Abstract
Secondary sclerosing cholangitis in critically ill patients (SSC-CIP) is a recently identified cholestatic liver disease occurring in patients without prior history of hepatobiliary disease, after receiving treatment in the intensive care unit (ICU) in different settings, including cardiothoracic surgery, infection, trauma, and burns. It is a rare entity, being estimated to occur in 1/2,000 patients in an ICU; however, it is a dismal condition, with up to half of the patients dying during the ICU stay and with rapid progression to liver cirrhosis over weeks to months. SSC-CIP should be considered in the differential diagnosis of cholestasis in the ICU, particularly when cholestasis persists after recovery from the critical event. Diagnosis is established with magnetic resonance cholangiopancreatography or endoscopic retrograde cholangiopancreatography showing dilations and stenoses of the intrahepatic bile ducts as well as biliary casts. No available treatment has been shown to slow the rapid progression of the disease, and liver transplant referral should be considered early after the diagnosis of SSC-CIP. Increased awareness and timely diagnosis are crucial in order to improve the current appalling outcome.
Keywords: Secondary sclerosing cholangitis, Critically ill patients, Intensive care unit
Resumo
A colangite esclerosante secundária em doentes críticos (CEP-DC) é uma doença colestàtica, identificada recentemente, que ocorre em doentes sem antecedentes de patologia hepato-biliar, após internamento em unidade de cuidados intensivos (UCI) por diferentes indicações, incluindo: cirurgia cárdio-torácica, infeção, trauma e queimaduras. É uma entidade rara, com uma incidência estimada de um em cada 2,000 doentes em UCI. Ainda assim, tratase de uma doença com um muito mau prognóstico, sendo que até metade dos doentes morre durante o internamento na UCI, e apresenta uma rápida progressão para cirrose em semanas a meses. A CEP-DC deve ser considerada no diagnóstico diferencial de colestase em UCI, particularmente se a colestase persistir após a recuperação do evento potencialmente fatal. O diagnóstico é estabelecido através de colangiopancreatografia por ressonância magnética ou colangiopancreatografia retrógrada endoscópica, que revelam estenoses e dilatações difusas dos canais biliares intra-hepáticos e cilindros biliares. Não está disponível uma terapêutica capazde atrasar a rápida progressão da doença e a referenciação para transplantação hepática deve ser considerada logo após o diagnóstico. Para melhorar o mau prognóstico atual, é essencial um maior reconhecimento e diagnóstico precoce.
Palavras Chave: Colangite esclerosante secundária, Doentes críticos, Unidade de cuidados intensivos
Introduction
Sclerosing cholangitis encompasses a group of progressive cholestatic diseases affecting the intra- and/or extrahepatic bile ducts that can progress to biliary cirrhosis. Primary sclerosing cholangitis (PSC) is an idiopathic disease characterized by a typical beaded appearance in cholangiographic studies, which has a strong association with inflammatory bowel disease [1, 2]. When we identify a cause for sclerosing cholangitis it is dubbed secondary sclerosing cholangitis (SSC). Examples of SSC are autoimmune IgG4-associated, infectious, drug-induced, ischemic, and obstructive [3]. SSC in critically ill patients (SSC-CIP) is a rare form of SSC that was first described by Scheppach et al. in 2001 [4]. SSC-CIP is believed to be ten times less frequent than PSC [5]; only 250 cases have been reported in the literature. Over half of the cases were published in the last 5 years, which reflects increasing awareness of this disease.
The pathogenesis of SSC-CIP is not fully understood, but the main pathogenic mechanisms seems to be ischemia of the bile ducts, changes in bile composition, and biliary infection [6, 7, 8].
SSC-CIP affects patients with no history of previous hepatobiliary disease, after treatment in an intensive care unit (ICU) for different underlying conditions including major surgery, sepsis, and trauma [5, 9]. SSC-CIP should be considered in the differential diagnosis of cholestasis in ICU patients when cholestasis persists beyond successful treatment of the underlying disease [6]. The diagnosis is made by endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP) revealing PSC-like diffuse strictures and dilations of the intrahepatic bile ducts, and filling defects translating biliary casts [8, 10].
SSC-CIP typically has two different presentations: (1) acute liver failure during ICU treatment or (2) persistent cholestasis rapidly progressing to cirrhosis [6, 9, 11, 12]. The prognosis is dire, with around half the patients dying in the ICU and the other half requiring liver transplantation in the following 3–4 years [5, 13].
This review aims to summarize the most recent evidence regarding the pathogenesis, diagnosis, and prognosis of SSC-CIP. Emphasis is placed on differential diagnosis to help in the early diagnosis, which might improve outcomes, allowing earlier palliative endoscopic therapy and referral for liver transplantation.
Epidemiology
Critically ill patients who develop SSC are a very heterogeneous group, which reflects the range of possible underlying diseases that lead to ICU admission. The unifying feature is the lack of previous hepatobiliary disease [5, 14, 15].
Reasons for ICU admission include burns, trauma, acute respiratory distress syndrome, infections, subarachnoid hemorrhage, and major surgery [5, 10, 14, 16]. Cardiothoracic surgery seems to confer a particularly high risk of developing SSC-CIP [9, 17]. The mean age of patients at the time of diagnosis is 50 years, but ranges from 19 to 79 years, which can be explained by the unpredictable nature of some of the reasons for ICU admission [6, 18]. The average length of ICU stay is 30–40 days, though in some cases it may be as short as 9 days [5, 11, 19]. Similarly to PSC, there is a male predominance, with studies reporting a male:female ratio ranging from 2.2:1 to 9:1 [6, 7, 8, 14, 16, 20]. All reported patients required mechanical ventilation during their ICU stay for an average of >30 days, and most patients presented severe hypotension requiring vasopressor treatment [6, 7, 14, 18, 21]. Weig et al. [22] evaluated patients with acute respiratory distress syndrome due to H1N1 pneumonia and found that obesity, increased visceral fat, and longer time spent in prone position were associated with a higher risk of developing SSC-CIP.
Although SSC-CIP is certainly rare, its true prevalence has not been ascertained. In fact, SSC-CIP is an underdiagnosed condition, with half of the patients dying during ICU stay before the diagnosis can be reached [9, 13]. Two retrospective studies found a similar prevalence of SSC-CIP, affecting about 1/2,000 patients admitted to an ICU [7, 14]. Leonhardt et al. [5] identified SSC-CIP as being responsible for 0.61% of all liver transplantations in one hospital, which was ten times less frequent than PSC accounting for 6.2%.
Pathophysiology
The exact mechanisms by which SSC-CIP develops are yet to be understood. Both critical illness and its intensive care treatment seem to contribute to the pathogenesis [7]. The most accepted theory is that ischemia (“ischemic cholangiopathy”) in combination with changes in bile composition (“toxic bile”) lead to necrosis of cholangiocytes and bile cast formation. The resulting biliary obstruction and biliary infection both participate in a process that leads to progressive and irreversible destruction and obliteration of the intrahepatic bile ducts, ultimately leading to secondary biliary cirrhosis [5, 6, 7, 23, 24].
Total parenteral nutrition is common in critically ill patients and can lead to steatosis and cholestatic liver dysfunction when used for >1 week [25, 26]. However, epidemiological studies do not support total parenteral nutrition as a risk factor for SSC-CIP [7].
