Short abstract
Objective
Diabetes is a risk equivalent for cardiovascular events. The increase in vascular inflammation with diabetes is believed to be responsible for increased risk of ischemic events in diabetic patients. Our goal was to assess whether knowledge of vascular inflammation alters cardiovascular risk over time, and how knowledge of vascular inflammation changes risk in non-diabetic, pre-diabetic and diabetic patients.
Methods
We retrospectively studied >100,000 primary-care patients per annum for 5 years (baseline in 2011 through 2015) with tests including lipoprotein profile, hemoglobin A1C and the vascular-specific inflammation risk marker myeloperoxidase. Results were obtained during the patient’s MD Value In Prevention (MDVIP) annual wellness program physical.
Results
We show that rates of patients with elevated myeloperoxidase levels were reduced from 14.4%, 15.2% and 21.3% to 4.0%, 4.0% and 6.7% in non-diabetic, pre-diabetic and diabetic patients, respectively, over the 5-year period. Decreases in vascular inflammation were achieved without decreases in the prevalence of pre-diabetes (hemoglobin A1C 5.7%–6.4%) or diabetes (hemoglobin A1C >6.4%) and were observed in patients below or above guideline low-density lipoprotein targets.
Conclusions
These data demonstrate that physicians informed of elevated markers of vascular inflammation can lower vascular inflammation correlating with biomarker-based decreased risk of cardiovascular events.
Keywords: Inflammation, prevention, atherosclerosis, diabetes, lipids, biomarkers, vulnerable plaque, myocardial infarction
Introduction
Cardiovascular disease continues to be the leading cause of mortality in the US, where nearly one-third of deaths can be directly attributed to a cardiovascular event. The growing increase in the prevalence of pre-diabetes and diabetes has changed the underlying risk profile of cardiovascular disease from one focused on cholesterol levels to one of vascular inflammation. Diabetes is a risk equivalent for cardiovascular disease because diabetics without a history of acute myocardial infarction (AMI) have the same likelihood of an AMI as non-diabetic patients with a history of AMI. Diabetes has been linked to an increased drive of vascular inflammation through multiple mechanisms including lipid oxidation, advanced glycation end-products and increased insulin levels.
Assessing risk factors of cardiovascular disease and screening for coronary artery disease (CAD) and its equivalents like diabetes have been the goal of the American College of Cardiology and the American Heart Association. They have tried to implement simple and evidence-based guidelines with high-sensitivity screening at a relatively low cost. After withdrawing the target goal for low-density lipoprotein (LDL) treatment in the new guidelines, it remains uncertain how successful we really are at controlling patients at risk and who would require further treatment and more aggressive strategies. This is important in light of recent data showing that approximately 50% of patients admitted with CAD and acute ischemic events have acceptable cholesterol levels.1
The link between atherosclerosis, clinical events and inflammation has been recognized for years and is well established.2 The recent findings of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) validated the concept that targeting inflammation is a good strategy to prevent clinical events in patients with atherosclerosis.3 There is growing recognition of the physiology represented by the measurement of novel markers of oxidation and inflammation.4 Two markers, lipoprotein-associated phospholipase A2 (Lp-PLA2) and myeloperoxidase (MPO)), are markers of vulnerable plaque and risk of clinical events.2,5–7 Although it is unclear that the inhibition of either of these enzymes would have clinical benefit,8,9 increased circulating levels of these markers are linked to increased risk of stroke and AMI.
In this study, we assessed whether the addition of a marker of vascular inflammation to advanced cholesterol measures annually in a primary-care population would lead to the long-term, down-regulation of cardiovascular risk. In recognition of the changing landscape of cardiovascular risk assessment, MD Value In Prevention (MDVIP) added MPO to their annual wellness panel in 2011. In this study, we present the findings of >100,000 patients who had these wellness panels yearly. Our objectives were to examine whether knowledge of vascular inflammation altered cardiovascular risk over time, and how knowledge of vascular inflammation changed the cardiovascular risk of non-diabetic, pre-diabetic and diabetic patients.
Methods
As part of its wellness services, MDVIP implements an annual screening panel to all enrolled patients. The wellness screening panel focuses on known risk factors for cardiovascular disease including diabetes (hemoglobin A1C; HbA1C), an advanced lipid panel, apolipoprotein A1 and MPO. Results from de-identified patients tested from 2011 to 2015 followed by 801 physician practices were captured from Cleveland HeartLab’s Laboratory Information System and encompassed all MDVIP wellness testing performed between January 2011 and December 2015. Results for LDL testing were provided by three laboratories (88% from Atherotech Vertical Auto Profile, 10% from Liposcience nuclear magnetic resonance profile and 2% from Cleveland HeartLab standard lipid panel).
