Abstract
Background
Sarcopenia is commonly observed in patients with advanced‐stage epithelial ovarian cancer (EOC). However, the effect of body composition changes—during primary debulking surgery (PDS) and adjuvant platinum‐based chemotherapy—on outcomes of patients with advanced‐stage EOC is unknown. This study aimed to evaluate the association between body composition changes and outcomes of patients with stage III EOC treated with PDS and adjuvant platinum‐based chemotherapy.
Methods
Pre‐treatment and post‐treatment computed tomography (CT) images of 139 patients with stage III EOC were analysed. All CT images were contrast‐enhanced scans and were acquired according to a standardized protocol. The skeletal muscle index (SMI), skeletal muscle radiodensity (SMD), and total adipose tissue index were measured using CT images obtained at the L3 vertebral level. Predictors of overall survival were identified using Cox regression models.
Results
The median follow‐up was 37.9 months. The median duration between pre‐treatment and post‐treatment CT was 182 days (interquartile range: 161–225 days). Patients experienced an average SMI loss of 1.8%/180 days (95% confidence interval: −3.1 to −0.4; P = 0.01) and SMD loss of 1.7%/180 days (95% confidence interval: −3.3 to −0.03; P = 0.046). SMI and SMD changes were weakly correlated with body mass index changes (Spearman ρ for SMI, 0.15, P = 0.07; ρ for SMD, 0.02, P = 0.82). The modified Glasgow prognostic score was associated with SMI loss (odds ratio: 2.42, 95% confidence interval: 1.03–5.69; P = 0.04). The median time to disease recurrence was significantly shorter in patients with SMI loss ≥5% after treatment than in those with SMI loss <5% or gain (5.4 vs. 11.2 months, P = 0.01). Pre‐treatment SMI (1 cm2/m2 decrease; hazard ratio: 1.08, 95% confidence interval: 1.03–1.11; P = 0.002) and SMI change (1%/180 days decrease; hazard ratio: 1.04, 95% confidence interval: 1.01–1.08; P = 0.002) were independently associated with poorer overall survival. SMD, body mass index, and total adipose tissue index at baseline and changes were not associated with overall survival.
Conclusions
Skeletal muscle index decreased significantly during treatment and was independently associated with poor overall survival in patients with stage III EOC treated with PDS and adjuvant platinum‐based chemotherapy. The modified Glasgow prognostic score might be a predictor of SMI loss during treatment.
Keywords: Ovarian cancer, Sarcopenia, Computed tomography, Body composition, Cancer cachexia, Systemic inflammation
Introduction
Ovarian cancer is the most lethal gynaecologic malignancy, with approximately 295 414 new cases and 184 799 deaths worldwide in 2018.1 Patients with advanced‐stage epithelial ovarian cancer (EOC) often develop large tumours and ascites that can affect dietary intake and bowel function; therefore, patients often appear malnourished or have cachexia at the time of presentation.2 Severe loss of muscle mass (i.e. sarcopenia) is a crucial component of cancer cachexia and was associated with poor outcomes in various malignancies.3, 4, 5, 6, 7, 8, 9, 10 Sarcopenia may also affect the outcomes of patients with advanced‐stage EOC; however, previous studies showed inconsistent associations between pre‐treatment sarcopenia and survival outcomes of patients with EOC.11, 12, 13, 14, 15, 16, 17
The mainstay of treatment for advanced‐stage EOC is primary debulking surgery (PDS) and adjuvant platinum‐based chemotherapy.18 During the course of cancer therapy, body composition may change and influence outcomes of cancer patients.5, 6, 7, 8, 9, 10 Body composition changes could be associated with systemic inflammation, treatment‐related toxicity, physical inactivity, malnutrition, cancer invasiveness, and cancer therapy.19, 20, 21, 22, 23, 24, 25 Therefore, evaluating the body composition at a single specific time point may not help in predicting survival. A longitudinal study evaluating the changes in body composition during cancer therapy provides a more comprehensive understanding about the influence of body composition on the outcomes of EOC.12 However, there is a paucity of data on the effect of body composition change during PDS and adjuvant chemotherapy on the outcomes of patients with advanced‐stage EOC.
Systemic inflammation could play a role in EOC genesis, growth, and progression.26, 27, 28, 29 Patients with EOC may have systemic inflammation; systemic inflammation may also potentiate muscle wasting through anabolic and catabolic pathways responsible for skeletal muscle physiology.19 Dolan et al. revealed that pre‐treatment skeletal muscle mass and quality were significantly associated with systemic inflammatory markers in patients with colorectal cancer.30 However, it is unclear whether systemic inflammation was associated with muscle loss during cancer therapy. Therefore, a longitudinal study would help evaluate the relationship between systemic inflammation and changes in skeletal muscle during cancer therapy.
Computed tomography (CT) images provide objective measures of the skeletal muscle and adipose tissue. The cross‐sectional areas of the skeletal muscle and adipose tissue on a single CT slice at the level of the third lumbar vertebra (L3) are strongly correlated with the total body skeletal muscle and adipose masses.31, 32 In addition, the use of contrast medium could influence the assessment of skeletal muscle radiodensity (SMD) and may affect the association with the outcomes.33, 34, 35, 36 Accordingly, evaluating the prognostic value of muscle measurement by using a standardized phase of contrast‐enhanced CT images may provide more comparable or consistent results in body composition studies.34
We hypothesized that body composition change during PDS and adjuvant platinum‐based chemotherapy would affect patient outcomes. Hence, the aims of this study were to assess the changes in body composition parameters using a standardized phase of contrast‐enhanced CT images and to determine whether body composition measures were associated with survival outcomes of patients with stage III EOC undergoing PDS and adjuvant platinum‐based chemotherapy.
Materials and methods
Patients
Our institutional review board approved this retrospective study and waived the need for informed consent from patients owing to the retrospective and observational nature of the study. Patients at our institution with International Federation of Gynecology and Obstetrics (FIGO) stage III EOC who had undergone PDS and adjuvant platinum‐based chemotherapy between 2008 and 2017 were reviewed. The inclusion criteria were as follows: (i) routine abdominal CT performed before PDS and a second CT scan obtained after adjuvant chemotherapy and (ii) sufficient quality of CT scans for accurate measurements of the tissue area. Patients who received neoadjuvant chemotherapy or refused to continue chemotherapy after receiving one or two courses of adjuvant chemotherapy were not enrolled. Patients were also excluded if relevant clinical data could not be obtained from the patient's medical records.
All surgical procedures were performed by accredited gynaecological oncologists. The outcomes of PDS were categorized into complete (no macroscopic residual tumour), optimal (largest residual tumour ≤1 cm), or suboptimal (largest residual tumour >1 cm). Adjuvant platinum‐based chemotherapy, every 3 weeks for 6 cycles, was recommended for all patients.18 Adjuvant chemotherapy was started within 3 weeks after PDS. A routine pre‐treatment CT image was obtained before PDS, and a post‐treatment CT image was obtained within 6 months of adjuvant chemotherapy completion.
Computed tomography‐based body composition analysis
The pre‐treatment and post‐treatment CT images were retrieved for analysis. Body weight and height were obtained from medical records within 2 weeks of the initial and follow‐up CT scans. In our institution, routine abdominal and pelvic CT images were obtained for women after intravenous administration of iohexol 300 (Omnipaque 300, GE Healthcare) or iopromide 300 (Ultravist 300, Bayer HealthCare) in a single uniphasic bolus dose of 80–100 mL via a power injector at 2 mL/s. The portal‐venous phase was obtained with a fixed delay of 70 s after the administration of the contrast material, and a pitch between 1.0 and 1.5 before the contrast medium was excreted into the bladder. The following CT image parameters included the following information: contrast‐enhanced, 5 mm slice thickness, 120 kVp, and approximately 290 mA.
