Short abstract
Objective
To analyze the clinical and laboratory features and determine the predictors of acute liver failure (ALF) among inpatients with anti-tuberculosis (TB) drug-induced liver injury (DILI).
Method
Patients diagnosed with anti-TB DILI from 2010 to 2016 at The First Affiliated Hospital of Zhejiang University were retrospectively included in this study. Demographic and clinical data were collected by reviewing electronic medical records.
Results
Among 155 inpatients with anti-TB DILI, 55 (35.48%) developed ALF, with an overall mortality of 9.68%. The median time to DILI onset was significantly longer in the ALF compared with the non-ALF group (51 versus 24 days). Eighty-three patients (53.55%) developed DILI (53.55%) within the first month of anti-TB treatment, and 60% of ALF cases occurred within 2 months. Multivariable models for ALF incorporating aspartate aminotransferase, total bilirubin, platelets, white blood cell count, and pre-existing hepatitis yielded a concordance (C-statistic) of 98.93%.
Conclusions
The results of this study suggest that approximately half of all cases of DILI occur within the first month, while 60% of ALF cases occur within 2 months. Elevated total bilirubin, aspartate aminotransferase, white blood cell count, pre-existing hepatitis, and low platelet count are independent risk factors for the development of anti-TB drug-induced ALF.
Keywords: Antitubercular agent, drug-induced liver injury, acute liver failure, risk factor, total bilirubin, aspartate aminotransferase, white blood cell count, hepatitis, platelet count
Introduction
The World Health Organization estimated that Mycobacterium tuberculosis was responsible for 6.3 million new active infections and 1.67 million deaths worldwide in 2016.1 China has the third-highest burden of tuberculosis (TB) cases after India and Indonesia. Early diagnosis and appropriate treatment are important for preventing TB-related deaths, and short-course chemotherapy including isoniazid, rifampin, pyrazinamide, and ethambutol has effectively prevented the spread of TB. However, mild or fatal liver dysfunction is a major adverse effect of the three most widely used drugs, potentially resulting in treatment discontinuation.2,3 Anti-TB drugs are the second leading cause of drug-induced liver injury (DILI) in China, after traditional Chinese herbal medicine,4,5 with anti-TB DILI accounting for 15.3%–31.3% of all cases of DILI.4–6 There have been few population-based studies of anti-TB DILI in China; however, studies in a TB-endemic region in India found that anti-TB DILI patients who developed acute liver failure (ALF) accounted for approximately 5%–25.7% of all ALF cases.7–9 In contrast, approximately 39% of all ALF cases in the United States resulted from paracetamol-induced liver injury.10 In terms of morbidity, the severity of anti-TB DILI cannot be ignored, given that anti-TB drug-induced ALF tends to result in the need for liver transplantation, or in death.
Few studies have assessed the abilities of clinical characteristics and laboratory tests to predict the risk of ALF among patients with anti-TB DILI. Hy’s Law suggests that DILI results in alanine aminotransferase (ALT) levels >3 times the upper limit of normal (ULN) and total bilirubin levels (TBL) >2 times the ULN, after excluding other potential recent causes, conferring a 10% mortality rate.11–14 However, its application to DILI is restricted by its low specificity.
This study therefore aimed to analyze clinical and laboratory features, evaluate the importance of several consequential factors of ALF, and identify appropriate predictors of ALF among inpatients with anti-TB DILI. We also examined the relationship between the criteria for Hy’s Law and a new model for predicting incident ALF.
Patients and methods
Patients
We conducted a retrospective survey from 2010 to 2016 to investigate the demographic, clinical, and laboratory features and outcomes of inpatients with anti-TB DILI who had received multidrug therapy for active TB at The First Affiliated Hospital of Zhejiang University. A total of 3376 inpatients were treated with anti-TB medication. The study complied with the relevant principles of the Declaration of Helsinki, and was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University. Written informed consent was obtained from each participant or his/her legal representatives.
Definitions
Patients were enrolled into the study if they met the following criteria: ALT or aspartate aminotransferase (AST) level >5 times the ULN, or alkaline phosphatase (ALP) or TBL >2 times the ULN, or an international normalized ratio >1.5 times the ULN with any elevation in AST, ALT, or ALP.15 The pattern of liver injury was classified based on R values (ALT × ULN/ALP × ULN).16 Hy’s Law cases were defined as ALT >3 times the ULN and bilirubin >2 times the ULN probably caused by drug, with no other potential causes.14 Risk factors such as sex, smoking, alcohol consumption, and pre-existing hepatitis were analyzed. Patients with hepatocellular carcinoma or who were pregnant were excluded. All eligible patients were estimated by the Roussel Uclaf Causality Assessment Method model (RUCAM)17 and Hy’s Law according to liver biochemistries of DILI determined by three specialists in hepatology. Finally, 155 patients who met the inclusion criteria were enrolled in the study.
