Abstract
In this study, whiteleg shrimp (Penaeus vannamei) imported from Vietnam were collected from South Korean markets, and examined for 2 viruses: infectious hypodermal and hematopoietic necrosis virus (IHHNV, recently classified as decapod penstyldensovirus-1), and white spot syndrome virus (WSSV). Among 58 samples, we detected IHHNV in 23 samples and WSSV in 2 samples, using polymerase chain reaction and sequencing analyses. This is the first report of IHHNV and WSSV detection in imported shrimp, suggesting that greater awareness and stricter quarantine policies regarding viruses infecting shrimp imported to South Korea are required.
Keywords: Infectious hypodermal and hematopoietic necrosis virus, white spot syndrome virus, South Korea, quarantine, Vietnam
INTRODUCTION
For several decades, shrimp have ranked as one of the most traded seafood in the world. Shrimp are being cultured in approximately 70 countries around the world, although approximately 80% of farmed shrimp are produced in China, Thailand, Indonesia, Vietnam, Ecuador, and India [1]. These countries now have a significant export trade in shrimp and shrimp products, generating thousands of new jobs and incalculable foreign currency earnings for their national economies [2]. Four species are commonly used in shrimp farming, namely tiger shrimp (Penaeus monodon), whiteleg shrimp (Penaeus vannamei), Atlantic-white shrimp (Penaeus setiferus), and Indian white shrimp (P. indicus). The production of whiteleg shrimp, in particular, has gradually increased, because this species offers numerous advantages, such as the fact that it is domesticated and can be subjected to genetic selection for growth rate and rapid maturation [3].
However, the shrimp trade has been a source of significant environmental disturbances. In addition, it has provided opportunities for the increased pathogenicity of existing infections, the rapid transmission of disease, and the exposure of different countries to new pathogens [4]. In the present study, we monitored 2 viruses; infectious hypodermal and hematopoietic necrosis virus (IHHNV) which was recently classified as decapod penstyldensovirus-1 [5], and white spot syndrome virus (WSSV), in shrimp imported from Vietnam into South Korea.
MATERIALS AND METHODS
For this study, 58 batches of frozen whiteleg shrimp imported from Vietnam (~6–19 g, 40–50 shrimp per batch) were collected from fishery markets in South Korea (3 markets in Inchon, 3 markets in Gwangju, and 3 markets in Busan areas), in 2019 and shipped on ice to the Laboratory of Aquatic Biomedicine, College of Veterinary Medicine, Kyungpook National University (Table 1).
Table 1. Shrimp samples and PCR results from this study.
| Samples | Viral nucleic acids detected by PCR assay | Samples | Viral nucleic acids detected by PCR assay |
|---|---|---|---|
| 19-005-a5 | -* | 19-006-1 | IHHNV |
| 19-005-b5 | IHHNV | 19-006-2 | - |
| 19-005-c5 | - | 19-006-3 | - |
| 19-005-d5 | - | 19-006-4 | IHHNV |
| 19-005-e5 | IHHNV | 19-006-5 | IHHNV |
| 19-005-f5 | - | 19-006-6 | - |
| 19-005-g5 | IHHNV | 19-006-7 | IHHNV |
| 19-005-h5 | IHHNV + WSSV† | 19-006-8 | IHHNV |
| 19-005-B5 | IHHNV | 19-006-9 | IHHNV |
| 19-005-C5 | - | 19-006-10 | - |
| 19-005-D4 | - | 19-006-11 | - |
| 19-005-E5 | IHHNV | 19-006-12 | - |
| 19-005-G5 | IHHNV | 19-006-13 | - |
| 19-005-H5 | IHHNV | 19-006-14 | - |
| 19-004-A1 | - | 19-006-15 | - |
| 19-004-B1 | - | 19-006-16 | - |
| 19-004-C1 | - | 19-006-17 | - |
| 19-004-D1 | IHHNV | 19-006-18 | - |
| 19-004-E1 | - | 19-006-19 | - |
| 19-004-F1 | - | 19-006-20 | IHHNV |
| 19-004-G1 | - | 19-006-21 | - |
| 19-004-H1 | - | 19-006-22 | IHHNV + WSSV |
| 19-004-I1 | - | 19-006-23 | IHHNV |
| 19-004-J1 | IHHNV | 19-006-24 | - |
| 19-004-A2 | - | 19-006-25 | - |
| 19-004-D2 | - | 19-006-26 | - |
| 19-004-F2 | IHHNV | 19-006-27 | IHHNV |
| 19-004-D3 | - | 19-006-28 | IHHNV |
| 19-004-F3 | - | 19-006-29 | IHHNV |
PCR, polymerase chain reaction; IHHNV, infectious hypodermal and hematopoietic necrosis virus; WSSV, white spot syndrome virus.
