Abstract
Background
There has been limited longitudinal assessment of the relationship between moderate‐to‐vigorous physical activity (MVPA) and sedentary behaviour (SB) with frailty, and no studies have explored the possibility of reverse causality. This study aimed to determine the potential bidirectionality of the relationship between accelerometer‐assessed MVPA, SB, and frailty over time in older adults.
Methods
Participants were from the Toledo Study for Healthy Aging. We analysed 186 older people aged 67 to 90 (76.7 ± 3.9; 52.7% female participants) over a 4‐year period. Time spent in SB and MVPA was assessed by accelerometry. Frailty Trait Scale was used to determine frailty levels. A cross‐lagged panel model design was used to test the reciprocal relationships between MVPA/SB and frailty.
Results
Frailty Trait Scale score changed from 35.4 to 43.8 points between the two times (P < 0.05). We also found a reduction of 7 min/day in the time spent on MVPA (P < 0.05), and participants tended to spend more time on SB (P = 0.076). Our analyses revealed that lower levels of initial MVPA predicted higher levels of later frailty [std. β = −0.126; confidence interval (CI) = −0.231, −0.021; P < 0.05], whereas initial spent time on SB did not predict later frailty (std. β = −0.049; CI = −0.185, 0.087; P = 0.48). Conversely, an initial increased frailty status predicted higher levels of later SB (std. β = 0.167; CI = 0.026, 0.307; P < 0.05) but not those of MVPA (std. β = 0.071; CI = −0.033, 0.175; P = 0.18).
Conclusions
Our observations suggest that the relationship between MVPA/SB and frailty is unidirectional: individuals who spent less time on MVPA at baseline are more likely to increase their frailty score, and individuals who are more frail are more likely to spent more time on SB at follow‐up. Interventions and policies should aim to increase MVPA levels from earlier stages to promote successful aging.
Keywords: Structural equation modelling, Longitudinal, Exercise, Sedentary time, Ageing, Functioning and disability
Introduction
Globally, the population aged 65 and over is growing faster than all other age groups1, 2. One of the most remarkable changes in body composition related to aging is the loss of skeletal muscle (i.e. sarcopenia).3 Frailty, defined as a condition of increased vulnerability associated with aging, and sarcopenia have been linked because both can lead to disability, hospitalization, and premature death.4, 5, 6 Sarcopenia has been considered both as the biological substrate for the development of physical frailty and the pathway through which adverse health‐related outcomes of physical frailty occur.7 Consequently, interventions to reduce the burden associated with frailty should be focused, among others, on skeletal muscle and its functionality.8
Increasing physical activity and reducing the levels of sedentary behaviour (SB) have been suggested to be a key strategy to attenuate the declines in muscle mass and physical function associated with aging and may also delay the clinical symptoms of frailty in older adults.9 Several cross‐sectional studies suggest that objectively assessed moderate‐to‐vigorous physical activity (MVPA) and SB are related with frailty in middle‐aged to older‐aged adults.10, 11, 12, 13 Nonetheless, a major limitation of the existing evidence is that it mainly relies on cross‐sectional designs, thus precluding us from making any causal inferences due to the inability of establishing the temporal sequence of the effects of MVPA/SB on frailty outcomes.
Therefore, longitudinal studies are essential because they provide an opportunity to explore in more detail the causal direction of the associations between MVPA/SB and frailty, knowledge which could then contribute to the development of intervention strategies to favour successful aging outcomes, including frailty and associated symptoms. To date, most of the existing longitudinal studies found a positive association between SB and frailty14, 15 and an inverse association for MVPA and frailty.16 For example, a longitudinal study in two Spanish cohorts of community‐dwelling older adults reported baseline television viewing time was also associated with frailty at 4‐year follow‐up.17 Nevertheless, all of these studies used self‐report methods to assess the movement behaviour of interest. Song et al.18 showed a relationship between objectively assessed sedentary time and development of physical frailty. However, an important caveat with this study is that used gait speed as a proxy measure of frailty thus could not capture the multidimensional nature of frailty.4 To our knowledge, there is no longitudinal study that has objectively measured both frailty and MVPA/SB.
The relationships between physical activity, sedentary time, and frailty are further complicated by the possibility of reverse causality.16, 19 In all previous longitudinal studies, whether using objective or subjective measures of the variables of interest, the authors investigated the prospective associations of MVPA/SB with frailty, not taking into account the potential reverse or temporal order in the causality chain. We cannot rule out the possibility that a high level of frailty can be associated with lower levels of physical activity and a greater amount of sedentary time at a future time. The reverse may also be true. Estimating the temporal ordering, and potential bidirectionality of the association of SB and MVPA with frailty would be advantageous to inform subsequent interventions aimed at reducing the burden of frailty among the older population. However, no studies exist investigating this issue.
With fill in this gap by examining the longitudinal association of accelerometer‐assessed MVPA and SB with frailty over a period of 4 years in a population sample of older adults from the Toledo Study for Healthy Aging (TSHA). In doing so, we applied a cross‐lagged panel model, a statistical technique appropriate for the context and aims of the current study.20, 21 Specifically, this study examined whether MVPA/SB predicted frailty in the future and whether frailty predicted subsequent movement behaviours (or both). As far as we know, this is the first study that has examined the potentially reciprocal relationships between movement behaviours and frailty in older adults. In the current study, four hypotheses were tested:
H0: Frailty does not predict changes in SB/MVPA, and SB/MVPA does not predict changes in frailty.
H1: Frailty predicts changes in SB/MVPA, but SB/MVPA does not predict changes in frailty.
H2: SB/MVPA predicts changes in frailty, but frailty does not predict changes in SB/MVPA.