Drug-induced ALF was defined as follows: TBL level ≥10 times the ULN or international normalized ratio ≥2.0 in the presence of signs of hepatic decompensation (ascites or encephalopathy) and/or other organ failure; the need for liver transplantation; or death due to liver failure,17,18 based on peak parameters of laboratory inspection in electronic medical records. According to this definition, we separated patients with anti-TB DILI into an ALF group (n = 55) and a non-ALF group (n = 100).
Statistical analysis
All data were analyzed using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, NY, USA). ALF events were considered as study endpoints. Normally distributed variables are summarized as the mean ± standard deviation, data from skewed distributions are shown as the median (range), and categorical variables are summarized as frequency and percentages. A (two-tailed) P value < 0.05 was considered to be significant. Univariate logistic regression analysis was performed to identify significant risk factors for ALF. Adjusted odds ratios (ORs) with 95% confidence intervals (CIs) were computed from the model coefficients. Receiver operating characteristic curves were plotted to measure the performances of Hy’s Law and the predictive model. All variables were evaluated using Student’s t-test, Wilcoxon’s rank-sum test, or Pearson’s χ2 test, as appropriate.
Results
Study population
A total of 155 inpatients with DILI received anti-TB chemotherapy from 2010 to 2016 (104 (67.10%) male; mean age 47.96±15.75 years). The demographic data and laboratory findings are shown in Tables 1 and 2. The median duration of treatment was 50 days (range, 2 to 330 days). Of the included patients, 70 patients (45.16%) developed frequent jaundice and required hospitalization, and nine patients (5.81%) presented with encephalopathy before or after hospitalization. The RUCAM scores were highly probable for DILI in 35 patients (22.58%), probable in 88 patients (56.77%), and possible in 32 patients (20.65%). Forty-four (28.39%) patients had a history of liver disease, including chronic viral hepatitis (n = 28), fatty liver disease (n = 12), and liver schistosomiasis (n = 4), and six patients (3.87%) had human immunodeficiency virus infection.
Table 1.
Demographic data for patients with drug-induced liver injury (n = 155).
| Variable | No ALF (n = 100) | ALF (n = 55) | P value |
|---|---|---|---|
| Age, year | 46.64 ± 15.57 | 50.36 ± 15.95 | 0.16 |
| Male, n (%) | 70 (70) | 34 (61.82) | 0.3 |
| Fluoroquinolone antibiotics used, n (%) | 19 (19) | 6 (10.91) | 0.19 |
| Pulmonary tuberculosis*, n (%) | 74 (74) | 43 (78.18) | 0.56 |
| Extrapulmonary tuberculosis*, n (%) | 39 (44) | 15 (27.27) | 0.14 |
| History of previous tuberculosis, n (%) | 10 (10) | 10 (18.18) | 0.15 |
| HIV, n (%) | 6 (6) | 0 (0) | 0.16 |
| Pre-existing hepatitis†, n (%) | 19 (19) | 25 (45.45) | 4.74E−04 |
| Fatty liver/alcohol liver, n (%) | 7 (7) | 5 (9.09) | |
| Chronic viral hepatitis, n (%) | 12 (12) | 16 (29.09) | |
| Liver schistosomiasis, n (%) | 0 (0) | 4 (7.27) | |
| Diabetes mellitus, n (%) | 8 (8) | 5 (9.09) | 0.82 |
| Pyrazinamide use, n (%) | 80 (80) | 39 (70.91) | 0.20 |
| Alcohol use, n (%) | 20 (20) | 14 (25.45) | 0.43 |
| Smoking, n (%) | 28 (28) | 14 (25.45) | 0.73 |
| Duration of treatment, days | 24 (2–228) | 51 (7–330) | 3.51E−04 |
| Hy’s law positive, n (%) | 15 (15) | 29 (52.73) | 6.21E−07 |
| Highly probable/probable/possible, n (%) | 26 (26)/56 (56)/18 (18) | 9 (16.36)/32 (58.18)/14 (25.45) | 0.30 |
| Type of liver injury (R), n (%) | |||
| Hepatocellular/cholestatic/mixed | 49 (49)/14 (14)/37 (27) | 30 (54.54)/16 (29.09)/9 (16.36) | 0.01 |
| Unknown treatment scheme, n (%) | 8 (8) | 3 (5.45) | 0.75 |
*Separate registration of pulmonary tuberculosis and extrapulmonary tuberculosis.