*Neither IHHNV and WSSV were detected; †The representative amplicons of IHHNV and WSSV were deposited in the GenBank database under accession No. MN481525 and MN481520, respectively.
For DNA extraction, 5 shrimp were randomly selected from each batch and the pleopods (30 mg) were pooled into a single tube. DNA was extracted using DNeasy Blood and Tissue kits (Qiagen, Germany). For IHHNV detection, a polymerase chain reaction (PCR) assay was carried out with primers IHHNV-389F (5′- CGG AAC ACA ACC CGA CTT TA -3′), and IHHNV-389R (5′- GGC CAA GAC CAA AAT ACG AA -3′) designed by Tang and Lightner [6]. For WSSV detection, a PCR assay was carried out with primers WSSV-WSI3 (5′-GTA ACT CCT TCC ATC TCC A -3′) and WSSV-WSI4 (5′-TAC GGC AGC TGC TGC ACC TTG T -3′) designed by Nunan and Lightner [7]. The obtained representative positive amplicons of IHHNV and WSSV were sequenced, and the phylogenetic analyses were respectively conducted using the datasets from different geographical origins with the maximum-likelihood method with 1,000 bootstrap replications using MEGAX software (ver. 10.0).
RESULTS AND DISCUSSION
For live and frozen shrimp sourced from foreign countries and imported into South Korea, the National Fishery Products Quality Management Service conducts quarantine, and checks for 6 viruses (IHHNV, WSSV, infectious myonecrosis virus, Taura syndrome virus, yellow head virus, and Macrobrachium rosenbergii nodavirus), and 1 fungus (Aphanomyces astaci) in accordance with the Aquatic Organism Disease Control Act [8].
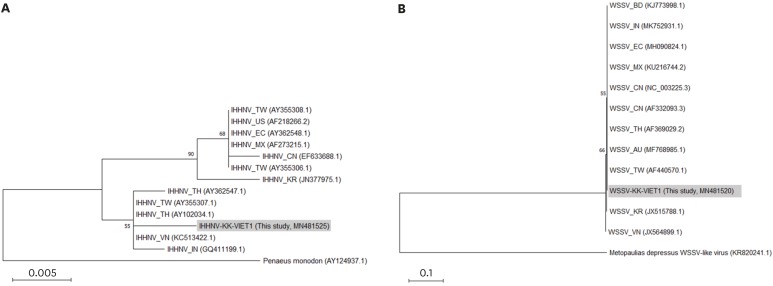
IHHNV causes runt-deformity syndrome in penaeid shrimp, which results in irregularly impaired growth and cuticular deformities [9]. This virus has been detected worldwide in cultured penaeid shrimp, and has been reported in whiteleg shrimp cultured in South Korea since 2010 [9]. However, there are no reports of IHHNV detection in shrimp that are sold in South Korea but originally imported from foreign countries. Interestingly, 39.6% of the tested samples collected in this study were confirmed to be positive for IHHNV (n = 23) when subjected to PCR assay and sequencing analysis (Table 1). The obtained sequences corresponding to the non-structural protein 1 of IHHNV were very similar (> 96% identity) to the reference strain of IHHNV (Penaeus stylirostris penstyldensovirus 1, NC_039043.1) and other IHHNVs detected from different geographical origins, including South Korea and Vietnam. Phylogenetic analysis of the obtained representative IHHNV sequence (IHHNV-KK-VIET1) indicated that the newly detected viral sequence was similar to those reported from Southeast Asian countries including Vietnam (KC513422.1), and clearly differentiated from the previously reported South Korean IHHNV isolate [9], thus suggesting that the IHHNV-positive shrimp samples collected in this study contained different types of IHHNV reported in South Korea (Fig. 1A). The representative sequence was deposited in the GenBank database under accession No. MN481525.
