Abstract
Dehydration, electrolyte disturbance, and acid-base imbalance are the most significant consequences of diarrhea in calves. We aimed to determine blood gas, hematological, electrolyte, and biochemical values and investigate the relationship between the physical status and blood parameters in Korean native calves (KNCs) with diarrhea. One hundred eighty KNCs with diarrhea (age < 75 days) were investigated. Blood samples were collected from the external jugular vein and analyzed using a portable clinical blood gas analyzer. The measured parameters were statistically compared according to the status of physical activity, dehydration, or prognosis. The mean values of parameters in the Calves with diarrhea showed metabolic acidosis, hyponatremia, and azotemia. The mean values of potassium, chloride, hematocrit, and hemoglobin were in the upper limit of their reference ranges. More than 75% of the calves had metabolic acidosis caused by bicarbonate loss, and 63.6% had high blood urea nitrogen (BUN) values. Moreover, BUN showed the highest correlation with the physical activity status and dehydration. pH, base excess of the extracellular fluid (BE), anion gap, potassium, hematocrit, bicarbonate, and hemoglobin were closely correlated with physical deterioration and dehydration (p < 0.001). BUN, pH, BE, and anion gap were closely correlated with physical deterioration and dehydration. These correlations between clinical symptoms and blood gas parameters can be clinically relevant in predicting the status of parameters according to clinical symptoms.
Keywords: Anion gap, blood gas analysis, BUN, dehydration, physical activity
INTRODUCTION
Traditionally, calf diarrhea has been a major cause of economic loss to the cattle industry worldwide owing to the cost of treatment, retarded growth, and death of calves [1,2,3]. The 2007 National Animal Health Monitoring System for US Dairy reported that 57% of calf mortality before weaning was due to diarrhea, with most mortality occurring within 1 month from birth [4]. According to a domestic report, 97.6% of calves had diarrhea more than once during the suckling period [5]. A recent Korean study reported that 53.4% of dairy calf deaths before weaning were due to diarrhea [6].
In calves, diarrhea increases the loss of water and electrolytes into the intestine, thereby compromising the compensatory ability of the lower intestine to reabsorb water and electrolytes [5,7,8]. Calves with diarrhea become depressed, lose the suck reflex, and become weak. If the disease progresses, recumbence, coma, and death may occur [2].
Generally, analysis of blood parameters is required for determining the acid-base imbalance of calves with diarrhea. In particular, blood gas parameters may help in treatment planning. No single laboratory test has been completely reliable for the prognosis of diarrhea in calves [9]. Moreover, accurate determination of the health status could only be achieved in a hospital setting. However, these days, the introduction of the point-of-care test in cattle is changing the way various diseases are diagnosed. Hand-held devices, such as the point-of-care analyzer, have allowed patient-side analysis of certain variables in various animal species [10,11]. The advantage of hand-held devices over laboratory analysis is that the results are usually more quickly available to guide treatment. The availability of faster test results expedites diagnosis and treatment initiation, while having a positive impact on the care of critically ill patients. The analytical performance of hand-held devices has been established in previous studies [10,11]. Some equipment are especially useful for predicting the need for fluid therapy based on the test results of calves with diarrhea [12]. Various reports have documented the treatment of calf diarrhea conducted on the basis of a clinicopathological diagnosis [13,14,15]. However, most veterinarians rely on the clinical assessment of calf diarrhea, and only a few reports are available on the determination of blood parameters in Korean native calves (KNCs).
Therefore, the objectives of this study were to determine the changes in blood parameters and to investigate the relationship between the physical status and values of blood parameters by using a portable blood gas analyzer in KNCs with diarrhea.
MATERIALS AND METHODS
Animals
To determine the values of blood parameters by using a portable blood gas analyzer and to investigate the relationship between the physical status and blood parameters in KNC with diarrhea, 180 KNCs with diarrhea aged under 75 days old from 96 farms were investigated between July 2014 and June 2016. The KNCs were selected from the Gangwon and Gyeongbuk areas on the basis of clinical signs of diarrhea. The mean and median ages of the KNCs were 19.78 ± 15.79 days and 15.00 ± 15.79 days, respectively.
Clinical evaluation
To collect clinical data, the patient's history, including farm, breed, sex, age, season, recovery period, and prognosis, were recorded. Physical examination of each patient was also conducted. Their physical activity was classified as bright, dull, dummy, and coma. Bright is a state of normal response to stimuli; dull is a state of sluggishness wherein the animal exhibits relative indifference to normal stimuli; dummy is a pronounced state of indifference in which the animal does not respond at all to external stimuli, but has the ability to stand and move; and coma is a terminal state of apathy or depression in which the animal is unconscious and cannot be roused. Dehydration scores were classified according to the degree of eyeball recession, which was estimated according to the distance between the globe and the palpebral conjunctiva. When the distance was less than 3 mm, dehydration was evaluated as less than 6%; when it was less than 4.5 mm, dehydration was evaluated as less than 8%; when it was less than 6 mm, dehydration was evaluated as less than 10%; when it was less than 7 mm, dehydration was evaluated as less than 12%; and when it was 7 mm or more, it was evaluated as 12% or more [16].