Extracted data were cleaned by transforming all test results that indicated a value above or below the test analytical range (using a “<” or “>”) to their numeric value. Records with test results indicating that the test was not performed (for any reason) were deleted. Clinical cutoffs for HbA1c used in the study were as follows: non-diabetic, <5.7%; pre-diabetic, 5.7%–6.4%; and diabetic, >6.4%. Based on guideline LDL targets for diabetics and non-diabetics without heart disease, analyses were stratified for LDL <100 mg/dL and <130 mg/dL.
Physician experience associated with each test result was calculated by determining the difference (in months) between the test order date and the date of the first test order for each ordering physician. The distribution of physician experience over the entire study was determined to be 24% with 0–12 months of experience, 17% with 12–24 months, 13% with 25–36 months, 13% with 37–48 months and 32% with 49–60 months.
Ethics committee review was not necessary as this study was a retrospective analysis of Laboratory Information System data with no patient identifiers or information available. Furthermore, all MDVIP patients in their agreement with MDVIP consent that their data can be used for blinded analyses.
Results
We studied >100,000 patients per year of the MDVIP annual wellness panels between 2011 and 2015. Overall, approximately 645,000 total patient visits from 285,901 unique patients were analyzed (2011: 104,608 patients; 2012: 115,864 patients; 2013: 125,193 patients; 2014: 140,764; 2015: 159,300 patients). These included approximately 623,000 LDL, 598,000 HbA1C and 603,000 MPO results. A total of 79.5% of patient visits were for patients who had testing done in multiple years.
The data in Figure 1 depict the trends for prevalence of diabetic, pre-diabetic and non-diabetic patients based on annual HbA1C. The prevalence of diabetes was 9%–11%, pre-diabetes was 41.1%–46.7% and non-diabetes was 42.3%–47.2%. Thus, in any year, >50% of the patient population were at risk of or diagnosed with diabetes.
Figure 1.
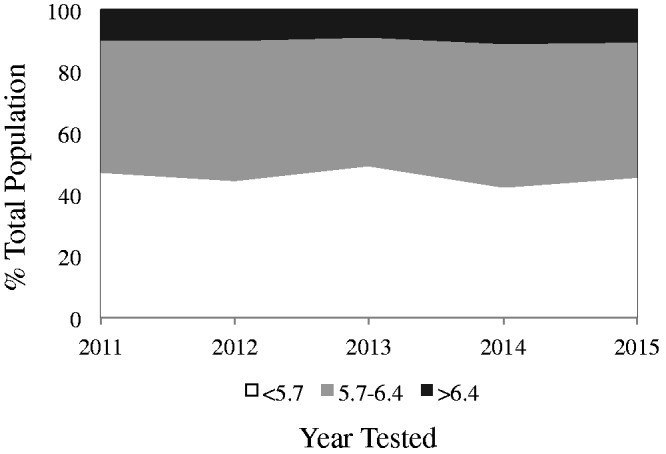
Population breakdown by hemoglobin A1C (HbA1C). Percentage of patients with pre-diabetes (gray) and diabetes (dark gray) as a function of year tested.
As seen in Figure 2, in the first year of testing diabetic patients we noted a 21.3% positivity rate for MPO compared with 14.4% for non-diabetics. The MPO positivity rate in pre-diabetics was 15.2%. Over the 5 years, the rate of MPO positivity steadily decreased to 6.7%, 4.0% and 4.0% for diabetics, pre-diabetics and non-diabetic patients, respectively, in 2015; i.e., a 68.5%, 73.7% and 72.2% reduction in risk annually for a cardiovascular event in these three groups, respectively.
Figure 2.
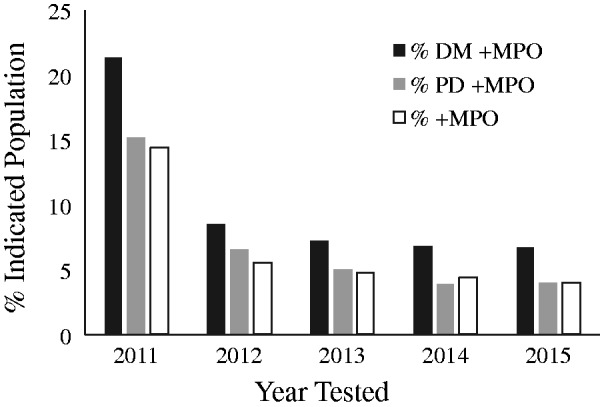
Risk based on myeloperoxidase (MPO) stratified by hemoglobin A1C (HbA1C). Percentage of patients with positive MPO test based on HbA1C status as a function of year tested.
