Abstract
Background
Frail older adults are at increased risk of post‐operative morbidity compared with robust counterparts. Simple methods testing frailty such as grip strength or gait speed have shown promising results for predicting post‐operative outcome, but there is a debate regarding the most appropriate and precise frailty assessment method. We compared the predictive value of multidimensional frailty score (MFS) with grip strength, gait speed, or conventional risk stratification tool for predicting post‐operative complications in older surgical patients.
Methods
From January 2016 to June 2017, 648 older surgical patients (age ≥ 65 years) were included for analysis. MFS was calculated based on the preoperative comprehensive geriatric assessment. Grip strength and gait speed were measured before surgery. The primary outcome was a composite of post‐operative complications (e.g. pneumonia, urinary tract infection, delirium, acute pulmonary thromboembolism, and unplanned intensive care unit admission). The secondary outcome was the 6 month all‐cause mortality.
Results
Among 648 patients (mean age 76.6 ± 5.4 years, 52.8% female), 66 (10.2%) patients experienced post‐operative complications, and the 6 month mortality was 3.9% (n = 25). Grip strength, gait speed, MFS, and American Society of Anesthesiologists (ASA) classification could predict post‐operative complication but only MFS (hazard ratio = 1.581, 95% confidence interval 1.276–1.959, P < 0.001) could predict 6 month mortality after adjustment. MFS (C‐index = 0.750) had a superior prognostic utility compared with age (0.638, P = 0.008), grip strength (0.566, P < 0.001), and ASA classification (0.649, P = 0.004). MFS improved the predictive value on age [C‐index of 0.638 (age) vs. 0.758 (age + MFS), P < 0.001] and ASA classification [C‐index of 0.649 (ASA) vs. 0.765 (ASA + MFS), P < 0.001] for post‐operative complication; however, gait speed or grip strength did not provide additional prognostic value in both age and ASA.
Conclusions
Multidimensional frailty score based on preoperative comprehensive geriatric assessment showed better utility than age, grip strength, gait speed, or ASA classification for predicting post‐operative complication and 6 month mortality. MFS also showed incremental predictive ability for post‐operative complications with the addition of age and ASA classification. Accordingly, MFS is superior to grip strength or gait speed for predicting complications among older surgical patients.
Keywords: Multidimensional frailty score, Grip strength, Gait speed, Surgery, Prognosis, Geriatrics
Introduction
Between 2015 and 2050, the proportion of the population aged more than 60 years will nearly double from 12% to 22%.1 This demographic change will influence the health care system in ways such as the increase of hospital utilization. As the increase of older population, the demand for surgical procedures is expected to rise.
Advanced age is considered an important risk factor for adverse post‐operative complications, and these adverse events have a negative impact on physical and functional ability in older patients.2, 3 Thus, better prediction of post‐operative complication in older adults is essential for deciding on surgical interventions or for recommending a perioperative management plan. However, conventional risk evaluation systems, including the American Society of Anesthesiologists (ASA) Physical Status Classification System and Goldman Cardiac Risk Index, are organ based or relatively subjective.4, 5 As for the age‐related phenotype, sarcopenia has been known to be associated with poor prognosis among older adults after surgery.6, 7 Preoperative risk stratification of older adults poses challenges because they are more likely to have multiple co‐morbidities, physical and cognitive impairments, and decreased reserve on multiple organ systems.
Frailty is a clinical state in which there is an increase in an individual's vulnerability for developing dependency and/or mortality when exposed to a stressor. Especially, physical frailty is an important medical syndrome with multiple causes and contributors. It is characterized by diminished strength, endurance, and reduced physiologic function.8 Previous studies showed that pre‐surgical frailty assessment is useful to identify high‐risk group for the morbidity and mortality after surgery.9 In our previous study, comprehensive geriatric assessment (CGA) was used to evaluate the status of physical frailty and predict adverse post‐operative event, and the multidimensional frailty score (MFS), which was derived from CGA, could predict adverse outcomes in patients undergoing general surgery or hip fracture surgery.10, 11, 12 However, CGA has been described as costly, time‐consuming, and labour‐intensive. Accordingly, gait speed and grip strength have been studied as substitute methods for rapidly assessing preoperative risk. Although gait speed and grip strength have been validated as reliable phenotypic indicators of physical performance and useful tools for predicting post‐operative complication, no study has compared these indicators with MFS with regard to predicting post‐operative adverse events or short‐term mortality.13, 14, 15
Thus, we aimed to compare the prognostic utility of age, physical frailty score (MFS), surrogate performance parameter for physical frailty (gait speed or grip strength), and other conventional risk factors (ASA classification) with respect to the occurrence of post‐operative complications or short‐term mortality in older adults who opt for elective general surgery. In addition, we examined the additive utility of MFS, gait speed, or grip strength on the predictive value of age or ASA classification for the prognostic assessment of post‐operative complication.