Blood gas analysis
A 0.5-mL sample of blood was collected from the external jugular vein by using a 23 G needle. Blood gas analysis was conducted immediately using an i-STAT Portable Clinical Analyzer (Abbott Point of Care Inc., USA) under farm conditions. This analyzer was chosen for the study because it is more stable than other manufacturers' blood analyzers are at low winter temperatures. The cartridge (i-STAT EC8+) can test the following variables: sodium (Na+, mmol/L), potassium (K+, mmol/L), chloride (Cl−, mmol/L), total carbon dioxide (tCO2, mmol/L), blood urea nitrogen (BUN, mg/dL), glucose (Glu, mg/dL), hematocrit (Hct, %), pH, partial pressure of carbon dioxide (pCO2, mmHg), bicarbonate (HCO3−, mmol/L), base excess of the extracellular fluid (BE, mmol/L), anion gap (AnGap, mmol/L), and hemoglobin (Hb, g/dL).
The reference ranges of all test variables were obtained from a previous study on healthy KNCs [17]. The values outside the test limits determined by the test equipment were calibrated as follows. The value of K+ above 9 mmol/L was corrected to 9 mmol/L; that of Cl− above 140 mmol/L was corrected to 140 mmol/L; that of tCO2 less than 5 mmol/L was corrected to 5 mmol/L; that of BUN above 140 mg/dL was corrected to 140 mg/dL; that of Glu above 700 mg/dL was corrected to 700 mg/dL, while that of Glu less than 20 mg/dL was corrected to 20 mg/dL; and that of BE less than −30 mmol/L was corrected to −30 mmol/L.
Statistical analysis
All results except for proteinuria and survival rate data are expressed as mean ± standard deviation (SD). Calves were categorized into four physical activity groups as bright (depression score 1), dull (depression score 2), dummy (depression score 3), and coma (depression score 4). Dehydration scores were classified according to the percentage of dehydration as score 1 in cases of dehydration less than 6%, score 2 in cases of dehydration less than 8%, score 3 in cases of dehydration less than 10%, and score 4 in cases of dehydration of 10% or more [16]. Blood parameters were compared among groups by using one-way analysis of variance (ANOVA) followed by Tukey's post hoc multiple-comparison tests. Pearson's correlations were used to evaluate the relationships. Correlations were defined as weakly positive (r = 0.1 to 0.3), moderately positive (r = 0.3 to 0.7), strongly positive (r = 0.7 to 1.0), weakly negative (r = −0.1 to −0.3), moderately negative (r = −0.3 to −0.7), or strongly negative (r = −0.7 to −1.0). The p values < 0.05 were considered statistically significant. All statistical analyses were conducted using IBM SPSS Statistics, Version 22.0 (IBM Corp., USA).
RESULTS
Blood parameter values of KNCs with diarrhea
The mean concentration of Na+, K+, Cl−, tCO2, BUN, Glu, Hct, pH, pCO2, HCO3−, BE, AnGap, and Hb are presented in Table 1. The mean values of parameters in the KNCs with diarrhea showed metabolic acidosis, hyponatremia, and azotemia. The mean values of K+, Cl−, Hct, and Hb were in the upper limit of their respective reference ranges.
Table 1. The blood parameters measured in Korean native calves with diarrhea (n = 180).
| Blood values | Number | Mean ± SD (Min–Max) | Reference range |
|---|---|---|---|
| Na+ (mmol/L) | 180 | 132.52 ± 10.56 (104.0–167.0) | 136.5–142.4 |
| K+ (mmol/L) | 180 | 5.55 ± 1.54 (2.0–9.0) | 4.1–5.6 |
| Cl− (mmol/L) | 172 | 103.06 ± 10.96 (75.0–140.0) | 94.5–105.2 |
| tCO2 (mmol/L) | 180 | 19.34 ± 8.49 (5.0–40.0) | 25.3–35.4 |
| BUN (mg/dL) | 176 | 58.99 ± 44.82 (4.0–140.0) | 6.0–30.0 |
| Glu (mg/dL) | 180 | 103.96 ± 66.78 (20.0–700.0) | 95.1–161.0 |
| Hct (%) | 180 | 36.03 ± 7.53 (10.0–59.0) | 24.3–36.6 |
| pH | 180 | 7.19 ± 0.17 (6.6–7.6) | 7.35–7.48 |
| pCO2 (mmHg) | 180 | 43.50 ± 12.64 (18.0–94.9) | 36.9–54.6 |
| HCO3− (mmol/L) | 179 | 18.10 ± 8.29 (3.7–38.3) | 24.0–33.9 |
| BE (mmol/L) | 180 | −10.13 ± 10.68 (−30.0–13.0) | −1.4–10.0 |
| AnGap (mmol/L) | 168 | 16.65 ± 4.61 (7.0–32.0) | 10.7–20.3 |
| Hb (g/dL) | 180 | 12.30 ± 2.65 (3.4–21.6) | 8.3–12.5 |
SD, standard deviation; BE, base excess of the extracellular fluid; tCO2, total carbon dioxide, HCO3−, bicarbonate; Na+, sodium; Glu, glucose; pCO2, partial pressure of carbon dioxide; Cl−, chloride; K+, potassium; Hct, hematocrit; Hb, hemoglobin; AnGap, anion gap; BUN, blood urea nitrogen.