Two consecutive transverse CT images extending from L3 to the iliac crest were analysed by using the Varian Eclipse software (Varian Medical Systems Inc., Palo Alto, CA, USA).31, 37 Predetermined Hounsfield unit (HU) thresholds were −29 to +150 HU for skeletal muscle, −50 to −150 HU for visceral adipose tissue, and −30 to −190 HU for subcutaneous and intermuscular adipose tissues.31, 32 The cross‐sectional areas (cm2) of the skeletal muscle (including the psoas, paraspinal, transversus abdominis, rectus abdominis, and internal and external oblique muscles) and adipose tissues were calculated. The mean radiation attenuation of the entire cross‐sectional area of the skeletal muscle was the SMD. The total adipose tissue (TAT) area was calculated as the sum of the areas of the subcutaneous, intermuscular, and visceral adipose tissues. The mean tissue areas were calculated by using two consecutive images. One researcher, blinded to the patient information, measured the body composition parameters. The intraobserver coefficients of variation were 0.8%, 0.8%, and 1.0% for the skeletal muscle area, SMD, and TAT area, respectively, in a sample of 60 patients randomly selected from this cohort. The cross‐sectional areas of the skeletal muscle and TAT were normalized based on the patients' height to determine the skeletal muscle index (SMI) and total adipose tissue index (TATI; cm2/m2).
As body composition varies greatly between regions, ethnicities, and cancer types,38, 39, 40, 41 we defined our own cut‐off values for defining sarcopenia, myosteatosis, and low TATI on the basis of previous studies with similar population sizes 7, 8, 9, 15. Cut‐off values were set at the lowest tertile for SMI and SMD and at the highest tertile for TATI. The post‐treatment body composition change was the difference between the pre‐treatment and post‐treatment CT images. In this study, the median duration to complete PDS and adjuvant chemotherapy was 127 days [interquartile range (IQR): 120–140 days]. The median duration between pre‐treatment and post‐treatment CT scans was 182 days (IQR: 161–225 days). To account for variations in the scan interval duration, body composition changes were calculated as the change per 180 days for providing a standardized unit for comparisons between patients. Per the current definition of cachexia,2 patients with a reduction in the weight, SMI, SMD, or TATI of ≥5% were classified as having ‘loss’;6 patients with a reduction in the weight, SMI, SMD, or TATI of <5% or gain in the weight, SMI, SMD, or TATI were classified as having ‘maintained’.
Markers of systemic inflammation
Differential blood cell counts and serum albumin were obtained prior to PDS. The neutrophil–lymphocyte ratio (NLR) was defined as the absolute neutrophil count divided by the absolute lymphocyte count. The NLR was categorized using a cut‐off value of 3 (i.e. ≤3 or >3).42, 43 The platelet–lymphocyte ratio (PLR) was defined as the absolute platelet count divided by the absolute lymphocyte count. The PLR was categorized using a cut‐off value of 150 (i.e. ≤150 or >150).42 Data of the serum C‐reactive protein (CRP) level were available for a subset of 127 patients. The modified Glasgow prognostic score (mGPS) is a useful inflammatory index for assessing the status of cachexia. This score includes the CRP levels, to reflect the systemic inflammation status, and serum albumin levels, to reflect the nutritional status.44 The mGPS was classified as follows: patients with both an elevated CRP level (>10 mg/L) and a reduced albumin level (<35 g/L) were given a score of 2; those with an elevated CRP level (>10 mg/L) and a non‐decreased albumin level (≥35 g/L) were given a score of 1; and those with a non‐elevated CRP level (≤10 mg/L), regardless of their albumin level, were given a score of 0.42
Statistical analysis
Continuous data were presented as the mean ± standard deviation or median and IQR, as applicable, while categorical data were presented as numbers and percentages. The distributions of patient characteristics were compared by using the chi‐square test for categorical variables and independent t‐test, Mann–Whitney U tests, or Kruskal–Wallis test for continuous variables as statistically appropriate. Paired t‐tests and the Wilcoxon signed‐rank test were used to assess body composition changes. The Spearman correlation coefficient was used to assess relationships between body composition parameters. Logistic regression models were used to test for associations between systemic inflammation markers and body composition parameters.
The primary endpoint was overall survival (OS), defined as the time from the date of surgery to the date of death from any cause; progression‐free survival (PFS) was defined as the time from the date of surgery to the date of disease recurrence, progression, or death from any cause. Survival curves were constructed using the Kaplan–Meier method with log‐rank tests. Cox proportional hazards models were used to estimate the hazard ratio and 95% confidence interval of body composition and risk of outcomes. All variables with a P < 0.10 on univariable analysis or with clinical relevance were added to the multivariable analysis. The data were analysed using IBM SPSS software (version 21.0; IBM Corp., Armonk, NY, USA). A P < 0.05 was considered statistically significant.
Results
A total of 139 patients met the inclusion criteria (Figure 1). The final analysis included 278 CT scans for the 139 patients. The baseline characteristics of the patients are presented in Table 1. The median duration between pre‐treatment and post‐treatment CT scans was 182 days (IQR: 161–225 days). The median duration between adjuvant chemotherapy completion and post‐treatment CT scans was 44 days (IQR: 26–73 days).
Figure 1.
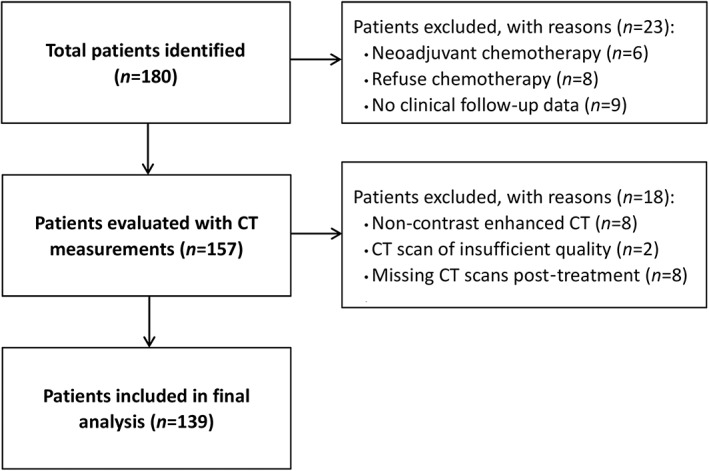
Flow chart for patient inclusion. CT, computed tomography.
Table 1.