Idiosyncratic drug-induced liver injury could also promote the development of SSC-CIP. When drug-induced liver injury manifests as a cholestatic or mixed-type pattern in hepatic tests it is dubbed drug-induced cholangiopathy, reflecting damage to the biliary epithelium. Patients with drug-induced cholangiopathy can develop SSC [27, 28]. Several drugs commonly used in the ICU setting have been implicated in drug-induced SSC, including antibiotics and anesthetics such as ketamine [28, 29, 30]. Interestingly, in one case series, 15 out of 16 patients received ketamine prior to developing SSC-CIP [7]. Predisposition to drug-induced cholangiopathy seems to be genetically determined [31].
Ischemic Cholangiopathy
The hepatic parenchyma is supplied by both the hepatic arteries and the portal vein, whereas the common bile duct receives a dual arterial blood supply from both the hepatic artery and branches of the gastroduodenal artery [32]. However, the intrahepatic biliary tree is supplied exclusively by branches of the hepatic artery, which form the intrahepatic peribiliary vascular plexus [33, 34]. This provides the anatomical grounds for intrahepatic bile duct ischemic susceptibility and could explain why the extrahepatic bile duct is usually spared in SSC-CIP [5, 8, 10].
Macrocirculatory compromise seems to be the cornerstone of ischemic bile duct injury, particularly when associated with microcirculatory disturbances directly affecting the peribiliary vascular plexus [7, 33]. Around 33% of ICU patients have hemodynamic instability requiring vasopressors at some time during their stay [35]. In patients who develop SSC-CIP, this number rises to 60–100% [6, 7, 10]. Moreover, the onset of hypotension seems to be temporarily associated with the onset of cholestasis [7]. Previous researchers have hypothesized that use of high-dose vasopressors could promote the development of SSC-CIP [6, 10, 14, 21]. However, this hypothesis is not substantiated by the available data. All vasopressors and inotropes increase systemic blood pressure and cardiac output, but this does not necessarily translate into improved hemodynamics in the hepatosplanchnic territory [36]. Norepinephrine, based on its α-adrenergic agonist effects, has been assumed to induce splanchnic ischemia. However, the experimental data are hard to interpret because studies are very heterogeneous regarding their population and hemodynamic endpoints [36]. For example, norepinephrine has been shown to have no effect on mesenteric blood flow in a septic sheep model, but it decreased mesenteric blood flow in a septic porcine model [37, 38]. In one study with 10 septic human patients, norepinephrine was associated with a higher hepatosplanchnic blood flow to cardiac output ratio as compared with dopamine, resulting in improved hepatocellular energy balance [39]. This result was unexpected since regional vasodilating properties of dopaminergic activation should lead to a higher hepatosplanchnic to cardiac output ratio in dopamine-treated patients. These findings translate the contradictions found in the literature as well as the unreliability of biological plausibility as the sole argument for vasopressor-induced biliary ischemia. More recently, two small retrospective studies failed to demonstrate an association between high-dose vasopressor use and a higher risk of SSC-CIP development [7, 22].
All patients with SSC-CIP received mechanical ventilation, which is believed to contribute to hepatosplanchnic ischemia [18, 21]. In fact, mechanical ventilation with positive end-expiratory pressure >10 cm H2O, prone position, and low tidal volumes were associated with negative effects on hepatosplanchnic blood flow in animal models [40, 41, 42]. Prone position in particular seems to be associated with the development of SSC-CIP in humans [22].
Leonhardt et al. [7] showed that all SSC-CIP patients presented at least one factor capable of disrupting the microcirculation blood flow, namely increased blood viscosity, red blood cell transfusions, and/or hypercoagulable states. Moreover, two different groups also supported the association with higher number of red blood cell units transfused [8, 10].
In summary, disturbances in the arterial supply of the peribiliary vascular plexus lead to necrosis of cholangiocytes with formation of biliary casts and inflammation/scarring of the bile ducts, which results in cholestasis [6, 17]. Biliary casts have also been described following orthotopic liver transplantation, in which two different types of casts have been identified based on their biochemical composition. One type is mainly composed of bilirubin (10–50%) and bile acids (10–15%) and is thought to arise due to mechanical obstruction. The other type is mainly composed of proteins, mostly collagen, and seems to be derived from necrotic cholangiocytes [43, 44]. Biliary casts in SSC-CIP are mainly composed of proteins and can be seen in the first weeks after the onset of cholestasis, further supporting ischemia as the primary insult in these patients [6].
The above considerations place SSC-CIP as a result of ischemic injury of the biliary tree. Whereas in the posttransplant setting and after chemoembolization biliary tree ischemia seems to be a result of local disturbances in the arterial supply of the biliary tree, in SSC-CIP, ischemia seems to be a consequence of systemic hemodynamic instability in critically ill patients.
Toxic Bile
Under physiological circumstances, cholangiocytes are protected from toxic hydrophobic bile salts through defense mechanisms that rely on hepatobiliary transporters. The formation of mixed micelles of bile salts is one such mechanism, and it is dependent on biliary phospholipid secretion by hepatocytes via MDR3/ABCB4 [24, 45]. Genetic defects with impaired MDR3/ABCB4 activity have been linked to cholestatic and ductopenic liver disease in humans [46]. It has been shown that MDR2 (human orthologue: MDR3) knockout mice develop sclerosing cholangitis secondary to complete absence of phospholipids from bile [47, 48]. Ischemia can negatively affect hepatobiliary transporters promoting cell injury and cholestasis [49, 50]. Trauner et al. [24] hypothesized that genetic variants with low MDR3 expression might predispose to the formation of toxic bile under ischemic or inflammatory conditions, explaining why some critically ill patients with cholestasis develop SSC-CIP. Another important mechanism of defense is the secretion of HCO3− via AE2, which maintains a high pH near the apical surface of cholangiocytes, capable of preventing permeation of protonated bile acids. Beuers et al. [45] theorized that loss of this protective mechanism due to ischemia is implicated in SSC-CIP. Indeed, proinflammatory cytokines inhibit AE2 activity in animal models [51].
Systemic inflammatory response syndrome seems to also play a role. It occurs in >50% of ICU patients [52]. Interestingly, Leonhardt et al. [7] found that all 16 of their SSC-CIP patients developed systemic inflammatory response syndrome prior to diagnosis. These findings suggest that inflammatory stress also promotes the development of toxic bile, which further contributes to cholangiocyte necrosis.
Biliary Infection
Few case reports have described SSC following a single severe episode of bacterial cholangitis; however, the development of SSC after recurrent bacterial cholangitis in the context of chronic biliary obstruction is much more frequent [53, 54].
Biliary obstruction is a prerequisite for bacterial cholangitis because it abrogates the antibacterial effects of bile flow and the biliary secretion of IgA [55]. In SSC-CIP, biliary obstruction is the result of the development of biliary casts, which are present since the first weeks of cholestasis. As the disease progresses, the obstruction is perpetuated due to the development of multifocal intrahepatic biliary strictures [5, 6]. A meta-analysis found that bacteria and/or Candida species are detectable in the bile collected during ERCP in 98% of SSC-CIP patients [21]. Enterococci and Candida albicans are the most common agents, which frequently present a high rate of antibiotic resistance, presumably a reflection of the high rate of previous antibiotic treatment in critically ill patients [56]. Recurrent bacterial cholangitis is common in SSC-CIP patients and is associated with the progression to cirrhosis [5, 6, 19, 21]. The role of the microbiome is also highlighted by the recently described association between NOD2 gene mutations and higher susceptibility for developing SSC-CIP [57]. NOD2 is a pattern recognition receptor that regulates the gut-microbiome homeostasis and plays a key role in bacterial translocation. NOD2 gene mutations had already been identified as risk factors for Crohn's disease and spontaneous bacterial peritonitis in patients with liver cirrhosis [57, 58]. The pathophysiology of SSC-CIP is summarized in Figure 1.