H3: SB/MVPA and frailty have a reciprocal relationship—frailty predicts changes in SB/MVPA, and SB/MVPA predicts changes in frailty.
Methods
Study design and participants
This is a longitudinal study consisting of two data collection waves separated by 4 years (3.8 ± 0.8 years). This investigation used data from the second and third waves of the TSHA. Details of the protocol of the TSHA are described elsewhere.22, 23 Briefly, the TSHA is a population prospective cohort study aimed at studying the determinants and consequences of frailty in institutionalized and community‐dwelling individuals older than 65 years living in the province of Toledo, Spain. In the current study, a subsample of the TSHA with accelerometer data was included. A total of 277 men and 351 women over 65 years of age at baseline, although 494 participants, concluded the three stages of assessment and provide with valid data for the analyses [224 men (45.3%)]. The first time point of assessment for this study started in July 2012 and lasted until June 2014. In the first stage, six psychologists conducted computer‐assisted interviews face to face with potential subjects. In the second stage, three nurses performed a physical examination followed by clinical and performance tests at the subject's home. In the third stage, the participants were invited to wear an accelerometer for a week. Participants were contacted again in 2015 and invited to participate in a follow‐up study conducted between May 2015 and July 2017.24 After the follow‐up, 200 participants (59.5% missing) completed the second evaluation. However, 186 subjects [88 men (47.3%) with complete data on all exposures, outcomes, and ≥80% covariates] were included in the final analyses of this study (see Figure 1 for the study participant flow diagram). Signed informed consent was obtained from all participants prior participation in the study. The study was approved by the Clinical Research Ethics Committee of the Toledo Hospital, which was conducted according to the ethical standards defined in the 1964 Declaration of Helsinki.
Figure 1.
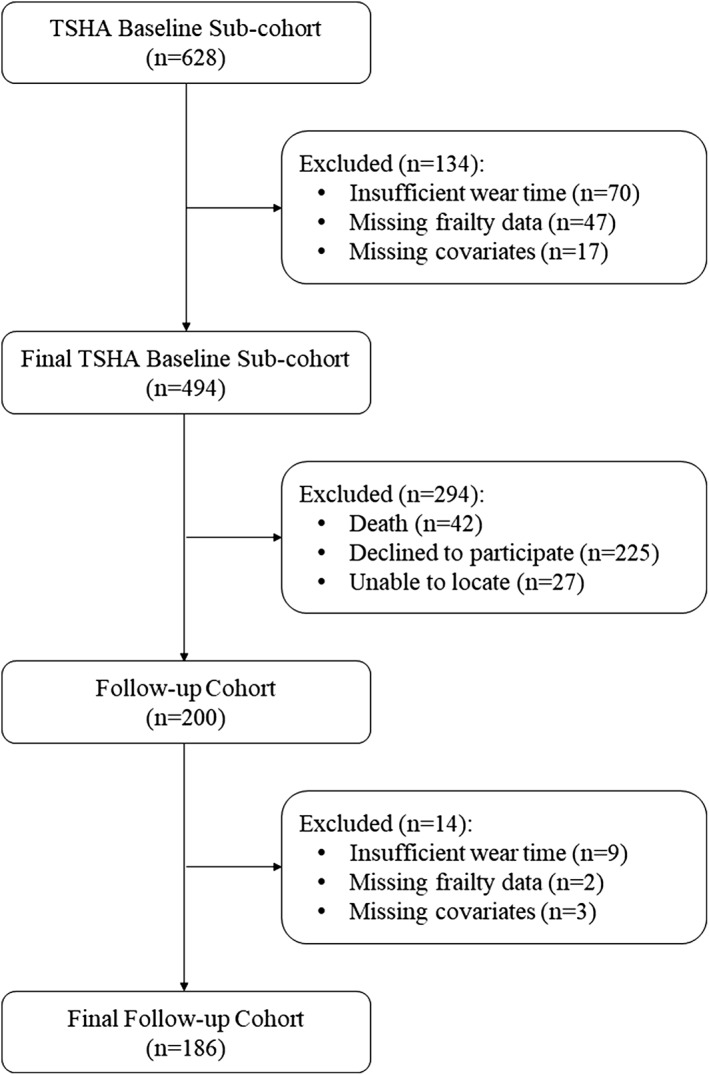
Flow diagram of the process for obtaining the final sample of the study.
Measurements
Frailty status
The Frailty Trait Scale (FTS)25 was used to assess frailty in this study. The FTS includes seven aspects: energy balance and nutrition, activity, nervous system, vascular system, weakness, endurance, and slowness. These domains become operational through 12 items:
Body mass index (BMI), central obesity (waist circumference), unintentional weight loss, and serum albumin level were used to assess energy balance and nutrition.
Activity was assessed using the total score of the Physical Activity Scale for the Elderly.26
The nervous system was calculated by considering verbal fluency and balance. Verbal fluency was estimated by asking the participants to give names of animals during 1 min.27
Balance was measured by Romberg test.28
The vascular system was measured by the brachial‐ankle index performed with Doppler ultrasound.29
Weakness was estimated assessing grip strength in the dominant arm and the knee extension strength.23
Endurance was assessed by the chair stand test, which measures the number of times that a person stands up in 30 s.30
Slowness was estimated by calculating the time to walk 3 m at a ‘normal pace’ according to a standard protocol.28
Each item score represents a biological trait and ranges from 0 (the best) to 4 (the worst), except in the ‘chair test’ where the range is from 0 to 5 points because of the necessity of scoring those unable to stand a single time. When appropriate, items are analysed according to the item's quintile distribution in the population.