†Pre-existing hepatitis including chronic viral hepatitis, fatty liver disease, liver schistosomiasis.
ALF, acute liver failure; HIV, human immunodeficiency virus; PZA, pyrazinamide.
Table 2.
Symptoms and laboratory findings in 155 patients with drug-induced liver injury.
| Variable | No ALF (n = 100) | ALF (n = 55) | P value |
|---|---|---|---|
| Jaundice, n (%) | 20 (20) | 50 (90.91) | 2.11E−17 |
| Nausea, n (%) | 20 (20) | 29 (52.73) | 2.76E−05 |
| Ascites, n (%) | 14 (14) | 23 (41.82) | 1.01E−04 |
| Vomiting, n (%) | 17 (17) | 19 (34.55) | 0.13 |
| Abdominal distention, n (%) | 15 (15) | 20 (36.36) | 2.34E−03 |
| Rash, n (%) | 18 (18) | 17 (30.91) | 0.07 |
| Itch, n (%) | 10 (10) | 13 (23.64) | 0.02 |
| Biochemical parameters of DILI recognition | |||
| ALT, U/L | 116 (8–924) | 243 (17–2426) | 0.03 |
| AST, U/L | 87.5 (17–599) | 134 (35–1645) | 0.01 |
| ALP, U/L | 85.5 (41–746) | 141 (49.6–600) | 2.75E−06 |
| Total bilirubin, µmol/L | 14.15 (4–158) | 265 (17–668) | 1.86E−21 |
| Direct bilirubin, µmol/L | 7 (1–112) | 151 (7–404) | 6.56E−21 |
| PT, seconds | 12.15 (9.8–16.3) | 18.5 (3.1–56.7) | 3.25E−16 |
| Albumin, g/L | 33.90 ± 6.26 | 29.28 ± 4.51 | 1.68E−06 |
| Serum creatinine, µmol/L, | 55 (25–107) | 56 (10–364) | 0.04 |
| Hemoglobin, g/L | 126.42 ± 18.59 | 115.24 ± 21.93 | 9.86E−04 |
| WBC, 109/L | 5.25 (1.9–29.6) | 6.1 (2.1–58.6) | 0.02 |
| Platelets, 109/L | 198.83 ± 89.48 | 150.13 ± 88.68 | 1.41E−03 |
| Alpha fetoprotein, ng/mL | 2.35 (0.4–3428.1) | 25.7 (1.4–1973) | 2.73E−07 |
| Eosinophil percent, % | 3.5 (0–28.2) | 2.7 (0–38.5) | 0.14 |
ALF, acute liver failure; ALT, alanine transaminase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; PT, prothrombin time; WBC, white blood cell count.
A total of 119 (76.77%) patients who developed DILI were treated with standard anti-TB regimens, 25 (16.13%) received combined use of fluoroquinolone antibiotics, and the treatment was unknown in 11 patients (7.10%).
Outcomes
A total of 55 patients developed ALF. On the basis of the patterns of liver injury, hepatocellular, cholestatic, and mixed hepatitis accounted for 54.54%, 29.09%, and 16.36% of ALF, respectively. Of these 55 patients, 15 patients died (9.68% overall; 12 males, 3 females), including eight (53.33%) who presented with encephalopathy (any grade). Patients with digestive symptoms at admission were more likely to be in the ALF group than patients without these symptoms, including nausea (52.73% versus 20%, P = 2.76E−05) and abdominal distention (36.36% versus 15%, P = 2.34E−03) (Table 2). No patient underwent transplantation, but 19 received assistance from a non-bioartificial liver support system (NBALSS). There was no significant difference in age, sex, history of previous TB, alcohol use, smoking, diabetes mellitus, or eosinophil percentage between the two groups.