Methods
Study population
This retrospective cohort study was conducted at Seoul National University Bundang Hospital, a 1300‐bed teaching hospital. Patients aged 65 years or older who planned to elective surgery and referred to a multidisciplinary geriatric team for pre‐surgical CGA were included in the study. Patients were consecutively recruited from January 2016 to June 2017. Patients were excluded if time interval between surgery and CGA exceeded 30 days, both gait speed and grip strength values were missing, and patients received antibiotics more than 1 week before surgery. Baseline demographic, anthropometric, laboratory, and ASA classification data were collected from electronic medical records.
The study protocol was reviewed and approved by the Seoul National University Bundang Hospital institutional review board, which waived the requirement for informed consent. In addition to this, all procedures were performed in accordance with the guideline for authorship and publishing in the journal and the Strengthening the Reporting of Observational Studies in Epidemiology statement and regulation of the institutional review board.16
Gait speed, grip strength, and multidimensional frailty score
We measured gait speed and grip strength before surgery as phenotypical characteristics of physical competence. Gait speed was calculated based on the time taken to travel 4.5 m following a 1 m unmeasured acceleration and deceleration distance. To minimize potential bias, an automated laser‐gated chronometer attached to the wall was used. Handgrip strength (kg) was measured using the Jamar Plus+ Digital Hand Dynamometer (Sammons Preston, Bolingbrook, IL, USA) in sitting position; a maximum of two measurements from the dominant hand were recorded. We adopted cut‐off values of handgrip strength (<28.6 and <16.4 kg in men and women, respectively) for the definition of low grip strength based on data from the Korea National Health and Nutrition Examination Survey VI.17 The slow gait speed group was defined as a cut‐off speed of ≤0.8 m/s, which was recommended by the Asian Working Group for Sarcopenia as an indicator of low physical performance.18 The patients whose gait speed data were missing (n = 51) were classified into the slow gait speed group (≤0.8 m/s).
Multidimensional frailty score was calculated with the data from CGA and laboratory test with their established cut‐off values.11 CGA was performed within 30 days before surgery. CGA consists of six domains: co‐morbid conditions, polypharmacy, physical function, psychological status, nutritional status, and risk of post‐operative delirium. Co‐morbidity status was assessed with Charlson's co‐morbidity index. Physical function was evaluated with activities of daily living (ADL) and instrumental ADLs (IADLs) using the modified Barthel Index and Lawton & Brody Index. Cognitive function and depressive mood were evaluated by the Korean version of the Mini‐Mental State Examination (MMSE‐KC) and Korea Geriatric Depression Scale (SGDS‐K). Nutritional status and risk of post‐operative delirium were analysed with Mini Nutritional Assessment and Nursing Delirium Screening Scale (NuDESC). Detailed practice and cut‐off values have been described previously.11 The cut‐off value for identifying a high risk of MFS score was defined as the upper 10 percentile of MFS score in the study population (>8 points).
Outcome
The primary endpoint was a composite of post‐operative complications including pneumonia, urinary tract infection, delirium, pulmonary thromboembolism, and unplanned intensive care unit (ICU) admission. Delirium was diagnosed by psychiatric consultation or by the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th edition. Pneumonia, urinary tract infection, and pulmonary thromboembolism were diagnosed using standard National Surgical Quality Improvement Program (NSQIP) definitions. Unplanned ICU admission was defined as admission to a medical or surgical ICU at least 72 h after surgery for close haemodynamic observation, respiratory failure, renal failure, infection, or bleeding.19
The secondary outcome was the 6 month all‐cause mortality after surgery. The mortality data were obtained by the National Statistical Office through 31 December 2017.
Statistical analysis
Continuous variables are presented as mean (standard deviation) or median [interquartile range (IQR)] for non‐normally distributed variables. The independent t‐test and chi‐square test were used for continuous and categorical variables, respectively, to compare the baseline characteristics of the patients according to the presence of post‐operative complication. The relationship between MFS, gait speed, grip strength, age, or other conventional risk factors and outcomes was determined using logistic regression or Cox proportional‐hazards model. Correlation was analysed using three models. Model 1 was adjusted by age, sex, and body mass index, and Model 2 was adjusted with Model 1 and type of surgery. Type of surgery was categorized as colorectal surgery, gastric surgery, breast surgery, hepatic surgery, pancreatobiliary surgery, vascular surgery, herniorrhaphy, and cholecystectomy, according to the organ system and invasiveness of surgery. Model 3, the final model, was adjusted by Model 2 and white blood cell count, haemoglobin, total cholesterol, protein, blood urea nitrogen, and creatinine.
We compared the model's predictive value for the primary outcome with age, ASA classification, gait speed, grip strength, and MFS using the comparison of the area under the curve (AUC) of the receiver operating characteristic curve. The additional prognostic utility of MFS, grip strength, and gait speed with regard to age or ASA classification for post‐operative complication was evaluated by comparing the C‐index, which measures goodness of fit for binary outcomes in a logistic regression model. The statistical analysis was performed using STATA 15.0 (StataCorp, College Station, TX, USA) and SPSS version 25.0 (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics
From January 2016 to June 2017, 692 patients aged more than 65 years underwent surgery and CGA. Thirty‐seven patients whose time interval between surgery and CGA exceeded 30 days and seven patients who received antibiotics more than 1 week before surgery were excluded. Ultimately, 648 patients were included in this analysis (Figure 1).