Fig. 1.
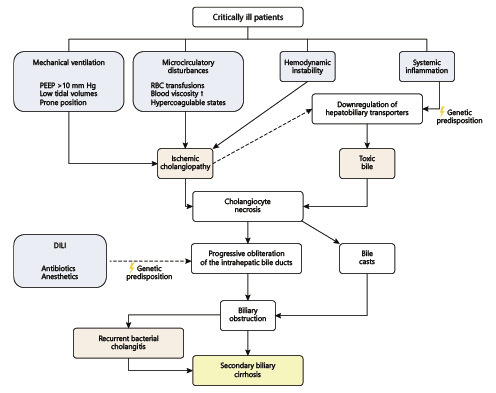
Schematic representation of the pathophysiology of SSC-CIP. DILI, drug-induced liver injury; PEEP, positive end-expiratory pressure; RBC, red blood cell; SSC-CIP, secondary sclerosing cholangitis in critically ill patients.
Diagnosis
The diagnosis of SSC-CIP is difficult for several reasons: (1) it is an underrecognized entity; (2) it is asymptomatic in the early stages, manifesting only as a laboratorial cholestatic pattern; (3) the differential diagnosis of cholestasis in ICU patients is vast, being secondary to SSC-CIP in only a minority of patients; (4) the diagnosis can only be established by MRCP/ERCP; (5) mortality is high during ICU treatment, often precluding a timely diagnosis [5, 10, 59].
Laboratory Parameters
SSC-CIP manifests with a pattern of cholestasis. Gamma-glutamyl transpeptidase increases first, around 7–9 days after the initial insult, and is followed a few days later by alkaline phosphatase elevation. Bilirubin is the last to rise, taking around 20 days. Gamma-glutamyl transpeptidase elevation is also more pronounced, peaking at around 20–50 times the upper limit of normal (ULN), while alkaline phosphatase and bilirubin elevation reach a maximum of 15 times the ULN [5, 8]. Conversely, alanine aminotransferase and aspartate aminotransferase show only a moderate increase of up to three times the ULN [18]. Interestingly, bilirubin levels may spontaneously decrease after 2–6 months while SSC-CIP keeps progressing [10]. In one study there was a significant increase of cholesterol levels in SSC-CIP patients, around 2.5 times the ULN, differentiating it from PSC, in which hypercholesterolemia is uncommon [5, 60].
The differential diagnosis for cholestasis in the ICU setting is extensive, most notably sepsis-induced cholestasis, total parenteral nutrition, choledocholithiasis, and drug-induced liver injury [25, 59]. Hypoxic liver injury, although mostly characterized by hepatocellular necrosis, still merits discussion. Indeed, hypoxic liver injury affects 10% of ICU patients, and it is also associated with shock and manifests as jaundice in one-third of patients [61]. The different characteristics of these diseases are shown in Table 1 and a diagnostic algorithm is presented in Figure 2.
Table 1.
Differential diagnosis of SSC-CIP in the ICU setting
| Diagnosis | Incidence in ICU patients | Clinical features and laboratory tests | Diagnosis | Treatment | Prognosis | References |
|---|---|---|---|---|---|---|
| Sepsis-induced cholestasis | 20% (most common cause of cholestasis in the ICU) | sepsis (mostly gram-negative); biphasic pattern: initial elevation of ALT/AST, followed by elevation of bilirubin; ALP and GGT may be normal | biphasic laboratory pattern in the setting of positive blood cultures (usually gram-negative) | aggressive antimicrobial treatment; circulatory and ventilatory support | two-fold increase in mortality compared to sepsis alone; cholestasis is reversible | 25, 73, 74 |
| TPN-associated cholestasis | 3% | TPN >1 week, RUQ and hepatomegaly; mixed pattern with cholestasis and hepato-cellular necrosis | cholestasis in the setting of TPN after exclusion of other causes | avoidance of excessive calories and appropriate dosing and formulation of lipids; discontinuation or cycling of TPN if feasible | liver dysfunction is self-limited but may progress to steatohepatitis and cirrhosis if TPN >6 months | 25, 26 |
| Choledocholithiasis | − | RUQ pain accompanied by nausea and vomiting; rise in ALT/AST followed by ALP and bilirubin; INR may be elevated; transient | US and CT scan may reveal dilated bile ducts and duct stones in the initial evaluation; diagnosis is usually confirmed by MRCP or EUS | ERCP with sphincterotomy and stone extraction | benign, but may complicate with acute pancreatitis and bacterial cholangitis | 62, 75 |
| DILI | − | idiosyncratic drug reaction (commonly antibiotics and anesthetics); hepatocellular, cholestatic, or mixed pattern; cholestatic pattern more common in patients aged >60 years, associated with antibiotics | establishment of causal relationship according to clinical scores such as RUCAM and Maria & Vitorino | rapid removal of the offending drug; UDCA may be beneficial in cholestatic DILI | mostly benign, but may lead to acute-liver failure requiring transplantation; cholestasis may persist for months | 76–79 |
| HLI | 10% | occurs in the setting of cardiac, respiratory, or circulatory failure, typically in the first 48 h after admission; rapid rise in ALT/AST >20× ULN with return to baseline within 1 week; cholestasis is seen in one-third of patients | clinical | correction of the underlying cause of ischemic injury; circulatory and ventilatory support | depends on underlying comorbidities, but mortality is >50% | 59, 61, 80 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CT, computed tomography; DILI, drug-induced liver injury; ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasound; GGT, gamma-glutamyl transpeptidase; HLI, hypoxic liver injury; ICU, intensive care unit; INR, international normalized ratio; MRCP, magnetic resonance cholangiopancreatography; RUCAM, Roussel Uclaf Causality Assessment Method; RUQ, right upper quadrant; SSC-CIP, secondary sclerosing cholangitis in critically ill patients; TPN, total parenteral nutrition; UDCA, ursodeoxycholic acid; ULN, upper limit of normal; US, ultrasound.
Fig. 2.
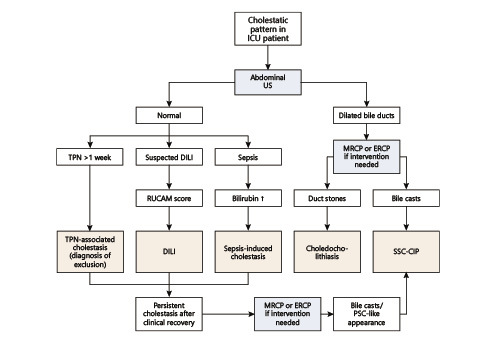
Diagnostic approach to cholestasis in the ICU. DILI, drug-induced liver injury; ERCP, endoscopic retrograde cholangiopancreatography; ICU, intensive care unit; MRCP, magnetic resonance cholangiopancreatography; PSC, primary sclerosing cholangitis; RUCAM, Roussel Uclaf Causality Assessment Method; SSC-CIP, secondary sclerosing cholangitis in critically ill patients; TPN, total parenteral nutrition; US, ultrasound.
The single most important aspect that distinguishes SSC-CIP is the persistence of cholestasis beyond clinical recovery, which reflects irreversible anatomical damage rather than transient functional impairment [25, 54].