Onset time of anti-TB ALF
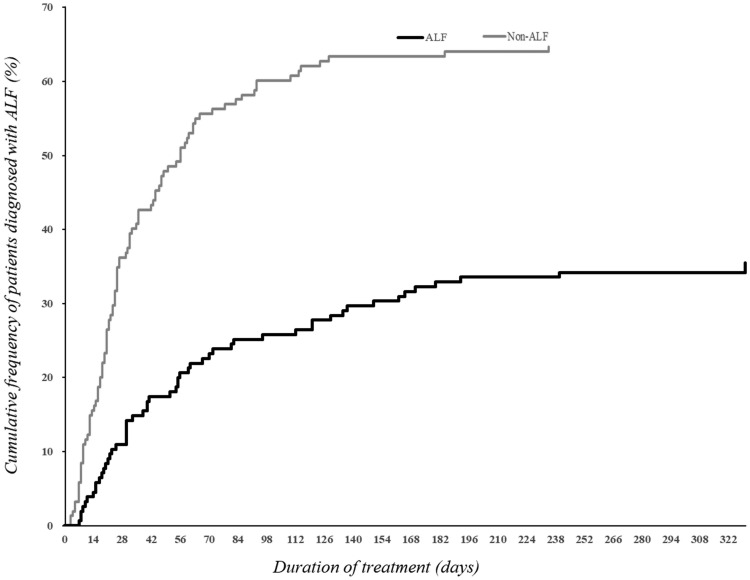
Among the 155 patients with anti-TB DILI, DILI appeared within the first month of treatment in 83 (53.55%) patients. The occurrence of anti-TB ALF was highest at days 15–30 of chemotherapy, followed by days 1–14. Of the 155 patients with anti-TB DILI, 21.29% developed ALF within the first 2 months (Figure 1). The median time to DILI onset was significantly longer in the ALF compared with the non-ALF group (51 versus 24 days, P = 3.51E−04) (Table 1). ALF patients diagnosed with or suspected of having DILI were instructed to stop taking potentially hepatotoxic medications (isoniazid, rifampin, and pyrazinamide).
Figure 1.
Time from beginning of treatment to onset of acute liver failure (ALF) in 155 patients with anti-tuberculosis drug-induced liver injury (DILI). Overall, 40% and 60% of the 55 patients who developed ALF were diagnosed at 1 and 2 months of initial chemotherapy, respectively. The median duration of treatment was 50 days (range 2 to 330 days). Among these patients, the cumulative frequencies of patients diagnosed with ALF at 1 and 2 months of initial chemotherapy were 14.19% (22/155) and 21.29% (33/155), respectively, and the cumulative frequencies of patients diagnosed with non-ALF were 39.35% (61/155) and 54.19% (84/155), respectively.
Overall, among the 55 patients presenting with ALF, 22 (40%) and 33 (60%) patients were diagnosed at 1 and 2 months of initial chemotherapy, respectively, with a median time of onset of 51 days (range 7 to 330 days).
Predictors of anti-TB ALF
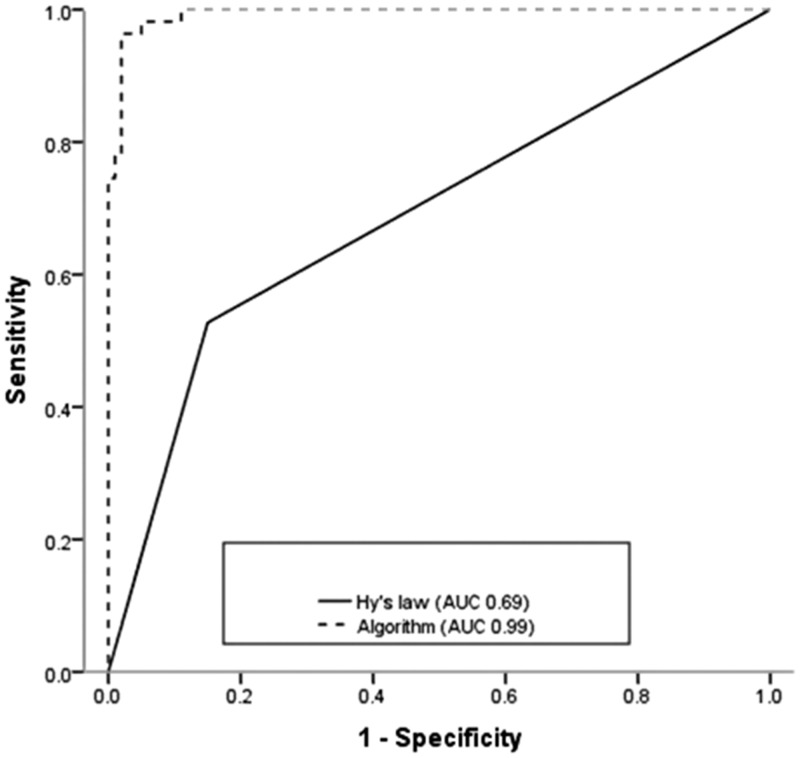
Univariate logistic regression analysis suggested that laboratory values at the time of DILI recognition, including transaminases, TBL, direct bilirubin, ALP, hemoglobin, platelets, albumin, and white blood cell count (WBC), as well as pre-existing hepatitis, symptoms (abdominal distension, nausea, vomiting, itching), ascites, and jaundice may be significant risk factors for the development of ALF during anti-TB treatment (Table 3). Serum levels of alpha fetoprotein were higher in the ALF compared with the non-ALF group (OR 1.76; 95% CI 1.41 to 2.18; P = 3.48E−07). Long latency may also be a potential predictor of ALF (OR 2; 95% CI 1.38 to 2.89; P = 2.25E−04). Multiple logistic regression analysis identified AST (OR 8.24; 95% CI 2.37 to 28.58; P = 8.95E−04), TBL (OR 50.72; 95% CI 8.82 to 291.61; P = 1.08E−05), platelets (OR 0.04; 95% CI 4.05E−03 to 0.43; P = 0.01), WBC (OR 14.00; 95% CI 1.72 to 