Distribution of KNCs with diarrhea according to the levels of blood parameters
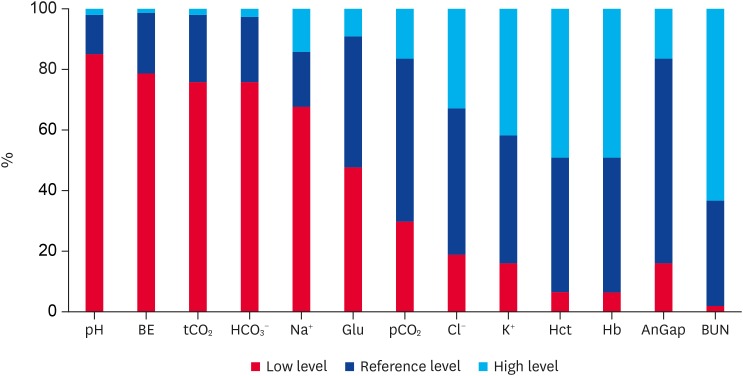
The distribution of the 180 KNCs with diarrhea according to the levels of blood parameters (i.e., within reference range, low, or high) is shown in Fig. 1. Of the 180 KNCs, 153 (85.0%) had a low pH (< 7.35), 23 (12.8%) had pH in the reference range, and 4 (2.2%) had a high pH (> 7.48). Of the 180 KNCs, 141 (78.3%) had a low BE (< −1.4 mmol/L), 36 (20.0%) had BE in the reference range, and 3 (1.7%) had a high BE (> 10.0 mmol/L). Of the 180 KNCs, 136 (75.6%) had a low tCO2 (< 25.3 mmol/L), 40 (22.2%) had tCO2 in the reference range, and 4 (2.2%) had a high tCO2 (> 35.4 mmol/L). Of the 179 KNCs, 135 (75.4%) had a low HCO3− level (< 24.0 mmol/L), 39 (21.8%) had HCO3− levels in the reference range, and 5 (2.8%) had a high HCO3− level (> 33.9 mmol/L). Hyponatremia (< 136.5 mmol/L) was present in 121 (67.2%) of the 180 KNCs, normonatremia in 33 (18.3%), and hypernatremia (> 142.4 mmol/L) in 26 (14.4%). Hypoglycemia (< 95.1 mg/dL) was present in 85 (47.2%) of the 180 KNCs, normoglycemia in 78 (43.3%), and hyperglycemia (> 161.0 mg/dL) in 17 (9.5%). Hypocapnia (< 36.9 mmHg) was present in 53 (29.4%) of the 180 KNCs, normocapnia in 97 (53.9%), and hypercapnia (> 54.6 mmHg) in 30 (16.7%). Hypochloremia (< 94.5 mmol/L) was present in 32 (18.6%) of 172 KNCs, normochloremia in 83 (48.3%), and hyperchloremia (> 105.2 mmol/L) in 57 (33.1%). Hypokalemia (< 4.1 mmol/L) was present in 27 (15%) of the 180 KNCs, normokalemia in 77 (42.8%), and hyperkalemia (> 5.6 mmol/L) in 76 (42.2%). Hct and Hb levels showed the same pattern, which was a good indicator of water balance. Of the 180 KNCs, 11 (6.1%) had low Hct (< 24.3%) and Hb (< 8.3 g/dL) levels, 80 (44.4%) had Hct and Hb levels in the reference range, and 89 (49.4%) had high Hct (> 36.6%) and Hb (> 12.5 g/dL) levels. Of 168 KNCs, 9 (5.4%) had a low AnGap level (< 10.7 mmol/L), 131 (78%) had AnGap levels in the reference range, and 28 (16.7%) had a high AnGap level (> 20.3 mmol/L). Of 176 KNCs, 3 (1.7%) had a low BUN level (< 6.0 mg/dL), 61 (34.7%) had BUN levels in the reference range, and 112 (63.6%) had had high BUN values (> 30.0 mg/dL); More than 75% of the KNCs with diarrhea had metabolic acidosis caused by the loss of HCO3−, and 63.6% had azotemia.
Fig. 1. Distribution of Korean native calves with diarrhea according to the levels of blood parameters (reference range, low, and high) (n = 180). BE, base excess of carbon dioxide; tCO2, total carbon dioxide; HCO3−, bicarbonate; Na+, sodium; Glu, glucose; pCO2, partial pressure of carbon dioxide; Cl−, chloride; K+, potassium; Hct, hematocrit; Hb, hemoglobin; AnGap, anion gap; BUN, blood urea nitrogen.
Relationship between the physical activity status and blood parameter values
To assess the relationship between the physical activity status and blood parameter values of the KNCs with diarrhea, 180 calves were classified into four groups according to their physical activity status (Table 2), with 15, 67, 58, and 40 calves being grouped into the bright, dull, dummy, and coma categories, respectively. The values of pH, tCO2, HCO3 −, and BE differed significantly according to the deterioration of the physical activity status (ANOVA, p < 0.001), and these values showed a moderate negative correlation with physical deterioration (Pearson, p < 0.001, Table 3). The values of K+, BUN, Hct, AnGap, and Hb also differed significantly according to the deterioration of the physical activity status (p < 0.001, Table 2), and these values showed a moderate positive correlation with physical deterioration (Table 3). The concentrations of Cl− also differed significantly according to the deterioration of the physical activity status, but they showed only a weak positive correlation with physical deterioration. The concentrations of Na+ remained unchanged regardless of the physical activity status. The concentrations of Glu and pCO2 decreased gradually according to the deterioration of the physical activity status, but it was not statistically significant.