Imaging Studies
The first diagnostic study in a patient with cholestasis is an abdominal ultrasound (US), which can rule out other diagnoses such as choledocholithiasis, although lacking sensitivity for SSC-CIP [62]. Indeed, abdominal US suggested the diagnosis of SSC-CIP in only 30–40% of patients [5, 9]. This low sensitivity is due to the fact that echogenic biliary casts assume the shape of the biliary tree, mimicking a normal bile duct system. Hence, a normal abdominal US should not exclude further testing when SSC-CIP is suspected (i.e., when cholestasis persists beyond recovery from the ICU or when cholangitis develops) [9].
MRCP is the imaging method of choice following abdominal US since it is noninvasive and can accurately diagnose SSC-CIP. In the early stages, MRCP presents defects in the intrahepatic biliary tree corresponding to biliary casts and biliary leakages, occasionally forming bilomas. At later stages, diffuse intrahepatic bile duct strictures are observed. Notably, the distal common bile duct is preserved at all stages [8]. MRCP has some limitations: (1) it does not allow for interventional procedures, and (2) it is limited in patients with heart medical devices [9].
ERCP is the gold standard for the diagnosis of SSC-CIP [5, 6, 10, 14]. However, most studies report a delay of around 60 days until ERCP is performed, though more recent studies reported lower delay of up to 25 days [5, 9, 14, 63]. This delay might be attributable to several factors: (1) lack of awareness of SSC-CIP, (2) dilated bile ducts on US (that would prompt the realization of ERCP) occur in <50% of cases, and (3) SSC-CIP is seldom misdiagnosed as sepsis-induced cholestasis (the most common cause of cholestasis in the ICU) [5, 54, 64]. As such, it is often not until cholestasis fails to resolve after clinical recovery that the clinical suspicion of SSC-CIP becomes significant enough to merit an ERCP, inevitably leading to a delayed diagnosis [9, 54].
Similarly to MRCP, early ERCP findings consist of intraductal filling defects in the intrahepatic bile ducts due to biliary casts. As the disease progresses, diffuse irregular strictures and dilations with a typical beaded appearance become evident. In later stages the peripheral intrahepatic bile ducts are completely obliterated, leaving only a central biliary system that Leonhardt et al. described as a “pruned tree” [5, 6]. Concomitant extrahepatic bile duct involvement occurs in around 20% of cases, but it is always mild. In 6% of cases the strictures are confined to the extrahepatic bile ducts [5, 9]. During ERCP it is important to collect bile samples for microbiological examination, since in 98% of patients a pathogen is identified, allowing for guided antimicrobial therapy [21].
The differential diagnosis based on radiographic findings includes PSC and other forms of SSC. Differentiation between SSC-CIP and other forms of sclerosing cholangitis heavily relies on the clinical history that allows for the identification of the primary insult. However, some radiological features suggest SSC-CIP, namely sparing of the extrahepatic bile ducts and presence of biliary casts [5, 65]. In fact, biliary casts seem to be exclusive to SSC-CIP and ischemic sclerosing cholangitis [3, 5]. Ischemic sclerosing cholangitis is mostly associated with post-orthotopic liver transplantation hepatic artery thrombosis and hepatic arterial infusion of floxuridine in the context of colorectal liver metastases [54]. The different forms of SSC and their typical features are shown in Table 2.
Table 2.
Different forms of SSC with typical clinical and imaging findings
| Etiology | Cause | Clinical features | US/CT scan | ERCP/MRCP | References |
|---|---|---|---|---|---|
| Obstructive | − choledocholithiasis − neoplasia − gastroduodenal/hepatic arterial aneurysms − biliary strictures following surgical trauma |
may occur as recurrent/persistent bacterial cholangitis; increased bilirubin | dilated bile ducts; CBD stones; pancreatic or cholangiocarcinoma | intraductal stones; evidence of extrinsic compression | 3, 54 |
| Infectious | AIDS cholangiopathy: − cryptosporidiosis − CMV − microsporidiosis |
CD4+ <100/mm3; other opportunistic infections; HAART with restoration of immune function is the only treatment, antimicrobials are ineffective | intra- and/or extrahepatic bile duct dilation, hyperechoic echogenic nodules at the distal end of the CBD | papillary stenosis; typical beaded appearance is seen in 20% | 54, 81 |
| Immunologic | IgG4-related disease | serum IgG4 ↑; associated with type 1 autoimmune pancreatitis in 90%; responds to glucocorticoids | bile duct wall thickening; pancreatic enlargement or other findings of IgG4-related disease | dilation following long and continuous stricture (>10 mm); narrowing of the main pancreatic duct | 82, 83 |
| Ischemic | − post-OLT hepatic artery thrombosis − hepatic intra-arterial chemotherapy (TACE) |
liver-transplanted patients; liver metastases in patients with colorectal cancer; BCLC stage B hepatocellular carcinoma | dilated bile ducts; US may reveal bilomas | biliary casts; middle third of the common bile duct > hepatic duct confluence > intrahepatic bile ducts | 33, 54 |
| Drug-induced | − amoxicillin clavulanate − ketamine − celecoxib − others |
extrahepatic manifestations of intolerance; mostly reversible with discontinuation of the offending drug | dilated bile ducts; hydronephrosis is commonly seen in ketamine abuse | mainly right, left, and common hepatic duct involvement | 3, 27, 30 |
| Critically ill patients | ischemic bile duct injury | persistent cholestasis in patients surviving ICU treatment | US is normal in >50% of cases; CT and US may reveal intrahepatic bile duct dilation | sparing of the extrahepatic bile ducts; biliary casts; “pruned tree” appearance | 5, 65 |
BCLC, Barcelona Clinic Liver Cancer; CBD, common bile duct; CMV, cytomegalovirus; CT, computed tomography; ERCP, endoscopic retrograde cholangiopancreatography; HAART, highly active antiretroviral therapy; ICU, intensive care unit; MRCP, magnetic resonance cholangiopancreatography; OLT, orthotopic liver transplantation; SSC, secondary sclerosing cholangitis; TACE, transarterial chemoembolization; US, ultrasound.
Histopathology
Liver histology has limited diagnostic value because the early features are nonspecific and consistent with chronic bile duct obstruction [6]. Liver biopsy suggests SSC-CIP in only one-third of patients [9]. Furthermore, there seems to be no correlation between histological findings and laboratory values. Nonetheless, liver biopsy can be useful by excluding other conditions [16].
Histological findings can be localized in the portal/periportal areas and in the acini. In the early stages only the portal/periportal areas are affected, with edema of the small and medium portal tracts, mild inflammatory infiltrates consisting mostly of lymphocytes with occasional neutrophils, and cytological changes in the interlobular bile ducts (cytoplasmic vacuolization and loss of polarization) [6, 16]. As the disease progresses, marginal ductular proliferation, ductular metaplasia of periportal hepatocytes, and portal/periportal fibrosis can occur. Some patients present bile thrombi [6, 10, 16]. Only in later stages can the acini be affected, with bilirubinostasis, hepatocellular rosette formation, and cholestatic necrosis. Eventually, it can progress to biliary fibrosis and secondary biliary cirrhosis [66]. Esposito et al. [16] hypothesized that portal bile duct damage is the primary insult, which leads to inflammation and ductular proliferation in the portal/periportal area and ultimately can induce the parenchymal changes.