114.26; P = 0.01), and pre-existing hepatitis (OR 8.66; 95% CI 1.13 to 66.09; P = 0.04) as strong predictive factors of ALF. The ALF score was calculated using the following equation: 2.11 × loge (AST in U/L) + 3.93 × loge (TBL in µmol/L) + 2.64 × loge (WBC in 109/L) − 3.18 × loge (platelets in 109/L) + 2.16 × (pre-existing hepatitis) − 17.14. Multivariable analysis including all the study variables studied yielded a concordance (C-statistic) of 98.93%, which outperformed Hy’s Law (C-statistic, 68.86%) (Figure 2).
Table 3.
Comparison of demographic, clinical, and laboratory variables in 155 patients with drug-induced liver injury with (n = 55) or without (n = 100) subsequent acute liver failure.
| Variable | Univariate OR (95% CI) | P value | Multivariate OR (95% CI) | P value | C-statistic |
|---|---|---|---|---|---|
| Loge (AST U/L) | 1.90 (1.30–2.77) | 9.16E−04 | 8.24 (2.37–28.58) | 8.95E−04 | 0.99 |
| Loge (TBL µmol/L) | 10.39 (4.95–21.80) | 5.92E−10 | 50.72 (8.82–291.61) | 1.08E−05 | |
| Loge (WBC 109/L) | 2.35 (1.22–4.54) | 0.04 | 14.00 (1.72–114.26) | 0.01 | |
| Loge (PLA 109/L) | 0.32 (0.17–0.62) | 6.84E−04 | 0.04 (4.05E−03–0.43) | 0.01 | |
| Pre-existing hepatitis* | 3.55 (1.71–7.36) | 0.01 | 8.66 (1.13–66.09) | 0.04 | |
| PT (seconds) | 1.82 (1.47–2.26) | 3.77E−08 | |||
| Loge (HGB g/L) | 0.05 (0.01–0.33) | 2.06E−03 | |||
| Loge (DBIL µmol/L) | 8.06 (4.16–15.41) | 4.76E−10 | |||
| Loge (AFP ng/mL) | 1.76 (1.41–2.18) | 3.48E−07 | |||
| Loge (ALB g/L) | 0.02 (3.33E−03–0.14) | 6.77E−05 | |||
| Loge (ALT U/L) | 2.40 (1.13–5.04) | 0.02 | |||
| Loge (ALP U/L) | 4.06 (2.02–8.14) | 8.34E−05 | |||
| Loge (duration of treatment, days) | 2 (1.38–2.89) | 2.25E−04 | |||
| Jaundice | 40 (14.11–113.37) | 0.01 | |||
| Abdominal distension | 3.24 (1.49–7.04) | 3.02E−03 | |||
| Nausea | 4.46 (2.17–9.18) | 4.83E−05 | |||
| Vomiting | 2.58 (1.20–5.52) | 0.02 | |||
| Itch | 2.79 (1.13–6.87) | 0.03 | |||
| Hy’s law positive | 6.32 (2.95–13.55) | 2.15E−06 | |||
| Age | 1.02 (0.99–1.04) | 0.16 | |||
| Male | 0.69 (0.35–1.39) | 0.30 | |||
| Alcohol use | 1.37 (0.63–2.98) | 0.43 | |||
| Smoking | 0.88 (0.42–1.85) | 0.73 |
*Pre-existing hepatitis including chronic viral hepatitis, fatty liver disease, liver schistosomiasis
AST, aspartate aminotransferase; TBL, total bilirubin; WBC, white blood cell count; PLA, platelets; PT, prothrombin time; HGB, hemoglobin; DBIL, direct bilirubin; AFP, alpha fetoprotein; ALB, albumin; ALT, alanine transaminase; ALT, alanine transaminase; ALP, alkaline phosphatase
Figure 2.