Table 2. Changes in blood parameter values according to the physical activity status (n = 180).
| Blood values | Bright (n = 15) | Dull (n = 67) | Dummy (n = 58) | Coma (n = 40) | F-value | p value |
|---|---|---|---|---|---|---|
| Na+ (mmol/L) | 133.13 ± 4.76 | 131.06 ± 8.25 | 133.45 ± 13.16 | 133.38 ± 11.37 | 0.678 | 0.567 |
| K+ (mmol/L) | 4.81 ± 0.86 | 5.13 ± 0.96 | 5.64 ± 1.65 | 6.41 ± 1.94 | 7.847 | < 0.001 |
| Cl− (mmol/L) | 100.40 ± 3.00 | 100.20 ± 7.79 | 105.22 ± 13.09 | 106.11 ± 13.08 | 3.606 | 0.015 |
| tCO2 (mmol/L) | 22.93 ± 4.22 | 22.10 ± 7.15 | 18.31 ± 8.90 | 14.85 ± 9.05 | 8.140 | < 0.001 |
| BUN (mg/dL) | 18.53 ± 11.90 | 41.02 ± 33.63 | 69.38 ± 45.74 | 90.05 ± 43.35 | 19.596 | < 0.001 |
| Glu (mg/dL) | 130.27 ± 51.85 | 101.18 ± 25.94 | 104.09 ± 64.45 | 98.58 ± 109.49 | 0.900 | 0.443 |
| Hct (%) | 31.20 ± 6.45 | 34.43 ± 6.44 | 36.26 ± 7.15 | 40.18 ± 8.32 | 7.953 | < 0.001 |
| pH | 7.25 ± 0.10 | 7.26 ± 0.12 | 7.20 ± 0.16 | 7.05 ± 0.19 | 17.276 | < 0.001 |
| pCO2 (mmHg) | 48.96 ± 9.59 | 44.54 ± 9.36 | 40.11 ± 11.18 | 44.63 ± 18.37 | 2.653 | 0.050 |
| HCO3− (mmol/L) | 21.56 ± 4.15 | 20.80 ± 7.00 | 17.08 ± 8.68 | 13.67 ± 8.85 | 8.098 | < 0.001 |
| BE (mmol/L) | −5.67 ± 5.45 | −6.30 ± 8.68 | −11.00 ± 10.99 | −16.95 ± 11.29 | 10.841 | < 0.001 |
| AnGap (mmol/L) | 16.07 ± 2.66 | 14.97 ± 3.35 | 17.29 ± 4.78 | 19.68 ± 4.79 | 10.341 | < 0.001 |
| Hb (g/dL) | 10.61 ± 2.19 | 11.70 ± 2.19 | 12.33 ± 2.43 | 13.89 ± 3.10 | 8.957 | < 0.001 |
Bright is a state of normal response to stimuli; dull is a state of sluggishness wherein the animal exhibits relative indifference to normal stimuli; dummy is a pronounced state of indifference in which the animal does not respond at all to external stimuli, but has the ability to stand and move; and coma is a terminal state of apathy or depression, in which the animal is unconscious and cannot be roused. Blood parameters were compared among groups by using one-way analysis of variance.
BE, base excess of the extracellular fluid; tCO2, total carbon dioxide, HCO3−, bicarbonate; Na+, sodium; Glu, glucose; pCO2, partial pressure of carbon dioxide; Cl−, chloride; K+, potassium; Hct, hematocrit; Hb, hemoglobin; AnGap, anion gap; BUN, blood urea nitrogen.
Table 3. Coefficients of correlation between the clinical and laboratory parameters.
| Blood values | Physical activity | Dehydration | ||
|---|---|---|---|---|
| Pearson, r | p value | Pearson, r | p value | |
| Na+ (mmol/L) | 0.066 | 0.380 | −0.052 | 0.491 |
| K+ (mmol/L) | 0.338 | < 0.001 | 0.519 | < 0.001 |
| Cl− (mmol/L) | 0.223 | 0.003 | 0.093 | 0.225 |
| tCO2 (mmol/L) | −0.341 | < 0.001 | −0.285 | < 0.001 |
| BUN (mg/dL) | 0.503 | < 0.001 | 0.571 | < 0.001 |
| Glu (mg/dL) | − 0.075 | 0.317 | −0.099 | 0.187 |
| Hct (%) | 0.340 | < 0.001 | 0.442 | < 0.001 |
| pH | −0.434 | < 0.001 | −0.438 | < 0.001 |
| pCO2 (mmHg) | −0.091 | 0.223 | 0.115 | 0.124 |
| HCO3− (mmol/L) | −0.340 | < 0.001 | −0.291 | < 0.001 |
| BE (mmol/L) | −0.380 | < 0.001 | −0.343 | < 0.001 |
| AnGap (mmol/L) | 0.355 | < 0.001 | 0.342 | < 0.001 |
| Hb (g/dL) | 0.355 | < 0.001 | 0.425 | < 0.001 |
Physical activity was classified into bright, dull, dummy, and coma. Dehydration scores were classified according to the percentage of dehydration as score 1 in cases of less than 6%, score 2 in cases of less than 8%, score 3 in cases of less than 10%, and score 4 in cases of 10% or more. Pearson's correlations were used to evaluate the relationships. Correlations were defined as weakly positive (r = 0.1 to 0.3), moderately positive (r = 0.3 to 0.7), strongly positive (r = 0.7 to 1.0), weakly negative (r = −0.1 to −0.3), moderately negative (r = −0.3 to −0.7), or strongly negative (r = −0.7 to −1.0).
BE, base excess of the extracellular fluid; tCO2, total carbon dioxide; HCO3−, bicarbonate; Na+, sodium; Glu, glucose; pCO2, partial pressure of carbon dioxide; Cl−, chloride; K+, potassium; Hct, hematocrit; Hb, hemoglobin; AnGap, anion gap; BUN, blood urea nitrogen.
BUN showed the highest correlation with the change in the physical activity status, and pH, BE, and AnGap were closely correlated with physical deterioration. Changes in blood parameter values due to the deterioration of physical activity were identified, and the values of HCO3−, BE, AnGap, K+, Cl−, Hct, and Hb could be useful in predicting the need for fluid therapy for correcting dehydration as well as electrolyte and acid-base imbalance in KNCs with diarrhea. In particular, the dose of HCO3− for correcting metabolic acidosis could be estimated on the basis of the measured BE.