To be included in the study, the participants had to overcome at least 75% (9 of the 12) of the items included in the FTS.25 The total score was calculated by adding all the scores in each item divided by total score for each individual and multiplying by 100, standardizing the measure to a range from 0 (best score) to 100 (worst score), according to the formula total score = (Σ items score/total score possible by individual)*100.
Physical activity and sedentary behaviour assessment
The ActiGraph accelerometer ActiTrainer and wGT3X‐BT (ActiGraph, LLC, Pensacola, FL) were used to assess the participants' physical activity and SB levels during a week as previously described.11 In brief, participants were instructed to wear an accelerometer on the left hip during waking hours, with exception for water activities. The devices were initialized to collect data using 1‐min epochs, and all data were collected using the vertical axis collection mode. Inclusion criteria comprised at least 4 days with at least 8 h recorded per day without excessive counts (i.e. >20,000 counts).31 Non‐wear time was defined as a minimum of 60 min with allowance of 1–2 min of counts below 100 counts.32 Daily average times spent in SB (<100 counts/min) and MVPA (≥1952 counts/min) were derived according to previous work.33 Although there is a lack of consensus on the use of cut‐off points to classify the intensity of the activity, the cut‐off points used in this study are the most commonly reported in this population group,34 and this makes our results comparable with other studies. Minutes spent in each of these three behaviours were tallied per day and averaged over all available valid days.
Anthropometrics and confounding variables
Height was measured to the nearest centimetre using a stadiometer (Seca 711 scales, Hamburg, Germany), and weight was measured with a Seca precision scale (Seca 711 scales, Hamburg, Germany). Individuals removed their shoes, socks, and heavy clothes prior to weighing. BMI was calculated as weight (kg) divided by height squared (m2).
Participants self‐reported their age, sex, and ethnicity. Education (no studies, primary school completed, secondary school completed, or more), marital status (single, married/living together, widowed, and divorced/separated), and income (it was coded into three categories ranging from any income to 3000€ per month) were also self‐reported in face‐to‐face interviews. We also evaluated objective cognitive function using the mini‐mental state examination.35
Statistical analysis
Preliminary analyses examined variable distributions, sample characteristics, and attrition using R software (R project version 3.5.1). Descriptive variables were compared between participants retained with those of participants not retained from wave1 to wave2 with an independent t‐test or χ2 test for continuous and categorical variables, respectively. Descriptive statistics [mean and standard deviation (SD) for continuous variables and as frequencies and percentages for categorical variables] were calculated for all outcome measurements. Comparison between baseline and follow‐up time continuous variables was performed using a paired sample t‐test.
We tested our hypotheses using structural equation modelling with maximum likelihood estimation using functions from the R package lavaan.36 Full information maximum likelihood was used to provide unbiased and efficient estimates of the parameters of interest missingness at random.37 Two cross‐lagged panel models were used to test the hypothesis of the study. A cross‐lagged panel model was implemented to test the relationships between SB and frailty status across the two time points for the present study (i.e. initial assessment and 4‐year follow‐up). The second cross‐lagged panel model was used to test the relationships between MVPA and frailty status. The null hypotheses would be supported if neither of the coefficients associated with the cross paths were significantly different from zero. If the cross path towards frailty in time 2, but not towards SB/MVPA in time 2, was significant, then H1 would be supported. If it were the reverse of the latter, then H2 would be supported. Finally, if both paths were significant, then H3 would be supported. Analyses included sex as time‐invariant variable; in addition, age, education, marital status, income, BMI, mini‐mental state examination, and accelerometer wear time were allowed to be time‐varying covariates (i.e. allowing for possible changes in these measures from initial assessment to follow‐up). Among the strengths of using a cross‐lagged panel approach is that it allows simultaneous analysis of the two dependent outcomes, thereby permitting the identification of possible bidirectional associations over time. Model fit was assessed using a selection of fit indices and criteria: root mean square error of approximation (RMSEA) (≤0.06), standardized root mean square residual (SRMR) (≤0.08), confirmatory fit index (CFI) (≥0.95), and Tucker‐Lewis index (TLI) (≥0.95).38
Results
Attrition/missing data across time points
Participants decreased from 494 with complete data at baseline to 186 with complete data at follow‐up assessment (see Figure 1). The causes and numbers who were lost to the follow‐up assessment were death (n = 42), refusal (n = 225), and could not be located (n = 27). Additional missing data were lost by insufficient accelerometer wear time data (n = 9), missing frailty data (n = 2), or losing more than 80% of the covariates (n = 3). Compared with the retained sample, participants who dropped the study at 4‐year follow‐up were significantly older, less educated, and spent more time on SB (Data S1). Also, MVPA had a trend toward significance reductions in those participants. Missing data were addressed using a full information maximum likelihood algorithm, as recommended elsewhere.39
Descriptive statistics
Means and standard deviations for MVPA, SB, and frailty as well as confounders at each of the two time‐points of assessment (viz. T1 and T2) for the present study are shown in Table 1. At baseline, participants had a mean age of 76.68 (SD = 3.90), a mean FTS of 35.35 (SD = 13.94), a mean time (min/day) spent on SB of 530.18 (SD = 84.86), and an MVPA of 20.12 (SD = 23.30). FTS score increased significantly between T1 and T2 (P < 0.05). It was found that participants tended to spend more time on SB (P = 0.076), and there was a significant reduction in the time spent on MVPA between the two times (P < 0.05). BMI and MSSE also decreased significantly at both time points (P < 0.05).
Table 1.
