [DOI] [PubMed] [Google Scholar]
- Park E.Y., Imazu H., Matsumura Y., Nakamura Y., Sato K. Effects of peptide fractions with different isoelectric points from wheat gluten hydrolysates on lipid oxidation in pork meat patties. J. Agric. Food Chem. 2012;60(30):7483–7488. doi: 10.1021/jf301532e. [DOI] [PubMed] [Google Scholar]
- Park S.Y., Ahn C.-B., Je J.-Y. Antioxidant and anti-inflammatory activities of protein hydrolysates from Mytilus edulis and ultrafiltration membrane fractions. J. Food Biochem. 2014;38(5):460–468. [Google Scholar]
- Pérez-González A., Alvarez-Idaboy J.R., Galano A. Free-radical scavenging by tryptophan and its metabolites through electron transfer based processes. J. Mol. Model. 2015;21(8):213. doi: 10.1007/s00894-015-2758-2. [DOI] [PubMed] [Google Scholar]
- Pizarro I., Gómez M., Cámara C., Palacios M.A. Arsenic speciation in environmental and biological samples: extraction and stability studies. Anal. Chim. Acta. 2003;495(1):85–98. [Google Scholar]
- Ryan L., Thondre P.S., Henry C.J.K. Oat-based breakfast cereals are a rich source of polyphenols and high in antioxidant potential. J. Food Compos. Anal. 2011 [Google Scholar]
- Sadiq Butt M., Tahir-Nadeem M., Khan M.K.I., Shabir R., Butt M.S. Oat: unique among the cereals. Eur. J. Nutr. 2008;47(2):68–79. doi: 10.1007/s00394-008-0698-7. [DOI] [PubMed] [Google Scholar]
- Sánchez-Rodas D., Luis Gómez-Ariza J., Oliveira V. Development of a rapid extraction procedure for speciation of arsenic in chicken meat. Anal. Bioanal. Chem. 2006;385(7):1172–1177. doi: 10.1007/s00216-006-0379-4. [DOI] [PubMed] [Google Scholar]
- Séby F., Charles S., Gagean M., Garraud H., Donard O.F.X. Chromium speciation by hyphenation of high-performance liquid chromatography to inductively coupled plasma-mass spectrometry—study of the influence of interfering ions. J. Anal. At. Spectrom. 2003;18(11):1386–1390. [Google Scholar]
- Shi X. Reduction of chromium (VI) and its relationship to carcinogenesis. J. Toxicol. Environ. Health, Part BOnline) Journal Xianglin Shi Journal of Toxicology and Environmental Health, Part B. 1999;2(1):1093–7404. doi: 10.1080/109374099281241. [DOI] [PubMed] [Google Scholar]
- Soyer A., Özalp B., Dalmiş Ü., Bilgin V. Effects of freezing temperature and duration of frozen storage on lipid and protein oxidation in chicken meat. Food Chem. 2010;120(4):1025–1030. [Google Scholar]
- Soylak M., Unsal Y.E. Chromium and iron determinations in food and herbal plant samples by atomic absorption spectrometry after solid phase extraction on single-walled carbon nanotubes (SWCNTs) disk. Food Chem. Toxicol. 2010;48(6):1511–1515. doi: 10.1016/j.fct.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Tsopmo A., Gao Q., Baakdah M.M. Reduction of hexavalent chromium by digested oat bran proteins. Food Chem. 2014;153:171–176. doi: 10.1016/j.foodchem.2013.12.049. [DOI] [PubMed] [Google Scholar]
- U.S. ATSDR . U.S. Agency for Toxic Substances and Diseases Registry. Arsenic toxicity what are the standards and regulation for arsenic exposure? In: Gehle K., editor. 2010. https://www.atsdr.cdc.gov/csem/csem.asp?csem=1&po=8 [Google Scholar]
- Uluozlu O.D., Tuzen M., Mendil D., Soylak M. Assessment of trace element contents of chicken products from turkey. J. Hazard Mater. 2009;163(2):982–987. doi: 10.1016/j.jhazmat.2008.07.050. [DOI] [PubMed] [Google Scholar]
- Van Hulle M., Zhang C., Zhang X., Cornelis R. Arsenic speciation in Chinese seaweeds using HPLC-ICP-MS and HPLC-ES-MS. Analyst. 2002;127(5):634–640. doi: 10.1039/b110940e. [DOI] [PubMed] [Google Scholar]
- Vanvi A., Tsopmo A. Pepsin digested oat bran proteins: separation, antioxidant activity, and identification of new peptides. J. Chem. 2016;2016:1–8. [Google Scholar]
- Walters M.E., Esfandi R., Tsopmo A. Potential of food hydrolyzed proteins and peptides to chelate iron or calcium and enhance their absorption. Foods. 2018;7(10):172. doi: 10.3390/foods7100172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters M.E., Udenigwe C.C., Tsopmo A. Structural characterization and functional properties of proteins from oat milling fractions. JAOCS (J. Am. Oil Chem. Soc.) 2018;95(8):991–1000. [Google Scholar]
- Wattanasiritham L., Theerakulkait C., Wickramasekara S., Maier C.S., Stevens J.F. Isolation and identification of antioxidant peptides from enzymatically hydrolyzed rice bran protein. Food Chem. 2016;192:156–162. doi: 10.1016/j.foodchem.2015.06.057. [DOI] [PubMed] [Google Scholar]
- Wiegand H.J., Ottenwälder H., Bolt H.M. The reduction of chromium (VI) to chromium (III) by glutathione: an intracellular redox pathway in the metabolism of the carcinogen chromate. Toxicology. 1984;33(3–4):341–348. doi: 10.1016/0300-483x(84)90050-7. http://www.ncbi.nlm.nih.gov/pubmed/6515663 [DOI] [PubMed] [Google Scholar]
- Wongekalak L., Sakulsom P., Jirasripongpun K., Hongsprabhas P. Potential use of antioxidative mungbean protein hydrolysate as an anticancer asiatic acid carrier. Food Res. Int. 2011;44(3):812–817. [Google Scholar]
- Zhou K., Canning C., Sun S. Effects of rice protein hydrolysates prepared by microbial proteases and ultrafiltration on free radicals and meat lipid oxidation. Lwt-Food Sci. Technol. 2013;50(1):331–335. [Google Scholar]