Figure 1.
Flow of patients through study. CGA, comprehensive geriatric assessment.
These 648 patients had a mean age of 76.6 years (standard deviation 5.4), and 52.8% (n = 342) were female. The median length of total hospital stay was 8.0 days (IQR 5–11). Sixty‐six (10.2%) patients experienced at least one post‐operative complication including pneumonia (12 patients), urinary tract infection (1 patient), pulmonary thromboembolism (1 patient), deep vein thrombosis (1 patient), delirium (47 patients), stroke (1 patient), and unplanned ICU admission (19 patients). Ten and three patients experienced two and three complications, respectively. The median follow‐up period was 13.0 (IQR 9.0–18.6) months. Overall mortality was 9.1% (n = 59) by 31 December 2017, and 6 month mortality after surgery was 3.9% (n = 25).
Patients who were male or with advanced age, slow gait speed, and weak grip strength tended to experience more post‐operative complications. Higher white blood cell count or ASA classification and lower haemoglobin, platelets, total cholesterol, protein, and albumin levels were also associated with an increased risk of post‐operative complication. Among the geriatric assessment domains, a low MMSE‐KC score, dependency in ADLs or IADL, a higher Charlson's co‐morbidity index, risk of delirium (NuDESC ≥2), malnutrition, and MFS were correlated with occurrence of the post‐operative complications (Table 1).
Table 1.
Comparison of demographic, laboratory, and comprehensive geriatric assessment components by post‐operative complication
| No complication (n = 582) | Complication (n = 66) | P values | |
|---|---|---|---|
| Demographic | |||
| Age (years) | 76.3 (5.3) | 79.0 (5.9) | <0.001 |
| Sex (male/female) | 261/321 | 45/21 | <0.001 |
| Body mass index (kg/m2) | 23.9 (3.5) | 23.5 (3.9) | 0.342 |
| ASA class (1/2/3/4)a | 61/405/97/2 | 2/34/27/1 | <0.001 |
| Laboratory | |||
| WBCs (×103/μL) | 6.5 (2.1) | 7.8 (2.7) | <0.001 |
| Haemoglobin (g/dL) | 12.6 (1.8) | 11.8 (2.2) | 0.003 |
| Platelets (×103/μL) | 252.1 (73.8) | 279.1 (105.9) | 0.048 |
| BUN (mg/dL) | 16.5 (6.4) | 17.7 (7.4) | 0.138 |
| Creatinine (mg/dL) | 0.87 (0.52) | 0.92 (0.30) | 0.485 |
| Total cholesterol (mg/dL)b | 171.2 (41.5) | 154.6 (40.7) | 0.002 |
| Protein (mg/dL) | 7.0 (0.6) | 6.7 (0.7) | 0.001 |
| Albumin (mg/dL) | 4.1 (0.5) | 3.6 (0.6) | <0.001 |
| AST (IU/L) | 26.5 (19.3) | 29.7 (30.5) | 0.417 |
| ALT (IU/L) | 22.0 (21.0) | 21.1 (18.3) | 0.743 |
| Comprehensive geriatric assessment | |||
| Charlson's co‐morbidity index | 2.5 (1.4) | 3.0 (1.3) | 0.016 |
| Polypharmacy (≥5) | 281 (48.0%) | 38 (57.6%) | 0.142 |
| ADL dependency (partial and full) | 5 (0.9%) | 6 (9.1%) | <0.001 |
| IADL dependency | 44 (7.6%) | 22 (33.3%) | <0.001 |
| MMSE‐KCc | 23.9 (4.5) | 19.8 (6.0) | <0.001 |
| SGDS‐Kd | 3.8 (3.6) | 4.6 (4.0) | 0.122 |
| NuDESC ≥2e | 17 (2.9%) | 14 (21.2%) | <0.001 |
| MNAe | 24.4 (3.8) | 21.8 (4.9) | <0.001 |
| Mid‐arm circumference (cm) | 25.3 (2.7) | 25.0 (2.8) | 0.354 |
| Grip strength (kg) | 24.4 (8.5) | 22.1 (9.7) | 0.039 |
| Gait speed (m/s)f | 1.03 (0.28) | 0.87 (0.28) | <0.001 |
| MFS | 4.81 (2.2) | 7.24 (2.9) | <0.001 |
Data are presented as mean (standard deviation) or number (%). ADL, activities of daily living; ALT, alanine aminotransferase; ASA, American Society of Anesthesiologists; AST, aspartate aminotransferase; BUN, blood urea nitrogen; IADL, instrumental activities of daily living; MFS, multidimensional frailty score; MMSE‐KC, Korean version of the Mini‐Mental Status Examination; MNA, Mini Nutritional Assessment; NuDESC, Nursing Delirium Screening Scale; SGDS‐K, short form of the Korean Geriatric Depression Scale; WBCs, white blood cells.
Data were missing for 19 patients.
Data were missing for four patients.
Data were missing for five patients.
Data were missing for 12 patients.