Sociodemographic and descriptive data.
| Variables | Baseline | Follow‐up |
|---|---|---|
| (n=186) | (n=186) | |
| Age (years)a | 76.68 ± 3.90 | 80.44 ± 4.24* |
| Sexb | ||
| Men | 88 (47.3) | 88 (47.3) |
| Women | 98 (52.7) | 98 (52.7) |
| BMI (kg/m2)a | 30.82 ± 4.62 | 30.33 ± 4.40* |
| Educationb | ||
| None | 139 (74.7) | 116 (62.4) |
| Primary school | 30 (16.1) | 51 (27.4) |
| Secundary or more | 14 (7.5) | 19 (10.2) |
| Missingc | 3 (1.6) | − |
| Incomeb | ||
| Low | 87 (46.8) | 75 (40.4) |
| Medium | 87 (46.8) | 70 (37.6) |
| High | 9 (4.8) | 6 (3.2) |
| Missingc | 3 (1.6) | 35 (18.8) |
| Marital statusb | ||
| Single | 7 (3.8) | 7 (3.8) |
| Married | 136 (73.1) | 125 (67.2) |
| Widower | 40 (21.5) | 51 (27.4) |
| Separated/Divorced | 1 (0.5) | 2 (1.1) |
| Missingc | 2 (1.1) | 1 (0.5) |
| MSSEa | 24.02 ± 3.73 | 23.32 ± 3.54* |
| Missingc | 15 (8.1) | 15 (8.1) |
| Frailty Trait Scale, pointsa | 35.35 ± 13.94 | 43.79 ± 13.86* |
| Accelerometer wear time, min/valid daya | 781.36 ± 83.14 | 777.61 ± 74.45 |
| Sedentary time, min/valid daya | 530.18 ± 84.86 | 542.61 ± 75.91Ŧ |
| MVPA, min/valid daya | 20.12 ± 23.30 | 13.21 ± 18.73* |
Abbreviations: BMI, body mass index; MSSE, mini‐mental scale examination; MVPA, moderate‐to‐vigorous physical activity.
Continuous variable; mean standard ± deviation.
Categorical variable; n (%).
Missing data; n (%).
Significant differences between baseline vs. follow‐up (p<0.05).
Trend toward significance between baseline vs. follow‐up (p<0.08>0.05)
Cross‐lagged panel model 1: moderate‐to‐vigorous physical activity
Figure 2 shows the final cross‐lagged model. The data fit the model well (RMSEA = 0.000; SRMR = 0.013; CFI = 1.000; TLI = 1.023). The largest effects on T2 MVPA and T2 frailty were those determined by the autoregressive pathways. That is, past MVPA and frailty scores predicted future MVPA and frailty scores, respectively. The cross‐lagged effect from MVPA at T1 to frailty status at T2 was statistically significant (standardized regression coefficient of −0.126; 95% CI = −0.231, −0.021; P < 0.05), indicating that higher levels of MVPA at baseline predicted lower frailty score 4 years later, adjusting for baseline frailty status. In contrast, the cross‐lagged effect from frailty status to MVPA was not statistically significant (standardized regression coefficient of −0.049; 95% CI = −0.185, 0.087; P = 0.48), suggesting that frailty did not predict future levels of MVPA.
Figure 2.
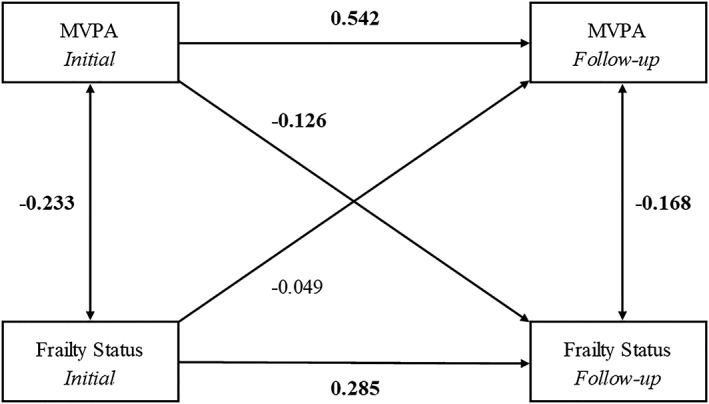
Cross‐lagged panel model 1: moderate‐to‐vigorous physical activity. MVPA, moderate‐to‐vigorous physical activity. Model adjusted for age, sex, body mass index, education, income, marital status, and mini‐mental scale examination. Bold indicates statistical significance (P < 0.05).
Cross‐lagged panel model 2: sedentary behaviour
Figure 3 shows the final cross‐lagged model. The data fit the model well (RMSEA = 0.012; SRMR = 0.018; CFI = 0.997; TLI = 0.992). Similar to the previous model, the autoregressive SB and frailty pathways were statistically significant. The cross‐lagged effect from frailty to SB levels was statistically significant (standardized regression coefficient of 0.167; 95% CI = 0.026, 0.307; P < 0.05), indicating that higher levels of frailty at baseline predicted higher SB 4 years later, adjusting for baseline SB. In contrast, the cross‐lagged effect from SB levels to frailty was not statistically significant (standardized regression coefficient of 0.071; 95% CI = −0.033, 0.175; P = 0.18), suggesting that SB levels did not predict future frailty status.
Figure 3.
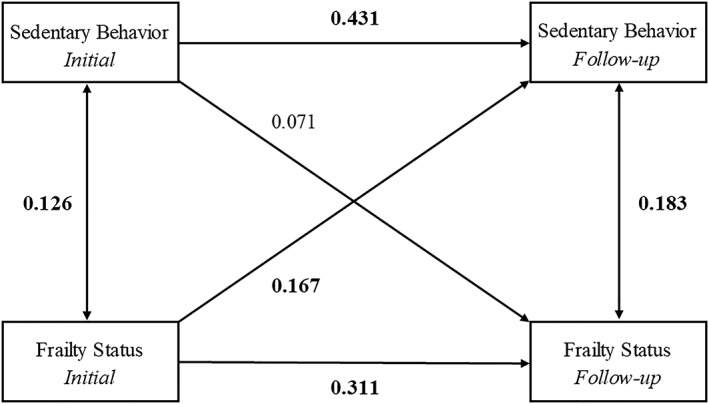
Cross‐lagged panel model 2: sedentary behaviour. Model adjusted for age, sex, body mass index, education, income, marital status, and mini‐mental scale examination. Bold indicates statistical significance (P < 0.05).