Comparison between the areas under the receiver operating characteristic curves for Hy’s law criteria and the prognostic algorithm.
Discussion
The current study showed a 35.48% incidence of ALF (55/155 patients) and an overall mortality of 9.68% among patients with anti-TB DILI. A long latency of jaundice appeared to have a marked effect on anti-TB ALF. A prognostic model including four main laboratory variables (AST, TBL, platelets, and WBC) and pre-existing hepatitis predicted incident ALF with a C-statistic of 98.93%, which was superior to the predictive ability of Hy’s Law (C-statistic 68.86%).
The primary cause of ALF in China is hepatitis virus, followed by traditional Chinese herbal medicines and anti-TB drugs.19 The incidence of anti-TB drug-induced ALF is similar across medical centers and regions, and was 35.48% in our study compared with 25.7% noted by Devarbhavi et al.8 and 28.8% by Shu et al.20 Notably however, the current study showed lower anti-TB DILI-related mortality (9.68%) than previous research, which reported 17.3% to 30% mortality in patients with drug-induced ALF,7,8,21,22 with especially high mortality in children (>50%)23,24 in India and the USA. The lower mortality in the current study may be attributable to the use of NBALSS to remove harmful substances and supply beneficial factors. We previously suggested that NBALSS reduced mortality (4-week survival rates of 42% for NBALSS versus 17% for non-NBALSS, P < 0.05) in patients with ALF mainly due to drug toxicity.25 NBALSS may thus be a potentially valuable treatment for patients with anti-TB drug-induced liver failure.
Among the 55 patients presenting with ALF, 22 (40%) and 33 (60%) patients were diagnosed at 1 and 2 months of initial chemotherapy, respectively. The RUCAM score was >3 in all patients, indicating a credible diagnosis. Although anti-TB DILI frequently occurred during the first months of exposure to medication, late-occurring DILI was significantly more likely to develop into ALF. We also aimed to identify the early predictors of ALF. Multivariate analysis identified high serum bilirubin, WBC, pre-existing hepatitis, AST, and low platelet count as independent predictors of early diagnosis. This also suggests that clinicians should consider universal testing every 2 weeks within the first 2 months in all patients receiving treatment,26 and should be aware of any history of chronic liver disease.27 Furthermore, in terms of symptom monitoring, our univariate analysis suggested that more attention should be paid to definitive diagnoses in clinical practice. Patients with DILI who presented with jaundice tended to have a poor outcome, in line with the results of earlier studies.7 Applying the current model at the patient’s first visit could identify more cases of anti-TB DILI at increased risk of ALF progression, especially in overlapping epidemic cases of TB and hepatitis B virus infection. Alpha fetoprotein is produced by proliferating hepatocytes during liver injury, and confounding factors such as cancer, active viral hepatitis, and pregnancy thus should be removed when considering the connection between drug-induced liver failure and alpha-fetoprotein levels.
Our study had some limitations. First, this was a retrospective, single-center study from a grade IIIA hospital that followed a strict triage system. Some patients with mild liver dysfunction may thus have been missed, and our findings may not be representative of patients from other regions and of other ethnicities. Second, the floating population is a matter for concern because these patients may stop anti-TB therapy or adjust their treatment once they have been released from hospital, and these changes would not be recorded. Further multicenter and prospective studies are needed to verify these results.
Conclusion
Among patients with DILI, 35.48% developed ALF and 9.68% died. The results of this study suggest that close monitoring of early signs, symptom, and laboratory test results during the first 2 months of anti-TB treatment is essential for identifying patients at increased risk of ALF. Patients with signs of severe liver damage, such as elevated AST and TBL, and decreased platelet count, should stop taking the medication to reduce their risk of developing ALF.