Data were missing for one patient.
Data were missing for 51 patients.
Predictive value of multidimensional frailty score and other conventional risk factors
The fully adjusted (Model 3) odds ratios of MFS (per point), grip strength (per kg), gait speed (per 0.1 m/s), and ASA classification for post‐operative complication were 1.594 [95% confidence interval (CI) 1.354–1.875, P < 0.001], 1.081 (95% CI 1.034–1.130, P = 0.001), 1.186 (95% CI 1.037–1.357, P = 0.013), and 2.499 (95% CI 1.420–4.399, P = 0.001), respectively. Low grip strength [defined as grip strength of <28.6 kg (male) and <16.4 kg (female), respectively] and slow gait speed (≤0.8 m/s) were also significantly associated with an increased risk of post‐operative complication [odds ratios 2.345 (95% CI 1.284–4.283) and 2.595 (95% CI 1.395–4.828)] (Table 2).
Table 2.
Adjusted odds ratios by grip strength, gait speed, and other risk factors for post‐operative complication
| Model 1a | Model 2b | Model 3c | |
|---|---|---|---|
| Continuous | |||
| Grip strength (per kg) | 1.101 (1.056–1.148)*** | 1.097 (1.05 2–1.144)*** | 1.081 (1.034–1.130)*** |
| Gait speed (per 0.1 m/s)d | 1.199 (1.066–1.349)** | 1.212 (1.069–1.375)** | 1.186 (1.037–1.357)* |
| MFS | 1.605 (1.409–1.827)*** | 1.614 (1.401–1.860)*** | 1.594 (1.354–1.875)*** |
| Categorical | |||
| Slow gait speed (≤0.8 m/s) | 2.880 (1.654–5.015)*** | 3.175 (1.777–5.674)*** | 2.595 (1.395–4.828)** |
| Low grip strengthe | 2.736 (1.575–4.755)*** | 2.711 (1.539–4.774)** | 2.345 (1.284–4.283)** |
| ASA classificationf | 2.550 (1.582–4.110)*** | 2.653 (1.592–4.422)*** | 2.499 (1.420–4.399)** |
| High‐risk MFS (>8) | 10.693 (5.033–22.716)*** | 10.009 (4.534–22.094)*** | 7.439 (3.186–17.366)*** |
Data are presented as odds ratio (95% confidence interval). ASA, American Society of Anesthesiologists; MFS, multidimensional frailty score.
Adjusted by age, sex, and body mass index.
Adjusted by age, sex, body mass index, and type of surgery.
Adjusted by age, sex, body mass index, type of surgery, and white blood cell count, haemoglobin, total cholesterol, protein, blood urea nitrogen, and creatinine.
Data were missing for 51 patients.
Low grip strength is defined as grip strength of <28.6 kg (male) and <16.4 kg (female).
Data were missing for 19 patients.
P < 0.05.
P < 0.01.
P < 0.001.
Multidimensional frailty score was more accurate than age, grip strength, and ASA classification in predicting post‐operative complication according to the AUC of receiver operating characteristic (P value for evaluating the difference between AUC of MFS and AUC of age, grip strength, and ASA classification was 0.008, <0.001, and 0.004, respectively). However, MFS did not show a statistically significant superiority to gait speed regarding the prediction of post‐operative complication (AUC 0.750 vs. 0.668, P = 0.248) (Table 3).
Table 3.
Comparison of prognostic utility of MFS with age or other risk factors in predicting postoperative complication
|
AUC of ROC (95% CI) |
P value | |
|---|---|---|
| MFS |
0.750 (0.687‐0.813) |
|
| Age |
0.638 (0.567‐0.710) |
0.008 |
| Gait speeda |
0.668 (0.589‐0.746) |
0.248 |
| Grip strength |
0.566 (0.486‐0.646) |
< 0.001 |
| ASA classificationb |
0.649 (0.586‐0.712) |
0.004 |
Data were missing for 51 patients.
Data were missing for 19 patients.
To assess the additional impact of MFS, gait speed, and grip strength with respect to the predictive ability of age and ASA classification, the C‐index of the logistic model of age and ASA classification alone was compared with their C‐index of their logistic models combined with MFS, gait speed, and grip strength. Compared with the C‐index of age (0.638) and ASA classification (0.649), that of age + MFS (0.758) and ASA + MFS (0.765) were statistically superior for predicting post‐operative complication (both P < 0.001). Gait speed improved the prognostic utility of ASA classification (C‐index: 0.612 vs. 0.685, P = 0.013) but did not improve that of age (C‐index: 0.643 vs. 0.694, P = 0.072). However, grip strength did not significantly improve the prognostic ability of age (C‐index: 0.638 vs. 0.646, P = 0.588) or ASA classification (C‐index: 0.649 vs. 0.666, P = 0.425) (Figure 2).
Figure 2.