Discussion
The present study investigated the longitudinal relationships between MVPA and SB with frailty status in a community‐based sample of older adults. As a novelty, we applied a cross‐lagged panel model to test for potential reciprocal relationships between MVPA/SB and frailty over a 4‐year period. The main finding in our study was that accelerometer‐assessed initial MVPA predicted frailty score at follow‐up. However, baseline sedentary time was not significantly related to frailty after the follow‐up. We further found that initial frailty status predicted subsequent sedentary time (i.e. more frailty status was related to posterior higher levels of SB), but not of MVPA. These results have the potential to inform future interventions that aim at reducing the burden associated with frailty among older adults.
Different cross‐sectional10, 11, 40 and longitudinal16 studies have linked MVPA with frailty. Blodgett et al.10 found that MVPA was associated with frailty in a group of community dwelling adults aged over 50 from the National Health and Nutrition Examination Survey. Other longitudinal studies such as Rogers et al.16 have also confirmed these results in 8649 adults aged 50 and over an average of 10 years of follow‐up. We also found that MVPA prospectively predicted frailty levels in our sample. There are numerous arguments supporting these findings.41, 42, 43, 44 It has been demonstrated that physical activity, particularly of moderate intensity, plays an important role on multiple components of the frailty syndrome including the frailty phenotype, physiologic dysregulation, and cellular function.9 Increases in MVPA seem to also preserve or even improve muscle function and structure, protein synthesis, glucose metabolism, or inflammation.43 Furthermore, regular physical activity can maintain a set of bioenergetically functional mitochondria that, by improving systemic mitochondrial function, contribute to reducing the risk of morbidity and mortality throughout life.41 Not surprisingly, MVPA is considered a cornerstone for the prevention, delay, or treatment of frailty among older adults.
On the other hand, our results did not support the hypothesis that initial frailty levels predict future MVPA levels. Several studies support the predictive ability of physical functioning on subsequent MVPA levels.45, 46, 47 However, it may also be plausible that other non‐biological mechanisms (e.g. behavioural) accounted for the observations of the current study. Different intervention studies have shown the possibility of increasing physical activity also in frail participants.48 For example, Yamada et al.49 found that is possible to promote exercise of moderate‐to‐vigorous intensity among very frail older adults. Future studies are warranted to clarify the role of frailty in subsequent MVPA levels in older adults.
SB has recently been considered as an important factor for numerous health outcomes.50, 51 A recent systematic review has shown that SB may be associated with increased levels of frailty, particularly among the most vulnerable population.52 Interestingly, our results indicate that SB was not a determinant of frailty but rather a consequence of an altered state of increased frailty. A smaller study by Edholm et al.53 with 60 older woman found that only activities of at least moderate intensity were associated with physical function in a subsequent follow‐up time but not activities of lighter intensity or sedentary activities. In addition, Marques et al.54 in a study conducted with 131 male and 240 female participants aged 65–103 years, suggested that sedentary time was not a significant predictor of loss of physical independence in later life. In a previous cross‐sectional study, we showed that engaging in high levels of MVPA (i.e. 27 min/day) could cancel out the detrimental effects of SB on frailty, which may partly explain our current observations.55 Given that the relationship between SB and frailty may go beyond total accumulated time,12 future studies should enquire whether or not the results of this and other studies are confirmed for different patterns of accumulation of SB.
According to our findings, the promotion of MVPA at earlier stages will translate into more MVPA and less frailty markers in the future. Also, the observations of the current study point out to the possibility that the detrimental effects on frailty are primarily defined by insufficient amounts of MVPA rather than an excessive amount of sedentary time. Public health organizations should target MVPA to reduce the burden associated with frailty in older adults.
Strengths and limitations
An important strength of our study is that it includes a relatively large sample of community‐dwelling older adults with longitudinal data separated by 4 years. It also includes accelerometer‐derived sedentary and physical activity behaviour estimations. Also, although there is no established gold standard to identify frailty, the FTS has been suggested as a measurement of frailty with superior predictive validity than previously validated scales such as the frailty phenotype56 and the frailty index.57 Additionally, a key strength of this study was that the statistical analysis deployed has allowed to explore the auto‐regressive and cross‐lagged pathways in exploring how frailty relates over time with both MVPA and SB. Despite the methodological rigour of this study, some limitations have to be acknowledged. First, we cannot rule out the possibility that our estimations could be influenced by the characteristics of the participants who did not provide valid data at follow‐up (i.e. older, less active, more sedentary, and less educated), and therefore, our results should be interpreted with caution. A further limitation of our work was that despite validity and widely used of accelerometers to assess physical activity in free living conditions, these devices are not able to discriminate between sitting and standing58 or activity type (e.g. running vs. muscle strength), which could potentially bias the estimations in our study. Finally, physical activity and SB have been examined separately from other lifestyle behaviours (e.g. diet, smoking, and alcohol consumption). However, lifestyle behaviours tend to cluster together. Therefore, it could be that our results rather reflect the synergistic consequences of different observed (i.e. physical activity and SB) and unobserved (e.g. diet quality) lifestyle behaviours.59, 60 Future studies may want to test this hypothesis.