Patient and tumour characteristics
| Characteristics | Overall (n = 139) | SMI loss (n = 48) | SMI maintained (n = 91) | P‐value |
|---|---|---|---|---|
| Age (years) | 54.4 ± 10.3 | 54.0 ± 8.7 | 54.6 ± 11.1 | 0.74 |
| ECOG performance status | ||||
| 0 | 130 (93.5) | 44 (91.7) | 86 (84.5) | 0.50 |
| 1 | 9 (6.5) | 4 (8.3) | 5 (5.5) | |
| Pre‐treatment BMI (kg/m2) | 22.3 ± 3.4 | 22.7 ± 3.4 | 22.0 ± 3.3 | 0.29 |
| Weight loss ≥5% | 11 (7.9) | 6 (12.5) | 5 (5.5) | 0.19 |
| Pre‐treatment SMI (cm2/m2) | 41.9 ± 7.0 | 42.4 ± 7.0 | 41.6 ± 7.1 | 0.56 |
| Pre‐treatment sarcopeniaa | 47 (33.8) | 14 (29.2) | 33 (36.3) | 0.40 |
| Post‐treatment SMI (cm2/m2) | 41.1 ± 7.5 | 38.4 ± 7.2 | 42.5 ± 7.2 | 0.002 |
| Post‐treatment sarcopeniaa | 55 (39.6) | 25 (52.1) | 30 (33.0) | 0.03 |
| Pre‐treatment SMD (HU) | 38.0 ± 7.3 | 36.8 ± 7.6 | 38.6 ± 7.0 | 0.17 |
| Pre‐treatment myosteatosisa | 46 (33.1) | 20 (41.7) | 26 (28.6) | 0.12 |
| SMD change (%/180 days) | −1.7 ± 9.8 | −4.8 ± 9.6 | −0.1 ± 9.6 | 0.007 |
| SMD maintained | 91 (65.5) | 25 (52.1) | 66 (72.5) | 0.02 |
| SMD loss ≥5% | 48 (34.5) | 23 (47.9) | 25 (27.5) | |
| Pre‐treatment TATI (cm2/m2) | 88.3 ± 44.8 | 93.3 ± 40.4 | 85.6 ± 47.0 | 0.34 |
| Pre‐treatment low TATIa | 93 (66.9) | 30 (62.5) | 63 (69.2) | 0.42 |
| TATI change (%/180 days) | 15.0 ± 34.7 | 8.2 ± 26.7 | 18.6 ± 38.0 | 0.09 |
| FIGO stage | 0.35 | |||
| IIIA–IIIB | 23 (16.5) | 6 (12.5) | 17 (18.7) | |
| IIIC | 116 (83.5) | 42 (87.5) | 74 (81.3) | |
| Histological grade | 0.15 | |||
| Grade 1 | 7 (5.0) | 1 (2.1) | 6 (6.6) | |
| Grade 2 | 45 (32.4) | 20 (41.7) | 25 (27.5) | |
| Grade 3 | 87 (62.6) | 27 (56.3) | 60 (65.9) | |
| Residual disease after PDS | 0.03 | |||
| No macroscopic residual disease | 73 (52.5) | 18 (37.5) | 55 (60.4) | |
| Residual disease ≤1 cm | 48 (34.5) | 21 (43.8) | 27 (29.7) | |
| Residual disease >1 cm | 18 (12.9) | 9 (18.8) | 9 (9.9) | |
| Ascites | 0.04 | |||
| No ascites | 16 (11.5) | 4 (8.3) | 12 (13.2) | |
| Non‐malignant ascites | 35 (25.2) | 7 (14.6) | 28 (30.8) | |
| Malignant ascites | 88 (63.3) | 37 (77.1) | 51 (56.0) | |
| Albumin (g/L) | 0.14 | |||
| ≥35 | 95 (68.3) | 29 (60.4) | 66 (72.5) | |
| <35 | 44 (31.7) | 19 (39.6) | 25 (27.5) | |
| CRP (mg/L) | 0.004 | |||
| ≤10 | 47 (33.8) | 11 (22.9) | 36 (39.6) | |
| >10 | 80 (57.6) | 36 (75.0) | 44 (48.4) | |
| Missing | 12 (8.6) | 1 (2.1) | 11 (12.1) | |
| mGPS | 0.04 | |||
| 0 | 47 (37.0) | 11 (23.4) | 36 (45.0) | |
| 1 | 45 (35.4) | 19 (40.4) | 26 (32.5) | |
| 2 | 35 (27.6) | 17 (36.2) | 18 (22.5) | |
| NLR | 0.15 | |||
| ≤3 | 52 (37.4) | 14 (29.2) | 38 (41.8) | |
| >3 | 87 (62.6) | 34 (70.8) | 53 (58.2) | |
| PLR | 0.25 | |||
| ≤150 | 31 (22.3) | 8 (16.7) | 23 (25.3) | |
| >150 | 108 (77.7) | 40 (83.3) | 68 (74.7) | |
| Median (IQR) duration between CT scans (days) | 182 (161–225) | 178 (159–224) | 186 (164–226) | 0.59 |
Data are mean ± standard error or number (%). Bolded P‐values are those significant with a P < 0.05. BMI, body mass index; CRP, C‐reactive protein; CT, computed tomography; ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Gynecology and Obstetrics; HU, Hounsfield unit; IQR, interquartile range; mGPS, modified Glasgow prognostic score; NLR, neutrophil–lymphocyte ratio; PDS, primary debulking surgery; PLR, platelet–lymphocyte ratio; SMD, skeletal muscle radiodensity; SMI, skeletal muscle index; TATI, total adipose tissue index.
SMI < 39.2 cm2/m2, SMD < 35.5 HU, and TATI < 100.8 cm2/m2 were defined as sarcopenia, myosteatosis, and low TATI, respectively.
Body composition at baseline and change after treatment
Table 2 summarizes the body composition characteristics at baseline and the changes after treatment. Overall, the body mass index (BMI) and TATI increased while SMI and SMD decreased after PDS and adjuvant chemotherapy. Eleven patients (7.9%) experienced weight loss of ≥5% while 48 patients (34.5%) each experienced SMI and SMD loss of ≥5%. SMI and SMD changes were not correlated with BMI change (Spearman ρ for SMI, 0.15; P = 0.07; ρ for SMD, 0.02; P = 0.82). TATI changes were moderately correlated with BMI change (Spearman ρ for TATI, 0.58; P < 0.001). SMI changes were weakly correlated with SMD change (Spearman ρ for SMD, 0.22; P = 0.01).
Table 2.
Change of body composition parameters during treatment
| Variable | First CT scan | Second CT scan | Absolute change per 180 days | Relative change per 180 days (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean | 95% CI | P‐value | Mean | 95% CI | P‐value | |
| BMI (kg/m2) | 22.3 ± 3.4 | 23.2 ± 3.3 | 0.8 | 0.6 to 1.1 | <0.001 | 4.1 | 2.9 to 5.3 | <0.001 |
| SMI (cm2/m2) | 41.9 ± 7.0 | 41.1 ± 7.5 | −0.7 | −1.3 to −0.2 | 0.005 | −1.8 | −3.1 to −0.4 | 0.01 |
| SMD (HU) | 38.0 ± 7.3 | 37.2 ± 7.3 | −0.8 | −1.4 to −0.2 | 0.009 | −1.7 | −3.3 to −0.03 | 0.046 |
| TATI (cm2/m2) | 88.3 ± 44.8 | 95.3 ± 43.6 | 6.7 | 3.2 to 10.2 | <0.001 | 15.0 | 9.2 to 20.8 | <0.001 |
Bolded P‐values are those significant with a P < 0.05. BMI, body mass index; CI, confidence interval; CT, computed tomography; HU, Hounsfield unit; SD, standard deviation; SMD, skeletal muscle radiodensity; SMI, skeletal muscle index; TATI, total adipose tissue index.
The cut‐off values for sarcopenia, myosteatosis, and low TATI were SMI <39.2 cm2/m2, SMD <35.5 HU, and TATI <100.8 cm2/m2, respectively. Patient and tumour characteristics according to pre‐treatment sarcopenia are summarized in Supporting Information, Table S1 . There were marginally more patients with malignant ascites in the pre‐treatment sarcopenia group than in the non‐sarcopenia group (P = 0.051). The patient characteristics according to SMI change are presented in Table 1. The pre‐treatment SMI was similar between the SMI‐loss and SMI‐maintained groups (P = 0.56); 14 (29.2%) and 33 (36.3%) patients in the SMI‐loss and SMI‐maintained groups, respectively, had pre‐treatment sarcopenia (P = 0.40). However, 25 (52.1%) and 30 (33.0%) patients in the SMI‐loss and SMI‐maintained groups, respectively, had post‐treatment sarcopenia (P = 0.03). The SMD decreased by a greater extent in patients in the SMI‐loss group compared with those in the SMI‐maintained group (P = 0.007); the TATI changes were not significantly different between the SMI‐loss and SMI‐maintained groups (P = 0.09). There were significantly more patients with residual disease after PDS or malignant ascites in the SMI‐loss group than in the SMI‐maintained group. Compared with patients treated with complete resection, patients with residual disease showed a greater decrease in the SMI (−2.9% vs. −0.7%; P = 0.03) and SMD (−2.5% vs. −1.0%; P = 0.37) during treatment. Patients with malignant ascites showed a greater decrease in SMI (−3.2% vs. 0.7%, respectively; P = 0.001) and SMD (−2.3% vs. −0.6%, respectively; P = 0.15) during treatment compared with patients with no ascites or non‐malignant ascites.