Fig. 1. Phylogenetic analyses of the representative IHHNV (A) and WSSV (B) detected in this study. Maximum-likelihood trees were reconstructed based on the obtained sequences of the IHHNV-positive sample (sample No. 19-005-h5 corresponding to the strain IHHNV-KK-VIET1, MN481525 [390 bp]) and WSSV-positive sample (sample No. 19-005-h5 corresponding to the strain WSSV-KK-VIET1, MN481520 [936 bp]), respectively. Tiger shrimp, Penaeus monodon (AY124937.1), and Jamaican Bromeliad Crab (Metopaulias depressus) WSSV-like virus (KR820241.1) were used as outgroup for IHHNV and WSSV phylogeny, respectively. Numbers at the branches indicate bootstrap values obtained with 1,000 replicates. The scale bar represents 0.005 and 0.1 nucleotide substitutions per site, respectively.
IHHNV, infectious hypodermal and hematopoietic necrosis virus; WSSV, white spot syndrome virus.
In shrimp, WSSV infection can cause up to 100% mortality within a few days under culture conditions, and this virus is responsible for severe economic loss in shrimp farming [10]. In South Korea, WSSV is regarded as the most dangerous shrimp virus, and has been causing mass mortalities with serious economic losses in Korean shrimp farms since 1998 [11]. However, there has been no report on WSSV detection in shrimp that are sold in South Korea, but imported from foreign countries, up to recent. In this study, 3.4% of the tested samples were confirmed to be positive for WSSV (n = 2) when subjected to PCR assay and sequencing analysis (Table 1). The obtained sequences corresponding to the VP664 (ORF 167) of WSSV were almost identical (> 99% identity) to the reference WSSV strain (CN1, NC_003225) and other WSSVs including South Korean (JX515788.1) and Vietnamese (JX564899.1) isolates reported to date, and thus, resultant phylogeny failed to discriminate the origin of the virus (Fig. 1B). The representative sequence was deposited in the GenBank database under accession No. MN481520.
In shrimp farming, co-infections are common. In this study, we found that the 2 samples infected with WSSV (19-006-22 and 19-005-h5) were also co-infected with IHHNV (Table 1). Multiple infections with WSSV and other viruses and, in particular, dual infection with WSSV and IHHNV in whiteleg shrimp, have been reported frequently in shrimp farms [12]. It was also confirmed that co-infection with several pathogens could cause faster and higher mortality in pond cultured shrimp than in those infected with WSSV alone [13]. In ponds, shrimp infected with WSSV at a low level did not always show clinical symptoms, but these shrimps were more susceptible to other pathogens [13].
To the best of our knowledge, this is the first report of IHHNV and WSSV detection in frozen shrimp imported from Vietnam into South Korea. Importantly, in South Korea, frozen shrimp imports have increased gradually to satisfy a growing shrimp demand [14]. However, virus infected-frozen shrimp still have the potential to spread disease, and can cause serious problems in shrimp aquaculture. There are possible virus transmission routes, such as the disposal of untreated liquid or solid wastes from imported shrimp, and the use of imported shrimp as bait by fishermen [15]. Therefore, accurate and strict monitoring of shrimp viruses is required for shrimp imported from foreign countries into South Korea.
Footnotes
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2019R1C1C1006212), the Korean Government Ministry of Trade, Industry, and Energy (N0001822), and DGIST R&D Program of the Ministry of Science and ICT (2020010096).
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Kim JH, Han JE.
- Data curation: Park SC, Park S, Jeon HJ.
- Formal analysis: Lee SC, Kim KY.
- Funding acquisition: Han JE, Choi S.
- Investigation: Park SC, Han S.
- Methodology: Park SC, Han JE.
- Project administration: Kim JH, Han JE.
- Resources: Lee YS.
- Supervision: Kim JH, Han JE.
- Visualization: Park SC, Han S.
- Writing - original draft: Park SC, Choi S, Han S, Kim JH.
- Writing - review & editing: Kim JH, Han JE.