Natural History of Disease and Prognosis
Persistent cholestasis in patients surviving a life-threatening event is what clinically defines SSC-CIP [10]. SSC-CIP has an unspecific presentation but can have a dramatic course, with mortality rates reaching 50% during ICU treatment. Mortality is associated with renal failure and higher Model for End-Stage Liver Disease (MELD) scores [9, 13]. The cause for ICU admission also affects mortality, with burns and trauma in previously healthy patients being associated with better outcomes [8].
Typical sclerosing cholangitis manifestations, such as jaundice, pruritus, and abdominal discomfort, only occur in advanced disease in patients who survived the ICU period [54]. Severe weight loss occurs in most patients within the first year, with an average loss of 18 kg, in contrast with PSC, where weight loss only occurs in one-third of patients [5, 67]. Recurrent episodes of bacterial cholangitis secondary to bile duct destruction are common, with biliary stenoses impairing bile flow and limiting the effect of antibiotic treatment. Indeed, biliary sepsis is an important cause of death in these patients [5, 17].
Progression to liver cirrhosis can occur rapidly over a period of months, and in some patients it takes as little as weeks [5, 10, 63]. This rapid progression translates into an exceptionally high mortality. Transplant-free survival is 55% after 1 year and only 14% after 6 years. SSC-CIP median transplant-free survival is 13–44 months, which contrasts with 89 months for PSC and 72 months for SSC in general [5, 17, 68]. On the other hand, while in PSC cholangiocarcinoma occurs in 7–13% of patients, there are no reports of cholangiocarcinoma in SSC-CIP patients [1, 68, 69, 70]. This might be explained by the short life expectancy and the short follow-up in the studies so far.
The most common cause of death is hepatic failure, which occurs in 36% of patients. Out of the 60% of surviving patients, approximately 40% develop biliary cirrhosis and remain in stable condition, while the other 20% progress to end-stage liver disease requiring liver transplantation [18].
Treatment
Endoscopic removal of biliary casts and sphincterotomy improve biliary drainage, resulting in transient clinical and biochemical improvement even when biliary cirrhosis has already occurred [6, 15, 23]. Endoscopic balloon dilation and intermittent stenting of dominant stenoses also seem to improve cholestasis. However, in most cases this approach is not feasible because of the multifocal and intrahepatic localization of the stenoses [10, 15, 23]. Repeated endoscopic interventions are often necessary as biliary casts may recur [5, 11, 65]. Despite the transient improvement, disease progression seems inevitable and the outcome of patients is not affected by endoscopic therapy [16, 17].
Ursodeoxycholic acid is commonly used in an effort to improve bile flow [6, 11, 15, 23, 65]. It has not been studied in clinical trials in SSC-CIP, but its efficacy seems to be limited [10, 17].
Recurrent episodes of cholangitis are treated with endoscopic therapy to alleviate obstruction and antimicrobials. The antimicrobial therapy should be adjusted based on microbiological analysis, and it should be extended for 2 weeks [21, 55]. In many cases, biliary drainage is inadequate because ERCP cannot access excluded peripheral bile ducts, limiting the effectiveness of the treatment [5].
When SSC-CIP progresses and biliary cirrhosis develops, orthotopic liver transplantation is the only curative treatment [23]. Urgent liver transplantation may be required during ICU stay due to acute liver failure [6]. Up to 75% of SSC-CIP patients are referred for liver transplantation within the first year after the diagnosis [5]. The MELD score is widely used in Europe to guide the allocation of liver grafts and has prognostic value in SSC-CIP [9, 71]. However, MELD alone may not be a good measurement of the urgency for liver transplantation, since many patients with SSC-CIP maintain stable coagulation and liver function despite a dismal evolution, thus delaying transplantation [13]. In the case of PSC, recurrent bacterial cholangitis confers higher priority in the form of MELD exception points [72]. In a study by Leonhardt et al. [5], 2 out of 16 patients died of biliary sepsis while on the transplant waiting list, so probably the same rationale should be applied for SSC-CIP. Survival rates after liver transplantation are comparable to those of patients transplanted due to alcoholic liver cirrhosis, with 1- and 3-year survival rates around 90 and 85%, respectively [5, 13]. Most deaths after liver transplantation occur within the first year, sepsis being the main cause of death. Previously healthy trauma patients seem to have a better prognosis after liver transplant [63].
Conclusion
SSC-CIP is an underdiagnosed entity that poses a great challenge to both intensivists and gastroenterologists. In SSC-CIP both critical illness and ICU treatment are responsible for ischemic injury of the biliary tree that, together with changes in bile composition, leads to the formation of biliary casts and stricturing, with subsequent persistent bacterial infection driving the rapid progression of the disease. SSC-CIP shows a typical cholestatic pattern that persists after recovery from the critical illness, which should be a hint to differentiate from other causes of cholestasis in the ICU. The diagnosis requires MRCP/ERCP, but it is often delayed due to unawareness of the disease and unspecific presentation. SSC-CIP has a dismal prognosis with high mortality rates during the ICU stay and rapid progression to liver cirrhosis requiring liver transplantation. Medical treatment is lacking, and endoscopic interventions allow only for palliative treatment, hence the diagnosis of SSC-CIP should prompt early referral for liver transplantation.
Disclosure Statement
The authors have no conflicts of interest to declare.
Author Contributions
P. Martins wrote the review. M. Verdelho Machado did the writing and corrected the review.
References
- 1.Williamson KD, Chapman RW. Primary sclerosing cholangitis: a clinical update. Br Med Bull. 2015 Jun;114((1)):53–64. doi: 10.1093/bmb/ldv019. [DOI] [PubMed] [Google Scholar]
- 2.Mohammad Alizadeh AH, Shahnazi A, Rasoulzadeh A, Shams E, Mohammadi M, Darabi F, et al. Characteristic Findings of Primary Sclerosing Cholangitis on Endoscopic Retrograde Cholangiography: Which is the Most Common Finding? Clin Med Insights Gastroenterol. 2011 Dec;5:1–4. doi: 10.4137/CGast.S7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooling J, Leal R. Secondary Sclerosing Cholangitis: a Review of Recent Literature. Curr Gastroenterol Rep. 2017 Sep;19((9)):44. doi: 10.1007/s11894-017-0583-8. [DOI] [PubMed] [Google Scholar]