Conclusion
In summary, our findings suggest that MVPA, but not SB, predicts frailty in older adults. In contrast, frailty seems to be a predictor of SB but not of MVPA. Efforts should be directed at increasing MVPA from earlier stages. Future experimental studies should examine the best strategies to include MVPA in the daily lives of older people.61
Conflict of interest
None to declared.
Funding
This work was supported by the Biomedical Research Networking Center on Frailty and Healthy Aging (CIBERFES) and FEDER funds from the European Union (CB16/10/00477), (CB16/10/00456), and (CB16/10/00464). It was further funded by grants from the Government of Castilla‐La Mancha (PI2010/020), Institute of Health Sciences, Ministry of Health of Castilla‐La Mancha (03031‐00), Spanish Government [Spanish Ministry of Economy, ‘Ministerio de Economía y Competitividad’, and Instituto de Salud Carlos III (PI10/01532, PI031558, and PI11/01068)], and by European Grants (Seventh Framework Programme: FRAILOMIC). Asier Mañas Bote received a PhD grant from the Universidad de Castilla‐La Mancha ‘Contratos predoctorales para la formación de personal investigador en el marco del Plan Propio de I+D+i, cofinanciados por el Fondo Social Europeo’ (2015/4062).
Supporting information
Data S1. Comparison of characteristics at baseline of participants retained with those of participants not retained from wave1‐wave2.
Acknowledgements
We are deeply thankful to Linda Fried, Professor (University of Columbia, USA), and Jack Guralnik, PhD (University of Maryland School of Medicine, USA), for their assistance in reviewing the final version of the manuscript. The authors would like to thank the cohort members, investigators, research associates, and team members. The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.62
Mañas A., del Pozo‐Cruz B., Rodríguez‐Gómez I., Losa‐Reyna J., Rodríguez‐Mañas L., García‐García F. J., and Ara I. (2020) Which one came first: movement behavior or frailty? A cross‐lagged panel model in the Toledo Study for Healthy Aging, Journal of Cachexia, Sarcopenia and Muscle, 11, 415–423. 10.1002/jcsm.12511.
Asier Mañas and Borja del Pozo‐Cruz contributed equally to this work.
References
- 1. Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet (London, England) 2017;389:1323–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oeppen J, Vaupel JW. Broken limits to life expectancy. Science. 2002;296:1029–1031. [DOI] [PubMed] [Google Scholar]
- 3. Rosenberg IH. Sarcopenia: origins and clinical relevance. The Journal of nutrition 1997;127:990s–991s. [DOI] [PubMed] [Google Scholar]
- 4. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet (London, England) 2013;381:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vermeiren S, Vella‐Azzopardi R, Beckwee D, Habbig AK, Scafoglieri A, Jansen B, et al. Frailty and the prediction of negative health outcomes: a meta‐analysis. Journal of the American Medical Directors Association 2016;17:1163.e1–e17. [DOI] [PubMed] [Google Scholar]
- 6. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower‐extremity function in persons over the age of 70 years as a predictor of subsequent disability. The New England journal of medicine. 1995;332:556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Calvani R, Marini F, Cesari M, Tosato M, Anker SD, von Haehling S, et al. Biomarkers for physical frailty and sarcopenia: state of the science and future developments. Journal of cachexia, sarcopenia and muscle. 2015;6:278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. St‐Jean‐Pelletier F, Pion CH, Leduc‐Gaudet JP, Sgarioto N, Zovile I, Barbat‐Artigas S, et al. The impact of ageing, physical activity, and pre‐frailty on skeletal muscle phenotype, mitochondrial content, and intramyocellular lipids in men. J Cachexia Sarcopenia Muscle. 2017;8:213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fried LP. Interventions for human frailty: physical activity as a model. Cold Spring Harb Perspect Med 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. The association between sedentary behaviour, moderate‐vigorous physical activity and frailty in NHANES cohorts. Maturitas. 2015;80:187–191. [DOI] [PubMed] [Google Scholar]
- 11. Manas A, Del Pozo‐Cruz B, Guadalupe‐Grau A, Marin‐Puyalto J, Alfaro‐Acha A, Rodriguez‐Manas L, et al. Reallocating accelerometer‐assessed sedentary time to light or moderate‐ to vigorous‐intensity physical activity reduces frailty levels in older adults: an isotemporal substitution approach in the TSHA study. Journal of the American Medical Directors Association 2018;19:185 e1–e6. [DOI] [PubMed] [Google Scholar]
- 12. Del Pozo‐Cruz B, Manas A, Martin‐Garcia M, Marin‐Puyalto J, Garcia‐Garcia FJ, Rodriguez‐Manas L, et al. Frailty is associated with objectively assessed sedentary behaviour patterns in older adults: evidence from the Toledo Study for Healthy Aging (TSHA). PLoS One. 2017;12:e0183911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kehler DS, Clara I, Hiebert B, Stammers AN, Hay JL, Schultz A, et al. The association between bouts of moderate to vigorous physical activity and patterns of sedentary behavior with frailty. Experimental gerontology. 2018;104:28–34. [DOI] [PubMed] [Google Scholar]
- 14. Soler‐Vila H, Garcia‐Esquinas E, Leon‐Munoz LM, Lopez‐Garcia E, Banegas JR, Rodriguez‐Artalejo F. Contribution of health behaviours and clinical factors to socioeconomic differences in frailty among older adults. Journal of epidemiology and community health. 2016;70:354–360. [DOI] [PubMed] [Google Scholar]