Systemic inflammation markers and body composition
The systemic inflammation markers of the patients according to pre‐treatment sarcopenia or SMI change are presented in Supporting Information, Table S1 and Table 1, respectively. There were significantly more patients with higher mGPS in the pre‐treatment sarcopenia group (P = 0.03) and SMI‐loss group (P = 0.04). Figure 2 showed the association between mGPS and skeletal muscle parameters. Patients with a higher mGPS had a significantly lower pre‐treatment SMD and a lower pre‐treatment SMI, with marginal significance (Figure 2A and 2B). Patients with a higher mGPS lost significant more SMI during treatment, but the changes in SMD were not significant among the mGPS groups (Figure 2C and 2D). When adjusted for age and BMI, an elevated mGPS was independently associated with pre‐treatment sarcopenia and myosteatosis (Tables 3 and 4). Because residual disease and malignant ascites were observed on PDS and were associated with SMI loss during treatment (Table 1), these factors were also adjusted on logistic regression analysis of systemic inflammation markers and changes in body composition after PDS and adjuvant chemotherapy. An elevated mGPS was independently associated with SMI loss during treatment (Table 3); the mGPS was not associated with SMD loss (Table 4). The NLR and PLR were not associated with skeletal muscle parameters at baseline or changes after treatment (Tables 3 and 4). Systemic inflammatory markers were also not associated with TATI at baseline and changes after treatment (Supporting Information, Table S2).
Figure 2.
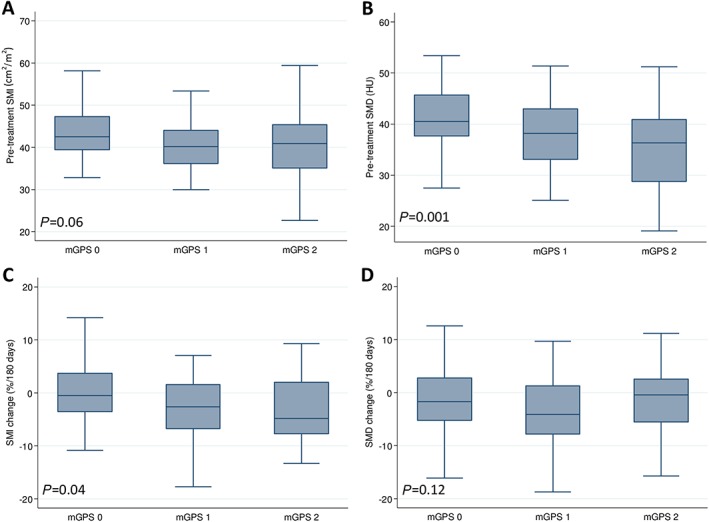
Association of modified Glasgow prognostic score with (A) pre‐treatment SMI, (B) pre‐treatment SMD, (C) SMI changes, and (D) SMD changes. mGPS, modified Glasgow prognostic score; SMD, skeletal muscle radiodensity; SMI, skeletal muscle index.
Table 3.
Logistic regression analysis of systemic inflammation markers, pre‐treatment sarcopenia, and skeletal muscle index loss
| Odds ratio (95% CI) | P‐value | |
|---|---|---|
| Pre‐treatment sarcopeniaa | ||
| mGPS | ||
| mGPS 0 | Reference | |
| mGPS 1–2 | 2.37 (1.01–5.54) | 0.04 |
| NLR | ||
| NLR ≤3 | Reference | |
| NLR >3 | 0.81 (0.35–1.88) | 0.63 |
| PLR | ||
| PLR ≤150 | Reference | |
| PLR >150 | 1.60 (0.59–4.37) | 0.36 |
| SMI loss ≥5%b | ||
| mGPS | ||
| mGPS 0 | Reference | |
| mGPS 1–2 | 2.42 (1.03–5.69) | 0.04 |
| NLR | ||
| NLR ≤3 | Reference | |
| NLR >3 | 1.66 (0.76–3.64) | 0.21 |
| PLR | ||
| PLR ≤150 | Reference | |
| PLR >150 | 1.57 (0.62–3.99) | 0.35 |
Bolded P‐values are those significant with a P < 0.05. CI, confidence interval; mGPS, modified Glasgow prognostic score; NLR, neutrophil–lymphocyte ratio; PLR, platelet–lymphocyte ratio; SMI, skeletal muscle index.
Model adjusted for age and body mass index.
Model adjusted for age, body mass index, residual disease after primary debulking surgery, and malignant ascites.
Table 4.
Logistic regression analysis of systemic inflammation markers, pre‐treatment myosteatosis, and skeletal muscle radiodensity loss
| Odds ratio (95% CI) | P‐value | |
|---|---|---|
| Pre‐treatment myosteatosisa | ||
| mGPS | ||
| mGPS 0 | Reference | |
| mGPS 1–2 | 3.47 (1.29–9.32) | 0.01 |
| NLR | ||
| NLR ≤3 | Reference | |
| NLR >3 | 1.37 (0.63–2.99) | 0.43 |
| PLR | ||
| PLR ≤150 | Reference | |
| PLR >150 | 2.01 (0.82–4.94) | 0.13 |
| SMD loss ≥5%b | ||
| mGPS | ||
| mGPS 0 | Reference | |
| mGPS 1–2 | 1.26 (0.56–2.81) | 0.58 |
| NLR | ||
| NLR ≤3 | Reference | |
| NLR >3 | 1.65 (0.77–3.55) | 0.20 |
| PLR | ||
| PLR ≤150 | Reference | |
| PLR >150 | 2.13 (0.83–5.49) | 0.12 |
Bolded P‐values are those significant with a P < 0.05. CI, confidence interval; mGPS, modified Glasgow prognostic score; NLR, neutrophil–lymphocyte ratio; PLR, platelet–lymphocyte ratio; SMD, skeletal muscle radiodensity.
Model adjusted for age and body mass index.
Model adjusted for age, body mass index, residual disease after primary debulking surgery, and malignant ascites.
Body composition at baseline and outcomes
The median follow‐up duration was 37.9 months (IQR, 22.6–64.2 months). The 5 year OS and PFS rates for the entire cohort were 60.3% and 32.9%, respectively. The 5 year OS rate was 54.7% in the sarcopenic group and 63.2% in the non‐sarcopenic group (P = 0.08); the corresponding 5 year PFS rates were 22.3% and 38.5%, respectively (P = 0.03). The 5 year OS rate was 48.4% in the myosteatosis group and 65.7% in the non‐myosteatosis group (P = 0.02); the corresponding 5 year PFS rates were 21.8% and 37.7%, respectively (P = 0.24). The 5 year OS rate was 57.5% in the low‐TATI group and 68.7% in the high‐TATI group (P = 0.33); the corresponding 5 year PFS rates were 29.1% and 34.4%, respectively (P = 0.64).
Body composition changes and outcomes
The median interval from post‐treatment CT scans to disease recurrence was 5.4 months (IQR, 0.8–12.4 months) in the SMI‐loss group and 11.2 months (IQR, 6.6–20.5 months) in the SMI‐maintained group (P = 0.01); 7.6 months (IQR, 3.4–14.7 months) in the SMD‐loss group and 10.3 months (IQR, 3.7–18.2 months) in the SMD‐maintained group (P = 0.64); and 10.4 months (IQR, 3.0–18.8 months) in the TATI‐loss group and 9.6 months (IQR, 3.7–15.8 months) in the TATI‐maintained group (P = 0.94).
The 5 year OS rate was 44.4% in the SMI‐loss group and 68.8% in the SMI‐maintained group (P = 0.001); the corresponding 5 year PFS rates were 10.4% and 43.4%, respectively (P < 0.001; Figure 3A). The 5 year OS rate was 49.8% in the SMD‐loss group and 67.2% in the SMD‐maintained group (P = 0.10); the corresponding 5 year PFS rates were 21.8% and 38.5%, respectively (P = 0.02; Figure 3B). There were no significant differences in the OS and PFS rates between the groups based on weight or TATI change (Supporting Information, Figure S1 ). In a subgroup analysis, patients with SMI loss had significantly poorer OS and PFS rates in both the pre‐treatment sarcopenia and non‐sarcopenia groups (Figure 4).