References
- 1.Food and Agriculture Organization of the United Nations. FishStatJ—Software for Fishery Statistical Time Series [Internet] Food and Agriculture Organization of the United Nations; 2016. [updated 2016 July 21]. [cited 2019 October 1]. Available from: http://www.fao.org/fishery/statistics/software/fishstatj/en. [Google Scholar]
- 2.Dastidar PG, Mallik A, Mandal N. Contribution of shrimp diseases research to the development of the shrimp aquaculture industry: an analysis of the research and innovation structure across the countries. Scientometrics. 2013;97:659–674. [Google Scholar]
- 3.Wyban J. World shrimp farming revolution: industry impact of domestication, breeding and widespread use of specific pathogen free Penaeus vannamei. In: Browdy CL, Jory DE, editors. The Rising Tide: Proceedings of the Special Session on Sustainable Shrimp Farming. Baton Rouge: World Aquaculture; 2009. pp. 12–21. [Google Scholar]
- 4.Walker PJ, Mohan CV. Viral disease emergence in shrimp aquaculture: origins, impact and the effectiveness of health management strategies. Aquaculture. 2009;1:125–154. doi: 10.1111/j.1753-5131.2009.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. San Diego: Elsevier Academic Press; 2012. [Google Scholar]
- 6.Tang KF, Lightner DV. Infectious hypodermal and hematopoietic necrosis virus (IHHNV)-related sequences in the genome of the black tiger prawn Penaeus monodon from Africa and Australia. Virus Res. 2006;118:185–191. doi: 10.1016/j.virusres.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Nunan LM, Lightner DV. Optimized PCR assay for detection of white spot syndrome virus (WSSV) J Virol Methods. 2011;171:318–321. doi: 10.1016/j.jviromet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 8.National Fishery Products Quality Management Service. Quarantine of Imported and Exported Aquatic Organisms [Internet] National Fishery Products Quality Management Service; 2019. [updated 2019]. [cited 2019 October 1]. Available from: http://www.nfqs.go.kr/foreign/en/sub1_1.html. [Google Scholar]
- 9.Kim JH, Choresca CH, Jr, Shin SP, Han JE, Jun JW, Han SY, Park SC. Detection of infectious hypodermal and hematopoietic necrosis virus (IHHNV) in Litopenaeus vannamei shrimp cultured in South Korea. Aquaculture. 2011;313:161–164. [Google Scholar]
- 10.Wang Q, White LB, Redman RM, Lightner DV. Per os challenge of Litopenaeus vannamei postlarvae and Farfantepenaeus duorarum juveniles with six geographic isolates of white spot syndrome virus. Aquaculture. 1999;170:179–194. [Google Scholar]
- 11.Park JH, Lee YS, Lee S, Lee Y. An infectious viral disease of penaeid shrimp newly found in Korea. Dis Aquat Organ. 1998;34:71–75. doi: 10.3354/dao034071. [DOI] [PubMed] [Google Scholar]
- 12.Yeh SP, Chen YN, Hsieh SL, Cheng W, Liu CH. Immune response of white shrimp, Litopenaeus vannamei, after a concurrent infection with white spot syndrome virus and infectious hypodermal and hematopoietic necrosis virus. Fish Shellfish Immunol. 2009;26:582–588. doi: 10.1016/j.fsi.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Han JE, Kim JE, Jo HY, Eun JS, Lee CR, Kim JH, Lee KJ, Kim JW. Increased susceptibility of white spot syndrome virus-exposed Penaeus vannamei to Vibrio parahaemolyticus causing acute hepatopancreatic necrosis disease. Aquaculture. 2019;512:734333 [Google Scholar]
- 14.De Silva BC, Hossain S, Wimalasena SH, Pathirana HN, Heo GJ. Putative virulence traits and antibiogram profile of Aeromonas spp. isolated from frozen white‐leg shrimp (Litopenaeus vannamei) marketed in Korea. J Food Saf. 2018;38:e12470 [Google Scholar]
- 15.Lightner DV, Redman RM, Poulos BT, Nunan LM, Mari JL, Hasson KW. Risk of spread of penaeid shrimp viruses in the Americas by the international movement of live and frozen shrimp. Rev Sci Tech. 1997;16:146–160. doi: 10.20506/rst.16.1.1010. [DOI] [PubMed] [Google Scholar]