Relationship between the dehydration status and blood parameter values
To assess the relationship between the dehydration status and blood parameter values, the 180 KNCs were classified in four groups according to the dehydration score (Table 4), with 65, 58, 37, and 20 calves being categorized into the score 1, score 2, score 3, and score 4 groups, respectively. The values of pH, tCO2, HCO3−, and BE differed significantly according to the deterioration of dehydration (p < 0.001, Table 4), and these values showed a moderate negative correlation with dehydration (Table 3). The values of K+, BUN, Hct, AnGap, pCO2, and Hb also differed significantly according to the deterioration of dehydration (p < 0.001), and these values showed a moderate positive correlation with dehydration (Table 3). The concentrations of Na+, Cl−, and Glu in each dehydration score group were not statistically significant.
Table 4. Evaluation of blood parameter values according to the dehydration status (n = 180).
| Blood values | Score 1 (4–6%) (n = 65) | Score 2 (6–8%) (n = 58) | Score 3 (8–10%) (n = 37) | Score 4 (10–12%) (n = 20) | F-value | p value |
|---|---|---|---|---|---|---|
| Na+ (mmol/L) | 132.75 ± 8.51 | 132.16 ± 11.64 | 135.05 ± 11.75 | 128.10 ± 10.21 | 1.943 | 0.124 |
| K+ (mmol/L) | 4.92 ± 0.86 | 5.14 ± 1.54 | 6.32 ± 1.53 | 7.41 ± 1.28 | 25.024 | < 0.001 |
| Cl− (mmol/L) | 100.92 ± 7.98 | 104.26 ± 12.80 | 106.06 ± 12.33 | 101.11 ± 10.23 | 2.155 | 0.095 |
| tCO2 (mmol/L) | 22.92 ± 7.16 | 17.35 ± 8.82 | 18.49 ± 8.96 | 15.05 ± 6.65 | 7.480 | < 0.001 |
| BUN (mg/dL) | 31.48 ± 31.79 | 60.16 ± 39.83 | 76.19 ± 42.75 | 112.75 ± 32.18 | 28.586 | < 0.001 |
| Glu (mg/dL) | 105.52 ± 36.69 | 115.41 ± 98.00 | 91.95 ± 49.13 | 87.90 ± 56.02 | 1.374 | 0.252 |
| Hct (%) | 33.08 ± 5.74 | 34.66 ± 7.77 | 39.68 ± 6.19 | 42.85 ± 7.67 | 15.300 | < 0.001 |
| pH | 7.27 ± 0.11 | 7.19 ± 0.18 | 7.14 ± 0.15 | 7.03 ± 0.18 | 14.373 | < 0.001 |
| pCO2 (mmHg) | 45.05 ± 10.28 | 37.94 ± 10.57 | 45.68 ± 12.62 | 50.55 ± 18.66 | 7.182 | < 0.001 |
| HCO3− (mmol/L) | 21.63 ± 7.02 | 16.18 ± 8.60 | 17.16 ± 8.63 | 13.76 ± 6.56 | 7.613 | < 0.001 |
| BE (mmol/L) | −5.26 ± 8.62 | −12.07 ± 11.29 | −11.84 ± 10.81 | −17.15 ± 8.45 | 9.518 | < 0.001 |
| AnGap (mmol/L) | 15.11 ± 4.02 | 16.92 ± 4.28 | 17.92 ± 4.43 | 19.78 ± 4.17 | 7.314 | < 0.001 |
| Hb (g/dL) | 11.25 ± 1.95 | 11.94 ± 2.94 | 13.49 ± 2.11 | 14.57 ± 2.61 | 13.443 | < 0.001 |
Dehydration scores were classified according to the percentage of dehydration as score 1 in cases of less than 6%, score 2 in cases of less than 8%, score 3 in cases of less than 10%, and score 4 in cases of 10% or more. Blood parameters were compared among groups by using one-way analysis of variance followed by Tukey's post hoc multiple-comparison tests.
BE, base excess of the extracellular fluid; tCO2, total carbon dioxide; HCO3−, bicarbonate; Na+, sodium; Glu, glucose; pCO2, partial pressure of carbon dioxide; Cl−, chloride; K+, potassium; Hct, hematocrit; Hb, hemoglobin; AnGap, anion gap; BUN, blood urea nitrogen.
BUN showed the highest correlation with the dehydration status. Moreover, K+, Hct, pH, and Hb were closely correlated with the dehydration status.
Relationship between blood parameter values and prognosis
To determine the difference in blood parameter values between the surviving and dead calves, KNCs with diarrhea were classified in to two groups, survival and death, and their blood parameters were compared. The values of BUN and AnGap were significantly higher in the death group than in the survival group (p < 0.001, Table 5). Thus, the values of BUN and AnGap can be good predictive indicators of survival.