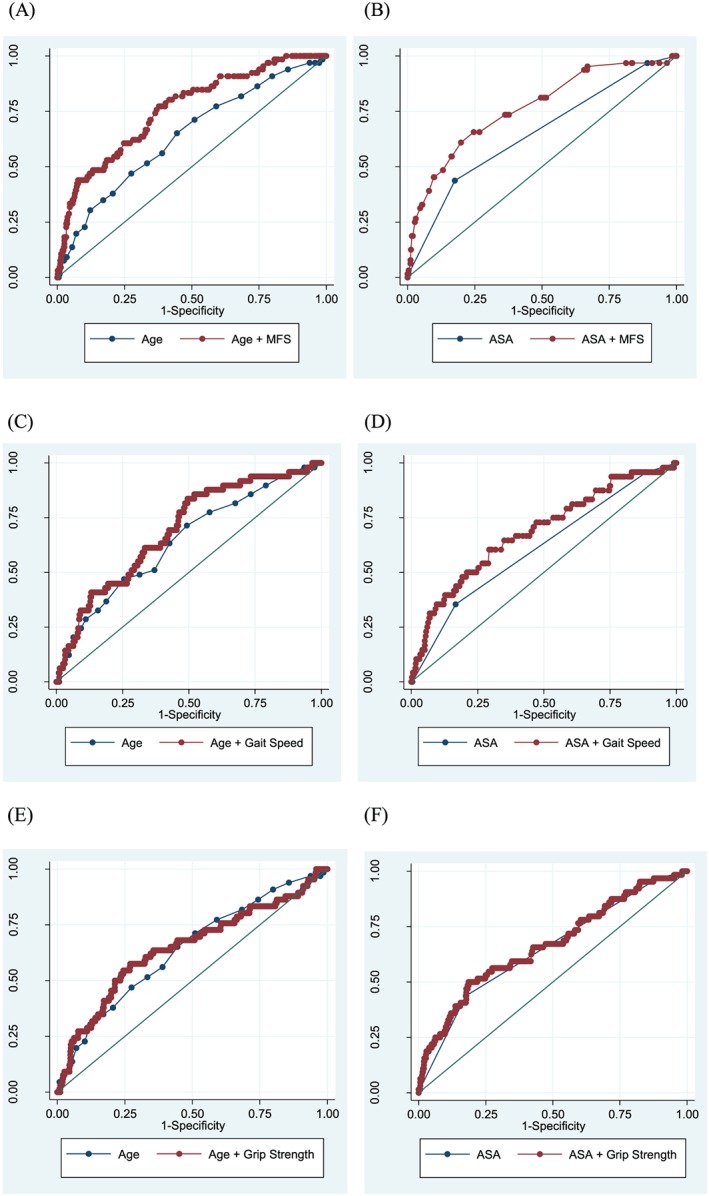
Area under the receiver operating characteristic curve of age or American Society of Anesthesiologists (ASA) classification and combination of age or ASA and multidimensional frailty score (MFS) (A and B), gait speed (C and D), and grip strength (E and F) for post‐operative complication.
The hazard ratio (HR) in the fully adjusted (Model 3) MFS for predicting the 6 month mortality after surgery was 1.581 (95% CI 1.276–1.959, P < 0.001). The other conventional risk factors including grip strength (HR 1.065, 95% CI 0.996–1.140, P = 0.067), gait speed (HR 0.996, 95% CI 0.813–1.221, P = 0.970), and ASA classification (HR 0.976, 95% CI 0.426–2.234, P = 0.953) could not predict the 6 month mortality after surgery in Model 3 (Table 4). Additional analysis was conducted to evaluate if MFS and other conventional risk factors could predict overall mortality during the study period. In the fully adjusted model (Model 3), MFS (HR 1.213, 95% CI 1.056–1.394, P = 0.003) but not grip strength (HR 1.131, 95% CI 0.989–1.075, P = 0.153), gait speed (HR 0.964, 95% CI 0.862–1.077, P = 0.518), and ASA classification (HR 1.149, 95% CI 0.699–1.888, P = 0.585) could predict overall mortality (Supporting Information, Table S1 ).
Table 4.
Adjusted HRs by grip strength, gait speed and other risk assessment tools for 6‐month mortality
| Model 1a | Model 2b | Model 3c | |
|---|---|---|---|
| Continuous | |||
| Grip strength (per kg) | 1.059 (0.998 – 1.124) | 1.071 (1.006 – 1.141)e | 1.065 (0.996 – 1.140) |
| Gait speed (per 0.1m/s)d | 0.993 (0.826 – 1.193) | 0.980 (0.812 – 1.184) | 0.996 (0.813 – 1.221) |
| MFS | 1.408 (1.209 – 1.638)*** | 1.511 (1.257 – 1.816)*** | 1.581 (1.276 – 1.959)*** |
| Categorical | |||
| Slow gait speed (≤0.8m/s) | 2.266 (0.981 – 5.231) | 2.720 (1.120 – 6.607)* | 2.494 (0.967 – 6.427) |
| Low grip strengthe | 1.351 (0.595 – 3.067) | 1.428 (0.623 – 3.275) | 1.336 (0.553 – 3.227) |
| ASA classificationf | 1.330 (0.644 – 2.745) | 1.204 (0.561 – 2.582) | 0.976 (0.426 – 2.234) |
| High risk MFS (>8) | 5.851 (2.320 – 14.754)*** | 6.372 (2.317 – 17.524)*** | 5.695 (2.002 – 16.200)*** |
Data are presented as odds ratio (95% confidence interval)
Adjusted by age, sex, body mass index,
Adjusted by age, sex, body mass index and type of surgery
Adjusted by age, sex, body mass index, type of surgery and white blood cell count, hemoglobin, Total cholesterol, protein, BUN and creatinine
Data were missing for 51 patients.