- 15. Stenholm S, Strandberg TE, Pitkala K, Sainio P, Heliovaara M, Koskinen S. Midlife obesity and risk of frailty in old age during a 22‐year follow‐up in men and women: the Mini‐Finland Follow‐up Survey. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69:73–78. [DOI] [PubMed] [Google Scholar]
- 16. Rogers NT, Marshall A, Roberts CH, Demakakos P, Steptoe A, Scholes S. Physical activity and trajectories of frailty among older adults: evidence from the English longitudinal study of ageing. PLoS One. 2017;12:e0170878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garcia‐Esquinas E, Andrade E, Martinez‐Gomez D, Caballero FF, Lopez‐Garcia E, Rodriguez‐Artalejo F. Television viewing time as a risk factor for frailty and functional limitations in older adults: results from 2 European prospective cohorts. The international journal of behavioral nutrition and physical activity. 2017;14:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Song J, Lindquist LA, Chang RW, Semanik PA, Ehrlich‐Jones LS, Lee J, et al. Sedentary behavior as a risk factor for physical frailty independent of moderate activity: results from the osteoarthritis initiative. American Journal of Public Health. 2015;105:1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dogra S, Ashe MC, Biddle SJH, Brown WJ, Buman MP, Chastin S, et al. Sedentary time in older men and women: an international consensus statement and research priorities. British journal of sports medicine. 2017;51:1526–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Selig JP, Little TD . Autoregressive and cross‐lagged panel analysis for longitudinal data. Handbook of Developmental Research Methods. New York: Guildford Press; 2012. p 265–278. [Google Scholar]
- 21. Newsom JT. Longitudinal Structural Equation Modeling: A Comprehensive Introduction. New York: Taylor & Francis; 2015. [Google Scholar]
- 22. Carcaillon L, Blanco C, Alonso‐Bouzon C, Alfaro‐Acha A, Garcia‐Garcia FJ, Rodriguez‐Manas L. Sex differences in the association between serum levels of testosterone and frailty in an elderly population: the Toledo Study for Healthy Aging. PLoS One. 2012;7:e32401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garcia‐Garcia FJ, Gutierrez Avila G, Alfaro‐Acha A, Amor Andres MS, De Los Angeles De La Torre Lanza M, Escribano Aparicio MV, et al. The prevalence of frailty syndrome in an older population from Spain. The Toledo Study for Healthy Aging. The Journal of Nutrition, Health & Aging. 2011;15:852–856. [DOI] [PubMed] [Google Scholar]
- 24. Rodríguez‐Gómez I, Mañas A, Losa‐Reyna J, Rodríguez‐Mañas L, Chastin SF, Alegre LM, et al. Compositional influence of movement behaviours on bone health during ageing. Medicine and science in sports and exercise 2019;51:1736–1744. [DOI] [PubMed] [Google Scholar]
- 25. Garcia‐Garcia FJ, Carcaillon L, Fernandez‐Tresguerres J, Alfaro A, Larrion JL, Castillo C, et al. A new operational definition of frailty: the Frailty Trait Scale. Journal of the American Medical Directors Association 2014;15:371.e7–e13. [DOI] [PubMed] [Google Scholar]
- 26. Schuit AJ, Schouten EG, Westerterp KR, Saris WH. Validity of the Physical Activity Scale for the Elderly (PASE): according to energy expenditure assessed by the doubly labeled water method. Journal of Clinical Epidemiology. 1997;50:541–546. [DOI] [PubMed] [Google Scholar]
- 27. del Ser QT, Sanchez Sanchez F, Garcia de Yebenes MJ, Otero Puime A, Zunzunegui MV, Munoz DG. Spanish version of the 7 minute screening neurocognitive battery. Normative data of an elderly population sample over 70. Neurologia (Barcelona, Spain) 2004;19:344–358. [PubMed] [Google Scholar]
- 28. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. Journal of gerontology. 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 29. Fowkes FG, Low LP, Tuta S, Kozak J. Ankle‐brachial index and extent of atherothrombosis in 8891 patients with or at risk of vascular disease: results of the international AGATHA study. European heart journal. 2006;27:1861–1867. [DOI] [PubMed] [Google Scholar]
- 30. Jones CJ, Rikli RE, Beam WC. A 30‐s chair‐stand test as a measure of lower body strength in community‐residing older adults. Research quarterly for exercise and sport. 1999;70:113–119. [DOI] [PubMed] [Google Scholar]
- 31. Rodríguez‐Gómez I, Mañas A, Losa‐Reyna J, Rodríguez‐Mañas L, Chastin SF, Alegre LM, et al. Associations between sedentary time, physical activity and bone health among older people using compositional data analysis. PLoS One. 2018;13:e0206013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Colley R, Connor Gorber S, Tremblay MS. Quality control and data reduction procedures for accelerometry‐derived measures of physical activity. Health reports. 2010;21:63–69. [PubMed] [Google Scholar]
- 33. Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Medicine and science in sports and exercise. 1998;30:777–781. [DOI] [PubMed] [Google Scholar]
- 34. Migueles JH, Cadenas‐Sanchez C, Ekelund U, Delisle Nystrom C, Mora‐Gonzalez J, Lof M, et al. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports medicine (Auckland, NZ) 2017;47:1821–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tombaugh TN, McIntyre NJ. The mini‐mental state examination: a comprehensive review. Journal of the American Geriatrics Society. 1992;40:922–935. [DOI] [PubMed] [Google Scholar]
- 36. Rosseel Y. Lavaan: an R package for structural equation modeling 2012.