Table 5. Blood parameters of Korean native calves with diarrhea classified into the survival and death groups.
| Blood values | Prognosis | p value | |||
|---|---|---|---|---|---|
| Survival | Death | ||||
| Mean ± SD | No. | Mean ± SD | No. | ||
| Na+ (mmol/L) | 132.05 ± 9.09 | 100 | 130.63 ± 14.03 | 41 | 0.553 |
| K+ (mmol/L) | 5.62 ± 1.53 | 100 | 5.88 ± 1.72 | 41 | 0.381 |
| Cl− (mmol/L) | 104.11 ± 10.17 | 97 | 103.50 ± 14.05 | 36 | 0.812 |
| tCO2 (mmol/L) | 17.42 ± 7.06 | 100 | 16.00 ± 6.55 | 41 | 0.270 |
| BUN (mg/dL) | 54.99 ± 42.88 | 99 | 93.08 ± 46.88 | 38 | < 0.001 |
| Glu (mg/dL) | 100.13 ± 32.89 | 100 | 124.34 ± 120.91 | 41 | 0.213 |
| Hct (%) | 36.11 ± 7.45 | 100 | 35.08 ± 8.84 | 41 | 0.835 |
| pH | 7.16 ± .15 | 100 | 7.12 ± .17 | 41 | 0.188 |
| pCO2 (mmHg) | 42.30 ± 12.18 | 100 | 42.71 ± 14.16 | 41 | 0.864 |
| HCO3− (mmol/L) | 16.18 ± 6.83 | 100 | 14.81 ± 6.39 | 40 | 0.277 |
| BE (mmol/L) | −12.52 ± 8.95 | 100 | −14.51 ± 8.52 | 41 | 0.226 |
| AnGap (mmol/L) | 16.90 ± 4.12 | 93 | 19.03 ± 5.05 | 35 | 0.016 |
| Hb (g/dL) | 12.37 ± 2.70 | 100 | 12.17 ± 3.01 | 41 | 0.705 |
Blood parameters were compared between two prognosis groups (survival vs death) by using Student's t-tests.
SD, standard deviation; BE, base excess of the extracellular fluid; tCO2, total carbon dioxide; HCO3−, bicarbonate; Na+, sodium; Glu, glucose; pCO2, partial pressure of carbon dioxide; Cl−, chloride; K+, potassium; Hct, hematocrit; Hb, hemoglobin; AnGap, anion gap; BUN, blood urea nitrogen.
DISCUSSION
Electrolyte disturbance, dehydration, and metabolic acidosis are the most significant consequences of diarrhea in calves [13]. In the clinical setting, accurate diagnosis of calf diarrhea is made through comprehensive clinical and laboratory tests, which are important for treatment and establishment of management measures for diarrhea. However, in large animal practice, performing laboratory tests on the farm is difficult. Therefore, it is necessary to develop a tool that can predict the degree of acid-base and electrolyte imbalance by evaluating the clinical symptoms without laboratory measurements. The objectives of this study were to determine blood gas, electrolyte, hematological, and biochemical values and to investigate the relationship between the physical status and values of blood parameters in KNCs with diarrhea.
Generally, calves with diarrhea show low blood pH, BE, and HCO3−. Constable et al. [18] reported the value of pH: 7.08 ± 0.12 and HCO3−: 13.7 ± 4.2 mmol/L in calves with acute diarrhea. Omole et al. [1] reported the mean values of pH: 7.17 ± 0.14, HCO3−: 18.0 ± 8.8 mmol/L, and BE: −10.3 ± 10.0 mmol/L. Sayer et al. [12] also reported the value of pH: 7.26, HCO3−: 20.3 mmol/L, and BE: −4.9 mmol/L in calves with diarrhea. In this study, the blood values of 180 KNCs with diarrhea aged less than 75 days old were as follows: pH: 7.19 ± 0.17, HCO3−: 18.10 ± 8.29 mmol/L, BE: −10.13 ± 10.68 mmol/L, and pCO2: 43.50 ± 12.64 mmHg. Thus, our results coincided with those of previous reports.
The low blood pH value indicates acidemia in calves with diarrhea [18]. Metabolic acidosis in these calves was originally attributed to intestinal HCO3− loss, as well as the presence of organic acids in plasma and a decrease in the glomerular filtration rate in response to severe dehydration [19]. The results obtained in our study were consistent with those of previous reports [11,20,21]. In this study, metabolic acidosis was detected in 153 of the 180 KNCs with diarrhea. However, 13 calves were classified as having respiratory acidosis, 5 as having respiratory alkalosis, and 3 as having metabolic alkalosis. Therefore, laboratory tests are needed to accurately diagnose the various acid-base imbalances in such calves [18,22].
Serum electrolyte concentrations in calves with diarrhea depend on the initial cause and severity of disease. In most cases of acute diarrhea, hyponatremia is usually present. The most common cause of hyponatremia is acute diarrhea caused by the loss of Na+ via secretion through the intestine [18]. In this study, the mean Na+ concentration showed hyponatremia (132.52 ± 10.56 mmol/L; reference range: 136.5 to 142.4 mmol/L) in the examined calves. Moreover, hyponatremia was observed in 67.2% of the calves, normonatremia in 18.3%, and hypernatremia in 14.4%. Other researchers reported various statuses of blood Na+ concentration in calves with diarrhea [1,12,18].
Calves with diarrhea may be hyperkalemic, normokalemic, or hypokalemic despite whole-body potassium depletion secondary to increased fecal loss; the serum levels of K+ are initially low, but as acidosis develops and becomes severe, hyperkalemia may occur [18,23]. Poor renal perfusion also limits the ability of the kidneys to correct hyperkalemia. Blood K+ and Na+ concentrations during metabolic acidosis could increase because of the cellular exchange of these ions with hydrogen ions entering the cells [19,24]. The mean concentration of K+ in this study was in the upper limit (5.55 ± 1.54 mmol/L) of the reference range. Moreover, hypokalemia was observed in 15.0% of the calves, normokalemia in 42.8%, and hyperkalemia in 42.2%. Other studies reported that the concentrations of K+ were 4.89 mmol/L [12], 5.4 ± 2.1 mmol/L [1], and 7.4 ± 1.6 mmol/L in calves with diarrhea [18].