- 4.Scheppach W, Druge G, Wittenberg G, Mueller JG, Gassel AM, Gassel HJ, et al. Sclerosing cholangitis and liver cirrhosis after extrabiliary infections: report on three cases. Crit Care Med. 2001 Feb;29((2)):438–41. doi: 10.1097/00003246-200102000-00042. [DOI] [PubMed] [Google Scholar]
- 5.Leonhardt S, Veltzke-Schlieker W, Adler A, Schott E, Eurich D, Faber W, et al. Secondary Sclerosing Cholangitis in Critically Ill Patients: Clinical Presentation, Cholangiographic Features, Natural History, and Outcome: A Series of 16 Cases. Medicine (Baltimore) 2015 Dec;94((49)):e2188. doi: 10.1097/MD.0000000000002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gelbmann CM, Rümmele P, Wimmer M, Hofstädter F, Göhlmann B, Endlicher E, et al. Ischemic-like cholangiopathy with secondary sclerosing cholangitis in critically ill patients. Am J Gastroenterol. 2007 Jun;102((6)):1221–9. doi: 10.1111/j.1572-0241.2007.01118.x. [DOI] [PubMed] [Google Scholar]
- 7.Leonhardt S, Veltzke-Schlieker W, Adler A, Schott E, Hetzer R, Schaffartzik W, et al. Trigger mechanisms of secondary sclerosing cholangitis in critically ill patients. Crit Care. 2015 Mar;19((1)):131. doi: 10.1186/s13054-015-0861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laurent L, Lemaitre C, Minello A, Plessier A, Lamblin G, Poujol-Robert A, et al. Cholangiopathy in critically ill patients surviving beyond the intensive care period: a multicentre survey in liver units. Aliment Pharmacol Ther. 2017 Dec;46((11-12)):1070–6. doi: 10.1111/apt.14367. [DOI] [PubMed] [Google Scholar]
- 9.Voigtländer T, Negm AA, Schneider AS, Strassburg CP, Manns MP, Wedemeyer J, et al. Secondary sclerosing cholangitis in critically ill patients: model of end-stage liver disease score and renal function predict outcome. Endoscopy. 2012 Nov;44((11)):1055–8. doi: 10.1055/s-0032-1325733. [DOI] [PubMed] [Google Scholar]
- 10.Benninger J, Grobholz R, Oeztuerk Y, Antoni CH, Hahn EG, Singer MV, et al. Sclerosing cholangitis following severe trauma: description of a remarkable disease entity with emphasis on possible pathophysiologic mechanisms. World J Gastroenterol. 2005 Jul;11((27)):4199–205. doi: 10.3748/wjg.v11.i27.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reichert MC, Jüngst C, Grünhage F, Lammert F, Krawczyk M. Secondary sclerosing cholangitis rapidly leading to liver cirrhosis: a possible post-ICU treatment sequel. QJM. 2016 Feb;109((2)):119–20. doi: 10.1093/qjmed/hcv119. [DOI] [PubMed] [Google Scholar]
- 12.Zack F, Nizze H, Blaas V, Port A, Büttner A. Secondary sclerosing cholangitis in critically ill patients after a traffic accident-a new entity that should be considered in death classification. Int J Legal Med. 2018 Nov;132((6)):1729–32. doi: 10.1007/s00414-018-1801-0. [DOI] [PubMed] [Google Scholar]
- 13.Voigtländer T, Jaeckel E, Lehner F, Manns MP, Lankisch TO. Liver transplantation for critically Ill patients with secondary sclerosing cholangitis: outcome and complications. Liver Transpl. 2015 Oct;21((10)):1295–9. doi: 10.1002/lt.24192. [DOI] [PubMed] [Google Scholar]
- 14.Schade I, Radakovic D, Hoffmann J, Sommer SP, Stefenelli U, Schimmer C, et al. Secondary sclerosing cholangitis in cardiac surgical patients: A complication with a dismal prognosis. J Thorac Cardiovasc Surg. 2017 Sep;154((3)):906–12. doi: 10.1016/j.jtcvs.2017.04.041. [DOI] [PubMed] [Google Scholar]
- 15.Jaeger C, Mayer G, Henrich R, Gossner L, Rabenstein T, May A, et al. Secondary sclerosing cholangitis after long-term treatment in an intensive care unit: clinical presentation, endoscopic findings, treatment, and follow-up. Endoscopy. 2006 Jul;38((7)):730–4. doi: 10.1055/s-2006-925241. [DOI] [PubMed] [Google Scholar]
- 16.Esposito I, Kubisova A, Stiehl A, Kulaksiz H, Schirmacher P. Secondary sclerosing cholangitis after intensive care unit treatment: clues to the histopathological differential diagnosis. Virchows Arch. 2008 Oct;453((4)):339–45. doi: 10.1007/s00428-008-0654-1. [DOI] [PubMed] [Google Scholar]
- 17.Kulaksiz H, Heuberger D, Engler S, Stiehl A. Poor outcome in progressive sclerosing cholangitis after septic shock. Endoscopy. 2008 Mar;40((3)):214–8. doi: 10.1055/s-2007-967024. [DOI] [PubMed] [Google Scholar]
- 18.Lin T, Qu K, Xu X, Tian M, Gao J, Zhang C, et al. Sclerosing cholangitis in critically ill patients: an important and easily ignored problem based on a German experience. Front Med. 2014 Mar;8((1)):118–26. doi: 10.1007/s11684-014-0306-6. [DOI] [PubMed] [Google Scholar]
- 19.Gudnason HO, Björnsson ES. Secondary sclerosing cholangitis in critically ill patients: current perspectives. Clin Exp Gastroenterol. 2017 Jun;10:105–11. doi: 10.2147/CEG.S115518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molodecky NA, Kareemi H, Parab R, Barkema HW, Quan H, Myers RP, et al. Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology. 2011 May;53((5)):1590–9. doi: 10.1002/hep.24247. [DOI] [PubMed] [Google Scholar]
- 21.Kirchner GI, Rümmele P. Update on Sclerosing Cholangitis in Critically Ill Patients. Viszeralmedizin. 2015 Jun;31((3)):178–84. doi: 10.1159/000431031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weig T, Schubert MI, Gruener N, Dolch ME, Frey L, Miller J, et al. Abdominal obesity and prolonged prone positioning increase risk of developing sclerosing cholangitis in critically ill patients with influenza A-associated ARDS. Eur J Med Res. 2012 Dec;17((1)):30. doi: 10.1186/2047-783X-17-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engler S, Elsing C, Flechtenmacher C, Theilmann L, Stremmel W, Stiehl A. Progressive sclerosing cholangitis after septic shock: a new variant of vanishing bile duct disorders. Gut. 2003 May;52((5)):688–93. doi: 10.1136/gut.52.5.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trauner M, Fickert P, Wagner M. MDR3 (ABCB4) defects: a paradigm for the genetics of adult cholestatic syndromes. Semin Liver Dis. 2007 Feb;27((1)):77–98. doi: 10.1055/s-2006-960172. [DOI] [PubMed] [Google Scholar]