Figure 3.
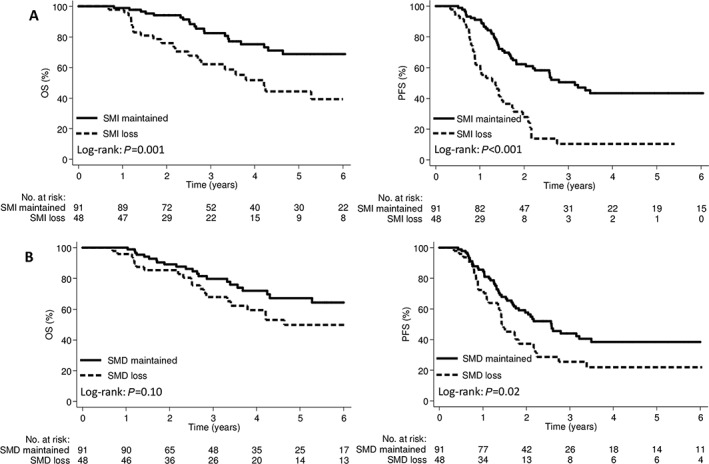
Kaplan–Meier curve demonstrating overall survival and progression‐free survival according to (A) SMI and (B) SMD change groups. OS, overall survival; PFS, progression‐free survival; SMD, skeletal muscle radiodensity; SMI, skeletal muscle index.
Figure 4.
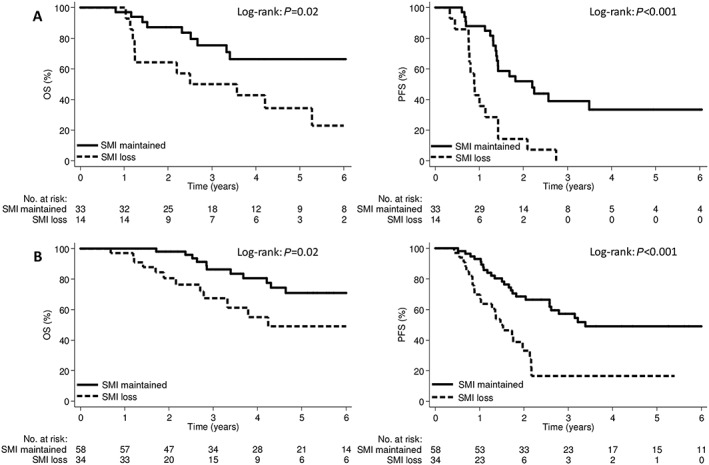
Kaplan–Meier curve demonstrating overall survival and progression‐free survival according to SMI change groups for the pre‐treatment (A) sarcopenia and (B) non‐sarcopenia patients. OS, overall survival; PFS, progression‐free survival; SMI, skeletal muscle index.
On univariable analysis, pre‐treatment SMI and SMD, SMI change, residual disease, and malignant ascites were predictors of OS and PFS (Supporting Information, Table S3). SMD change and FIGO stage were predictors of PFS, but not of OS. On multivariable analysis (Table 5), pre‐treatment SMI and SMI change were independently associated with OS. Pre‐treatment SMI and SMD as well as SMI change were independently associated with PFS. The baseline BMI and TATI and changes in BMI and TATI during treatment were not associated with OS or PFS.
Table 5.
Multivariable Cox proportional hazards model for overall survival and progression‐free survival
| Variable | OS | PFS | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | |
| Age | 1.01 (0.98–1.05) | 0.51 | 1.02 (0.99–1.04) | 0.17 |
| Pre‐treatment SMI (1 cm2/m2 decrease) | 1.08 (1.03–1.11) | 0.002 | 1.03 (1.01–1.06) | 0.04 |
| SMI change (per 1%/180 days decrease) | 1.04 (1.01–1.08) | 0.002 | 1.04 (1.01–1.06) | 0.003 |
| Pre‐treatment SMD (1 HU decrease) | 1.04 (0.99–1.10) | 0.13 | 1.04 (1.01–1.09) | 0.03 |
| SMD change (per 1%/180 days decrease) | — | — | 1.02 (0.99–1.04) | 0.11 |
| FIGO stage | ||||
| IIIA–B | Reference | Reference | ||
| IIIC | 1.47 (0.56–3.85) | 0.43 | 1.23 (0.58–2.63) | 0.59 |
| Residual disease | ||||
| No macroscopic residual disease | Reference | Reference | ||
| Residual disease >0 mm | 1.70 (0.85–3.40) | 0.14 | 1.51 (0.92–2.48) | 0.10 |
| Ascites | ||||
| No ascites or non‐malignant ascites | Reference | Reference | ||
| Malignant ascites | 2.14 (0.93–4.93) | 0.07 | 1.70 (0.98–2.95) | 0.06 |
Bolded P‐values are those significant with a P < 0.05. CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; HU, Hounsfield unit; mGPS, modified Glasgow prognostic score; OS, overall survival; PFS, progression‐free survival; SMD, skeletal muscle radiodensity; SMI, skeletal muscle index.
Discussion
To the best of our knowledge, this is the first study that longitudinally assessed body composition changes by using a standardized phase of CT in patients with stage III EOC undergoing PDS and adjuvant platinum‐based chemotherapy. We found that SMI and SMD decreased during treatment; the changes in SMI and SMD were not correlated with BMI change, suggesting that muscle loss was occult and occurred independent of BMI change. In addition, we found that the mGPS was associated with pre‐treatment sarcopenia and myosteatosis as well as SMI loss during treatment. Patients with SMI loss experienced a significantly shorter time to disease recurrence. SMI at baseline and changes during treatment were independently associated with poor survival outcomes.
Previous studies have reported conflicting results regarding the effect of pre‐treatment sarcopenia in patients with advanced‐stage EOC.11, 12, 13, 14, 15, 16, 17 These differences in the results might be attributed to the muscle changes that may occur during treatment, possibly influencing the outcomes.12 Among patients with advanced‐stage EOC treated with neoadjuvant chemotherapy and interval debulking surgery, SMI loss during neoadjuvant chemotherapy was associated with poorer OS, while low SMI at a specific time point was not.12 In the present study, all patients were treated with PDS and adjuvant platinum‐based chemotherapy.18 We found that SMI at baseline and changes during treatment were independently associated with poorer OS and PFS after adjusting for FIGO stage, PDS outcome, and malignant ascites. Complete resection is the goal of PDS and one of the most important prognostic factors for advanced‐stage EOC.45 The complete resection rate in the current study was 52.5%, similar to the rate obtained in previous large prospective randomized trials.45, 46, 47
It is of interest that the SMI at baseline was independently associated with PFS in this study. Previous studies also showed conflicting results of pre‐treatment sarcopenia on PFS in EOC.11, 13 The possible reason for our findings might be cancer aggressiveness;22 we found that there were marginally more patients with malignant ascites in the pre‐treatment sarcopenia group than in the non‐sarcopenia group. In addition, we found that there were more patients with a higher mGPS in the pre‐treatment sarcopenia group. Systemic inflammation may also play a role in the processes associated with tumour progression 26. Interleukin‐6 plays an important role in the development of ascites as well as the spread of ovarian cancer through its induction of tumour angiogenesis, thus leading to rapid progression and short survival 22, 26, 29. However, the measurement of interleukin‐6 levels is not a part of current routine care for EOC 18; accordingly, interleukin‐6 levels could not be analysed in this retrospective study. Future studies are hence required to validate our findings.