Low grip strength is defined as grip strength of <28.6kg (male), <16.4kg (female)
Data were missing for 19 patients.
P < 0.05.
P < 0.01.
P < 0.001.
Discussion
In this retrospective cohort study, MFS, gait speed, grip strength, and ASA classification could predict the occurrence of post‐operative complications after general surgery, but the prognostic utility of MFS was superior to that of age, grip strength, or ASA classification. Patients who had slow gait speed and low handgrip strength had an increased risk of post‐operative complication. Although AUC of MFS for predicting post‐operative complication was greater than that of gait speed, the difference was not statistically significant. Only MFS showed an incremental predictive value with both age and ASA classification regarding the incidence of post‐operative complication.
In predicting post‐operative 6 month and overall mortality, only MFS had a predictive value after full adjustment. Grip strength, gait speed, and ASA classification failed to predict mortality after full adjustment. Although grip strength and gait speed are known to be associated with frailty status, these single performance measures were not as sensitive as MFS, which represents physiologic frailty and is able to predict short‐term mortality after general surgery.14, 20 This may be because MFS comprises multiple domains, including co‐morbidity, nutritional status, cognitive function, and independence of daily living obtained by comprehensive multidimensional assessment, while grip strength and gait speed represent single physical performance parameters.11
This study suggests that the comprehensive frailty assessment for the older surgical patients could predict adverse outcomes more accurately than conventional risk factors or single performance parameters and only the multidimensional evaluation of older people had an additional prognostic value in predicting post‐operative complication as well as mortality. The results of our study were comparable with those of a previous guideline or systematic review indicating that the evaluation of frailty could identify older surgical patients who had an increased risk of adverse event and that preoperative CGA was likely to have a positive impact on outcomes in older patients through a more accurate assessment.21, 22 Proper assessment and optimization before surgery could also help to shorten length of hospital stay, lower complications, and reduce high level of dependency on discharge.23
In this study, 7.9% (n = 51) of patients could not measure gait speed due to the reasons of ‘unable to walk’ (n = 40, 78.4% of missing patients) or ‘general medical conditions’ (n = 11, 21.6% of missing patients). Therefore, we categorized the patients whose gait speed was missing to slow gait speed group. As expected, the incidence of post‐operative complication in patients who could not measure gait speed was higher than those who were able to measure gait speed, which means that the missing of the gait speed was not a random (8.0% vs. 33.3%, P < 0.001). With our additional sensitivity analysis to evaluate uncertain effect of the missing values, the results were not significantly changed on prediction of complication and 6 month mortality with replacement of missing value with mean 20 values of multiple imputation of age, sex, body mass index, ADL, and IADL (multiple imputation Model 1) or Model 1 plus grip strength (multiple imputation Model 2) or with the mean value (0.61 m/s) of slow gait speed group (single imputation with mean), compared with the original model that excluded the missing values (deletion method) (Supporting Information, Table S2 ).
Considering that post‐operative complications could influence functional recovery, surgical outcome, survival, and ultimately deteriorate patients' quality of life and raise total medical costs, the accurate prediction of preoperative risk for older surgical patients is important for surgeons, patients, and policymakers; thus, the result obtained in this study seems helpful. ASA, APACHE II, NSQIP‐PMP, SURPAS, and modified Frailty Index were commonly used for pre‐surgical risk assessment tool.24 However, most of them are not universally used or are specific to certain interventions. Compared with these available risk stratification tools, in the light of the two main purposes, risk stratification and identification of modifiable factors, MFS based on CGA is simple, feasible, and intuitive frailty assessment tool. Precise risk stratification and identification of modifiable domains could help surgeons decide whether surgery is to be performed and select patients who need a proactive approach to medical optimization, rehabilitation before or after surgery, and establishment of a preemptive discharge plan.
To determine whether predictive ability of MFS differs according to the age groups, we conducted subgroup analysis among young–old group (age 65–74, n = 235) and old–old group (age ≥ 75, n = 395). The young–old group showed lower incidence of low grip strength (19.0% vs. 44.8%, P < 0.001), slow gait speed (13.8% vs. 32.7%, P < 0.001), and high‐risk MFS (3.6% vs. 11.9%, P < 0.001) than old–old group. Old–old group also experienced more post‐operative complications (12.9% vs. 5.9%, P = 0.004) and 6 month mortality (5.8% vs. 0.8%, P = 0.001) than young–old group. In both age groups, MFS was useful for predicting post‐operative morbidity and mortality (Supporting Information, Table S3 ). Thus, we believed that the frailty is an important prognostic indicator in both young–old and old–old surgical patients.