- 25.Aronsohn A, Jensen D. Hepatobiliary manifestations of critically ill and postoperative patients. Clin Liver Dis. 2011 Feb;15((1)):183–97. doi: 10.1016/j.cld.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Grau T, Bonet A, Rubio M, Mateo D, Farré M, Acosta JA, et al. Working Group on Nutrition and Metabolism of the Spanish Society of Critical Care Liver dysfunction associated with artificial nutrition in critically ill patients. Crit Care. 2007;11((1)):R10. doi: 10.1186/cc5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Visentin M, Lenggenhager D, Gai Z, Kullak-Ublick GA. Drug-induced bile duct injury. Biochim Biophys Acta Mol Basis Dis. 2018 Apr;1864((4 4 Pt B)):1498–506. doi: 10.1016/j.bbadis.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 28.Gudnason HO, Björnsson HK, Gardarsdottir M, Thorisson HM, Olafsson S, Bergmann OM, et al. Secondary sclerosing cholangitis in patients with drug-induced liver injury. Dig Liver Dis. 2015 Jun;47((6)):502–7. doi: 10.1016/j.dld.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Turkish A, Luo JJ, Lefkowitch JH. Ketamine abuse, biliary tract disease, and secondary sclerosing cholangitis. Hepatology. 2013 Aug;58((2)):825–7. doi: 10.1002/hep.26459. [DOI] [PubMed] [Google Scholar]
- 30.Lo RS, Krishnamoorthy R, Freeman JG, Austin AS. Cholestasis and biliary dilatation associated with chronic ketamine abuse: a case series. Singapore Med J. 2011 Mar;52((3)):e52–5. [PubMed] [Google Scholar]
- 31.Padda MS, Sanchez M, Akhtar AJ, Boyer JL. Drug-induced cholestasis. Hepatology. 2011 Apr;53((4)):1377–87. doi: 10.1002/hep.24229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Douglass TC, Cutter WW. Arterial blood supply of the common bile duct. Arch Surg. 1948 Oct;57((4)):599–612. doi: 10.1001/archsurg.1948.01240020606015. [DOI] [PubMed] [Google Scholar]
- 33.Deltenre P, Valla DC. Ischemic cholangiopathy. Semin Liver Dis. 2008 Aug;28((3)):235–46. doi: 10.1055/s-0028-1085092. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi S, Nakanuma Y, Matsui O. Intrahepatic peribiliary vascular plexus in various hepatobiliary diseases: a histological survey. Hum Pathol. 1994 Sep;25((9)):940–6. doi: 10.1016/0046-8177(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 35.Sakr Y, Reinhart K, Vincent JL, Sprung CL, Moreno R, Ranieri VM, et al. Does dopamine administration in shock influence outcome? Results of the Sepsis Occurrence in Acutely Ill Patients (SOAP) Study. Crit Care Med. 2006 Mar;34((3)):589–97. doi: 10.1097/01.CCM.0000201896.45809.E3. [DOI] [PubMed] [Google Scholar]
- 36.Woolsey CA, Coopersmith CM. Vasoactive drugs and the gut: is there anything new? Curr Opin Crit Care. 2006 Apr;12((2)):155–9. doi: 10.1097/01.ccx.0000216584.72427.e4. [DOI] [PubMed] [Google Scholar]
- 37.Di Giantomasso D, May CN, Bellomo R. Norepinephrine and vital organ blood flow during experimental hyperdynamic sepsis. Intensive Care Med. 2003 Oct;29((10)):1774–81. doi: 10.1007/s00134-003-1736-9. [DOI] [PubMed] [Google Scholar]
- 38.Krejci V, Hiltebrand LB, Sigurdsson GH. Effects of epinephrine, norepinephrine, and phenylephrine on microcirculatory blood flow in the gastrointestinal tract in sepsis. Crit Care Med. 2006 May;34((5)):1456–63. doi: 10.1097/01.CCM.0000215834.48023.57. [DOI] [PubMed] [Google Scholar]
- 39.Guérin JP, Levraut J, Samat-Long C, Leverve X, Grimaud D, Ichai C. Effects of dopamine and norepinephrine on systemic and hepatosplanchnic hemodynamics, oxygen exchange, and energy balance in vasoplegic septic patients. Shock. 2005 Jan;23((1)):18–24. doi: 10.1097/01.shk.0000150549.45338.6c. [DOI] [PubMed] [Google Scholar]
- 40.Fujita Y. Effects of PEEP on splanchnic hemodynamics and blood volume. Acta Anaesthesiol Scand. 1993 May;37((4)):427–31. doi: 10.1111/j.1399-6576.1993.tb03742.x. [DOI] [PubMed] [Google Scholar]
- 41.Putensen C, Wrigge H, Hering R. The effects of mechanical ventilation on the gut and abdomen. Curr Opin Crit Care. 2006 Apr;12((2)):160–5. doi: 10.1097/01.ccx.0000216585.54502.eb. [DOI] [PubMed] [Google Scholar]
- 42.Paramythiotis D, Kazamias P, Grosomanidis V, Kotzampassi K. Splanchnic ischemia during mechanical ventilation. Ann Gastroenterol. 2008;21((1)):45–52. [Google Scholar]
- 43.Shah JN, Haigh WG, Lee SP, Lucey MR, Brensinger CM, Kochman ML, et al. Biliary casts after orthotopic liver transplantation: clinical factors, treatment, biochemical analysis. Am J Gastroenterol. 2003 Aug;98((8)):1861–7. doi: 10.1111/j.1572-0241.2003.07617.x. [DOI] [PubMed] [Google Scholar]
- 44.Gor NV, Levy RM, Ahn J, Kogan D, Dodson SF, Cohen SM. Biliary cast syndrome following liver transplantation: predictive factors and clinical outcomes. Liver Transpl. 2008 Oct;14((10)):1466–72. doi: 10.1002/lt.21492. [DOI] [PubMed] [Google Scholar]
- 45.Beuers U, Hohenester S, de Buy Wenniger LJ, Kremer AE, Jansen PL, Elferink RP. The biliary HCO(3)(-) umbrella: a unifying hypothesis on pathogenetic and therapeutic aspects of fibrosing cholangiopathies. Hepatology. 2010 Oct;52((4)):1489–96. doi: 10.1002/hep.23810. [DOI] [PubMed] [Google Scholar]
- 46.Gotthardt D, Runz H, Keitel V, Fischer C, Flechtenmacher C, Wirtenberger M, et al. A mutation in the canalicular phospholipid transporter gene, ABCB4, is associated with cholestasis, ductopenia, and cirrhosis in adults. Hepatology. 2008 Oct;48((4)):1157–66. doi: 10.1002/hep.22485. [DOI] [PubMed] [Google Scholar]
- 47.Fickert P, Fuchsbichler A, Wagner M, Zollner G, Kaser A, Tilg H, et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004 Jul;127((1)):261–74. doi: 10.1053/j.gastro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993 Nov;75((3)):451–62. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- 49.Hoekstra H, Tian Y, Jochum W, Stieger B, Graf R, Porte RJ, et al. Dearterialization of the liver causes intrahepatic cholestasis due to reduced bile transporter expression. Transplantation. 2008 Apr;85((8)):1159–66. doi: 10.1097/TP.0b013e31816b2465. [DOI] [PubMed] [Google Scholar]
- 50.Fouassier L, Beaussier M, Schiffer E, Rey C, Barbu V, Mergey M, et al. Hypoxia-induced changes in the expression of rat hepatobiliary transporter genes. Am J Physiol Gastrointest Liver Physiol. 2007 Jul;293((1)):G25–35. doi: 10.1152/ajpgi.00175.2006. [DOI] [PubMed] [Google Scholar]
- 51.Spirlì C, Nathanson MH, Fiorotto R, Duner E, Denson LA, Sanz JM, et al. Proinflammatory cytokines inhibit secretion in rat bile duct epithelium. Gastroenterology. 2001 Jul;121((1)):156–69. doi: 10.1053/gast.2001.25516. [DOI] [PubMed] [Google Scholar]
- 52.Robertson CM, Coopersmith CM. The systemic inflammatory response syndrome. Microbes Infect. 2006 Apr;8((5)):1382–9. doi: 10.1016/j.micinf.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 53.Hoffmeister B, Ockenga J, Schachschal G, Suttorp N, Seybold J. Rapid development of secondary sclerosing cholangitis due to vancomycin-resistant enterococci. J Infect. 2007 Feb;54((2)):e65–8. doi: 10.1016/j.jinf.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 54.Ruemmele P, Hofstaedter F, Gelbmann CM. Secondary sclerosing cholangitis. Nat Rev Gastroenterol Hepatol. 2009 May;6((5)):287–95. doi: 10.1038/nrgastro.2009.46. [DOI] [PubMed] [Google Scholar]