The serum level of Cl− may be normal or subnormal in acute diarrhea. Other studies reported that the concentrations of Cl− were 101.3 mmol/L [12], 105.8 ± 10.9 mmol/L [1], and 101.4 ± 7.5 mmol/L in calves with diarrhea [18]. In this study, the concentration of Cl− was 103.06 ± 10.96 mmol/L. Moreover, hypochloremia was observed in 18.6% of the calves, normochloremia in 48.3%, and hyperchloremia in 33.1%.
Dehydration is defined as a loss of body water, and hence, it is an important clinical sign of diarrhea. The Hct (packed cell volume), concentration of Hb, and total plasma protein are reliable guides of dehydration. In this study, the calves with diarrhea showed mean Hct and mean Hb concentration that were in the upper limits of the respective reference ranges. Interestingly, these calves had very high levels of BUN, and the values for BUN, Hct, and Hb showed moderate positive correlations with the deterioration of dehydration (p < 0.001). BUN showed the highest correlation with the dehydration status. Fayet and Overwater [9] and Constable et al. [18] also reported abnormally high levels of BUN in calves with diarrhea.
The AnGap can be an extremely helpful tool for categorizing causal factors in acid-base imbalances and may prove a useful prognostic guide in animals with severe calf diarrhea. The AnGap normally is 12 to 16 and provides an approximation of the so-called unmeasured anions such as lactate, ketones, plasma proteins, phosphate, sulfate, and inorganic anions. In this study, the mean AnGap was 16.65 ± 4.61 mmol/L; however, 16.7% of the calves showed an abnormally high level of AnGap.
In this study, hypoglycemia was observed in 47.2% and hyperglycemia in 9.5% of the calves with diarrhea. The mortality of such calves was higher in both the hypoglycemia (40.6%) and hyperglycemia (70%) groups. In particular, a high mortality rate of 66.7% was observed in case of severe hypoglycemia (Glu ≤ 40 mg/dL). Trefz et al. [25] reported that severe hypoglycemia was associated with a poor survival rate of 20.6% vs. 74.0% in calves with initial normoglycemia.
In this study, the expected pCO2 was 35.0 ± 12.57 mmol/L (Winter's formula: expected pCO2 = HCO3 × 1.5 + 8), but the actual measured value was 43.50 ± 12.64 mmol/L in calves with metabolic acidosis (p < 0.001). In general, metabolic acidosis may be a compensatory decrease in pCO2 due to hyperventilation. However, since calves with severe acidosis have already lost much respiratory function, hypoventilation does not serve a compensatory function [18].
We also investigated the relationship between the physical activity status and blood parameter values of calves with diarrhea. The values of pH, tCO2, HCO3−, and BE differed significantly according to the deterioration of the physical activity status, and these values showed a moderate negative correlation with physical deterioration. Further, the values for K+, BUN, Hct, AnGap, and Hb differed significantly according to the deterioration of the physical activity status, and these values showed a moderate positive correlation with physical deterioration. BUN showed the highest correlation with the change in the physical activity status, and pH, BE, and AnGap were closely correlated with physical deterioration.
The relationship between the dehydration status and blood parameter values of calves with diarrhea was also evaluated. The values for pH, tCO2, HCO3−, and BE differed significantly according to the deterioration of dehydration, and these values showed a moderate negative correlation with dehydration. The values for K+, BUN, Hct, AnGap, pCO2, and Hb also differed significantly according to the deterioration of dehydration, and these values showed a moderate positive correlation with dehydration. BUN showed the highest correlation with the dehydration status. K+, Hct, pH, and Hb were also closely correlated with the dehydration status.
This study revealed correlations between clinical symptoms and blood gas measurements, which can be used in the clinical field because these can help predict the status of each measurement according to the clinical symptoms. Blood gas analysis is a very useful tool in clinical practice, especially when combined with history taking and physical examination. However, since the values of blood parameters according to changes in the physical status can be predicted, these values can be applied for the clinical treatment of calves with diarrhea without necessarily conducting laboratory tests.
In conclusion, i) hand-held devices have made it possible to quickly and accurately assess and treat calf diarrhea; ii) BUN and AnGap can be used as useful indicators for predicting death; and iii) if hand-held devices are not available, clinical symptoms can be used in the clinical field because they can predict the status of each measurement according to the clinical symptoms.
Footnotes
Other: This study is a part of PhD thesis of Sung-Hwan Lee.
Funding: This work was supported by the Institute of Veterinary Science, Kangwon National University, Korea.
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Kim D.
- Data curation: Lee SH.
- Formal analysis: Choi EW, Kim D.
- Investigation: Lee SH
- Writing - original draft: Lee SH.
- Writing - review & editing: Lee SH, Choi EW, Kim D.