The mechanism of how muscle loss results in poorer survival outcomes is not well understood. A possible explanation might be cancer‐associated muscle loss 21, 22, 23, 24. In the current study, patients with residual disease after PDS or malignant ascites showed a significantly higher decrease in SMI compared with patients without these factors. Moreover, patients with SMI loss had a significantly shorter time to disease recurrence. Pin et al. evaluated the effect of intraperitoneal implantation of ES‐2 human ovarian cancer cells on muscle wasting in an animal model. They implanted ES‐2 cells intraperitoneally to simulate disseminated abdominal disease and malignant ascites formation and found progressive muscle wasting via enhanced muscle protein catabolism, abnormal mitochondrial homoeostasis, and elevated tumour‐derived interleukin‐6 levels.22 Therefore, our clinical findings may be explained by their results in the animal model. However, it is unknown if the body composition is causally related to the OS of patients with advanced‐stage EOC. It is also unknown whether muscle deterioration can be reversed in cancer patients and if this reversal can affect prognosis. In patients with advanced‐stage EOC, the performance status at baseline and at 3 months after adjuvant chemotherapy were associated OS, suggesting the relevance of optimal supportive care during cancer therapy.48 Lifestyle therapy and pharmacotherapy may also offer benefits for the management of body composition in patients with EOC;49, 50, 51, 52 however, their effectiveness for advanced‐stage EOC remains unclear. Hence, future studies are warranted to determine if the effects of body composition on survival outcomes are both causal and, more importantly, reversible through intervention for advanced‐stage EOC.
Systemic inflammation was a risk factor for muscle loss and may be a useful marker of catabolic drive.19, 30 Dolan et al. reported that both pre‐treatment low SMI and SMD were significantly associated with the systemic inflammatory response in patients with colorectal cancer.30 Two previous longitudinal studies revealed that pre‐treatment systemic inflammation was associated with muscle mass loss over time.43, 53 In the current study, we found that an elevated pre‐treatment mGPS was associated with pre‐treatment sarcopenia and myosteatosis, and SMI loss during treatment. However, we found that the SMD loss was not associated with systemic inflammatory markers including the mGPS, NLR, or PLR. SMD is a marker of lipid content in skeletal muscles and could be affected by physiological and pathological variations.54 It is interesting that systemic inflammation may have different effects on SMI and SMD. Although the current study evaluated the changes in SMI and SMD using contrast‐enhanced CT scans with a standardized phase, the study included only a small number of patients, which may not be sufficient to draw a definite conclusion about the relationship between systemic inflammation and SMD loss. In addition, the food intake, lifestyle, or physical function could also confound the relationship between systemic inflammation and skeletal muscle; however, data about these factors were not comprehensively available for analysis given the retrospective nature of this study. It is also unknown whether moderation of the systemic inflammatory response could improve SMI and SMD during treatment.19, 30 Hence, we suggest further longitudinal studies with a larger number of patients to elucidate the relationship between systemic inflammation and skeletal muscle change during cancer therapy.
Sarcopenia has long been associated with ageing and is common in elderly people. Beyond the age of 50 years, loss of leg muscle mass (1–2% per year) and loss of strength (1.5–5% per year) have been reported.55, 56 In the current study, we found that age at diagnosis was not associated with pre‐treatment sarcopenia or SMI loss during treatment. Age at diagnosis was also not associated with survival outcomes on the multivariable Cox model (Table 5). The possible explanation might be that we only evaluated body composition on pre‐treatment and post‐treatment CT images with a median duration of 182 days (IQR: 161–225 days). Data from more number of CT images during a longer follow‐up might reveal the effect of age on muscle loss. However, in the current study, more number of CT images after post‐treatment CT were not available for analysis because CT scans during follow‐up were acquired only when clinically indicated. The small number of patients might also limit the statistical power to detect the effect of age on sarcopenia. Notably, cancer‐associated muscle loss might be the major cause of muscle loss in this study; patients with SMI loss experienced a significantly shorter time to disease recurrence. Although age‐associated muscle loss with time may potentially affect the Cox proportional hazards model, this effect could not be evaluated in the current study due to the short duration of CT images.
We used a standardized phase of contrast‐enhanced CT images to assess body compositions in the current study. Contrast‐enhanced CT images could confound the assessment of SMD, but their influence on the assessment of SMI may be clinically insignificant.33, 34, 35, 36 van Vugt et al. revealed that the mean SMI was significantly lower for the unenhanced phase (42.5 ± 9.9 cm2/m2) than for the arterial (42.8 ± 9.9 cm2/m2; P = 0.021) and portal‐venous (43.6 ± 9.9 cm2/m2; P < 0.001) phases.34 Paris et al. reported that the mean SMI increased in the arterial (54.9 ± 9.6 cm2/m2; P = 0.007) and 3 min phases (55.0 ± 9.6 cm2/m2; P < 0.001) than in the pre‐contrast phase (54.3 ± 9.6 cm2/m2), with no differences between the arterial and 3 min phases.35 Rollins et al. revealed that the overall mean SMI was not significantly different between the different phases of the CT scans.33 Although the difference in SMI measurements may not be clinically relevant, these studies still recommend the evaluation of skeletal muscle parameters with a standardized phase of contrast‐enhanced CT scans because of the influence of contrast on SMD33, 34, 35. Moreover, documentation of the phase of CT analysed would assist in the interpretation of the effects of skeletal muscle on outcomes of patients with cancer.33, 34, 35
The current definition of cachexia is based on weight loss without considering body composition changes;2 however, weight change might not be representative of muscle change.5, 6, 7, 8, 57 Kays et al. reported that CT‐based body composition analysis revealed muscle loss of >5% in 81% of patients, while the traditional definition of >5% weight loss was observed in 56.6%.6 In the current study, 11 patients (7.9%) had weight loss of ≥5% while 48 (34.5%) patients had SMI loss of ≥5%. The BMI change was moderately correlated with TATI change but not with SMI or SMD changes. Overall, the BMI increased during treatment; the BMI increase was mainly attributed to TATI gain while muscle loss occurred. Moreover, weight loss was not associated with poorer survival outcomes while SMI was. Therefore, CT‐based body composition could help define distinct cachexia phenotypes more precisely rather than using weight alone. Nevertheless, these findings should be validated in a large patient cohort.
The current study has several limitations. This study was a retrospective investigation involving a small number of patients, which possibly limited our statistical power to evaluate the relationship among the skeletal muscle parameters, systemic inflammation, and survival. Selection bias and residual and unmeasured confounding are potential limitations of this retrospective study. In addition, this study only analysed pre‐treatment and post‐treatment CT images acquired during routine cancer care. A longitudinal study with CT images of more time points may provide more comprehensive information; however, the CT images during the course of cancer therapy or follow‐up would be acquired only when clinically indicated in real‐world clinical practice.18 Hence, more CT images at different time points could not be analysed in this study. The information regarding food intake, muscle strength (e.g. handgrip strength or chair stand test), or physical performance (e.g. timed‐up‐and‐go test or gait speed) was not available in the present study. The revised definition of sarcopenia by EWGSOP2 indicates low muscle strength as a key characteristic of low muscle quality and requires the presence of low muscle quantity to confirm sarcopenia diagnosis. If a person also has functional impairment, confirmed with a physical performance measure, this is characterized as severe sarcopenia.25, 56 Moreover, our study included only Asian patients. Despite these limitations, the strength of our study is that patients received very similar treatments and consistently underwent pre‐treatment and post‐treatment CT with a standardized protocol. The treatment outcomes were comparable with those obtained in previous studies,11, 12, 13, 14, 15, 16, 46 and the follow‐up period was adequate.
In conclusion, SMI loss occurred in patients with stage III EOC during PDS and adjuvant platinum‐based chemotherapy; the mGPS might be a predictor of SMI loss during treatment. SMI at baseline and changes during treatment were independently associated with poor outcomes. Patients with SMI loss after treatment had a significantly shorter time to recurrence. Moreover, the changes in SMI were not correlated with changes in BMI, suggesting that body composition measurements using CT images acquired for routine cancer care should be incorporated into clinical practice, as they might help predict outcomes and potentially guide cancer therapy. Future studies are required to evaluate the value of cancer therapy and supportive care based on individual body composition phenotypes and systemic inflammatory markers; this would serve to improve the survival outcomes of patients with advanced‐stage EOC.