- 55.Zimmer V, Lammert F. Acute Bacterial Cholangitis. Viszeralmedizin. 2015 Jun;31((3)):166–72. doi: 10.1159/000430965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Voigtländer T, Leuchs E, Vonberg RP, Solbach P, Manns MP, Suerbaum S, et al. Microbiological analysis of bile and its impact in critically ill patients with secondary sclerosing cholangitis. J Infect. 2015 May;70((5)):483–90. doi: 10.1016/j.jinf.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 57.Jüngst C, Stadlbauer V, Reichert MC, Zimmer V, Weber SN, Ofner-Ziegenfuß L, et al. NOD2 gene variants confer risk for secondary sclerosing cholangitis in critically ill patients. Sci Rep. 2017 Aug;7((1)):7026. doi: 10.1038/s41598-017-06268-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al Nabhani Z, Dietrich G, Hugot JP, Barreau F. Nod2: the intestinal gate keeper. PLoS Pathog. 2017 Mar;13((3)):e1006177. doi: 10.1371/journal.ppat.1006177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horvatits T, Trauner M, Fuhrmann V. Hypoxic liver injury and cholestasis in critically ill patients. Curr Opin Crit Care. 2013 Apr;19((2)):128–32. doi: 10.1097/MCC.0b013e32835ec9e6. [DOI] [PubMed] [Google Scholar]
- 60.Sinakos E, Abbas G, Jorgensen RA, Lindor KD. Serum lipids in primary sclerosing cholangitis. Dig Liver Dis. 2012 Jan;44((1)):44–8. doi: 10.1016/j.dld.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 61.Jenniskens M, Langouche L, Van den Berghe G. Cholestatic Alterations in the Critically Ill: Some New Light on an Old Problem. Chest. 2018 Mar;153((3)):733–43. doi: 10.1016/j.chest.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 62.Molvar C, Glaenzer B. Choledocholithiasis: Evaluation, Treatment, and Outcomes. Semin Intervent Radiol. 2016 Dec;33((4)):268–76. doi: 10.1055/s-0036-1592329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kirchner GI, Scherer MN, Obed A, Ruemmele P, Wiest R, Froh M, et al. Outcome of patients with ischemic-like cholangiopathy with secondary sclerosing cholangitis after liver transplantation. Scand J Gastroenterol. 2011 Apr;46((4)):471–8. doi: 10.3109/00365521.2010.537683. [DOI] [PubMed] [Google Scholar]
- 64.Chand N, Sanyal AJ. Sepsis-induced cholestasis. Hepatology. 2007 Jan;45((1)):230–41. doi: 10.1002/hep.21480. [DOI] [PubMed] [Google Scholar]
- 65.Kwon ON, Cho SH, Park CK, Mun SH. Biliary cast formation with sclerosing cholangitis in critically ill patient: case report and literature review. Korean J Radiol. 2012 May-Jun;13((3)):358–62. doi: 10.3348/kjr.2012.13.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pollheimer MJ, Fickert P, Stieger B. Chronic cholestatic liver diseases: clues from histopathology for pathogenesis. Mol Aspects Med. 2014 Jun;37:35–56. doi: 10.1016/j.mam.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 67.Wiesner RH, Grambsch PM, Dickson ER, Ludwig J, MacCarty RL, Hunter EB, et al. Primary sclerosing cholangitis: natural history, prognostic factors and survival analysis. Hepatology. 1989 Oct;10((4)):430–6. doi: 10.1002/hep.1840100406. [DOI] [PubMed] [Google Scholar]
- 68.Gossard AA, Angulo P, Lindor KD. Secondary sclerosing cholangitis: a comparison to primary sclerosing cholangitis. Am J Gastroenterol. 2005 Jun;100((6)):1330–3. doi: 10.1111/j.1572-0241.2005.41526.x. [DOI] [PubMed] [Google Scholar]
- 69.Rahman M, Chapel H, Chapman RW, Collier JD. Cholangiocarcinoma complicating secondary sclerosing cholangitis from cryptosporidiosis in an adult patient with CD40 ligand deficiency: case report and review of the literature. Int Arch Allergy Immunol. 2012;159((2)):204–8. doi: 10.1159/000337457. [DOI] [PubMed] [Google Scholar]
- 70.Zhang YA, Shen XZ, Zhu JM, Liu TT. Extensive Metastatic Cholangiocarcinoma Associated With IgG4-Related Sclerosing Cholangitis Misdiagnosed as Isolated IgG4-Related Sclerosing Cholangitis: A Case Report and Literature Review. Medicine (Baltimore) 2015 Nov;94((45)):e2052. doi: 10.1097/MD.0000000000002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu Electronic address eee. EASL Clinical Practice Guidelines: liver transplantation. J Hepatol. 2016 Feb;64((2)):433–85. [Google Scholar]
- 72.Khungar V, Goldberg DS. Liver Transplantation for Cholestatic Liver Diseases in Adults. Clin Liver Dis. 2016 Feb;20((1)):191–203. doi: 10.1016/j.cld.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brienza N, Dalfino L, Cinnella G, Diele C, Bruno F, Fiore T. Jaundice in critical illness: promoting factors of a concealed reality. Intensive Care Med. 2006 Feb;32((2)):267–74. doi: 10.1007/s00134-005-0023-3. [DOI] [PubMed] [Google Scholar]
- 74.Strnad P, Tacke F, Koch A, Trautwein C. Liver - guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 2017 Jan;14((1)):55–66. doi: 10.1038/nrgastro.2016.168. [DOI] [PubMed] [Google Scholar]
- 75.Copelan A, Kapoor BS. Choledocholithiasis: diagnosis and Management. Tech Vasc Interv Radiol. 2015 Dec;18((4)):244–55. doi: 10.1053/j.tvir.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 76.Alempijevic T, Zec S, Milosavljevic T. Drug-induced liver injury: do we know everything? World J Hepatol. 2017 Apr;9((10)):491–502. doi: 10.4254/wjh.v9.i10.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kullak-Ublick GA, Andrade RJ, Merz M, End P, Benesic A, Gerbes AL, et al. Drug-induced liver injury: recent advances in diagnosis and risk assessment. Gut. 2017 Jun;66((6)):1154–64. doi: 10.1136/gutjnl-2016-313369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bjornsson ES, Bergmann OM, Bjornsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013 Jun;144((7)):1419–25. doi: 10.1053/j.gastro.2013.02.006. 1425 e1-3; quiz e19-20. [DOI] [PubMed] [Google Scholar]
- 79.Wree A, Dechêne A, Herzer K, Hilgard P, Syn WK, Gerken G, et al. Steroid and ursodesoxycholic Acid combination therapy in severe drug-induced liver injury. Digestion. 2011;84((1)):54–9. doi: 10.1159/000322298. [DOI] [PubMed] [Google Scholar]
- 80.Lightsey JM, Rockey DC. Current concepts in ischemic hepatitis. Curr Opin Gastroenterol. 2017 May;33((3)):158–63. doi: 10.1097/MOG.0000000000000355. [DOI] [PubMed] [Google Scholar]
- 81.Naseer M, Dailey FE, Juboori AA, Samiullah S, Tahan V. Epidemiology, determinants, and management of AIDS cholangiopathy: A review. World J Gastroenterol. 2018 Feb;24((7)):767–74. doi: 10.3748/wjg.v24.i7.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kamisawa T, Zen Y, Nakazawa T, Okazaki K. Advances in IgG4-related pancreatobiliary diseases. Lancet Gastroenterol Hepatol. 2018 Aug;3((8)):575–85. doi: 10.1016/S2468-1253(18)30121-3. [DOI] [PubMed] [Google Scholar]
- 83.Zen Y, Kawakami H, Kim JH. IgG4-related sclerosing cholangitis: all we need to know. J Gastroenterol. 2016 Apr;51((4)):295–312. doi: 10.1007/s00535-016-1163-7. [DOI] [PubMed] [Google Scholar]