Acknowledgements
We thank all subjects for their contributions to this study. We also thank the staff members of the TB laboratories in The First Affiliated Hospital, School of Medicine, Zhejiang University.
Author contributions
WST, SGYW, and LPC participated in the design of the study, searched the literature, and drafted the manuscript. DC, YSG, and WJ participated in the data analysis. JZK, SJD, FH, YMF, SP, and RJJ contributed cases and collected follow-up information on the therapeutic effects and prognosis. YJ, SYZ, and LJN participated in the literature search. LLJ and XKJ wrote and revised the manuscript. All authors have read and approved the final manuscript.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
The work was supported by a National Science and Technology Major project of the 13th Five-Year Plan (2017ZX10105001006001; 2017ZX10105001006002) and the Medical and Health Science and Technology Project of Zhejiang province (2015KYA102).
References
- 1.WHO: Global tuberculosis report, 2017. In Geneva: World Health Organization. Available at: http://www.who.int/tb/publications/global_report/gtbr2017_main_text.pdf
- 2.Yee D, Valiquette C, Pelletier M, et al. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med 2003; 167: 1472–1477. Epub 2003/02/06. doi: 10.1164/rccm.200206-626OC. PubMed PMID: 12569078 [DOI] [PubMed] [Google Scholar]
- 3.Guo N, Marra F, Fitzgerald JM, et al. Impact of adverse drug reaction and predictivity of quality of life status in tuberculosis. Eur Respir J 2010; 36: 206–208. Epub 2010/07/03. doi: 10.1183/09031936.00159409. PubMed PMID: 20595167 [DOI] [PubMed] [Google Scholar]
- 4.Li L, Jiang W, Wang J. Clinical analysis of 275 cases of acute drug-induced liver disease. Front Med China 2007; 1: 58–61. Epub 2007/02/01. doi: 10.1007/s11684-007-0012-8. PubMed PMID: 24557619 [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y, Yang L, Liao Z, et al. Epidemiology of drug-induced liver injury in China: a systematic analysis of the Chinese literature including 21,789 patients. Eur J Gastroenterol Hepatol 2013; 25: 825–829. Epub 2013/03/21. doi: 10.1097/MEG.0b013e32835f6889. Pub Med PMID: 23510965 [DOI] [PubMed] [Google Scholar]
- 6.Yang LX, Liu CY, Zhang LL, et al. Clinical characteristics of patients with drug-induced liver injury. Chin Med J (Engl) 2017; 130: 160–164. Epub 2017/01/17. doi: 10.4103/0366-6999.197995. PubMed PMID: 28091407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar R, Bhatia V, Khanal S, et al. Antituberculosis therapy-induced acute liver failure: magnitude, profile, prognosis, and predictors of outcome. Hepatology 2010; 51: 1665–1674. Epub 2010/03/03. doi: 10.1002/hep.23534. PubMed PMID: 20196116 [DOI] [PubMed] [Google Scholar]
- 8.Devarbhavi H, Singh R, Patil M, et al. Outcome and determinants of mortality in 269 patients with combination anti-tuberculosis drug-induced liver injury. J Gastroenterol Hepatol 2013; 28: 161–167. Epub 2012/10/02. doi: 10.1111/j.1440-1746.2012.07279.x. PubMed PMID: 23020522 [DOI] [PubMed] [Google Scholar]
- 9.Singh J, Garg PK, Tandon RK. Hepatotoxicity due to antituberculosis therapy. Clinical profile and reintroduction of therapy. J Clin Gastroenterol 1996; 22: 211–214. Epub 1996/04/01. PubMed PMID: 8724260 [DOI] [PubMed] [Google Scholar]
- 10.Ostapowicz G, Fontana RJ, Schiodt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002; 137: 947–954. Epub 2002/12/18. PubMed PMID: 12484709 [DOI] [PubMed] [Google Scholar]
- 11.Maddrey WC. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. Gastroenterology 2000; 118: 984–985. doi: 10.1016/S0016-5085(00)70192-210784603 [DOI] [Google Scholar]
- 12.Reuben A. Hy’s law. Hepatology 2004; 39: 574–578. Epub 2004/02/10. doi: 10.1002/hep.20081. PubMed PMID: 14768020 [DOI] [PubMed] [Google Scholar]