We investigated the prevalence of patients at typical LDL targets based on National Cholesterol Education Program Adult Treatment Panel (ATP) III (<100 mg/dL for diabetics and <130 mg/dL for pre-diabetics and non-diabetics; see Figure 3). The number of diabetic patients at LDL goal was always >57% of the population and in 2014 reached a high of 71%. For non-diabetics and pre-diabetics with the higher LDL goal of 130 mg/dL, the percentage of patients at LDL goal was between 76% and 84%. Importantly, there was no correlation between lowering of LDL and lowering of the prevalence of patients with positive MPO in diabetics, pre-diabetics or non-diabetics, suggesting that modulation of risk associated with presence of a positive marker of vascular inflammation was distinct from simply lowering cholesterol levels.
Figure 3.
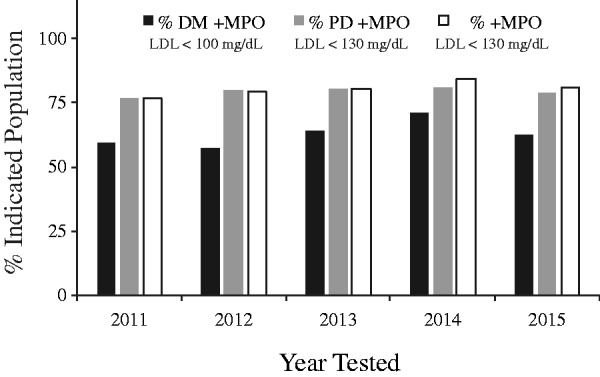
Patients with guideline target low-density lipoprotein (LDL) levels still have risk based on inflammation. Percentage of patients at guideline-based LDL goal with positive myeloperoxidase (MPO) stratified by hemoglobin A1C (HbA1C) status as a function of year tested.
Although the prevalence of patients with evidence of vascular inflammation decreased from 2011 to 2015 (Figure 4), the percentage of patients with a positive MPO and controlled cholesterol levels did not. In 2011, 59.2%, 77.0% and 77.1% of diabetics, pre-diabetics and non-diabetic patients, respectively, who had a positive MPO also had cholesterol levels at goal. In 2015, those percentages were 61.2%, 80.0% and 80.0%, and not significantly changed from 2011 relative to the significant decrease in the overall prevalence of positive MPO.
Figure 4.
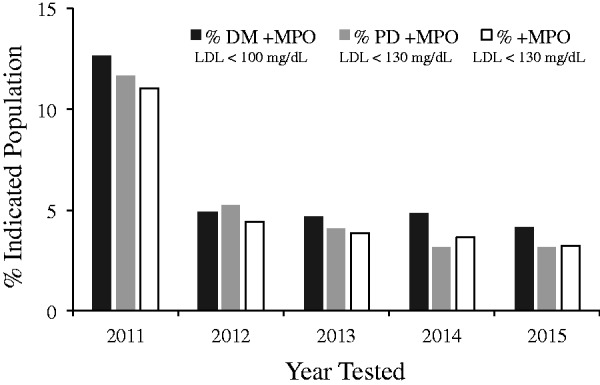
Identification of risk in patients with target low-density lipoprotein (LDL) levels. Percentage of patients at guideline LDL target with positive myeloperoxidase (MPO) stratified by hemoglobin A1C (HbA1C) as a function of year tested.
The data in Figure 5 explore the relationship between the number of years a physician was aware of and ordered an MPO test and the prevalence of positive results. Although the prevalence of diabetes and patients with LDL levels above goal does not appear to change, the prevalence of positive MPO levels decreases within the year after the physician begins to measure vascular inflammation.
Figure 5.