Conflict of interest
None declared.
Funding
None.
Authors contributions
Chueh‐Yi Huang, Jie Lee, and Chih‐Long Chang contributed to the conception and design of the study. All authors contributed to the acquisition, analysis, or interpretation of data. Ya‐Ting Jan performed imaging data analysis. Chueh‐Yi Huang and Jie Lee drafted the manuscript. All authors performed critical revision of the manuscript for important intellectual content. Jie Lee performed statistical analysis.
Supporting information
Table S1. Patient and tumour characteristics according to pre‐treatment sarcopenia groups (n = 139)
Table S2. Logistic regression of systemic inflammation markers and pre‐treatment low TATI and TATI loss
Table S3. Univariable Cox proportional hazards model for overall survival and progression‐free survival
Figure S1. Kaplan–Meier curve demonstrating overall survival and progression‐free survival according to (A) weight and (B) TATI change groups. OS, overall survival; PFS, progression‐free survival; TATI, total adipose tissue index.
Acknowledgements
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.58
Huang C.‐Y., Yang Y.‐C., Chen T.‐C., Chen J.‐R., Chen Y.‐J., Wu M.‐H., Jan Y.‐T., Chang C.‐L., and Lee J. (2020) Muscle loss during primary debulking surgery and chemotherapy predicts poor survival in advanced‐stage ovarian cancer, Journal of Cachexia, Sarcopenia and Muscle, 11, 534–546. 10.1002/jcsm.12524.
Contributor Information
Chih‐Long Chang, Email: clchang@mmc.edu.tw.
Jie Lee, Email: sinus125125@gmail.com, Email: sinus5706@mmh.org.tw.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 3. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol. 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 4. Daly LE, Ni Bhuachalla EB, Power DG, Cushen SJ, James K, Ryan AM. Loss of skeletal muscle during systemic chemotherapy is prognostic of poor survival in patients with foregut cancer. J Cachexia Sarcopenia Muscle. 2018;9:315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basile D, Parnofiello A, Vitale MG, Cortiula F, Gerratana L, Fanotto V, et al. The IMPACT study: early loss of skeletal muscle mass in advanced pancreatic cancer patients. J Cachexia Sarcopenia Muscle. 2019;10:368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kays JK, Shahda S, Stanley M, Bell TM, O'Neill BH, Kohli MD, et al. Three cachexia phenotypes and the impact of fat‐only loss on survival in FOLFIRINOX therapy for pancreatic cancer. J Cachexia Sarcopenia Muscle. 2018;9:673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Dijk DP, Bakens MJ, Coolsen MM, Rensen SS, van Dam RM, Bours MJ, et al. Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J Cachexia Sarcopenia Muscle. 2017;8:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee J, Lin JB, Wu MH, Jan YT, Chang CL, Huang CY, et al. Muscle radiodensity loss during cancer therapy is predictive for poor survival in advanced endometrial cancer. J Cachexia Sarcopenia Muscle. 2019;10:814–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee J, Chang CL, Lin JB, Wu MH, Sun FJ, Jan YT, et al. Skeletal muscle loss is an imaging biomarker of outcome after definitive chemoradiotherapy for locally advanced cervical cancer. Clin Cancer Res. 2018;24:5028–5036. [DOI] [PubMed] [Google Scholar]
- 10. Brown JC, Caan BJ, Meyerhardt JA, Weltzien E, Xiao J, Cespedes Feliciano EM, et al. The deterioration of muscle mass and radiodensity is prognostic of poor survival in stage I‐III colorectal cancer: a population‐based cohort study (C‐SCANS). J Cachexia Sarcopenia Muscle. 2018;9:664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumar A, Moynagh MR, Multinu F, Cliby WA, McGree ME, Weaver AL, et al. Muscle composition measured by CT scan is a measurable predictor of overall survival in advanced ovarian cancer. Gynecol Oncol. 2016;142:311–316. [DOI] [PubMed] [Google Scholar]
- 12. Rutten IJ, van Dijk DP, Kruitwagen RF, Beets‐Tan RG, Olde Damink SW, van Gorp T. Loss of skeletal muscle during neoadjuvant chemotherapy is related to decreased survival in ovarian cancer patients. J Cachexia Sarcopenia Muscle. 2016;7:458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bronger H, Hederich P, Hapfelmeier A, Metz S, Noel PB, Kiechle M, et al. Sarcopenia in advanced serous ovarian cancer. Int J Gynecol Cancer. 2017;27:223–232. [DOI] [PubMed] [Google Scholar]
- 14. Aust S, Knogler T, Pils D, Obermayr E, Reinthaller A, Zahn L, et al. Skeletal muscle depletion and markers for cancer cachexia are strong prognostic factors in epithelial ovarian cancer. PLoS One. 2015;10:e0140403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rutten IJ, Ubachs J, Kruitwagen RF, van Dijk DP, Beets‐Tan RG, Massuger LF, et al. The influence of sarcopenia on survival and surgical complications in ovarian cancer patients undergoing primary debulking surgery. Eur J Surg Oncol. 2017;43:717–724. [DOI] [PubMed] [Google Scholar]
- 16. Ataseven B, Luengo TG, du Bois A, Waltering KU, Traut A, Heitz F, et al. Skeletal muscle attenuation (sarcopenia) predicts reduced overall survival in patients with advanced epithelial ovarian cancer undergoing primary debulking surgery. Ann Surg Oncol. 2018;25:3372–3379. [DOI] [PubMed] [Google Scholar]
- 17. Ubachs J, Ziemons J, Minis‐Rutten IJG, Kruitwagen R, Kleijnen J, Lambrechts S, et al. Sarcopenia and ovarian cancer survival: a systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle. 2019;10(6):1165–74 10.1002/jcsm.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Comprehensive Cancer Network . Clinical practice guidelines in oncology: Ovarian Cancer [Version 2.2018]. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. .