The strength of our study lies in its comparison of the frailty score model with age, simple performance parameters, and conventional risk stratification tools for predicting post‐operative adverse events after general surgery not only on a one‐to‐one basis but also on an add‐on basis. Although physicians (non‐geriatricians) or surgeons prefer methods that entail minimal cost, space, time, or manpower, single‐parameter or conventional risk stratification methods may not be appropriate to assess the complex co‐morbidity and frailty status of the geriatric population. In our study, single physical performance measures (gait speed and grip strength) could predict post‐operative complication; however, as solitary indicators, their ability was modest for predicting mortality. Thus, we suggest that gait speed or grip strength could be used for screening, to identify frail patients and target those who may benefit from CGA.
There are several limitations to this study. First, because the patients were recruited retrospectively and from only one hospital, our study might not be generalizable and needs verification in multiple institutions. Second, the lack of statistical significance in the all‐cause mortality (6 months or overall) of our study might be attributed to the relatively short follow‐up (median, 13 months) period. However, we thought that this was a sufficient period to see the short‐term result of insult, the surgery. Third, 4 m is commonly used distance for gait speed measurement, but we used 4.5 m following a 1 m acceleration and deceleration distance. Previous studies reported that protocols for gait speed measurement were widely variable and the distance was not significantly associated with mean gait velocity.25, 26 Besides, Afilalo research group used 5 m gait speed for pre‐surgical evaluation, and we published a paper using 4.5 m gait speed for frailty assessment.13, 27 We need more studies for establishing optimal protocol of gait speed measurement. Fourth, although this study proved that MFS is a good risk stratification indicator and geriatricians (J. Y. C., M. G. K., K. I. K., and C. H. K.) of our institution routinely present the recommendations through outpatient clinic and CGA reports preoperatively, we were not able to conduct a prospective randomized clinical trial to verify whether tailored recommendations could reduce post‐operative adverse events. Therefore, further prospective studies using standardized method including multiple institutions and individualized treatment strategies, with longer observation periods, are warranted.
Conclusions
Multidimensional frailty score, gait speed, grip strength, and ASA classification could predict post‐operative complication, but only MFS could predict the 6 month or all‐cause mortality after full adjustment. Compared with other risk stratification indicators, MFS, a frailty score derived from CGA, showed the best predictive power in both complication and 6 month mortality and had incremental predictive utility for post‐operative complication with the addition of age or ASA classification. Considering this, we recommend the use of gait speed or grip strength for screening to identify frail patients and target those who may benefit from further CGA.
Conflict of interest
None declared.
Supporting information
Table S1. Adjusted HRs by grip strength, gait speed and other risk factors for overall mortality
Table S2. Sensitivity analysis to identify uncertain effect on missing values of gait speed after full adjustment
Table S3. Adjusted ORs by grip strength, gait speed and other risk factors for postoperative complication according to the age groups
Acknowledgements
This study was supported by a grant from the Seoul National University Bundang Hospital (SNUBH) Research Fund (grant number 14‐2017‐023). The funder of this study had no involvement in the study design, collection of data, interpretation of data, or writing of the manuscript.
Choi J.‐Y., Kim K.‐i., Choi Y. R., Ahn S.‐H., Kang E., Oh H.‐K., Kim D.‐W., Kim E.‐K., Yoon Y.‐S., Kang S.‐B., Kim H.‐H., Han H.‐S., and Kim C.‐H. (2020) Comparison of multidimensional frailty score, grip strength, and gait speed in older surgical patients, Journal of Cachexia, Sarcopenia and Muscle, 11, 432–440. 10.1002/jcsm.12509.
[Correction added on 23 January 2020, after first online publication: The affiliations of the Corresponding author Kwang‐il Kim and co‐authors Jung‐Yeon Choi, Yoo‐Seok Yoon, Sung‐Bum Kang and Cheol‐Ho Kim were previously incorrect and have been corrected in this current version.]
References
- 1. Ageing and health (2018, February 5) . From https://www.who.int/news-room/fact-sheets/detail/ageing-and-health.