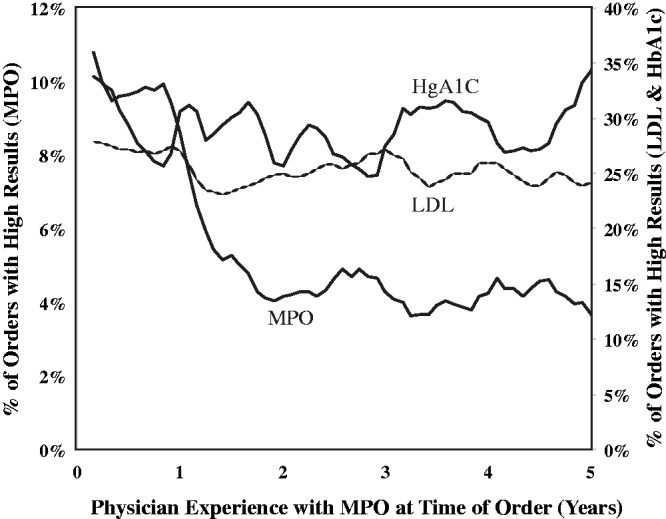
Physician experience with inflammation testing and risk reduction. Time from initiation of inflammation testing with myeloperoxidase (MPO) and decrease in incidence of MPO within a physician practice.
Discussion
The response-to-injury hypothesis of atherosclerosis proposes that the atherosclerotic process is initiated through arterial injury and is propagated in response to subsequent inflammation.10–12 Periods of episodic inflammation lead to the degradation of stable atherosclerotic plaque and then to vulnerable plaque formation. Rupture of vulnerable plaque can lead to growth of the atherosclerotic plaque or intra-arterial thrombosis and end organ ischemia.13 Multiple studies have shown that knowledge of both the inflammatory state and lipid status allows additive information for risk.14,15
The work of Ridker and colleagues has demonstrated the utility of measuring arterial inflammation in patients with a narrow range of the non-specific marker high-sensitivity C-reactive protein (hsCRP; <10 mg/L) as a marker of cardiovascular risk, even after correcting for lipoprotein levels, age, and other accepted cardiovascular risk factors. The innovative Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) randomized double blind trial demonstrated that risk reduction in patients based on inflammatory markers leads to reduction of events and mortality.16
Multiple studies have demonstrated the utility of measuring the circulating of free myeloperoxidase as a correlate of the risk of presence of vulnerable plaque.2,5,17 Heslop et al.2 showed that patients who underwent elective coronary angiography had a 5-fold increased hazard ratio for mortality if either MPO or hsCRP was elevated, but they had a further 4.33-fold increase in hazard ratio if both MPO and hsCRP were elevated.
We have previously modeled the potential return on investment for a commercial health insurance plan with one million members. An estimated 43,693 cardiovascular events were estimated to occur over 5 years under a standard-of-care scenario, whereas a biomarker testing approach using hsCRP, MPO and Lp-PLA2 could yield an estimated reduction of events by 8.9% (3,908 events) over 5 years.18 The similarity of the decline in the prevalence of patients with positive MPO in Figure 2 is reminiscent of the decline in hsCRP seen in the CANTOS trial with the interleukin 1 beta monoclonal antibody canakinumab.3 Thus, our findings suggest that vascular inflammation can be significantly lowered in patients without the need for expensive biologics.19
The goal of this study was to determine the extent to which a patient’s risk could be reduced through the measurement of MPO and the knowledge of inflammatory risk. Our data demonstrate that within one year of using MPO as a marker of cardiovascular risk, the number of patients at risk decreased. Not surprisingly, our data demonstrate that the probability of an abnormal MPO is greater in patients with HbA1C >6.4%. We demonstrate that, based on inflammatory risk as measured by MPO, by year 5 patients with HbA1C between 5.7% and 6.4% was reduced to that of patients with HbA1C <5.7%. Furthermore, the percentage of patients positive for MPO in these two groups was decreased by 73% over the 5-year period of our study. Patients with HbA1C >6.4% had residual risk based on MPO, but the percentage of patients with positive MPO was reduced by 69% over the 5 years studied. Finally, the trends of positive MPO we observed in different groups of patients based on HbA1C were similar whether or not patients had LDL levels at target based on ATPIII.
These data demonstrate that primary-care physicians can and will respond to increased cardiovascular risk based on inflammatory markers. Future studies need to focus on strategies implemented by these physicians that led to decreases in MPO. Because the percentage of patients with LDL levels greater than goal did not change over the 5 years studied, it is unlikely that the medical approach had to do with simply increasing the use or dosing of lipid-lowering therapies.
Conclusion
There is growing recognition of the need to identify novel strategies to risk stratify patients for cardiovascular events. With at least 50% of patients presenting with acute coronary syndromes having LDL levels at guideline levels based on ATP III or on statin therapy based on current guidelines, there is a growing need to identify biomarkers other than lipoproteins. Biomarkers based on vascular inflammation seem to be a rational approach given the increasing prevalence of obesity and diabetes, both of which are linked to increased levels of inflammation. Our study shows that vascular inflammation as measured by MPO is modifiable in this MDVIP population of patients. Future studies will need to define the strategies implemented to lower vascular inflammation and whether modification of MPO in this patient group led to a reduction in cardiovascular events.