- 19. Cole CL, Kleckner IR, Jatoi A, Schwarz EM, Dunne RF. The role of systemic inflammation in cancer‐associated muscle wasting and rationale for exercise as a therapeutic intervention. JCSM Clin Rep. 2018;3. [PMC free article] [PubMed] [Google Scholar]
- 20. Greimel E, Kristensen GB, van der Burg ME, Coronado P, Rustin G, del Rio AS, et al. Quality of life of advanced ovarian cancer patients in the randomized phase III study comparing primary debulking surgery versus neo‐adjuvant chemotherapy. Gynecol Oncol. 2013;131:437–444. [DOI] [PubMed] [Google Scholar]
- 21. Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14:58–74. [DOI] [PubMed] [Google Scholar]
- 22. Pin F, Barreto R, Kitase Y, Mitra S, Erne CE, Novinger LJ, et al. Growth of ovarian cancer xenografts causes loss of muscle and bone mass: a new model for the study of cancer cachexia. J Cachexia Sarcopenia Muscle. 2018;9:685–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pin F, Barreto R, Couch ME, Bonetto A, O'Connell TM. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J Cachexia Sarcopenia Muscle. 2019;10:140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brown JL, Lee DE, Rosa‐Caldwell ME, Brown LA, Perry RA, Haynie WS, et al. Protein imbalance in the development of skeletal muscle wasting in tumour‐bearing mice. J Cachexia Sarcopenia Muscle. 2018;9:987–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bauer J, Morley JE, Schols A, Ferrucci L, Cruz‐Jentoft AJ, Dent E, et al. Sarcopenia: a time for action. An SCWD Position Paper. J Cachexia Sarcopenia Muscle. 2019;10:956–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maccio A, Madeddu C. Inflammation and ovarian cancer. Cytokine. 2012;58:133–147. [DOI] [PubMed] [Google Scholar]
- 27. Lin HW, Tu YY, Lin SY, Su WJ, Lin WL, Lin WZ, et al. Risk of ovarian cancer in women with pelvic inflammatory disease: a population‐based study. Lancet Oncol. 2011;12:900–904. [DOI] [PubMed] [Google Scholar]
- 28. Clendenen TV, Lundin E, Zeleniuch‐Jacquotte A, Koenig KL, Berrino F, Lukanova A, et al. Circulating inflammation markers and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lo CW, Chen MW, Hsiao M, Wang S, Chen CA, Hsiao SM, et al. IL‐6 trans‐signaling in formation and progression of malignant ascites in ovarian cancer. Cancer Res. 2011;71:424–434. [DOI] [PubMed] [Google Scholar]
- 30. Dolan RD, Almasaudi AS, Dieu LB, Horgan PG, McSorley ST, McMillan DC. The relationship between computed tomography‐derived body composition, systemic inflammatory response, and survival in patients undergoing surgery for colorectal cancer. J Cachexia Sarcopenia Muscle. 2019;10:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 32. Shen W, Punyanitya M, Wang Z, Gallagher D, St‐Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross‐sectional image. J Appl Physiol (1985) 2004;97:2333–2338. [DOI] [PubMed] [Google Scholar]
- 33. Rollins KE, Javanmard‐Emamghissi H, Awwad A, Macdonald IA, Fearon KCH, Lobo DN. Body composition measurement using computed tomography: does the phase of the scan matter? Nutrition. 2017;41:37–44. [DOI] [PubMed] [Google Scholar]
- 34. van Vugt JLA. Coebergh van den Braak RRJ, Schippers HJW, Veen KM, Levolger S, de Bruin RWF et al. Contrast‐enhancement influences skeletal muscle density, but not skeletal muscle mass, measurements on computed tomography. Clin Nutr. 2018;37:1707–1714. [DOI] [PubMed] [Google Scholar]
- 35. Paris MT, Furberg HF, Petruzella S, Akin O, Hotker AM, Mourtzakis M. Influence of contrast administration on computed tomography‐based analysis of visceral adipose and skeletal muscle tissue in clear cell renal cell carcinoma. JPEN J Parenter Enteral Nutr. 2018;42:1148–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morsbach F, Zhang YH, Nowik P, Martin L, Lindqvist C, Svensson A, et al. Influence of tube potential on CT body composition analysis. Nutrition. 2018;53:9–13. [DOI] [PubMed] [Google Scholar]
- 37. McDonald AM, Swain TA, Mayhew DL, Cardan RA, Baker CB, Harris DM, et al. CT Measures of bone mineral density and muscle mass can be used to predict noncancer death in men with prostate cancer. Radiology. 2017;282:475–483. [DOI] [PubMed] [Google Scholar]
- 38. Wigodski S, Carrasco F, Bunout D, Barrera G, Hirsch S, de la Maza MP. Sarcopenia: the need to establish different cutoff points of fat‐free mass for the Chilean population. Nutrition. 2019;57:217–224. [DOI] [PubMed] [Google Scholar]
- 39. Hilmi M, Jouinot A, Burns R, Pigneur F, Mounier R, Gondin J, et al. Body composition and sarcopenia: the next‐generation of personalized oncology and pharmacology? Pharmacol Ther. 2019;196:135–159. [DOI] [PubMed] [Google Scholar]
- 40. Martin L, Gioulbasanis I, Senesse P, Baracos VE. Cancer‐associated malnutrition and CT‐defined sarcopenia and myosteatosis are endemic in overweight and obese patients. JPEN J Parenter Enteral Nutr. 2019; 10.1002/jpen.1597. [DOI] [PubMed] [Google Scholar]
- 41. Ni Bhuachalla EB, Daly LE, Power DG, Cushen SJ, MacEneaney P, Ryan AM. Computed tomography diagnosed cachexia and sarcopenia in 725 oncology patients: is nutritional screening capturing hidden malnutrition? J Cachexia Sarcopenia Muscle. 2018;9:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dolan RD, McSorley ST, Park JH, Watt DG, Roxburgh CS, Horgan PG, et al. The prognostic value of systemic inflammation in patients undergoing surgery for colon cancer: comparison of composite ratios and cumulative scores. Br J Cancer. 2018;119:40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Malietzis G, Currie AC, Johns N, Fearon KC, Darzi A, Kennedy RH, et al. Skeletal muscle changes after elective colorectal cancer resection: a longitudinal study. Ann Surg Oncol. 2016;23:2539–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McMillan DC. The systemic inflammation‐based Glasgow prognostic score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39:534–540. [DOI] [PubMed] [Google Scholar]
- 45. du Bois A, Reuss A, Pujade‐Lauraine E, Harter P, Ray‐Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO‐OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO). Cancer. 2009;115:1234–1244. [DOI] [PubMed] [Google Scholar]
- 46. Oza AM, Cook AD, Pfisterer J, Embleton A, Ledermann JA, Pujade‐Lauraine E, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16:928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Walker JL, Brady MF, Wenzel L, Fleming GF, Huang HQ, DiSilvestro PA, et al. Randomized trial of intravenous versus intraperitoneal chemotherapy plus bevacizumab in advanced ovarian carcinoma: an NRG Oncology/Gynecologic Oncology Group study. J Clin Oncol. 2019;37:1380–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carey MS, Bacon M, Tu D, Butler L, Bezjak A, Stuart GC. The prognostic effects of performance status and quality of life scores on progression‐free survival and overall survival in advanced ovarian cancer. Gynecol Oncol. 2008;108:100–105. [DOI] [PubMed] [Google Scholar]
- 49. Brown JC, Cespedes Feliciano EM, Caan BJ. The evolution of body composition in oncology‐epidemiology, clinical trials, and the future of patient care: facts and numbers. J Cachexia Sarcopenia Muscle. 2018;9:1200–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Golan T, Geva R, Richards D, Madhusudan S, Lin BK, Wang HT, et al. LY2495655, an antimyostatin antibody, in pancreatic cancer: a randomized, phase 2 trial. J Cachexia Sarcopenia Muscle. 2018;9:871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tao JJ, Cangemi NA, Makker V, Cadoo KA, Liu JF, Rasco DW, et al. First‐in‐human phase I study of the activin A inhibitor, STM 434, in patients with granulosa cell ovarian cancer and other advanced solid tumors. Clin Cancer Res. 2019;25:5458–5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pettersen K, Andersen S, van der Veen A, Nonstad U, Hatakeyama S, Lambert C, et al. Autocrine activin A signalling in ovarian cancer cells regulates secretion of interleukin 6, autophagy, and cachexia. J Cachexia Sarcopenia Muscle. 2019; 10.1002/jcsm.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wallengren O, Iresjo BM, Lundholm K, Bosaeus I. Loss of muscle mass in the end of life in patients with advanced cancer. Support Care Cancer. 2015;23:79–86. [DOI] [PubMed] [Google Scholar]
- 54. Aubrey J, Esfandiari N, Baracos VE, Buteau FA, Frenette J, Putman CT, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf). 2014;210:489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Keller K, Engelhardt M. Strength and muscle mass loss with aging process. Age and strength loss. Muscles Ligaments Tendons J. 2013;3:346–350. [PMC free article] [PubMed] [Google Scholar]
- 56. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee J, Chang CL, Lin JB, Wu MH, Sun FJ, Wu CJ, et al. The effect of body mass index and weight change on late gastrointestinal toxicity in locally advanced cervical cancer treated with intensity‐modulated radiotherapy. Int J Gynecol Cancer. 2018;28:1377–1386. [DOI] [PubMed] [Google Scholar]
- 58. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019.J Cachexia Sarcopenia Muscle 2019. 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient and tumour characteristics according to pre‐treatment sarcopenia groups (n = 139)
Table S2. Logistic regression of systemic inflammation markers and pre‐treatment low TATI and TATI loss
Table S3. Univariable Cox proportional hazards model for overall survival and progression‐free survival
Figure S1. Kaplan–Meier curve demonstrating overall survival and progression‐free survival according to (A) weight and (B) TATI change groups. OS, overall survival; PFS, progression‐free survival; TATI, total adipose tissue index.