- 37. Enders CK, Bandalos DL. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling: A Multidisciplinary Journal. 2001;8:430–457. [Google Scholar]
- 38. Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6:1–55. [Google Scholar]
- 39. Allison PD. Missing data techniques for structural equation modeling. Journal of Abnormal Psychology. 2003;112:545–557. [DOI] [PubMed] [Google Scholar]
- 40. Rodríguez‐Gómez I, Mañas A, Losa‐Reyna J, Rodríguez‐Mañas L, Chastin SF, Alegre LM, et al. The impact of movement behaviors on bone health in elderly with adequate nutritional status: compositional data analysis depending on the frailty status. Nutrients. 2019;11:582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rebelo‐Marques A, De Sousa LA, Andrade R, Ribeiro CF, Mota‐Pinto A, Carrilho F, et al. Aging hallmarks: the benefits of physical exercise. Frontiers in endocrinology. 2018;9:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xue QL, Bandeen‐Roche K, Mielenz TJ, Seplaki CL, Szanton SL, Thorpe RJ, et al. Patterns of 12‐year change in physical activity levels in community‐dwelling older women: can modest levels of physical activity help older women live longer? American Journal of Epidemiology. 2012;176:534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zampieri S, Pietrangelo L, Loefler S, Fruhmann H, Vogelauer M, Burggraf S, et al. Lifelong physical exercise delays age‐associated skeletal muscle decline. The journals of gerontology Series A, Biological sciences and medical sciences. 2015;70:163–173. [DOI] [PubMed] [Google Scholar]
- 44. Sourial N, Bergman H, Karunananthan S, Wolfson C, Guralnik J, Payette H, et al. Contribution of frailty markers in explaining differences among individuals in five samples of older persons. The journals of gerontology Series A, Biological sciences and medical sciences. 2012;67:1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim Y, White T, Wijndaele K, Sharp SJ, Wareham NJ, Brage S. Adiposity and grip strength as long‐term predictors of objectively measured physical activity in 93 015 adults: the UK Biobank study. International Journal of Obesity. 2017;41:1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cooper A, Lamb M, Sharp SJ, Simmons RK, Griffin SJ. Bidirectional association between physical activity and muscular strength in older adults: results from the UK Biobank study. International journal of epidemiology. 2017;46:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Metti AL, Best JR, Shaaban CE, Ganguli M, Rosano C. Longitudinal changes in physical function and physical activity in older adults. Age and ageing 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Puts MTE, Toubasi S, Andrew MK, Ashe MC, Ploeg J, Atkinson E, et al. Interventions to prevent or reduce the level of frailty in community‐dwelling older adults: a scoping review of the literature and international policies. Age and ageing. 2017;46:383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yamada M, Arai H, Sonoda T, Aoyama T. Community‐based exercise program is cost‐effective by preventing care and disability in Japanese frail older adults. Journal of the American Medical Directors Association. 2012;13:507–511. [DOI] [PubMed] [Google Scholar]
- 50. de Rezende LF, Rey‐Lopez JP, Matsudo VK, do Carmo Luiz O. Sedentary behavior and health outcomes among older adults: a systematic review. BMC public health 2014;14:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Manas A, Del Pozo‐Cruz B, Garcia‐Garcia FJ, Guadalupe‐Grau A, Ara I. Role of objectively measured sedentary behaviour in physical performance, frailty and mortality among older adults: a short systematic review. European journal of sport science. 2017;17:940–953. [DOI] [PubMed] [Google Scholar]
- 52. Kehler DS, Hay JL, Stammers AN, Hamm NC, Kimber DE, Schultz ASH, et al. A systematic review of the association between sedentary behaviors with frailty. Experimental gerontology. 2018;114:1–12. [DOI] [PubMed] [Google Scholar]
- 53. Edholm P, Nilsson A, Kadi F. Physical function in older adults: impacts of past and present physical activity behaviors. Scandinavian journal of medicine & science in sports. 2018. [DOI] [PubMed] [Google Scholar]
- 54. Marques EA, Baptista F, Santos DA, Silva AM, Mota J, Sardinha LB. Risk for losing physical independence in older adults: the role of sedentary time, light, and moderate to vigorous physical activity. Maturitas. 2014;79:91–95. [DOI] [PubMed] [Google Scholar]
- 55. Manas A, Pozo‐Cruz BD, Rodriguez‐Gomez I, Losa‐Reyna J, Rodriguez‐Manas L, Garcia‐Garcia FJ, et al. Can physical activity offset the detrimental consequences of sedentary time on frailty? A moderation analysis in 749 older adults measured with accelerometers. Journal of the American Medical Directors Association. 2019. [DOI] [PubMed] [Google Scholar]
- 56. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. The journals of gerontology Series A, Biological sciences and medical sciences. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 57. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ: Canadian Medical Association Journal 2005;173:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. An HS, Kim Y, Lee JM. Accuracy of inclinometer functions of the activPAL and ActiGraph GT3X+: a focus on physical activity. Gait & posture. 2017;51:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kvaavik E, Batty GD, Ursin G, Huxley R, Gale CR. Influence of individual and combined health behaviors on total and cause‐specific mortality in men and women: the United Kingdom health and lifestyle survey. Archives of internal medicine. 2010;170:711–718. [DOI] [PubMed] [Google Scholar]
- 60. McCullough ML, Patel AV, Kushi LH, Patel R, Willett WC, Doyle C, et al. Following cancer prevention guidelines reduces risk of cancer, cardiovascular disease, and all‐cause mortality. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2011;20:1089–1097. [DOI] [PubMed] [Google Scholar]
- 61. Stamatakis E, Johnson NA, Powell L, Hamer M, Rangul V, Holtermann A. Short and sporadic bouts in the 2018 US physical activity guidelines: is high‐intensity incidental physical activity the new HIIT? British Journal of Sports Medicine 2019; bjsports‐2018‐100397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. von Haehling S, Morley JE, Coats AJ, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. Journal of cachexia, sarcopenia and muscle. 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Comparison of characteristics at baseline of participants retained with those of participants not retained from wave1‐wave2.