Acknowledgements
This work was funded by Cleveland HeartLab, Inc. and MDVIP, Inc.. Dr. Alcivar-Franco is a recipient of the Rice Fellowship.
Declaration of conflicting interests
Dr. Penn is a co-founder of Cleveland HeartLab Inc. and serves as the Chief Medical Officer of Cleveland HeartLab Inc. and as such receives consulting fees and equity. He is the inventor named on patents associated with a portion of the testing discussed and receives royalties for these inventions. Dr. Klemes is the Chief Medical Officer of MDVIP, Inc. for which she receives salary and equity. Dr. Purvis is an employee of Cleveland HeartLab Inc. for which he receives a salary and equity. Dr. Alcivar-Franco declares no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
References
- 1.Sachdeva A, Cannon CP, Deedwania PC, et al. Lipid levels in patients hospitalized with coronary artery disease: an analysis of 136,905 hospitalizations in Get With The Guidelines. Am Heart J 2009; 157: 111–117. [DOI] [PubMed] [Google Scholar]
- 2.Heslop CL Frohlich JJ andHill JS.. Myeloperoxidase and C-reactive protein have combined utility for long-term prediction of cardiovascular mortality after coronary angiography. J Am Coll Cardiol 2010; 55: 1102–1109. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 2017; 377: 1119–1131. [DOI] [PubMed] [Google Scholar]
- 4.Penn MS andKlemes AB.. Multimarker approach for identifying and documenting mitigation of cardiovascular risk. Future Cardiol 2013; 9: 497–506. [DOI] [PubMed] [Google Scholar]
- 5.Brennan ML, Penn MS, Van Lente F, et al. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003; 349: 1595–1604. [DOI] [PubMed] [Google Scholar]
- 6.Wei L, Ke Z, Zhao Y, et al. The elevated lipoprotein-associated phospholipase A2 activity is associated with the occurrence and recurrence of acute cerebral infarction. NeuroReport. 2017; 28: 325–330. [DOI] [PubMed] [Google Scholar]
- 7.Garg PK, Jorgensen NW, McClelland RL, et al. Lipoprotein-associated phospholipase A2 and risk of incident peripheral arterial disease in a multi-ethnic cohort: The Multi-Ethnic Study of Atherosclerosis. Vasc Med. 2017; 22: 5–12. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal U zhou X Weber K Dadabayev AR andPenn MS.. Critical role for white blood cell NAD(P)H oxidase-mediated plasminogen activator inhibitor-1 oxidation and ventricular rupture following acute myocardial infarction. J MolCell Cardiol. 2011; 50: 426–432. [DOI] [PubMed] [Google Scholar]
- 9.O'Donoghue ML, Braunwald E, White HD, et al. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA 2014; 312: 1006–1015. [DOI] [PubMed] [Google Scholar]
- 10.Ross R Glomset J andHarker L.. Response to injury and atherogenesis. Am J Pathol 1977; 86: 675–684. [PMC free article] [PubMed] [Google Scholar]
- 11.Jang IK Lassila R andFuster V.. Atherogenesis and inflammation. Eur Heart J 1993; 14(Suppl K): 2–6. [PubMed] [Google Scholar]
- 12.Libby P. Changing concepts of atherogenesis. J Intern Med 2000; 247: 349–358. [DOI] [PubMed] [Google Scholar]
- 13.Teng N, Maghzal GJ, Talib J, et al. The roles of myeloperoxidase in coronary artery disease and its potential implication in plaque rupture. Redox Rep. 2017; 22: 51–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ridker PM, Rifai N, Rose L, et al. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 2002; 347: 1557–1565. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005; 352: 20–28. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359: 2195–2207. [DOI] [PubMed] [Google Scholar]
- 17.O'Donoghue ML, Morrow DA, Cannon CP, et al. Multimarker Risk Stratification in Patients With Acute Myocardial Infarction. J Am Heart Assoc 2016; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penn MS, Yenikomshian MA, Cummings AK, et al. The economic impact of implementing a multiple inflammatory biomarker-based approach to identify, treat, and reduce cardiovascular risk. J Med Econ 2015; 18: 483–491. [DOI] [PubMed] [Google Scholar]
- 19.Harrington RA. Targeting inflammation in coronary artery disease. N Engl J Med 2017; 377: 1197–1198. [DOI] [PubMed] [Google Scholar]