- 13.Temple R. Hy’s law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf 2006; 15: 241–243. Epub 2006/03/23. doi: 10.1002/pds.1211. PubMed PMID: 16552790 [DOI] [PubMed] [Google Scholar]
- 14.Guidance for Industry Drug-Induced Liver Injury: Premarketing Clinical Evaluation, 2009. Center for Drug Evaluation and Research (CDER). Silver Spring, Maryland. Available at: http://www.fda.gov/downloads/Drugs/GuidanceCompliance Regulatory Information/Guidances/UCM174090.pdf
- 15.Hayashi PH, Rockey DC, Fontana RJ, et al. Death and liver transplantation within 2 years of onset of drug-induced liver injury. Hepatology 2017; 66: 1275–1285. Epub 2017/05/26. doi: 10.1002/hep.29283. PubMed PMID: 28543844; PubMed Central PMCID: PMCPmc5605419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aithal GP, Watkins PB, Andrade RJ, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther 2011; 89: 806–815. doi: 10.1038/clpt.2011.58. PubMed PMID: WOS:000290786000019 [DOI] [PubMed] [Google Scholar]
- 17.Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG clinical guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol 2014; 109: 950–966; quiz 67. Epub 2014/06/18. doi: 10.1038/ajg.2014.131. PubMed PMID: 24935270 [DOI] [PubMed] [Google Scholar]
- 18.Chalasani N, Bonkovsky HL, Fontana R, et al. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology 2015; 148: 1340–52.e7. Epub 2015/03/11. doi: 10.1053/j.gastro.2015.03.006. PubMed PMID: 25754159; PubMed Central PMCID: PMCPmc4446235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Punzalan CS, Barry CT. Acute liver failure: diagnosis and management. J Intensive Care Med 2016; 31: 642–653. Epub 2015/10/09. doi: 10.1177/0885066615609271. Pub Med PMID: 26446105 [DOI] [PubMed] [Google Scholar]
- 20.Shu CC, Lee CH, Lee MC, et al. Hepatotoxicity due to first-line anti-tuberculosis drugs: a five-year experience in a Taiwan medical centre. Int J Tuberc Lung Dis 2013; 17: 934–939. Epub 2013/06/08. doi: 10.5588/ijtld.12.0782. PubMed PMID: 23743313 [DOI] [PubMed] [Google Scholar]
- 21.Devarbhavi H, Dierkhising R, Kremers WK, et al. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol 2010; 105: 2396–2404. Epub 2010/07/22. doi: 10.1038/ajg.2010.287. PubMed PMID: 20648003 [DOI] [PubMed] [Google Scholar]
- 22.Lo Re V, Haynes K, Forde KA, et al. Risk of acute liver failure in patients with drug-induced liver injury: evaluation of Hy’s law and a new prognostic model. Clin Gastroenterol Hepatol 2015; 13: 2360–2368. doi: 10.1016/j.cgh.2015.06.020. PubMed PMID: WOS:000365191300028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devarbhavi H, Singh R, Adarsh CK, et al. Factors that predict mortality in children with Wilson disease associated acute liver failure and comparison of Wilson disease specific prognostic indices. J Gastroenterol Hepatol 2014; 29: 380–386. doi: 10.1111/jgh.12356. PubMed PMID: WOS:000336252400026 [DOI] [PubMed] [Google Scholar]
- 24.Wu SS, Chao CS, Vargas JH, et al. Isoniazid-related hepatic failure in children: a survey of liver transplantation centers. Transplantation 2007; 84: 173–179. doi: 10.1097/01.tp.0000269104.22502.d2. PubMed PMID: WOS:000248381700007 [DOI] [PubMed] [Google Scholar]
- 25.Xia Q, Dai X, Huang J, et al. A single-center experience of non-bioartificial liver support systems among Chinese patients with liver failure. Int J Artif Organs 2014; 37: 442–454. Epub 2014/07/11. doi: 10.5301/ijao.5000341. PubMed PMID: 25008954 [DOI] [PubMed] [Google Scholar]
- 26.Thompson NP, Caplin ME, Hamilton MI, et al. Anti-tuberculosis medication and the liver: dangers and recommendations in management. Eur Respir J 1995; 8: 1384–1388. Epub 1995/08/01. PubMed PMID: 7489806 [DOI] [PubMed] [Google Scholar]
- 27.Teschke R, Danan G. Drug-induced liver injury: is chronic liver disease a risk factor and a clinical issue? Expert Opin Drug Metab Toxicol 2017; 13: 425–438. doi: 10.1080/17425255.2017.1252749. PubMed PMID: WOS:000399492600006 [DOI] [PubMed] [Google Scholar]