References
- 1.Omole OO, Nappert G, Naylor JM, Zello GA. Both L- and D-lactate contribute to metabolic acidosis in diarrheic calves. J Nutr. 2001;131:2128–2131. doi: 10.1093/jn/131.8.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunn AA, Naylor JA, House JK. Diarrhea. In: Smith BP, editor. Large Animal Internal Medicine. 4td ed. St Louis: Mosby; 2009. pp. 340–363. [Google Scholar]
- 3.Torsein M, Lindberg A, Sandgren CH, Waller KP, Törnquist M, Svensson C. Risk factors for calf mortality in large Swedish dairy herds. Prev Vet Med. 2011;99:136–147. doi: 10.1016/j.prevetmed.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleul U, Götz E. Evaluation of the i-STAT portable point-of-care analyzer for determination of blood gases and acid-base status in newborn calves. J Vet Emerg Crit Care (San Antonio) 2014;24:519–528. doi: 10.1111/vec.12228. [DOI] [PubMed] [Google Scholar]
- 5.Kim D, Lyoo YS, Lyoo HS, Yoon CK. Etiology and clinical aspects of diarrhea of Korean native calves during the suckling period. Korean J Vet Res. 1990;30:255–260. [Google Scholar]
- 6.Hur TY, Jung YH, Choe CY, Cho YI, Kang SI, Lee HJ, Ki KS, Baek KS, Suh GH. The dairy calf mortality: the causes of calf death during ten years at a large dairy farm in Korea. Korean J Vet Res. 2013;53:103–108. [Google Scholar]
- 7.Trefz FM, Lorch A, Feist M, Sauter-Louis C, Lorenz I. Metabolic acidosis in neonatal calf diarrhea-clinical findings and theoretical assessment of a simple treatment protocol. J Vet Intern Med. 2012;26:162–170. doi: 10.1111/j.1939-1676.2011.00848.x. [DOI] [PubMed] [Google Scholar]
- 8.Lim KG, Kang MI, Kim SK, Nam KW, Park HJ, Park JR, Cho KO, Lee BJ. Identification and characterization of Shiga toxin-producing Escherichia coli isolated from diarrhea in calves. Korean J Vet Res. 2006;46:135–142. [Google Scholar]
- 9.Fayet JC, Overwater J. Prognosis of diarrhoea in the newborn calf: statistical analysis of blood biochemical data. Ann Rech Vet. 1978;9:55–61. [PubMed] [Google Scholar]
- 10.Bardell D, West E, Mark Senior J. Evaluation of a new handheld point-of-care blood gas analyser using 100 equine blood samples. Vet Anaesth Analg. 2017;44:77–85. doi: 10.1111/vaa.12392. [DOI] [PubMed] [Google Scholar]
- 11.Iwabuchi S, Suzuki K, Abe I, Asano R. Comparison of the effects of isotonic and hypertonic sodium bicarbonate solutions on acidemic calves experimentally induced by ammonium chloride administration. J Vet Med Sci. 2003;65:1369–1371. doi: 10.1292/jvms.65.1369. [DOI] [PubMed] [Google Scholar]
- 12.Sayers RG, Kennedy A, Krump L, Sayers GP, Kennedy E. An observational study using blood gas analysis to assess neonatal calf diarrhea and subsequent recovery with a European Commission-compliant oral electrolyte solution. J Dairy Sci. 2016;99:4647–4655. doi: 10.3168/jds.2015-10600. [DOI] [PubMed] [Google Scholar]
- 13.Naylor JM. Severity and nature of acidosis in diarrheic calves over and under one week of age. Can Vet J. 1987;28:168–173. [PMC free article] [PubMed] [Google Scholar]
- 14.Peiró JR, Borges AS, Gonçalves RC, Mendes LC. Evaluation of a portable clinical analyzer for the determination of blood gas partial pressures, electrolyte concentrations, and hematocrit in venous blood samples collected from cattle, horses, and sheep. Am J Vet Res. 2010;71:515–521. doi: 10.2460/ajvr.71.5.515. [DOI] [PubMed] [Google Scholar]
- 15.Izzo MM, Kirkland PD, Mohler VL, Perkins NR, Gunn AA, House JK. Prevalence of major enteric pathogens in Australian dairy calves with diarrhoea. Aust Vet J. 2011;89:167–173. doi: 10.1111/j.1751-0813.2011.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Constable PD, Walker PG, Morin DE, Foreman JH. Clinical and laboratory assessment of hydration status of neonatal calves with diarrhea. J Am Vet Med Assoc. 1998;212:991–996. [PubMed] [Google Scholar]
- 17.Lee S, Ok S, Kwon H, Kim D. Arterial and venous blood gas, electrolytes, biochemical and hematological values in healthy Korean native calves. J Vet Clin. 2015;32:499–503. [Google Scholar]
- 18.Constable PD, Hinchcliff KW, Done SH, Grünberg W. Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs, and Goats. 11th ed. St Louis: Elsevier; 2017. pp. 384–409. [Google Scholar]
- 19.Klein P, Kleinová T, Volek Z, Simůnek J. Effect of Cryptosporidium parvum infection on the absorptive capacity and paracellular permeability of the small intestine in neonatal calves. Vet Parasitol. 2008;152:53–59. doi: 10.1016/j.vetpar.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naylor JM. A retrospective study of the relationship between clinical signs and severity of acidosis in diarrheic calves. Can Vet J. 1989;30:577–580. [PMC free article] [PubMed] [Google Scholar]
- 21.Aldridge BM, Garry FB, Adams R. Neonatal septicemia in calves: 25 cases (1985-1990) J Am Vet Med Assoc. 1993;203:1324–1329. [PubMed] [Google Scholar]
- 22.Abutarbush SM, Petrie L. Treatment of hypernatremia in neonatal calves with diarrhea. Can Vet J. 2007;48:184–187. [PMC free article] [PubMed] [Google Scholar]
- 23.Constable PD, Grünberg W. Hyperkalemia in diarrheic calves: implications for diagnosis and treatment. Vet J. 2013;195:271–272. doi: 10.1016/j.tvjl.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Hendrix CM, Sirois M. Laboratory Procedures for Veterinary Technicians. 5th ed. Kansas: Mosby, St. Louis; 2007. p. 416. [Google Scholar]
- 25.Trefz FM, Feist M, Lorenz I. Hypoglycaemia in hospitalised neonatal calves: Prevalence, associated conditions and impact on prognosis. Vet J. 2016;217:103–108. doi: 10.1016/j.tvjl.2016.10.001. [DOI] [PubMed] [Google Scholar]