- 2. Hamel MB, Henderson WG, Khuri SF, Daley J. Surgical outcomes for patients aged 80 and older: morbidity and mortality from major noncardiac surgery. J Am Geriatr Soc. 2005;53:424–429. [DOI] [PubMed] [Google Scholar]
- 3. Couwenberg AM, de Beer FSA, Intven MPW, Burbach JPM, Smits AB, Consten ECJ, et al. The impact of postoperative complications on health‐related quality of life in older patients with rectal cancer; a prospective cohort study. J Geriatr Oncol. 2018;9:102–109. [DOI] [PubMed] [Google Scholar]
- 4. Menke H, Klein A, John KD, Junginger T. Predictive value of ASA classification for the assessment of the perioperative risk. Int Surg. 1993;78:266–270. [PubMed] [Google Scholar]
- 5. Prause G, Ratzenhofer‐Comenda B, Pierer G, Smolle‐Juttner F, Glanzer H, Smolle J. Can ASA grade or Goldman's cardiac risk index predict peri‐operative mortality? A study of 16,227 patients. Anaesthesia. 1997;52:203–206. [DOI] [PubMed] [Google Scholar]
- 6. Mayr R, Gierth M, Zeman F, Reiffen M, Seeger P, Wezel F, et al. Sarcopenia as a comorbidity‐independent predictor of survival following radical cystectomy for bladder cancer. J Cachexia Sarcopenia Muscle. 2018;9:505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang M, Hu X, Wang H, Zhang L, Hao Q, Dong B. Sarcopenia predicts readmission and mortality in elderly patients in acute care wards: a prospective study. J Cachexia Sarcopenia Muscle. 2017;8:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Hert S, Staender S, Fritsch G, Hinkelbein J, Afshari A, Bettelli G, et al. Pre‐operative evaluation of adults undergoing elective noncardiac surgery: updated guideline from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2018;35:407–465. [DOI] [PubMed] [Google Scholar]
- 10. Kim KI, Park KH, Koo KH, Han HS, Kim CH. Comprehensive geriatric assessment can predict postoperative morbidity and mortality in elderly patients undergoing elective surgery. Arch Gerontol Geriatr. 2013;56:507–512. [DOI] [PubMed] [Google Scholar]
- 11. Kim SW, Han HS, Jung HW, Kim KI, Hwang DW, Kang SB, et al. Multidimensional frailty score for the prediction of postoperative mortality risk. JAMA Surg. 2014;149:633–640. [DOI] [PubMed] [Google Scholar]
- 12. Choi JY, Cho KJ, Kim SW, Yoon SJ, Kang MG, Kim KI, et al. Prediction of mortality and postoperative complications using the hip‐multidimensional frailty score in elderly patients with hip fracture. Sci Rep. 2017;7:42966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Afilalo J, Eisenberg MJ, Morin JF, Bergman H, Monette J, Noiseux N, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56:1668–1676. [DOI] [PubMed] [Google Scholar]
- 14. Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sultan P, Hamilton MA, Ackland GL. Preoperative muscle weakness as defined by handgrip strength and postoperative outcomes: a systematic review. BMC Anesthesiol. 2012;12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoo JI, Choi H, Ha YC. Mean hand grip strength and cut‐off value for sarcopenia in Korean adults using KNHANES VI. J Korean Med Sci. 2017;32:868–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. [DOI] [PubMed] [Google Scholar]
- 19. Khuri SF, Daley J, Henderson W, Hur K, Demakis J, Aust JB, et al. The Department of Veterans Affairs' NSQIP: the first national, validated, outcome‐based, risk‐adjusted, and peer‐controlled program for the measurement and enhancement of the quality of surgical care. National VA Surgical Quality Improvement Program. Ann Surg. 1998;228:491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Syddall H, Cooper C, Martin F, Briggs R, Aihie SA. Is grip strength a useful single marker of frailty? Age Ageing. 2003;32:650–656. [DOI] [PubMed] [Google Scholar]
- 21. Partridge JS, Harari D, Martin FC, Dhesi JK. The impact of pre‐operative comprehensive geriatric assessment on postoperative outcomes in older patients undergoing scheduled surgery: a systematic review. Anaesthesia. 2014;69:8–16. [DOI] [PubMed] [Google Scholar]
- 22. Chow WB, Rosenthal RA, Merkow RP, Ko CY, Esnaola NF. American College of Surgeons National Surgical Quality Improvement Program, et al. Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society. J Am Coll Surg. 2012;215:453–466. [DOI] [PubMed] [Google Scholar]
- 23. Partridge JS, Harari D, Martin FC, Peacock JL, Bell R, Mohammed A, et al. Randomized clinical trial of comprehensive geriatric assessment and optimization in vascular surgery. Br J Surg. 2017;104:679–687. [DOI] [PubMed] [Google Scholar]
- 24. Eamer G, Al‐Amoodi MJH, Holroyd‐Leduc J, Rolfson DB, Warkentin LM, Khadaroo RG. Review of risk assessment tools to predict morbidity and mortality in elderly surgical patients. Am J Surg. 2018;216:585–594. [DOI] [PubMed] [Google Scholar]
- 25. Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: a systematic review. J Gerontol A Biol Sci Med Sci. 2013;68:39–46. [DOI] [PubMed] [Google Scholar]
- 26. Graham JE, Ostir GV, Kuo YF, Fisher SR, Ottenbacher KJ. Relationship between test methodology and mean velocity in timed walk tests: A review. Arch Phys Med Rehabil. 2008;89:865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Choi JY, Kang MG, Park KU, Park WB, Kim KI, Kim ES, et al. Immunogenicity of the varicella‐zoster vaccine in community‐dwelling non‐robust elderly compare to robust elderly: a prospective cohort study. J Gerontol A Biol Sci Med Sci. 2019;74:1225–1230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Adjusted HRs by grip strength, gait speed and other risk factors for overall mortality
Table S2. Sensitivity analysis to identify uncertain effect on missing values of gait speed after full adjustment
Table S3. Adjusted ORs by grip strength, gait speed and other risk factors for postoperative complication according to the age groups


